Abstract
Case summary
A 4-year-old female spayed, indoor/outdoor domestic mediumhair cat presented with multiple bleeding puncture wounds and hemorrhagic shock. The cat was diagnosed with suspected pit viper envenomation based on the location and appearance of the bite wounds, as well as the presence of severe coagulopathy with prolonged activated coagulation time (762 s), which responded to antivenom administration. The clinical course of the cat was unique owing to the prolonged clinical signs of envenomation that appeared as intermittent coagulopathy and hemorrhage over a 2 week period. Five vials of antivenom were administered and three units of packed red blood cells were transfused over a 7 day period. The cat made a complete recovery with cessation of hemorrhage and normalization of clotting times.
Relevance and novel information
This is the first report of persistent pit viper venom-induced coagulopathy in the feline veterinary literature.
Introduction
Compared with the number of dogs presented for suspected pit viper envenomation, cats are presented infrequently. There is a lack of documentation for prevalence, morbidity and mortality in cats with suspected pit viper envenomation. In one retrospective study of 18 cats evaluated,1 15 survived and three did not, allowing for a 16% mortality rate. In this study, 10 bite sites (56%) involved a forelimb. In another retrospective study describing Vipera palaestinae envenomation in 18 cats,2 the mortality rate was 22%. Bites were localized to the forelimbs in 61% of the cats and hindlimbs in 17% of the cases. In these two studies,1,2 prolongation of at least one of the clotting times was noted in almost all patients in which coagulation testing was monitored.
‘Venom-induced coagulopathy’ is caused by proteolytic enzymes (fibrinolytics), and it may lead to pure defibrination and increased clotting times.2 The coagulation abnormalities observed in people treated for pit viper envenomation have traditionally been considered short-lived; however, prolonged or recurrent coagulopathy may occur after envenomation by North American pit vipers.3 Hypofibrinogenemia, prolonged prothrombin time (PT), activated partial thromboplastin time (aPTT) and thrombocytopenia may persist in humans with pit viper envenomation for as long as 2 weeks following the incident. Very few reports of late or persistent coagulopathy have been published in the human and veterinary literature.3,4 To our knowledge, this is the first case report that describes a persistent venom-induced coagulopathy in a cat with a suspected pit viper envenomation. The term ‘persistent envenomation’ is characterized by the temporary alleviation of clinical signs owing to the neutralizing effects of antivenom, followed by continued absorption of venom from the tissue deposit site after the antivenom leaves the bloodstream and the reappearance of clinical signs.3,5
Case description
A 4-year-old female spayed, domestic mediumhair cat was examined at the University of Florida, College of Veterinary Medicine, small animal emergency service for evaluation of multiple bleeding puncture wounds and severe hemorrhage. This was an indoor/outdoor cat that lived in a wooded residential neighborhood in Gainesville, Florida, where pit vipers were known to be present. The owner first noticed the draining puncture wounds on the right forelimb and left hindlimb 36 h prior to evaluation that were presumed to be caused by bite injuries from another carnivore. The cat was treated initially at a local clinic where it was hospitalized overnight and its wounds were cleansed and the left rear paw bandaged. The cat was given meloxicam (Metacam; Boehringer) at 0.1 mg/kg subcutaneously (SC) once, long-acting buprenorphine (Symbadol; Zoetis) at 0.24 mg/kg SC once and an injection of cefovecin sodium (Convenia; Zoetis) at 8 mg/kg SC once. The next morning the cat was found in its kennel, bleeding profusely from its puncture wounds and severely weak and lethargic. Packed cell volume (PCV; reference interval [RI] 0.34–0.51) and total solids (TS; RI 60–75 g/l) showed severe anemia at 0.15 and 50 g/l, respectively, secondary to blood loss. At this point, the cat was referred to the emergency service for evaluation.
