Abstract
Design and objectives
To identify and compare how intensive care unit specialists in the United Kingdom and Australia and New Zealand self-reportedly define, assess and manage fluid overload in critically ill patients using a structured online questionnaire.
Results
We assessed 219 responses. Australia and New Zealand and United Kingdom intensive care unit specialists reported using clinical examination findings, bedside tools and radiological features to assess fluid status, diagnose fluid overload and initiate fluid removal in the critically ill. An elevated central venous pressure is not regarded as helpful in diagnosing fluid overload and targeting a clinician-set fluid balance is the most popular management strategy. Renal replacement therapy is used ahead of more diuretic therapy in patients who are oligo/anuric, or when diuretic therapy has not generated an adequate response.
Conclusions
This self-reported account of practice by United Kingdom and Australia and New Zealand intensivists demonstrates that fluid overload remains poorly defined with variability in both management and practice.
Keywords: Fluid overload, fluid removal, renal replacement therapy, assessment, critical care
Introduction
Pathological fluid overload1 is common in the critically ill. However, the definition and optimal management of fluid overload remain poorly defined.2 An increasing body of evidence links fluid overload during intensive care unit (ICU) stay with increased duration of mechanical ventilation, longer length of hospital stay and increased mortality.1,3–8 In contrast, a negative fluid balance has been associated with improved organ function and survival.9,10
Fluid overload in the critically ill often occurs secondary to increased fluid administration, decreased urinary output or both.2,11 Its potential harm means that attention is increasingly being paid to the available techniques for fluid removal.12 Pharmacological means (diuretics) and mechanical modes of fluid removal (ultrafiltration via renal replacement therapy (RRT) techniques) are both used to improve and restore optimal volume status.13–15 To date, however, there is no evidence supporting the use of one technique over the other. Thus, the choice of therapy for fluid removal remains at the discretion of the treating intensivist, and appears influenced by renal function, urine output and the severity of fluid overload.1,2
Given existing uncertainties, the perception that international clinical practice might vary, and the desire to develop interventional trials, we aimed to ascertain how Intensive Care Specialists in Australia and New Zealand (ANZ) and the United Kingdom (UK) define, assess and manage fluid overload in critically ill patients. This data will describe current self-reported practice and inform the design of future studies targeted at optimizing fluid management in the critically ill.
Methods
Survey design
We designed a succinct, multiple-choice online questionnaire. The questionnaire was piloted at two centres in the UK and Australia and refined prior to distribution. We obtained approval from the Austin Health Human Research Ethics Committee for the study (approval no. LNR/15/Austin 402).
Questionnaire
The survey consisted of 12 questions (see online supplementary material). Information on location, scope of practice and postgraduate qualifications in intensive care medicine (ICM) was obtained. Methods used to assess fluid status, features which were felt to support a diagnosis of fluid overload, indications for commencing fluid removal, specific approaches used in the management of fluid overload and preference for pharmacological versus mechanical means for fluid removal were then sought. The survey was constructed using an online survey design and reporting tool (https://www.surveymonkey.com).
Survey distribution
All 71-member units of the Australian and New Zealand Intensive Care Society Clinical Trials Group were approached and asked to invite their ICU Specialists to participate. An invitation was sent by e-mail containing a link to the survey. In the UK, the survey was publicised via the Intensive Care Society (ICS) website with the invitation to participate containing a link to the survey. A link to the survey was also distributed by e-mail to representatives of the UK specialty-lead groups in ICM for regional distribution. Participation was voluntary and anonymous. Responses were obtained over a four-month period from October 2015 to January 2016. A response to the invitation was taken as implied consent for participation.
Statistical methods
Statistical analysis was performed using GraphPad QuickCalcs (https://www.graphpad.com/quickcalcs/). Categorical variables were compared using Fischer’s exact test with responses to questions from ANZ and UK specialists being grouped into strongly agree, agree versus neutral, disagree and strongly disagree for comparison and statistical significance defined by a P-value of <0.05.
Results
Respondent demographics
In total, 219 responses were received (Table S1). Sixty per cent of respondents were from the UK, 40% from ANZ. Nearly two-thirds of respondents (63%) held a post-graduate qualification in ICM and an allied specialty, more UK respondents held a postgraduate qualification solely in only an allied specialty (P = 0.0001) and more ANZ respondents held a postgraduate qualification in ICM alone. Overall, more than half (58%) of respondents worked in a tertiary hospital/specialist centre, but this was more common amongst the respondents from ANZ (P ≤ 0.001). Almost all respondents (99%) treated medical and general surgical patients in their ICUs.
