Abstract
Background:
Following injury to the rotator cuff and anterior cruciate ligament, a direct enthesis is not regenerated, and healing occurs with biomechanically inferior fibrous tissue. Demineralized bone matrix (DBM) is a collagen scaffold that contains growth factors and is a promising biological material for tendon and ligament repair because it can regenerate a direct fibrocartilaginous insertion via endochondral ossification.
Purpose:
To provide a comprehensive review of the literature investigating the use of DBM to augment tendon-bone healing in tendon repair and anterior cruciate ligament reconstruction (ACLR).
Study Design:
Systematic review.
Methods:
Electronic databases (MEDLINE and EMBASE) were searched for preclinical and clinical studies that evaluated the use of DBM in tendon repair and ACLR. Search terms included the following: (“demineralized bone matrix” OR “demineralized cortical bone”) AND (“tissue scaffold” OR “tissue engineering” OR “ligament” OR “tendon” OR “anterior cruciate ligament” OR “rotator cuff”). Peer-reviewed articles written in English were included, and no date restriction was applied (searches performed February 10, 2017). Methodological quality was assessed with peer-reviewed scoring criteria.
Results:
The search strategy identified 339 articles. After removal of duplicates and screening according to inclusion criteria, 8 studies were included for full review (tendon repair, n = 4; ACLR, n = 4). No human clinical studies were identified. All 8 studies were preclinical animal studies with good methodological quality. Five studies compared DBM augmentation with non-DBM controls, of which 4 (80%) reported positive findings in terms of histological and biomechanical outcomes.
Conclusion:
Preclinical evidence indicates that DBM can improve tendon-bone healing, although clinical studies are lacking. A range of animal models of tendon repair and ACLR showed that DBM can re-create a direct fibrocartilaginous enthesis, although the animal models are not without limitations. Before clinical trials are justified, research is required that determines the best source of DBM (allogenic vs xenogenic) and the best form of DBM (demineralized cortical bone vs DBM paste) to be used in them.
Keywords: ACL, biologic healing enhancement, demineralized bone matrix, demineralized cortical bone, rotator cuff, tendon-bone healing
Tendon and ligament insert into bone at a specialized site called the enthesis, which allows efficient stress distribution from muscle to bone during joint motion.2 “Direct” entheses occur at anatomic sites that are subject to high loading and cyclic stress and exhibit a 4-zone gradient in tissue, with varying tissue composition having different mechanical properties.5 The first zone is tendon, similar to that found in the tendon midsubstance, with a composition of well-aligned collagen I fibers; the second zone is fibrocartilage, with a composition of type II and III collagen; the third zone is calcified fibrocartilage, with a composition predominately of type II collagen; and the fourth zone is bone, characterized by type I collagen and mineral content.47 The tidemark refers to the mechanical boundary between the calcified and uncalcified fibrocartilaginous zones, and it is important for reducing damage to soft tissues during joint movement.1 The anterior cruciate ligament (ACL) and rotator cuff insertion are examples of direct entheses that develop via endochondral ossification.46 “Indirect” entheses are typically found at metaphyseal insertions and are characterized by dense fibrous tissue that attaches to bone indirectly through the periosteum.4
Direct entheses are susceptible to overuse injuries and trauma.4 Following injury, the body is unable to regenerate a direct-type fibrocartilaginous enthesis but instead forms an indirect-type enthesis with inferior biomechanical properties that can lead potentially damaging stress concentrations.8,17,42 In the context of rotator cuff injuries following tendon repair, studies have shown that the defect is replaced by biomechanically inferior fibrovascular tissue,8,17,26,41,44 and high rates of rerupture have been reported.16 When associated with osteopenia of the greater tuberosity, this appears to be associated with compromised suture anchorage for operative repair and reduced pullout strength.7,50 Similarly, following ACL reconstruction (ACLR), the graft heals by fibrovascular scar tissue at the graft-tunnel interface,24,39 which might contribute rerupture rates of up to 6% at 10-year follow-up.11 Augmentation of healing at damaged insertion sites remains a surgical challenge for orthopaedic surgeons, and research investigating biological methods to enhance tendon-bone healing is ongoing.15,28
Demineralized bone matrix (DBM) is an osteoinductive agent that consists of a collagen scaffold containing several growth factors, such as bone morphogenetic proteins (BMPs), insulin growth factor, transforming growth factor, and fibroblast growth factor.48 The manufacture of DBM was first described by Urist,48 and it involves acid extraction of mineral component from bone tissue.13 Urist described how the implantation of DBM into a soft tissue site led to the formation of bone through endochondral ossification, and this article led to the discovery of BMPs. Allogenic DBM poses minimal risk of foreign-body immunogenic reaction and is in current use clinically for treating bone defects in cases of nonunion.56 The promise behind DBM for tendon-bone healing in tendon and ligament injuries is that it can induce new bone formation via endochondral ossification38 and produce a direct-type fibrocartilaginous insertion similar to the native enthesis.43 In the context of rotator cuff and ACL injuries, the hope is that DBM will promote differentiation of the newly formed tendon-bone junction into a direct-type insertion instead of fibrovascular tissue, which could provide physiological distribution of tensile forces onto the bone insertion site and improve surgical outcomes.
The purpose of this systematic review was to (1) critique preclinical and clinical evidence regarding the use of DBM to biologically augment tendon-bone healing; (2) provide a descriptive summary of the current evidence for DBM use in tendon repair and ACLR, as this is the first systematic review on the topic; and (3) highlight areas of future research to facilitate clinical translation.
