Abstract
Background
Cartilage degeneration has been reported after recurrent patellar dislocation. However, effects of surgical stabilization in childhood have not yet been described.
Purpose
To examine the cartilage quality in very young adults operated with a patellar stabilizing procedure due to recurrent patellar dislocation in childhood, and evaluate if cartilage quality correlates with clinical parameters and patient-reported outcomes.
Material and Methods
Seventeen patients were investigated ≥ 5 years (mean = 11.6 years) after patellar stabilizing surgery in childhood. Pre-contrast T2 relaxation times were analyzed in four superficial and four deep patellar cartilage regions of both knees. Two hours after 0.2 mM/kg Gd-DTPA2 i.v., post-contrast T1 (T1(Gd)) was analyzed in the same regions. Patient-reported outcomes (KOOS, Kujala, and Tegner scores) and recurrence rates were evaluated.
Results
Comparing operated to healthy side, neither T2 nor dGEMRIC differed between the operated and the reference knee regarding the superficial half of the cartilage. In the deep half of the cartilage, T1(Gd) was shorter in the central part of the cartilage, whereas T2 was longer medially (P < 0.05). A low score in the KOOS subscales Symptom and Sports & Recreation, was correlated to the degenerative changes detected by T1(Gd) (r = 0.5, P = 0.041).
Conclusion
In general, our findings demonstrate good cartilage quality 12 years after patellar stabilizing surgery during childhood. The subtle changes in T2 and T1(Gd) in the deep cartilage layer may be a result of altered biomechanics, although very early degenerative changes cannot be excluded. The short T1(Gd) centrally may reflect lower glycosaminoglycan content, whereas the increase in T2 medially indicates increased cartilage hydration.
Keywords: dGEMRIC, T2, patellar dislocation, post-traumatic osteoarthritis
Introduction
Recurrent patellar dislocation in childhood is a complex condition, related to both anatomical and general risk factors for patellar instability. In healthy children, the annual incidence of first time traumatic patellar dislocation is 0.6–1.2:1000, and it is the most common cause of traumatic hemarthrosis in the age group 9–15 years (1). Despite adequate non-surgical treatment, the recurrence rate is high. Approximately 50% of the children are at risk of recurrence and it seems that the risk of recurrence is higher in younger children (2).
In recurrent patellar dislocation, the cartilage of the patella is exposed to repeated shear stress with the potential to initiate degenerative changes and future osteoarthritis (OA). Using delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC), Watanabe et al. have shown that the severity of cartilage degeneration increases with time in adult patients with recurrent patellar dislocation (3). Combining dGEMRIC and T2 mapping, Bengtsson Moström et al. have recently demonstrated that also children with recurrent dislocation show signs of superficial cartilage degeneration on the patella, already in early adulthood (4). Both these magnetic resonance imaging (MRI) metrics reflect two important features in early OA: loss of glycosaminoglycans (GAG) and disruption of the collagen network (5–7). In dGEMRIC, the negatively charged contrast agent Gd-DTPA2- distributes in an inverse relationship to the negatively charged GAG in the cartilage. Consequently, a short T1 relaxation time when gadolinium is present (usually referred to as T1(Gd) or the dGEMRIC index) corresponds to a low cartilage GAG content (8,9).
Longer T2 relaxation time indicates disruption of the collagen network with increased water content, which is seen in osteoarthritic cartilage (10,11).
Several surgical methods have been described to treat recurrent patellar dislocation, including reconstruction of the soft-tissue stabilizers, and correction of malalignment and dysplasia through skeletal procedures (12,13). In the growing child, preservation of the physes is important in order to avoid asymmetric and premature closure that might cause secondary deformities. The outcome of surgical treatment is good regarding recurrence rate, but the knee function is not fully restored (14). Furthermore, the stabilizing surgery may alter the biomechanical load of the patellofemoral joint (15). In adults, even though surgery reduces the recurrence rate, a high incidence of degenerative changes in the patellofemoral joint, i.e. early OA, has been reported at long-term follow-up (16). Whether cartilage degeneration also occurs if patellar stabilizing surgery is performed before skeletal maturity is not yet described.
The purpose of the present study was to examine the cartilage quality, using dGEMRIC and T2-mapping, in young adults who had been operated due to recurrent patellar dislocation in childhood. We also wanted to investigate if clinical parameters correlate with MRI findings.
Material and Methods
The study was approved by the Human Ethics Committee of the local research institute and was conducted according to the Declaration of Helsinki (D.nr. 2009/610-31/2, 2013/1473-32). The patients received oral and written information before inclusion in the study. Participation was voluntary and written consent was obtained from all patients.
