Abstract
Objectives
Though controversial, onychectomy remains a commonly performed distal thoracic limb surgical procedure in cats. Peripheral nerve block techniques have been proposed in cats undergoing onychectomy but evidence of efficacy is lacking. Preliminary tests of the described technique using cadavers resulted in incomplete staining of nerves. The aim of this study was to develop nerve block methods based on cadaveric dissections and test these methods with cadaveric dye injections.
Methods
Ten pairs of feline thoracic limbs (n = 20) were dissected and superficial branches of the radial nerve (RSbr nn.), median nerve (M n.), dorsal branch of ulnar nerve (UDbr n.), superficial branch of palmar branch of ulnar nerve (UPbrS n.) and deep branch of palmar branch of ulnar nerve (UPbrDp n.) were identified. Based on these dissections, a four-point block was developed and tested using dye injections in another six pairs of feline thoracic limbs (n = 12). Using a 25 G × 5/8 inch needle and 1 ml syringe, 0.07 ml/kg methylene blue was injected at the site of the RSbr nn., 0.04 ml/kg at the injection site of the UDbr n., 0.08 ml/kg at the injection site of the M n. and UPbrS n., and 0.01 ml/kg at the injection site of the UPbrDp n. The length and circumference of each nerve that was stained was measured.
Results
Positive staining of all nerves was observed in 12/12 limbs. The lengths stained for RSbr nn., M n., UDbr n., UPbrS n. and UPbrDp n. were 34.9 ± 5.3, 26.4 ± 4.8, 29.2 ± 4.0, 39.1 ± 4.3 and 17.5 ± 3.3 mm, respectively. The nerve circumferences stained were 93.8 ± 15.5, 95.8 ± 9.7, 100 ± 0.0, 100 ± 0.0 and 93.8 ± 15.5%, respectively.
Conclusions and relevance
This described four-point injection method may be an effective perioperative analgesia technique for feline distal thoracic limb procedures.
Introduction
Distal thoracic limb surgery in cats, including digit amputation, biopsy and fracture repair, is performed for a variety of reasons, for example for treatment of neoplasia, infected nail beds and trauma. Onychectomy is probably the most commonly performed surgery of the distal thoracic limb in cats.
Scratching is a normal behavior of cats for the purpose of territory marking, stretching forelimbs and conditioning claws. 1 However, for many owners, this behavior is reported to be undesirable owing to the damage inflicted on furniture and belongings. 2 Options available to owners to limit the damage from scratching cats include non-surgical and surgical methods. 3 Onychectomy is becoming increasingly controversial and has been banned in at least 22 countries and in eight California cities, 4 primarily because it is considered an unnecessary cosmetic procedure associated with pain and distress. Another reason for the controversy is a belief that onychectomized cats are more likely to develop behavioral problems. 2 Regardless, in North America it is still a commonly performed procedure,2–5 with an estimated 15.5 million cats undergoing onychectomy every year. 4 Owner satisfaction with this procedure is reported to be high. 3 However, it is generally agreed that onychectomy is associated with pain. 6 Many cats display overt lameness at the time of discharge from the hospital, 2 and objective gait analysis has shown that limb function does not return to preoperative values 12 days after onychectomy. 6 Furthermore, chronic pain/neuropathic pain following surgical onychectomy has been described. 2
The best way to avoid pain associated with onychectomy is to not perform the procedure, but when it is performed, it is incumbent on veterinarians to use the most effective means of providing comprehensive analgesia. Multiple methods for the alleviation of perioperative pain associated with this procedure have been described, including irrigation of surgical incisions with bupivacaine before closure.6,7 Local analgesics are commonly utilized preoperatively in an attempt to block nociceptive input, avoid central sensitization, reduce the amount of systemic analgesic required and decrease hospitalization time.8–10 In the case of onychectomy and indeed any other anticipated painful procedure of the distal thoracic limb, this would involve effective blockade of the nerves that innervate the manus, including the superficial branches of the radial nerve (RSbr nn.), the median nerve (M n.), the dorsal branch of the ulnar nerve (UDbr n.), the superficial branch of the palmar branch of the ulnar nerve (UPbrS n.) and the deep branch of the palmar branch of the ulnar nerve (UPbrDp n.). Use of bupivacaine administered as a four-point regional nerve block prior to onychectomy in cats has been proposed. 11 However, in one study, no additional benefit from this block over systemic buprenorphine administration was found in cats undergoing forelimb onychectomy. 7
The most commonly used technique for local anesthetic block in cats undergoing onychectomy appears to stem from a single case report. 11 Preliminary data that we obtained from critically testing this technique using cadaver limbs and injections of dye indicated frequent incomplete staining of targeted nerves (see supplementary material). Given that a successfully performed nerve block is one of the most effective means to manage pain, 12 we re-evaluated the injection techniques. The aims of this study were to review the anatomical location of the RSbr nn., M n., UDbr n., UPbrS n. and UPbrDp n. in relation to palpable anatomical landmarks and identify clearly defined injection sites that may provide a comprehensive blockade of the distal thoracic limb. An additional aim of this study was to test the defined sites using volumetrically relevant injections of dye in cadaver limbs. Our ultimate goal was to establish a peripheral nerve block technique that may provide effective analgesia for cats undergoing distal thoracic limb surgery.
