Abstract
Background and aims
Lung cancer has the highest mortality rate of all cancers worldwide. Non-small-cell lung cancer (NSCLC) accounts for 85% of all lung cancers and has an extremely poor prognosis. Afatinib is an irreversible ErbB family blocker designed to suppress cellular signaling and inhibit cellular growth and is approved in Europe after platinum-based therapy for squamous NSCLC. The objective of the present analysis was to evaluate the cost-effectiveness of afatinib after platinum-based therapy for squamous NSCLC in France.
Methods
The study population was based on the LUX-Lung 8 trial that compared afatinib with erlotinib in patients with squamous NSCLC. The analysis was performed from the perspective of all health care funders and affected patients. A partitioned survival model was developed to evaluate cost-effectiveness based on progression-free survival and overall survival in the trial. Life expectancy, quality-adjusted life expectancy and direct costs were evaluated over a 10-year time horizon. Future costs and clinical benefits were discounted at 4% annually. Deterministic and probabilistic sensitivity analyses were performed.
Results
Model projections indicated that afatinib was associated with greater life expectancy (0.16 years) and quality-adjusted life expectancy (0.094 quality-adjusted life years [QALYs]) than that projected for erlotinib. The total cost of treatment over a 10-year time horizon was higher for afatinib than erlotinib, EUR12,364 versus EUR9,510, leading to an incremental cost-effectiveness ratio of EUR30,277 per QALY gained for afatinib versus erlotinib. Sensitivity analyses showed that the base case findings were stable under variation of a range of model inputs.
Conclusion
Based on data from the LUX-Lung 8 trial, afatinib was projected to improve clinical outcomes versus erlotinib, with a 97% probability of being cost-effective assuming a willingness to pay of EUR70,000 per QALY gained, after platinum-based therapy in patients with squamous NSCLC in France.
Keywords: cost, cost-effectiveness, afatinib, lung cancer
Introduction
Non-small-cell lung cancer (NSCLC) represents a substantial clinical and economic burden for health care systems. It accounts for 85% of all new lung cancers worldwide.1 In Europe, lung cancer has the highest mortality rate of all cancers and accounts for 20% of all cancer-related deaths.2 NSCLC can be grouped into three common histologies: adenocarcinoma, squamous cell cancer and large cell carcinoma. Approximately 15–30% of all NSCLC patients present with squamous histology.3,4 The 5-year survival rate for patients with advanced NSCLC is low, ~25% for stage III and <1% for stage IV.1,5 Effective treatments are required to prolong patient survival and increase quality of life. Prognosis of patients diagnosed with NSCLC is poor witĥ80% of patients having advanced disease and a survival time of roughly 1 year.4
Traditional first-line treatments include platinum doublet therapy.6 However, successful response is only observed in 30–40% of patients.7 Once disease progression occurs on a platinum doublet, further second-line therapy is dependent on the first-line treatment used and any co-morbidities that the patient may have.8 Current international recommendations for second-line therapy use in squamous NSCLC include docetaxel, erlotinib (epidermal growth factor receptor [EGFR] blocker), ramucirumab (monoclonal antibody, inhibits angiogenesis) and more recently two monoclonal antibodies that inhibit the activation of the PD-1 protein, nivolumab and pembrolizumab (it should be noted that these two immunotherapies have greatest success in patients with tumor PD-L1 expression ≥5%, and no clear survival benefit has been found in EGFR mutation-positive patients).9–13
On the basis of international, randomized, Phase III trials (LUX-Lung 3 and 6), afatinib has been approved in EGFR mutation-positive patients and from March 2016 in patients with squamous histology (LUX-Lung 8).14–16 Afatinib is an irreversible ErbB family blocker that functions by inhibiting signaling from homo- and heterodimers including HER2/ErbB3, resulting in prolonged suppression of signaling and therefore inhibition of cellular growth.17 Irreversible binding is achieved through covalent bonding and is able to induce apoptosis and subsequently enable tumor shrinkage as a result.18
Afatinib has recently been directly compared with erlotinib, an EGFR, as second-line therapy in patients with advanced, squamous NSCLC in the LUX-Lung 8 trial.16 The aim of the present analysis was to determine the cost-effectiveness of afatinib versus erlotinib after platinum-based therapy in patients with advanced squamous NSCLC in the French setting based on the findings of the LUX-Lung 8 trial.
Methods
LUX-Lung 8 trial
The LUX-Lung 8 trial compared the efficacy and safety of afatinib versus erlotinib as second-line treatment in squamous advanced NSCLC patients who experienced disease progression during or following treatment with platinum-based chemotherapy. The primary end point of the trial was progression-free survival (PFS) and the secondary end point was overall survival (OS). Patients were randomly allocated 1:1 to afatinib or erlotinib. Median follow-up at the time of the primary analysis was 6.7 months. PFS at the primary analysis was significantly longer with afatinib than erlotinib (median 2.4 months [95% confidence interval {CI} 1.9–2.9] versus 1.9 months [95% CI 1.9–2.2]; hazard ratio {HR} 0.82 [95% CI 0.68–1.00], p=0.0427). At the time of the primary analysis of OS (median follow-up of 18.4 months), OS was significantly greater in the afatinib group than in the erlotinib group (median 7.9 months [95% CI 7.2–8.7] versus 6.8 months [95% CI 5.9–7.8], HR 0.81 [95% CI 0.69–0.95], p=0.0077). Data from the LUX-Lung 8 trial were used to develop a cost-effectiveness model of afatinib in the French setting.16
Model structure
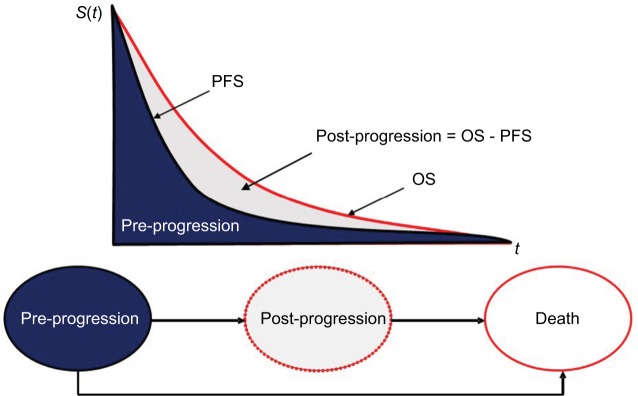
The cost-effectiveness model was a state transition model (specifically a partitioned survival model) developed in Micro-soft Excel® containing three health states: pre-progression, post-progression and death (Figure 1). Patients began in the pre-progression state, where they received second-line therapy (with either afatinib or erlotinib). During this period, patients either responded to therapy and remained in pre-progression, transitioned to post-progression or directly to the death state. In the post-progression state, patients were assumed to receive best supportive care (BSC, with options for additional nivolumab or chemotherapy explored in sensitivity analysis). Patients in the post-progression state were also exposed to the risk of mortality (and transition to the death state). Time spent in the pre-progression state matched the duration of treatment with second-line therapy in LUX-Lung 8. This was also the case for the time spent in the post-progression state for third-line treatment in the sensitivity analysis.
Figure 1.
Overview of model structure depicting transition from pre-progression to post-progression and death health states.
Abbreviations: PFS, progression-free survival; OS, overall survival; S(t), survival function (probability of survival beyond time t); t, time.