The study consisted of patients with unilateral traumatic patellar dislocation who were operated in childhood (age range = 9–16 years) due to persistent patellar instability after non-surgical treatment. The minimum follow-up time was five years after surgery (proximal realignment combined with a distal realignment procedure in patients with an increased quadriceps angle [> 20°]. The non-injured knee of each participant served as control. The exclusion criteria were osteochondral injury at index patellar dislocation, a history of other significant knee injuries, medical conditions influencing knee function, or re-dislocation ≤ 3 months before the follow-up examination.
Sample size calculation was based on the results from an earlier study of patellar cartilage in conservatively treated patients with recurrent patellar dislocations (3), which would require 15 patients for a power of 80%, a type-I error (α) of 0.05, and an estimated effect size of 0.8.
We could establish contact with 23 patients that had been operated between 1998–2008. Eighteen of these patients accepted participation, but one patient had to be excluded from the analysis due to motion artifacts in the MR images. Thus, the study group consists of 17 unilaterally operated patients. Detailed information regarding the patients is presented in Table 1.
Table 1.
Operatively treated patients with recurrent patellar dislocation, n = 17.
| Patient-reported outcome | Demographics | Mean | ±1 SD | Range |
|---|---|---|---|---|
| KOOS pain | F: 92, M: 92* | 81 | 14 | 53–94 |
| KOOS symptom | F: 89, M: 87* | 72 | 24 | 14–100 |
| KOOS ADL | F: 95, M: 94* | 84 | 17 | 51–100 |
| KOOS sports & recreation | F: 86, M: 85* | 58 | 28 | 10–90 |
| KOOS quality of life | F: 84, M: 85* | 44 | 24 | 6–81 |
| Kujala | 99.9† | 77 | 14 | 51–94 |
| BMI | 25 | 4 | 19–34 | |
| Number of re-dislocations before surgery | 8 | 6 | 2–22 | |
| Tegner activity level | 4 | 2 | 2–7 | |
| Age at index injury (years) | 12 | 2 | 8–15 | |
| Age at follow-up (years) | 25 | 3 | 18–29 | |
| Follow-up time from index injury (years) | 13 | 2 | 11–19 | |
| Time from index dislocation to operation (years) | 1.8 | 1.4 | 0.4–5.7 | |
| Sex F:M | 12:5 |
Normal reference values of KOOS (18): Knee injury and Osteoarthritis Outcome Score, range 0–100; higher scores indicate a better outcome. Values in parentheses are the mean values in men and women, aged 18–34 years in a population-based study by Paradowski et al. (22).
†Normal reference values of the Kujala score (17), Kujala Anterior Knee pain score, range 0–100; higher scores indicate a better outcome. Values in parentheses are the mean normal value in healthy adults.
Patient-reported outcome (PRO)
PRO was assessed by the Kujala score (17) and the Knee injury and Osteoarthritis Outcome Score (KOOS) (18), and the activity level by Tegner activity score (19).
Imaging protocol
MRI examinations of both knees were performed in a Philips Achieva® 1.5-T scanner (Philips Medical Systems, Best, The Netherlands) with a dedicated knee coil. The operated knee was scanned first and the non-injured knee directly thereafter. The individuals were examined in accordance with a previously published study of non-operatively treated patients with recurrent patellar dislocation (4). Because patients were investigated both pre-contrast and post-contrast, great care was taken to ensure identical positioning of the knee in-between investigations, including marking of the apex, the proximal pole, and the center of the patella. Apex patella was placed in the central line of the knee coil. Anatomical landmarks were matched, and the slice selection positioned centrally over the patella in the transverse plane (Fig. 1) by the same researcher (EBM). The quality of the MR images was independently evaluated by two experienced evaluators (TF and EL) and when motion artifacts was observed in either the T2 or the T1Gd images the patient was excluded from further analysis and excluded from the study.
Fig. 1.
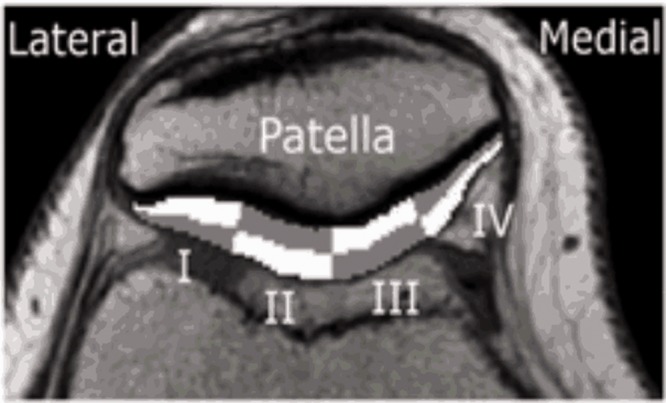
Regions of interest.
