Abstract
Radiotherapy resistance is an enduring major setback in lung cancer therapy, and is responsible for a large proportion of treatment failures. In previous years, cyclooxygenase-2 (COX-2) has frequently been reported to promote tumor occurrence and development, suggesting a potential role in radiotherapy resistance. To investigate whether COX-2 inhibitors can be applied in radiosensitization, an MTT assay was performed to examine cell viability after X-ray radiation in the presence or absence of the specific COX-2 inhibitor Celecoxib. Cell apoptosis and cell cycle changes were also detected through laser confocal scanning microcopy and flow cytometry. X-ray treatment only caused mild cell death in lung cancer A549 cells. However, combination treatment using celecoxib and X-ray radiation exhibited improved inhibitory effects and significantly suppressed cell proliferation. Therefore, COX-2 inhibitors combined with radiotherapy can counteract radiation-induced high COX-2 expression, demonstrating that celecoxib can function as a radiosensitizer of lung cancer cells. It is therefore reasonable to predict COX-2 inhibitors to be potential clinical radiotherapy synergists.
Keywords: cyclooxygenase-2, lung cancer, synergist, synthetic lethal
Introduction
Lung cancer is a severe form malignant disease, with ~80% of patients diagnosed with non-small cell lung cancer (1). Radio- or chemotherapy prolongs the overall survival time of the majority of patients with advanced or relapsed lung cancer and improves their quality of life (2). However, several phase III randomized clinical trials have revealed that the most effective radio- or chemotherapy can only achieve an overall response rate of 20–40% and a 1-year overall survival rate of 35–45% (3–5), accompanied by severe side effects and toxicity that elderly patients are intolerant to (6). As radiosensitization can enhance the efficiency and mitigate the side effects of radiotherapy, it has been one of the most active fields of cancer radiotherapy research (7). An ideal radiosensitizer should be tumor-specific but have low or absent toxicity to normal cells. However, the most widely used radiosensitizers, are nitroimidazoles, fluorouracil, cisplatin and Taxol, which exhibit a sensitizing action on radiotherapy, remain limited in their clinical use due to high toxicity to normal tissues (8). Molecular targeted drugs (MTDs) are extensively studied in cancer therapy (9). By harnessing the distinctions between cancer cells and normal cells, MTDs target tumor-specific structures and signal transduction-associated receptors or other enzymes, proteins and cytokines in order to kill cancer cells and suppress tumor development (10,11).
Previous studies have suggested that the overexpression of COX-2 is associated with oncogenesis, and that COX-2 inhibition or prostaglandin-endoperoxide synthase-2 knockout can reduce tumor occurrence (12,13). In addition, there is a negative correlation between COX-2 expression levels and prognosis (14,15). With its differing expression patterns in tumor and normal tissues, COX-2 has become a novel target in cancer therapy for its role in tumor occurrence and development (12–15). Selective COX-2 inhibitors (SCIs) are also effective in cancer prophylaxis and tumor therapy, exhibiting a high efficiency and a good safety profile with few side effects (16,17).
Representative SCIs, including celecoxib and NS-398, have exerted notable anticancer effects in several prior in vitro studies (18,19); however, the results were not as expected in clinical trials, possibly as SCIs do not kill cancer cells directly (20,21). Therefore, a combination of SCIs and radiotherapy or chemotherapy may be necessary to improve the treatment efficacy. In particular, it has been reported that radiotherapy stimulates COX-2 expression in a dose-dependent manner (22), which provides a rational basis for the combined treatment of SCIs and radiotherapy.
The present study aimed to treat lung cancer A549 cells using celecoxib combined with radiotherapy. Through analysis of cell cycle progression, cell growth, proliferation and apoptosis, the efficacy of this combination therapy was evaluated in cell culture.
Materials and methods
Reagents
Celecoxib was purchased from Selleck Chemicals (Houston, TX, USA). X-ray radiation was conferred by Radsource 2000 from Radsource, LLC (Brentwood, TN, USA). TRIzol® and primers were purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the ThermoScript RT reverse transcription-polymerase chain reaction (RT-PCR) kit was purchased from Fermentas (Thermo Fisher Scientific, Inc.). The PCR Amplification kit was obtained from Takara Bio, Inc., (Otsu, Japan). The Annexin V-Fluorescein Isothiocyanate (FITC) kit (cat. no. KFG001) was purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Hoechst 33258 was purchased from Beyotime Institute of Biotechnology (Haimen, China). The laser confocal scanning microscope, flow cytometer, PCR thermocycler, gel electrophoresis imaging system and cell culturing equipment were all obtained from The Second Affiliated Hospital, Suzhou University (Suzhou, China).
Cell culture
The human A549 lung adenocarcinoma and H292 lung mucoepidermoid carcinoma cell lines were purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China) and cultured using RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA, USA). Cultures were maintained in a 5% CO2 incubator at 37°C.