Transitions between health states were dependent on treatment-specific hazard rates observed in the trial. The proportion of patients in the PFS and OS states were calculated using various distributions (Weibull, loglogistic and lognormal) to obtain curves. These curves were estimated using the Kaplan–Meier estimates of PFS and OS to distribute patient numbers over time. The Kaplan–Meier OS curves demonstrated the comparison of patient proportion at several time points fitted using the extrapolation method (Figure 1). The proportion of patients in the post-progression health state can be defined as the difference between the proportion of patients in the pre-progression state and the proportion of alive patients (post-progression = OS − PFS). For the base case analysis, loglogistic functions were used to model PFS, and Weibull functions were used to model OS for afatinib and erlotinib based on a best fit analysis. To investigate the uncertainty of the extrapolation method of clinical data, additional sensitivity analyses were performed where lognormal and Weibull functions were used. The model used monthly cycles with half-cycle correction. Monthly cycle length was 30.4 days (365/12 = 30.4).
Model inputs
To determine the transferability of results to the French population, the characteristics of patients from the LUX-Lung 8 trial were compared with data extracted from epidemiological studies in French patients with NSCLC stage IIIb/IV.19 Characteristics were found to be comparable in terms of gender (>80% men), mean age (65 years in LUX-Lung 8 trial and 66 years in extracted data) and smoking status (>90% were smokers or former smokers in both). In addition, 9% of the LUX-Lung 8 patient population were French, 57% were from other European countries and 22% were from east Asian countries.
Erlotinib was selected as the comparator intervention as it is recommended by the Institut National Du Cancer as a second-line treatment, and the LUX-Lung 8 trial comparing erlotinib to afatinib is the only trial in which afatinib is directly compared to another treatment. The recommended starting dose of afatinib used in this study was 40 mg once daily and 150 mg once daily for erlotinib.
Post-progression treatments included only in additional sensitivity analysis were selected with the help of an expert in pneumology (CC) and are in line with clinical practice within France. Docetaxel, paclitaxel, nivolumab and BSC were selected as the post-progression strategies, recommended for either of the two compared arms. Post-progression treatments were not considered in the base case to ensure that the model outcomes directly reflected the comparison of afatinib with erlotinib only, the affected costs were captured in the sensitivity analysis.
The rates of grade 3 and 4 adverse events included in this analysis were taken directly from the LUX-Lung 8 trial, and the list of relevant adverse events included was validated by a clinical expert and comprised adverse events that had an impact on costs and quality of life data in NSCLC patients. The adverse events included were grade 3 and 4 diarrhea, rash/acne, grade 3 stomatitis, grade 3 fatigue and grade 3 nausea. The probability of an adverse event occurring during the pre-progression health state was distributed over time. The corresponding costs were therefore also evenly distributed over time. The probabilities of grade 3 and 4 adverse events used in the model for each arm are described in Table 1. The occurrence of adverse events did not affect state transitions in the model but did influence the estimated costs and quality of life utilities. As post-progression therapies were not considered in the base case, adverse event rates and costs associated with post-progression therapies were not included. During the sensitivity analysis, post-progression therapies were considered, however, the associated adverse event rates were not.
Table 1.
Proportion of patients who experienced grade 3 and 4 AE for each treatment arm
| Grade 3/4 AE | Proportion of patients who experienced afatinib AE (95% CI) | Proportion of patients who experienced erlotinib AE (95% CI) |
|---|---|---|
| Diarrhea grade 3 | 9.9% (7.1; 13.0) | 2.3% (1.1; 4.0) |
| Diarrhea grade 4 | 0.5% (0.1; 1.4) | 0.3% (0.0; 1.0) |
| Rash/acne | 5.9% (3.8; 8.4) | 10.4% (7.6; 13.6) |
| Stomatitis grade 3 | 4.1% (2.4; 6.3) | 0.0% |
| Fatigue grade 3 | 1.5% (0.5; 2.9) | 1.8% (0.7; 3.3) |
| Nausea grade 3 | 1.0% (0.3; 2.2) | 0.8% (0.2; 1.9) |
Notes: The data were taken directly from the Clinical Study Report of the LUX-Lung 8 trial, which was made available by the study sponsor to HEVA-HEOR (unpublished data, 2015). These data were reported for additional outcomes but in less detail by Soria et al.16
Abbreviations: AE, adverse event; CI, confidence interval.
Costs and quality of life utilities
The study was performed from the perspective of all French health care funders and affected patients. Drug acquisition and administration costs, monitoring costs, transportation costs and adverse event costs were accounted for in the model. The post-progression treatments used in additional sensitivity analysis are administered intravenously and are currently only available within the hospital setting in France. Docetaxel and paclitaxel are included in the diagnosis-related group (DRG) costs in France, therefore only the administration cost is included whereas nivolumab is not included in DRG and therefore its costs were captured separately. Drug acquisition costs are summarized in Table S1. Administration costs in France are captured in DRG estimates and for treatments that require intravenous administration, the cost of DRG 28Z07Z was used from 2014. The cost of hospital administration was considered in public hospitals only, and it was assumed that all patients were treated in outpatient care (this was the case for >90% of patients who received chemotherapy during 2015).20 The administration cost estimate used in the base case was EUR378.26 in 2016 (inflated from the 2014 value of EUR374.56 using the consumer price index).
Transportation costs were estimated from the Cour des Comptes report.21 In 2010, transportation costs were estimated to be EUR1.9 billion for 50.1 million round trips with an average cost of EUR75.84 per round trip. For the base case analysis, this cost was inflated to the 2016 value of EUR77.06 per round trip in 2016.
Monitoring costs consider medical consultations and biological and radiographic examinations. To estimate the monitoring costs for patients with squamous NSCLC, four clinical experts based in France were requested to complete a survey. A summary of the blood and imaging tests along with their corresponding costs is presented in Table S2. The costs of blood tests were estimated from the Table nationale de biologie, imaging tests from Classification commune des actes médicaux and nursing procedures from Nomenclature générale des actes professionnels.22–24 A summary of these costs is presented in Table S2. Monthly post-progression monitoring costs were estimated to be EUR253.63 (2016). Monthly monitoring costs for the pre-progression health state were also estimated to be EUR253.63. Resource consumption was assumed to be the same for all patients (in pre- and post-progression health states) based on an expert in pneumology’s opinion.
The costs of management of adverse events were calculated using rates reported in the LUX-Lung 8 trial. These were combined with an estimate of management costs based on a study performed in four European countries (including France) that compared cost of management of grade 3/4 adverse events in patients with NSCLC who received erlotinib and pemetrexed as therapy.25 The estimated cost of each adverse event included any additional treatment and hospital visit costs that were incurred. Adverse event costs included in the model were grade 3 and 4 diarrhea (EUR2,802), rash/acne (EUR226), grade 3 stomatitis (EUR469), grade 3 fatigue (EUR571) and grade 3 nausea (EUR1,997).
Utility values were obtained from the LUX-Lung 8 trial using the EuroQol 5 dimension (EQ-5D) health status self-assessment questionnaire. The French value set by the EQ-5D was applied to patient data of the LUX-Lung 8 trial to obtain scores from the French setting.26 Health-state utility values of 0.65 (95% CI 0.63–0.67) and 0.58 (95% CI 0.55–0.61) were estimated for the pre-progression and post-progression states, respectively. Disutility values were incorporated for the rate of adverse events. Values were sourced from the literature.27 Grade 3 and 4 diarrhea were each assigned a disutility value of 0.050 (95% CI 0.02–0.10), rash/acne had a value of 0.030 (95% CI 0.00–0.08), grade 3 stomatitis 0.131 (95% CI 0.11–0.15), grade 3 fatigue 0.070 (95% CI 0.04–0.11) and grade 3 nausea 0.050 (95% CI 0.02–0.10). The utility values used for adverse event rates were equal for both treatment arms.
