Abstract
The rat is a commonly used model for the study of lower urinary tract function before and after spinal cord injury. We have previously reported that in unanesthetized freely moving rats, although phasic external urethral sphincter (EUS) activity (bursting) is most common during micturition, productive voiding can occur in the absence of bursting, which differs from results seen in anesthetized or unanesthetized restrained animals. The purpose of the present study was to characterize EUS behavior in unanesthetized, freely moving rats before and after mid-thoracic (T8) or thoraco-lumbar (T13-L1) spinal transection to determine how EUS behavior after spinal cord injury differs from that seen in anesthetized or unanesthetized restrained rats. Several abnormalities became evident that were comparable after transection at either level, including the following: repetitive non-voiding EUS contractions; increased prevalence, intensity, and duration of EUS bursting; decreased rate of urine evacuation during bursting; increased void size and decreased number of daily voids; shorter inter-burst silent period and increased frequency of bursting; and loss of the direct linear relationships that are evident in intact animals between void size and bursting silent period. These data suggest that transection-induced delayed initiation of EUS bursting allows co-contraction of the bladder and the EUS that prevents or limits urine evacuation, resulting in a detrusor-sphincter dyssynergia-like phenomenon. In addition, the higher-than-normal frequency at which EUS bursting occurs after transection is associated with shorter silent periods during which urine typically flows, which interferes with voiding by slowing the rate of urine evacuation. That results were comparable after either transection suggests that the central pattern generator responsible for EUS bursting is located caudal to the L1 spinal segment.
Keywords: : EMG, external urethral sphincter, implanted electrodes, longitudinal study, spinal cord
Introduction
The external urethral sphincter (EUS) muscle plays a vital role in lower urinary tract (LUT) function; it contracts to keep urine stored in the bladder and at the appropriate time and place for voiding, it relaxes to allow passage of urine through the urethra to the body exterior.1 Spinal reflexes regulated by multiple sites in the brain coordinate the activity of the EUS with that of the bladder detrusor muscle.2 Injury to the spinal cord interrupts supraspinal control of these reflexes, frequently resulting in voiding impairment.3,4 This impairment commonly manifests as bladder over-activity and detrusor-sphincter dyssynergia (DSD) in which the EUS fails to relax while the bladder contracts.4–6 DSD can result in urine retention, elevated bladder pressure, and vesicoureteral reflux, and if untreated, can cause renal failure.4,7
Studies using rats have contributed much to the current understanding of LUT function, despite an important difference in micturition reflexes between rats and humans; while EUS relaxation is required for efficient micturition in both human and rats, brief bursts of EUS muscle activity rapidly alternate with periods of relaxation during voiding in rats. This phasic activation (bursting) is thought to be essential for efficient voiding.8–10 Early studies of micturition reflexes elicited during bladder cystometry in urethane-anesthetized rats demonstrated that complete spinal transection abolishes EUS bursting.3,11 These rats exhibited tonic EUS activity instead of bursting during LUT reflexes, leading to the conclusion that rats develop a DSD-like pattern of EUS activation similar to that seen in humans after spinal cord injury (SCI). This belief persisted until subsequent studies showed that anesthesia was a confounding factor. At reduced levels of urethane anesthesia, EUS bursting after mid-thoracic spinal transection was seen occasionally.12 Studies in unanesthetized rats with SCI demonstrated that bursting also can occur during micturition, but was inconsistently expressed.13,14 Cystometric recordings in these studies were performed shortly after recovery from anesthesia for implantation surgery, which, given the influence of stress on LUT function,15–20 may have contributed to this variable expression of bursting in unanesthetized rats.
The finding that bursting can recover in rats with complete spinal transections demonstrated the existence of spinal circuitry that can function as a pattern generator for phasic EUS activity independent of supraspinal control. Bursting has been reported in anesthetized rats transected at T8-T9, but not at L3-L4, suggesting that the pattern generator is located between T8 and L4.21 Thus, the level of injury would be expected to influence the pattern of EUS activity after SCI.
The purpose of the present study was 1) to assess the extent to which rats recovered EUS bursting during voiding after SCI without the complications introduced by anesthesia, restraint, and post-surgical stress and 2) to determine the influence of the putative EUS bursting pattern generator on the recovery of urinary function after SCI. To accomplish this, we chronically implanted rats with electrodes for recording EUS electromyography (EMG), and subsequently recorded and analyzed this activity during spontaneous voiding over 24-h epochs from unanesthetized rats before and after two different levels of spinal cord transection. The lesions were originally planned for mid-thoracic and mid-lumbar levels to assess the role of the EUS bursting pattern generator on EUS activity patterns and urinary function after SCI, but technical difficulties forced the caudal lesions to be adjusted to the low-thoracic to upper-lumbar levels (thoraco-lumbar lesions; see “Spinal transections” in the Methods section for details).
Methods
Animal subjects
Subjects were 14 female Sprague-Dawley rats (8–12 weeks old at time of entry into the study) housed individually with a 12 h light/12 h dark cycle with food and water available ad libitum. All of these animals were part of a larger study of EUS activity during spontaneous voiding in rats with intact spinal cords.22 All animal procedures were in accord with the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (National Academy Press, Washington, DC, 2010), and had been reviewed and approved by the Wadsworth Center Institutional Animal Care and Use Committee.