Assessment of fluid status
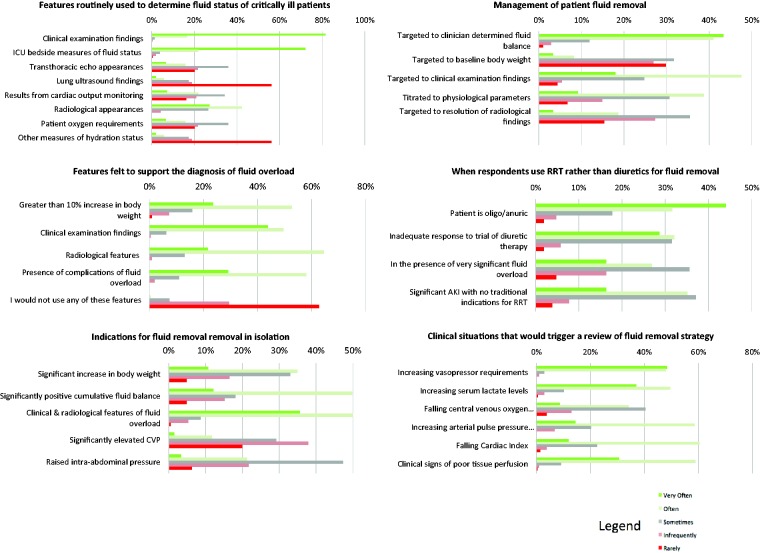
ICU specialists from ANZ and the UK are most likely (very often or often) to use clinical examination (98% respondents) and bedside measures of fluid status (e.g. weight and cumulative fluid balance) (94% respondents) to assess the fluid status (Table 1). Radiological appearances (69%) and patient oxygen requirements (72%) are also commonly used ‘very often or often’. Measures of fluid responsiveness from invasive cardiac output monitoring devices are predominantly used ‘sometimes, infrequently or rarely’, although the latter are more likely to be used by intensivists in the UK than in ANZ (P ≤ 0.001).
Table 1.
Features routinely used by specialists to determine fluid status of critically ill patients.
| UK (n = 124) N (% of UK respondents answering) | ANZ (n = 87) N (% of ANZ respondents answering) | Overall (n = 211) N (% of all respondents answering) | P comparison ANZ vs. UKa | |
|---|---|---|---|---|
| Clinical examination findings: | ||||
| Very often/often | 123 (99.2) | 84 (x16.9) | 207 (98.1) | 0.31 |
| Sometimes/infrequently/rarely | 1 (0.8) | 3 (3.4) | 4 (1.9) | |
| ICU bedside measures of fluid status: | ||||
| Very often/often | 117 (94.4) | 81 (93.1) | 198 (93.8) | 0.78 |
| Sometimes/infrequently/rarely | 7 (5.6) | 6 (4) | 13 (6.2) | |
| Transthoracic echo appearances: | ||||
| Very often/often | 27 (21.7) | 20 (23) | 47 (22.3) | 1.00 |
| Sometimes/infrequently/rarely | 95 (76.6) | 67 (77) | 162 (76.8) | |
| Lung ultrasound findings: | ||||
| Very often/often | 13 (10.5) | 3 (3.4) | 16 (7.6) | 0.07 |
| Sometimes/infrequently/rarely | 109 (87.9) | 83 (95.4) | 192 (91) | |
| Results from cardiac output monitoring devices: | ||||
| Very often/often | 49 (39.5) | 12 (13.8) | 61 (28.9) | 0.0001b |
| Sometimes/infrequently/rarely | 75 (60.5) | 75 (86.2) | 150 (71.1) | |
| Radiological appearances: | ||||
| Very often/often | 81 (65.3) | 65 (74.7) | 146 (69.2) | 0.17 |
| Sometimes/infrequently/rarely | 43 (34.7) | 22 (25.3) | 65 (30.8) | |
| Patient oxygen requirements: | ||||
| Very often/often | 92 (74.2) | 60 (69) | 152 (72) | 0.53 |
| Sometimes/infrequently/rarely | 32 (25.8) | 26 (29.9) | 58 (27.5) | |
| Other measures of hydrations status | ||||
| Very often/often | 4 (3.2) | 0 (0) | 4 (1.9) | 0.14 |
| Sometimes/infrequently/rarely | 118 (95.2) | 86 (98.9) | 204 (96.7) | |
UK: United Kingdom; ANZ: Australia and New Zealand; ICU: intensive care unit.