Model assumptions
For the base case analysis, a time horizon of 10 years was assumed as patients with advanced or metastatic disease, who have previously received one line of treatment, will not survive a follow-up period >10 years. Sensitivity analyses were run with 1-, 2- and 5-year options. Treatment costs were calculated based on the treatments received and the cost of resource use associated with each health state. Future costs and clinical benefits were discounted at a rate of 4% per annum for the base case as recommended by the Haute Autorité de Santé (HAS).
Sensitivity analyses
One-way deterministic sensitivity analysis (DSA) was performed to identify key drivers of modeled outcomes. The following parameters were varied in one-way sensitivity analysis: adverse event rates, utility scores, transportation costs, monitoring costs, adverse event costs and discount rate. High and low values for each of the parameters used in sensitivity analysis are summarized in Table S3. Probabilistic sensitivity analysis (PSA) was also conducted by sampling from distributions around the same parameters. Five thousand Monte Carlo iterations were performed for the PSA. Beta distributions were assigned to safety parameters and utilities while gamma distributions were assigned to cost estimates. Scenario analyses were included to determine the level of uncertainty associated with other parameters including time horizon, the inclusion of post-progression treatments, survival extrapolations and health state utility data sources (Table S4).
Scenario analyses
Scenario analyses were performed to identify the impact on outcomes of inclusion of post-progression treatments in the analysis, of altering the method of adverse event consideration, of altering health utility data source, of inclusion of various time horizons and of altering the survival extrapolation methods. The following survival extrapolations were explored: independently assessed PFS with Weibull regression, independently assessed PFS with lognormal regression, investigator-assessed PFS with Weibull regression, investigator-assessed PFS with loglogistic regression and investigator-assessed PFS with lognormal regression. Parameters for OS function were also evaluated with lognormal and Weibull regressions applied. The parameters are summarized in Table S4.
Results
Clinical outcomes
Patients treated with afatinib as second-line therapy for squamous advanced NSCLC benefitted from longer life expectancy than those treated with erlotinib (0.94 years versus 0.78 years, respectively) translating in an increase of 0.16 years. Quality-adjusted life expectancy was also projected to be greater in patients treated with afatinib with an increase of 0.094 quality-adjusted life years (QALYs; 0.567 QALYs versus 0.473 QALYs) for afatinib and erlotinib, respectively (Table 2).
Table 2.
Clinical and cost-effectiveness outcomes
| Health outcome | Afatinib | Erlotinib | Difference |
|---|---|---|---|
| Progression-free survival time (months) | 5.05 | 3.85 | 1.2 |
| Post-progression survival time (months) | 6.84 | 6.12 | 0.72 |
| Life expectancy (years) | 0.94 | 0.78 | 0.16 |
| Quality-adjusted life expectancy (QALY) | 0.567 | 0.473 | 0.094 |
| Total costs (EUR) | 12,364 | 9,510 | 2,854 |
| ICER based on life expectancy | EUR18,568 per LY gained | ||
| ICER based on quality-adjusted life expectancy | EUR30,277 per QALY gained |
Abbreviations: ICER, incremental cost-effectiveness ratio; LY, life year; QALY, quality-adjusted life year.
Long-term costs and cost-effectiveness
Mean direct costs were higher for afatinib than erlotinib, EUR12,364 versus EUR9,510, respectively (Table 3), corresponding to a difference of EUR2,854 over the 10-year time horizon.
Table 3.
Breakdown of costs
| Health outcome | Afatinib
|
Erlotinib
|
||
|---|---|---|---|---|
| Cost (EUR) | Proportion of total costs (%) | Cost (EUR) | Proportion of total costs (%) | |
| Drug acquisition cost in pre-progression | 9,158 | 74 | 7,007 | 74 |
| Monitoring cost in pre-progression (including transportation costs) | 1,143 | 9 | 846 | 9 |
| Monitoring cost in post-progression (including transportation costs) | 1,715 | 14 | 1,535 | 16 |
| Adverse events costs | 348 | 3 | 122 | 1 |
| Total costs | 12,364 | 100 | 9,510 | 100 |
Note: Total costs for drug acquisition and administration and monitoring are shown in bold.
Mean drug acquisition costs in the pre-progression state were higher for afatinib (EUR9,158) than for erlotinib (EUR7,007) and accounted for 74% of the total costs incurred by afatinib and erlotinib (Table 3). Monitoring costs were associated with a mean EUR297 increase in total costs for afatinib versus erlotinib, with individual monitoring cost values of EUR1,143 and EUR846, respectively. Monitoring costs in the post-progression health state were also higher for afatinib than erlotinib, with mean values of EUR1,715 versus EUR1,535, corresponding to a difference of EUR180. Adverse event costs accounted for 3% of the total costs of afatinib with a cost of EUR348 and 1% of the total costs of erlotinib, EUR122. Adverse event costs for patients treated with afatinib were EUR226 greater than that with erlotinib. Longer survival times on afatinib contributed to higher monitoring and adverse event costs.
Evaluation of cost-effectiveness demonstrated that afatinib was associated with incremental cost-effectiveness ratios (ICERs) of EUR18,568 per life year gained and EUR30,277 per QALY gained versus erlotinib (Table 2).
Sensitivity analysis and scenario analyses
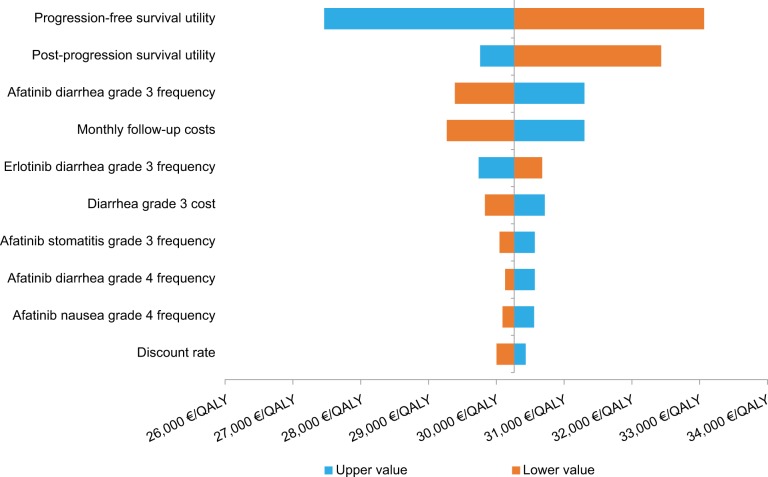
DSA and scenario analysis showed that variation in the PFS and OS extrapolation parameters had the greatest impact on results (application of Weibull regression function to OS for afatinib and lognormal regression for erlotinib led to an increased ICER of EUR62,546 per QALY gained). The inclusion of post-progression treatments during scenario analysis led to an increased ICER of EUR37,338 per QALY gained. Application of investigator assessed PFS functions resulted in only modest changes with application of loglogistic regression for afatinib and erlotinib increasing the ICER to EUR31,025 per QALY gained. A decreased ICER of EUR20,919 per QALY gained was evaluated when a lognormal regression was applied to the OS function for afatinib and erlotinib. DSA evaluated the main driver of outcomes to be PFS utility with a low value of EUR27,263 per QALY gained and a high value of EUR30,973 per QALY gained (Figure 2). Post-progression survival utility had the second greatest influence on model outcomes ranging between EUR32,440 and EUR29,759 per QALY gained. Variation in monthly follow-up costs produced ICERs between EUR29,266 and EUR31,310 per QALY gained and had the third largest impact on outcomes (Table S5). Variation in inputs including afatinib grade 3 stomatitis frequency, afatinib grade 3 nausea frequency, afatinib grade 4 diarrhea frequency and post-progression monthly follow-up costs had little impact on cost-effectiveness outcomes (Figure 2).