Pre-contrast investigations: Diagnostic proton density weighted images and T2 mapping was performed pre-contrast.
The following sequence was used for T2 mapping: multi-slice multi-echo spin echo (field of view [FOV] = 12 cm; matrix = 256 × 256; TR = 2000 ms; eight TEs in the range of 9–72 ms; ETL = 8; slice thickness = 3 mm; number of averages = 1).
Contrast medium injection: To assess renal function, serum creatinine was analyzed before the MRI examination and normal creatinine levels (below 100 µmol/L) was confirmed in all participants. After the T2 mapping and diagnostic MRI sequences, an intravenous injection of Gd-DTPA2 (0.2 mM/kg [0.4 mL/kg] of Magnevist®, Bayer Schering AG, Berlin, Germany) was administered to the individuals. Starting 5 min after contrast injection, participants walked up and down stairs for 10 min in order to facilitate the diffusion of the contrast agent into the cartilage (7,8).
Post-contrast investigations: The post-contrast series was performed 2 h after the contrast injection. The sequence for T1 post-contrast mapping of the patellar cartilage was: 2D inversion recovery fast spin echo (FOV = 12 cm; matrix = 256 × 256; TR = 2000 ms; TE = 15 ms; six TIs of 1600, 800, 400, 200, 100, and 50 ms; ETL = 5; slice thickness = 3 mm; number of averages = 2). The mean time between scanning of the operated and the non-injured control knee was 17 min.
Regions of interest (ROIs): Segmentation of ROIs for T1(Gd) and T2 calculations was done using an in-house MATLAB application (MATLAB R2010, MathWorks Inc, Natick, MA, USA).
The patellar cartilage was divided in four sections from medial to lateral (sectors I–IV), which were further divided into a deep and a superficial half, resulting in eight ROIs: four deep and four superficial (Fig. 1)
To standardize the procedure, all ROI segmentations were done manually by one researcher (EBM) and re-evaluated by another researcher (EL). In cases of discrepancies between the two investigators, segments were re-evaluated and consensus was reached.
In order to compensate for dosing bias due to differences in BMI, we used the correction factor recommended by Tiderius et al. (20).
Conventional diagnostic images were evaluated by a senior radiologist (TF), and the patellar cartilage was classified according to the modified Outerbridge classification system (21) by two independent evaluators (TF and EBM).
Correlation between the macroscopic cartilage status and the functional imaging parameters of the patellar cartilage were assessed in all knees.
Statistical analysis
IBM SPSS Statistics® (version 22) (SPSS IBM, Armonk, NY, USA) was used for the statistical analysis. The level of significance was set to P ≤ 0.05. The Shapiro Wilks test of normality was performed. When analyzing MR parameters, the paired T-test was used. When analyzing PRO measure and the correlation between MR parameters and PRO, non-parametric tests were used: Wilcoxon signed rank test, Spearman rho correlation, and Mann Whitney U test, respectively.
Results
The mean age at index injury was 11.8 years (age range = 8.0–15.0 years) and the mean time from index injury to operation was 1.8 years (range = 0.4–5.7 years). The mean follow-up time from index injury was 13.4 years (range = 10.7–19.3 years) and from surgery to follow-up was 11.6 years (range = 8.8–15.0) (Table 1).
The mean number of re-dislocations after index injury and prior to the operation was eight (range = 2–22). There were no re-dislocations requiring medical attention, however, six patients experienced one or two subluxations within the first 12 months after surgery. No subluxations were reported thereafter. Three patients had proximal realignment surgery only, and in 14 patients the distal alignment was also in need of correction. The recurrence rate or the type of surgery was not correlated to PRO, T2, or T1(Gd) findings at follow-up.
PROs
In all five subscales of the KOOS, the patients scored lower than a comparative age group of a normal population of asymptomatic individuals (22). The Kujala score demonstrated problems pertaining to anterior knee pain. Despite the KOOS and Kujala scores, the patients participated in recreational sports activities one to five times a week. The activity level according to Tegner was 4. The results are presented in Table 1.
