Abstract
In an era of considerable advances in anaesthesiology and pain medicine, chronic pain after major surgery continues to be problematic. This article briefly reviews the known psychological risk and protective factors associated with the development of chronic postsurgical pain (CPSP). We begin with a definition of CPSP and then explain what we mean by a risk/protective factor. Next, we summarize known psychological risk and protective factors for CPSP. Psychological interventions that target risk factors and may impact postsurgical pain are reviewed, including the acceptance and commitment therapy (ACT)-based approach to CPSP prevention and management we use in the Transitional Pain Service (TPS) at the Toronto General Hospital. Finally, we conclude with recommendations for research in risk factor identification and psychological interventions to prevent CPSP. Several pre-surgical psychological risk factors for CPSP have been consistently identified in recent years. These include negative affective constructs, such as anxiety symptoms, depressive symptoms, pain catastrophizing and general psychological distress. In contrast, relatively few studies have examined psychological protective factors for CPSP. Psychological interventions that target known psychological risk factors while enhancing protective psychological factors may reduce new incidence of CPSP. The primary goal of our ACT intervention is to teach patients a mindful way of responding to their postsurgical pain that empowers them to interrupt the negative cycle of pain, distress, behavioural avoidance and escalating opioid use that can limit functioning and quality of life while paradoxically amplifying pain over time. Early clinical outcome data suggest that patients who receive care from TPS physicians reduce their pain and opioid use, yet patients who also receive our ACT intervention have a larger decrease in daily opioid dose while reporting less pain interference and lower depression scores.
Keywords: Chronic postsurgical pain, risk factors, protective factors, psychological interventions, acceptance and commitment therapy
In an era of considerable advances in anaesthesiology and pain medicine, chronic pain after major surgery continues to be problematic. An early study on the prevalence of chronic postsurgical pain (CPSP) reported that 22.5% of 5130 chronic pain patients seen in 10 UK clinics indicated major surgery as its source.1 Studies since then have specified that between 10% and 70% of major surgery patients go on to develop CPSP depending on the surgery type (e.g. cardiac, thoracic, gynaecological, spinal).2,3 CPSP places considerable physical and psychological burdens on individuals initially hoping to improve or recover from medical conditions for which major surgery had been indicated. Given the proliferation of major, oftentimes necessary, surgeries for various medical illnesses over the years, there are potentially millions of individuals globally suffering from, and at risk for, CPSP on an annual basis. The burden of CPSP also falls under the purview of the opioid public health crisis.4 Increasing numbers of chronic pain and major surgery patients remain on long-term, high-dose prescription opioids5 with little to no medical assistance for tapering and incur risks for opioid dependence, misuse, addiction and even fatal overdoses.4,5 The first step in addressing this pain, disability and suffering is to identify the causal, modifiable risk and protective factors that predict CPSP.
This article briefly reviews the known psychological risk and protective factors associated with the development of CPSP. We begin with a definition of CPSP and explain what we mean by a risk/protective factor. We then summarize known psychological risk and protective factors for CPSP. Psychological interventions that target risk factors and may impact postsurgical pain are reviewed, including the approach to CPSP prevention and management we use in the Transitional Pain Service (TPS) at the Toronto General Hospital. Finally, we conclude with recommendations for research in risk factor identification and psychological interventions to prevent CPSP.
Chronic postsurgical pain
Although there is considerable variability in surgical procedures and the time required to recover, the following 6-point definition appears to capture the most important aspects of CPSP.6,7 The pain (1) developed after a surgical procedure, (2) is at least 2 months in duration, (3) interferes significantly with health-related quality of life, (4) is a continuation of acute postsurgical pain or develops after an asymptomatic period, (5) is localized to the surgical field and/or projected to territory or dermatome innervated by a nerve in the surgical field and (6) is not caused by other factors (e.g. pre-op pain, recurrence after surgery for cancer, chronic infection have been ruled out).