On physical examination, the cat was obtunded and in lateral recumbency. It had weak femoral pulses, pale oral mucous membranes, sinus tachycardia, tachypnea and hypersalivation. There was active hemorrhage from the puncture wounds and hemorrhage in the surrounding soft tissues on the right antebrachium and the fifth digit of the left pelvic limb (Figures 1 and 2). The right forelimb was painful on palpation and had diffuse swelling. Doppler blood pressure measurement (Model 811-B, Doppler Ultrasound; Parks Medical Electronics) was immeasurably low. Initial diagnostics included a venous blood gas analysis (Stat Profile, pHOx Ultra; Nova Biomedical), which showed severe hyperlactatemia at 7.9 mmol/l (RI 0–2.5 mmol/l), hypokalemia at 3.0 mmol/l (RI 3.5–5.5 mmol/l) and hyperglycemia at 20.3 mmol/l (RI 3.9–6.7 mmol/l). An activated clotting time (ACT) (ACT-II; Medtronics) was markedly elevated at 762 s (RI 62–133 s) and the hemogram showed a normal platelet count and severe normocytic normochromic anemia with echinocytes in conjunction with a decreased plasma protein level (Table 1).
Figure 1.
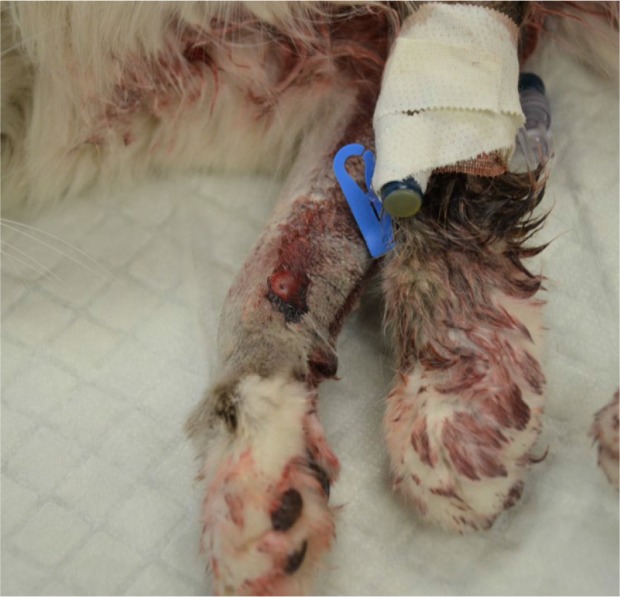
Puncture wound on the right antebrachium of a cat with active hemorrhage due to persistent pit viper envenomation
Figure 2.
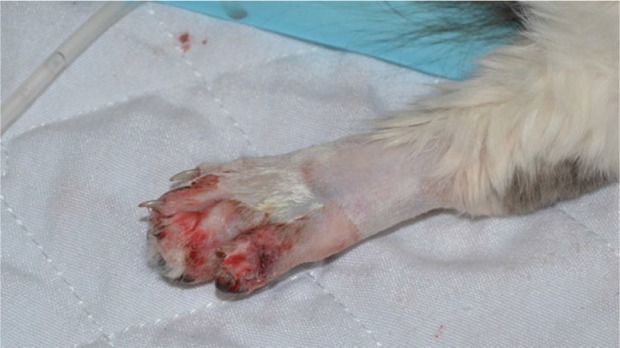
Puncture wound on the fifth digit of the left pelvic limb of a cat with active hemorrhage due to persistent pit viper envenomation
Table 1.