Methods
This systematic review was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. This review included original peer-reviewed studies based on the following criteria: (1) publication in an English-language journal and (2) an in vivo animal study or clinical study that evaluated the use of DBM in the treatment of tendon repair or ACLR. Studies evaluating biological interventions with recombinant pharmaceutical agents (eg, BMPs in bone defects, spinal injuries, and meniscal injuries) were excluded because the purpose of this review was to focus on DBM applications for tendon-bone healing. Studies reporting in vitro work without in vivo analysis were excluded. Only original peer-reviewed articles were included, so letters, editorials, and review articles were excluded.
The search engines used to identify studies were MEDLINE and EMBASE. The following search terms were used for the literature search: (“demineralized/demineralised bone matrix” OR “demineralized/demineralised cortical bone”) AND (“tissue scaffold” OR “tissue engineering” OR “ligament” OR “tendon” OR “anterior cruciate ligament” OR “rotator cuff”). Searches were performed on February 10, 2017, with no date limit applied.
Studies were first screened to assess suitability for inclusion according to the criteria. Then, full article review was performed by 2 authors (A.T.H. and G.B.) to extract the following information: details of the animal model, the groups investigated, the methods of evaluation, and the main findings. Assessment of the study methodological quality was undertaken by 2 authors (A.T.H. and G.B.) using criteria modified from those proposed by Hooijmans et al,18 as shown in Table 1. The clinical relevance of outcome measures used in animal studies was scored with a peer-reviewed system15 as follows: A, clinically useful quantitative outcome measures (eg, anterior-posterior knee laxity); B, biomechanical testing (eg, ultimate tensile strength of the graft); C, quantitative biochemical outcomes (eg, immunohistochemistry); D, semiquantitative histological analysis; and E, simple nonquantitative histological analysis. Interrater reliability was calculated with intraclass correlation coefficient mean measures (SPSS Statistics, v 22; IBM).
TABLE 1.
Scoring Criteria for Methodological Quality of Animal Studies of Tendon-Bone Healinga
| Criteria | Scores | Comments |
|---|---|---|
| Unit of sample | Unilateral, 1; bilateral, 0 | Studies with bilateral operation may regard limbs as independent samples and assign them to different treatment groups |
| Standardization of surgical procedure | Yes, 1; no, 0 | Descriptions about graft harvest, surgical approach, drilling tunnels, graft tensioning, and fixation method are important |
| Description of surgical complications | Yes, 1; no, 0 | Details such as wound infection and postoperative morbidity and mortality |
| Biomechanical testing | Yes, 1; no, 0 | Mechanical testing is a useful outcome when assessing tendon-bone healing |
| Variation (ratio of SD to mean) | <50%, 1; >50%, 0 | Large SD may imply poor precision or large intragroup variations |
| Statistical method and control group | Appropriate, 1; inappropriate, 0 | Appropriate statistical tests were used, such as analysis of variance or Kruskal-Wallis test |
| Description of tendon-bone interface | Yes, 1; no, 0 | During histological analysis, sampling description for region of interest is important |
| Semiquantitative histological analysis | Yes, 1; no, 0 | During histological analysis, the use of scoring systems indicates better study quality |
aModified from Hooijmans et al.18
Results
Search Results
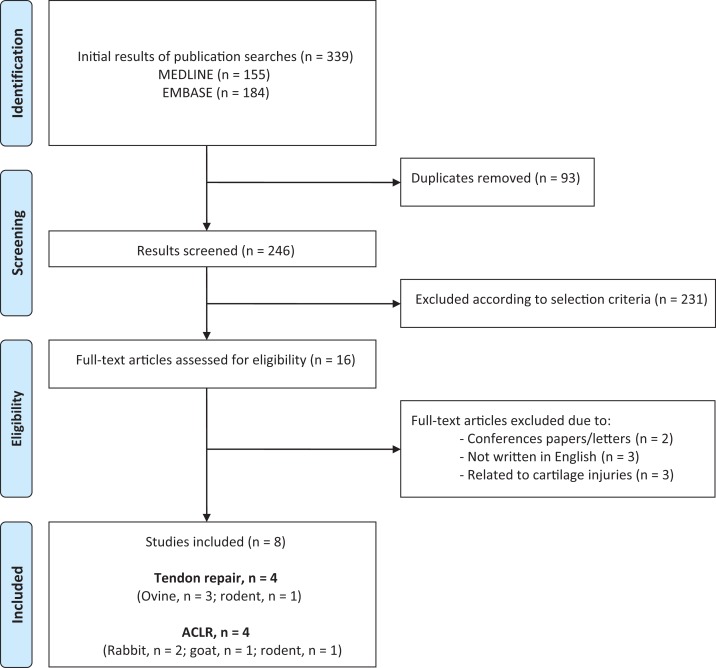
Figure 1 outlines the process for evaluating studies for inclusion in the systematic review. A total of 339 articles were identified through our search literature search. After removal of duplicates and screening to exclude irrelevant studies, 16 studies underwent full review. Of these, 3 were excluded because they were not written in English, 2 because they were conference papers, and 3 because they were related to tissue engineering of cartilage defects.31,34,53 Eight articles met inclusion criteria: the characteristics of the 4 studies14,43–45 that evaluated tendon injuries are shown in Table 2, and the characteristics of the 4 studies19,20,22,27 that evaluated ACLR are shown in Table 3. No clinical studies were identified, and all 8 studies were preclinical animal studies. The median length of follow-up was 12 weeks (used in 4 studies) and ranged from 6 weeks to 1 year. Six studies19,22,27,43,44,45 evaluated DBM in paste form, and 2 studies14,20 evaluated DBM in the form of demineralized cortical bone (DCB). Five studies14,20,22,43,44 evaluated allogenic DBM, and 2 studies19,27 evaluated xenogenic DBM, with 1 study45 comparing the 2 forms directly. One study45 evaluated a combination of biological interventions, which combined mesenchymal stem cells with DBM.