An understanding of the aetiology of CPSP begins with consideration of intraoperative factors, such as ongoing inflammatory processes arising from tissue damage, and neuropathic pain associated with intraoperative nerve damage, both of which may mark the beginning of the process by which acute time-limited pain transitions to chronic, pathological pain.2,8–11 In addition, peripheral sensitization and central sensitization have been proposed as risk factors for the development of CPSP. Sensitization by way of injury discharge in the intraoperative and acute postoperative stages may cause neuroplastic changes resulting in the reduction in the threshold of nociceptor peripheral terminals (peripheral sensitization) or an increase in the excitability of brain and spinal cord neurons (central sensitization).12–14 These changes contribute to the long-term amplification and maintenance of pain in individuals who develop CPSP and also help to explain the greater vulnerability of people who present for surgery with a pre-existing chronic pain condition.2,3 The clinical presentation of CPSP likely involves a combination of the aforementioned mechanisms and other possibilities.2 Further complicating the clinical picture are psychological factors, namely, perioperative depressive–anxiety symptoms and cognitive, emotional and traumatic reactions to pain that can modulate the experience of pain and play a contributing role in the manifestation of CPSP.2,3,15
Psychological risk and protective factors for CPSP
Risk/protective factor definition
A risk or protective factor is a measurable construct used to classify participants along a continuum of risk of the probability of developing an outcome of interest.16 Risk/protective factors must be measured before the outcome has manifested. Participants are typically classified into a high-risk and a low-risk group using a common metric (e.g. odds ratio, relative risk ratio). Causal risk factors are distinguished from correlated risk factors on the basis of their likelihood to alter the outcome when modified. Modification of a causal risk factor reduces the risk of the outcome, whereas modification of a correlated risk factor has no effect on the outcome. In the case of CPSP, if intervening on a risk or protective factor prior to surgery, or in the acute post-surgical period, reduces the likelihood of transition to CPSP, then it can be considered a clinically important causal risk factor. The main aim of research on risk factor identification is to establish the causal, modifiable risk factors that confer a greater (or lesser) risk of developing CPSP. Identification of psychological risk and protective factors for CPSP is integral for the development and implementation of timely psychological interventions to prevent and/or manage CPSP.
Psychological risk factors
Several pre-surgical psychological risk factors for CPSP have been consistently identified in recent years. These include negative affective constructs such as anxiety symptoms,17–19 depressive symptoms,20–22 pain catastrophizing18,23,24 and general psychological distress.25,26 Systematic reviews of psychosocial risk factors for CPSP indicate a high level of evidence (i.e. Grade of Association 1: Association Likely) for the predictive value of pre-surgical depression, psychological vulnerability and chronic stress on risk of persistent pain following surgery.27 Pooled odds ratios (ORs) for pre-surgical anxiety (OR = 1.76, 95% confidence interval (CI): 1.07–2.90) and pre-surgical pain catastrophizing (OR = 2.37, 95% CI: 1.32–4.28) indicate that patients who present with these issues before surgery are approximately twice as likely to go on to develop CPSP compared with controls.28 Newer constructs such as perceived injustice24 and sensitivity to pain traumatization29,30 also predict CPSP, but, with so few studies conducted to date, these findings require replication.
Psychological protective factors
Relatively few studies have examined psychological protective factors for CPSP. Psychological robustness, a composite variable comprising high dispositional optimism, high positive affect and low emotional distress, has been found to predict a lower incidence of CPSP at 4 months in women undergoing breast cancer surgery (OR = 0.70, 95% CI: 0.49–0.99).31 In a study of patients undergoing coronary artery bypass graft, greater pre-operative optimism was found to be significantly associated with lower pain intensity and fewer physical symptoms following surgery, after controlling for demographic, clinical and behavioural covariates, including negative affectivity.32 Both studies suggest that having an optimistic outlook with respect to outcomes of major surgery may help patients navigate their post-surgical recovery period. However, maintaining an optimistic outlook is not always possible when facing an uncertain future mired with health problems. Moreover, the recovery stage of major surgery oftentimes requires patients to endure significant pain, impairment and setbacks that can limit re-engagement with important daily activities. In this respect, protective factors pertaining to behavioural principles may lend more clarity to how patients may take an active role in their post-surgical recovery. Interestingly, a study has found lower pre-operative pain self-efficacy scores, which assess a person’s confidence in performing general activities despite pain, to be a significant predictor of greater functional limitations, but not pain, at 1 year after total knee arthroplasty.33 It is possible that pain self-efficacy assessed in the post-operative period would be an even stronger predictor of functional ability than pre-operative pain self-efficacy, due to the shift in expectations about function and perceived abilities one is likely to incur after undergoing a major operation. By contrast, another study has shown that patients with a higher pre-surgical score on the Patient Activation Measure (reflecting the propensity to engage in adaptive health behaviours) experienced better pain relief at 6 months after total knee arthroplasty.34 Taken altogether, these studies indicate that fostering optimism and enhancing self-efficacy and adaptive behaviours in the perioperative period could improve outcomes for patients undergoing major surgeries.
Targeting psychological risk factors for CPSP using behavioural interventions
Psychological interventions that target known psychological risk factors while enhancing protective psychological factors may reduce new incidence of CPSP.2 Psychological interventions for people living with chronic pain have a long track record of efficacy as part of multidisciplinary chronic pain treatment,35,36 so it is reasonable to expect that the same tools and techniques could minimize acute post-operative pain intensity and improve quality of life by reducing distress, disability and chronic opioid use for patients who sustain neuropathic injury during surgery and are at risk for CPSP. Despite the long-standing evidence for psychological risk factors in CPSP, until quite recently there has been relatively little investigation into the efficacy of behavioural interventions aimed at targeting these risk factors perioperatively with the goal of preventing CPSP.
