Abstract
Aim
Endometriosis is defined as the presence of endometrial glandular and stromal cells outside of the uterine cavity. A previous study reported that microRNA (miR)‐542‐3p plays a critical role in eutopic endometrial decidualization. This study aims to clarify the potential role of miR‐542‐3p and the target gene, IGFBP‐1 (insulin‐like growth factor‐binding protein 1), in the impairment of the decidualizing capacity of human ectopic endometrial stromal cells (HEcESCs).
Methods
In vitro analysis of primary undifferentiated and decidualizing human eutopic endometrial stromal cells (HEuESCs) and HEcESCs was conducted. The primary HEuESCs or HEcESCs were expanded in culture and decidualized with 8‐bromo‐cyclic adenosine monophosphate (8‐bromo‐cAMP) and medroxyprogesterone acetate (MPA).
Results
The morphological and biological differentiating capacities of the HEcESCs were markedly impaired. In contrast to the HEuESCs, the HEcESCs that were treated with the decidual stimulus retained the mesenchymal phenotype and capacity for migration. The down‐regulation of miR‐542‐3p in the HEcESCs treatment with 8‐bromo‐cAMP and MPA was much weaker than that of the HEuESCs. High expression of miR‐542‐3p led to a significant decrease in the expression of IGFBP1 in the HEcESCs.
Conclusion
Impairment of the differentiating capacity by the overexpression of miR‐542‐3p could influence the capacity for migration and invasion of endometriotic cells in an ectopic environment.
Keywords: decidualization, endometriosis, mesenchymal‐to‐epithelial transition, microRNA, migration
1. Introduction
Endometriosis is one of the most prevalent gynecological disorders and affects ~10% of women of reproductive age and has a prevalence of as high as 35%‐50% of women with endometriosis‐associated infertility and/or pain.1 Several theories have been proposed in order to explain the etiology of endometriosis, but the most common acceptable theory on the origins of endometriosis is the retrograde reflux of menstrual blood that contains endometrial tissue via the fallopian tubes into the peritoneal cavity, where it attaches to the peritoneum, proliferates, and differentiates and eventually invades the underlying tissue.2 Although 90% of women of reproductive age have retrograde menstruation, endometriosis is only diagnosed in ~10% of them.3 Retrograde menstrual flow is a common event but does not explain why only some women develop endometriosis. Therefore, other pathological factors are required to establish this disease. The eutopic endometrium of women with endometriosis is believed to be abnormal, thereby predisposing these women to the establishment of ectopic diseases. It has been reported that the phenotype for the differentiation capacity of the ectopic endometrium is significantly varied, in comparison with the eutopic endometrium.4 However, the pathogenesis of endometriosis has not been fully characterized.
MicroRNAs (miRNAs) are single‐stranded, small, endogenous non‐coding RNAs that functionally regulate gene expression by blocking the translation or decreasing the stability of mRNA.5 In mammals, miRNAs are predicted to control the activity of ~50% of all protein‐coding genes.6, 7 The expression of many of these miRNAs has been identified in several mammalian cells and tissues, where they play important roles in the regulation of development and homeostasis of diverse cellular processes. Aberrant miRNA expression is a feature of many pathological conditions, including cancer, viral infections, metabolic diseases, and neurological disorders.8 In addition, there is growing evidence that miRNAs contribute to the pathogenesis of endometriosis by regulating abnormal cell differentiation, invasion, and inflammation.9, 10, 11
To date, several studies have demonstrated that ectopic endometrial stromal cells display an impaired differentiation (decidualization) response.4, 12 However, the mechanism that underlies this limited response to the decidualization process in vitro remains unclear. The authors recently demonstrated that the overexpression of miR‐542‐3p inhibited the decidualization process and it was suggested that the down‐regulation of miR‐542‐3p plays an important role in endometrial decidualization by regulating IGFBP1 expression.13 Hence, it is postulated that the overexpression of miR‐542‐3p contributes to the impairment of the dicidualization capacity of ectopic endometrial stromal cells. This study aims to clarify the potential role of miR‐542‐3p and a decidual marker of the target gene that encodes insulin‐like growth factor‐binding protein 1 (IGFBP‐1) in the impaired decidualization of ectopic endometrial stromal cells.
2. Materials and Methods
2.1. Tissue collection and isolation of human eutopic and ectopic endometrial stromal cells
The protocol of the current study was approved by the Institutional Review Board of Saitama Medical University Hospital (11‐017‐1), Saitama, Japan. Primary undifferentiated and decidualizing eutopic human endometrial stromal cells (HEuESCs) were obtained at the time of hysterectomy for uterine fibroids from normally cycling premenopausal women. None of the patients was undergoing hormonal treatment at the time of surgery. All the HEuESCs were obtained during the proliferative phase of the menstrual cycle. Human ectopic endometrial stromal cells (HEcESCs) were obtained at the time of ovarian cystectomy or oophorectomy of women with ovarian endometriomas. The diagnosis of ovarian endometrioma was confirmed by histological examination.
