Abstract
Study design:
Narrative review.
Method:
Eight bibliographic databases were searched for studies published in the (last five years up until Feb 2017). For the two database searches (Cochrane and DARE), the time frame was unlimited. The review involved keyword searches of the term ‘Amputation’ AND ‘chronic pain’. Studies selected were interrogated for any association between peri-operative factors and the occurrence of chronic post amputation pain (CPAP).
Results:
Heterogeneity of study populations and outcome measures prevented a systematic review and hence a narrative synthesis of results was undertaken. The presence of variation in two gene alleles (GCH1 and KCNS1) may be relevant for development of CPAP. There was little evidence to draw conclusions on the association between age, gender and CPAP. Pre-operative anxiety and depression influenced pain intensity post operatively and long-term post amputation pain (CPAP). The presence of pre-amputation pain is correlated to the development of acute and chronic post amputation pain while evidence for the association of post-operative pain with CPAP is modest. Regional anaesthesia and peri-neural catheters improve acute postoperative pain relief but evidence on their efficacy to prevent CPAP is limited. A suggested whole system pathway based on current evidence to optimize peri-operative amputation pain is described.
Conclusion:
The current evidence suggests that optimized peri-operative analgesia reduces the incidence of acute peri-operative pain but no firm conclusion can be drawn on reducing risk for CPAP.
Keywords: Amputation, chronic pain, phantom limb pain, stump pain, persistent post-surgical pain
Introduction
The Hospital Episode Statistics for 2009/2010 show that 5498 amputations were performed in England alone, with 95% of them for vascular disease (peripheral arterial disease (PAD)). These vascular patients are usually American Society of Anesthesiology (ASA) grading III/IV patients with multiple co-morbidities and have a disproportionately high mortality (38%–48% will die within the year).1
In those who survive, pain management following a limb amputation is a major issue. Post-amputation pain is a broad term that includes residual limb or stump pain, phantom limb pain (PLP) and phantom limb sensation (PLS). The estimated incidence of chronic post-surgical pain (CPSP) following amputation (chronic pain after amputation (CPAP)) is between 30% and –80% up to 20 years after amputation.2,3 This includes data from amputations due to trauma, cancer and peripheral vascular disease. The causes of CPAP are multifactorial and largely unknown despite a plethora of work concentrating on peripheral, spinal and supra-spinal mechanisms, and its treatment is universally accepted as difficult and challenging.4,5 This translates into a significant long-term health service burden.
There are a variety of other factors (genetics, pre-operative pain, intra-operative variables, and psychological factors) that influence the development of CPSP and need to be considered. The aim of this is to identify factors (pre, intra and post-surgery) that predict the development of CPAP. Elucidation of modifiable predictors could inform peri-operative management of amputation surgery in clinical practice.
Methods
The review involved keyword searches of the term ‘Amputation’ AND ‘chronic pain’. The Scottish Knowledge network (http://www.knowledge.scot.nhs.uk) was used as a search engine. This is a composite database that searches multiple databases for the keywords entered (see Table 1 below). Databases included in the search on the knowledge network include MEDLINE (general medicine), CINAHL (nursing and allied health), PsycINFO (psychology and related behavioural and social sciences), CDSR, HMIC and clinical knowledge summaries. The search was limited to the last 5 years. Additional searches using the above keywords and criteria were carried out in PubMed and Google Scholar, the Cochrane Library and DARE database. Relevant articles were extracted from this search for the review. A hand search of references of the relevant retrieved articles was performed. Eligible studies included adults with CPAP, and this included both chronic stump pain and PLP. Studies selected were interrogated for association between peri-operative factors and the occurrence of CPAP.
Table 1.
Search methodology.
| Database | Years searched | Search terms | Hits |
|---|---|---|---|
| Knowledge.scot.nhs.uk | 2012–2017 | Amputation AND Chronic pain | 493 |
| PubMed | 2012–2017 | Amputation AND Chronic pain | 201 |
| Cochrane Library | 2012–2017 | Amputation AND Chronic pain | 6 |
| Google Scholar | 2012–2017 | Amputation AND Chronic pain | 7 |
| DARE | 2012–2017 | Amputation AND Chronic pain | 1 |
This narrative review includes theoretical and opinion pieces, case studies, descriptive studies (case control and prospective cohort), frameworks and systematic reviews describing processes and overview articles on the topic of amputation and chronic pain. The search process is shown in Table 1.
Classification of amputation pain
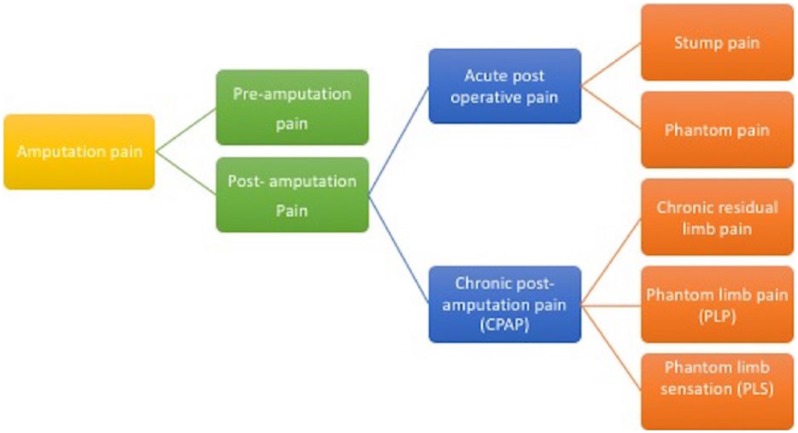
It is important to classify the pain phenotypes (Figure 1), and the definitions of the terms frequently used in amputation pain literature are listed in Table 2.
Figure 1.
The classification of amputation pain.
Table 2.