Behavioural interventions in the perioperative domain have largely targeted acute post-surgical outcomes such as pain intensity, anxiety, opioid use in the hours or days immediately after surgery and time to hospital discharge.37 Pre-operative education (including information about the surgery, expected pain and pain medication, with content sometimes also addressing coping strategies) has long been known to reduce pre-surgical anxiety, leading to improved post-surgical outcomes, including reduced opioid consumption in hospital and shorter length of stay following surgery.38–41 Relaxation, guided imagery and clinical hypnosis have been shown to reduce pain, use of analgesic medication and negative affect in the acute post-operative period.37,42
Cognitive-behavioural approaches common to chronic pain interventions have also been used with surgical patients. Scheel et al.43 examined the impact of a brief cognitive-behavioural therapy (CBT) intervention for 48 young men undergoing surgical correction of a chest malformation. The intervention consisted of pain education, cognitive restructuring challenging pain catastrophizing, practice redirecting attention from pain to other sensory channels of perception and relaxation with a personalized audio recording. The intervention was delivered for 1 hour the day before surgery and for an additional hour 1–3 days after surgery. The intervention group reported lower acute pain intensity than the control group 1 week after surgery and less disability (but not significantly less pain intensity) 3 months later. At 6 and 12 months, there was no difference between groups in pain intensity or pain-related disability; however, at 12 months, when patients were anticipating a second surgery to remove the trans-sternal metal implant, a re-emergence of pain anxiety was found only in the control group, suggesting that the intervention group felt more empowered to deal with the pain of their upcoming surgery 1 year later.
CBT programmes can be combined with physical interventions for surgical patients, in the same way that CBT and physiotherapy are integrated in multidisciplinary chronic pain clinics. Archer et al.44 randomized 86 patients undergoing laminectomy to either a cognitive-behavioural-based physical education programme (cognitive-behavioural play therapy) or an education programme at 6 weeks after surgery. At 3-month follow-up, play therapy participants had significantly greater decreases in pain and disability compared to the education group.
The TPS: targeting CPSP with acceptance and commitment therapy
In addition to controlled research trials, novel clinical initiatives have been developed to address psychological risk factors for CPSP. The innovative post-surgical TPS at Toronto General Hospital offers comprehensive, multidisciplinary pain management to patients at high risk of CPSP from pre-admission up to 6 months after surgery.15 Clinical services include (1) multimodal medication optimization by anaesthesiologists, (2) post-surgical physiotherapy and (3) a pain psychology intervention consisting of pain education, mindfulness training and a form of cognitive-behavioural treatment called acceptance and commitment therapy (ACT).
ACT is part of a new wave of cognitive-behavioural approaches45 that seamlessly integrates mindfulness.46 ACT protocols have garnered substantial support as an intervention for chronic pain in a relatively short period of time.47–49 In terms of CPSP risk factors, mindfulness and acceptance-based interventions have demonstrated efficacy in the treatment of depression and anxiety,50–53 and although ACT does not target pain catastrophizing per se, mindfulness and acceptance-based interventions have been shown to lead to reduced pain catastrophizing.54,55 Furthermore, brief ACT interventions for pain and other medical problems have been effective for, and acceptable to, medical patients.56 ACT has shown promise in the treatment of opioid misuse, including higher rates of completed detoxification, reduced fear of detoxification and lower opiate and total drug use at follow-up than methadone alone or in combination with drug counselling.57,58 There have been recent calls to expand the scope of ACT interventions to the treatment of post-surgical pain that may persist.59
In the TPS, the primary goal of the peri-surgical ACT intervention is to teach patients a mindful way of responding to their post-surgical pain that empowers them to interrupt the negative cycle of pain, distress, behavioural avoidance and escalating opioid use that can limit functioning and quality of life while paradoxically amplifying pain over time.60 The overarching goal is to expand the post-surgical patient’s capacity to experience pain – and the thoughts and feelings that come with pain – without rigidly engaging in problematic avoidance behaviour. Instead, pain sensations – as well as the mind’s reactions to pain sensations – are observed neutrally and non-reactively, while motivation and commitment to engage in personally meaningful, goal-oriented activities are enhanced.61 The end result is greater ‘psychological flexibility’, the ability to persist or adapt one’s behaviour in the service of living a rich and purposeful life while in open psychological contact with internal experience, including pain. Our conceptualization of the six core ACT processes thought to underlie psychological flexibility and how they are at play in the post-surgical context can be seen in Figures 1 and 2. ACT clinicians use a variety of techniques to intervene on these six processes of change, including teaching metaphors, mindfulness practice and adaptations of familiar CBT tools such as exposure and behavioural activation.