The HEuESCs were isolated and cultured as previously described.14, 15, 16 For the isolation and culture of the HEcESCs, ectopic endometrial tissues were collected in Dulbecco's modified Eagle medium with nutrient mixture F‐12 (DMEM/F‐12) containing 1% antibiotic–antimycotic solution (Life Technologies/Gibco, Carlsbad, CA, USA). The isolation of the HEcEScs from the tissue samples had been processed with collagenase Ia (Sigma‐Aldrich Corporation, St. Louis, MO, USA) and DNase I (Sigma‐Aldrich Corporation). After enzymatic digestion, the cells were passed through a 100 μm nylon mesh filter (BD Falcon; BD Biosciences, Franklin Lakes, NJ, USA). Subsequently, the ectopic cell suspension was passed through a 70 μm nylon mesh filter (BD Falcon). The isolated HEcESCs cells then were cultured.
The HEuESCs and HEcESCs were cultured in a maintenance medium that was composed of 10% DMEM/F‐12 containing 10% dextran‐coated, charcoal‐treated fetal bovine serum and 1% antibiotic–antimycotic solution (Life Technologies/Gibco). The confluent monolayers of the HEuESCs and HEcESCs were treated with or without a final concentration of 10−6 mol L−1 medroxy‐progesterone acetate (MPA) (Sigma M1629; Sigma‐Aldrich Corporation) and 0.5 mmol L−1 8‐bromo‐cyclic adenosine monophosphate (8‐br‐cAMP) (Sigma B7880; Sigma‐Aldrich Corporation) in 2% DMEM/F12 (1:1 v/v) for 6 days. The culture medium was changed every 3 days. All the experiments were conducted before the third passage of the cultures.
2.2. RNA extraction
The total RNA was purified from the HEuESCs or HEcESCs by using the miRNAeasy Mini kit (Qiagen, Hilden, Germany), according to the manufacturer's protocol. The RNA's quality was verified by measuring the absorbance at 230 nm, 260 nm, and 280 nm with a spectrometer (NanoDrop Technologies, Inc., Wilmington, DE, USA).
2.3. Transfection of the microRNA mimics
Immediately prior to transfection, the culture medium of the HEuESCs and HEcESCs was changed to antibiotic‐free decidualization medium that contained 0.5 mmol L−1 8‐br‐cAMP and 10–6 mol L−1 MPA. The control cultures were maintained in a standard medium. mirVana™ miRNA mimic (Applied Biosystems, Foster City, CA, USA) for hsa‐miR‐542‐3p and hsa‐miR‐negative control (NC; Applied Biosystems) was mixed with lipofectamine 2000 transfection reagent (Invitrogen Corporation, Carlsbad, CA, USA), according to the manufacturer's protocol, and was added to the HEuESCs and HEcESCs in six‐well culture plates at a density of 80% confluence. The final concentration of the miRNA was 30 nmol L−1. After 3 days and 6 hours, the cells were subjected to a change in the antibiotic medium in order to induce decidualization with 0.5 mmol L−1 8‐br‐cAMP and 10−6 mol L−1 MPA or the control medum. A morphological evaluation, RNA extraction, and quantitative real‐time polymerase chain reaction (qRT‐PCR) analysis were performed on day 6 of decidualization.
2.4. Quantitative real‐time polymerase chain reaction amplification of the microRNA
The total RNA of each sample was reverse‐transcribed by using Bio Script reverse transcriptase (Bioline, London, UK) with oligo dT primers. The obtained cDNA was mixed with gene‐specific primers and power SYBER Green PCR Master Mix (Applied Biosystems) and subjected to the qPCR analysis by using the 7900 HT Fast Real Time PCR System (Applied Biosystems) or the PikoReal 96Real‐Time PCR system (Thermo Fisher Scientific, Waltham, MA, USA). The primer sequences for each gene were: 5′‐CGA CCA CTT TGT CAA GCT CA‐3′ (glyceraldehyde phosphate dehydrogenase [GAPDH]‐forward), 5′‐AGG GGT CTA CAT GGC AAC TG‐3′ (GAPDH‐reverse); 5′‐CTG CGT GCA GGA GTC TGA‐3′ (IGFBP1‐forward), 5′‐CCCAAAGGATGGAATGATCC‐3′ (IGFBP1‐reverse); and 5′‐CTA CAT CCA TAA CCT CTC CTC A‐3′ (prolactin [PRL]‐forward), 5′‐GGG CTT GCT CCT TGT CTT C‐3′ (PRL‐reverse). The relative mRNA expression was calculated by using the 2⊿⊿Ct method13 and is presented as the mean±standard error normalized to GAPDH.