Definitions of frequently used terms in chronic post amputation pain.
| Type of pain | Description |
|---|---|
| Pre-amputation pain | Pre-existing pain prior to surgery. Could be due to vascular ischaemia (and inflammation), diabetes or other causes. Nociceptive plus neuropathic components present usually. Critical ischaemia patients suffer moderate-to-severe pain with an average pain score of 7.5/10. Management of pre-amputation pain was found to be good in only 22.8% of UK hospitals in a recent NCEPOD review1. |
| Acute post-operative pain | The pain is localised to the residual stump and is sharp and aching. This pain is usually nociceptive in nature and usually resolves in a few weeks but occasionally neuropathic features are present. |
| Chronic post-amputation pain (CPAP) | Pain that persists for more than 2–3 months after amputation surgery. |
| Chronic residual limb pain or stump pain | Pain in the remaining limb stump after surgery. In the majority of patients, this resolves with tissue healing. If stump pain persists, it may be a risk factor for CPAP. Clarke et al.12 and Buchheit et al.7 further subdivided stump pain into somatic or neuropathic. The neuropathic causes of stump pain include neuroma, Complex Regional Pain Syndrome (CRPS) and Mosaic neuralgia. |
| Phantom limb pain (PLP) | Pain is experienced in the limb that is no longer there. Frequently described in neuropathic terms, that is, burning, aching, stabbing, throbbing, pins and needles. PLP usually develops in the first week in about 25% of the amputees. |
| Phantom limb sensation (PLS) | Sensation felt in a limb that is no longer there. Patients also describe ‘telescoping’, that is, the distal limb parts (toes for example) are felt to be closer to the amputated stump. |
| Heterotrophic ossification | Deposition of calcium in the stump soft tissues (heterotrophic ossification) has been observed in 65% of traumatic amputees and has been recognized as a cause of persistent stump pain. |
PAD is diagnosed epidemiologically by measuring the ankle–brachial index. This is the ratio of (1) the higher systolic blood pressure between the posterior tibial artery and dorsalis pedis artery to the (2) higher systolic blood pressure between both arms. A value of less than 0.9 is an indicator of PAD. Symptomatic PAD patients present with intermittent claudication pain. Intermittent claudication is defined as reproducible lower extremity muscular pain induced by exercise and relieved by short periods of rest. Amputation is often preceded by a variable period of limb ischaemia in patients with vascular disease. This is termed as pre-amputation pain. However, these patients frequently have multiple co-morbidities like diabetes, and hence, the pain mechanism may include nociceptive, inflammatory and neuropathic components. However, not all patients with intermittent claudication will require amputation surgery. Only 20% of these patients will deteriorate over a 5-year period, and of these, only 5% require amputation.8
Uncontrolled pre-amputation pain results in an increased stress response and activation of the autonomic nervous system.9 As detailed later in this review, pre-amputation pain has been correlated with an increased incidence of acute post-operative pain and CPAP.
Pain after amputation surgery (Figure 1) has two components – acute post-operative pain and chronic post-amputation pain (CPAP). Two types of acute post-operative pain may occur. The first is the pain in the amputated stump (called stump pain or residual limb pain (RLP)) and the second is the occurrence of PLP and/or PLS. Both conditions may become chronic and they may occur in more than 50% of patients after a surgical amputation according to a recent estimate.6 Long-term RLP may have somatic or neuropathic mechanisms. Neuropathic mechanisms include the presence of a neuroma, development of Complex Regional Pain Syndrome, heterotrophic ossification or mosaic neuralgia.7
Pain vulnerability: risk factors for chronic pain following limb amputation surgery
The elucidation of risk factors for chronic post-operative pain (CPSP) in general is a still evolving field and there is limited evidence for specific risk factors for CPAP. The following paragraphs highlight current evidence of risk factors for CPAP specifically.
Genetics
Chronic pain in general has a heritability ranging from 30% to 70%.10 The heritability of chronic pain is thought to be the result of an interaction between multiple mutated genes and environmental disorders.11 It is surmised that a variety of genetic variants combine to influence the risk of transition from acute post-surgical acute pain to chronic pain.12 About two thirds of genetic variants are thought to reside in the exons on genes and exome analysis/genome wide analysis (GWAS) is the method used to uncover these variant genes.
Mogil et al.13 reported that in bred mouse studies, 50% of the neuropathic pain sensitivity is heritable. Specifically, for CPAP, single-nucleotide polymorphism (SNP) in two genes may be relevant. These are the GCH1 and KCNS1 gene alleles.
GCH1 gene codes for synthesis of tetrahydrobiopterin, an essential co-factor for catecholamine, serotonin and nitric oxide production. GCH1 blockers are potential analgesics and are currently in development.14
Potassium (K+) channels play an important role in the propagation of action potentials. Neuronal hyper-excitability is a key feature of pathological pain. Opening of K+ channels hyperpolarize the neuron, thus decreasing conductivity. A K+ channel genotype has been found to predict acute pain thresholds, risk of developing chronic pain and analgesic effectiveness.15 Genetic variants coding for the various sub units of the tetrameric K+ channel (KCNA1, KCND2, etc.) have been found to be associated with CPSP after breast surgery and limb amputation.16,17
No GWAS has been reported for amputation pain to the best of our knowledge, though a few GWAS studies have been reported for complex regional pain syndrome18 and fibromyalgia.19
Age and gender
The German PAIN OUT registry collects data from 115,775 patients from 578 surgical wards in 105 hospitals. Data from this registry for 30 surgical procedures were analysed by Gerbershagen20 who found that irrespective of duration and type of surgery, patients with pre-operative chronic pain, of female sex and younger age reported more post-operative pain. However, these data do not include amputations.