T2
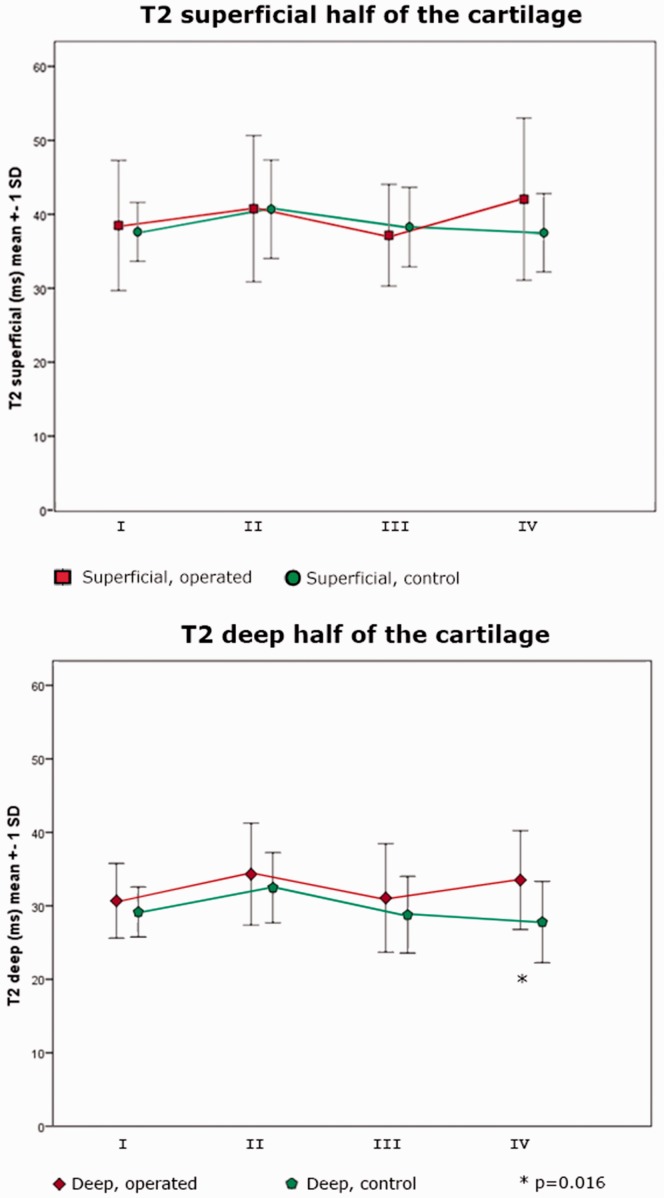
In general, the T2 values were longer in the superficial compared to the deep half of the cartilage in all knees (Table 2). Comparing operated and control knees, T2 was longer in the operated knee in the deep half of sector IV, 34 ± 7 ms and 28 ± 6 ms, respectively (P = 0.016). No significant difference could be seen in the superficial half of the cartilage (Fig. 2).
Table 2.
MR parameters and the results of paired t-test, operated compared to healthy control in the different sectors (I–IV).
| Sector | I Operated | I Healthy | II Operated | II Healthy | III Operated | III Healthy | IV Operated | IV Healthy |
|---|---|---|---|---|---|---|---|---|
| T1(Gd) Superficial half (ms) | 462 (± 69) | 464 (± 64) | 451 (± 71) | 462 (± 71) | 416 (± 66) | 438 (± 68) | 380 (± 40) | 392 (± 43) |
| P | 0.880 | 0.370 | 0.264 | 0.342 | ||||
| T1(Gd) Deep half (ms) | 587 (± 75) | 574 (± 89) | 588 (± 76) | 595 (± 84) | 497 (± 94) | 562 (± 96) | 426 (± 65) | 442 (± 62) |
| P | 0.437 | 0.633 | 0.016 | 0.381 | ||||
| T2 Superficial half (ms) | 38.5 (± 8.8) | 37.6 (± 3.7) | 40.8 (± 9.9) | 40.7 (± 6.7) | 37.2 (± 6.9) | 38.3 (± 5.4) | 42.0 (± 11.0) | 37.5 (± 5.3) |
| P | 0.690 | 0.976 | 0.531 | 0.156 | ||||
| T2 Deep half (ms) | 30.7 (± 5.1) | 29.2 (± 3.4) | 34.3 (± 6.9) | 32.5 (± 4.8) | 31.1 (± 7.4) | 28.8 (± 5.2) | 33.5 (± 6.7) | 27.8 (± 5.5) |
| P | 0.228 | 0.252 | 0.219 | 0.016 |
Fig. 2.
T2 in the superficial and the deep half of the cartilage, respectively. A significant difference between operated and control was seen in the deep half of the cartilage in sector IV (P = 0.016).