Fischer’s exact test (very often and often vs. sometimes, infrequently and rarely).
Statistically significant difference.
Diagnosing fluid overload
UK and ANZ intensivists selected a number of features to support a diagnosis of fluid overload (Table 2). These included an increase in estimated or measured body weight >10% above baseline (76%); clinical examination findings (93%); recognized radiological features (85%) and the presence of potential complications (such as increasing oxygen requirements, difficulty weaning from invasive ventilation and intra-abdominal hypertension) (87%).
Table 2.
Features which specialists felt support the diagnosis of fluid overload in the critically ill patient.
| UK (n = 123) N (% of respondents answering) | ANZ (n = 87) N (% of respondents answering) | Overall (n = 210) N (% of respondents answering) | P comparison ANZ vs. UKa | |
|---|---|---|---|---|
| Documented fluid accumulation with >10% in BW above baseline | ||||
| Strongly agree/agree | 93 (75.6%) | 66 (75.9%) | 159 (75.7%) | 0.87 |
| Neutral/disagree/strongly disagree | 30 (24.4%) | 20 (23%) | 50 (23.8%) | |
| Presence of clinical examination findings consistent with fluid overload | ||||
| Strongly agree/agree | 114 (92.) | 82 (94.3) | 196 (93.3) | 0.78 |
| Neutral/disagree/strongly disagree | 9 (7.3) | 5 (5.7) | 14 (6.7) | |
| Radiological features of fluid overload | ||||
| Strongly agree/agree | 106 (86.2) | 73 (83.9) | 179 (85.2) | 0.69 |
| Neutral/disagree/strongly disagree | 16 (13) | 13 (14.9) | 29 (13.8) | |
| Presence of potential complications of fluid overload | ||||
| Strongly agree/agree | 112 (91.1) | 70 (80.5) | 182 (86.7) | 0.06 |
| Neutral/disagree/strongly disagree | 11 (8.9) | 16 (18.4) | 27 (12.9) | |
| I would not use any of these features | ||||
| Strongly agree/agree | 50 (40.7) | 44 (50.6) | 94 (44.8) | 0.12 |
| Neutral/disagree/strongly disagree | 72 (58.5) | 40 (46) | 112 (53.3) | |
UK: United Kingdom; ANZ: Australia and New Zealand; BW: body weight.
Fischer’s exact test (strongly agree and agree vs. neutral disagree and strongly disagree).
Indications for fluid removal
Respondents ‘agreed’ or ‘strongly agreed’ that in isolation, a significant increase in body weight above baseline (45%), a significantly positive fluid balance (61%), and clinical and radiological features suggestive of fluid overload (86%) were indications to remove fluid in a critically ill patient (Table S2). A significantly elevated central venous pressure (CVP) was not considered helpful as a guide for initiating fluid removal (86% neutral/disagree/strongly disagreed with this indication).
Managing fluid removal in the critically ill
The most popular approaches (used often or very often) for managing fluid removal included targeting a clinician-set fluid balance (84%), which was more popular amongst UK respondents (P ≤ 0.001), fluid removal targeted to clinical examination findings (64%) and fluid removal titrated to physiological parameters such as cardiac output measurements, blood pressure and gas exchange (48%) (Table 3).
Table 3.
Approaches to management of patient with fluid overload in normal clinical practice.
| UK (n = 123) N (% of respondents answering) | ANZ (n = 87) N (% of respondents answering) | Overall (n = 210) N (% of respondents answering) | Pcomparison ANZ vs. UKa | |
|---|---|---|---|---|
| Fluid removal targeted to clinician determined daily net fluid balance | ||||
| Very often/often | 113 (91.9) | 64 (73.6) | 177 (84.3) | 0.0005b |
| Sometimes/infrequently/rarely | 10 (8.1) | 23 (26.4) | 33 (15.7) | |
| Fluid removal targeted to baseline body weight | ||||
| Very often/often | 15 (12.2) | 9 (10.3) | 24 (11.4) | 0.83 |
| Sometimes/infrequently/rarely | 106 (86.2) | 78 (90) | 184 (87.6) | |
| Fluid removal targeted to clinical examination findings | ||||
| Very often/often | 78 (63.4) | 57 (65.5) | 135 (64.3) | 0.88 |
| Sometimes/infrequently/rarely | 42 (34.1) | 29 (33.3) | 71 (33.8) | |
| Fluid removal titrated to physiological parameters (cardiac output measurements, blood pressure, gas exchange) | ||||
| Very often/often | 59 (48) | 41 (33.3) | 100 (47.6) | 0.89 |
| Sometimes/infrequently/rarely | 63 (51.2) | 46 (52.9) | 109 (51.9) | |
| Fluid removal targeted to resolution of radiological findings of fluid overload | ||||
| Very often/often | 25 (20.3) | 21 (24.1) | 46 (21.9) | 0.61 |
| Sometimes/infrequently/rarely | 97 (78.9) | 66 (75.9) | 163 (77.6) | |
UK: United Kingdom; ANZ: Australia and New Zealand.