2.5. Quantitative real‐time polymerase chain reaction amplification of the microRNAs
In order to confirm the relative expression level of the objective miRNA, the total RNA was subjected to a qRT‐PCR analysis. The TaqMan microRNA RT kit (Applied Biosystems) was used to synthesize cDNA from 5 ng of RNA. The PikoReal 96 Real‐Time PCR system (Thermo Fisher Scientific) and TaqMan Universal Master Mix II with UNG (Applied Biosystems) were used to perform the qRT‐PCR in duplicate. The TaqMan MicroRNA assays (Applied Biosystems) for miR‐542‐3p (Assay ID: MC 001284) and U6 (as an endogenous control) were used as the primers for the reverse transcription and amplification. The mature sequences of hsa‐miR‐542‐3p were 5′‐UGU GAC AGA UUG AUA ACU GAA A‐3′ (miRBase accession no: MIMAT0003389). The expression level of miR‐542‐3p, relative to U6, was calculated by the 2∆∆Ct method.17
2.6. Actin staining analysis
The HEuESCs or HEcESCs were cultured on glass cover slips up to subconfluence. Subsequently, the cells were exposed to decidualization treatment with or without 0.5 mmol L−1 8‐bromo‐cAMP and/or 10–6 mol L−1 MPA for 6 days. The cells were fixed with 4% paraformaldehyde in phosphate‐buffered saline (PBS) (Nacalai Tesque, Inc., Kyoto, Japan) for 10 minutes, washed in PBS, and permeabilized with 0.5% Triton X‐100 at room temperature. The actin was stained with the fluorescent marker, Acti‐stain™ 555 phalloidin (Cytoskeleton, Inc., Denver, CO, USA). The cells were mounted with mounting media that contained 4,6‐diamidino‐2‐pheniylindole (DAPI) (Vector Laboratories, Inc., Burlingame, CA, USA) and visualized by using a fluorescent laser microscope (Axiocam MRm; Carl Zeiss AG, Oberkochen, Germany).
2.7. Scratch wound assay
The HEuESCs or HEcESCs were seeded on culture dishes and cultured up to 100% confluence. Monolayers of the HEuESCs or HEcESCs in each dish were wounded by scratching with sterile plastic 200 μL pipette tips. At 24 hours after wounding, the cells were observed under a phase‐contrast microscope. The assays were performed independently in triplicate.
2.8. Immunofluorescent staining
The HEuESCs and HEcESCs were plated onto glass cover slips up to 60% confluence and then subjected to a decidualization stimulus with or without 0.5 mmol L−1 8‐bromo‐cAMP and/or 10–6 mol L−1 MPA for 3 days. Afterwards, the cells were fixed with 4% paraformaldehyde in PBS (Nacalai Tesque, Inc.) for 15 minutes at room temperature. After washing with PBS, the cells were blocked with 2% bovine serum albumin in PBS for 1 hour and then incubated with primary antibodies against N‐cadherin (1:250 dilution; Abcam, Cambridge, MA, USA) and E‐cadherin (1:500 dilution; Cell Signaling, Beverly, MA, USA) at 4°C overnight, followed by Alexa Fluor 488 that was conjugated with anti‐rabbit immunoglobulin G (1:10,000 dilution; Invitrogen) in PBS for 1 hour at room temperature. Afterwards, the cells were mounted with mounting media that contained DAPI and visualized by using a fluorescent laser microscope (Axiocam MRm).
2.9. Statistical analyses
All the experiments were repeated a minimum of three times by using independent primary cell cultures. The statistical analyses were performed with the two‐tailed Student's t test. The data are expressed as the mean±SE. A P‐value of <.05 was considered to be significant.
3. Results
3.1. Ectopic endometrial stromal cells show a markedly impaired morphological and biological decidualization process
The authors investigated whether HEcESCs preserve the capacity for differentiation in response to 8‐bromo‐cAMP and MPA, as assessed by their morphology and the measurement of the decidual markers, IGFBP1 and PRL. In the absence of hormonal treatment, the primary HEuESCs and HEcESCs have an elongated spindle fibroblast‐like morphology under a light microscope (Figure 1A,C). The treatment with 8‐bromo‐cAMP and MPA induced morphological changes in the decidual phenotype of the HEuESCs, characterized as cells that were larger and rounder than normal cells (Figure 1B). In contrast, the treatment of the HEcESCs with 8‐bromo‐cAMP and MPA had no discernible effect on their morphological appearance (Figure 1D).
Figure 1.