MTT assay
A549 cells were cultured on 96-well plates at 5,000 per well and allowed to grow to ~70% confluence. The medium was discarded and replaced following cell adherence and the cells were subjected to dimethyl sulfoxide (DMSO) or celecoxib treatment (100, 200 or 400 µM), with/without prior exposure to 6 Gy X-ray radiation for 5 min. A total of five replicates were set for each group and the cells were cultured for 24, 48, 72, 96 and 120 h, respectively. The medium was renewed for each group every 24 h. MTT solution (5 g/l) was added to each well prior to the chromogenic reaction and subsequently incubated for an additional 4 h, following which the incubation was stopped and the medium was carefully aspirated with a sterile pipette. DMSO (100 µl) was added to each well, followed by oscillation with a micro-oscillator for 10 min to dissolve the crystals completely. The optical density (OD) value of each well was detected under a wavelength of 490 nm, and the survival ratio was calculated according to following equation: Survival ratio = (mean OD value of the experimental group-background)/(mean OD value of the control group-background).
Detection of cell apoptosis by laser confocal scanning microscopy (LSCM)
A549 cells were cultured on 6-well plates with a coverslip in each well (50,000/well). Cells were treated with DMSO (0.1%), Celecoxib (200 µM), X-ray irradiation (6 Gy for 5 min) or Celecoxib (200 µM) combined with prior X-ray irradiation (6 Gy for 5 min). Serum-free medium and celecoxib-containing medium was refreshed every 24 h, and the cells were incubated at 37°C for a total of 48 h. Cells were fixed in 4% formaldehyde in PBS containing 0.1% TritonX-100 for 15 min at room temperature. Hoechst 33258 staining and mounting were performed according to the protocol provided by the Beyotime Institute of Biotechnology. Images were visualized and captured on an Olympus FV3000 microscope (Olympus Corporation, Tokyo, Japan) using magnification, ×400.
Detection of cell cycle and apoptosis by flow cytometry
A549 or H292 cells were seeded onto a 6-well plate (50,000/well) and allowed to grow to ~70% confluence. Cells were treated as aforementioned for 48 h. Prior to harvesting, cells were washed twice with PBS. In total, ~1×105 cells were collected for each group and centrifuged at 500 × g for 5 min at 4°C. For cell cycle analysis, cells were fixed in 70% ethanol at 4°C for 3 h. 100 µl Ribonuclease A (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added and the system was incubated in a water bath at 37°C for 15 min. Subsequently, 400 µl propidium iodide (PI) was added, mixed well and incubate in a dark chamber at room temperature for 30 min. Samples were detected at an excitation wavelength of 488 nm (Cytomics FC 500 MCL; Beckman Coulter, Inc., Brea, CA, USA). Results were from three replicate experiments. The percentages of cells in the G0/G1 phase, S phase and G2/M phase were statistically analyzed using CXP software (Beckman Coulter, Inc.). The detection of apoptosis was performed using the Annexin V-FITC kit, according to its recommended protocol. Early phase apoptosis, which is characterized by Annexin V-positive staining, was recorded to assess the pro-apoptotic effect of indicated treatment.
COX-2 expression levesl measured by RT-PCR
A549 cells (50,000/well) were cultured on a 6-well plate with 3 technical repeats and grown to ~70% confluence. Cells were then irradiated with 6 Gy X-ray for 5 min, and subsequently treated with 100, 200 or 400 µM celecoxib. Total RNA extraction and target RNA amplification by RT-PCR was performed according to the protocol provided with the kit under the following conditions: 37°C for 15 min; 85°C for 5 sec and 4°C for 5 min. cDNA products were subjected to PCR amplification as follows: 94°C for 5 min followed by 30 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C for 30 min followed by a final extension step of 72°C for 5 min. The following primers were used: COX-2, forward, 5′-CTGGCGCTCAGCCATACAG-3′ and reverse, 5′-CGCACTTATACTGGTCAAAT-CCC-3′; β-actin forward, 5′-GGGACCTGACTGACTACCTC-3′ and reverse, 5′-TCATACTCCTGCTTGCTGAT-3′. PCR products were separated on a 1% agarose gel at 160 V for 20 min.
