Abstract
Solute carrier family 19 (thiamine transporter), member 3 (SCL19A3) gene defect produces an autosomal recessive neurodegenerative disorder associated with different phenotypes and acronyms. One of the common presentations is early infantile lethal Leigh-like syndrome. We report a case of early infantile Leigh-like SLC19A3 gene defects of patients who died at 4 months of age with no response to a high dose of biotin and thiamine. In addition, we report a novel mutation that was not reported previously. Finally, we review the literature regarding early infantile Leigh-like SLC19A3 gene defects and compare the literature with our patient.
Keywords: SCL19A3 gene defect, SLC19A3 gene, Leigh syndrome, Leigh-like, biotin, thiamine
Introduction
Solute carrier family 19 (thiamine transporter), member 3 (SCL19A3) gene defect produces an autosomal recessive neurodegenerative disorder associated with different phenotypes and acronyms. The known phenotypes include early infantile Leigh-like, classical childhood, and Wernicke-like encephalopathy. In early infantile lethal Leigh-like syndrome, the probands usually present in the first 3 months of life with Leigh-like syndrome: poor feeding, vomiting, acute encephalopathy, and lactic acidosis. Magnetic resonance imaging (MRI) of patients’ brains usually shows high T2 signals involving the perirolandic area, bilateral putamen, and medial thalamic nuclei, with the spectroscopy showing lactate peaks.1 The second phenotype is the classical childhood onset biotin-thiamine responsive basal ganglia disease. In this disorder, the symptoms usually start between 3 and 7 years of age with subacute encephalopathy and confusion, dysarthria, and dysphagia, with occasional central facial palsy or external ophthalmoplegia that progresses to severe cogwheel rigidity, dystonia, seizure, quadriparesis, and even death if left untreated with biotin and thiamine.2–4 The third phenotype is the adult Wernicke-like encephalopathy-SLC19A3 gene defect, which was reported in 2 Japanese men, both of whom presented in their second decade of life with status epilepticus, diplopia, nystagmus, ptosis, ophthalmoplegia, and ataxia. Brain MRI showed high-intensity signals in the bilateral medial thalamus and periaqueductal gray region. The patients showed a dramatic response to a high dose of thiamine.5 The disease is caused by a defect in thiamine transporter 2 (hTHTR2), which is encoded by the SLC19A3 gene. Diagnosis is usually made after molecular testing for the SLC19A3 gene defect, and measurement of the free thiamine level could be a potential biomarker for monitoring and diagnosis of this disorder.6 The treatment consists of thiamine alone or in combination with biotin for life.2,3,7 In this report, we describe a case of early infantile Leigh-like-SLC19A3 gene defects who died at 4 months of age with no response to a high dose of biotin and thiamine. In addition, we report a novel mutation that was not reported previously. Finally, we review the literature regarding early infantile Leigh-like-SLC19A3 gene defects and compare it with our patient.
Case Report
A 2-month-old infant, full-term, through a normal vaginal delivery, was born to healthy Saudi consanguineous parents with appropriate growth parameters and no significant antenatal history. This first child of this couple was discharged on the second day of life with no complications. He presented to our center with a 3-day history of low-grade fever, poor feeding, decreased activity, and complex partial seizures in the form of turning the head to the right with eye staring; then, the patient was admitted to the pediatric intensive care unit, where he was started on parenteral phenobarbital and antibiotics after performing all necessary investigations, including a septic workup. Then, the patient’s condition deteriorated as he became encephalopathic and required mechanical ventilation. On examination, his length was 56 cm (5th percentile), weight was 4 kg (5th percentile), and head circumference was 38.5 cm (25th percentile).