Material and methods
Cadaver dissections
Ten pairs of cadaveric thoracic limbs were obtained from domestic shorthair cats (nine castrated males and one spayed female) that had been euthanized with an intravenous overdose of pentobarbital (Fatal Plus solution; Vortech Pharmaceuticals) for reasons unrelated to this study. Mean ± SD body weight was 4.0 ± 0.9 kg. The limbs were disarticulated at their muscular attachment to the thoracic wall and stored immediately at −18 °C, then thawed at room temperature overnight prior to use. Anatomic dissection of the RSbr nn., M n., UDbr n., UPbrS n. and UPbrDp n. from the level of the mid-antebrachium distally was conducted to determine palpable landmarks, anatomic variations, fascial planes and associated structures. During dissection, potential injection points and landmarks that would guide injection were recorded.
The accessory carpal bone (ACb) was established as a reference landmark because it is relatively fixed, and an easily palpable landmark in the live animal. The location of each putative injection point was recorded using measurements taken relative to the ACb in the transverse and sagittal planes by the same person (ME) (see ‘Measurements’ and Figure 1), and simultaneously the descriptive relationship to anatomical landmarks was recorded. Based on these findings, a subcutaneous, perineural injection technique using four injection sites was developed for the RSbr nn., M n., UDbr n., UPbrS n. and UPbrDp n.
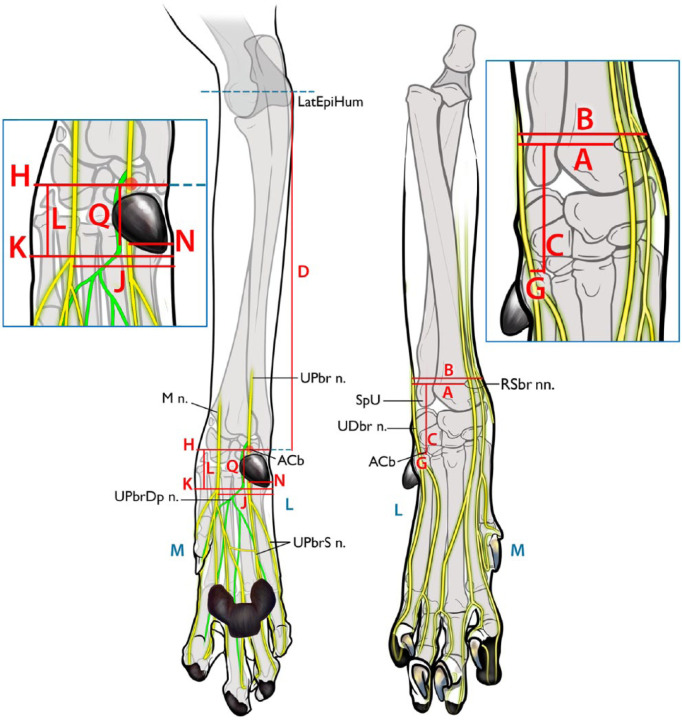
Figure 1.