Statistical analysis
All data are presented as the mean ± standard deviation from three independent experiments. Drug doses and inhibitory rates were subjected to normality tests and accorded with a normal distribution. Single factor analysis of variance (ANOVA) was used. A two-way ANOVA test was adopted for analysis of the cell cycle and apoptosis. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed with SPSS v.13.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Untreated A549 cells are partially resistant to X-ray radiation
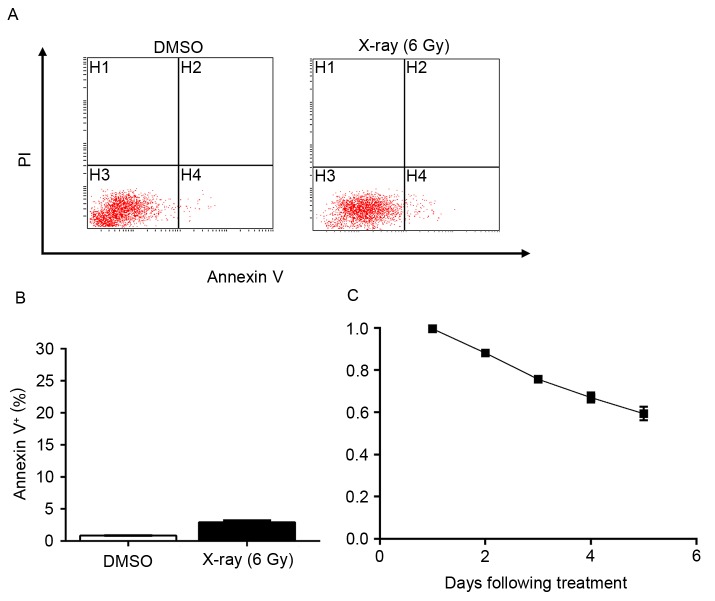
To evaluate the inhibitory effects of radiation on lung cancer A549 cells, assessment of apoptosis was performed subsequent to exposing A549 cells to X-ray radiation (6 Gy). The results demonstrated that radiation alone mildly induced apoptosis (Fig. 1A and B). As demonstrated by an MTT assay, ~50% of A549 cells remained proliferative five days following exposure (Fig. 1C). These results indicated that a large portion of A549 cells harbored resistance to radiation treatment, which necessitates a synergist to provide improved efficiency.
Figure 1.
Lung cancer cells are resistant to X-ray-mediated cell death. (A) Apoptosis of A549 cells exposed to 6 Gy X-ray radiation was detected by flow cytometry. (B) Quantification of (A). Data are presented as the mean ± standard deviation of three independent experiments. (C) Growth inhibition curve of A549 cells following exposure to 6 Gy X-ray radiation. PI, propidium iodide; DMSO, dimethyl sulfoxide.
COX-2 inhibitors enhance radiation-induced growth inhibition
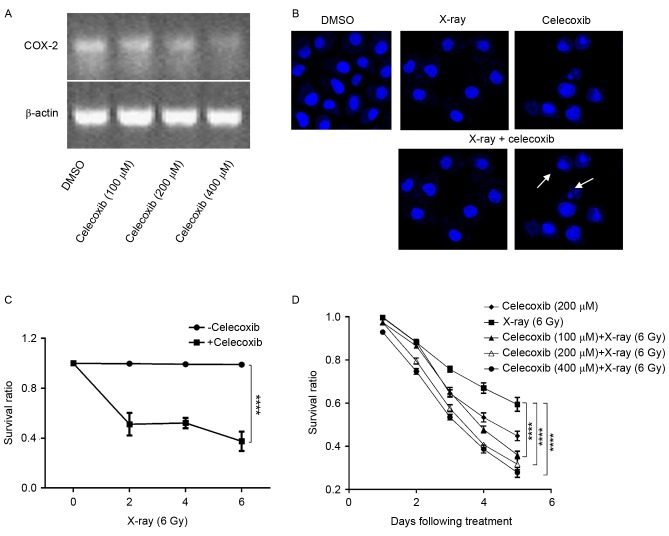
Studies have demonstrated that COX-2 is associated with tumorigenesis (12,13), and that the expression of COX-2 is elevated in lung cancer (23). Furthermore, radiotherapy is a stimulus of COX-2 expression (22,24), which indicates that COX-2 may be involved in the resistance of A549 cells to X-ray radiation. Therefore, COX-2 inhibitors might be able to potentiate the efficacy of radiotherapy. Based on this radiosensitizing potential, a cell counting assay was performed using A549 cells treated with celecoxib, X-ray radiation or celecoxib combined with irradiation. First, it was revealed that COX-2 expression was sufficiently inhibited when COX-2 inhibitors were added (Fig. 2A). Nuclear staining was then performed using Hoechst 33528. Using LSCM, clear apoptosis-associated morphological changes were observed, including anachromasis, pyknosis and karyorrhexis, as well as apoptotic bodies, particularly when celecoxib and X-ray radiation were combined (Fig. 2B). Furthermore, an MTT assay was conducted to confirm whether the killing effect of X-rays was enhanced by COX-2 inhibitors. The results demonstrated that celecoxib combined with X-ray irradiation had an improved efficacy compared with X-rays or celecoxib alone, in a time and dose-dependent manner (Fig. 2C and D).
Figure 2.
COX-2 inhibition increases the sensitivity of lung cancer cells to X-ray radiation. (A) Total RNA extract was amplified by reverse transcription-polymerase chain reaction and separated by agarose gel electrophoresis. Upper and lower lanes are COX-2 and β-actin, respectively. (B) Nuclear morphology of A549 cells treated with DMSO, X-rays, celecoxib or celecoxib plus X-ray. Nucleic changes, including anachromasis, pyknosis and karyorrhexis are indicated by the white arrows. Nuclei were stained with Hoechst 33258; image magnification, ×400. (C) A549 cells were irradiated with various doses of X-ray radiation, then treated with/without 200 µM celecoxib for three days. The growth inhibition curve is presented. Data are presented as the mean and standard error of five replicates. ****P<0.0001, two-way ANOVA. (D) Growth inhibition of A549 cells under indicated treatment. Data were pooled from three parallels and are presented as the mean ± standard deviation. Survival ratio at day 5 was subjected to statistical analysis. ****P<0.0001, two-way ANOVA. COX-2, cyclooxygenase-2; DMSO, dimethyl sulfoxide; ANOVA, analysis of variance.