Figure 2.
Tornado diagram of DSA results.
Abbreviations: DSA, deterministic sensitivity analysis; QALY, quality-adjusted life year.
Additional sensitivity analyses were conducted to determine the level of uncertainty generated by variation in other parameters including: time horizon, post-progression treatment use, health state utility data source, survival extrapolations and the adverse event consideration method (Table S4). Variation of time horizon had the biggest impact on cost-effectiveness with ICERs of EUR55,064 per QALY gained at 1 year and EUR30,123 per QALY gained at 5 years. Altering the health state utilities included in the analysis to match National Institute for Health and Clinical Excellence (NICE) Nivolumab Guidance resulted in a decrease in the ICER to EUR27,315 per QALY gained while altering the utilities included to those reported by Nafees et al,27 increased the ICER to EUR31,609 per QALY gained.
PSA
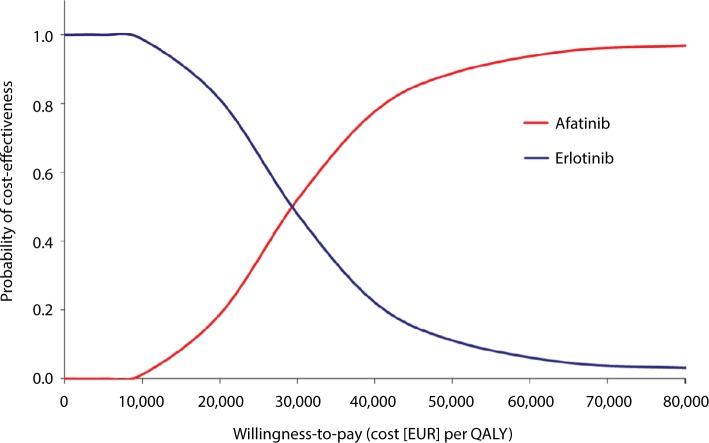
Scatter plot analysis of PSA with 5,000 iterations showed the majority of data points were in the upper right quadrant of the cost-effectiveness plane, indicating that treatment with afatinib was more costly but had greater clinical benefit than erlotinib (Figure 3). A mean ICER of EUR29,164 per QALY gained (95% CI [27,697; 30,632]) was evaluated. The acceptability curve generated (Figure 4) from the same data demonstrated that afatinib had a probability of 97% of being cost-effective at a willingness-to-pay threshold of EUR70,000 per QALY gained (at a threshold of EUR30,000 per QALY gained the probability of afatinib being cost-effective was 52%).
Figure 3.
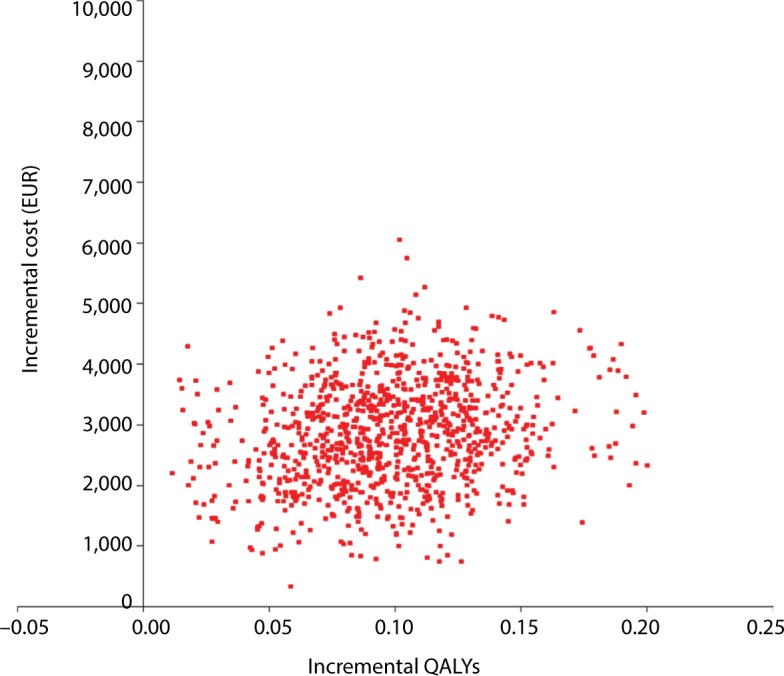
PSA outcomes, cost-effectiveness plane.
Note: Willingness-to-pay (cost per QALY) for afatinib.
Abbreviations: PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year.
Figure 4.
PSA outcomes, cost-effectiveness acceptability curve.
Note: Willingness-to-pay (cost per QALY) for afatinib versus erlotinib.
Abbreviations: PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year.
Discussion
The present analysis indicated that afatinib is cost-effective versus erlotinib after platinum-based therapy for advanced squamous NSCLC, with ICERs of EUR18,568 per life year gained and EUR30,277 per QALY gained. Direct costs of afatinib were calculated to be EUR12,364 versus EUR9,510 for erlotinib, a difference of EUR2,854, over the 10-year modeling analysis. Assuming a willingness-to-pay threshold of EUR30,000 per QALY gained, the probability of afatinib being cost-effective was 52%. At a threshold of EUR70,000 per QALY gained, the probability of afatinib being cost-effective versus erlotinib was estimated to be 97%.
In addition to PSA, the main drivers of model outcomes were identified using sensitivity analysis (comprised of DSA and scenario analyses). Variation in the PFS and OS extrapolation parameters had the greatest impact on results, with application of a Weibull regression function to OS for afatinib and lognormal regression for erlotinib leading to an ICER of approximately EUR62,546 per QALY gained. From the DSA, variation in adverse event rates, in particular grade 3 diarrhea had the greatest impact on outcomes with ICERs ranging from EUR24,948 to EUR26,851 per QALY gained for afatinib versus erlotinib. The majority of parameters assessed using DSA did not significantly influence the model outcomes, indicating a low level of uncertainty in the model results.
To determine the generalizability of the findings from the present analysis to the French population, the characteristics of patients from the LUX-Lung 8 trial were compared with data extracted from epidemiological studies in French patients with NSCLC stage IIIb/IV.19 Characteristics were found to be comparable in terms of gender (>80% men), mean age (65 years in LUX-Lung 8 trial and 66 years in extracted data) and smoking status (>90% were smokers or former smokers in both). In the LUX-Lung 8 trial, the results in terms of PFS and OS were consistent across all the tested subgroups showing efficacy results in favor of afatinib, suggesting that the outcomes may well be similar in populations where baseline characteristics are not identical to those modeling the present base case analysis. Patient characteristics in the LUX-Lung 8 trial were comparable to those of the recently published PEPiTA study: a real-world observational study of stage IIIB/IV squamous NSCLC, providing additional evidence that the finding of the present analysis may well be generalizable to the French population.28
LUX-Lung 8 is currently the largest prospective trial comparing two established tyrosine kinase inhibitors for second-line treatment of patients with squamous cell carcinoma of the lung. It therefore could be considered to make an appropriate basis for health economic analysis in second-line therapy. However, it could also be considered to represent a potential limitation of the analysis as other second-line treatments, such as docetaxel, are not taken into consideration. A 2014 meta-analysis of trials that assessed second-line treatment with EGFR tyrosine kinase inhibitors versus chemotherapy demonstrated better tolerability in the EGFR tyrosine kinase group and confirmed comparable OS between groups, both in unselected patients with NSCLC and in an EGFR wild-type population.29 Along with observation that gefitinib and ceritinib are not currently recommended for second-line therapy by the European Society for Medical Oncology (ESMO), and with ceritinib indicated for ALK translocation and not EGFR mutation tumor types, these data indicated that erlotinib can be considered to be the current standard of care and the most appropriate comparator for the present analysis.