Fischer’s exact test (very often and often vs. sometimes infrequently and rarely).
Statistically significant difference.
Modality for fluid removal
RRT was most likely to be used ahead of diuretic therapy, if a fluid-overloaded critically ill patient was oligo/anuric (76% respondents) (Table 4). It was also likely to be used if there was an inadequate response to a trial of diuretic therapy (60%) and if there was a significant acute kidney injury even when no traditional indications for RRT existed (51%). When significant fluid overload was present, almost half of respondents (43%) said they would use RRT rather than diuretics in the first instance. Continuous RRT was the most popular modality of RRT being employed by 88% of respondents overall; intermittent haemodialysis was used more frequently by UK intensivists (P = 0.05).
Table 4.
RRT modality and situations in which respondents would choose to use RRT rather than diuretics to remove fluid from a patient in the ICU.
| UK (n = 123) N (% of respondents answering) | ANZ (n = 86) N (% of respondents answering) | Overall (n = 209) N (% of respondents answering) | P Value comparison ANZ vs. UKa | |
|---|---|---|---|---|
| RRT modality | ||||
| Continuous renal replacement therapy | 111 (84.7) | 81 (92) | 192 (87.7) | 0.44 |
| Intermittent haemodialysis | 9 (6.9) | 1 (1.1) | 10 (4.6) | 0.05b |
| Hybrid therapy | 3 (2.3) | 6 (6.8) | 9 (4.1) | 0.17 |
| Team responsible for prescription and management of RRT | ||||
| ICM medical and nursing staff | 118 (90.1) | 81 (92) | 199 (90.9) | 0.74 |
| External team | 2 (1.5) | 0 (0) | 2 (0.9) | 0.51 |
| Both ICM and external team | 4 (3.1) | 5 (5.7) | 9 (4.1) | 0.49 |
| Patient is oligo/anuric | ||||
| Very often/often | 95 (77.2) | 63 (73.3) | 158 (75.6) | 0.52 |
| Sometimes/infrequently/rarely | 28 (22.8) | 23 (26.7) | 51 (24.4) | |
| Inadequate response to trial of diuretic therapy | ||||
| Very often/often | 71 (57.7) | 54 (62.8) | 125 (59.8) | 0.56 |
| Sometimes/infrequently/rarely | 50 (40.7) | 31 (36) | 81 (38.8) | |
| In the presence of very significant fluid overload | ||||
| Very often/often | 57 (46.3) | 33 (38.4) | 90 (43.1) | 0.26 |
| Sometimes/infrequently/rarely | 65 (52.8) | 53 (61.6) | 118 (56.4) | |
| Significant acute kidney injury in the absence of traditional indications for commencing RRT | ||||
| Very often/often | 65 (52.8) | 42 (48.8) | 107 (51.2) | 0.57 |
| Sometimes/infrequently/rarely | 57 (46.3) | 44 (51.2) | 101 (48.3) | |
UK: United Kingdom; ANZ: Australia and New Zealand; RRT: renal replacement therapy.
Fischer’s exact test (very often, often and sometimes vs. infrequently and rarely).
Statistically significant difference.
Review of fluid removal goals
In critically ill patients, UK and ANZ intensivists ‘agreed’ or ‘strongly agreed’ that circumstances which would trigger a review of their planned fluid removal strategy were an increase in vasopressor requirements (96%), clinical signs of poor tissue perfusion (90%), increasing serum lactate levels (86%) and a falling cardiac index (72%) (Figure 1).
Figure 1.
Frequency with which respondents use clinical features to assess fluid status, diagnose fluid overload and the frequency with which methods are chosen to initiate fluid removal in the critically ill.
RRT: renal replacement therapy.