Figure 1.
A flowchart showing the selection of studies for inclusion in the systematic review. ACLR, anterior cruciate ligament reconstruction.
TABLE 2.
Animal Studies Investigating DBM in Tendon Repaira
| Publication | Animal Model | DBM Form | Follow-up | Groups (No.) | Methods (wk) | Findings | Positive Findingb | Quality Scorec | Evidence Level |
|---|---|---|---|---|---|---|---|---|---|
| Thangarajah et al44 (2017) | Rat model of chronic rotator cuff tear | DBM (allogenic) | 6 wk | DBM (6), dermal scaffold (6), control (no augmentation) (6) | SQ histological analysis (6), pQCT (6) | The application of DBM did not improve the composition of the healing enthesis when compared with nonaugmented controls and a commercially available scaffold. Nonaugmented repairs exhibited a significantly higher bone mineral density than DBM | No | 7 | D |
| Elnikety et al14 (2016) | Ovine patellar tendon model (large defect) | DCB (allogenic) | 12 wk | DCB (6), no control group | Gait analysis (3, 9, 12), radiographs (12), ROM (12), pQCT (12), histological analysis (12) | Functional weightbearing significantly increased from 44% at week 3 to 79% at week 12. The formation of a neoenthesis with the presence of fibrocartilage and mineralized fibrocartilage was seen in all specimens. Collagen remodeling with “ligamentization” of the DCB was observed | n/a | 6 | A |
| Thangarajah et al45 (2016) | Ovine patellar tendon model | DBM (allogenic, xenogenic) | 12 wk | Allograft DBM and mmMSCs (5), xenogenic DBM and mmMSCs (5) | Gait analysis (6, 9, 12), pQCT (12), SQ histological analysis (12) | The allograft was associated with significantly higher functional weightbearing throughout. The allogenic group showed greater remodeling of the DBM into tendon-like tissue in the region of the defect, with the presence of direct enthesis associated with more fibrocartilage | n/a | 5 | A |
| Sundar et al43 (2009) | Ovine patellar tendon model | DBM (allogenic) | 12 wk | DBM (8), control (11) | Gait analysis, (3, 6, 9, 12), radiographs, (3, 6, 9, 12), mechanical/UTS (0, 12), SQ histological analysis (6, 12) | Tendon repairs failed at a rate of 33% and 0% for the control and DBM groups, respectively. DBM augmentation resulted in significantly improved functional weightbearing and increased amounts of fibrocartilage and mineralized fibrocartilage on histology | Yes | 8 | A |
aDBM, demineralized bone matrix; DCB, demineralized cortical bone; DCM, demineralized cortical bone; mmMSCs, minimally manipulated mesenchymal stem cells; n/a, not applicable; pQCT, peripheral quantitative computer topography; ROM, range of motion; SQ, semiquantitative; UTS, ultimate tensile strength.
bWe define a positive finding as a study where DBM augmentation provided superior histological scores when compared with a nonaugmented control group.
cOut of 8.
TABLE 3.
Animal Studies Investigating DBM in ACLRa
| Publication | Animal Model | DBM Form | Follow-up | Groups (No.) | Methods (wk/mo) | Findings | Positive Findingb | Quality Scorec | Evidence Level |
|---|---|---|---|---|---|---|---|---|---|
| Hsu and Wang et al19 (2014) | Rabbit | DBM (xenogenic) | 12 wk | ACLR with DBM in the tibial tunnel (5), ACLR alone (5) | Radiographs (4, 8, 12 wk), histological analysis (12 wk), immunohistochemistry (12 wk; VEGF and BMP-2) | DBM group showed less displacement of tendon in tibial tunnel and showed better integration between tendon and bone. The DBM group showed significantly higher expressions of VEGF and BMP-2 | Yes | 5 | C |
| Lovric et al27(2012) | Rat | DBM (xenogenic) | 6 wk | ACLR with DBM on graft/bone tunnel (28), ACLR alone (28) | SQ histological analysis (2, 4, 6 wk), immunohistochemistry (2, 4, 6 wk; BMP-2, BMP-7, Smad4, VEGF, CTSK), mechanical/UTS (4, 6 wk), pQCT (4, 6 wk) | The DBM group showed increased woven bone formation and enhanced bone remodeling. The DBM group had a larger peak load to failure of the tendon-bone interface | Yes | 8 | B |
| Kilicoglu et al22 (2012) | Rabbit | DBM (allogenic) | 9 wk | ACLR alone (6), ACLR with DBM in bone tunnel (6) | SQ histological analysis (3, 6, 9 wk) | The DBM group showed a higher number of Sharpey-like fibers, increased fibrocartilage formation, and new bone formation scores than the control group in the third week. However, all histological scores were similar in both groups in the sixth and ninth weeks | Yes (early) | 6 | D |
| Jackson et al20 (1996) | Goat | DCB (allogenic) | 1 y | ACLR with DBM (14), the contralateral limb acted as control | MRI (6, 12 mo), histological analysis (6, 12 mo), examination for AP laxity (12 mo), mechanical/UTS (0, 12 mo) | AP testing was stable at 1-y follow-up with no failures. Bone replaced the DBM in the bone tunnels, and a fibrocartilage transition was seen with no long-term inflammatory response. The time-zero structural properties of a collagen matrix increased to more desired values after 12 mo, although was less than controls | n/a | 7 | A |
aACLR, anterior cruciate ligament reconstruction; AP, anteroposterior; BMP2, bone morphogenetic protein 2; BMP7, bone morphogenetic protein 7; CTSK, cathepsin; DBM, demineralized bone matrix; DCB, demineralized cortical bone; DCM, demineralized cortical bone; MRI, magnetic resonance imaging; n/a, not applicable; pQCT, peripheral quantitative computer topography; SQ, semiquantitative; UTS, ultimate tensile strength; VEGF, vascular endothelial growth factor.