Psychological factors
There is limited published evidence to highlight the effect of psychological factors on the trajectory of CPAP. Niraj and Rowbotham21 reported on the psychological factors that have been linked to an increased incidence of CPSP in general. They found that the presence of anxiety, depression, catastrophizing, illness perception, poor coping strategy, low sense of control, poor social control, poor social support and high expectations correlated with the development of CPSP. Hinrichs–Rocker et al.22 conducted a systematic review to identify psychological correlates of CPSP. Depression, psychological vulnerability, stress and late return to work showed likely correlation with CPSP (grade of association = 1). Montes et al.23 attempted to devise a clinical risk prediction model for CPSP after hernia repair, hysterectomy and thoracotomy in their study of 2929 patients. Six clinical predictors – age, SF12 physical (Short-Form 12 Health Survey questionnaire), SF12 mental, pre-operative pain in surgical area, pre-operative pain in other areas, along with type of surgery – identified 73% of patients who subsequently developed CPSP.
More specifically for CPAP, Raichle et al.6 examined the relationship between pre-operative anxiety and acute post-operative stump and phantom pain in amputees undergoing amputation due to trauma. This study reported that the degree of pre-operative anxiety was correlated with the intensity of PLP in the post-operative period. An earlier study by Ephraim et al.24 found that depressive symptom was a significant predictor of level of pain intensity and bothersomeness after amputation long term.
Disease states
Multiple disease states (fibromyalgia, migraine, irritable bowel syndrome, irritable bladder and Reynaud syndrome) have been shown to be associated with CPSP.25
A small case control study of lower limb amputees showed no correlation between those with fibromyalgia and neuropathic pain scores.
Pre-operative chronic pain
Sustained and intense nociceptive input prior to amputation may influence post-amputation acute and long-term pain (CPAP) by inducing central sensitization to pain.26 Recent literature supports the assertion that the presence of pre-operative pain, regardless of the relationship to surgical site, significantly increases the risk of developing surgically induced chronic neuropathic pain or CPSP.27
The relationship between pre-amputation pain and CPAP is however not simple. Melzack et al.28 suggested in 2001 that PLP was more likely to occur in amputees who had pain in their limb prior to amputation. Jensen et al.,29 in a prospective study of 58 amputees, studied pre-amputation limb pain and its relationship to chronic PLP in non-traumatic amputees. PLP was reported by 65% patients at 6 months and stump pain by 22% at 6 months. Phantom pain was significant more frequent in those who had pre-amputation pain lasting more than 1 month. Pre-amputation pain was a significant predictor of both acute post-operative pain and chronic PLP at both 6 and 12 months in a 2-year prospective study carried out in a large trauma centre3 However, Nikolajsen et al.30 reported that several patients with severe pre-amputation pain never developed CPAP (PLP). Equally, patients who undergo traumatic amputation with no pre-amputation pain develop CPAP to the same extent as those with longstanding pain who undergo vascular disease–related amputations.
The above studies suggest that the presence of pre-amputation pain is correlated to the development of acute and chronic post-amputation pain, but the relationship is not concordant all the time.
Pre-operative opioids
Patients with pre-operative pain may also be on opioids prior to their surgery. As per the ‘Opponent Process Theory’,31 opioid therapy leads to activation of both anti-nociception and pro-nociception systems. Opioid-induced analgesia and hyperalgesia are due to the interaction of these two opposing processes. Prolonged exposure to opioids results in reduction of the analgesic effect via either decrease in anti-nociception effect or hyperalgesia due to activation of the pro-nociceptive system.
Roullet et al.32 reported that pre-operative opioid consumption was related to increased morphine requirement after amputation. In all, 22 patients were included in this observational study with 12 in the pre-op opioids group and 10 in the opioid naïve group. Average consumption of opioids on day 3 post-operatively was greater in the opioid group (52 (13–133) mg morphine equivalents) versus 0 (0–26 mg) in the opioid naïve group. This difference was sustained at day 7 (10 mg (8–25) vs 0 mg (0–0)). A similar finding of increased opioid requirement post surgery in those on pre-operative opioids has been reported by Armaghani et al.33 Thus, the amount of opioids on board may influence the degree and intensity of immediate post-amputation pain and perhaps CPAP indirectly.
Inadequate intra-operative analgesia
Surgical technique that results in nerve injury is a recognized risk factor for CPSP.34 Maladaptive nerve injury is one of the many factors necessary for development of CPSP following amputation. If adequate analgesia is not provided both intra- and post-operatively, the central nervous system continues to receive continuous nociceptive input from the periphery due to either sensitization of neurons, ectopic activity or phenotypic switch.35,36 This highlights the necessity of efficacious intra-operative analgesia.
Acute post-operative pain
High levels of pain in the acute post-operative period can predict CPSP. This has been seen across a range of surgeries (hernia, breast, thoracic and cholecystectomy).24 For CPAP specifically, evidence to demonstrate relation between efficacy of post-operative pain relief and prevention of CPAP is mixed.3,37 Issues such as the duration of high-quality post-operative analgesia that is needed to prevent CPSP/CPAP are at present unaddressed.
Prevention
Population-level prevention of vascular disease
A lack of effective treatments for PLP/CPAP forces us to review methods for its occurrence in the first place, that is, prevention. This is important as the disease burden of vascular disease and trauma continue to rise. For preventing amputation due to vascular disease, there needs to be a continued focus on primary and secondary prevention cardiovascular disease and vascular disease (specifically on the high-risk segments).
Whole system approach necessary to prevent/manage CPAP
It is quite clear that to prevent CPAP, a whole system approach is necessary. This approach has been shown to be successful in reducing the incidence of moderate/severe pain in 22 Veteran Health Administration hospitals.38 For amputation, the principal recommendation of the National Confidential Enquiry into Patient Outcome and Death (NCEPOD) report ‘Lower limb amputation: working together’ has been that hospitals should develop a best-practice clinical care pathway incorporating the findings of the report.1 Other recommendations in this document include involving a multi-disciplinary team pre- and post-operatively. The service design needs to include all stakeholders in the amputation pain pathway wherever possible. This may include Anaesthetists, Vascular surgeons, Acute Pain nurses, Physiotherapists and Pharmacists.
Peri-operative strategy: three-phase system approach
Given that the modifiable risk factors for CPAP start pre-operatively and extend to the post-operative period, it is useful to segment our prevention strategy into three phases: (1) pre-operative, (2) intra-operative and (c) post-operative.