Discussion
Key findings
We surveyed intensive care specialists in the UK and ANZ to understand their approach to the assessment and management of fluid overload in critically ill patients. We identified that they most frequently use a combination of clinical examination, bedside tools and radiological features to assess fluid status and diagnose fluid overload.
The most popular approach adopted in the management of fluid overload was to target a clinician-set fluid balance. RRT was likely to be used ahead of diuretic therapy if a fluid-overloaded critically ill patient was oligo/anuric or to deal with an inadequate response to a trial of diuretic therapy. A significantly elevated CVP was not regarded as being an indication for fluid removal in the critically ill. An increase in vasopressor requirements was the most common reason clinicians reviewed their fluid removal strategy.
UK specialists were more likely to use measures of fluid responsiveness and target fluid removal to a clinician-determined daily net fluid balance in their diagnosis and management of fluid overload, but overall there were few statistically significant differences in the answers given in this survey between intensivists from the UK and ANZ.
Relationship to previous findings
The association between fluid balance and adverse outcomes in critical illness is now well established and investigators are beginning to prospectively evaluate benefit from strategies to minimize or treat fluid overload in critical illness.16,17 However, there is no clear consistency regarding definition of fluid overload, monitoring of volume status or preferred method of fluid removal.
Numerous methods now exist to assess intravascular volume status.18,19 However, we found that clinicians reported using a combination of the available methods rather than relying on one alone.2
Studies examining diuretic therapy in the ICU have specifically focused on the role of diuretics in critically ill patients with AKI.20–22 In AKI, diuretics have been associated with improved outcome if their use achieves a negative fluid balance in patients with AKI.23 This underlines the fact that diuretics are a tool to manipulate salt and water balance, not renal function. A multinational survey of diuretic use by intensive care physicians and nephrologists which generated 331 responses from 16 countries, found that the use of furosemide in AKI was common (67.1%), delivered primarily intravenously and by bolus dosing.24 However, no studies have considered the use of RRT for prevention or resolution of fluid overload in the critically ill either as an adjunct, or as an alternative, to diuretic therapy, while studies of mechanical ultrafiltration in the setting of decompensated chronic cardiac failure have given inconsistent results.25,26
Study implications
This study has shown that UK and ANZ intensivists assess fluid status and diagnose fluid overload based on clinical examination, bedside tools and radiological findings, while ultrasound techniques and information provided by invasive intravascular devices are not routinely preferred. Thus, it appears that in most ICUs in the UK and ANZ fluid status is being assessed in various inconsistent ways using a variety of non-evidence based, poorly specific and poorly sensitive techniques. However, such a stance is difficult to criticise given the absence of good quality evidence to support more complex monitoring and the evidence against the use of historically important measures such as CVP and pulmonary artery catheter measurements.
We have found that in practice, in those patients with significant AKI, oliguria or inadequate response to diuretics extra corporeal fluid removal was an acceptable therpaeutic option. These findings suggest that many clinicians would have equipoise for participation in studies examining early use of RRT or ultrafiltration in the critically ill prompted by a fluid balance threshold.
Strengths and Limitations
This study has several strengths. To our knowledge, it is the first study to document the self-reported practice of specialists practicing in ICM with regard to the assessment, diagnosis and management of fluid overload in the critically ill. Secondly, the survey has allowed us to define the situations in which ICU practitioners are more likely to use RRT than diuretics for fluid removal. Thirdly, we subjected our survey to robust assessment before its distribution, using both expert review and a pilot version.
The study also has limitations. First, as a survey of self-reported practice, our results do not necessarily represent actual clinical practice. However, ICM is a well-established specialty in both the UK and ANZ, and hence the extremes of practice, particularly given the similarities in medical education and postgraduate training, are unlikely. Secondly, there is an imbalance in sub-specialty representation between the UK and ANZ. A greater proportion of ANZ respondents worked in tertiary referral centres. Thirdly, we only surveyed a proportion of the critical care practitioners who were motivated to respond to the survey. However, the size of our response from 219 ANZ and UK ICU specialists suggests our findings may be representative of the general view of ICU practitioners in these countries. Finally, our findings may not represent practice in other countries justifying the need for a larger, multinational study to determine this.
Conclusions
This survey provides the first self-reported account of practice with regards to fluid status assessment and the diagnosis and management of fluid overload in critically ill patients by ICU specialists in the UK and ANZ. It found that fluid overload remains poorly defined and there is some variability in both management and practice, including the use of RRT. Our results justify the need for observational studies of actual practice in the management of fluid overload.