Surgical preparation and peri-operative care
During all surgeries, aseptic technique was used and animals were deeply anesthetized with isoflurane gas (4% for induction, 1.5–2.5% for maintenance). Hydration during surgical procedures was maintained by subcutaneous saline administration. Local anesthetic (bupivacaine) and topical antibiotic ointment (nitrofurazone) were applied to all skin incisions after closure. For 5–10 days after implantation, animals received antibiotics (gentamicin 4 mg/kg, intraperitoneally [i.p.] twice daily) and penicillin (50,000 units/kg, i.p. every other day) and an analgesic (carprofen, 10 mg/kg, subcutaneously [s.c.] immediately after surgery, and subsequently 5 mg/kg s.c. or 1 mg p.o. [half of a 5-g tablet] twice daily). After transection, animals received antibiotics until the return of spontaneous bladder reflex function (see below). Transected animals also received a high-calorie nutrient supplement (Nutri-Cal) p.o. two times per day until the end of the study.
Each rat received two surgical procedures. The first procedure was for implantation of EUS EMG electrodes. After a 2- to 3-week recovery period and subsequent 24-h EUS EMG and urinary output recording sessions using spinal-intact rats, a second surgical procedure was performed for spinal cord transection, and the recordings were repeated. These procedures and recordings are described below.
EUS EMG electrode implantation
Rats were implanted with wires for recording EUS EMG as described in LaPallo and colleagues.22 Briefly, anesthetized rats received a midline incision over the skull through which EUS EMG electrodes were routed to a midline abdominal incision. The pubic bone was exposed by blunt dissection. The EUS EMG electrodes were passed through a pair of holes drilled in the pubic bone just lateral to the midline about 5 mm caudal to the rostral bone edge and secured with suture to a pair of holes drilled near the rostral bone edge. After flushing the surgical field with saline and closing the abdominal incision with absorbable suture and the skin with wound clips, the connector to which the EUS EMG wires connected was secured to the skull with bone screws and dental cement.
Spinal transections
Animals received either mid-thoracic or thoraco-lumbar complete spinal cord transections. For the mid-thoracic transection, rats were anesthetized and a 3-cm skin incision was made over the vertebral column extending caudally from the level of the caudal aspect of the scapulae. Bilateral incisions were made along the sides of the T7 to T9 spinous processes and the muscle and connective tissue were cleared from the vertebral laminae. After removing the T8 spinous process and lamina with rongeurs, the periosteum was carefully pulled from the surface of the dura and the dura was cut to expose the spinal cord. A drop of local anesthetic was placed on the spinal cord and after 3–4 minutes, it was transected with fine spring scissors. Visual observation of retraction of the cut ends of the spinal cord allowed confirmation of complete transection. After inserting a small piece of absorbable gelatin sponge (Gelfoam) between the cut ends of the spinal cord to control bleeding, the incised muscle over the transection area was closed with absorbable suture and the skin incision was closed with wound clips. The surgical site was flushed with sterile saline at each stage of the procedure. Analgesics and antibiotics were administered as described above.
The procedure for thoraco-lumbar transections differed from that of the mid-thoracic transections only in the location of the surgical site, laminectomy, and the spinal segmental location of the transection. The skin incision centered over the T12 vertebra and subsequent incision and clearance of muscle and connective tissue exposed the T11 to T13 vertebrae. The T12 spinous process and lamina were removed and the dura was incised along the midline and reflected. Initial transections of the lumbar spinal cord targeted to the L3-L4 level resulted in near-total gray matter atrophy and loss of EUS EMG activity. This was likely due to ischemia caused by interruption of the main arterial supply to the lumbar spinal cord, given that the most caudal ventral radicular artery enters the spinal cord typically at or rostral to L1, occasionally at L2, but never more caudally.23,24 Thus, subsequent transections were performed just cranial to the most caudal large thoraco-lumbar blood vessels, typically in the lower thoracic or upper lumbar spinal segment.
Spinal transection renders the LUT areflexive for a variable length of time. During this period, the bladders of rats were manually expressed two to three times per day until spontaneous micturition reflexes re-emerged and once per day thereafter. In transected rats, post-operative antibiotics were continued until the re-emergence of spontaneous micturition reflexes.
Urine output and EUS EMG recording
On recording days, rats were housed in a metabolic cage (Tecniplast) during 24-h sessions for recording EUS EMG and accumulated urine weight as described in LaPallo and colleagues.22 Briefly, a flexible armored cable attached to the pedestal mounted on the rat's skull connected the implanted wires to recording equipment via a commutator, which permitted the rat to move freely about its cage during the recording session. Urine and feces fell through the cage grid floor onto funnels that separated and diverted the urine into a cup mounted on a force transducer (FT03; Grass Technologies) that weighed the accumulating urine. Force oscillations were dampened with a mineral oil-filled dashpot. A small amount of mineral oil added to the collection cup prevented evaporation of the accumulating urine. Metabolic cage efficiency evaluated by applying saline aliquots of various amounts revealed that an average of 97% of the applied fluid was recovered below the cage. Void size was not adjusted for this small loss because the percent lost with each fluid application varied with location of the applied fluid.