Pre-operative phase preventive measures
High-risk patients for CPAP need to be identified, pre-operative analgesia needs to be optimized, patients educated and expectations managed.
Treat pre-operative pain
It is important to treat pre-operative pain as this has been consistently shown to have a concordant association with CPAP. Early involvement of the acute pain service (APS) is more likely to address the pre-operative pain issues of the patient and tag them onto the APS radar of patients to follow. One of the key recommendations of the NCEPOD report1 is that the pain team should be involved within 12 h of admission for all major lower limb amputation. This may not be always possible in case of emergency amputations.
Education of foundation doctors and ward nurses is key to achieving pre-emptive analgesia as they are often the first responders. Emphasis needs to be placed on identification of the primary pain mechanism (nociceptive, inflammation or neuropathic) for optimizing pain relief.
Pre-emptive analgesia
Pre-emptive analgesia is an attractive proposition to reduce noxious sensitization of the central nervous system. Epidural analgesia has been studied the most in this regard. Karanikolos et al.39 and colleagues investigated whether pre-emptive analgesia reduced PLP/CPAP at 6 months; 65 patients were assigned to five treatment groups. Epi/Epi/Epi group received epidural analgesia starting 48 h before surgery and continuing till 48 h post-surgery. PCA/Epi/Epi group received fentanyl patient-controlled analgesia (PCA) pre-operatively for 48 h and the epidural till 48 h post-operatively. PCA/Epi/PCA group received PCA fentanyl for 48 h pre-operatively and intra-operative epidural and then PCA fentanyl for 48 h post-operatively. PCA/GA/PCA group received pre- and post-operative analgesia for 48 h with PCA fentanyl and intra-operative general anaesthesia (GA) with propofol, sevoflurane and an intravenous infusion of remifentanil. The control group received meperidine (pethidine) intramuscularly 50 mg 4–6 times a day and codeine/acetaminophen (paracetamol) 30/500 mg tablets three to five times a day pre- and post-operatively. Intra-operatively, they received GA. All the groups had pain scores >70 mm on Visual Analogue Scale (VAS) at initiation.
After 48 h of pre-operative analgesia, immediately prior to surgery, the pre-operative epidural group (Epi/Epi/Epi) had pain scores of 0 whereas the PCA groups (PCA/Epi/Epi, PCA/Epi/PCA and PCA/GA/PCA) had pain scores 20/20/10 out of 100. At 6 months, PLP was present in 1 of 13 Epi/Epi/Epi; 4 of 13 PCA/Epi/Epi; 3 of 13 PCA/GA/PCA; 7 of 12 in PCA/Epi/PCA patients versus 9 of 12 control patients.
This study highlights the fact that epidurals placed 48 h before amputations and PCA fentanyl are both effective in treating pre-operative pain as well as preventing PLP. However, the control group patients received meperidine (pethidine) and the VAS scores after 48 h of the analgesic protocol was 60/100. This represents suboptimal pain relief in the control group and the sample size was small.
Earlier studies on epidurals placed pre-operatively support the conclusions of Karanikolos et al. that epidurals may prevent CPAP/PLP. Bach et al.40 randomized 25 patients to two groups. The epidural group (11 patients) received an epidural 72 h before amputation, while the conventional analgesics group (14 patients) did not receive an epidural. At 6 months, the incidence of phantom limb was lower in epidural group. Jahangiri et al.41 compared the effect of epidural infusion of diamorphine, bupivacaine and clonidine (13 patients) with opioid analgesia on demand (11 patients) on post-amputation stump and phantom pain. The incidence of severe phantom pain was lower in the epidural group 1 year after amputation. This study was not a randomized control trial.
Nikolajsen et al.37 randomized 60 patients into two groups. The blockade group received epidural bupivacaine and morphine, whereas the control group received epidural saline and oral or intramuscular morphine. The epidural was placed 18 h before the amputation and continued for 3–5 days post-operatively. Both groups had GA for amputation. There was no difference in PLP at 12 months between the groups. However, pre-amputation pain was better controlled with epidural (0 mm VAS) compared to control (31 mm VAS).
Ypsilantis and Tang42 performed a systematic review of pre-emptive analgesia to prevent CPAP; 11 studies were analysed. Most of the studies had small sample sizes and a loss of up to 52% of patients to follow-up, mainly due to high mortality in this patient group. The authors could not demonstrate robust evidence (level 1 Oxford Centre for Evidence-based Medicine) for any peri-operative analgesia technique (epidural bupivacaine plus morphine/diamorphine or peri-neural bupivacaine) to prevent CPAP but did report level 2b evidence for the role of epidural pre-emptive analgesia in preventing acute post-amputation pain.
The inference from the above studies is that evidence of pre-emptive analgesia with epidural infusions of local anaesthetics to prevent long-term PLP/CPAP is mixed or equivocal. However, optimal pre-operative and post-operative acute pain relief is possible with epidural analgesia and this may reduce the incidence of PLP and CPAP. However, pre-emptive analgesic relief is not restricted to the use of epidurals and there is evidence to show that the type of analgesia technique may be secondary to the consideration that the therapy should be able to stop nociceptive impulses from reaching the spinal cord.43
Gabapentin – pre-emptive analgesia
A recent combined systematic review and meta-analysis by Clarke et al.44 concluded that peri-operative administration of gabapentin and pregabalin is effective in reducing CPSP. This review, however, did not include amputations.
Nikolajsen et al.45 conducted a randomized control trial on the efficacy of gabapentin on chronic post-amputation pain (CPAP); 46 patients were randomized to receive either gabapentin (23) or placebo (23). An epidural (bupivacaine) was sited for the surgery and continued 2–3 days post-operatively. Gabapentin was started on day 1 post-operatively and increased to 2400 mg between days 13 and 30. There was no difference in PLP at 6 months after surgery between the groups.