COX-2 inhibitors promote radiation-induced apoptosis and cell cycle arrest
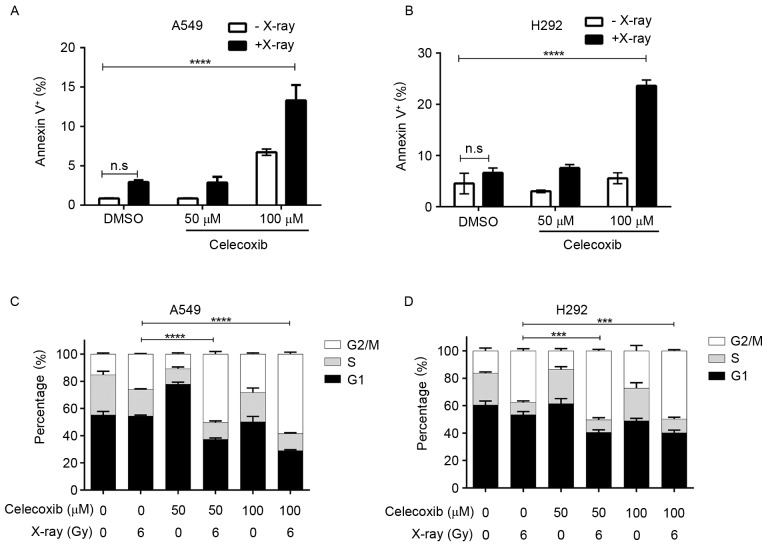
To quantify the pro-apoptotic function of the combination treatment, an Annexin-V and PI dual labeling kit was used to detect cell apoptosis, from which the apoptotic rate was calculated. The results demonstrated that 100 µM celecoxib or 6 Gy radiation induced apoptosis in lung cancer A549 cells, but a combination of the two had an improved pro-apoptotic effect (Fig. 3A). Comparable results were also obtained using the H292 lung cancer cell line (Fig. 3B). The number of normally cycling cells substantially determines the viability of a cell population, and COX-2 inhibitors have been reported to hamper cell cycle progression, causing a G0/G1 or G2/M stage arrest depending on the type of cells (25). Therefore, it was investigated whether cell cycle alterations caused by celecoxib contributed to the improved efficiency of X-ray irradiation. Cell cycle analysis was conducted under designated conditions. As shown in Fig. 3C, in A549 cells, X-ray treatment induced G2/M arrest, and this cell cycle blockage was enhanced significantly by the addition of celecoxib (Fig. 3C, Table I). The same effect was also observed in H292 cells (Fig. 3D). It was concluded that COX-2 inhibitors sensitize lung cancer cells to X-rays, not only via suppressing the tumor-promoting effects of COX-2 that are stimulated by radiotherapy, but also due to enhanced rates of cell cycle arrest and apoptosis.
Figure 3.
Cyclooxygenase-2 inhibition enhances X-ray-induced apoptosis and cell cycle arrest. Percentage of Annexin V-positive (A) A549 and (B) H292 cells following the indicated treatments for 48 h. Cell cycle stages of (C) A549 and (D) H292 cells treated as indicated for 48 h. Data are presented as the mean ± standard deviation of three independent experiments. ***P<0.001, ****P<0.0001, two-way analysis of variance. DMSO, dimethyl sulfoxide; n.s., not significant.
Table I.
Cell cycle analysis for each experimental group.
| Group | Celexcoxib, µM | X-ray, Gy | G0/G1, %a | S, %a | G2/M, %a |
|---|---|---|---|---|---|
| Control | 0 | 0 | 50.01±1.86 | 34.33±1.30 | 15.88±2.05 |
| Celecoxib | 200 | 0 | 56.24±1.53 | 29.17±1.27 | 14.52±1.83 |
| Radiation | 0 | 6 | 63.19±1.87 | 24.17±1.23 | 12.82±1.57 |
| Combination treatment | 100 | 6 | 67.21±1.93 | 23.27±1.34 | 9.65±1.67 |
| 200 | 6 | 73.18±1.89 | 18.36±1.87 | 8.53±1.42 | |
| 400 | 6 | 78.24±2.05 | 14.25±1.47 | 7.62±1.72 |
Data presented as mean ± standard deviation.