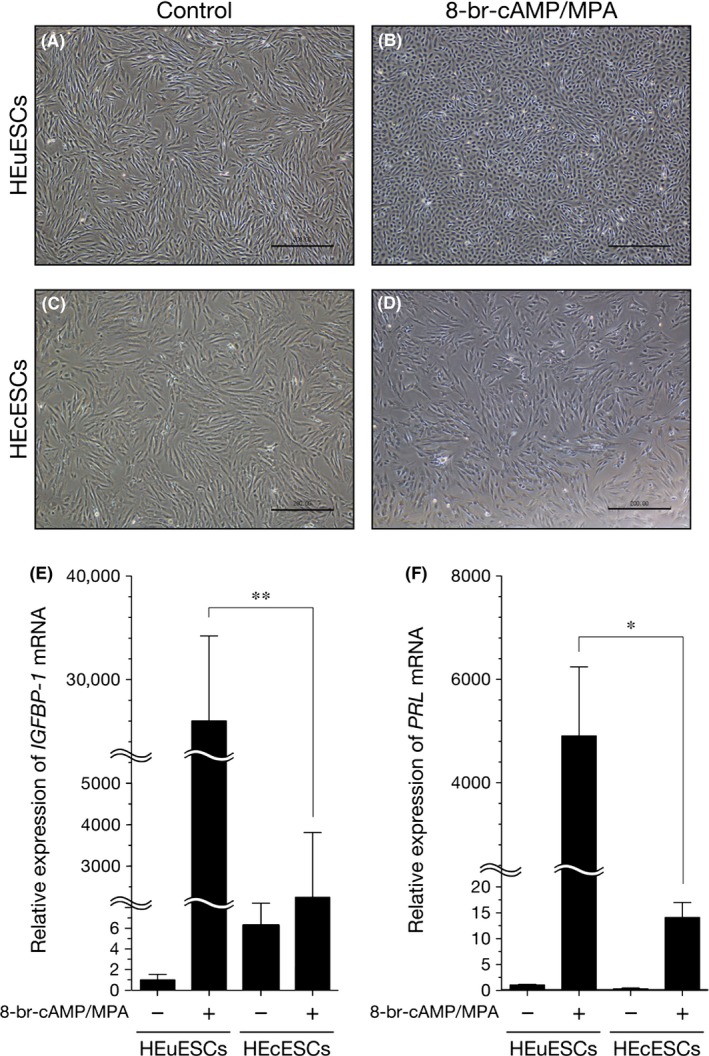
Effect of dedidual stimulation on the phenotype of the human eutopic endometrial stromal cells (HEuESCs) and the human ectopic endometrial stromal cells (HEcESCs). Light microscopic images of the decidual transformation of (A,B) the HEuESCs and (C,D) the HEcESCs in vitro. The confluent monolayers were treated with or without 8‐bromo‐cyclic adenosine monophosphate (8‐bromo‐cAMP) (0.5 mmol L−1) and medroxyprogesterone acetate (MPA) (10–6 mol L−1) for 6 days. The medium was changed on day 3. The expression levels of (E) insulin‐like growth factor‐binding protein 1 (IGFBP1) and (F) prolactin (PRL) microRNA were measured by quantitative real‐time polymerase chain reaction amplification. The confluent HEuESCs or HEcESCs were treated as indicated for 6 days. The medium was changed on day 3. The data are presented as the mean±standard error of the mean. *P<.05 and **P<.01. Scale bar, 200 μm
The expression levels of IGFBP1 and PRL were used to monitor the decidual response. In line with previous studies,18, 19 IGFBP1 and PRL expression of the HEuESCs markedly increased in response to the treatment with 8‐bromo‐cAMP and MPA. The inducible effect of IGFBP1 and PRL expression on the decidual response of the HEcESCs was dramatically reduced, as compared with the HEuESCs (Figure 1E,F). These observations suggest that the morphological and biological differentiating capacities of the HEcESCs were markedly impaired.
3.2. Alternation of F‐actin localization and the cytoskeleton on the decidualization of the human eutopic endometrial stromal cells and the human ectopic endometrial stromal cells
A previous study reported that the actin‐based cytoskeleton contributes to both functional and morphological endometrial decidualization.20 Therefore, the authors investigated the subcellular distribution of the actin stress fibers in the HEuESCs and HEcESCs that had been treated with or without 8‐bromo‐cAMP and MPA by using Acti‐stain™ 555 phalloidin. Well‐stretched F‐actin was distributed throughout the cytoplasm in the undifferentiated HEuESCs and HEcESCs (Figure 2A,C). In response to decidual stimulation, F‐actin was located in the periphery of the HEuESCs (Figure 2B). However, the alternation of F‐actin localization in the HEcESCs in response to decidual stimulation (Figure 2D) was unable to be observed.
Figure 2.
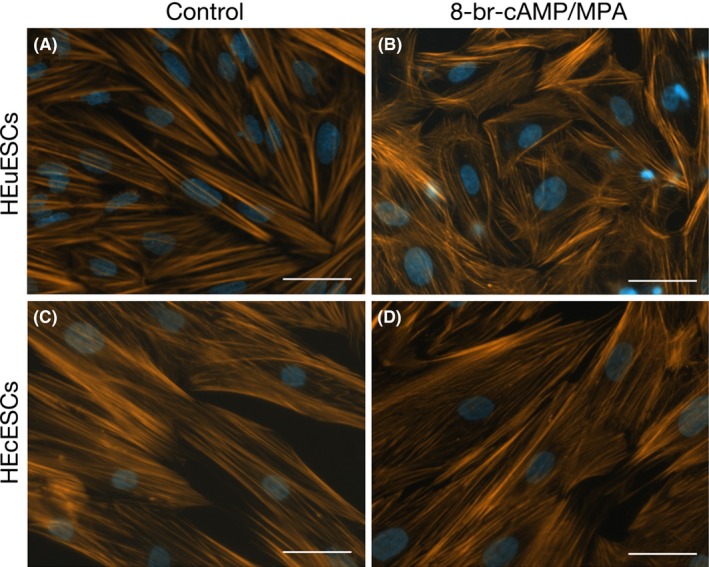
Alternations to F‐actin localization in the human eutopic endometrial stromal cells (HEuESCs) and the human ectopic endometrial stromal cells (HEcESCs) on decidualization. The actin filaments of (A,B) the HEuESCs and (C,D) the HEcESCs were visualized with Acti‐stain™ 555 phalloidin (Cytoskeleton) after treatment with or without 8‐bromo‐cyclic adenosine monophosphate (8‐br‐cAMP) and medroxyprogesterone acetate (MPA) for 6 days. Scale bar, 50 μm
3.3. Decidual stimulation did not inhibit the cell motility or induce a cadherin switch in the human ectopic endometrial stromal cells
An immunofluorescence study was conducted in order to assess alternations in the expression and localization of E‐cadherin and N‐cadherin in the HEuESCs and HEcESCs in response to treatment with 8‐bromo‐cAMP and MPA. The expression of N‐cadherin was down‐regulated (Figure 3C,D), whereas the expression of E‐cadherin was up‐regulated (Figure 3A,B) in the HEuESCs on decidualization. Although the staining of N‐cadherin was strong (Figure 3G,H), the staining of E‐cadherin was faint in the HEcESCs that had been treated with or without 8‐bromo‐cAMP and MPA (Figure 3E‐H). These observations suggest that MET occurred in the HEuESCs, but not in the HEcESCs, during the decidualization process.