Description of the measured parameters used to evaluate the proposed injection sites for distal limb nerves. (Right) Craniocaudal view. (A) Distance from the lateral aspect of the limb to the superficial branches of the radial nerve (RSbr nn.); (B) width of the limb at the level of the injection point of the RSbr nn.; (C) Distance proximally from the accessory carpal bone (ACb) to the RSbr nn.; (G) distance from the accessory carpal bone to the dorsal branch of the ulnar nerve (UDbr n.). (Left) Caudocranial view. (D) distance from the accessory carpal bone to the lateral epicondyle of the humerus (LatEpiHum); (H) width of the carpus at the level of the accessory carpal bone; (J) distance from the lateral aspect of the limb to the median nerve (M n.); (K) width of the limb at the level of the injection point of the M n. and the superficial branch of palmar branch of ulnar nerve (UPbrS n.); (L) proximodistal distance from the accessory carpal bone to the level of the M n. and UPbrS n.; (N) distance from the lateral aspect of the limb to the UPbrS n.; (Q) distance from the accessory carpal bone to the point where the deep branch of palmar branch of ulnar nerve (UPbrDp n.) turns to a deeper location. L (blue) = lateral; M (blue) = medial; SpU = styloid process of the ulna; UPbr n. = palmar branch of the ulnar nerve
Dye studies
Based on the results of the cadaver dissection study, a perineural four-point injection technique was used for ‘blockade’ of the RSbr nn., M n., UDbr n., UPbrS n. and UPbrDp n. Twelve additional thoracic limbs from six cats (three intact male and three intact females) that were euthanized for reasons unrelated to this study were used. Mean ± SD body weight was 4.0 ± 0.9 kg, and individual body weights were used to calculate the volume for a 0.5% bupivacaine dose of 1 mg/kg per limb. Using a 25 G × 5/8 inch needle and a 1 ml syringe, an equivalent volume (0.2 ml/kg per limb) of new methylene blue (NMB [New Methylene Blue Stain; Jorgensen Laboratories]) distributed as 0.07 ml/kg was administered at the injection site for RSbr nn., 0.04 ml/kg for UDbr n., 0.08 ml/kg for M n. and UPbrS n., and 0.01 ml/kg for UPbrDP n. Approximately 10 mins after the injections, dissection was performed to expose each nerve. The length and circumference of the stained portion of nerves were measured as previously described. 13 The proximity of dye to unstained or poorly stained nerves was noted.
Measurements
All measurements were made using Dial Calipers (Dial caliper 505-633-50; Mitutoyo). To minimize error by limb positioning, all measurements were made with the carpus extended to 180º and the elbow at an angle of approximately 45º. The measurements made are detailed in Figure 1. The confluence point of the cephalic vein and accessory cephalic vein was established as the target injection point of the RSbr nn. in order to block these nerves before they branched distally into the digits. The distance from the lateral aspect of the limb to the RSbr nn. (A), the width of the limb at the level of the injection point of the RSbr nn. (B) and the distance proximally from the ACb to the RSbr nn. (C) were measured. The distance from the ACb to the lateral epicondyle of the humerus (D) was also measured. The ratios of A/B (E) and C/D (F) were calculated. The optimal injection point for the UDbr n. was determined to be at the level of the ACb, on the lateral aspect, because at this point the nerve is not covered with fascia. The distance from the ACb to the UDbr n. (dorsal view) (G) and the width of the carpus at the level of the ACb (palmar view) (H) were measured. The ratio of G/H (I) was calculated. The area distal to the flexor retinaculum where the M n. and UPbrS n. descend, parallel to each other, was determined to be an optimal injection point. The distance from the lateral aspect of the limb to the M n. (J), the width of the limb at the level of the injection point of the M n. and UPbrS n. (K), and the proximodistal distance from the ACb to the level of the M n. and UPbrS n. (L) were measured. The ratio of J/K (M) was calculated. The distance from the lateral aspect of the limb to the UPbrS n. (N) was measured and J-N (O) and the ratio of N/K (P) were calculated. The distance from the ACb to the point where the UPbrDp n. turns deeply was measured (Q). For all measurements, the measurement point on the ACb was defined as the center of the attachment of the flexor carpi ulnaris, humeral head. Measurements of nerve location were made using the center of the nerve.