Figure 1.
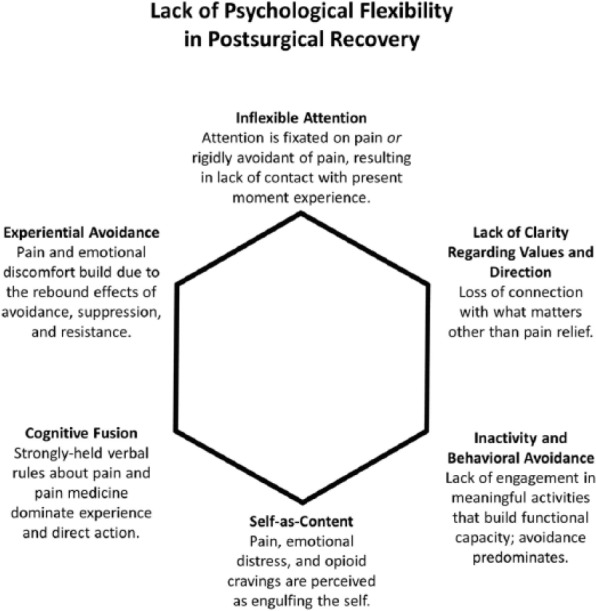
This clinical conceptualization of six psychological processes thought to underlie poor adjustment and recovery after surgery is based on the treatment model of acceptance and commitment therapy (ACT), as well as our clinical experience working with post-surgical patients in the Transitional Pain Service at Toronto General Hospital, a novel multidisciplinary pain service targeting the prevention and early comprehensive management of CPSP.
Figure 2.
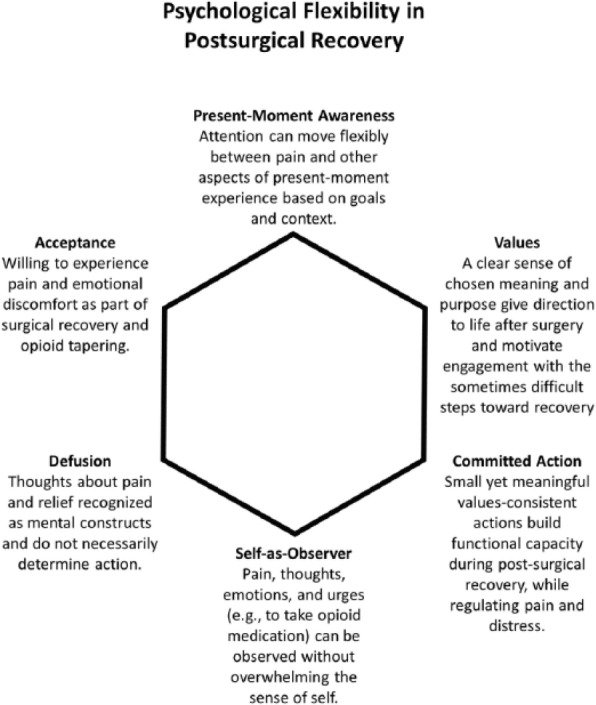
The Transitional Pain Service ACT intervention aims to develop these six facets of psychological flexibility in order to reduce distress, foster new skills for the self-management of pain (e.g. mindfulness skills), promote behavioural activation within functional limits and reduce psychological barriers to opioid weaning.
Early clinical outcome data suggest that patients who receive care from TPS physicians reduce their pain and opioid use, yet patients who also receive our ACT intervention have a larger decrease in daily opioid dose while reporting less pain interference and lower depression scores.62 In a preliminary, non-randomized study, 91 patients received ACT plus care from the TPS physicians, whereas 252 patients received care from only the TPS physicians. The following outcomes were compared between the two groups at the first and last TPS visits after surgery: pain intensity, pain interference, sensitivity to pain traumatization, pain catastrophizing, anxiety, depression and opioid use. The results showed that compared to patients who did not receive ACT, those who did were more likely to report a mental health condition pre-operatively, had higher opioid use at the first post-surgical visit, and also reported higher sensitivity to pain traumatization and higher anxiety scores at the first and last TPS visits. Despite these group differences, compared to the patients who did not receive ACT, those who did demonstrated greater reductions in opioid use and pain interference and showed reductions in depressed mood by the end of treatment.62 These outcomes suggest ACT was effective in reducing opioid use while pain interference and mood improved, but they require replication in a randomized, controlled study.