Figure 3.
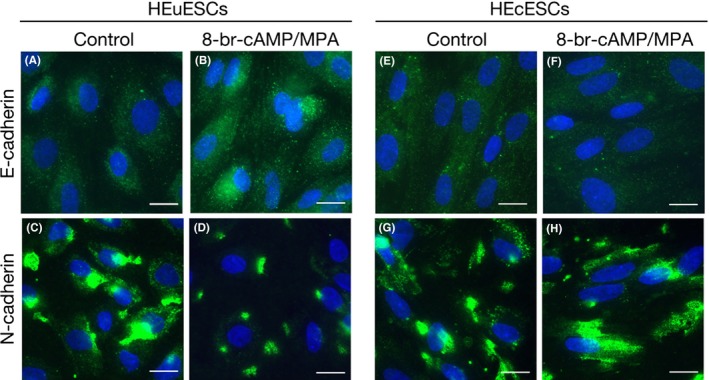
Suppression of the mesenchymal–epithelial transition in the decidualized human ectopic endometrial stromal cells (HEcESCs). Localization of the immunofluorescent (A,B,E,F) E‐cadherin and (C,D,G,F) N‐cadherin proteins is shown. The confluent monolayers of the human eutopic endometrial stromal cells (HEuESCs) (A‐D) and the HEcESCs (E–H) were treated with or without 8‐bromo‐cyclic adenosine monophosphate (8‐br‐cAMP) and medroxyprogesterone acetate (MPA) for 3 days. Scale bar, 20 μm
The motility of the HEuESCs and HEcESCs that had been treated with or without 8‐bromo‐cAMP and MPA was examined by using a wound closure assay. As shown in Figure 4E, the level of cell motility was dramatically inhibited in the decidualized HEuESCs, in comparison with the non‐decidualized HEuESCs (Figure 4A,B). In contrast, the motility of the HEcESCs that had been treated with 8‐bromo‐cAMP and MPA was only slightly inhibited, in comparison with the non‐decidualized HEcESCs (Figure 4C,D).
Figure 4.
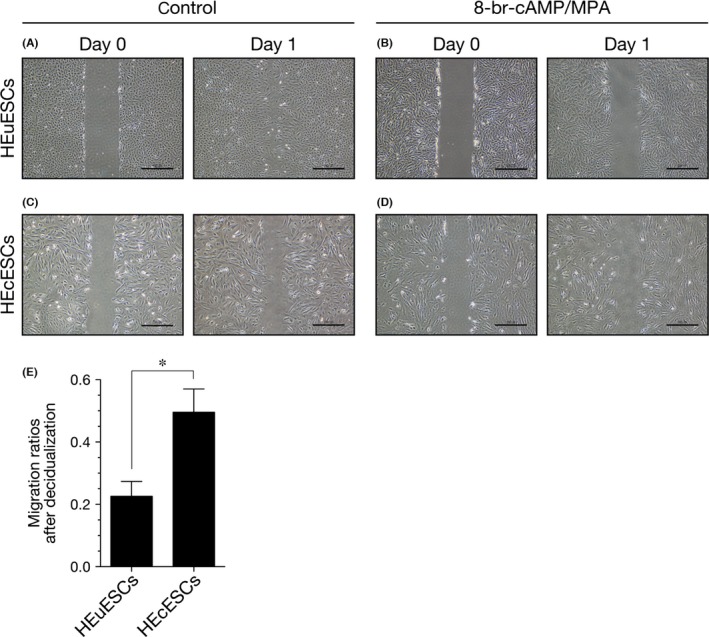
Decidual stimulation did not inhibit the motility of the human ectopic endometrial stromal cells (HEcESCs). The confluent monolayers of (A,B) the human eutopic endometrial stromal cells (HEuESCs) and (C,D) the HEcESCs were cultured with (A,C) or without 8‐bromo‐cyclic adenosine monophosphate (8‐br‐cAMP) and medroxyprogesterone acetate (MPA) (B,D) for 6 days. The HEuESCs and HEcESCs were photographed at 0 hour and 24 hours after scratching of the monolayer. (E) Each wound area was measured. Each gray bar represents the mean±standard error of the mean, relative to the closed area at 24 hours that had been obtained from three independent experiments. *P<.05. Scale bar, 200 μm
3.4. Overexpression of microRNA‐542‐3p induces an aberrant decidual response in endometriotic stromal cells
Following the authors’ previous report,13 the role of miR‐542‐3p in the impairment of the decidualization process in HEcESCs was investigated. In order to account for interpatient variability, the expression of miRNAs was calculated as the fold change relative to the HEuESCs or HEcESCs. The expression of miR‐542‐3p in both the HEuESCs and HEcESCs that had been treated with 8‐bromo‐cAMP and MPA was dramatically decreased. Interestingly, the down‐regulation of miR‐542‐3p by a decidualization stimulus in the HEcESCs was much weaker than that in the HEuESCs (Figure 5A). In addition, miR‐542‐3p overexpression induced a significant decrease in IGFBP1 expression in the HEcESCs (Figure 5B). These observations suggest that the overexpression of miR‐542‐3p in HEcESCs caused the aberrant expression of the decidual marker, IGFBP1, in response to decidual stimulation.