As additional evidence on afatinib becomes available, including synthesized evidence such as indirect comparison and meta-analysis, it will be important to confirm the finding of the present health economic analysis based on single study, albeit currently representing the best available evidence. In addition, future studies could address some of the limitations of the present analysis. For example, in the base case adverse event rates were not captured in the post-progression state nor was the use of post-progression therapies. Although there is no evidence to suggest that inclusion of these costs (and the potential impact on quality of life) would substantially alter the findings of the base case analysis, they may offer a more complete estimate of the overall direct costs associated with second-line therapy. The present study did not capture treatment discontinuation due to adverse events. It is noteworthy, however, that treatment discontinuation can reduce both costs and effectiveness and therefore may have little impact on overall cost-effectiveness. In the absence of detailed follow-up data on discontinued patients, it is challenging to accurately model the impact of treatment discontinuation in any health economic analysis.
Conclusion
The increased duration of both PFS and post-progression survival observed with afatinib makes it an attractive second-line therapy for patients with advanced squamous NSCLC in comparison to erlotinib. Improved PFS requires a longer period of therapy and therefore increased costs associated with afatinib therapy making cost-effectiveness analysis important. The present analysis showed that afatinib was likely to be cost-effective by generally accepted standards versus erlotinib with a base case ICER of EUR30,277 per QALY gained and a probability of 97% of being cost-effective at a willingness-to-pay threshold of EUR70,000 per QALY gained.
Supplementary materials
Table S1.
Drug acquisition costs
| Product | Dosage | Price per package (including all taxes) (EUR) | Dispensing fee (EUR) | Monthly treatment cost (EUR) |
|---|---|---|---|---|
| Compared treatments | ||||
| Afatinib (GIOTRIF®; Boehringer Ingelheim, Ingelheim, Germany) 40 mg; 28 tablets CIP: 3400927565878 |
40 mg/day orally | 1,870.48 | 1.02/pack | 2,033.04 |
| Erlotinib (TARCEVA®; Roche, Basel, Switzerland) 150 mg; 30 tablets CIP: 3400936923522 |
150 mg/day orally | 2,071.92 | 1.02/pack | 2.072.94 |
| Post-progression treatments for additional sensitivity analyses | ||||
| Nivolumab (OPDIVO®; Bristo-Myers Squibb, New York, NY, USA) 10 mg/mL; vial of 10 mL* |
3 mg/kg every 2 weeks intravenously until death | 1,344.33 | N/A | 8,757.66 |
| Docetaxel | 75 mg/m2 every 3 weeks intravenously | Cost included in DRG cost | Cost included in DRG cost | Cost included in DRG cost |
| Paclitaxel | 200 mg/m2 every 3 weeks intravenously | Cost included in DRG cost | Cost included in DRG cost | Cost included in DRG cost |
| Best supportive care | N/A | N/A | N/A | N/A |
Note:
UK cost estimated in euros using an exchange rate of 1.19474 (July 2016).
Abbreviations: CIP, Club Inter Pharmaceutique (ID code for pharmaceuticals in France); DRG, diagnosis-related group; N/A, not applicable.
Table S2.
Unit costs of resources
| Resource | Data source | Unit cost (EUR, 2016) |
|---|---|---|
| Medical follow-up (with excess fees) | 45.88 | |
| Specialist visit (oncologist or lung specialist) | French medical insurance and physician fees (updated to 2016 using a CPI).1,2 | 45.88 |
| Blood tests | 52.11 | |
| Sedimentation rate | TNB 11245 | 1.89 |
| C-reactive protein | TNB 18045 | 2.70 |
| Blood count (with platelets) | TNB 11045 | 7.83 |
| Creatinine with GFR estimation | TNB 05925 | 1.89 |
| Serum electrolytes | TNB 16105 | 7.29 |
| Liver function tests | 9.45 | |
| Transaminases (ALT, AST) | TNB 05225 | 2.97 |
| Alkaline phosphatase | TNB 05145 | 1.89 |
| GammaGT | TNB 05195 | 1.89 |
| Total bilirubin | TNB 16105 | 2.70 |
| Hemostasis screening tests | 21.06 | |
| Quick’s time | TNB 01255 | 5.40 |
| Activated cephalin time | TNB 11275 | 5.40 |
| Fibrinogen concentrate | TNB 01745 | 4.86 |
| Bleeding time | TNB 01715 | 5.40 |
| Sampling act by direct venipuncture | NGAP-Titre XVI, chapter 17 | 4.73 |
| Security package for management of a blood sample | TNB 91055 | 1.35 |
| Package of pre-analytical management of a blood sample | TNB 90055 | 4.05 |
| Imaging tests | 70.49 | |
| Chest radiography | CCAM LJQK0026 | 45.22 |
| CT scan with injection | CCAM ZBQH0016 | 25.27 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CCAM, Classification Commune des Actes Médicaux [general classification of medical procedures]; CPI, consumer price index; CT, computed tomography; GFR, glomerular filtration rate; NGAP, nomenclature générale des actes professionnels [general nomenclature for professional procedures]; TNB, Table Nationale de codage de biologie [national table for diagnostic codes].
Table S3.
Parameters included in the DSA
| Parameters | Base case value | Low value | High value | Justification of tested values |
|---|---|---|---|---|
| Discounting (%) | 4 | 0 | 6 | HAS recommendations |
| Safety (reported probability of event) | ||||
| Afatinib – diarrhea grade 3 | 0.099 | 0.071 | 0.130 | 95% CI |
| Afatinib – diarrhea grade 4 | 0.005 | 0.001 | 0.014 | 95% CI |
| Afatinib – rash/acne | 0.059 | 0.038 | 0.084 | 95% CI |
| Afatinib – stomatitis grade 3 | 0.041 | 0.024 | 0.063 | 95% CI |
| Afatinib – fatigue grade 3 | 0.015 | 0.005 | 0.029 | 95% CI |
| Afatinib – nausea grade 3 | 0.010 | 0.003 | 0.022 | 95% CI |
| Erlotinib – diarrhea grade 3 | 0.023 | 0.011 | 0.040 | 95% CI |
| Erlotinib – diarrhea grade 4 | 0.003 | 0.0001 | 0.010 | 95% CI |
| Erlotinib – rash/acne | 0.104 | 0.076 | 0.136 | 95% CI |
| Erlotinib – fatigue grade 3 | 0.018 | 0.007 | 0.033 | 95% CI |
| Erlotinib – nausea grade 3 | 0.008 | 0.002 | 0.019 | 95% CI |
| Utilities and disutilities | ||||
| Progression-free survival utility | 0.65 | 0.63 | 0.75 | Low value: low value of 95% CI of two data sources (LUX-Lung 8 trial3 and Nafees et al4) High value: high value of 95% CI of LUX-Lung 8 trial3 data source |
| Post-progression survival utility | 0.58 | 0.47 | 0.61 | Low value: Nafees et al4 High value: high value of 95% CI of LUX-Lung 8 trial3 data source |
| Diarrhea grade 3 disutility | 0.050 | 0.019 | 0.096 | 95% CI |
| Diarrhea grade 4 disutility | 0.050 | 0.019 | 0.096 | 95% CI |
| Rash/acne disutility | 0.030 | 0.004 | 0.080 | 95% CI |
| Stomatitis grade 3 disutility | 0.131 | 0.112 | 0.151 | 95% CI |
| Fatigue grade 3 disutility | 0.070 | 0.036 | 0.114 | 95% CI |
| Nausea grade 3 disutility | 0.050 | 0.019 | 0.096 | 95% CI |
| Costs (EUR) | ||||
| Mean transportation cost per round-trip | 77.06 | 64.76 | 89.41 | Low value: estimated VSL cost High value: estimated taxi cost |
| Unit cost for diarrhea grade 3 | 2,801.76 | 1,813.15 | 4,002.05 | 95% CI |
| Unit cost for diarrhea grade 4 | 2,801.76 | 1,813.15 | 4,002.05 | 95% CI |
| Unit cost for rash/acne | 225.67 | 146.04 | 322.35 | 95% CI |
| Unit cost for stomatitis grade 3 | 569.44 | 303.80 | 670.56 | 95% CI |
| Unit cost for fatigue grade 3 | 570.57 | 369.24 | 815.01 | 95% CI |
| Unit cost for nausea grade 3 | 1,997.00 | 1,292.35 | 2,852.52 | 95% CI |
| Monthly monitoring costs in pre-progression and post-progression for all arms | 253.63 | 202.90 | 304.36 | ±20% |
Abbreviations: CI, confidence interval; DSA, deterministic sensitivity analysis; HAS, Haute Autorité de Santé [French National Authority for Health]; VSL, véhicule sanitaire léger [ambulance transport].