Injection technique
Each limb was shaved on the dorsal aspect from the distal third of the antebrachium to just distal to the carpus; on the lateral aspect at the level of the ACb; and on the palmar aspect between the metacarpal pad and the carpal pad. The blocks were performed as described below and the technique is described and illustrated in Figure 2(a–c).
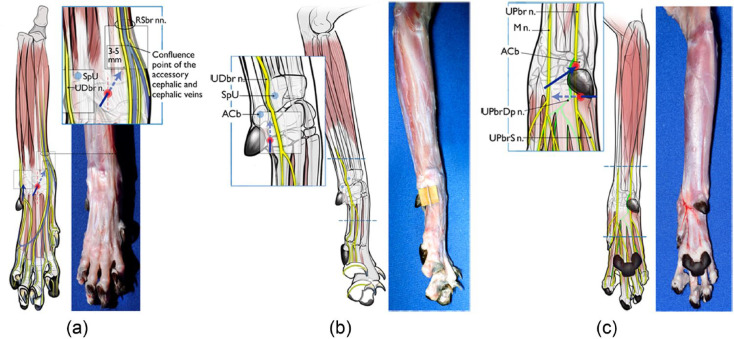
Figure 2.
Diagrams illustrating the developed and tested injection technique for each nerve. (a) Superficial branches of radial nerve (RSbr nn.). A 25 G × 5/8 inch needle is inserted subcutaneously (SC) from a point at the center of the limb at the level of the antebrachiocarpal joint. The needle is advanced approximately 10 mm SC at a 10–20° angle to the long axis of the limb with the bevel facing up. Once the tip of the needle is 3–5 mm from the confluence point of the accessory cephalic and cephalic veins, the injection is made (0.07 ml/kg). (b) Dorsal branch of ulnar nerve (UDbr n.). A point is located lateral to and at the same level as the accessory carpal bone (ACb), positioned between the ACb and the styloid process of the ulna (SpU). A 25 G × 5/8 inch needle is inserted SC distal to proximal so the tip lies at the midpoint of the groove formed between ACb and the SpU, and then the injection is made (0.04 ml/kg). (c) Median nerve (M n.) and superficial branch of palmar branch of ulnar nerve (UPbrS n.): a 25 G × 5/8 inch needle is inserted SC at the distal border of the carpal pad and approximately 5 mm lateral to it, perpendicular to the long axis of the metacarpus, with the bevel facing up. The needle is inserted SC until the point is located two-thirds of the distance from the lateral aspect of the limb to the medial aspect. Two-thirds of the injectate volume is deposited at this point and the remaining volume is injected while the needle is withdrawn (0.08 ml/kg total). Gentle massage is applied to the injected volume under the skin for 5 s. Deep branch of palmar branch of ulnar nerve (UPbrDp n.): a 25 G × 5/8 inch needle is inserted almost perpendicular to the ACb in a mediolateral direction such that the needle contacts the medial palmar aspect of the midpoint of the ACb with the bevel facing up. The needle is then redirected dorsally and advanced on the medial side of the ACb 2–3 mm until it penetrates the flexor retinaculum. At this point the injection is performed (0.01 ml/kg of injectate). Red dots indicate the site of needle insertion. The blue arrows represent the direction of needle insertion to reach the desired injection site
RSbr nn
The limb was positioned with the dorsal aspect facing upwards and the carpus in 180º of extension. A point on the cranial antebrachium was identified that was about 80% of the distance from lateral to medial and about 18 mm proximal to the level of the ACb (approximately the point of confluence of the accessory cephalic and cephalic veins). A 25 G × 5/8 inch needle was inserted subcutaneously (SC) from a point at the center of the limb at the level of the antebrachiocarpal joint. The needle was advanced approximately 10 mm SC at a 10°–20°angle to the long axis of the limb with the bevel facing up. Once the tip of the needle was 3–5 mm from the confluence point of the accessory cephalic and cephalic veins, 0.07 ml/kg NMB was injected.
UDbr n
The limb was positioned with the lateral aspect facing upwards. A point was located lateral to and at the same level as the ACb, positioned between the ACb and the styloid process of the ulna. A 25 G × 5/8 inch needle was inserted SC from distal to proximal so that the tip lay at the midpoint of the groove formed between the ACb and the styloid process of the ulna, and then injection of 0.04 ml/kg NMB was performed.