EMG and force signals were routed to recording apparatus in an adjacent room. EMG data were band-pass filtered (10–300 Hz), digitized at 600 Hz, full-wave rectified digitally, down-sampled to 200 Hz by averaging sequences of three adjacent points, and stored. Urine weight recordings were low-pass filtered (12 Hz) to further remove oscillations from the signal. At the end of each experiment, rats were euthanized and the vertebral column with spinal cord surrounding the lesion site were stored in 4% paraformaldehyde in phosphate-buffered saline. Bladders were removed, cleaned, blotted dry, and weighed in six of seven rats in each of the two transection groups.
Data analysis
Urine weight and EUS EMG data were analyzed using custom software (Igor Pro, Wavemetrics). Power spectra of EMG signals were calculated using the fast Fourier transform (FFT) algorithm. Time-frequency spectrograms were obtained by repeating FFTs every 0.1 sec on overlapping 1-sec epochs of Hanning-windowed EMG data, and were corrected for temporal overlap of these repeated spectral calculations and for power loss due to windowing.
Although data were collected continuously in each 24-h session, analysis focused on voiding epochs. Voids were detected as a urine weight increase of at least 0.04 g. Void duration was calculated as the time during which urine flowed in each void, ignoring intra-void pauses in flow (see LaPallo and colleagues22 for details of addressing voids containing late-falling drops). Determination of EUS EMG properties during voiding was based on the onset and offset of urine accumulation (as determined above) and the pattern of EUS EMG. Because EUS bursting was not consistently evident during voiding, the transition from guarding to voiding (defined as EUS EMG onset) was calculated using an empirical method based on spectral analysis of EMG activity during voids that was validated using voids with visually identified EUS bursting.22 Voiding EUS EMG offset was defined as the EUS EMG onset time plus void duration.
The following void-associated EMG properties were calculated between void EMG onset and void EMG offset: 1) average EMG amplitude; bursting power (Pburst), defined as the average signal power in the EUS bursting-frequency range expressed as a percentage of the total average power; 2) low-frequency bursting power (P3-5), defined as the average EUS EMG power in the 3- to 5-Hz range expressed as a percentage of the total average power; 3) high-frequency bursting power (P8-12), defined as the average EUS EMG power in the 8- to 12-Hz range expressed as a percentage of the total average power; 4) bursting time, defined as the cumulative time during which the bursting power was above a threshold value defined as the mean Pburst in amplitude-matched non-voiding epochs +2 × standard deviations (SD) of amplitude-matched 2-sec non-void epochs); and 5) bursting productivity, defined as the ratio of the void size (normalized to 200 g body weight) to bursting time. The EUS bursting frequency range used to calculate Pburst was defined using five to 10 (median of eight) 1-Hz wide consecutive frequency bins. The range used was always the same within each animal, but varied between animals (see “Intra-void EUS EMG” in the Results section). Values of Pburst were first divided by the number of 1-Hz wide frequency bins that comprised the bursting range, and then normalized to a 7-Hz wide frequency range to enable comparisons with our previous study, in which the bursting frequency ranges used were 4–10 Hz or 3–9 Hz.22
The inter-burst timing of EUS EMG activity was analyzed in a subset of voids (n = 308 from the 14 animals) that showed clearly alternating bursts of EMG activity with minimal tonic activity between the active phases by calculating bursting silent period (defined as the time during a single bursting cycle in which the EUS EMG was not active); bursting active period (defined as the time during a single bursting cycle in which the EUS EMG was active); and bursting period (defined as the duration of a single bursting cycle, i.e., active period + silent period).
Repetitive non-voiding contractions (NVCs) of the bladder are common after SCI, and are associated with co-contraction of the EUS and impaired urinary output.12 Although bladder pressure was not measured in the present study, a similar pattern of EUS activations were observed prior to many voids in rats with spinal cord lesions (see Repetitive non-voiding contraction in Results), but not in rats with intact spinal cords. Thus, these EUS contractions were considered to reflect reflex activity in response to SCI-induced bladder NVCs and thus are identified here as repetitive non-voiding EUS contractions (rNVECs; this acronym's parallel structure to NVC emphasizes that it is EUS activity that is distinct from, but still serves as a marker for NVCs of the bladder). Based on spectral analysis of inter-void EUS EMG activity, rNVECs are defined as oscillations in EUS EMG occurring at one to eight cycles per minute that exceed average EMG power from the same rat under intact conditions in the one to eight cycles per minute range by 2 × SD. rNVECs were characterized by calculating: rNVEC amplitude, defined as the peak amplitude of individual rNVEC contractions; rNVEC duration, defined as the width of individual rNVEC contractions at one-half peak amplitude; total rNVEC duration, defined as the sum of time of all rNVEC epochs during the inter-void interval; and inter-rNVEC interval, defined as the time between peaks of successive contractions.
Statistical analysis
Evaluations of voiding and EUS properties before and after spinal transection were performed using analyses of variance (ANOVAs) with repeated measures (equivalent to paired t-test for comparisons made between intact and transected animals, in which the latter group was formed by pooling data from the 3rd and 4th week post-transection). Statistical significance of relationships between void size and other properties was determined by simple or multiple regression analysis. In preparation for regression analysis of the relationship between urine weight per void and other urodynamic and EUS EMG-related factors, data for each parameter were normalized by z-transformation for each individual animal by subtracting the mean value and then dividing by its standard deviation.