Recommendations for future research
Risk/protective factor identification
Progress in risk factor identification research will be facilitated by attention to several interrelated methodological issues:
Selection of risk factors. Potential risk factors may be selected based on currently established risk factors for CPSP (e.g. depressive symptoms, anxiety), including those targeted by the leading, evidence-based behavioural pain management intervention for chronic pain, CBT (e.g. pain catastrophizing). Further investigation may gather evidentiary support for alternative models such as the ACT model, adding additional risk factors to the literature.63 The identification of pre-operative risk factors that can be altered by clinical interventions is especially useful in the prevention of CPSP.
Timing of risk factor assessment. Ideally, risk factors should be assessed at least twice: once before and once after major surgery (e.g. in the days or weeks after). This will ensure that the issue of changing baselines does not confound assessment and lead to non-representative risk factor estimate.64 Instances have been reported in which risk factors assessed pre-operatively do not predict CPSP, but assessment of the same factors assessed in the days or weeks after are predictive.65 The same has been shown for early (days) and late (weeks) acute post-operative pain intensity.66 Timing of risk factor assessment is critical and may be surgery-specific.
Timing of outcome assessment. Studies rarely follow patients for more than a year after surgery and many assess CPSP only at 3 or 6 months when the pain has just transitioned to chronicity. Elsewhere, we have shown that the risk and protective factors involved in the transition of acute pain to chronic pain differ from the factors involved in maintaining the pain once it has become established.66–68 The transition to CPSP and related psychosocial dysfunction is a dynamic process that evolves over time, necessitating outcome assessment at more than one point after surgery. Assessing outcomes at a single point in time after surgery will not permit an examination of whether the factors involved in the transition to chronicity differ from those involved in the maintenance of already established chronic pain. Moreover, modifying a time-dependent, causal risk factor that predicts CPSP early after surgery (e.g. 3 months), but not later on (e.g. 1 year), will have no effect on the later outcome.
Patient-centred data analysis. The traditional approach to studying CPSP has involved a single measure (e.g. pre-operative anxiety) or an average of several measurements across the perioperative period as predictors of CPSP. Examination of patient trajectories after major surgery is an emerging, novel approach to studying the dynamic, evolving nature of the development and maintenance of CPSP. Use of latent trajectories allows for the clustering of patient subgroups that share similar trajectory characteristics and therefore take into account the heterogeneity of psychological experiences over time.69,70 Trajectory-based analyses of post-surgical pain may help identify patients in need of post-surgical intervention, such as those with consistent levels of acute post-operative pain and elevated anxiety symptoms.
Intervention research
Substantial research is needed to establish how psychological and behavioural interventions can be maximally effective in addressing psychological risk factors for CPSP:
Research on behavioural treatment modalities. Research is needed on a range of intervention options, including psycho-education, hypnosis, CBT, mindfulness and ACT. These psychological interventions could be offered separately or in treatment packages where they are combined with one another or with other interventions such as physiotherapy.
How much behavioural treatment is needed and when can it help the most? The timing (pre-surgical, acute post-surgical and/or long-term ‘booster sessions’) and ‘dosing’ of the amount of behavioural treatment needed are not yet understood (i.e. number of hours of contact with behavioural clinician, as well as ‘homework’ practice including relaxation, mindfulness and/or self-hypnosis).
Measuring risk factors and key outcomes longitudinally. Controlled intervention trials are needed that prospectively and longitudinally track key psychological risk factors (anxiety, depression, pain catastrophizing or other constructs associated with poor pain coping, and substance misuse history) and clinical outcomes (such as pain, disability, mood and opioid use) at key time points up to a minimum of 1 year after major surgery. Extending outcome assessment to 5 years after surgery would provide a much clearer idea of the natural course of CPSP.
Assessing interventions across a wide range of surgeries. There is great diversity in the surgeries that are conducted and in their long-term impact. Research should be conducted across a range of surgeries, including surgeries understood to confer greater risk of CPSP due to high risk of nerve injury (e.g. thoracic surgery, breast cancer surgery and amputations).9–11,71
Targeting subgroups of patients. Interventions may be developed to specifically meet the needs of specific subtypes of patients, such as those with a history of chronic pain or those with a history of substance misuse.
From risk factors to effective interventions
It is time to translate our growing understanding of the psychological risk factors predictive of CPSP into effective psychological interventions that improve patient quality of life. In 2017, the International Association for the Study of Pain’s (IASP) Global Year Against Pain After Surgery, we have the tools that we need to move forward: awareness of key psychological risk factors for CPSP; effective psychological interventions for acute post-surgical pain, including education and hypnosis;37,42 and a large body of research on the efficacy of CBT, mindfulness training and ACT for a variety of chronic pain conditions.36,48 The time is ripe for a leap forward in experimental investigations as well as clinical care for the millions of people each year who undergo necessary, often live-saving surgeries and are surprised to find their recovery marred by unexpected and debilitating long-term pain.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: KAB is supported by a Fellowship from the Canadian Institutes of Health Research (CIHR) held at the Lawrence S. Bloomberg Faculty of Nursing, University of Toronto. LCB was supported by a CIHR Doctoral Research Award - Frederick Banting and Charles Best Canada Graduate Scholarship held at York University. HC is supported by a Merit award from the Department of Anesthesia, University of Toronto, Faculty of Medicine. JK is supported by a Canadian Institutes of Health Research Tier 1 Canada Research Chair in Health Psychology held at York University.