bWe define a positive finding as a study where DBM augmentation provided superior histological scores when compared with a nonaugmented control group.
cOut of 8.
Assessment of Methodological Quality
The scores for methodological quality (out of 8) for all studies were as follows: 5 for 2 studies, 6 for 2 studies, 7 for 2 studies, and 8 for 2 studies. Acceptable interrater reliability was seen between the 2 reviewers (intraclass correlation coefficient mean measures, 0.792). Consensus on final scores was reached following mutual discussion, with the majority of differences between reviewers seen in their evaluation of the robustness of the statistical analysis used in the studies. Studies lost points for not performing biomechanical testing (n = 5), conducting bilateral operations in 1 animal (n = 2), and not having an appropriate control group/statistics (n = 4).
In terms of outcome measures, 4 studies ranked A, 1 study ranked B, 1 study ranked C, and 2 studies ranked D. All studies used histological outcomes, with quantitative histological analysis performed in 6 studies.19,22,27,43,44,45 Immunohistochemistry was utilized in 2 studies,19,27 with both measuring BMPs and vascular endothelial growth factor. Biomechanical testing was performed in 3 studies,20,27,43 consisting of various tensile failure tests. Peripheral quantitative computed tomography was used in 4 studies14,27,44,45 to assess bone formation at the graft-tunnel interface. Four studies14,20,43,45 evaluated quantitative outcome measures analogous to clinical outcome measures, such as gait analysis and knee laxity.
Appraisal of Study Outcomes
In this systematic review, we defined a positive finding as a study where the use of DBM augmentation provided superior results in terms of histological scores as compared with a nonaugmented control group. Out of the 8 studies, 519,22,27,43,44 compared DBM with a nonaugmented control group. Of these 5, 419,22,27,43 demonstrated positive results for DBM augmentation, and 144 observed no difference. Of the 3 studies with no nonaugmented control, 145 compared 2 types of DBM (allogenic and xenogenic) and showed that allogenic DBM was superior in terms of histological and gait analysis. The other 2 studies14,20 reported favorable findings of DBM-augmented constructs in terms of histological, biomechanical, and radiological outcomes but did not compare their findings with a control group.
Discussion
DBM is an osteoinductive scaffold that has potential in augmentation of tendon-bone healing. This systematic review found that the level of evidence pertaining to the use of DBM in tendon-bone healing is low and remains at the preclinical stage. In this section, we appraise the existing 8 animal studies, critique the representativeness of animal models, and highlight areas for future research if clinical translation is to be achieved.
DBM in Tendon Repair
Four studies evaluating DBM in tendon repair were reported, all of which were from the same laboratory, consisting of 3 studies with a sheep animal model and 1 with a rodent animal model. The first study to evaluate the use of DBM at a healing enthesis was based on an ovine patellar tendon model,43 which allows functional recovery to be directly evaluated because the entire extensor mechanism depends on the tendon-bone interface. Ovine patellar tendon was detached from its insertion on the tibia and repaired with suture anchors alone (control group) or with allogenic DBM interposed between the tendon and bone (intervention group). After 12 weeks, the DBM group demonstrated better functional weightbearing than the controls and with no failures, compared with 33% failure in the control group. Histologically, the control group demonstrated a largely fibrous insertion, whereas in the DBM group, the tendon-bone interface had remodeled into an organized fibrocartilaginous insertion with mineralization.
Elnikety et al14 reported that DBM successfully repaired “large tendon” defects in their work, where allogenic DBM in the form of a strip (ie, DCB) was used to replace a large patellar tendon defect in 6 sheep. After 12 weeks, there was evidence of mature remodeling with the formation of a neoenthesis composed of fibrocartilage and mineralized fibrocartilage. Polarized microscopy confirmed that the haversian system arrangement of the bone was lost and that the collagen had remodeled into a longitudinal arrangement, indicating the process of “ligamentization.”35
The only study identified in this systematic review with a negative finding used a chronic rotator cuff tear model in rats.44 In this study, when compared with nonaugmented repair and augmented repair with commercially available dermal matrix, allogenic DBM was not associated with improved histological remodeling. In all animals, histological analysis showed that the tendon-bone gap closed with a fibrocartilaginous insertion, although the degeneration of the tendon was not reversed. Bone mineral density was measured with peripheral quantitative computed tomography at the humeral head, as this is thought to influence pullout strengths,7 and nonaugmented repairs had higher bone mineral density than both augmented groups.