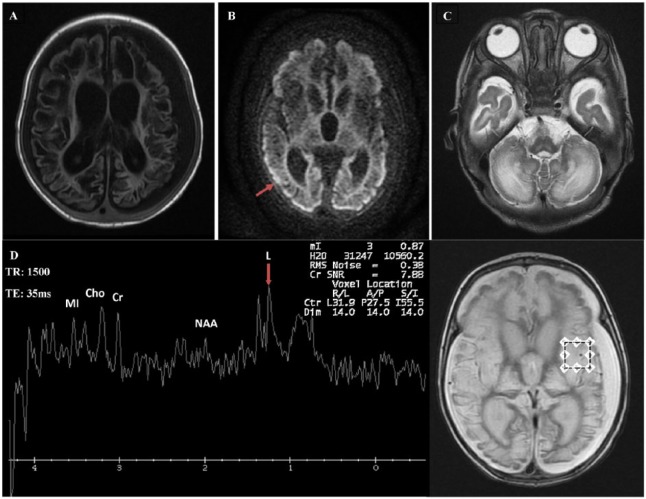
He had poor eye contact, horizontal nystagmus, axial hypotonia with appendicular hypertonia, hyperreflexia, and clonus in both upper and lower limbs. Other system examinations were unremarkable. Investigations showed the following results. Brain MRI showed cystic changes in both cerebral hemispheres and signal abnormality in the brain stem and cerebellum. There were abnormalities in the bilateral basal ganglia and thalamus, with volume loss. There was diffuse volume loss of both cerebral hemispheres with ex vacuo enlargement of the ventricles. There were bilateral moderate subdural effusions (Figure 1). Magnetic resonance spectroscopy confirmed a mild lactate peak at 1.3 ppm (Figure 1). Electroencephalogram demonstrated a slow diffuse background indicating nonspecific encephalopathy. There was low voltage activity with periods of suppression, followed by a burst of low-voltage multifocal epileptic discharges. The epileptic discharge was greater over the right temporal head region. The auditory brain stem response, echocardiogram, and ophthalmology evaluation were normal.
Figure 1.
Brain MRI and MRS. (A)-T1 weighted axial section showing extensive brain damage with cystic encephalomalacia, bilateral subdural effusion, and ex vacuo enlargement of the ventricles. (B) Diffusion-weighted image, axial section, showing restricted diffusion in the margin of the cerebral cortices bilaterally (arrow). (C) T2-weighted axial section showing abnormal signaling in the cerebellum and volume loss in brain stem. (D) MRS showing mild lactate peak at 1.3 ppm (arrow). TE: 35 ms. Ch indicates choline; Cr, creatine; L, lactate; MI, myoinositol; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NAA, N-acetylaspartate; TE, echo time.
Lab investigations showed mildly elevated lactic acid in the blood, ranging from 2.6 to 3.0 mmol/L (normal values are 1.2-2.2 mmol/L). Blood gas showed mild metabolic acidosis as follows (venous pH: 7.30, PCO2: 52 mm Hg, base excess: −1.7). Plasma amino acids showed mildly elevated alanine levels. All other biochemical investigations in the serum, urine, and cerebrospinal fluid were unremarkable.
Whole exome sequencing revealed a novel homozygous frameshift duplication in the SLC19A3 gene (NM_025243.3; c. 91dupT, p. Val65Glyfs*160). The parents were tested, and they are heterozygous for this mutation. The patient was started on high doses of biotin 20 mg twice a day (10 mg/kg/d) and thiamine 75 mg twice a day (37.5 mg/kg/d). Despite maximal support, the patient’s condition deteriorated, and he succumbed at 4 months of life.
Discussion
Table 1 summarizes the clinical characteristics of the early infantile form of SLC19A3 gene defects and compares it with our patient. Interestingly, before 2013, Leigh-like SLC19A3 gene defects were not yet a known phenotype. However, during that year, 19 infants were described from different origins but were mainly Moroccans (Table 1).1,8,9 The clinical pictures were almost the same, with an acute devastating course between the first and third months of life. The reported patients started to have poor feeding and vomiting that progressed to seizure and acute encephalopathy after a period of febrile illness. The MRI findings showed a picture of Leigh syndrome with extensive signaling abnormalities in the brain stem and cerebellum. There were abnormalities in the bilateral basal ganglia and thalamus with brain atrophy. Magnetic resonance spectroscopy demonstrated a lactate peak in most of these patients. Subsequently, different probands from Mexico, Turkey, Sweden, and Poland were reported.10–13 The current report is the first from Saudi Arabia with Leigh-like SLC19A3 gene defect.
Table 1.