References
- 1. Crombie IK, Davies HTO, Macrae WA. Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain 1998; 76: 167–171. [PubMed] [Google Scholar]
- 2. Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother 2009; 9: 723–744. [DOI] [PubMed] [Google Scholar]
- 3. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 4. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health 2015; 36: 559–574. [DOI] [PubMed] [Google Scholar]
- 5. Gan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin 2014; 30: 149–160. [DOI] [PubMed] [Google Scholar]
- 6. Werner M, Kongsgaard UI. Defining persistent post-surgical pain: is an update required? Br J Anaesth 2014; 113: 1–4. [DOI] [PubMed] [Google Scholar]
- 7. Macrae W, Davies H. Chronic postsurgical pain. Epidemiol Pain 1999; 125: e142. [Google Scholar]
- 8. Macrae W. Chronic pain after surgery. Br J Anaesth 2001; 87: 88–98. [DOI] [PubMed] [Google Scholar]
- 9. Buchheit T, Pyati S. Prevention of chronic pain after surgical nerve injury: amputation and thoracotomy. Surg Clin North A 2012; 92: 393.x–407.x. [DOI] [PubMed] [Google Scholar]
- 10. Haroutiunian S, Nikolajsen L, Finnerup NB, et al. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain 2013; 154: 95–102. [DOI] [PubMed] [Google Scholar]
- 11. Johansen A, Romundstad L, Nielsen CS, et al. Persistent postsurgical pain in a general population: prevalence and predictors in the Tromso study. Pain 2012; 153: 1390–1396. [DOI] [PubMed] [Google Scholar]
- 12. Cregg R, Anwar S, Farquhar-Smith P. Persistent postsurgical pain. Curr Opin Support Palliat Care 2013; 7: 144–152. [DOI] [PubMed] [Google Scholar]
- 13. Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000; 288: 1765–1768. [DOI] [PubMed] [Google Scholar]
- 14. Woolf CJ, Chong M-S. Preemptive analgesia-treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 1993; 77: 362–379. [DOI] [PubMed] [Google Scholar]
- 15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res 2015; 8: 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kraemer HC, Kazdin AE, Offord DR, et al. Coming to terms with the terms of risk. Arch Gen Psychiatry 1997; 54: 337–343. [DOI] [PubMed] [Google Scholar]
- 17. Masselin-Dubois A, Attal N, Fletcher D, et al. Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. J Pain 2013; 14: 854–864. [DOI] [PubMed] [Google Scholar]
- 18. Pinto PR, McIntyre T, Nogueira-Silva C, et al. Risk factors for persistent postsurgical pain in women undergoing hysterectomy due to benign causes: a prospective predictive study. J Pain 2012; 13: 1045–1057. [DOI] [PubMed] [Google Scholar]
- 19. Singh JA, Lewallen DG. Medical and psychological comorbidity predicts poor pain outcomes after total knee arthroplasty. Rheumatology 2013; 52: 916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papakostidou I, Dailiana ZH, Papapolychroniou T, et al. Factors affecting the quality of life after total knee arthroplasties: a prospective study. BMC Musculoskel Dis 2012; 13: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinikallio S, Airaksinen O, Aalto T, et al. Coexistence of pain and depression predicts poor 2-year surgery outcome among lumbar spinal stenosis patients. Nord J Psychiatry 2010; 64: 391–396. [DOI] [PubMed] [Google Scholar]
- 22. Sinikallio S, Lehto SM, Aalto T, et al. Depressive symptoms during rehabilitation period predict poor outcome of lumbar spinal stenosis surgery: a two-year perspective. BMC Musculoskel Dis 2010; 11: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riddle DL, Wade JB, Jiranek WA, et al. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res 2010; 468: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yakobov E, Scott W, Stanish W, et al. The role of perceived injustice in the prediction of pain and function after total knee arthroplasty. Pain 2014; 155: 2040–2046. [DOI] [PubMed] [Google Scholar]
- 25. Lingard EA, Riddle DL. Impact of psychological distress on pain and function following knee arthroplasty. J Bone Joint Surg Am 2007; 89: 1161–1169. [DOI] [PubMed] [Google Scholar]
- 26. Mejdahl MK, Mertz BG, Bidstrup PE, et al. Preoperative distress predicts persistent pain after breast cancer treatment: a prospective cohort study. J Natl Compr Canc Netw 2015; 13: 995–1003. [DOI] [PubMed] [Google Scholar]
- 27. Hinrichs-Rocker A, Schulz K, Järvinen I, et al. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) – a systematic review. Eur J Pain 2009; 13: 719–730. [DOI] [PubMed] [Google Scholar]