Table S4.
Summarized results from the additional sensitivity analyses in comparison to the base case
| Parameters | Base case | Additional sensitivity analyses | Results (EUR per QALY gained) |
|---|---|---|---|
| Time horizon | 10 years | 1 year | 55,064 |
| 2 years | 33,947 | ||
| 5 years | 30,123 | ||
| Post-progression treatments included | No | Yes | 37,338 |
| Progression-free survival function | Afatinib independent review – loglogistic Erlotinib independent review – loglogistic |
Afatinib independent review – Weibull Erlotinib independent review – Weibull |
24,842 |
| Afatinib independent review – lognormal Erlotinib independent review – lognormal |
29,876 | ||
| Afatinib investigator choice – Weibull Erlotinib investigator choice – Weibull |
25,687 | ||
| Afatinib investigator choice – loglogistic Erlotinib investigator choice – loglogistic |
31,025 | ||
| Afatinib investigator choice – lognormal Erlotinib investigator choice – lognormal |
29,291 | ||
| Overall survival function | Afatinib: Weibull Erlotinib: Weibull |
Afatinib: lognormal Erlotinib: lognormal (extreme scenario) |
20,919 |
| Afatinib: Weibull Erlotinib: lognormal (extreme scenario) |
62,546 | ||
| Health state utility data source | LUX-Lung 83 | NICE Nivolumab guidance | 27,315 |
| Nafees et al27 | 31,609 | ||
| AE consideration method | Considering a distribution of the probability of each AE over the time spent in pre- progression | Considering that the probability of each AE is applied one time at the first cycle of the model for all patients | 29,040 |
Abbreviations: AE, adverse event; NICE, National Institute for Health and Care Excellence; QALY, quality-adjusted life year.
Table S5.
ICER for low and high values for parameters included in the DSA
| Main parameters (base case: EUR30,277 per QALY gained) | Low value | High value | Low cost per QALY gained (EUR per QALY gained) | High cost per QALY gained (EUR per QALY gained) |
|---|---|---|---|---|
| Progression-free survival utility | 0.63 | 0.75 | 30,973 | 27,463 |
| Post-progression survival utility | 0.47 | 0.61 | 32,440 | 29,759 |
| Afatinib diarrhea grade 3 frequency | 0.071 | 0.130 | 29,378 | 31,310 |
| Monthly follow-up costs | EUR202.90 | EUR304.36 | 29,266 | 31,288 |
| Erlotinib diarrhea grade 3 frequency | 0.011 | 0.040 | 30,675 | 29,733 |
| Diarrhea grade 3 cost | EUR1,813.15 | EUR4,002.05 | 29,832 | 30,722 |
| Afatinib stomatitis grade 3 frequency | 0.024 | 0.063 | 30,041 | 30,577 |
| Afatinib diarrhea grade 4 frequency | 0.001 | 0.014 | 30,133 | 30,572 |
| Afatinib nausea grade 3 frequency | 0.003 | 0.022 | 30,100 | 30,568 |
| Discounting | 0% | 6% | 30,000 | 30,432 |
Abbreviations: DSA, deterministic sensitivity analysis; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
References
- 1.National Healthcare Insurance Online (AMELI) Activity of physicians by specialty and region. 2014. [Accessed October 6, 2017]. [cited Feburary 9, 2016]. Available from: https://www.ameli.fr/fileadmin/user_upload/documents/Activ-ite_des_medecins_par_departement_en_2014.xls. French.
- 2.National Healthcare Insurance Online (AMELI) Salaries of health-care professionals, by specialty and region. 2014. [Accessed October 6, 2017]. [cited February 9, 2016]. Available from: https://www.ameli.fr/fileadmin/user_upload/documents/Honoraires_des_professionnels_de_sante_APE_par_region_en_2014.xls. French.
- 3.Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as secondline treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16(8):897–907. doi: 10.1016/S1470-2045(15)00006-6. [DOI] [PubMed] [Google Scholar]
- 4.Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. doi: 10.1186/1477-7525-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Healthcare Insurance for Employees (CNAMTS) National table for diagnostic codes. 2016. [Accessed October 6, 2017]. [cited October 5, 2016]. Available from: http://www.codage.ext.cnamts.fr/codif/nabm/index.php?p_site=AMELI. French.
- 6.National Healthcare Insurance Classification of medical procedures Version 43. 2016. [Accessed October 6, 2017]. [cited January 10, 2017]. Available from: http://www.ameli.fr/accueil-de-la-ccam/telechargement/version-actuelle/index.php. French.
- 7.National Healthcare Insurance Online (AMELI) General nomenclature for professional procedures (NGAP) [Accessed October 6, 2017]. Version dated March 11, 2015 [cited October 5, 2016]. Available from: http://www.ameli.fr/professionnels-de-sante/directeurs-d-etablissements-de-sante/codage/ngap.php. French.
Acknowledgments
Grant support: the analysis was supported by funding from Boehringer Ingelheim.
Footnotes
Disclosure
MP and SR are employees of HEVA-HEOR, which received financial support from Boehringer Ingelheim to perform the health economic analysis. CC has received consulting fees from Boehringer Ingelheim. KLL and JG are employees of Boehringer Ingelheim, manufacturer of afatinib. CMC is an employee of Ossian Health Economics and Communications, which received consulting fees from HEVA-HEOR. The authors report no other conflicts of interest in this work.
References
- 1.Le Chevalier T. Adjuvant chemotherapy for resectable non-small-cell lung cancer: where is it going? Ann Oncol. 2010;21(suppl 7):vii196–vii198. doi: 10.1093/annonc/mdq376. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16(3):481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society Detailed Guide: Lung Cancer (Non-Small Cell) 2017. [Accessed October 6, 2017]. [cited October 6, 2017]. Available from: https://www.cancer.org/cancer/non-small-cell-lung-cancer.html.
- 4.Leighl NB. Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol. 2012;19(suppl 1):S52–S58. doi: 10.3747/co.19.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Research UK. Lung Cancer Survival Statistics. 2016. [Accessed October 4, 2016]. [cited October 4, 2016]. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/survival.