Results
Lesion status and recovery
Inspection of the fixed spinal cords under a dissecting microscope verified that no spinal cords had residual connections between the rostral and caudal stumps. Six of seven mid-thoracic transections were at the junction of the T8 and T9 spinal segments; the other transection was slightly more rostral but within the T8 segment. For thoraco-lumbar transections, the exact location depended on the spinal vasculature of each rat (see “Spinal transections” in the Methods section), ranging from the T12-T13 to the L1-L2 junctions (one at the T12-13 junction, two within T13, one at the T13-L1 junction, and three at the L1-2 junction). All animals recovered from the implantation and spinal transection surgeries without incident.
During post-mortem assessments, the pubic bone was carefully removed to allow inspection of the implant recording wires and removal of the bladder for weighing. All implants had remained secure in their original positions and the recording sites were free of connective tissue. Bladders of both groups were comparable in weight, and larger (244 ± 40 and 180 ± 10 mg per 200 g body weight for thoraco-lumbar– and mid-thoracic–transected rats, respectively; n = 6 for each group) than those of implanted rats that were not transected (62 ± 2 mg per 200 g body weight, as reported in LaPallo and colleagues22).
Recordings
Recordings of EUS EMG and urine output included 168 24-h sessions from 14 rats (9–16 recordings per rat (mean = 12 ± 2 SD) before and after transection in each rat. Recordings were performed 1–4 times per week for each rat (mean recordings per week = 1.9 ± 0.3 SD) for up to 4 weeks before and up to 4 weeks after complete spinal transection, excluding the first 6 post-operative days. The need to manually express areflexive bladders during the initial post-transection week precluded 24-h recording sessions during this time. Recording sessions typically restarted 7–9 days after spinal transection upon recovery of spontaneous voiding. In two of 14 animals, recordings began before post-transection Day 7 (one at Day 3 and one at Day 6) because spontaneous voiding reflexes emerged earlier. These data were not included in subsequent analyses.
Urine output
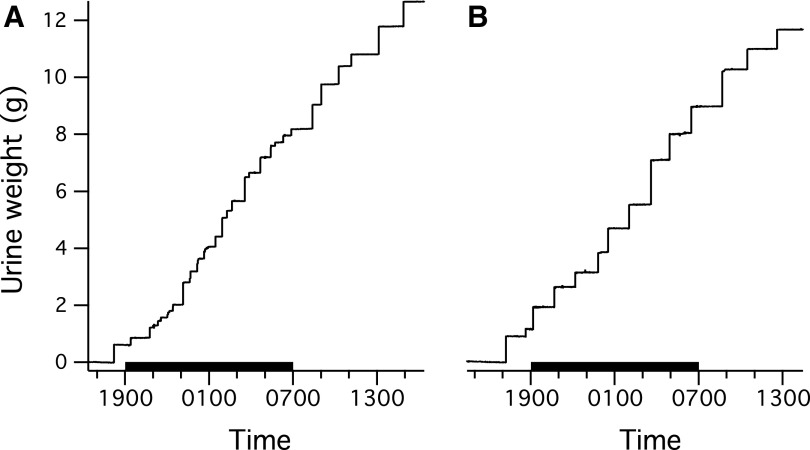
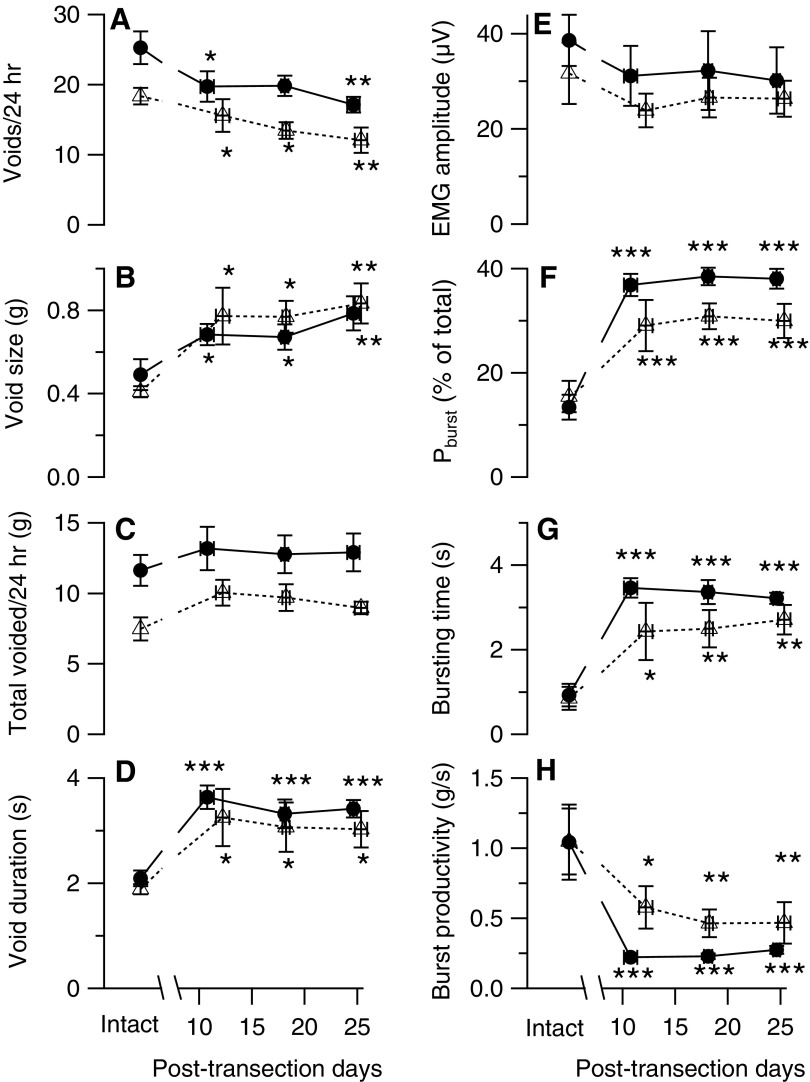
Figure 1 shows examples of urine weight traces recorded over 24 h from a single rat before (A) or after (B) mid-thoracic spinal transection. At this time scale, each void appears as step increase in urine weight whose height indicates void size. These recordings illustrate that voids are typically larger and less frequent in transected animals. Figure 2 (panels A-D) shows the time course of the changes in voiding properties after mid-thoracic (solid symbols and lines) and thoraco-lumbar (open symbols and dashed lines) transection. Because voiding properties were stable over time in intact implanted animals (except from the first recording session, whose data have been excluded from these analyses), properties from intact animals were averaged together for comparison with values from transected animals, which are presented as weekly averages during the 3 recording weeks after transection (i.e., from post-transection Day 7 to post-transection Day 28). The data show that the number of voids per 24-h session decreased (Fig. 2A) as void size (i.e., urine weight per 200 g of body weight) increased (Fig. 2B); daily urine voided (normalized to 200 g of body weight) tended to increase after either transection, but not significantly (Fig. 2C). In addition, void duration increased after both transections (Fig. 2D).