Discussion
Surgery, radiotherapy and chemotherapy are the three main treatments for malignant tumors. Although the response rate of these therapies has improved over previous years, even higher efficacy is challenging to achieve due to their concomitant disadvantages. Radiotherapy is widely adopted for malignant tumors as a local treatment. However, unfavorable responses are frequently observed due to tumor resistance to radiation and the intolerance of patients to severe toxicity and side effects (26–28). Furthermore, uncontrolled distant metastasis also contributes to treatment failures. Contemporarily, a combination of radiotherapy with chemotherapy is the predominant approach of cancer therapy, but due to toxicity and adverse reactions the effects are frequently unsatisfactory (26,29). Therefore, it is essential for oncologists to identify methods of improving the therapeutic efficacy and local control rate, and to control the rate of distant metastasis and reduce the impairment of healthy tissues.
COX is a key enzyme catalyzing the synthesis of prostaglandins (PG) and thromboxane A2 from arachidonic acid (30–32). Several types of PG have comprehensive functions in body, and are involved in a number of physiological reactions, including cruor, ovulation, parturition, renal function maintenance and immune response (32,33). Furthermore, PG performs a pivotal role in inflammation (31). COX includes two subtypes, COX-1 and COX-2, which are distinct in their biological characteristics. COX-1 maintains normal physiological functions of the human body, while COX-2 is associated with inflammation, pain and tumorigenesis (34,35). Expression of COX-2 is elevated in numerous tumor tissues, including colon and lung cancer (23,36). A number of studies have confirmed that COX inhibitors can suppress tumor occurrence and development (37–40); however, previous non-specific COX inhibitors have been gradually aborted due to their gastrointestinal toxicity (41). Thus, selective COX-2 inhibitors have become an alternative that is being intensively studied. The antitumor activity of COX-2 inhibitors may contribute to their functions to promote apoptosis, to suppress angiogenesis, to inhibit synthesis of PG, to enhance immunity and to prevent tumor invasion and metastasis (42,43). Radiotherapy is a stimulus of COX-2 overexpression, which functions in a dose-dependent manner (22,24), indicating a potential mechanism of tumor reoccurrence and metastasis. This provides a theory basis for the combination treatment of radiotherapy and COX-2 inhibitors.
To investigate whether COX-2 inhibitors combined with radiotherapy yield enhanced antitumor effects, lung cancer A549 cells overexpressing COX-2 were treated with celecoxib, radiation or a combination of celecoxib and radiation. Through different methods it was concluded that, under the same conditions, proliferation of A549 cells is inhibited more evidently by celecoxib combined with radiotherapy than by the drug or irradiation alone. Within the combination groups, growth inhibition became more potent when the concentration of celecoxib was increased or the exposure time was longer, indicating that this combination treatment works in a dose- and time-dependent manner. The results also demonstrated that treatment with 100 µM celecoxib enhanced the cell cycle arrest and apoptosis caused by radiation. Consistent outcomes have been reported using other types of cancer cells. Using mouse fibrosarcoma cells, Raju et al (25) identified that COX-2 inhibitors triggered an accumulation of cells in the G2/M phase, and the expression of cyclin A, cyclin B and cyclin dependent kinase 1/2 (CDK1/2) was also decreased. However, a study by Grösch et al (44) on colon cancer cells revealed a G0/G1 arrest caused by COX-2 inhibitors, which lowered the expression levels of cyclin A, cyclin B and and CDK1/2, and the expression of cell cycle inhibitory proteins, including p21/waf1 and p27/Kip1, was upregulated. Therefore, the cell cycle arrest caused by celecoxib may depend on the type of cancer cells or other unknown factors.
Numerous studies have demonstrated that COX-2 inhibitors induce apoptosis in tumor cells (45–47). A previous study by Kern et al (45) involving four hepatoma cell lines expressing COX-2 determined that COX-2 inhibitors induced apoptosis independent of the phosphorylation state of B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein, protein kinase B and Bcl-2-associated death promoter, but associated with the activation of caspase-9, caspase-3 and caspase-6. Li et al (46) revealed that, in esophageal carcinoma cells, COX-2 inhibitors induced the apoptosis of those expressing COX-2 through a cytochrome c-dependent pathway. It was also reported that the apoptosis induced by COX-2 inhibitors is not evident in human hepatoma cells, and COX-2 inhibitor induces apoptosis in tumor cells is independent of COX-2 expression (47). In the present study, cell apoptosis induced by a combination therapy of X-ray irradiation and COX-2 inhibitors was significantly increased.