- 6.Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(25):2969–2979. doi: 10.1200/JCO.2016.66.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calikusu Z, Yildirim Y, Akcali Z, et al. The effect of HER2 expression on cisplatin-based chemotherapy in advanced non-small cell lung cancer patients. J Exp Clin Cancer Res. 2009;28:97. doi: 10.1186/1756-9966-28-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Consensus Guidelines ESMO. Non-Small-Cell Lung Cancer First-Line/Second and Further Lines in Advanced Disease. 2014. [Accessed October 4, 2016]. [cited October 4, 2016]. Available from: http://www.esmo.org/Guidelines/Lung-and-Chest-Tumours/Consensus-Guidelines-Non-small-cell-lung-cancer-first-line-second-and-further-lines-in-advanced-disease.
- 9.Hoelzer D, Bassan R, Dombret H, et al. Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v69–v82. doi: 10.1093/annonc/mdw025. [DOI] [PubMed] [Google Scholar]
- 10.Shu CA, Rizvi NA. Into the clinic with nivolumab and pembrolizumab. Oncologist. 2016;21:527–528. doi: 10.1634/theoncologist.2016-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 13.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 14.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 15.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 16.Soria J-C, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16(8):897–907. doi: 10.1016/S1470-2045(15)00006-6. [DOI] [PubMed] [Google Scholar]
- 17.Modjtahedi H, Cho BC, Michel MC, Solca F. A comprehensive review of the preclinical efficacy profile of the ErbB family blocker afatinib in cancer. Naunyn Schmiedebergs Arch Pharmacol. 2014;387(6):505–521. doi: 10.1007/s00210-014-0967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsh V. Next-generation covalent irreversible kinase inhibitors in NSCLC: focus on afatinib. BioDrugs. 2015;29(3):167–183. doi: 10.1007/s40259-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantar Health Database. [Accessed January 1, 2017]. [cited January 1, 2017]. Available from: http://www.kantarhealth.com/services/epidemiology.
- 20.Technical Agency for Information on Hospitalization (ATIH) Analysis of hospital activity 2015. 2015. [Accessed October 10, 2017]. [cited February 24, 2017]. Available from http://www.atih.sante.fr/sites/default/files/public/content/3020/atih_synthese_activite_hospitaliere_2015_0.pdf. French.
- 21.Court of Audit Social Insurance 2012. Patient transports reimbursed by healthcare insurance. 2012. [Accessed October 6, 2017]. [cited October 5, 2016]. Available from: https://www.ccomptes.fr/fr/documents/23069 Cited September 13, 2012. French.
- 22.National Healthcare Insurance for Employees (CNAMTS) National table for diagnostic codes. 2016. [Accessed October 6, 2017]. [cited October 5, 2016]. Available from: http://www.codage.ext.cnamts.fr/codif/nabm/index.php?p_site=AMELI. French.
- 23.National Healthcare Insurance Online (AMELI) General nomenclature for professional procedures (NGAP) [Accessed October 6, 2017]. Version dated March 11, 2015 [cited October 5, 2016. Available from: http://www.ameli.fr/professionnels-de-sante/directeurs-d-etablissements-de-sante/codage/ngap.php. French.
- 24.Ameli.fr Nomenclature générale des actes professionnels – NGAP. [Accessed October 6, 2017]. Version dated March 11, 2015 [cited October 5, 2016]. Available from: http://www.ameli.fr/professionnels-de-sante/directeurs-d-etablissements-de-sante/codage/ngap.php.
- 25.Banz K, Bischoff H, Brunner M, et al. Comparison of treatment costs of grade 3/4 adverse events associated with erlotinib or pemetrexed maintenance therapy for patients with advanced non-small-cell lung cancer (NSCLC) in Germany, France, Italy, and Spain. Lung Cancer. 2011;74(3):529–534. doi: 10.1016/j.lungcan.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Chevalier J, de Pouvourville G. Valuing EQ-5D using time trade-off in France. Eur J Health Econ. 2013;14(1):57–66. doi: 10.1007/s10198-011-0351-x. [DOI] [PubMed] [Google Scholar]
- 27.Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. doi: 10.1186/1477-7525-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monnet I, Audigier-Valette C, Girard N, et al. Real-life effectiveness of erlotinib as second-line treatment of stage IIIB/IV squamous non-small cell lung cancer: results of the PEPiTA observational study. Lung Cancer. 2016;98:84–90. doi: 10.1016/j.lungcan.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Li N, Yang L, Ou W, Zhang L, Zhang SL, Wang SY. Meta-analysis of EGFR tyrosine kinase inhibitors compared with chemotherapy as second-line treatment in pretreated advanced non-small cell lung cancer. PLoS One. 2014;9:e102777. doi: 10.1371/journal.pone.0102777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Drug acquisition costs
| Product | Dosage | Price per package (including all taxes) (EUR) | Dispensing fee (EUR) | Monthly treatment cost (EUR) |
|---|---|---|---|---|
| Compared treatments | ||||
| Afatinib (GIOTRIF®; Boehringer Ingelheim, Ingelheim, Germany) 40 mg; 28 tablets CIP: 3400927565878 |
40 mg/day orally | 1,870.48 | 1.02/pack | 2,033.04 |
| Erlotinib (TARCEVA®; Roche, Basel, Switzerland) 150 mg; 30 tablets CIP: 3400936923522 |
150 mg/day orally | 2,071.92 | 1.02/pack | 2.072.94 |
| Post-progression treatments for additional sensitivity analyses | ||||
| Nivolumab (OPDIVO®; Bristo-Myers Squibb, New York, NY, USA) 10 mg/mL; vial of 10 mL* |
3 mg/kg every 2 weeks intravenously until death | 1,344.33 | N/A | 8,757.66 |
| Docetaxel | 75 mg/m2 every 3 weeks intravenously | Cost included in DRG cost | Cost included in DRG cost | Cost included in DRG cost |
| Paclitaxel | 200 mg/m2 every 3 weeks intravenously | Cost included in DRG cost | Cost included in DRG cost | Cost included in DRG cost |
| Best supportive care | N/A | N/A | N/A | N/A |
Note:
UK cost estimated in euros using an exchange rate of 1.19474 (July 2016).
Abbreviations: CIP, Club Inter Pharmaceutique (ID code for pharmaceuticals in France); DRG, diagnosis-related group; N/A, not applicable.
Table S2.
Unit costs of resources
| Resource | Data source | Unit cost (EUR, 2016) |
|---|---|---|
| Medical follow-up (with excess fees) | 45.88 | |
| Specialist visit (oncologist or lung specialist) | French medical insurance and physician fees (updated to 2016 using a CPI).1,2 | 45.88 |
| Blood tests | 52.11 | |
| Sedimentation rate | TNB 11245 | 1.89 |
| C-reactive protein | TNB 18045 | 2.70 |
| Blood count (with platelets) | TNB 11045 | 7.83 |
| Creatinine with GFR estimation | TNB 05925 | 1.89 |
| Serum electrolytes | TNB 16105 | 7.29 |
| Liver function tests | 9.45 | |
| Transaminases (ALT, AST) | TNB 05225 | 2.97 |
| Alkaline phosphatase | TNB 05145 | 1.89 |
| GammaGT | TNB 05195 | 1.89 |
| Total bilirubin | TNB 16105 | 2.70 |
| Hemostasis screening tests | 21.06 | |
| Quick’s time | TNB 01255 | 5.40 |
| Activated cephalin time | TNB 11275 | 5.40 |
| Fibrinogen concentrate | TNB 01745 | 4.86 |
| Bleeding time | TNB 01715 | 5.40 |
| Sampling act by direct venipuncture | NGAP-Titre XVI, chapter 17 | 4.73 |
| Security package for management of a blood sample | TNB 91055 | 1.35 |
| Package of pre-analytical management of a blood sample | TNB 90055 | 4.05 |
| Imaging tests | 70.49 | |
| Chest radiography | CCAM LJQK0026 | 45.22 |
| CT scan with injection | CCAM ZBQH0016 | 25.27 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CCAM, Classification Commune des Actes Médicaux [general classification of medical procedures]; CPI, consumer price index; CT, computed tomography; GFR, glomerular filtration rate; NGAP, nomenclature générale des actes professionnels [general nomenclature for professional procedures]; TNB, Table Nationale de codage de biologie [national table for diagnostic codes].