FIG. 1.
Examples of 24-h recordings of voided urine acquired from a rat before (A) and 4 weeks after (B) thoraco-lumbar transection. Time is shown on the abscissa in military format with the black bar denoting the 12-h dark cycle. Step-like increases in weight indicate the occurrence of voiding. Fewer but larger voids tend to occur in the spinal-transected than in the spinal-intact rat.
FIG. 2.
Time course of urodynamic and external urethral sphincter electromyography (EUS EMG) variables recorded before and up to 4 weeks after thoraco-lumbar (open symbols with dashed lines) or mid-thoracic (filled symbols with solid lines) transections. Data recorded in intact animals were stable over time,22 and thus were averaged over all pre-transection recordings. Weekly averages (± standard error) are shown as the number of days after spinal transection. Data are shown for: (A) number of voids per 24 h; (B) void size (urine weight/void/200 g of body weight); (C) total voided/24 h (total urine weight/200 g of body weight per 24 h); (D) void duration (duration of urine weight accumulation in seconds); (E) EMG amplitude (average intra-void rectified EMG amplitude); (F) bursting power (Pburst; average percent of total power in bursting frequency bands normalized to a 7-Hz wide frequency range; see “Data analysis” in the Methods section for details); (G) bursting time (cumulative time during a void when Pburst exceeded the power in the same frequency range of amplitude-matched non-voiding epochs by 2 standard deviations); and (H) bursting productivity (urine weight/void/200 g of body weight divided by the bursting time). Significant difference from intact:*p < 0.05; **p < 0.01; ***p < 0.001 by repeated measures analysis of variance and post hoc contrast.
The time course of changes in voiding parameters was comparable after the two lesions. No significant differences were found in any voiding variable among the 3 post-transection weeks studied. Table 1 shows final values of voiding variables for pre-transection (intact) and post-transection (TX) conditions for the thoraco-lumbar and mid-thoracic groups. Post-transection values were calculated as the average of data from post-transection Weeks 3 and 4. The two lesions elicited comparable change in voiding parameters in that no significant difference was found between the two lesion groups when expressed as % transection-induced change (Table 1). Because of the lack of difference in the effects of the two lesions, voiding data were pooled (all transections; Table 1). Results were comparable to those seen with the two transection groups individually, although the pooled data did show a significant increase in the daily urine voided, which is consistent with the polyuria reported after spinal contusion or transection.25,26
Table 1.