- 28. Theunissen M, Peters ML, Bruce J, et al. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain 2012; 28: 819–841. [DOI] [PubMed] [Google Scholar]
- 29. Kleiman V, Clarke H, Katz J. Sensitivity to pain traumatization: a higher-order factor underlying pain-related anxiety, pain catastrophizing and anxiety sensitivity among patients scheduled for major surgery. Pain Res Manag 2011; 16: 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katz J, Fashler SR, Wicks C, et al. Sensitivity to Pain Traumatization Scale: development, validation, and preliminary findings. J Pain Res 2017; 10: 1297–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bruce J, Thornton AJ, Powell R, et al. Psychological surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain 2014; 155: 232–243. [DOI] [PubMed] [Google Scholar]
- 32. Ronaldson A, Poole L, Kidd T, et al. Optimism measured pre-operatively is associated with reduced pain intensity and physical symptom reporting after coronary artery bypass graft surgery. J Psychosom Res 2014; 77: 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wylde V, Dixon S, Blom A. The role of preoperative self-efficacy in predicting outcome after total knee replacement. Musculoskeletal Care 2012; 10: 110–118. [DOI] [PubMed] [Google Scholar]
- 34. Andrawis J, Akhavan S, Chan V, et al. Higher preoperative patient activation associated with better patient-reported outcomes after total joint arthroplasty. Clin Orthop Relat Res 2015; 473: 2688–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flor H, Fydrich T, Turk DC. Efficacy of multidisciplinary pain treatment centers: a meta-analytic review. Pain 1992; 49: 221–230. [DOI] [PubMed] [Google Scholar]
- 36. Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999; 80: 1–13. [DOI] [PubMed] [Google Scholar]
- 37. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016; 17: 131–157. [DOI] [PubMed] [Google Scholar]
- 38. Egbert LD, Battit GE, Welch CE, et al. Reduction of postoperative pain by encouragement and instruction of patients. A study of doctor-patient rapport. N Engl J Med 1964; 270: 825–827. [DOI] [PubMed] [Google Scholar]
- 39. Langer E, Janis I, Wolfer J. Reduction of psychological stress in surgical patients. J Exp Soc Psychol 1975; 11: 115–165. [Google Scholar]
- 40. Anderson EA. Preoperative preparation for cardiac surgery facilitates recovery, reduces psychological distress, and reduces the incidence of acute postoperative hypertension. J Consult Clin Psychol 1987; 55: 513–520. [DOI] [PubMed] [Google Scholar]
- 41. Butler G, Hurley C, Buchanan K, et al. Prehospital education: effectiveness with total hip replacement surgery patients. Patient Educ Couns 1996; 29: 189–197. [DOI] [PubMed] [Google Scholar]
- 42. Montgomery GH, David D, Winkel G, et al. The effectiveness of adjunctive hypnosis with surgical patients: a meta-analysis. Anesth Analg 2002; 94: 1639–1645, table of contents. [DOI] [PubMed] [Google Scholar]
- 43. Scheel J, Parthum A, Dimova V, et al. Psychologisches prophylaxetraining zur bewaltigung postoperativer schmerzen. Der Schmerz 2014; 28: 513–519. [DOI] [PubMed] [Google Scholar]
- 44. Archer KR, Devin CJ, Vanston SW, et al. Cognitive-behavioral-based physical therapy for patients with chronic pain undergoing lumbar spine surgery: a randomized controlled trial. J Pain 2016; 17: 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hayes SC, Villatte M, Levin M, et al. Open, aware, and active: contextual approaches as an emerging trend in the behavioral and cognitive therapies. Annu Rev Clin Psychol 2011; 7: 141–168. [DOI] [PubMed] [Google Scholar]
- 46. Fletcher L, Hayes SC. Relational frame theory, acceptance and commitment therapy, and a functional analytic definition of mindfulness. J Ration-Emot Cognit-Behav Ther 2005; 23: 315–336. [Google Scholar]
- 47. Scott W, McCracken LM. Psychological flexibility, acceptance and commitment therapy, and chronic pain. Curr Opin Psychol 2015; 2: 91–96. [Google Scholar]
- 48. Veehof MM, Oskam MJ, Schreurs KM, et al. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain 2011; 152: 533–542. [DOI] [PubMed] [Google Scholar]
- 49. McCracken LM, Vowles KE. Acceptance and commitment therapy and mindfulness for chronic pain: model, process, and progress. Am Psychol 2014; 69: 178–187. [DOI] [PubMed] [Google Scholar]