M n. and UPbrS n
The limb was positioned with the palmar aspect facing upwards. A point was identified 11 mm distal to the ACb, approximately level with the distal extent of the carpal pad. A 25 G × 5/8 inch needle was inserted SC at the distal border of the carpal pad and approximately 5 mm lateral to it, perpendicular to the long axis of the metacarpus, with the bevel facing up. The needle was inserted SC until the tip was located two-thirds of the distance from the lateral aspect of the limb to the medial aspect. Two-thirds of the injectate volume was deposited at this point and the remaining volume was injected while the needle was being withdrawn. A total volume of 0.08 ml/kg was deposited. Gentle massage was applied to the injected volume under the skin for 5 s.
UPbrDp n
The limb was positioned with the palmar aspect facing upwards. A 25 G × 5/8 inch needle was inserted with the bevel facing up, almost perpendicular to the ACb in a mediolateral direction such that the needle contacted the medial palmar aspect of the midpoint of the ACb. The needle was then redirected dorsally and advanced on the medial side of the ACb 2–3 mm until it penetrated the flexor retinaculum. At this point 0.01 ml/kg NMB was injected.
Statistics
Statistical analyses were performed using JMP software (JMP Pro 11; SAS). Wilcoxon’s signed rank tests were used to evaluate pairwise difference in measurements A through Q between left and right limbs and to compare body weights between cadaver dissection and dye injection parts of the study. Correlations between body weight and measurements A through Q were determined using Spearman’s rank correlation coefficient. Differences were considered significant at P <0.05.
Results
Cadaver dissections
There were no significant differences between right limb and left limb for any measurements (A–Q). The mean, SD, median, minimum and maximum of the measured and calculated parameters for the 20 limbs are shown in Table 1. The correlation coefficient of the measured or calculated parameter with body weight is given for each parameter. There were significant positive correlations between body weights and A, B, C, D, H, I, K, L, M and Q. There was no significant relationship between body weight and the measurements E, F, G, J, N, O and P.
Table 1.
Measured and calculated parameters for the location of proposed injection sites for distal limb nerves
| Mean | Maximum | Minimum | Median | SD | CC | |
|---|---|---|---|---|---|---|
| Superficial branches of radial nerve | ||||||
| (A) Distance from the lateral aspect of the limb (mm) | 13.0 | 14.7 | 9.3 | 13.6 | 1.38 | 0.69* |
| (B) Width of the limb at the level of injection point (mm) | 15.7 | 17.8 | 12.8 | 16.0 | 1.47 | 0.65* |
| (C) Distance proximally from the accessory carpal bone (mm) | 17.8 | 20.9 | 14.1 | 18.2 | 1.62 | 0.71* |
| (D) Distance from the accessory carpal bone to lateral epicondyle of the humerus (cm) | 9.6 | 11.1 | 8.4 | 9.7 | 0.91 | 0.91* |
| (E) Width% (the ratio of A/B expressed as %) | 82.5 | 87.5 | 72.7 | 83.3 | 4.23 | 0.29 |
| (F) Length% (the ratio of C/D expressed as %) | 18.5 | 21.1 | 16.2 | 18.7 | 1.32 | −0.34 |
| Dorsal branch of ulnar nerve | ||||||
| (G) Distance from the accessory carpal bone (mm) | 4.0 | 4.6 | 3.0 | 4.0 | 0.42 | −0.12 |
| (H) Width of the carpus at the level of accessory carpal bone (mm) | 17.4 | 20.2 | 14.5 | 17.2 | 1.63 | 0.76* |
| (I) Width% (the ratio of G/H expressed as a %) | 23.2 | 30.0 | 18.1 | 23.1 | 3.15 | −0.55* |
| Median nerve | ||||||
| (J) Distance from lateral aspect of the limb (mm) | 11.5 | 14.2 | 9.8 | 11.2 | 1.14 | 0.04 |
| (K) Width of the limb at the level of injection point (mm) | 17.6 | 19.2 | 15.2 | 17.6 | 1.24 | 0.68* |
| (L) Proximodistal distance from the accessory carpal bone (mm) | 11.5 | 13.5 | 10.0 | 11.5 | 0.90 | 0.55* |
| (M) Width% (the ratio of J/K expressed as a %) | 65.5 | 74.6 | 55.7 | 65.3 | 5.59 | −0.54* |
| Superficial and deep branch of palmar branch of ulnar nerve | ||||||
| (N) Distance from the lateral aspect of the limb (mm) | 6.8 | 9.5 | 5.5 | 6.4 | 1.19 | −0.10 |
| (O) Distance from the median nerve (mm) | 4.8 | 5.1 | 4.3 | 4.8 | 0.27 | 0.39 |
| (P) Width% (the ratio of N/K expressed as %) | 38.4 | 50.8 | 29.7 | 36.3 | 5.89 | −0.43 |
| (Q) Distance from the accessory carpal bone (mm) | 11.5 | 12.5 | 10.3 | 11.6 | 0.59 | 0.56* |
Significant correlation to body weight (P <0.05)
CC = correlation coefficient
Dye test
There was no significant difference in body weight between cats in the dissection study and cats in the dye study. All the cats were of an unknown immature or early mature adult age.