Thangarajah et al45 reported the first and only study to date that utilized a combination of stem cells with DCM to evaluate tendon-bone healing. In this work, a 1-cm transverse patellar tendon defect in an ovine model was repaired with suture anchors and either allogenic or xenogenic DBM combined with minimally manipulated mesenchymal stem cells isolated by centrifugation of iliac crest bone marrow aspiration during surgery. After 12 weeks, a 4-zone direct neoenthesis was observed in both groups, but the allograft DBM group was associated with more mature fibrocartilaginous and higher functional weightbearing scores. The authors attributed the better outcome seen in the allograft group to the fact that ovine bone has a higher density of bone when compared with porcine bone, thus allowing better tolerance of the mechanical forces and microtrauma across the graft. A limitation of this study is that it did not have a group without minimally manipulated mesenchymal stem cells, which makes the influence of the stem cells on the outcome difficult to determine.
DBM in ACL Reconstruction
The longest in vivo follow-up of the use of DBM in ACL-bone healing was in 1996 when Jackson et al20 used allogenic DBM in DCB form in a goat animal model of ACLR. DCB strips were trimmed to fit through a femoral and tibial tunnel to reconstruct the ACL. Histological analysis showed replacement of DCB with bone within the osseous tunnels and the presence of a more physiological fibrocartilage transition at the graft insertion site. Magnetic resonance image scans showed that the osseous tunnels were nearly obliterated with a signal similar to bone. There was a 550% increase in graft strength at 1 year, although the ultimate tensile strength was statistically significantly less than control ACLs.
Kilicoglu et al22 evaluated the effect of allogenic DBM within a tibial osseous tunnel in a rabbit model of ACL injury and in the first 6 weeks observed slightly increased fibrocartilage formation and more bone formation in the DBM group than the nonaugmented control. However, the superiority was short-term, and by 9 weeks, there were no differences in histology, with both groups showing replacement of fibrocartilage with calcified cartilage and new bone. Conversely, Hsu and Wang19 found that rabbits that had xenogenic (human) DBM applied to the tibial tunnel showed superiority versus nonaugmented repair at 12 weeks. Histological examination showed more intimate contact between tendon and bone in the DBM group with more mineralized fibrocartilage between tendon and bone, which corresponded to higher levels of expression of BMP-2 and vascular endothelial growth factor.
Lovric et al27 performed a study with a rodent model where the intervention group had xenogenic (human) DBM inserted into the femoral and tibial bone tunnels before reconstruction. When compared with a control group, the DBM group had considerably more woven bone formation at the interface, as confirmed on histology and micro–computed tomography, which was associated with statistically higher levels of peak load to failure of the tendon-bone interface. Immunohistochemistry revealed higher levels of BMPs throughout, such as BMP-2 and BMP-7, which were thought to be responsible for the higher level of new bone formation.
Representativeness of Animal Models
Animal models are a useful way to investigate interventions and to see if it is worthwhile to take them into human clinical trials.9 Requirements of animal models in translational orthopaedic research into tendon-bone healing include the following: the model must be comparable to the human environment; the surgery must be feasible, affordable, and ethically acceptable; and animals need to be able to tolerate the rehabilitation and have suitable facilities available.30 A range of small-animal models (rodents, rabbits)19,22,27,44 and large-animal models (goats, sheep)20,43,45 has been used to evaluate the effect of DBM on tendon-bone healing; nevertheless, they are not without limitations. Considerable anatomic differences exist between animals and humans, especially in terms of gait and posture, which can lead to different biomechanical stresses.37 The use of rodent animal models makes the interpretation of results for humans difficult. Rodent models seem to produce significant amounts of fibrous tissue that are not seen in humans, and the pathology associated with tendon damage is different from humans.6 The limited surface area in rodent models might not create an environment conducive to healing owing to high shear forces, which brings into question the suitability of rodent models for assessing tendon-bone healing44 and might account for the rodent animal model being unable to reproduce promising results seen with DBM in the large-animal models. Further doubt over the suitability of rodent models of rotator cuff pathology is that spontaneous bone healing of the humerus occurs, which is not thought to happen in humans.44
There have been 3 large-animal studies (ovine) investigating tendon injury, but the use of DBM for ACLR has been investigated in only 1 large-animal model (goat). There have been 3 large-animal studies (ovine) investigating tendon injury, but the use of DBM for ACLR has been investigated in only 1 large-animal model (goat). A limitation of the studies using large-animal models is that they have used a relatively low number of animals with no reported power calculation. Reasons why studies using small animals generally evaluate larger numbers of animals include small animals (eg, rabbits) having simpler requirements for animal maintenance and housing when compared with larger animals, as well as their potential for faster postoperative recovery.3 Dogs33,40 and pigs10,23 are recognized large-animal models of ACLR that have not been used to evaluate DBM modulation of ACLR, despite some evidence that pigs best reflect human knee in situ forces.55 In the context of tendon injuries, large-animal models have only simulated acute injuries, with the only chronic model of disease being a study in rodents. Acute injury models are useful when investigating the formation of the enthesis, but they do not consider the condition of the tendon, which, in human clinical situations at the time of surgery, is retracted with fatty infiltration.9,17 Similarly, in models of ACLR, the creation of the injury and the surgical reconstruction have always occurred in the same operation, which does not reflect the delay seen in human patients between injury and surgery owing to the preoperative workup. This might have implications because during acute injury there is a release of cytokines and a change in gene expression, which might create a biological environment different from that seen in human patients undergoing ACLR.49,54
The published animals models reported here are also relatively short term, and in this analysis, most of the studies were conducted for <6 months. While this length of time may be appropriate for investigating bone-graft integration, remodeling and ligamentization may take longer. In addition, the surgical procedures for the ACL animal models have all been open, which does not reflect the current surgical practice of arthroscopic reconstruction.