Summary of all published cases of the early infantile SLC19A3 gene defect.
| S. no. | References | No. of cases | Origin | M:F | Age of onset | SLC19A3 gene mutation | Treatment with biotin | Treatment with thiamine | Prognosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Kevelam et al8 | 7 | Canada Europe Lebanon Morocco |
NA | Mean: 2.7 mo | Canadian: [c.68G>T(p.Gly23Val); r.1173_1314del(p.Gln393*)] European: [c.541T>C(p.Ser181Pro); c.1154T>G(p.Leu385Arg)] European: [c.507C>G(p.Tyr169*); c.527C>A(p.Ser176Tyr)] Lebanese: c.895_925del(p.Val299fs) Moroccan: c.1332C>G(p.Ser444Arg) |
Yes | No | All died |
| 2 | Gerards et al9 | 11 | Morocco | 8:3 | 1 mo | c.20C>A(p. p.Ser7*) | Yes | Yes | All died |
| 3 | Pérez-Dueñas et al1 | 1 | Morocco | M | 4 wk | c.68G>T(p.Gly23Val) | 10 mg/d | 20 mg/kg/d | Excellent response to biotin and thiamine |
| 4 | Sremba et al10 | 1 | Mexico (mixed ancestry) | F | 6 wk | c.74dupT(p. Ser26LeufsX19) c.81_82dup(p.Met28fs) |
No | 10 mg/kg/d | Died at 12 y |
| 5 | Haack et al11 | 2 | Turkey | M | 18 d | c. 982del(p. Ala328Leufs*10) | 10 mg/kg/d | 15 mg/kg/d | Improved dramatically |
| 6 | Ygberg et al12 | 2 | Sweden | M | 5 wk | c.74dupT/p. Ser26LeufsX19 c.1403delA |
10 mg/kg/d | 60 mg/kg/d | First patient died, whereas second survived with dystonic symptoms |
| 7 | Pronicka et al13 | 1 | Poland | M | Birth | c.74dupT(p.Ser26LeufsX19) | Yes | Yes | Died |
| 8 | Alfadhel3 | 1 | Saudi | M | 2 mo | c. 91dupT, p. Val65Glyfs*160 | Yes | Yes | Died |
| Total | 26 | 13:4 | 1-3 mo | ||||||
Abbreviations: F, female; M, male; NA, not available.
Strikingly, Yamada et al14 reported different phenotypes of early infantile SLC19A3 gene defects that are infantile spasm. They reported 4 male Japanese patients with poor response to biotin and thiamine.
Unlike the juvenile form of the SLC19A3 gene defect, the prognosis of early infantile Leigh-like SLC19A3 gene defect seems to be poor despite treatment with biotin and thiamine. Indeed, 22/26 (85%) of the reported children died; 1 survived with dystonic symptoms, and only 3 patients had good response to treatment with biotin and thiamine.1,8,9,11,12
In addition, the early infantile Leigh-like SLC19A3 gene defect is associated with some biochemical abnormalities, which include high lactate and alanine, increased leucine and isoleucine, and increased excretion of α-ketoglutarate, whereas the juvenile form has normal biochemical profiles.1 This result could be explained by a deficiency of the thiamine active form (thiamine pyrophosphate), which is an important cofactor for 3 mitochondrial enzymes (pyruvate dehydrogenase complex, branched chain α-ketoacid dehydrogenase complex, and α-ketoglutarate dehydrogenase).8
The poor response to treatment in this phenotype supports the conclusion from functional studies that the effectiveness of treatment mainly depends on whether the transport capacity is reduced at physiological levels, whereas it seems unlikely to be beneficial in cases where the transporter function is completely abolished when there is a null mutation in the early-onset form.15
Conclusions
We reported the first early infantile Leigh-like SLC19A3 gene defect from Saudi Arabia, with a novel mutation not described previously. We confirmed the poor prognosis of this a phenotype despite maximizing treatment with biotin and thiamine. We also alert clinicians to consider the SLC19A3 gene defect in any infant presenting early in life with Leigh-like syndrome. The poor response to treatment and outcome warrants thorough genetic counseling for the families and proper planning for future pregnancies.
Acknowledgments
The author is grateful to the patient and his family for their genuine support.