T1 post contrast, T1(Gd)
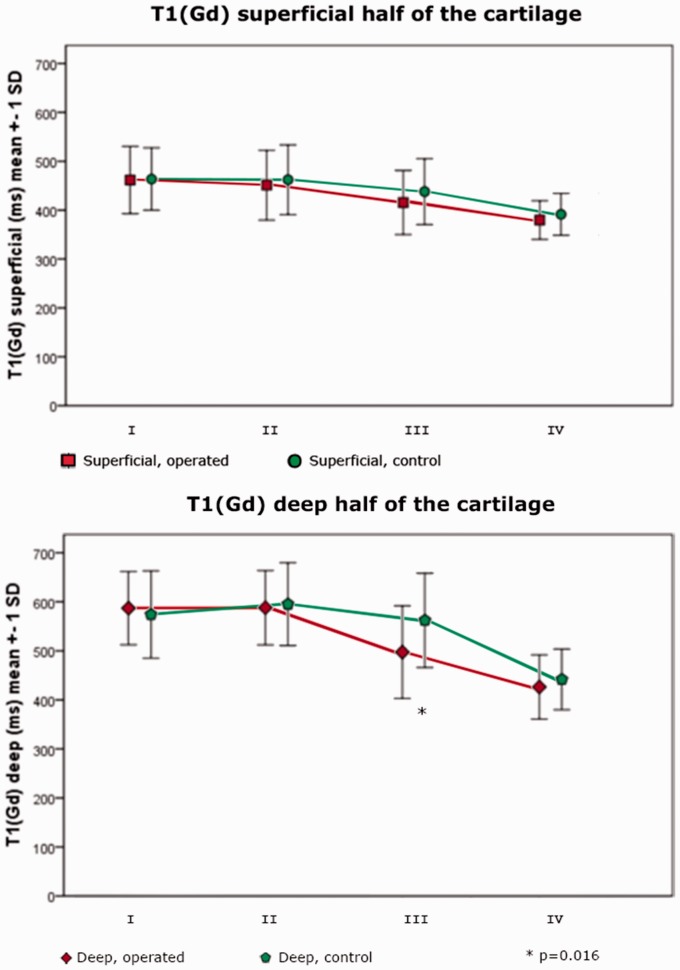
T1(Gd) was longer in the deep compared to the superficial half of the cartilage in both injured and asymptomatic knees (Table 2, Fig. 3).
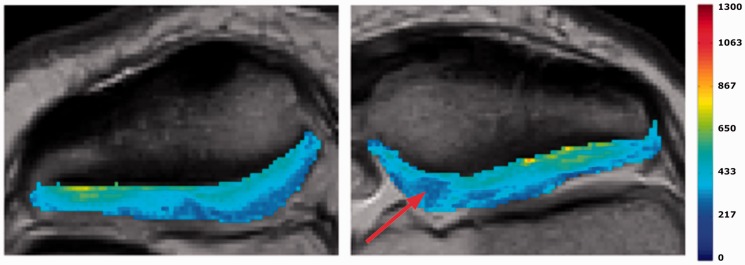
Fig. 3.
Example of T1(Gd) of the non- injured patella and the operated patella. Note the low dGEMRIC index in the central region of the operated knee (arrow).
Comparing T1(Gd) of the operated with the control knee, we found a significant decrease in T1(Gd) in the deep half of sector III. In the deep cartilage T1(Gd) was 497 ± 94 ms in the operated knee compared to 562 ± 96 ms in the healthy knee (P = 0.016). No difference could be detected in the superficial half of the cartilage (Fig. 4).
Fig. 4.
T1(Gd) of the superficial and the deep half of the cartilage, respectively. A significant difference between operated and control was seen in the deep half of the cartilage in sector III (P = 0.016).
Outerbridge classification and correlation with quantitative MRI
The modified Outerbridge classification of cartilage lesions revealed two patients with grade 1 lesions (abnormal signal in the cartilage, without contour defects) and four patients with grade 2 lesions (contour defects of less than or equal to 50% of the cartilage thickness) of the patella. Inter-rater agreement was 0.808 according to Cohen’s kappa, indicating a strong agreement between the evaluators.
No correlations were found between the T1(Gd) or the T2 values and the grade of the cartilage lesions.
PRO and correlations to quantitative MRI
The T1(Gd) of the deep half of the cartilage in sector III in the operated knee was correlated to both the KOOS symptom (r = 0.501, P = 0.041) and KOOS sports and recreation (r = 0.500, P = 0.041), but not to sex, age at injury, recurrence rate, activity level, or follow-up time.
There was no correlation between sex, age at injury, number of re-dislocations, PRO, activity level, and the T2 values.
Discussion
To the best of our knowledge, this is the first study using both dGEMRIC and T2 mapping to describe how the cartilage quality is affected by patellar stabilizing surgery in childhood.