It is important to note that the study on amputation patients by Nikolajsen et al. was performed in 2006 and that recent evidence by Clarke et al. (2012) points towards a role for gabapentin in reducing the incidence of CPSP in general.
Ketamine pre-emptive analgesia
Ketamine is a N-Methyl-d-aspartate (NMDA) antagonist and analgesic in sub-anaesthetic doses. Ketamine also has a profound anti-inflammatory action46 and may help diminish surgical and post-surgical inflammation; 45 patients undergoing amputation were randomized by Hayes et al.47 to receive a ketamine 0.5 mg/kg bolus pre-induction or placebo followed by an intravenous infusion of ketamine 0.5 mg/kg/h or normal saline for 72 h post-operatively. Both groups received GA and PCA morphine. There was no difference in the incidence of CPAP/PLP 6 months after surgery. However, low-dose ketamine has shown efficacy in reducing post-operative opioid requirements in a recent systematic review by Jouguelet-Lacoste et al.48 The authors studied the use of low-dose intravenous infusion of ketamine for the management of peri-operative pain. In this study, five meta-analyses and 39 clinical trials met the inclusion criteria. This study concluded that use of low-dose ketamine infusions (less than 1.2 mg/kg/hour) for 24–48 h post-operatively reduced post-operative opioid consumption by 40%. The effect was less marked for pain scores post-operatively. However, the authors also reported that ketamine infusions for at least 24 h post-operatively were efficacious in reducing long-term CPAP/PLP.
The inference from the above studies is that there is no firm evidence for ketamine’s role in reducing long-term CPAP but it does have a role in reducing acute post-operative pain.
Education of patients
Clear explanations with leaflets about PLP should be offered. It may be important to manage expectations at this stage.
Intra-operative phase
Amputations should be performed on an elective list during normal working hours, and a consultant vascular surgeon and consultant anaesthetist should be present in theatre.1 It is preferable that amputations are performed on elective lists where possible.
Epidural, spinal or GA. As discussed earlier, epidural analgesia has efficacy in improving pre- and post-operative pain scores and reducing PLP/CPAP. Regional anaesthesia may provide superior analgesia as compared to GA in the immediate post-operative period but evidence on their efficacy in preventing CPAP is limited. A recent systematic review by Humble et al.49 reported that regional analgesia was beneficial in reducing peri-operative pain. A cross-sectional survey of 150 amputees by Ong et al.50 and Sahin et al.51 noted an improvement in acute post-operative pain with epidural or spinal techniques but did not prevent long-term CPAP including PLP.
Peri-neural catheters
Peri-neural catheters are placed adjacent to the nerves and local anaesthetic infusion administered through them. Elizaga et al.52 in a retrospective study compared those who had a peri-neural sciatic nerve block with bupivacaine for at least 72 h after amputation (n = 9) with a control group who had systemic analgesia (n = 12). There was no difference in incidence of PLP at 6 months. Pinzur et al. in a prospective study of 16 patients randomized patients to receive peri-neural catheter infusions of bupivacaine (n = 9) or normal saline (n = 12) for 72 h post-operatively. There was a decrease in post-operative morphine requirement in the peri-neural catheter group but there was no decrease in the incidence of long-term PLP/CPAP.
In a retrospective review of 64 patients, Grant and Wood53 noted that post-operative morphine requirements were less (10 mg vs 74 mg) in the intra-neural infusion group (n = 33) than in the routine analgesia group (n = 31). The study also reported a reduction in prescription of amitriptyline for PLP in the intra-neural infusion group (4 vs 11 patients).
Borghi et al.54 in an observational study with no control group placed peri-neural catheters and started a 0.5% ropivacaine infusion at 5 mL/h for a median 30 days in 71 patients. The reported incidence of severe or intolerable PLP at 12 months was found to be 3%. Ilfeld et al.55 reported a good outcome in a case series (n = 3) treating refractory PLP with 6 days of peri-neural infusion with a 0.4% ropivacaine infusion. This highlights the role of continued peripheral sensitization post-amputation. Ayling et al.56 conducted a retrospective chart review of 198 patients who underwent amputation; 102 received peri-neural catheter post-operatively versus 96 patients who did not. Use of peri-neural catheters resulted in a 40% reduction in opioid use in the first 72 h post-operatively.
Bosanquet et al.57 in a recent meta-analysis of trials for nerve catheters placed intra-operatively and kept for 72 h found evidence of significantly reduced opioid consumption (50%) post-operatively in amputation patients. There was considerable heterogeneity among the studies, and the quality of studies was not high with four retrospective studies, two small RCTs and a case report included in the analysis. A variety of local anaesthetics were used in the peri-neural catheters in the seven studies included in the meta-analysis (0.25% bupivacaine, 0.2% ropivacaine, and 1% lignocaine).
The above evidence suggests that peri-neural catheter local anaesthetic infusions are useful in reducing post-operative opioid requirements and may have a role in long-term reduction of PLP/CPAP. The neural catheter should be placed as close to the sciatic nerve as possible as the nerve retracts after transection. An additional stump wound catheter may be placed but this can result in the plaster cast getting wet from the soakage post-operatively.
Post-operative phase
In this phase, follow-up and review by the acute pain team is important. Post-operative analgesia is optimized and individualised according to the pain mechanisms.
Stump pain is common post-operatively. Stump infection often increases analgesia requirements and should be excluded. The other causes of stump pain such as continuing vascular insufficiency, osteomyelitis, haematoma, inadequate wound flap or a poorly fitting prosthesis should be considered. Patients should be prescribed regular opioids orally post-operatively. Occasionally, a switch of opioids may be necessary as might be the use of PCA morphine. Recent evidence points to the increasing role of drugs such as tapentadol or antidepressants in establishing the equilibrium of descending inhibitory pathways for pain.58 Occasionally, it may be useful to use ketamine, either orally or intravenously.