Footnotes
Peer review:Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 325 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MA performed all the work associated with preparing, writing, and submitting the manuscript and contributed to the clinical diagnosis and management of the patients.
References
- 1. Pérez-Dueñas B, Serrano M, Rebollo M, et al. Reversible lactic acidosis in a newborn with thiamine transporter-2 deficiency. Pediatrics. 2013;131:e1670–e1675. [DOI] [PubMed] [Google Scholar]
- 2. Tabarki B, Al-Hashem A, Alfadhel M. Biotin-thiamine-responsive basal ganglia disease. In: Pagon RA, Adam MP, Ardinger HH, et al. eds. GeneReviews. Seattle, WA: University of Washington; 1993. [PubMed] [Google Scholar]
- 3. Alfadhel M, Almuntashri M, Jadah RH, et al. Biotin-responsive basal ganglia disease should be renamed biotin-thiamine-responsive basal ganglia disease: a retrospective review of the clinical, radiological and molecular findings of 18 new cases. Orphanet J Rare Dis. 2013;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozand PT, Gascon GG, Al Essa M, et al. Biotin-responsive basal ganglia disease: a novel entity. Brain. 1998;121:1267–1279. [DOI] [PubMed] [Google Scholar]
- 5. Kono S, Miyajima H, Yoshida K, Togawa A, Shirakawa K, Suzuki H. Mutations in a thiamine-transporter gene and Wernicke’s-like encephalopathy. N Engl J Med. 2009;360:1792–1794. [DOI] [PubMed] [Google Scholar]
- 6. Ortigoza-Escobar JD, Molero-Luis M, Arias A, et al. Free-thiamine is a potential biomarker of thiamine transporter-2 deficiency: a treatable cause of Leigh syndrome. Brain. 2016;139:31–38. [DOI] [PubMed] [Google Scholar]
- 7. Tabarki B, Alfadhel M, AlShahwan S, Hundallah K, AlShafi S, AlHashem A. Treatment of biotin-responsive basal ganglia disease: open comparative study between the combination of biotin plus thiamine versus thiamine alone. Eur J Paediatr Neurol. 2015;19:547–552. [DOI] [PubMed] [Google Scholar]
- 8. Kevelam SH, Bugiani M, Salomons GS, et al. Exome sequencing reveals mutated SLC19A3 in patients with an early-infantile, lethal encephalopathy. Brain. 2013;136:1534–1543. [DOI] [PubMed] [Google Scholar]
- 9. Gerards M, Kamps R, van Oevelen J, et al. Exome sequencing reveals a novel Moroccan founder mutation in SLC19A3 as a new cause of early-childhood fatal Leigh syndrome. Brain. 2013;136:882–890. [DOI] [PubMed] [Google Scholar]
- 10. Sremba LJ, Chang RC, Elbalalesy NM, Cambray-Forker EJ, Abdenur JE. Whole exome sequencing reveals compound heterozygous mutations in SLC19A3 causing biotin-thiamine responsive basal ganglia disease. Mol Genet Metab Rep. 2014;1:368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haack TB, Klee D, Strom TM, et al. Infantile Leigh-like syndrome caused by SLC19A3 mutations is a treatable disease. Brain. 2014;137:e295. [DOI] [PubMed] [Google Scholar]
- 12. Ygberg S, Naess K, Eriksson M, et al. Biotin and thiamine responsive basal ganglia disease—a vital differential diagnosis in infants with severe encephalopathy. Eur J Paediatr Neurol. 2016;20:457–461. [DOI] [PubMed] [Google Scholar]
- 13. Pronicka E, Piekutowska-Abramczuk D, Ciara E, et al. New perspective in diagnostics of mitochondrial disorders: two years’ experience with whole-exome sequencing at a national paediatric centre. J Transl Med. 2016;14:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamada K, Miura K, Hara K, et al. A wide spectrum of clinical and brain MRI findings in patients with SLC19A3 mutations. BMC Med Genet. 2010;11:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schanzer A, Doring B, Ondrouschek M, et al. Stress-induced upregulation of SLC19A3 is impaired in biotin-thiamine-responsive basal ganglia disease. Brain Pathol. 2014;24:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]