The present study corroborates that COX-2 inhibitors can enhance the killing effect of radiation on lung cancer cells, which is consistent with the results of prior studies (25,48). However, the mechanisms underlying the radiosensitizing effect of COX-2 inhibitors require further investigation. Raju et al (25) reported that radiosensitization may be attributed to inhibited sub-lethal DNA damage repair or an increased percentage of cells in the radiosensitive G2/M phase. Contradictory results were recorded in U2251 brain glioma cells, in which COX-2 inhibitors boosted radiosensitivity without expanding the cell number of the G2/M phase (49). In the present study, evident apoptosis was observed in the combination therapy group, supported by the formation of apoptotic bodies, which was observed using LSCM, thereby confirming the pro-apoptotic effects of COX-2 inhibitors combined with radiotherapy on lung cancer A549 cells. Studies have demonstrated that radiation is responsible for elevated COX-2 expression (22,24), and this can be reversed by the addition of a COX-2 inhibitor such as NS398 (48), which also acts as a radiosensitizer. Therefore, it was speculated that NS398 may act upstream COX-2 to exert a radiosensitizing effect and inhibit the radiation-induced upregulation of COX-2 expression. In the present study, COX-2 inhibitors were also identified to be a synergist to radiotherapy, of which the mechanism may be associated with decreased intracellular expression of COX-2, accompanied by their effects on improving immunity and inhibiting angiogenesis (50).
To conclude, compared with other intervention groups and the control, COX-2 inhibitors combined with radiotherapy exhibit a synergistic effect. This combination significantly suppressed the growth and proliferation of lung cancer cells and promoted apoptosis. The present in vitro study provides a novel insight into the treatment of advanced lung cancer. However, whether COX-2 inhibitors combined with X-rays are effective in vivo or in clinical use remains unknown. Therefore, the optimal dose and toxicity of celecoxib requires further study.
References
- 1.Gottfried M, Keizman D, Mishaeli M, Rabinovich Maimon N. The Current Approach To Advanced Lung Cancer. Harefuah. 2015;154(521–524):539–540. (In Hebrew) [PubMed] [Google Scholar]
- 2.Xu P, Le Pechoux C. Chemoradiotherapy for stage III non-small cell lung cancer: Have we reached the limit? Chin Clin Oncol. 2015;4:45. doi: 10.3978/j.issn.2304-3865.2015.11.04. [DOI] [PubMed] [Google Scholar]
- 3.Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK, Lee KC, Chen M, Kim DW, Kim HK, Min YJ, et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol. 2015;33:2660–2666. doi: 10.1200/JCO.2014.60.0130. [DOI] [PubMed] [Google Scholar]
- 4.Shen WY, Ji J, Zuo YS, Pu J, Xu YM, Zong CD, Tao GZ, Chen XF, Ji FZ, Zhou XL, et al. Comparison of efficacy for postoperative chemotherapy and concurrent radiochemotherapy in patients with IIIA-pN2 non-small cell lung cancer: An early closed randomized controlled trial. Radiother Oncol. 2014;110:120–125. doi: 10.1016/j.radonc.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Sun JM, Ahn YC, Choi EK, Ahn MJ, Ahn JS, Lee SH, Lee DH, Pyo H, Song SY, Jung SH, et al. Phase III trial of concurrent thoracic radiotherapy with either first-or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann Oncol. 2013;24:2088–2092. doi: 10.1093/annonc/mdt140. [DOI] [PubMed] [Google Scholar]
- 6.Bourgier C, Lacombe J, Solassol J, Mange A, Pèlegrin A, Ozsahin M, Azria D. Late side-effects after curative intent radiotherapy: Identification of hypersensitive patients for personalized strategy. Crit Rev Oncol Hematol. 2015;93:312–319. doi: 10.1016/j.critrevonc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi Y, Nishimura R, Kawara H, Omori K, Matsumoto K, Tokuyama Y, Uchiyama K, Shimizu Y. Survey of current status of adverse ocular reactions to paclitaxel and a retrospective analysis for aiding in early detection of adverse reactions. Gan To Kagaku Ryoho. 2013;40:819–822. [PubMed] [Google Scholar]
- 8.Linam J, Yang LX. Recent developments in radiosensitization. Anticancer Res. 2015;35:2479–2485. [PubMed] [Google Scholar]
- 9.Sheppard DW, MacRitchie JA. Building in molecular diversity for targeted libraries. Drug Discov Today Technol. 2013;10:e461–e466. doi: 10.1016/j.ddtec.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Milano GA. Targeted therapy in non-small cell lung cancer: A focus on epidermal growth factor receptor mutations. Chin Clin Oncol. 2015;4:47. doi: 10.3978/j.issn.2304-3865.2015.12.04. [DOI] [PubMed] [Google Scholar]