The fact that no significant differences were found in the superficial half of the patellar cartilage indicates absence of early OA in these patients, despite a mean follow-up of 12 years after surgery. In a previously investigated cohort of slightly younger patients (mean age = 22 years compared to 25 years in the present study) with persistent patellar dislocations, a low T1(Gd) was found in the medial part of the superficial cartilage, consistent with GAG loss in that area. Also, Watanabe et al. report signs of early cartilage degeneration in recurrent, non-operated patients of similar age as in the present study. In that study, only full thickness cartilage ROIs were analyzed. Hence, in addition to regaining patellar stability (only two early subluxations were reported after surgery), the patellar stabilizing surgery may have been beneficial for the cartilage integrity, at least in the superficial cartilage where OA usually starts.
The shorter T1(Gd) in the deep half of the patellar cartilage in sector III indicates less GAG in the operated knee compared to the healthy contralateral knee (23,24). The localization of these changes is in accordance with previous findings in young adult and middle-aged patients (3,4).
An upregulation of the GAG synthesis has been reported as a response to dynamic pressure (25), On the contrary, static compression inhibits the synthesis of proteins and proteoglycans (26,27). Also, excessive mechanical stress can alter the balance between synthesis and degradation (28). Since our patients had been clinically stable after surgery, it could be assumed that the shear stress on the patellar cartilage has decreased. However, the axial load, i.e. the pressure between the patella and the femur may have increased. Several studies have demonstrated a shift in load from lateral to medial (corresponding to our sector III) after patellar stabilizing surgery (15,29,30). It could be hypothesized that increased focal strain in sector III after surgery has impeded the synthesis of proteoglycans in the deep cartilage layer (25,27,31).
Both in the operated and the contralateral knee, we found longer T1(Gd) in the deep compared to the superficial part of the cartilage. This result is well in accordance with previous studies of both femoral and patella cartilage (4,32). The high dGEMRIC index in the deep cartilage may to some extent depend on a high cartilage GAG content (33). However, another important explanation for this finding is a slower diffusion of the contrast medium into deep cartilage regions (32). This effect increases with cartilage thickness and is therefore particularly important to recognize when investigating patella cartilage, the thickest articular cartilage in the human body.
In the growing child, the histological pattern, and thereby also the T2 relaxation time, will gradually mature and resemble the adult T2 pattern; a gradual lengthening of the T2 towards the surface (34). In general, T2 values were longer in the superficial compared to the deep half of the cartilage in both operated and control knee, indicating a mature and organized collagen fiber structure (4,34,35). Though, most medially, in sector IV, we found a longer T2 in the deep half of the cartilage compared to the healthy side.
The T2 relaxation of articular cartilage is influenced by several parameters such as integrity and orientation of the collagen network, GAG concentration, and macromolecular interactions (23,36). Most published data report longer T2 values in early stages of OA, due to cartilage swelling secondary to disruption of the collagen network (23). Moreover, longer T2 values has been correlated to progression of cartilage degeneration, which indicates a prognostic value of T2 mapping in terms of OA development (37).
As in previous studies, the correlation between T1(Gd) and KOOS indicates a clinical relevance of the dGEMRIC findings (38). In patients with pre-radiographic cartilage changes on the femoral condyle, a low dGEMRIC index was related to OA at a six-year follow-up (39). In our patients, the surgical procedure almost eliminated the patellar instability, but the PROs were not restored to normal for their age. Furthermore, when comparing the present results to previously published results from a cohort of non-operatively treated patients with recurrent patellar dislocation, the operatively treated patients had lower values in KOOS ADL, Sports and Recreation, and Quality of life (4). A change in KOOS subscale of 8–10 p is often considered clinically significant (18,22). Unfortunately, we do not have PROs from the time of surgery to compare with the present results.
Our study has limitations, the relatively small number of patients being the most obvious. However, several dGEMRIC studies have found clinically relevant results in small series of patients (8,9,38,39). Another issue relates to using the contralateral, asymptomatic knee as a healthy control. Watanabe et al. found a slightly decreased T1(Gd) also in the asymptomatic knee in patients with non-operated unilateral patellar instability. Importantly, our patients do not have any persistent instability after surgery, which may have eliminated this potential error. Another reason for using the contralateral knee as a reference is the fact that T1(Gd) differs between individuals. Also, differences in BMI also affects T1(Gd), a source of error that we have eliminated by using the contralateral knee as reference.