Positive dye staining of all nerves was observed in 12/12 limbs (Table 2). When targeting the RSbr nn., the dye was deposited too laterally in 1/12 limbs, resulting in patchy staining of the nerve. When targeting the UDbr n., the dye was located in the groove between the styloid process of the ulna and the ACb, and stained the nerve well in all cases. For the M n. and UPbrS n., the dye appeared to spread under the superficial digital flexor tendon in 2/12 limbs owing to the injection being made too deeply. This resulted in the injection being deposited dorsal to the M n. in one case, and therefore only the dorsal aspects of that nerve were stained well. The nerves were stained well in the other case. The injection was deposited intramuscularly (into the medial edge of the ulnar head of the deep digital flexor immediately proximal to the continuing deep digital flexor tendon) when targeting the UPbrDp n. in 1/12 limbs owing to the injection being made too medially, and not directly adjacent the ACb. This resulted in patchy staining of the nerve in this case. The dye stained the nerve well in the other case. Overall, it was determined that all nerves targeted were stained well and consistently with this developed four-point injection method (Figure 2).
Table 2.
Degree of new methylene blue distribution on the surface (length and circumference stained) of each nerve
| Mean | Maximum | Minimum | Median | SD | |
|---|---|---|---|---|---|
| Superficial branches of radial nerve | |||||
| Length (mm) | 34.9 | 44.3 | 25.4 | 34.6 | 5.25 |
| Circumference (%) | 93.8 | 100.0 | 50.0 | 100.0 | 15.54 |
| Dorsal branch of ulnar nerve | |||||
| Length (mm) | 29.2 | 38.8 | 24.8 | 28.3 | 4.00 |
| Circumference (%) | 100.0 | 100.0 | 100.0 | 100.0 | 0.00 |
| Median nerve | |||||
| Length (mm) | 26.4 | 32.2 | 14.9 | 27.4 | 4.78 |
| Circumference (%) | 95.8 | 100.0 | 75.0 | 100.0 | 9.73 |
| Superficial branch of palmar branch of ulnar nerve | |||||
| Length (mm) | 39.1 | 45.0 | 30.6 | 39.8 | 4.33 |
| Circumference (%) | 100.0 | 100.0 | 100.0 | 100.0 | 0.00 |
| Deep branch of palmar branch of ulnar nerve | |||||
| Length (mm) | 17.5 | 22.6 | 11.4 | 17.9 | 3.25 |
| Circumference (%) | 93.8 | 100.0 | 50.0 | 100.0 | 15.54 |
Discussion
Preoperatively administered local anesthetics affect postoperative pain relief by blocking the transmission of nociceptive signals, thereby intercepting the nociceptive message. 14 This should help avoid cellular wind up and subsequent central sensitization that appears to contribute to postoperative pain in clinical cases. 15 A local nerve block together with systemic analgesia is recommended for cats undergoing onychectomy. 16 However, neither the described techniques of nerve blockade nor irrigation of the wound with bupivacaine before skin closure have been shown to be associated with beneficial analgesic effects, using subjective or objective outcome measures.6,7 In our preliminary testing, NMB dye injection using the currently described four-point block technique resulted in incomplete nerve staining. 11 The currently accepted injection technique has not been described in depth, 11 and this lack of a detailed description might be partially responsible for the inaccuracy of the injections performed. The most obvious deficit of the original technique is that the injectate is likely to be deposited more medially than the optimal injection point when targeting the M n., and this could result in intramuscular injection and poor blockade of the M n. In this report, we describe in detail both the anatomical location of nerves in relation to palpable landmarks and a proposed nerve block technique. In our dye injection study using our proposed injection technique, all branches of nerves responsible for innervating the feline thoracic