Figure 5.
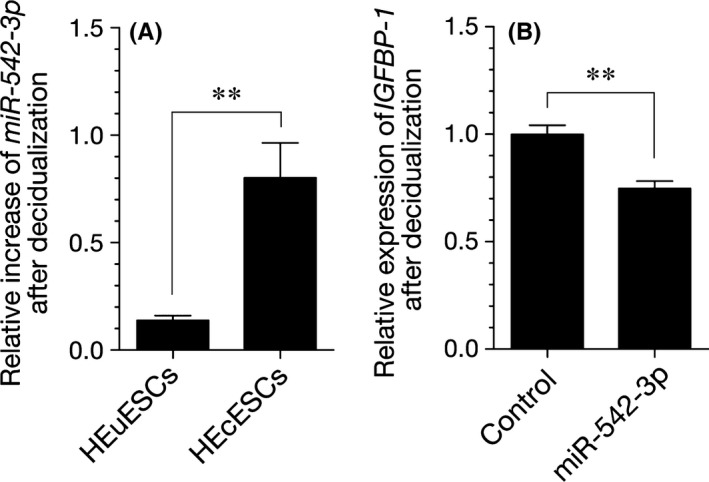
Impact of microRNA (miR)‐542‐3p overexpression on the decidual phenotype. A, A quantitative real‐time polymerase chain reaction amplification (qRT‐PCR) analysis of the miR‐542‐3p expression levels in the human eutopic endometrial stromal cells (HEuESCs) and the human ectopic endometrial stromal cells (HEcESCs) that had been treated with or without 8‐bromo‐cyclic adenosine monophosphate (8‐br‐cAMP) and medroxyprogesterone acetate (MPA) for 6 days. The expression of miR542‐3p was calculated as the fold change, relative to the HEuESCs or HEcESCs. B, A qRT‐PCR analysis of the insulin‐like growth factor‐binding protein 1 (IGFBP1) transcript levels in the HEcESCs that were transfected with a miR‐542‐3p mimic or NC mimic and decidualized for 6 days. The expression levels were normalized to glyceraldehyde 3‐phosphate dehydrogenase. The data are presented as the mean±standard error of the mean of three independent experiments. **P<.01
4. Discussion
Although the causes of endometriosis remain unknown, growing evidence points to phenotypical change of the endometrial cells, including their differentiation, proliferation, migration, and invasion capacities, as an important factor that is involved in the pathophysiology of this condition. The eutopic endometrium of women with endometriosis is believed to behave differently, thereby predisposing them to the establishment of ectopic diseases. The normal decidualization process of the upper (functional) endometrial layer is initiated during the mid‐secretory phase of the menstrual cycle. In the absence of pregnancy, luteolysis and falling ovarian progesterone levels induce a cascade of events in the endometrium of menstruating species, leading to proteolytic breakdown, shedding of the functional layer, and bleeding. The notion that decidualization in the absence of pregnancy is causally linked to menstruation is supported by several other lines of evidence.21 The behavior of the endometrium in patients with endometriosis could be attributed to the aberrant transport of the decidual phenotype endometrium into the peritoneal cavity.
The findings of the present study demonstrated that the morphological and biochemical decidualization capacities of ectopic endometrial stromal cells were remarkably impaired. Interestingly, in contrast to the HEuESCs, decidual stimulation sustained migration activity that was accompanied by the mesenchymal phenotype of HEcESCs. These observations imply that the aberrant differentiating capacity of endometrial cells, which actively migrate, could result in the establishment and development of endometriosis.