Steps Toward Clinical Translation
This systematic review aimed to review preclinical and clinical evidence behind the use of DBM to augment tendon-bone healing. There were no clinical studies identified, which means that general statements about the effectiveness of DBM to augment tendon-bone healing cannot be made.
More preclinical data are required to justify the use of DBM in clinical trials, and the following areas need to be addressed in particular. First, the best source and method of manufacturing DBM for clinical applications need to be determined,12 reflected by the fact that the strength and biological activity of DBM that is currently available are variable. The biological activity of bone obtained from a young donor compared with that from an older individual would be different; therefore, there is not a consistent supply of DCB. Xenogeneic DCB might be advantageous, as the biological activity of the graft material would be reproducible and there would be an unlimited supply of graft material, although there might be problems with immunogenic activity. Thangarajah et al45 showed that allograft has superior results to xenograft in tendon-bone healing, but further studies comparing xenograft and allograft, with long-term follow-up assessing for immunological reactions, are needed before DBM can be used in humans.
Second, DBM can be created in the form of DCB,44 although this is not commercially available at present. Theoretically, instead of being used to augment tendon reconstruction, the DCB could be used as the primary graft material for reconstruction, with the promise of forming a tendon enthesis via endochondral ossification. Future large-animal studies of ACLR are required that compare DCB reconstruction with DBM augmentation, but these need to have follow-up for at least 6 months to provide time for ligamentization to occur.35 In addition, longer-term studies are needed to clarify if the benefit of DBM is just in the first 3 weeks, as suggested by 1 study,22 or longer lasting, as suggested by others.19,43,45
Finally, as tendon healing involves different stages, such as inflammation, vascularization, and remodeling, it might be possible to enhance tendon-bone healing by using a combination of biological interventions. Future animal studies that combine DBM with other biological approaches to augment tendon-bone healing merit investigation, such as tissue scaffolds,25,36,57 gene therapy,21,29,32 and biophysical interventions.51,52 Before biological interventions achieve clinical translation in addition to demonstrating safety profile and clinical efficacy, they will need to be cost-effective given the current environment of cost containment.
Conclusion
The enthesis has a complex composition, structure, and mechanical behavior that effectively transfer stress from tendon to bone. Injuries to the rotator cuff and ACL are common sports injuries that have high rates of early failure, which is thought to be due in part to inadequate healing at the tendon-bone interface. DBM is a promising biomaterial because it provides a protein scaffold with its own supply of growth factors capable of repairing small/large tendon defects and it augments ACLR by creating a 4-zone fibrocartilaginous enthesis and enhancing osseointegration. DBM shows promise in animal studies, but the use of DBM in tendon-bone healing remains in the exploratory stage with no clinical research undertaken. Future research is required to determine the best source (allogenic versus xenogenic) and best form (augmentation versus reconstruction) of DBM to take forward into clinical trials.
Acknowledgments
The authors thank Heather Chesters and the team at UCL Great Ormond Street Institute of Child Health Library for their support with the literature search.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1. Angeline ME, Rodeo SA. Biologics in the management of rotator cuff surgery. Clin Sports Med. 2012;31(4):645–663. [DOI] [PubMed] [Google Scholar]
- 2. Apostolakos J, Durant TJ, Dwyer CR, et al. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014;4(3):333. [PMC free article] [PubMed] [Google Scholar]
- 3. Bachy M, Sherifi I, Zadegan F, Petrover D, Petite H, Hannouche D. Anterior cruciate ligament surgery in the rabbit. J Orthop Surg Res. 2013;8(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benjamin M, Kumai T, Milz S, Boszczyk B, Boszczyk A, Ralphs J. The skeletal attachment of tendons—tendon “entheses.” Comp Biochem Physiol A Mol Integr Physiol. 2002;133(4):931–945. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin M, Toumi H, Ralphs J, Bydder G, Best T, Milz S. Where tendons and ligaments meet bone: attachment sites (“entheses”) in relation to exercise and/or mechanical load. J Anat. 2006;208(4):471–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchmann S, Walz L, Sandmann GH, et al. Rotator cuff changes in a full thickness tear rat model: verification of the optimal time interval until reconstruction for comparison to the healing process of chronic lesions in humans. Arch Orthop Trauma Surg. 2011;131(3):429–435. [DOI] [PubMed] [Google Scholar]