Urine Output and EMG Properties before (Intact) and 3–4 Weeks after Complete Spinal Transection (TX) at Thoracolumbar or Mid-Thoracic Levels
| Thoraco-lumbar transection only | Mid-thoracic transection only | All transections | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Properties | Intact | TX | %TX | Intact | TX | %TX | Intact | TX | %TX |
| Number of voids/24 h | 18 ± 1 | 13 ± 1** | 69 ± 5 | 25 ± 2 | 19 ± 1* | 77 ± 8 | 22 ± 2 | 16 ± 1*** | 73 ± 5 |
| Void size (g/200 g body weight) | 0.41 ± 0.03 | 0.81 ± 0.08*** | 203 ± 27 | 0.49 ± 0.07 | 0.72 ± 0.07* | 158 ± 19 | 0.45 ± 0.04 | 0.77 ± 0.05*** | 181 ± 17 |
| Urine voided/24 h (g/200 g body weight) | 7.4 ± 0.8 | 9.3 ± 0.7 | 133 ± 16 | 11.6 ± 1.1 | 12.8 ± 1.3 | 112 ± 10 | 9.6 ± 0.9 | 11.1 ± 0.8 | 123 ± 9 |
| Void duration (sec) | 1.9 ± 0.1 | 3.1 ± 0.4* | 168 ± 28 | 2.1 ± 0.2 | 3.4 ± 0.2** | 169 ± 20 | 2.0 ± 0.1 | 3.2 ± 0.2*** | 168 ± 17 |
| EMG amplitude (μV) | 32 ± 6 | 26 ± 4 | 101 ± 22 | 39 ± 5 | 31 ± 8 | 78 ± 10 | 35 ± 4 | 29 ± 4 | 90 ± 12 |
| Pburst (%) | 16 ± 3 | 30 ± 3** | 230 ± 39 | 13 ± 2 | 38 ± 2*** | 324 ± 40 | 14 ± 2 | 34 ± 2*** | 277 ± 30 |
| Bursting time (sec) | 0.9 ± 0.3 | 2.6 ± 0.4** | 575 ± 210 | 0.9 ± 0.3 | 3.3 ± 0.2*** | 535 ± 133 | 0.9 ± 0.2 | 2.9 ± 0.2*** | 554 ± 120 |
| Number of voids with bursting (% of total) | 70 ± 8 | 93 ± 3* | 145 ± 20 | 79 ± 6 | 100 ± 0* | 130 ± 9 | 75 ± 5 | 96 ± 2*** | 137 ± 11 |
| Bursting productivity (g/200 g body weight/sec) | 1.05 ± 0.23 | 0.47 ± 0.11* | 60 ± 14 | 1.04 ± 0.27 | 0.25 ± 0.02* | 37 ± 10 | 1.05 ± 0.17 | 0.38 ± 0.07** | 51 ± 10 |
All values are means ± standard error of the mean.
%TX, post-transection values expressed as % of intact values for each animal; Pburst, power in bursting frequencies as a percent of total; bursting time, cumulative time during void when power in bursting frequencies exceeds the power in the same range expressed as a percent of total power or amplitude-matched non-voiding epochs by 2 × standard deviation; bursting productivity, void size/bursting time. Comparisons of intact and post-transection data were performed by paired t-test, where transection differed from intact at *p ≤ 0.05, **p < 0.01, and ***p < 0.001. Comparison of the %TX between the two lesion levels by Student's t-test revealed no significant differences for any variable.
EMG, electromyography.
Due to variability in the spinal vascular branching pattern (see “Spinal transections” in the Methods section), thoraco-lumbar lesions were performed at different locations ranging from the T11-T12 junction to the L1-L2 junction. Inclusion of the exact level of transection in the analyses described above revealed that the observed effects of thoraco-lumbar transection did not vary with the lesion level within this narrow range.
Intra-void EUS EMG
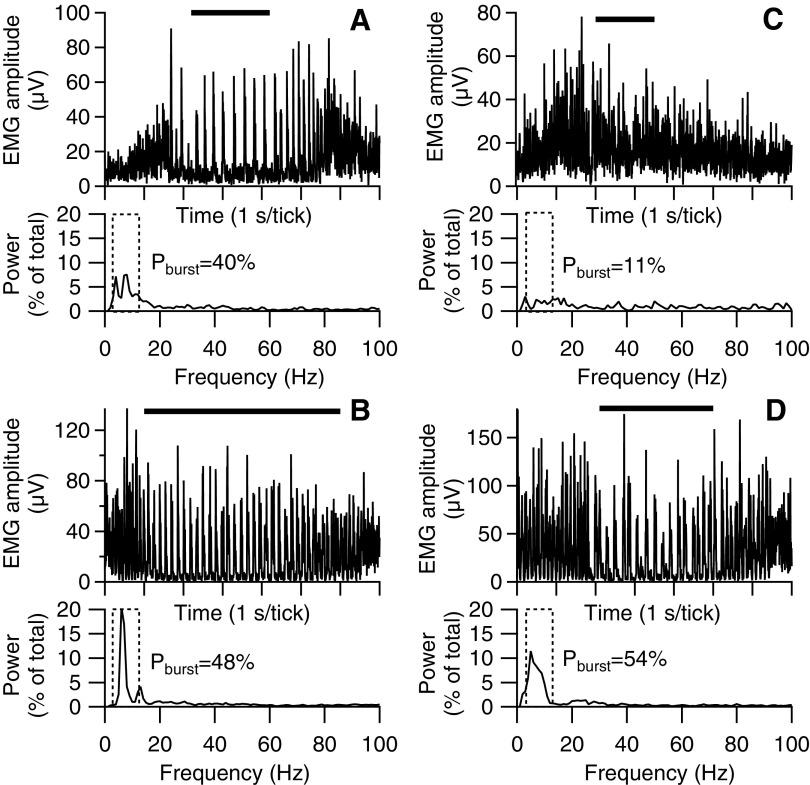
Figure 3 shows examples of EUS EMG activity during voiding in two rats before (A, C) and after (B, D) thoraco-lumbar (left panels) or mid-thoracic (right panels) transection. As described in the previous study of intact unanesthetized rats, three-fourths of voids were accompanied by phasic activation pattern of alternating high and low amplitude EMG at ∼3–10 Hz (i.e., bursting; Table 1), which is illustrated in the upper trace of Figure 3A. This bursting manifests in the corresponding power spectrum (lower trace in Fig. 3A) as peaks in power in frequencies corresponding to the rate of bursting (enclosed within dashed box). Spectral analysis reveals that in this example, the power in the bursting-frequency range during voiding (lower trace in Fig. 3A) as a percent of the total power in the EUS EMG (Pburst) is substantial. The upper panel in Figure 3C illustrates the other one-fourth of the voids recorded in this study in which no EUS bursting was observed; the value of Pburst calculated from the corresponding power spectrum (lower panel in Fig. 3C) in the bursting is low. After spinal transection in each of these examples, EUS bursting is enhanced, which is reflected in larger values of Pburst than was present in the spinally intact rats.