As small, non‐coding RNAs, miRNAs are classified as regulatory RNAs and have been reported to regulate diverse biological process, including cell growth, proliferation, and differentiation.22 As each miRNA is predicted to involve a broad range of mRNAs, based on the degree of sequence homology, the expression of ~50% of all protein‐coding genes are estimated to be potential targets of miRNA regulation.6, 7 The inverse relationship between the expression of miRNAs and their target genes is complex and evolving. There is accumulating data that miRNAs contribute to the pathogenesis of endometriosis by regulating abnormal cell differentiation, invasion, and inflammation.23 It has been reported that the expression of several miRNAs differs between the eutopic and ectopic endometrial tissues of women with and without endometriosis.9, 24 The authors recently reported that the down‐regulation of miR‐542‐3p expression in eutopic endometrial stromal cells enabled morphological and biological differentiation of the endometrium.13 Also, other studies have showed that the decidualization capacity of ectopic endometrial stromal cells was remarkably impaired.4, 12 Therefore, the authors postulated that the overexpression of miR‐542‐3p contributes to the impairment of the decidualization capacity of ectopic endometrial stromal cells in endometriosis. The authors demonstrated that the expression of miR‐542‐3p in HEcESCs in response to decidual stimulation was dramatically higher, in comparison with the decidualized HEuESCs, and that the overexpression of miR‐542‐3p significantly decreased the expression of IGFBP1 mRNA in the HEcESCs that had been treated with 8‐bromo‐cAMP and MPA. These observations suggest that a high expression of miR‐542‐3p impaired the decidualization capacity of the HEcESCs.
It has been reported that the overexpression of miR‐542‐3p can inhibit cell growth and prevent tumor formation in vivo.25 This report suggested that miR‐542‐3p is a promising target for cancer therapy. In contrast, it was demonstrated that miR‐542‐3p suppresses osteogenic differentiation by repressing bone morphogenetic protein 7 and its downstream signaling.26 The authors’ findings raise the possibility that miR‐542‐3p has potential as a biomarker or therapeutic target in endometriosis.
The epithelial‐to‐mesenchymal transition (EMT), a developmental process in which epithelial cells reduce intercellular adhesion and acquire fibroblastic properties, plays critical roles in the conversion to motile mesenchymal cells for the invasive and metastatic potential that is associated with cancer progression.27, 28 The MET is the reverse process of the EMT. Increasing evidence suggests that the EMT of endometrial epithelial cells contributes to the establishment of endometriotic lesions.29, 30 In contrast, recent studies have provided compelling evidence that decidualization is associated with the MET of HEuESCs.31, 32 The authors demonstrated that decidualized stimulation down‐regulated N‐cadherin expression and up‐regulated E‐cadherin expression in the HEuESCs. In contrast, although N‐cadherin expression was strong, E‐cadherin expression was relatively weak in the HEcESCs that had been treated with or without 8‐bromo‐cAMP and MPA. These observations suggest that the differentiated HEuESCs acquired an epithelial phenotype and lost their capacity for migration. In contrast, the HEcESCs that had been treated with decidual stimulation fairly retained the mesenchymal phenotype and capacity for migration. Therefore, the authors speculate that this, in turn, could influence the capacity for proliferation and invasion of the cells in an ectopic environment.
During MET, the mesenchymal cells shift from a mesenchymal to an epithelial phenotype via reorganization of the cytoskeleton. The cytoskeleton plays an important role in various cellular processes, including mitosis, growth, motility, aging, and apoptosis.33 The organization and plasticity of the cytoskeleton are characterized primarily by forces that are generated by actin–myosin interactions.34 Previous studies demonstrated that F‐actin was located in the periphery of decidualized HEuESCs.20, 33 These observations are consistent with the current microscopic findings. Meanwhile, the localization of F‐actin was maintained in the HEuESCs that had been treated with or without 8‐bromo‐cAMP and MPA. It is possible that the actin‐based cytoskeleton might actively contribute to sustain the mesenchymal phenotype and cell motility of HEcESCs against decidualization stimulation.
There were several limitations to this study that should be addressed. Although the use of the stromal cells of an endometriotic cyst is a well‐established model, the absence of other cellular constituents in the endometrial tissue (eg, epithelial, vascular, or immune cells) might have impacted the results. Furthermore, all the primary cultures were established from ovarian endometriotic cysts. Therefore, it is necessary to perform the same experiments in the future with eutopic endometrial stromal cells that have been collected from women with and without endometriosis.
In conclusion, the present study demonstrated that the morphological and biochemical differentiating capacities of HEcESCs were remarkably impaired against decidualization stimulation. In contrast to the HEuESCs, HEcESCs retained the mesenchymal phenotype and capacity for migration against decidualization stimulation. The high expression of miR‐542‐3p might impair the differentiating capacity of HEcESCs. Therefore, impairment of the differentiating capacity by the overexpression of miR‐542‐3p influences the capacity for the migration, proliferation, and invasion of endometriotic cells in an ectopic environment.
Disclosures
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: All the procedures were followed in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all the patients who were included in the study. This article does not contain any study with animal participants that was performed by any of the authors.
Acknowledgements
The authors thank Ms. S. Mitsui and Ms. K. Komatsu for their expert technical assistance. SS is a recipient of the Saitama Medical University Research Fellowship, Saitama, Japan.