Acknowledgements
We would like to thank our consultant colleagues from the Adult Critical Care Unit at the Royal London Hospital and the Intensive Care Unit at the Austin Hospital whom piloted the survey. We would also like to thank the Intensive Care Society and the Australian and New Zealand Intensive Care Society for their help with the distribution of the survey.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Michael E O’Connor was a National Institute of Health Research Fellow. Rinaldo Bellomo has received travel support and consultancy fees from Gambro, Braun and Baxter Healthcare. John R Prowle has received honoraria or research support from Nikkiso GmBH and Baxter Healthcare.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Neil J Glassford is supported by a scholarship grant from The Avant Doctors-In-Training.
References
- 1.O'Connor ME, Prowle JR. Fluid overload. Crit Care Clin 2015; 31: 803–821. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R. Issue and challenges of fluid removal in the critically ill. Br J Anaesth 2014; 113: 734–735. [DOI] [PubMed] [Google Scholar]
- 3.Payen D, de Pont AC, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 2008; 12: R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 2009; 76: 422–427. [DOI] [PubMed] [Google Scholar]
- 5.Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003; 238: 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toraman F, Evrenkaya S, Yuce M, et al. Highly positive intraoperative fluid balance during cardiac surgery is associated with adverse outcome. Perfusion 2004; 19: 85–91. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg AL, Dechert RE, Park PK, et al. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intens Care Med 2009; 24: 35–46. [DOI] [PubMed] [Google Scholar]
- 8.Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39: 259–265. [DOI] [PubMed] [Google Scholar]
- 9.Alsous F, Khamiees M, DeGirolamo A, et al. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest 2000; 117: 1749–1754. [DOI] [PubMed] [Google Scholar]
- 10.National Heart Lung Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354: 2564–2575. [DOI] [PubMed]
- 11.Rosner MH, Ostermann M, Murugan R, et al. Indications and management of mechanical fluid removal in critical illness. Br J Anaesth 2014; 113: 764–771. [DOI] [PubMed] [Google Scholar]
- 12.Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 2014; 10: 37–47. [DOI] [PubMed] [Google Scholar]
- 13.Ostermann M, Alvarez G, Sharpe MD, et al. Frusemide administration in critically ill patients by continuous compared to bolus therapy. Nephron Clin Pract 2007; 107: c70–c76. [DOI] [PubMed] [Google Scholar]
- 14.Singh NC, Kissoon N, al Mofada S, et al. Comparison of continuous versus intermittent furosemide administration in postoperative pediatric cardiac patients. Crit Care Med 1992; 20: 17–21. [DOI] [PubMed] [Google Scholar]
- 15.Bagshaw SM, Bellomo R, Kellum JA. Oliguria, volume overload, and loop diuretics. Crit Care Med 2008; 36(4 Suppl): S172–S178. [DOI] [PubMed] [Google Scholar]
- 16.Hjortrup PB, Haase N, Bundgaard H, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intens Care Med 2016; 42: 1695–1705. [DOI] [PubMed] [Google Scholar]
- 17.Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intens Care Med 2017; 43: 155–170. [DOI] [PubMed] [Google Scholar]
- 18.Kalantari K, Chang JN, Ronco C, et al. Assessment of intravascular volume status and volume responsiveness in critically ill patients. Kidney Int 2013; 83: 1017–1028. [DOI] [PubMed] [Google Scholar]
- 19.Claure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol 2016; 17: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchino S, Doig GS, Bellomo R, et al. Diuretics and mortality in acute renal failure. Critical Care Med 2004; 32: 1669–1677. [DOI] [PubMed] [Google Scholar]
- 21.Nadeau-Fredette AC, Bouchard J. Fluid management and use of diuretics in acute kidney injury. Adv Chronic Kidney Dis 2013; 20: 45–55. [DOI] [PubMed] [Google Scholar]
- 22.Mehta RL, Pascual MT, Soroko S, et al. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA 2002; 288: 2547–2453. [DOI] [PubMed] [Google Scholar]
- 23.Grams ME, Estrella MM, Coresh J, et al. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol 2011; 6: 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagshaw SM, Delaney A, Jones D, et al. Diuretics in the management of acute kidney injury: a multinational survey. Contrib Nephrol 2007; 156: 236–249. [DOI] [PubMed] [Google Scholar]
- 25.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367: 2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49: 675–683. [DOI] [PubMed] [Google Scholar]