Complete blood cell count results of a cat with persistent pit viper envenomation*
| Test | Day 1 | Day 5 | Day 10 | RI |
|---|---|---|---|---|
| WBCs (109/l) | 14.18 | 9.01 | 15.15 | 5.4–15.4 |
| RBCs (1012/l) | 3.32 | 4.75 | 4.68 | 7.4–10.4 |
| HGB (g/l) | 45 | 72 | 72 | 110–160 |
| Hematocrit | 0.14 | 0.24 | 0.23 | 0.34–0.51 |
| PCV | 0.14 | 0.24 | 0.23 | 0.34–0.51 |
| MCV (fl) | 42.1 | 49.9 | 48.6 | 42–52 |
| MCH (pg) | 13.5 | 15.2 | 15.4 | 13–17 |
| MCHC (g/l) | 320 | 305 | 317 | 300–330 |
| RDW (%) | 17.3 | 27.3 | 21.5 | 13–16 |
| HDW (g/l) | 241 | 272 | 268 | 140–200 |
| Platelets (109/l) | 242 | 65 | 175 | 160–502 |
| MPV (fl) | 16.8 | 22.7 | 20.8 | 10–22 |
| Icterus index (U) | <5 | <5 | <5 | 0–5 |
| Plasma protein (g/l) | 55 | 80 | 68 | 62–80 |
| Fibrinogen (μmol/l) | 2.94 | 2.94 | 2.94 | 2.94–8.82 |
| Bands (109/μl) | 0.13 | 0.17 | 0.28 | |
| Neutrophils (109/μl) | 10 | 5 | 8.8 | 2.3–9.8 |
| Lymphocytes (109/μl) | 2 | 2.4 | 3.9 | 0.9–5.5 |
| Monocytes (109/μl) | 0.4 | 0.17 | 0.43 | 0–0.8 |
| Eosinophils (109/μl) | 0.67 | 0.58 | 0.85 | 0–1.8 |
| Basophils (109/μl) | 0.13 | 0 | 0 | 0–0.2 |
| RBC morphology | 2+ anisocytosis, 1+ polychromia, occasional echinocytes | 2+ anisocytosis, 2+ polychromia, occasional elliptocytes, schistocytes; clumped platelets | 2+ anisocytosis, 2+ polychromia, 2+ echinocytes | |
| Reticulocyte count (punctate, aggregate) (μl) | 477,360; 192,560 | 0–500,000 0–30,000 |
Automated hematology analyzer (Siemens Advia 120)
RI = reference interval; WBCs = white blood cells; RBCs = red blood cells; HGB = hemoglobin; PCV = packed cell volume; MCV = mean cell volume; MCH = mean cell hemoglobin; MCHC = mean cell hemoglobin concentration; RDW = red cell distribution width; HDW = hemoglobin distribution width; MPV = mean platelet volume
Owing to the appearance of the cat, the nature of the wounds in combination with the active bleeding and prolonged ACT, the most likely diagnosis was pit viper envenomation. It was given one vial of antivenom (VenomVet, F(ab)2; Antivenin Crotalidae Polyvalent, Instituto Biológico Argentino SAIC) intravenously over 30 mins and one unit of blood-typed feline packed red blood cells (pRBCs) at 8 ml/kg over 1 h without complication. A repeat ACT was 192 s (RI 62–133 s) after completion of the antivenom infusion, so a second vial of antivenom was administered at a constant rate infusion over 2 h without adverse reactions. The ACT normalized (32 s, RI 62–133 s) after administration of the two vials of antivenom, and hemorrhage from the puncture wounds subsided. The cat’s cardiovascular status improved based on attitude and signs of improved peripheral perfusion (pulse quality and capillary refill time). Repeat blood work showed resolved hyperlactatemia, hypokalemia, hyperglycemia and improved PCV (0.2; RI 0.34–0.51) and TS (60 g/l; RI 60–75 g/l). The cat was hospitalized overnight and no further signs of spontaneous bleeding were noted. It received 0.01 mg/kg of buprenorphine hydrochloride intravenously every 12 h during its stay in the intensive care unit.