- 11.Ricciuti B, Leonardi GC, Metro G, Grignani F, Paglialunga L, Bellezza G, Baglivo S, Mencaroni C, Baldi A, Zicari D, Crinò L. Targeting the KRAS variant for treatment of non-small cell lung cancer: Potential therapeutic applications. Expert Rev Respir Med. 2016;10:53–68. doi: 10.1586/17476348.2016.1115349. [DOI] [PubMed] [Google Scholar]
- 12.Müller-Decker K. Cyclooxygenase-dependent signaling is causally linked to non-melanoma skin carcinogenesis: Pharmacological, genetic, and clinical evidence. Cancer Metastasis Rev. 2011;30:343–361. doi: 10.1007/s10555-011-9306-z. [DOI] [PubMed] [Google Scholar]
- 13.Tsujii M. Cyclooxygenase, cancer stem cells and DNA methylation play important roles in colorectal carcinogenesis. Digestion. 2013;87:12–16. doi: 10.1159/000343898. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, Myung SK, Song YS. Prognostic role of cyclooxygenase-2 in epithelial ovarian cancer: A meta-analysis of observational studies. Gynecol Oncol. 2013;129:613–619. doi: 10.1016/j.ygyno.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Magnowska M, Zaborowski M, Surowiak P, Nowak-Markwitz E, Zabel M, Spaczyński M. COX-2 expression pattern is related to ovarian cancer differentiation and prognosis, but is not consistent with new model of pathogenesis. Ginekol Polska. 2014;85:335–341. doi: 10.17772/gp/1733. [DOI] [PubMed] [Google Scholar]
- 16.Liu R, Xu KP, Tan GS. Cyclooxygenase-2 inhibitors in lung cancer treatment: Bench to bed. Eur J Pharmacol. 2015;769:127–133. doi: 10.1016/j.ejphar.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Regulski M, Regulska K, Prukala W, Piotrowska H, Stanisz B, Murias M. COX-2 inhibitors: A novel strategy in the management of breast cancer. Drug Discov Today. 2016;21:598–615. doi: 10.1016/j.drudis.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Amir M, Agarwal H. Role of COX-2 selective inhibitors for prevention and treatment of cancer. Pharmazie. 2005;60:563–570. [PubMed] [Google Scholar]
- 19.Kalgutkar AS, Zhao Z. Discovery and design of selective cyclooxygenase-2 inhibitors as non-ulcerogenic, anti-inflammatory drugs with potential utility as anti-cancer agents. Curr Drug Targets. 2001;2:79–106. doi: 10.2174/1389450013348830. [DOI] [PubMed] [Google Scholar]
- 20.Liu R, Xu KP, Tan GS. Cyclooxygenase-2 inhibitors in lung cancer treatment: Bench to bed. Eur J Pharmacol. 2015;769:127–133. doi: 10.1016/j.ejphar.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Mohammadinejad P, Arya P, Esfandbod M, Kaviani A, Najafi M, Kashani L, Zeinoddini A, Emami SA, Akhondzadeh S. Celecoxib versus diclofenac in mild to moderate depression management among breast cancer patients: A double-blind, placebo-controlled, randomized trial. Ann Pharmacother. 2015;49:953–961. doi: 10.1177/1060028015592215. [DOI] [PubMed] [Google Scholar]
- 22.Steinauer KK, Gibbs I, Ning S, French JN, Armstrong J, Knox SJ. Radiation induces upregulation of cyclooxygenase-2 (COX-2) protein in PC-3 cells. Int J Radiat Oncol Biol Phys. 2000;48:325–328. doi: 10.1016/S0360-3016(00)00671-4. [DOI] [PubMed] [Google Scholar]
- 23.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 24.Isoherranen K, Punnonen K, Jansen C, Uotila P. Ultraviolet irradiation induces cyclooxygenase-2 expression in keratinocytes. Br J Dermatol. 1999;140:1017–1022. doi: 10.1046/j.1365-2133.1999.02897.x. [DOI] [PubMed] [Google Scholar]
- 25.Raju U, Nakata E, Yang P, Newman RA, Ang KK, Milas L. In vitro enhancement of tumor cell radiosensitivity by a selective inhibitor of cyclooxygenase-2 enzyme: Mechanistic considerations. Int J Radiat Oncol Biol Phys. 2002;54:886–894. doi: 10.1016/S0360-3016(02)03023-7. [DOI] [PubMed] [Google Scholar]
- 26.Blanco R, Maestu I, de la Torre MG, Cassinello A, Nuñez I. A review of the management of elderly patients with non-small-cell lung cancer. Ann Oncol. 2015;26:451–463. doi: 10.1093/annonc/mdu268. [DOI] [PubMed] [Google Scholar]
- 27.Guy JB, Rancoule C, Méry B, Espenel S, Wozny AS, Simonet S, Vallard A, Alphonse G, Ardail D, Rodriguez-Lafrasse C, Magné N. Radiosensitivity and/or radioresistance of head and neck cancers: Biological angle. Bull Cancer. 2016;103:41–47. doi: 10.1016/j.bulcan.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Chang L, Graham PH, Ni J, Hao J, Bucci J, Cozzi PJ, Li Y. Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit Rev Oncol Hematol. 2015;96:507–517. doi: 10.1016/j.critrevonc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Chang L, Graham P, Hao J, Bucci J, Malouf D, Gillatt D, Li Y. Proteomics discovery of radioresistant cancer biomarkers for radiotherapy. Cancer Lett. 2015;369:289–297. doi: 10.1016/j.canlet.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Spector AA, Kim HY. Discovery of essential fatty acids. J Lipid Res. 2015;56:11–21. doi: 10.1194/jlr.R055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frungieri MB, Calandra RS, Mayerhofer A, Matzkin ME. Cyclooxygenase and prostaglandins in somatic cell populations of the testis. Reproduction. 2015;149:R169–R180. doi: 10.1530/REP-14-0392. [DOI] [PubMed] [Google Scholar]