limb digits were successfully stained with NMB. This detailed description and associated diagrams, we believe, will allow veterinarians to perform a more successful nerve blockade. However, whether this described technique results in a more successful nerve blockade needs to be clinically tested. We included calculations of ratios when developing the technique, assuming that the ratios we calculated would not be affected by body weight. However, several were, and so the described technique uses descriptions based on anatomical landmarks instead of calculated ratios. A shortcoming of this study was the lack of knowledge of the specific age of the feline cadavers. However, we know the cadavers were young animals that had almost completed their maturity or were young adults and it is most typical for onychectomy to be performed in young cats. 17 We therefore believe our results are generalizable to the target population of cats undergoing declawing procedures. Furthermore, we also believe this nerve block technique is potentially useful for cats undergoing other distal thoracic limb surgeries such as complete digit amputation, biopsy and fracture repair.
Bupivacaine is known to cause cardiac and neurological side effects, and doses of bupivacaine recommended for perineural administration in dogs and cats are 1–2 mg/kg. 18 Bupivacaine should not be administered intravascularly to cats. 18 Although we did not detect any vascular trauma or intravascular injection, precautions should be taken to avoid this, including syringe aspiration to confirm an absence of intravascular needle position before injection is performed. From our observations during dissections, we believe that the highest risk for intravascular injection is into the median artery, which courses in association with the M n. When targeting the M n., keeping the needle as superficial as possible may help to avoid the median artery, because the vessel lies deep to the nerve. Our observations suggest that keeping the injection superficial will also minimize the chance of a poor block of the M n.
Limitations of this study include the use of cadavers and the use of NMB dye for injections. Because of the possible uptake of the local anesthetic solution by the lymph and blood vessels the distribution of injectate in a clinical patient may be different than that in cadavers. 19 We used NMB as a substitute for local anesthetic solution, but NMB distribution might not reflect the action of local anesthetics in vivo, where the action on the nerve fiber depends on several factors, including drug lipid solubility, tissue pH and molecular weight. 20 Additionally, although we estimated the circumference of the nerve that was stained, this is not easily assessed accurately, as previously reported. 13
Conclusions
All branches of nerves responsible for innervation of the feline thoracic limb digits were successfully stained with NMB using the described four-point injection technique. To assess the utility and effectiveness of this distal limb nerve block injection technique it should be evaluated in clinical patients.
Supplemental Material
Degree of new methylene blue distribution on the surface of each nerve using a four-point block
Acknowledgments
We are grateful to Alice Harvey for the medical illustrations and to Wendy Savage for the photography. We also thank Dr Margaret Gruen for comments on the manuscript. The authors of this report do not support feline onychectomy; however, we do believe cats undergoing this procedure should be provided effective pain relief of an appropriate duration.
Footnotes
Supplementary material: Degree of new methylene blue distribution on the surface of each nerve using a four-point block.
The authors do not have any potential conflicts of interest to declare.