- 50. Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol 2010; 78: 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roemer L, Orsillo SM, Salters-Pedneault K. Efficacy of an acceptance-based behavior therapy for generalized anxiety disorder: evaluation in a randomized controlled trial. J Consult Clin Psychol 2008; 76: 1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arch JJ, Eifert GH, Davies C, et al. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol 2012; 80: 750–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Forman EM, Herbert JD, Moitra E, et al. A randomized controlled effectiveness trial of acceptance and commitment therapy and cognitive therapy for anxiety and depression. Behav Modif 2007; 31: 772–799. [DOI] [PubMed] [Google Scholar]
- 54. Turner JA, Anderson ML, Balderson BH, et al. Mindfulness-based stress reduction and cognitive behavioral therapy for chronic low back pain: similar effects on mindfulness, catastrophizing, self-efficacy, and acceptance in a randomized controlled trial. Pain 2016; 157: 2434–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trompetter HR, Bohlmeijer ET, Fox JP, et al. Psychological flexibility and catastrophizing as associated change mechanisms during online Acceptance & Commitment Therapy for chronic pain. Behav Res Ther 2015; 74: 50–59. [DOI] [PubMed] [Google Scholar]
- 56. Dindo L. One-day acceptance and commitment training workshops in medical populations. Curr Opin Psychol 2015; 2: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hayes SC, Wilson K, Gifford E, et al. A preliminary trial of twelve-step facilitation and acceptance and commitment therapy with polysubstance-abusing methadone-maintained opiate addicts. Behavior Thrapy 2006; 35: 667–668. [Google Scholar]
- 58. Stotts AL, Green C, Masuda A, et al. A stage I pilot study of acceptance and commitment therapy for methadone detoxification. Drug Alcohol Depend 2012; 125: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wicksell RK, Olsson GL. Predicting and preventing chronic postsurgical pain and disability. Anesthesiology 2010; 113: 1260–1261. [DOI] [PubMed] [Google Scholar]
- 60. Lee M, Silverman SM, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011; 14: 145–161. [PubMed] [Google Scholar]
- 61. Weinrib AZ, Burns LC, Mu A, et al. A case report on the treatment of complex chronic pain and opioid dependence by a multidisciplinary transitional pain service using the ACT Matrix and buprenophine/naloxone. J Pain Res 2017; 10: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Azam MA, Weinrib AZ, Montbriand JJ, et al. Acceptance and commitment therapy to manage pain and opioid use after major surgery: preliminary outcomes from the Toronto General Hospital Transitional Pain Service. Can J Pain 2017; 1(1): 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scott W, McCracken LM. Psychological assessment to identify patients at risk of postsurgical pain: the need for theory and pragmatism. Br J Anaesth 2016; 117: 546–548. [DOI] [PubMed] [Google Scholar]
- 64. Katz J. Establishment of a new pain catastrophizing baseline after pediatric major surgery? J Pain 2015; 16: 388. [DOI] [PubMed] [Google Scholar]
- 65. Pinto PR, McIntyre T, Ferrero R, et al. Risk factors for moderate and severe persistent pain in patients undergoing total knee and hip arthroplasty: a prospective predictive study. PLoS ONE 2013; 8: e73917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pagé MG, Stinson J, Campbell F, et al. Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J Pain Res 2013; 6: 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Katz J. One man’s risk factor is another man’s outcome: difference in risk factor profiles for chronic postsurgical pain maintenance vs transition. Pain 2012; 153: 505–506. [DOI] [PubMed] [Google Scholar]
- 68. Katz J, Asmundson GJ, McRae K, et al. Emotional numbing and pain intensity predict the development of pain disability up to one year after lateral thoracotomy. Eur J Pain 2009; 13: 870–878. [DOI] [PubMed] [Google Scholar]
- 69. Pagé MG, Katz J, Escobar EMR, et al. Distinguishing problematic from nonproblematic postsurgical pain: a pain trajectory analysis after total knee arthroplasty. Pain 2015; 156: 460–468. [DOI] [PubMed] [Google Scholar]
- 70. Pagé MG, Watt-Watson J, Choinière M. Do depression and anxiety profiles over time predict persistent post-surgical pain? A study in cardiac surgery patients. Eur J Pain 2017; 21: 965–976. [DOI] [PubMed] [Google Scholar]
- 71. Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008; 101: 77–86. [DOI] [PubMed] [Google Scholar]