FIG. 3.
Examples of external urethral sphincter electromyography (EUS EMG) activity during voiding in two rats before (A, C) and after (B, D) thoraco-lumbar (left panels) or mid-thoracic (right panels) transection. Within each panel, the upper traces show rectified EUS EMG (1-sec time axis divisions) during single voids (time of urine flow indicated by horizontal bars above each trace), and the lower traces show EUS EMG spectral power (expressed as % of total power) as a function of frequency. The pattern of EUS activity is highly variable in intact rats (upper panels). Some voids exhibit strong phasic activity (i.e., bursting; illustrated in A) associated with peaks in EMG power in frequencies corresponding to the rate of bursting. Within the entire bursting frequency range (indicated by dashed rectangle), the percent of total power associated with bursting (Pburst; shown to right of range) is high. Other voids of intact rats exhibit little or no phasic EUS EMG activity (illustrated in C), having no distinct peaks in the power spectrum and a low value of Pburst. After transection (lower panels), bursting is seen in the vast majority of voids after thoraco-lumbar or mid-thoracic transection and is associated with large values of Pburst.
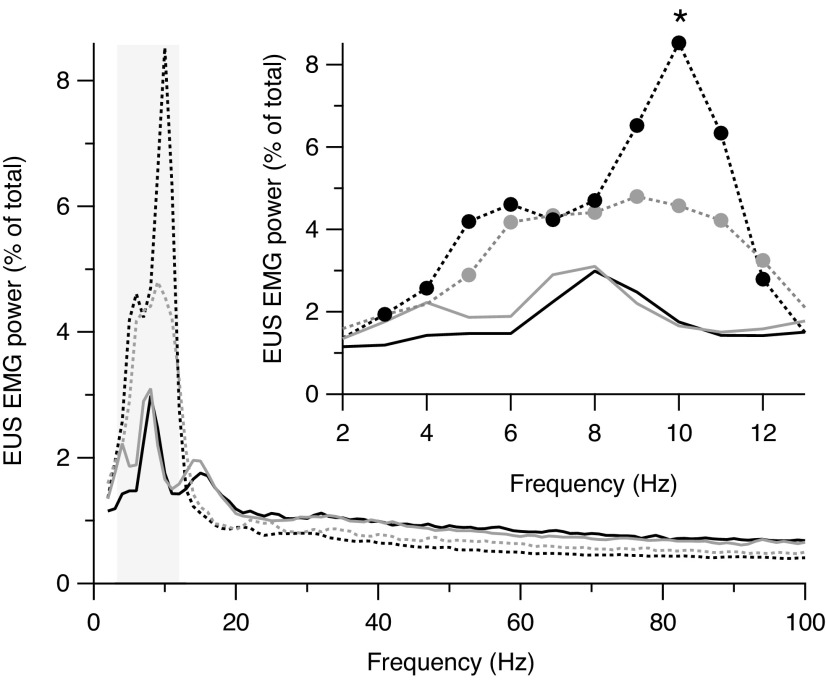
Figure 4 shows power spectra from all animals averaged by spinal state and transection group. The percentage of power in the band spanning putative EUS bursting frequencies (indicated by gray box) of transected animals is increased relative to that of intact rats. The inset in Figure 4 shows power spectra expanded in the bursting frequency range. This band ranged from 5–12 Hz and 3–12 Hz in mid-thoracic– and thoraco-lumbar–transected rats, respectively (filled circles indicate frequencies at which Pburst of transected rats is significantly different from that of intact rats). For individual animals, the number of bursting frequencies with enhanced power ranged from 7–10 frequencies (median = 8). The lower end of the bursting frequencies ranged from 3–6 Hz (median = 4) and the upper end ranged from 11–12 Hz (median = 11). In intact rats, power in the bursting frequencies was comparable in the mid-thoracic and thoraco-lumbar groups (black and gray solid lines, respectively, between 3 and 12 Hz). After both transections, power in the bursting frequencies increased with respect to pre-transection values, although the increase was greater after mid-thoracic transection (dashed black line) than after thoraco-lumbar transection (dashed gray line), most notably at 10-Hz (mid-thoracic ≠ thoraco-lumbar power at 10 Hz; p = 0.048 by t-test).
FIG. 4.