- 7. Cadet ER, Hsu JW, Levine WN, Bigliani LU, Ahmad CS. The relationship between greater tuberosity osteopenia and the chronicity of rotator cuff tears. J Shoulder Elbow Surg. 2008;17(1):73–77. [DOI] [PubMed] [Google Scholar]
- 8. Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7(6):599–605. [DOI] [PubMed] [Google Scholar]
- 9. Carpenter JE, Thomopoulos S, Soslowsky LJ. Animal models of tendon and ligament injuries for tissue engineering applications. Clin Orthop Relat Res. 1999;367:S296–S311. [DOI] [PubMed] [Google Scholar]
- 10. Cho S, Li H, Chen C, Jiang J, Tao H, Chen S. Cationised gelatin and hyaluronic acid coating enhances polyethylene terephthalate artificial ligament graft osseointegration in porcine bone tunnels. Int Orthop. 2013;37(3):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crawford SN, Waterman MBR, Lubowitz JH. Long-term failure of anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(9):1566–1571. [DOI] [PubMed] [Google Scholar]
- 12. Dinopoulos HT, Giannoudis PV. Safety and efficacy of use of demineralised bone matrix in orthopaedic and trauma surgery. Expert Opin Drug Saf. 2006;5(6):847–866. [DOI] [PubMed] [Google Scholar]
- 13. Drosos GI, Kazakos KI, Kouzoumpasis P, Verettas D-A. Safety and efficacy of commercially available demineralised bone matrix preparations: a critical review of clinical studies. Injury. 2007;38:S13–S21. [DOI] [PubMed] [Google Scholar]
- 14. Elnikety S, Pendegrass CJ, de Godoy RF, Holden C, Blunn GW. Augmentation and repair of tendons using demineralised cortical bone. BMC Musculoskelet Disord. 2016;17(1):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fu SC, Cheuk YC, Yung SH, Rolf CG, Chan KM. Systematic review of biological modulation of healing in anterior cruciate ligament reconstruction. Orthop J Sports Med. 2014;2(3):23259 67114526687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219–224. [DOI] [PubMed] [Google Scholar]
- 17. Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff: correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982–989. [PubMed] [Google Scholar]
- 18. Hooijmans CR, Leenaars M, Ritskes-Hoitinga M. A gold standard publication checklist to improve the quality of animal studies, to fully integrate the three Rs, and to make systematic reviews more feasible. Altern Lab Anim. 2010;38(2):167–182. [DOI] [PubMed] [Google Scholar]
- 19. Hsu SL, Wang CJ. The use of demineralized bone matrix for anterior cruciate ligament reconstruction: a radiographic, histologic, and immunohistochemical study in rabbits. J Surg Res. 2014;187(1):219–224. [DOI] [PubMed] [Google Scholar]
- 20. Jackson DW, Simon TM, Lowery W, Gendler E. Biologic remodeling after anterior cruciate ligament reconstruction using a collagen matrix derived from demineralized bone: an experimental study in the goat model. Am J Sports Med. 1996;24(4):405–414. [DOI] [PubMed] [Google Scholar]
- 21. Kawakami Y, Takayama K, Matsumoto T, et al. Anterior cruciate ligament-derived stem cells transduced with BMP2 accelerate graft-bone integration after ACL reconstruction [published online December 14, 2016]. Am J Sports Med. [DOI] [PubMed] [Google Scholar]
- 22. Kilicoglu OI, Dikmen G, Koyuncu O, Bilgic B, Alturfan AK. Effects of demineralized bone matrix on tendon-bone healing: an in vivo, experimental study on rabbits. Acta Orthopaedica et Traumatologica Turcica. 2012;46(6):443–448. [DOI] [PubMed] [Google Scholar]
- 23. Kouroupis D, Kyrkou A, Triantafyllidi E, et al. Generation of stem cell-based bioartificial anterior cruciate ligament (ACL) grafts for effective ACL rupture repair. Stem Cell Res. 2016;17(2):448–457. [DOI] [PubMed] [Google Scholar]
- 24. Lazarides AL, Eward WC, Green K, Cardona DM, Brigman BE, Taylor DC. Histological evaluation of tendon-bone healing of an anterior cruciate ligament hamstring graft in a 14-year-old boy. Am J Sports Med. 2015;43(8):1935–1940. [DOI] [PubMed] [Google Scholar]
- 25. Lee AJ, Chung WH, Kim DH, et al. Anterior cruciate ligament reconstruction in a rabbit model using canine small intestinal submucosa and autologous platelet-rich plasma. J Surg Res. 2012;178(1):206–215. [DOI] [PubMed] [Google Scholar]
- 26. Liu SH, Panossian V, al-Shaikh R, et al. Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res. 1997(339):253–260. [DOI] [PubMed] [Google Scholar]
- 27. Lovric V, Chen D, Yu Y, Oliver RA, Genin F, Walsh WR. Effects of demineralized bone matrix on tendon-bone healing in an intra-articular rodent model. Am J Sports Med. 2012;40(10):2365–2374. [DOI] [PubMed] [Google Scholar]
- 28. Lui PP-Y, Zhang P, Chan K-M, Qin L. Biology and augmentation of tendon-bone insertion repair. J Orthop Surg Res. 2010;5(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Madry H, Kohn D, Cucchiarini M. Direct FGF-2 gene transfer via recombinant adeno-associated virus vectors stimulates cell proliferation, collagen production, and the repair of experimental lesions in the human ACL. Am J Sports Med. 2013;41(1):194–202. [DOI] [PubMed] [Google Scholar]
- 30. Madry H, Ochi M, Cucchiarini M, Pape D, Seil R. Large animal models in experimental knee sports surgery: focus on clinical translation. J Exp Orthop. 2015;2(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Man Z, Hu X, Liu Z, et al. Transplantation of allogenic chondrocytes with chitosan hydrogel-demineralized bone matrix hybrid scaffold to repair rabbit cartilage injury. Biomaterials. 2016;108:157–167. [DOI] [PubMed] [Google Scholar]