Table S3.
Parameters included in the DSA
| Parameters | Base case value | Low value | High value | Justification of tested values |
|---|---|---|---|---|
| Discounting (%) | 4 | 0 | 6 | HAS recommendations |
| Safety (reported probability of event) | ||||
| Afatinib – diarrhea grade 3 | 0.099 | 0.071 | 0.130 | 95% CI |
| Afatinib – diarrhea grade 4 | 0.005 | 0.001 | 0.014 | 95% CI |
| Afatinib – rash/acne | 0.059 | 0.038 | 0.084 | 95% CI |
| Afatinib – stomatitis grade 3 | 0.041 | 0.024 | 0.063 | 95% CI |
| Afatinib – fatigue grade 3 | 0.015 | 0.005 | 0.029 | 95% CI |
| Afatinib – nausea grade 3 | 0.010 | 0.003 | 0.022 | 95% CI |
| Erlotinib – diarrhea grade 3 | 0.023 | 0.011 | 0.040 | 95% CI |
| Erlotinib – diarrhea grade 4 | 0.003 | 0.0001 | 0.010 | 95% CI |
| Erlotinib – rash/acne | 0.104 | 0.076 | 0.136 | 95% CI |
| Erlotinib – fatigue grade 3 | 0.018 | 0.007 | 0.033 | 95% CI |
| Erlotinib – nausea grade 3 | 0.008 | 0.002 | 0.019 | 95% CI |
| Utilities and disutilities | ||||
| Progression-free survival utility | 0.65 | 0.63 | 0.75 | Low value: low value of 95% CI of two data sources (LUX-Lung 8 trial3 and Nafees et al4) High value: high value of 95% CI of LUX-Lung 8 trial3 data source |
| Post-progression survival utility | 0.58 | 0.47 | 0.61 | Low value: Nafees et al4 High value: high value of 95% CI of LUX-Lung 8 trial3 data source |
| Diarrhea grade 3 disutility | 0.050 | 0.019 | 0.096 | 95% CI |
| Diarrhea grade 4 disutility | 0.050 | 0.019 | 0.096 | 95% CI |
| Rash/acne disutility | 0.030 | 0.004 | 0.080 | 95% CI |
| Stomatitis grade 3 disutility | 0.131 | 0.112 | 0.151 | 95% CI |
| Fatigue grade 3 disutility | 0.070 | 0.036 | 0.114 | 95% CI |
| Nausea grade 3 disutility | 0.050 | 0.019 | 0.096 | 95% CI |
| Costs (EUR) | ||||
| Mean transportation cost per round-trip | 77.06 | 64.76 | 89.41 | Low value: estimated VSL cost High value: estimated taxi cost |
| Unit cost for diarrhea grade 3 | 2,801.76 | 1,813.15 | 4,002.05 | 95% CI |
| Unit cost for diarrhea grade 4 | 2,801.76 | 1,813.15 | 4,002.05 | 95% CI |
| Unit cost for rash/acne | 225.67 | 146.04 | 322.35 | 95% CI |
| Unit cost for stomatitis grade 3 | 569.44 | 303.80 | 670.56 | 95% CI |
| Unit cost for fatigue grade 3 | 570.57 | 369.24 | 815.01 | 95% CI |
| Unit cost for nausea grade 3 | 1,997.00 | 1,292.35 | 2,852.52 | 95% CI |
| Monthly monitoring costs in pre-progression and post-progression for all arms | 253.63 | 202.90 | 304.36 | ±20% |
Abbreviations: CI, confidence interval; DSA, deterministic sensitivity analysis; HAS, Haute Autorité de Santé [French National Authority for Health]; VSL, véhicule sanitaire léger [ambulance transport].
Table S4.
Summarized results from the additional sensitivity analyses in comparison to the base case
| Parameters | Base case | Additional sensitivity analyses | Results (EUR per QALY gained) |
|---|---|---|---|
| Time horizon | 10 years | 1 year | 55,064 |
| 2 years | 33,947 | ||
| 5 years | 30,123 | ||
| Post-progression treatments included | No | Yes | 37,338 |
| Progression-free survival function | Afatinib independent review – loglogistic Erlotinib independent review – loglogistic |
Afatinib independent review – Weibull Erlotinib independent review – Weibull |
24,842 |
| Afatinib independent review – lognormal Erlotinib independent review – lognormal |
29,876 | ||
| Afatinib investigator choice – Weibull Erlotinib investigator choice – Weibull |
25,687 | ||
| Afatinib investigator choice – loglogistic Erlotinib investigator choice – loglogistic |
31,025 | ||
| Afatinib investigator choice – lognormal Erlotinib investigator choice – lognormal |
29,291 | ||
| Overall survival function | Afatinib: Weibull Erlotinib: Weibull |
Afatinib: lognormal Erlotinib: lognormal (extreme scenario) |
20,919 |
| Afatinib: Weibull Erlotinib: lognormal (extreme scenario) |
62,546 | ||
| Health state utility data source | LUX-Lung 83 | NICE Nivolumab guidance | 27,315 |
| Nafees et al27 | 31,609 | ||
| AE consideration method | Considering a distribution of the probability of each AE over the time spent in pre- progression | Considering that the probability of each AE is applied one time at the first cycle of the model for all patients | 29,040 |
Abbreviations: AE, adverse event; NICE, National Institute for Health and Care Excellence; QALY, quality-adjusted life year.
Table S5.
ICER for low and high values for parameters included in the DSA
| Main parameters (base case: EUR30,277 per QALY gained) | Low value | High value | Low cost per QALY gained (EUR per QALY gained) | High cost per QALY gained (EUR per QALY gained) |
|---|---|---|---|---|
| Progression-free survival utility | 0.63 | 0.75 | 30,973 | 27,463 |
| Post-progression survival utility | 0.47 | 0.61 | 32,440 | 29,759 |
| Afatinib diarrhea grade 3 frequency | 0.071 | 0.130 | 29,378 | 31,310 |
| Monthly follow-up costs | EUR202.90 | EUR304.36 | 29,266 | 31,288 |
| Erlotinib diarrhea grade 3 frequency | 0.011 | 0.040 | 30,675 | 29,733 |
| Diarrhea grade 3 cost | EUR1,813.15 | EUR4,002.05 | 29,832 | 30,722 |
| Afatinib stomatitis grade 3 frequency | 0.024 | 0.063 | 30,041 | 30,577 |
| Afatinib diarrhea grade 4 frequency | 0.001 | 0.014 | 30,133 | 30,572 |
| Afatinib nausea grade 3 frequency | 0.003 | 0.022 | 30,100 | 30,568 |
| Discounting | 0% | 6% | 30,000 | 30,432 |
Abbreviations: DSA, deterministic sensitivity analysis; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.