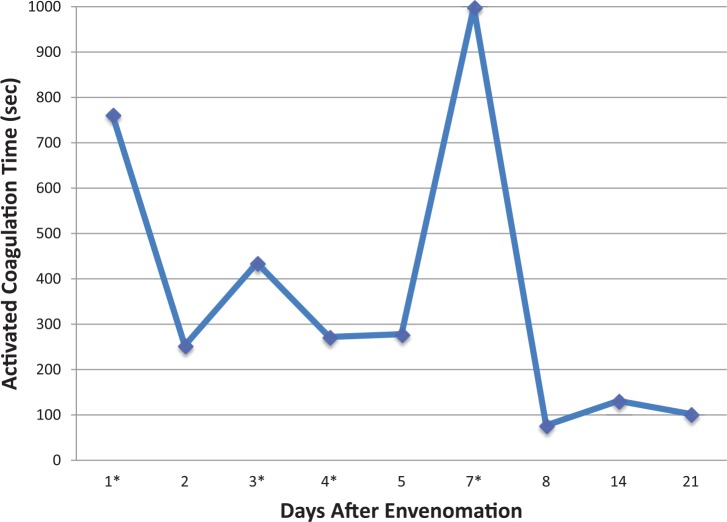
Approximately 12 h later, the ACT was elevated at 253 s (RI 62–133 s), but owing to the absence of clinical bleeding, no additional antivenom was administered, and the cat was discharged to be monitored at home under the care of its owner, who was a veterinarian. On the morning of day 3, the owner noted that the puncture wounds had resumed bleeding. Upon recheck examination, the ACT was elevated at 435 s (RI 62–133 s), and external bleeding was noted from the puncture wounds present on the cat’s left hindlimb. The PCV and TS were 0.26 and 68 g/l, respectively. The cat was alert and otherwise stable, and was given a third vial of antivenom. Six hours later, the cat received a fourth vial of antivenom, as well as a second unit of cross-matched pRBCs owing to persistent bleeding and declining PCV and TS (0.18 and 52 g/l) with persistently prolonged ACT (604 s). By day 4 spontaneous bleeding ceased, but the ACT remained prolonged (Figure 3), which prompted the administration of the fifth vial of antivenom over 3 h (Antivenin Crotalidae; Boehringer Ingelheim). A different type of antivenom was used because of its IgG immunoglobulin composition, allowing for its longer duration of action in the plasma space.5 Despite a persistently prolonged ACT, the cat was clinically normal and was kept overnight for observation. A hemogram (Table 1) on day 5 showed a decreased platelet count, but clumping of platelets on the blood smear questioned the actual presence of thrombocytopenia. As the cat remained asymptomatic during the day, the decision was made to send it home again where it was to be confined to a restricted indoor environment.
Figure 3.
Changes in activated coagulation time over the course of 21 days in a cat with persistent pit viper envenomation. The asterisks (*) represent days when antivenom was administered
The cat was stable at home for the next 3 days but was returned to the hospital on day 9 because of relapsing hemorrhage from the puncture wounds, progressive lethargy and pale mucous membranes. At admission, the PCV and TS were 0.14 and 58 g/l, respectively, the ACT was >999 s and the platelet count was noted as adequate and without clumping on the blood smear. A third unit of cross-matched pRBCs and one empirical dose of phytonadione (6 mg/kg PO) was administered at the discretion of a new shift of emergency veterinarians. The following day (day 10), the PT and aPTT were >70 s and 83.9 s, respectively, and the hemogram (Table 1) showed platelet count was 175,000 ×109/l (RI 160,000–502,000 ×109/l). Owing to the cat’s overall good appearance and normal appetite, it was sent home with phytonadione (6 mg/kg PO q24h) and gabapentin (25 mg/kg PO q12h) for sedation. The owner chose not to give either drug after the discharge, and the cat continued to improve, showing no further signs of bleeding. Repeat ACT, and PCV and TS showed improved values on days 14 and 21 with continued normal status thereafter.
Discussion
The clinical course of the cat described in this report was unique because of the prolonged clinical signs of suspected envenomation that appeared as intermittent coagulopathy over a 2 week time period following the suspected snakebite incident. The accumulated dose of administered antivenom (five vials) was relatively large for a cat, based on our clinical experience. Although the actual snakebite incident in this cat was not observed, the clinical signs (toxidrome) were typical of other similar regional cases of known pit viper envenomation in the cat, including bleeding puncture wounds on extremities, obtundation, hypotension, coagulopathy, hemorrhagic lymphedema, hypersalivation, presence of echinocytes and hypokalemia. The hyperglycemia on day 1 was transient and attributed to a stress response. There is no proven explanation for the hypokalemia, but this abnormality is not uncommon to pit viper victims presented to our emergency facility. There is also no definite explanation for the absence of echinocytes on day 2, despite their presence on days 1 and 8. Perhaps, they were cleared from the blood on day 2 and reappeared after the continued absorption of venom from the peripheral deposit site. In previously published retrospective studies,1,2 the most common physical and clinical pathological findings included lameness, hemorrhagic edema at the envenomation site, mental depression, tachypnea, abnormal body temperature, prolonged clotting times and thrombocytopenia. Although different species of pit vipers have their characteristic pathophysiologic effects on the victim,5 those with venom containing metalloproteinases share similar clinical effects. The snakes indigenous to this cat’s location include the eastern diamondback rattlesnake, timber (canebrake) rattlesnake, water moccasin and pygmy rattlesnake. The coagulopathy eliminated the likelihood of the pygmy rattlesnake. The single dose of meloxicam that was given to this cat prior to admission could have contributed to the initial hemorrhage owing to the potential ability of non-steroidal anti-inflammatory drugs (NSAIDs) to inhibit platelet function.6 However, administration of NSAIDs does not cause prolongation of the clotting times.7,8
Crotaline snake envenomation can cause a spectrum of adverse hematologic effects, which consist primarily of fibrinogen degradation, thrombocytopenia and morphologic changes involving the red blood cells as were seen in this cat.9 In this case report, the cat required three units of feline pRBCs over a 7 day period. Previously, there was only one description of a cat with pit viper venom-induced coagulopathy requiring blood transfusion.1 The anemia in this cat was mainly attributed to blood loss. Increased red blood cell destruction or hemolytic transfusion reactions might have contributed to the anemia, although the characteristic gross pigmenturia and hemoglobinemia were not evident.