Funding: Internally funded by the Comparative Pain Research Laboratory. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Accepted: 29 June 2015
References
- 1. Landsberg GM. Feline scratching and destruction and the effects of declawing. Vet Clin North Am Small Anim Pract 1991; 21: 265–279. [DOI] [PubMed] [Google Scholar]
- 2. Patronek GJ. Assessment of claims of short- and long-term complications associated with onychectomy in cats. J Am Vet Med Assoc 2001; 219: 932–937. [DOI] [PubMed] [Google Scholar]
- 3. Swiderski J. Onychectomy and its alternatives in the feline patient. Clin Tech Small Anim Pract 2002; 17: 158–61. [DOI] [PubMed] [Google Scholar]
- 4. Lockhart LE, Motsinger-Reif AA, Simpson WM, et al. Prevalence of onychectomy in cats presented for veterinary care near Raleigh, NC and educational attitudes toward the procedure. Vet Anaesth Analg 2014; 41: 48–53. [DOI] [PubMed] [Google Scholar]
- 5. Jankowski AJ, Brown DC, Duval J, et al. Comparison of effects of elective tenectomy or onychectomy in cats. J Am Vet Med Assoc 1998; 213: 370–373. [PubMed] [Google Scholar]
- 6. Romans CW, Gordon WJ, Robinson DA, et al. Effect of postoperative analgesic protocol on limb function following onychectomy in cats. J Am Vet Med Assoc 2005; 227: 89–93. [DOI] [PubMed] [Google Scholar]
- 7. Curcio K, Bidwell LA, Bohart GV. Evaluation of signs of postoperative pain and complications after forelimb onychectomy in cats receiving buprenorphine alone or with bupivacaine administered as a four-point regional nerve block. J Am Vet Med Assoc 2006; 228: 65–68. [DOI] [PubMed] [Google Scholar]
- 8. Melzack R, Coderre TJ, Katz J. Central neuroplasticity and pathological pain. Ann N Y Acad Sci 2001; 933: 157–174. [DOI] [PubMed] [Google Scholar]
- 9. Naja ZM, Raf M, El Rajab M, et al. Nerve stimulator-guided paravertebral blockade combined with sevoflurane sedation versus general anesthesia with systemic analgesia for postherniorrhaphy pain relief in children: a prospective randomized trial. Anesthesiology 2005; 103: 600–605. [DOI] [PubMed] [Google Scholar]
- 10. Siddiqui ZI, Cepeda MS, Denman W, et al. Continuous lumbar plexus block provides improved analgesia with fewer side effects compared with systemic opioids after hip arthroplasty: a randomized controlled trial. Reg Anesth Pain Med 2007; 32: 393–398. [DOI] [PubMed] [Google Scholar]
- 11. Ringwood PB, Smith JA. Anesthesia case of the month. J Am Vet Med Assoc 2000; 217: 1633–1635. [DOI] [PubMed] [Google Scholar]
- 12. Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg 2006; 102: 248–257. [DOI] [PubMed] [Google Scholar]
- 13. Trumpatori BJ, Carter JE, Hash J, et al. Evaluation of a midhumeral block of the radial, ulnar, musculocutaneous and median (RUMM block) nerves for analgesia of the distal aspect of the thoracic limb in dogs. Vet Surg 2010; 39: 785–796. [DOI] [PubMed] [Google Scholar]
- 14. Buback JL, Boothe HW, Carroll GL, et al. Comparison of three methods for relief of pain after ear canal ablation in dogs. Vet Surg 1996; 25: 380–385. [DOI] [PubMed] [Google Scholar]
- 15. Lascelles BD, Cripps PJ, Jones A. Post-operative central hypersensitivity and pain: the pre-emptive value of pethidine for ovariohysterectomy. Pain 1997; 73: 461–471. [DOI] [PubMed] [Google Scholar]
- 16. Steagall PV, Monteiro-Steagall BP. Multimodal analgesia for perioperative pain in three cats. J Feline Med Surg 2013; 15: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clark K, Bailey T, Rist P, et al. Comparison of 3 methods of onychectomy. Can Vet J 2014; 55: 255–262. [PMC free article] [PubMed] [Google Scholar]
- 18. Robertson SA, Taylor PM. Pain management in cats – past, present and future. Part 2. Treatment of pain – clinical pharmacology. J Feline Med Surg 2004; 6: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kull K, Baer GA, Samarutel J, et al. Distribution of local anesthetic solution in retromediastinal block. Preliminary experimental results. Reg Anesth 1997; 22: 308–312. [PubMed] [Google Scholar]
- 20. Portela DA, Otero PE, Tarragona L, et al. Combined paravertebral plexus block and parasacral sciatic block in healthy dogs. Vet Anaesth Analg 2010; 37: 531–541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Degree of new methylene blue distribution on the surface of each nerve using a four-point block