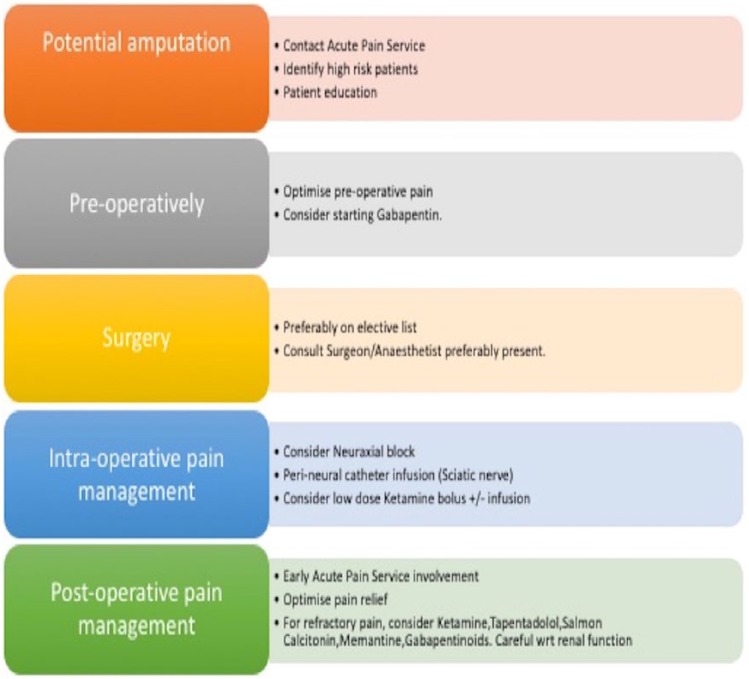
Diabetes needs to be controlled and diabetic neuropathic pain treatment with duloxetine may need to be started. For patients who develop established PLP despite preventive measures, a recent Cochrane review59 suggests starting NMDA blockers like ketamine or memantine, gabapentinoids, amitriptyline, opioids or a calcitonin infusion. Calcitonin infusion of 200 IU is effective in the treatment of PLP developing in the first week post-operatively and not for long-term PLP. Lidocaine infusion at 4 mg/kg was found effective for stump pain but not for PLP. A detailed discussion on treatment of long-term PLP/CPAP is outside the remit of this review and readers are referred to the Cochrane review on this topic. A suggested peri-operative pathway for management of amputation-related pain is provided in Figure 3.
Figure 3.
A suggested peri-operative pathway for management of amputation pain.
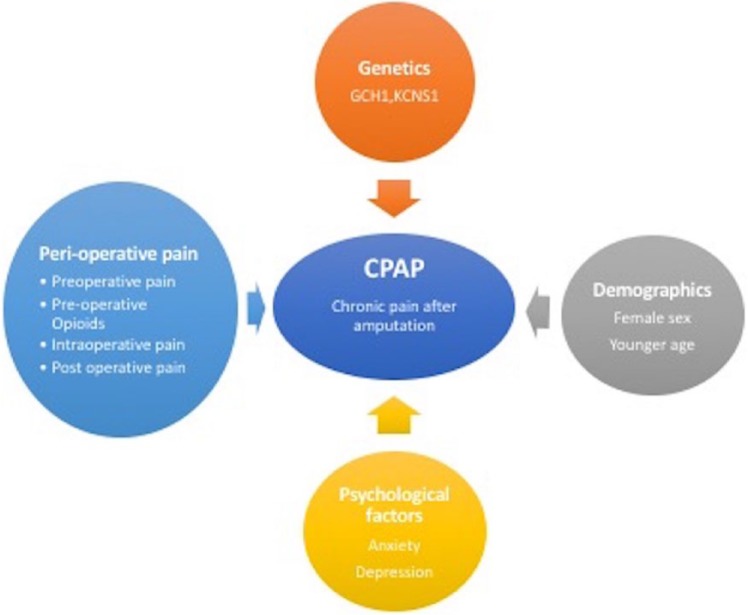
Figure 2.
Risk factors for development of chronic pain after amputation (CPAP)13,20,21,22,6,23,28,29,30,32,35,36,37.
Conclusion
Classification of peri-operative amputation phenotypes is important for the prevention, identification, and treatment of CPAP. The presence of variation in two gene alleles (GCH1 and KCNS1) may be relevant for development of CPAP. Identification of the high-risk patients, that is, younger age group, female, pre-operative chronic ischaemic pain in the surgical area, pre-operative chronic pain elsewhere, depression, anxiety and use of high opioids pre-operatively, is recommended. Pre- and post-operative pain control should be optimized to prevent CPAP/PLP. Peri-operative epidural and peri-neural catheter infusions of local anaesthetics have been found to be efficacious for optimal peri-operative pain control and may reduce the incidence of CPAP/PLP. There is limited evidence for pre-emptive analgesia with gabapentin for CPAP. A low-dose ketamine infusion peri-operatively reduces post-operative morphine requirement. The evidence seems to suggest that optimized peri-operative analgesia may reduce the incidence of CPAP. More research is needed on the type, timing and duration of peri-operative analgesia to prevent CPAP.
Footnotes
Declaration of conflicting interests: The author declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
References
- 1. National Confidential Enquiry into Patient Outcome and Death. Lower limb amputation: working together, 2014, http://www.ncepod.org.uk/2014report2/downloads/WorkingTogetherFullReport.pdf (accessed 25 February 2017).