The coagulopathy in this cat was initially diagnosed and monitored with the ACT test. While both the ACT and the aPTT tests screen for defects in the intrinsic and common pathways, there are some important differences. The ACT test will only detect a factor abnormality when there is a ⩾95% or more decrease in single factor activity (<5% normal factor activity). The aPTT test can detect a factor abnormality with a ⩾70% decrease in single-factor activity (<30% normal factor activity).10 Although PT and aPTT tests are considered the gold standards for assessing coagulation by most clinicians, the ACT is frequently used by many emergency clinicians as a rapid screening coagulation test owing to its low cost, ready availability and rapid results.
Although there have been no reports published in the veterinary literature documenting persistent or recurrent coagulopathy in cats following pit viper envenomation, there is a paper describing persistent envenomation in the dog.4 In the human literature, the phenomena of symptom ‘recurrence’ have been mainly observed with Fab antivenom from a variety of source animals.3,11 Recurrence of venom effects in Fab-treated patients appears to be the result of a pharmacokinetic and pharmacodynamic mismatch between the short-lived persistence of antivenom in the circulation and target venom components.11
The cat in this report received four vials of F(ab)2 equine antivenom (VenomVet; Antivenin Crotalidae Polyvalent, Instituto Biológico Argentino) and one vial of Crotalidae Polyvalent whole IgG equine antivenin (ACP; Boehringer Ingelheim). One vial of VenomVet is standardized to neutralize at least the following venom quantities: 20 mg Crotalus simus venom, 30 mg Bothrops asper venom and 30 mg Lachesis muta venom (JC López, 2017, personal communication). The manufacturer’s product insert states that the antivenom effectively treats envenomations caused by North American pit vipers despite the product being derived from South American species.12 This benefit is caused by shared antigens across the species. The neutralizing capacity of an antivenom known to be effective against a particular species of snake depends on the species of the snake and amount of venom injected,13 which is impossible to determine in this case and most other locations in the western hemisphere where multiple species are found in any particular habitat.
F(ab)2 antivenom has a molecular mass of approximately 100 kDa and is not eliminated by the renal route, and therefore has a prolonged half-life in the patient ranging between 2 and 4 days vs a Fab product that has a short half-life elimination, lasting between 4 and 24 h.12 The half-life of ACP is 61–194 h.3 After venom is deposited in the tissues, the diffusion of the venom into the plasma space begins with a phase of very steep rise. After about 10 mins the venom absorption level becomes less steep and soon reaches a plateau.13 The process of resorption extends over 72 h, and this contributes to the maintaining of high plasma venom levels.13 The phenomenon of a slow venom absorption from the deposit site has been confirmed by many experimental and clinical studies.3 This hypothesis could explain the reappearance of the venom in the circulation 7–10 days after the bite, observed by many clinicians after the treatment had been stopped, similar to what happened in this cat.