Also, it would be interesting to evaluate patients at earlier time-points to study a potential progression of cartilage changes. However, that was not possible considering the limited number of patients available. In addition, a shorter follow-up period would increase the risk of a more heterogeneous population since the youngest patients would still be skeletally immature.
In conclusion, our findings demonstrate good cartilage quality 12 years after patellar stabilizing surgery during childhood, at least in the superficial cartilage layer where OA initially occurs. The increase in T2 in the deep cartilage layer indicates increased hydration but may also be a result of different collagen orientation after surgery. The shorter T1(Gd) in the deep cartilage of the medial facet is consistent with lower GAG content in that area. The relevance of these subtle changes in the deep cartilage with respect to OA development require future follow-up.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from: H.R.H. King Oscar II’s and H.R.H. Queen Sophia’s Golden Wedding Foundation, Capio Research Foundation, Sophiahemmet Research Foundation and Swedish National Centre for Research in Sports, Skandia Research Foundation, Promobilia Research Foundation, and Trygg-Hansa Research Foundation.
References
- 1.Askenberger M, Ekström W, Finnbogason T, et al. Occult intra-articular knee injuries in children with hemarthrosis. Am J Sports Med 2014; 42: 1600–1606. [DOI] [PubMed] [Google Scholar]
- 2.Lewallen L, Mcintosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg 2014; 1: 1–7. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe A, Obata T, Ikehira H, et al. Degeneration of patellar cartilage in patients with recurrent patellar dislocation following conservative treatment: evaluation with delayed gadolinium-enhanced magnetic resonance imaging of cartilage. Osteoarthr Cartil 2009; 17: 1546–1553. [DOI] [PubMed] [Google Scholar]
- 4.Bengtsson Moström E, Lammentausta E, Finnbogason T, et al. Pre- and postcontrast T1 and T2 mapping of patellar cartilage in young adults with recurrent patellar dislocation. Magn Reson Med 2015; 74: 1363–1369. [DOI] [PubMed] [Google Scholar]
- 5.Eagle S, Potter HG, Koff MF. Morphologic and quantitative magnetic resonance imaging of knee articular cartilage for the assessment of post-traumatic osteoarthritis. J Orthop Res 2017; 35: 412–423. [DOI] [PubMed] [Google Scholar]
- 6.Gray ML, Burstein D. Molecular (and functional) imaging of articular cartilage. J Musculoskelet Neuronal Interact 2004; 4: 365–368. [PubMed] [Google Scholar]
- 7.Burstein D, Velyvis J, Scott KT, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med 2001; 45: 36–41. [DOI] [PubMed] [Google Scholar]
- 8.Tiderius CJ, Jessel R, Kim Y-J, et al. Hip dGEMRIC in asymptomatic volunteers and patients with early osteoarthritis: the influence of timing after contrast injection. Magn Reson Med 2007; 57: 803–805. [DOI] [PubMed] [Google Scholar]
- 9.Tiderius CJ, Olsson LE, Leander P, et al. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med 2003; 49: 488–492. [DOI] [PubMed] [Google Scholar]
- 10.David-Vaudey E, Ghosh S, Ries M, et al. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging 2004; 22: 673–682. [DOI] [PubMed] [Google Scholar]
- 11.Dunn TC, Lu Y, Jin H, et al. T2 relaxation time of cartilage at MR imaging. Radiology 2004; 232: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arendt EA, Dejour D. Patella instability: building bridges across the ocean a historic review. Knee Surg Sports Traumatol Arthrosc 2013; 21: 279–293. [DOI] [PubMed] [Google Scholar]
- 13.Andrish J. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc 2007; 15: 82–88. [DOI] [PubMed] [Google Scholar]
- 14.Moström EB, Mikkelsen C, Weidenhielm L, et al. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. Sci World J 2014; 2014: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elias JJ, Cosgarea AJ. Patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med 2006; 34: 1478–1485. [DOI] [PubMed] [Google Scholar]
- 16.Sillanpää PJ, Mattila VM, Visuri T, et al. Patellofemoral osteoarthritis in patients with operative treatment for patellar dislocation: a magnetic resonance-based analysis. Knee Surg Sports Traumatol Arthrosc 2011; 19: 230–235. [DOI] [PubMed] [Google Scholar]
- 17.Kujala UM, Jaakkola LH, Koskinen SK, et al. Scoring of patellofemoral disorders. Arthrosc J Arthrosc Relat Surg 1993; 9: 159–163. [DOI] [PubMed] [Google Scholar]