- 32.Claar D, Hartert TV, Peebles RS., Jr The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev Respir Med. 2015;9:55–72. doi: 10.1586/17476348.2015.992783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugimoto Y, Inazumi T, Tsuchiya S. Roles of prostaglandin receptors in female reproduction. J Biochem. 2015;157:73–80. doi: 10.1093/jb/mvu081. [DOI] [PubMed] [Google Scholar]
- 34.Tjandrawinata RR, Dahiya R, Hughes-Fulford M. Induction of cyclo-oxygenase-2 mRNA by prostaglandin E2 in human prostatic carcinoma cells. Br J Cancer. 1997;75:1111–1118. doi: 10.1038/bjc.1997.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 36.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and-2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 37.Azer SA. Overview of molecular pathways in inflammatory bowel disease associated with colorectal cancer development. Eur J Gastroenterol Hepatol. 2013;25:271–281. doi: 10.1097/MEG.0b013e32835b5803. [DOI] [PubMed] [Google Scholar]
- 38.Harris RE, Beebe J, Alshafie GA. Reduction in cancer risk by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. J Exp Pharmacol. 2012;4:91–96. doi: 10.2147/JEP.S23826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cathcart MC, O'Byrne KJ, Reynolds JV, O'Sullivan J, Pidgeon GP. COX-derived prostanoid pathways in gastrointestinal cancer development and progression: Novel targets for prevention and intervention. Biochim Biophys Acta. 2012;1825:49–63. doi: 10.1016/j.bbcan.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Baek SJ, Eling T. COX inhibitors directly alter gene expression: Role in cancer prevention? Cancer Metastasis Rev. 2011;30:641–657. doi: 10.1007/s10555-011-9301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nalamachu S, Pergolizzi JV, Raffa RB, Lakkireddy DR, Taylor R., Jr Drug-drug interaction between NSAIDS and low-dose aspirin: A focus on cardiovascular and GI toxicity. Expert Opin Drug Saf. 2014;13:903–917. doi: 10.1517/14740338.2014.924924. [DOI] [PubMed] [Google Scholar]
- 42.Masferrer JL, Isakson PC, Seibert K. Cyclooxygenase-2 inhibitors: A new class of anti-inflammatory agents that spare the gastrointestinal tract. Gastroenterol Clin North Am. 1996;25:363–372. doi: 10.1016/S0889-8553(05)70252-1. [DOI] [PubMed] [Google Scholar]
- 43.Pairet M, Engelhardt G. Distinct isoforms (COX-1 and COX-2) of cyclooxygenase: Possible physiological and therapeutic implications. Fundam Clin Pharmacol. 1996;10:1–17. doi: 10.1111/j.1472-8206.1996.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 44.Grösch S, Tegeder I, Niederberger E, Bräutigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 45.Kern MA, Schubert D, Sahi D, Schöneweiss MM, Moll I, Haugg AM, Dienes HP, Breuhahn K, Schirmacher P. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36:885–894. doi: 10.1016/S0270-9139(02)00094-0. [DOI] [PubMed] [Google Scholar]
- 46.Li M, Wu X, Xu XC. Induction of apoptosis by cyclo-oxygenase-2 inhibitor NS398 through a cytochrome C-dependent pathway in esophageal cancer cells. Int J Cancer. 2001;93:218–223. doi: 10.1002/ijc.1322. [DOI] [PubMed] [Google Scholar]
- 47.Elder D, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: Independence from COX-2 protein expression. Clin Cancer Res. 1997;3:1679–1683. [PubMed] [Google Scholar]
- 48.Amirghahari N, Harrison L, Smith M, Rong X, Naumann I, Ampil F, Shi R, Glass J, Nathan CO. NS 398 radiosensitizes an HNSCC cell line by possibly inhibiting radiation-induced expression of COX-2. Int J Radiat Oncol Biol Phys. 2003;57:1405–1412. doi: 10.1016/S0360-3016(03)01577-3. [DOI] [PubMed] [Google Scholar]
- 49.Kang KB, Wang TT, Woon CT, Cheah ES, Moore XL, Zhu C, Wong MC. Enhancement of glioblastoma radioresponse by a selective COX-2 inhibitor celecoxib: Inhibition of tumor angiogenesis with extensive tumor necrosis. Int J Radiat Oncol Biol Phys. 2007;67:888–896. doi: 10.1016/j.ijrobp.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 50.Lang S, Lauffer L, Clausen C, Löhr I, Schmitt B, Hölzel D, Wollenberg B, Gires O, Kastenbauer E, Zeidler R. Impaired monocyte function in cancer patients: Restoration with a cyclooxygenase-2 inhibitor. FASEB J. 2003;17:286–288. doi: 10.1096/fj.02-0417fje. [DOI] [PubMed] [Google Scholar]