- 2. Luo Y, Anderson TA. Phantom limb pain: a review. Int Anesthesiol Clin 2016; 54(2): 121–139. [DOI] [PubMed] [Google Scholar]
- 3. Hanley MA, Jensen MP, Smith DG, et al. Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J Pain 2007; 8(2): 102–109. [DOI] [PubMed] [Google Scholar]
- 4. Lenggenhager B, Arnold CA, Giummarra MJ. Phantom limbs: pain, embodiment, and scientific advances in integrative therapies. Wiley Interdiscip Rev Cogn Sci 2014; 5(2): 221–231. [DOI] [PubMed] [Google Scholar]
- 5. Kern U, Busch V, Muller R, et al. Phantom limb pain in daily practice – still a lot of work to do! Pain Med 2012; 13(12): 1611–1626. [DOI] [PubMed] [Google Scholar]
- 6. Raichle KA, Osborne TL, Jensen MP, et al. Preoperative state anxiety, acute postoperative pain, and analgesic use in persons undergoing lower limb amputation. Clin J Pain 2015; 31(8): 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchheit T, Van de, Ven T, Hsia HL, et al. Pain Phenotypes and Associated Clinical Risk Factors Following Traumatic Amputation: Results from Veterans Integrated Pain Evaluation Research (VIPER). Pain Med 2016; 17(1): 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fowkes FG, Aboyans V, Fowkes FJ, et al. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol 2017; 14(3): 156–170. [DOI] [PubMed] [Google Scholar]
- 9. Seretny M, Colvin LA. Pain management in patients with vascular disease. Br J Anaesth 2016; 117(Suppl. 2): ii95–ii106. [DOI] [PubMed] [Google Scholar]
- 10. Young EE, Lariviere WR, Belfer I. Genetic basis of pain variability: recent advances. J Med Genet 2012; 49(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schoeps A, Rudolph A, Seibold P, et al. Identification of new genetic susceptibility loci for breast cancer through consideration of gene-environment interactions. Genet Epidemiol 2014; 38(1): 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarke H, Katz J, Flor H, et al. Genetics of chronic post-surgical pain: a crucial step toward personal pain medicine. Can J Anaesth 2015; 62(3): 294–303. [DOI] [PubMed] [Google Scholar]
- 13. Mogil JS, Wilson SG, Bon K, et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 1999; 80(1–2): 67–82. [DOI] [PubMed] [Google Scholar]
- 14. Latremoliere A, Costigan M. GCH1, BH4 and pain. Curr Pharm Biotechnol 2011; 12(10): 1728–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsantoulas C. Emerging potassium channel targets for the treatment of pain. Curr Opin Support Palliat Care 2015; 9(2): 147–154. [DOI] [PubMed] [Google Scholar]
- 16. Langford DJ, Paul SM, West CM, et al. Variations in potassium channel genes are associated with distinct trajectories of persistent breast pain after breast cancer surgery. Pain 2015; 156(3): 371–380. [DOI] [PubMed] [Google Scholar]
- 17. Hoofwijk DM, van Reij RR, Rutten BP, et al. Genetic polymorphisms and their association with the prevalence and severity of chronic postsurgical pain: a systematic review. Br J Anaesth 2016; 117(6): 708–719. [DOI] [PubMed] [Google Scholar]
- 18. Janicki PK, Alexander GM, Eckert J, et al. Analysis of common single nucleotide polymorphisms in complex regional pain syndrome: Genome Wide Association study approach and pooled DNA strategy. Pain Med 2016; 17(12): 2344–2352. [DOI] [PubMed] [Google Scholar]
- 19. Ablin JN, Buskila D. Update on the genetics of the fibromyalgia syndrome. Best Pract Res Clin Rheumatol 2015; 29(1): 20–28. [DOI] [PubMed] [Google Scholar]
- 20. Gerbershagen HJ. Procedure-specific postoperative pain treatment, 2015, https://dspace.library.uu.nl/handle/1874/311168 (accessed 26 February 2017).
- 21. Niraj G, Rowbotham DJ. Persistent postoperative pain: where are we now? Br J Anaesth 2011; 107(1): 25–29. [DOI] [PubMed] [Google Scholar]
- 22. Hinrichs-Rocker A, Schulz K, Jarvinen I, et al. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) – a systematic review. Eur J Pain 2009; 13(7): 719–730. [DOI] [PubMed] [Google Scholar]
- 23. Montes A, Roca G, Sabate S, et al. Genetic and clinical factors associated with chronic postsurgical pain after hernia repair, hysterectomy, and thoracotomy: a two-year multicenter cohort study. Anesthesiology 2015; 122(5): 1123–1141. [DOI] [PubMed] [Google Scholar]
- 24. Ephraim PL, Wegener ST, MacKenzie EJ, et al. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil 2005; 86(10): 1910–1919. [DOI] [PubMed] [Google Scholar]
- 25. Pozek JP, Beausang D, Baratta JL, et al. The acute to chronic pain transition: can chronic pain be prevented? Med Clin North Am 2016; 100(1): 17–30. [DOI] [PubMed] [Google Scholar]
- 26. Coderre TJ, Katz J. Peripheral and central hyperexcitability: differential signs and symptoms in persistent pain. Behav Brain Sci 1997; 20(3): 404–419; discussion 435–513. [DOI] [PubMed] [Google Scholar]
- 27. Borsook D, Kussman BD, George E, et al. Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg 2013; 257(3): 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Melzack R, Coderre TJ, Katz J, et al. Central neuroplasticity and pathological pain. Ann N Y Acad Sci 2001; 933: 157–174. [DOI] [PubMed] [Google Scholar]
- 29. Jensen TS, Krebs B, Nielsen J, et al. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain 1985; 21(3): 267–278. [DOI] [PubMed] [Google Scholar]
- 30. Nikolajsen L, Ilkjaer S, Kroner K, et al. The influence of preamputation pain on postamputation stump and phantom pain. Pain 1997; 72(3): 393–405. [DOI] [PubMed] [Google Scholar]
- 31. Koppert W, Schmelz M. The impact of opioid-induced hyperalgesia for postoperative pain. Best Pract Res Clin Anaesthesiol 2007; 21(1): 65–83. [DOI] [PubMed] [Google Scholar]
- 32. Roullet S, Nouette-Gaulain K, Biais M, et al. Preoperative opioid consumption increases morphine requirement after leg amputation. Can J Anaesth 2009; 56(12): 908–913. [DOI] [PubMed] [Google Scholar]