Sultana S, Kajihara T, Mizuno Y, et al. Overexpression of microRNA‐542‐3p attenuates the differentiating capacity of endometriotic stromal cells. Reprod Med Biol. 2017;16:170–178. https://doi.org/10.1002/rmb2.12028
References
- 1. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sampson J. The development of the implantation theory for the origin of peritoneal endometriosis. Am J Obstet Gynecol. 1940;40:549‐556. [Google Scholar]
- 3. Bulun SE. Endometriosis. N Engl J Med. 2009;360:268‐279. [DOI] [PubMed] [Google Scholar]
- 4. Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85:564‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11:1753‐1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engels BM, Hutvagner G. Principles and effects of microRNA‐mediated post‐transcriptional gene regulation. Oncogene. 2006;25:6163‐6169. [DOI] [PubMed] [Google Scholar]
- 8. Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro‐RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13:797‐806. [DOI] [PubMed] [Google Scholar]
- 9. Petracco R, Grechukhina O, Popkhadze S, Massasa E, Zhou Y, Taylor HS. MicroRNA 135 regulates HOXA10 expression in endometriosis. J Clin Endocrinol Metab. 2001;96:E1925‐E1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. 2010;16:142‐165. [DOI] [PubMed] [Google Scholar]
- 11. Naqvi H, Mamillapalli R, Krikun G, Taylor HS. Endometriosis located proximal to or remote from the uterus differentially affects uterine gene expression. Reprod Sci. 2016;23:186‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsuno A, Nasu K, Yuge A, Matsumoto H, Nishida M, Narahara H. Decidualization attenuates the contractility of eutopic and ectopic endometrial stromal cells: implications for hormone therapy of endometriosis. J Clin Endocrinol Metab. 2009;94:2516‐2523. [DOI] [PubMed] [Google Scholar]
- 13. Tochigi H, Kajihara T, Mizuno Y, et al. Loss of miR‐542‐3p enhances IGFBP1 expression in decidualising human endometrial stromal cells. Sci Rep. 2017;7:40001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leitao B, Jones MC, Fusi L, et al. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J. 2010;24:1541‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809‐4820. [DOI] [PubMed] [Google Scholar]
- 16. Christian M, Zhang X, Schneider‐Merck T, et al. Cyclic AMP‐induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer‐binding protein beta in differentiating human endometrial stromal cells. J Biol Chem. 2002;277:20825‐20832. [DOI] [PubMed] [Google Scholar]
- 17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods. 2001;25:402‐408. [DOI] [PubMed] [Google Scholar]
- 18. Kajihara T, Tochigi H, Prechapanich J, et al. Androgen signaling in decidualizing human endometrial stromal cells enhances resistance to oxidative stress. Fertil Steril. 2012;97:185‐191. [DOI] [PubMed] [Google Scholar]
- 19. Cloke B, Huhtinen K, Fusi L, et al. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149:4462‐4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maruyama T, Yoshimura Y, Sabe H. Tyrosine phosphorylation and subcellular localization of focal adhesion proteins during in vitro decidualization of human endometrial stromal cells. Endocrinology. 1999;140:5982‐5990. [DOI] [PubMed] [Google Scholar]
- 21. Labied S, Kajihara T, Madureira PA, et al. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20:35‐44. [DOI] [PubMed] [Google Scholar]
- 22. Mizuno Y, Yagi K, Tokuzawa Y, et al. miR‐125b inhibits osteoblastic differentiation by down‐regulation of cell proliferation. Biochem Biophys Res Commun. 2008;368:267‐272. [DOI] [PubMed] [Google Scholar]
- 23. Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array‐based analysis. Fertil Steril. 2016;106:402‐409. [DOI] [PubMed] [Google Scholar]
- 24. Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, et al. MicroRNA‐regulated pathways associated with endometriosis. Mol Endocrinol. 2009;23:265‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oneyama C, Morii E, Okuzaki D, et al. MicroRNA‐mediated upregulation of integrin‐linked kinase promotes Src‐induced tumor progression. Oncogene. 2012;31:1623‐1635. [DOI] [PubMed] [Google Scholar]
- 26. Kureel J, Dixit M, Tyagi AM, et al. miR‐542‐3p suppresses osteoblast cell proliferation and differentiation, targets BMP‐7 signaling and inhibits bone formation. Cell Death Dis. 2014;5:e1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumor progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415‐428. [DOI] [PubMed] [Google Scholar]
- 28. Tsuji T, Ibaragi S, Hu GF. Epithelial–mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69:7135‐7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartley J, Jülicher A, Hotz B, Mechsner S, Hotz H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch Gynecol Obstet. 2014;289:871‐881. [DOI] [PubMed] [Google Scholar]
- 30. Proestling K, Birner P, Gamperl S, et al. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod Biol Endocrinol. 2015;13:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang XH, Liang X, Liang XH, et al. The mesenchymal–epithelial transition during in vitro decidualization. Reprod Sci. 2013;20:354‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu J, Berga SL, Johnston‐MacAnanny EB, et al. Endometrial stromal decidualization responds reversibly to hormone stimulation and withdrawal. Endocrinology. 2016;157:2432‐2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ihnatovych I, Hu W, Martin JL, Fazleabas AT, de Lanerolle P, Strakova Z. Increased phosphorylation of myosin light chain prevents in vitro decidualization. Endocrinology. 2007;148:3176‐3184. [DOI] [PubMed] [Google Scholar]
- 34. Elson EL. Cellular mechanics as an indicator of cytoskeletal structure and function. Annu Rev Biophys Biophys Chem. 1998;17:397‐430. [DOI] [PubMed] [Google Scholar]