- 32. Martinek V, Latterman C, Usas A, et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am. 2002;84(7):1123–1131. [DOI] [PubMed] [Google Scholar]
- 33. Matsumoto T, Kubo S, Sasaki K, et al. Acceleration of tendon-bone healing of anterior cruciate ligament graft using autologous ruptured tissue. Am J Sports Med. 2012;40(6):1296–1302. [DOI] [PubMed] [Google Scholar]
- 34. Meng Q, Man Z, Dai L, et al. A composite scaffold of MSC affinity peptide-modified demineralized bone matrix particles and chitosan hydrogel for cartilage regeneration. Sci Rep. 2015;5:17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muller B, Bowman KF, Bedi A. ACL graft healing and biologics. Clin Sports Med. 2013;32(1):93–109. [DOI] [PubMed] [Google Scholar]
- 36. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19(4):493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reddi AH, Anderson WA. Collagenous bone matrix-induced endochondral ossification hemopoiesis. J Cell Biol. 1976;69(3):557–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel: a biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795–1803. [DOI] [PubMed] [Google Scholar]
- 40. Sasaki K, Kuroda R, Ishida K, et al. Enhancement of tendon-bone osteointegration of anterior cruciate ligament graft using granulocyte colony-stimulating factor. Am J Sports Med. 2008;36(8):1519–1527. [DOI] [PubMed] [Google Scholar]
- 41. St Pierre P, Olson EJ, Elliott JJ, O’Hair KC, McKinney LA, Ryan J. Tendon-healing to cortical bone compared with healing to a cancellous trough: a biomechanical and histological evaluation in goats. J Bone Joint Surg Am. 1995;77(12):1858–1866. [DOI] [PubMed] [Google Scholar]
- 42. Stolarz M, Ficek K, Binkowski M, Wróbel Z. Bone tunnel enlargement following hamstring anterior cruciate ligament reconstruction: a comprehensive review [published online November 11, 2016]. Phys Sportsmed. [DOI] [PubMed] [Google Scholar]
- 43. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115–122. [DOI] [PubMed] [Google Scholar]
- 44. Thangarajah T, Henshaw F, Sanghani-Kerai A, Lambert SM, Blunn GW, Pendegrass CJ. The effectiveness of demineralized cortical bone matrix in a chronic rotator cuff tear model [published online February 2, 2017]. J Shoulder Elbow Surg. [DOI] [PubMed] [Google Scholar]
- 45. Thangarajah T, Shahbazi S, Pendegrass CJ, Lambert S, Alexander S, Blunn GW. Tendon reattachment to bone in an ovine tendon defect model of retraction using allogenic and xenogenic demineralised bone matrix incorporated with mesenchymal stem cells. PLoS One. 2016;11(9):e01614 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thomopoulos S, Genin GM, Galatz LM. The development and morphogenesis of the tendon-to-bone insertion: what development can teach us about healing. J Musculoskelet Neuronal Interact. 2010;10(1):35. [PMC free article] [PubMed] [Google Scholar]
- 47. Thomopoulos S, Marquez JP, Weinberger B, Birman V, Genin GM. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech. 2006;39(10):1842–1851. [DOI] [PubMed] [Google Scholar]
- 48. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893–899. [DOI] [PubMed] [Google Scholar]
- 49. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–1542. [DOI] [PubMed] [Google Scholar]
- 50. Waldorff EI, Lindner J, Kijek TG, et al. Bone density of the greater tuberosity is decreased in rotator cuff disease with and without full-thickness tears. J Shoulder Elbow Surg. 2011;20(6):904–908. [DOI] [PubMed] [Google Scholar]
- 51. Walsh WR, Stephens P, Vizesi F, Bruce W, Huckle J, Yu Y. Effects of low-intensity pulsed ultrasound on tendon-bone healing in an intra-articular sheep knee model. Arthroscopy. 2007;23(2):197–204. [DOI] [PubMed] [Google Scholar]
- 52. Wang CJ, Ko JY, Chou WY, et al. Shockwave therapy improves anterior cruciate ligament reconstruction. J Surg Res. 2014;188(1):110–118. [DOI] [PubMed] [Google Scholar]
- 53. Wang X, Li Y, Han R, et al. Demineralized bone matrix combined bone marrow mesenchymal stem cells, bone morphogenetic protein-2 and transforming growth factor-beta3 gene promoted pig cartilage defect repair. PLoS One. 2014;9(12):e11606 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Y, Tang Z, Xue R, et al. TGF-β1 promoted MMP-2 mediated wound healing of anterior cruciate ligament fibroblasts through NF-κB. Connect Tissue Res. 2011;52(3):218–225. [DOI] [PubMed] [Google Scholar]
- 55. Xerogeanes JW, Fox RJ, Takeda Y, et al. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Eng. 1998;26(3):345–352. [DOI] [PubMed] [Google Scholar]
- 56. Zhai X. Value of bone repair materials in the treatment of fractures and bone defects. Journal of Clinical Rehabilitative Tissue Engineering Research. 2011;15(51):9655–9658. [Google Scholar]
- 57. Zhang W, Pan W, Zhang M, Wei Y. In vivo evaluation of two types of bioactive scaffold used for tendon-bone interface healing in the reconstruction of anterior cruciate ligament. Biotechnol Lett. 2011;33(4):837–844. [DOI] [PubMed] [Google Scholar]