- 33. Armaghani SJ, Lee DS, Bible JE, et al. Increased preoperative narcotic use and its association with postoperative complications and length of hospital stay in patients undergoing spine surgery. Clin Spine Surg 2016; 29(2): E93–E98. [DOI] [PubMed] [Google Scholar]
- 34. Reddi D, Curran N. Chronic pain after surgery: pathophysiology, risk factors and prevention. Postgrad Med J 2014; 90(1062): 222–227; quiz 226. [DOI] [PubMed] [Google Scholar]
- 35. Neumann S, Doubell TP, Leslie T, et al. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 1996; 384(6607): 360–364. [DOI] [PubMed] [Google Scholar]
- 36. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152(3 Suppl.): S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nikolajsen L, Ilkjaer S, Christensen JH, et al. Randomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower-limb amputation. Lancet 19978; 350(9088): 1353–1357. [DOI] [PubMed] [Google Scholar]
- 38. Cleeland CS, Reyes-Gibby CC, Schall M, et al. Rapid improvement in pain management: the Veterans Health Administration and the institute for healthcare improvement collaborative. Clin J Pain 2003; 19(5): 298–305. [DOI] [PubMed] [Google Scholar]
- 39. Karanikolas M, Aretha D, Tsolakis I, et al. Optimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: a prospective, randomized, clinical trial. Anesthesiology 2011; 114(5): 1144–1154. [DOI] [PubMed] [Google Scholar]
- 40. Bach S, Noreng MF, Tjellden NU. Phantom limb pain in amputees during the first 12 months following limb amputation, after preoperative lumbar epidural blockade. Pain 1988; 33(3): 297–301. [DOI] [PubMed] [Google Scholar]
- 41. Jahangiri M, Jayatunga AP, Bradley JW, et al. Prevention of phantom pain after major lower limb amputation by epidural infusion of diamorphine, clonidine and bupivacaine. Ann R Coll Surg Engl 1994; 76(5): 324–326. [PMC free article] [PubMed] [Google Scholar]
- 42. Ypsilantis E, Tang TY. Pre-emptive analgesia for chronic limb pain after amputation for peripheral vascular disease: a systematic review. Ann Vasc Surg 2010; 24(8): 1139–1146. [DOI] [PubMed] [Google Scholar]
- 43. Rathmell JP, Kehlet H. Do we have the tools to prevent phantom limb pain? Anesthesiology 2011; 114(5): 1021–1024. [DOI] [PubMed] [Google Scholar]
- 44. Clarke H, Bonin RP, Orser BA, et al. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg 2012; 115(2): 428–442. [DOI] [PubMed] [Google Scholar]
- 45. Nikolajsen L, Finnerup NB, Kramp S, et al. A randomized study of the effects of gabapentin on postamputation pain. Anesthesiology 2006; 105(5): 1008–1015. [DOI] [PubMed] [Google Scholar]
- 46. Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg 2011; 62(1): 47–58. [PubMed] [Google Scholar]
- 47. Hayes C, Armstrong-Brown A, Burstal R. Perioperative intravenous ketamine infusion for the prevention of persistent post-amputation pain: a randomized, controlled trial. Anaesth Intensive Care 2004; 32(3): 330–338. [DOI] [PubMed] [Google Scholar]
- 48. Jouguelet-Lacoste J, La Colla L, Schilling D, et al. The use of intravenous infusion or single dose of low-dose ketamine for postoperative analgesia: a review of the current literature. Pain Med 2015; 16(2): 383–403. [DOI] [PubMed] [Google Scholar]
- 49. Humble SR, Dalton AJ, Li L. A systematic review of therapeutic interventions to reduce acute and chronic post-surgical pain after amputation, thoracotomy or mastectomy. Eur J Pain 2015; 19(4): 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ong BY, Arneja A, Ong EW. Effects of anesthesia on pain after lower-limb amputation. J Clin Anesth 2006; 18(8): 600–604. [DOI] [PubMed] [Google Scholar]
- 51. Sahin SH, Colak A, Arar C, et al. A retrospective trial comparing the effects of different anesthetic techniques on phantom pain after lower limb amputation. Curr Ther Res Clin Exp 2011; 72(3): 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elizaga AM, Smith DG, Sharar SR, et al. Continuous regional analgesia by intraneural block: effect on postoperative opioid requirements and phantom limb pain following amputation. J Rehabil Res Dev 1994; 31(3): 179–187. [PubMed] [Google Scholar]
- 53. Grant AJ, Wood C. The effect of intra-neural local anaesthetic infusion on pain following major lower limb amputation. Scott Med J 2008; 53(1): 4–6. [DOI] [PubMed] [Google Scholar]
- 54. Borghi B, D’Addabbo M, White PF, et al. The use of prolonged peripheral neural blockade after lower extremity amputation: the effect on symptoms associated with phantom limb syndrome. Anesth Analg 2010; 111(5): 1308–1315. [DOI] [PubMed] [Google Scholar]
- 55. Ilfeld BM, Moeller-Bertram T, Hanling SR, et al. Treating intractable phantom limb pain with ambulatory continuous peripheral nerve blocks: a pilot study. Pain Med 2013; 14(6): 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ayling OG, Montbriand J, Jiang J, et al. Continuous regional anaesthesia provides effective pain management and reduces opioid requirement following major lower limb amputation. Eur J Vasc Endovasc Surg 2014; 48(5): 559–564. [DOI] [PubMed] [Google Scholar]
- 57. Bosanquet DC, Glasbey JC, Stimpson A, et al. Systematic review and meta-analysis of the efficacy of perineural local anaesthetic catheters after major lower limb amputation. Eur J Vasc Endovasc Surg 2015; 50(2): 241–249. [DOI] [PubMed] [Google Scholar]
- 58. Coluzzi F, Fornasari D, Pergolizzi J, et al. From acute to chronic pain: tapentadol in the progressive stages of this disease entity. Eur Rev Med Pharmacol Sci 2017; 21(7): 1672–1683. [PubMed] [Google Scholar]
- 59. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev 2016; 10: CD006380. [DOI] [PMC free article] [PubMed] [Google Scholar]