- 18.Roos EM, Roos HP, Lohmander LS, et al. Knee Injury and Osteoarthritis Outcome Score (KOOS)–development of a self-administered outcome measure. J Orthop Sports Phys Ther 1998; 28: 88–96. [DOI] [PubMed] [Google Scholar]
- 19.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 1985; 198: 43–49. [PubMed] [Google Scholar]
- 20.Tiderius C, Hori M, Williams A, et al. dGEMRIC as a function of BMI. Osteoarthritis Cartilage 2006; 14: 1091–1097. [DOI] [PubMed] [Google Scholar]
- 21.Potter H, Linklater J, Allen A, et al. Magnetic resonance imaging of articular cartilage in the knee. J Bone Jt Surg 1998; 80: 1276–1284. [DOI] [PubMed] [Google Scholar]
- 22.Paradowski PT, Bergman S, Sundén-Lundius A, et al. Knee complaints vary with age and gender in the adult population. Population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskelet Disord 2006; 7: 38–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burstein D, Bashir A, Gray ML. MRI techniques in early stages of cartilage disease. Invest Radiol 2000; 35: 622–638. [DOI] [PubMed] [Google Scholar]
- 24.Allen RG, Burstein D, Gray ML. Monitoring glycosaminoglycan replenishment in cartilage explants with gadolinium-enhanced magnetic resonance imaging. J Orthop Res 1999; 17: 430–436. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y-JJ, Sah RL, Grodzinsky AJ, et al. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys 1994; 311: 1–12. [DOI] [PubMed] [Google Scholar]
- 26.Buschmann MD, Hunziker EB, Kim YJ, et al. Altered aggrecan synthesis correlates with cell and nucleus structure in statically compressed cartilage. J Cell Sci 1996; 109: 499–508. [DOI] [PubMed] [Google Scholar]
- 27.Grodzinsky AJ, Levenston ME, Jin M, et al. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng 2000; 2: 691–713. [DOI] [PubMed] [Google Scholar]
- 28.Buckwalter JA, Anderson DD, Brown TD, et al. The roles of mechanical stresses in the pathogenesis of osteoarthritis: implications for treatment of joint injuries. Cartilage 2013; 4: 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mani S, Kirkpatrick MS, Saranathan A, et al. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med 2011; 39: 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuroda R, Kambic H, Valdevit A, et al. Articular cartilage contact pressure after tibial tuberosity transfer. A cadaveric study. Am J Sports Med 2001; 29: 403–409. [DOI] [PubMed] [Google Scholar]
- 31.Sambasivarao SV, Saranathan A, Kirkpatrick MS, et al. The effect of tibial tuberosity realignment procedures on the patellofemoral pressure distribution. Knee Surg Sport Traumatol Arthrosc 2012; 20: 2050–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawezi ZK, Lammentausta E, Svensson J, et al. In vivo transport of Gd-DTPA(2-) in human knee cartilage assessed by depth-wise dGEMRIC analysis. J Magn Reson Imaging 2011; 34: 1352–1358. [DOI] [PubMed] [Google Scholar]
- 33.Müller C, Khabut A, Dudhia J, et al. Quantitative proteomics at different depths in human articular cartilage reveals unique patterns of protein distribution. Matrix Biol 2014; 40: 34–45. [DOI] [PubMed] [Google Scholar]
- 34.Shiraj S, Kim HK, Anton C, et al. Spatial variation of T2 relaxation times of patellar cartilage and physeal patency: an in vivo study in children and young adults. Am J Roentgenol 2014; 202: W292–297. [DOI] [PubMed] [Google Scholar]
- 35.Dardzinski BJ, Laor T, Schmithorst VJ, et al. Mapping T2 relaxation time in the pediatric knee: feasibility with a clinical 1.5-T MR imaging system. Radiology 2002; 225: 233–239. [DOI] [PubMed] [Google Scholar]
- 36.Apprich S, Mamisch TC, Welsch GH, et al. Quantitative T2 mapping of the patella at 3.0T is sensitive to early cartilage degeneration, but also to loading of the knee. Eur J Radiol 2012; 81: e438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prasad AP, Nardo L, Schooler J, et al. T1ρ and T2 relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage 2013; 21: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ericsson YB, Tjörnstrand J, Tiderius CJ, et al. Relationship between cartilage glycosaminoglycan content (assessed with dGEMRIC) and OA risk factors in meniscectomized patients. Osteoarthritis Cartilage 2009; 17: 565–570. [DOI] [PubMed] [Google Scholar]
- 39.Owman H, Tiderius CJ, Neuman P, et al. Association between findings on delayed gadolinium-enhanced magnetic resonance imaging of cartilage and future knee osteoarthritis. Arthritis Rheum 2008; 58: 1727–1730. [DOI] [PubMed] [Google Scholar]