In one human study of 38 patients, 20 (53%) had recurrent, persistent or late coagulopathy 2–14 days after envenomation. No patient in that study experienced significant spontaneous bleeding. Eventual resolution of the coagulopathy occurs through the combined effects of the complete resorption of the deposited venom over the subsequent 2 weeks plus further neutralization of circulating venom by additional doses of antivenom.3 A second mechanism for recurrent coagulopathy may be reversible binding of antivenom to venom proteins, with dissociations resulting in the reappearance of free circulating venom components.3
The clinical signs of coagulopathy in this cat spontaneously resolved by day 9, whereas clotting times normalized by day 21. During the first week after envenomation, the bleeding from the puncture wound sites had been responding to antivenom administration, but the clotting times were intermittently prolonged. This is consistent with previously published human studies describing a roughly 2 week subacute phase of the disease during which the ongoing presence of venom may result in serious delayed or recurrent coagulation defects.14 The vitamin K was administered only once by another on-duty clinician while the cat was hospitalized in the intensive care unit, but was subsequently discontinued after further consideration for the unlikelihood of anticoagulant rodenticide toxicosis.
Conclusions
The cat in this report showed signs of persistent venom-induced coagulopathy and the need for extensive therapeutic interventions, which included relatively high doses of antivenom and transfusion of three units of pRBCs administered over a 7 day period. We recommend close monitoring of coagulation and hematologic profiles in cats and other species suffering from similar pit viper envenomations during the first 14 days owing to the possibility of persistent envenomation.
Footnotes
Accepted: 4 September 2017
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Hoose JA, Carr A. Retrospective analysis of clinical findings and outcome of cats with suspected rattlesnake envenomation in Southern California: 18 cases (2007–2010). J Vet Emerg Crit Care 2013; 23: 314–320. [DOI] [PubMed] [Google Scholar]
- 2. Lenchner I, Aroch I, Segev G, et al. A retrospective evaluation of Vipera palaestinae envenomation in 18 cats: (2006–2011). J Vet Emerg Crit Care 2014; 24: 437–443. [DOI] [PubMed] [Google Scholar]
- 3. Boyer LV, Seifert SA, Clark RF, et al. Recurrent and persistent coagulopathy following pit viper envenomation. Arch Intern Med 1999; 159: 706–710. [DOI] [PubMed] [Google Scholar]
- 4. Schaer M, Buckley G, Conner BJ, et al. Severe pit viper envenomation with extended clinical signs and treatment complications in a dog. J Am Anim Hosp Assoc 2015; 51: 329–337. [DOI] [PubMed] [Google Scholar]
- 5. Fry BG. Venomous reptiles and their toxins. New York: Oxford University Press, 2015. [Google Scholar]
- 6. Schafer AI. Effects of nonsteroidal anti-inflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol 1995; 35: 209–219. [DOI] [PubMed] [Google Scholar]
- 7. Cathcart CJ, Brainard BM, Reynolds LR, et al. Lack of inhibitory effect of acetylsalicylic acid and meloxicam on whole blood platelet aggregation in cats. J Vet Emerg Crit Care 2012; 22: 99–106. [DOI] [PubMed] [Google Scholar]
- 8. Gunew MN, Menrath VH, Marshall RD. Long-term safety, efficacy and palatability of oral meloxicam at 0.01–0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg 2008; 10: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med 2002; 347: 347–356. [DOI] [PubMed] [Google Scholar]
- 10. Horton S, Augustin S. Activated clotting time (ACT). Methods Mol Biol 2013; 992: 155–167. [DOI] [PubMed] [Google Scholar]
- 11. Seifert SA, Boyer LV. Recurrence phenomena after immunoglobulin therapy for snake envenomations: part 1. Pharmacokinetics and pharmacodynamics of immunoglobulin antivenoms and related antibodies. Ann Emerg Med 2001; 37: 189–195. [DOI] [PubMed] [Google Scholar]
- 12. VenomVet, Instituto Biológico Argentino, SAIC Product information. http://venomvet.com/venom-vet-fact-sheet/ (2016, accessed July 12, 2017).
- 13. Chippaux JP. Snake venoms and envenomations. Malabar, FL: Krieger, 2006, pp 112–115. [Google Scholar]
- 14. Boyer LV, Chase PB, Degan JA, et al. Subacute coagulopathy in a randomized, comparative trial of Fab and F(ab’)2 antivenoms. Toxicon 2013; 74: 101–108. [DOI] [PubMed] [Google Scholar]