Average power spectra during voiding before (solid lines) and after (dashed lines) mid-thoracic (black lines) or thoraco-lumbar (gray lines) spinal transection. Both transections enhance power in frequencies corresponding to the rate of external urethral sphincter (EUS) bursting outlined by the gray rectangle. In the enlargement of this region (inset), the filled circles indicate individual frequencies with statistically significantly differences (p < 0.05) between mid-thoracic–transected (black) or thoraco-lumbar–transected (gray) rats and intact rats. The asterisk indicates that the power at 10 Hz is greater in mid-thoracic– than thoraco-lumbar– transected rats.
Figure 2 (panels E-H) shows analysis of the time course of EUS EMG activity before and after spinal transection. Both transections induce a rapid and sustained enhancement of Pburst and in the duration of bursting during voiding (bursting time; Fig. 2G), without a significant effect on the magnitude of the EUS EMG (EMG amplitude; Fig. 2E). EUS bursting is thought to be essential for efficient voiding in rats,8,10 and yet Figure 2H shows the rapid and sustained decrease in urine produced during bursting (bursting productivity, calculated as the ratio of void size to bursting time, which indicates how much urine is eliminated per unit time of bursting). The final transection values for these EUS EMG variables averaged over the third and fourth post-transection weeks (Table 1) show results comparable to those shown in the time courses of Figure 2. In addition, the percentage of voids with EUS EMG bursting increased significantly after both lesions (Table 1). Examination of the effects of both transections on an animal-by-animal basis revealed that the increases in Pburst, bursting time and percentage of voids with EUS bursting were consistent across all animals, indicating a robust effect of transection at either level. All of the final changes in EUS EMG variables were similarly expressed after each type of transection (%TX columns under thoraco-lumbar and mid-thoracic transection only; Table 1), and thus these data were pooled into a single group (“All transected,” Table 1). Analysis of these pooled data revealed transection-induced effects similar to those seen for the individual transection-level groups.
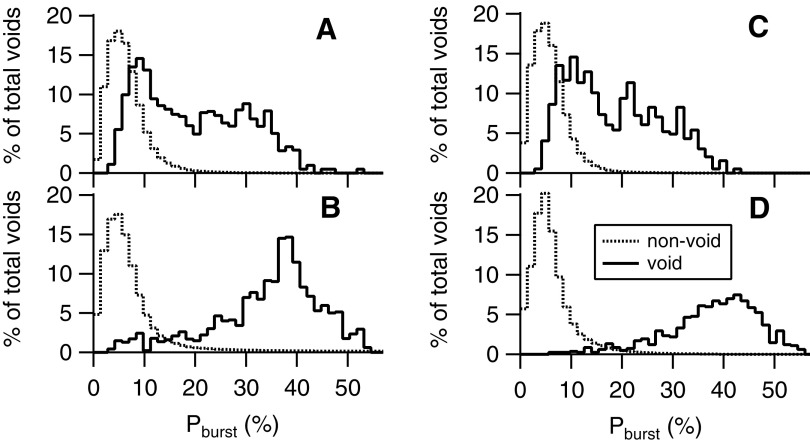
Figure 5 shows histograms of the distributions of Pburst for all voids and non-voids (calculated from 2-sec EMG epochs at least 60 sec away from voids) pooled by transection location group and spinal state. In intact animals (upper panels), mean Pburst during voids (Table 1) was significantly higher in both transection groups than Pburst values from non-void epochs (mean ± SD = 6.4 ± 0.8% and 5.9 ± 0.4% for thoraco-lumbar [Fig. 5A] and mid-thoracic [Fig. 5C] groups, respectively; p = 0.02 for both comparisons by paired t-test). This is consistent with the occurrence of bursting during the majority of voids and tonic activity during non-void epochs. However, consistent with the variable incidence of bursting, the range of intra-void Pburst (3–52% and 3–43% for the thoraco-lumbar and mid-thoracic transection groups, respectively) overlapped with that of non-void EMG epochs (0–50% and 0–47%, for thoraco-lumbar and mid-thoracic transection groups, respectively). The % overlap between void and non-void distributions (calculated as the percentile at which the distribution of voiding Pburst values had the same proportion of higher values as the non-void distribution had of lower values) was 21% for intact rats in both the thoraco-lumbar and mid-thoracic transection groups.
FIG. 5.
Distribution of power in the bursting frequency range expressed as a percent of the total power (Pburst) during epochs of external urethral sphincter electromyography (EUS EMG) associated with voiding (black lines) or not associated with voiding (gray lines) in two animals before (A, C) and after (B, D) thoraco-lumbar (left panels) mid-thoracic (right panels) spinal transection. The variable contribution of both tonic and phasic voiding patterns of EUS activity during voiding in rats with intact neuraxes (upper panels) results in a broad distribution of bursting power. This distribution is higher than but still overlaps considerably that of bursting power during non-voiding EMG epochs. Spinal transection at either level (lower panels) results in robust EUS bursting during the vast majority of voids, such that the distribution of bursting power is consistently high and minimally overlaps that of non-voiding EMG epochs.
Figure 5 also shows that the increase in bursting after transection at either level (lower panels) was evident in the EMG of the vast majority of voids and manifest as increased power in bursting frequencies of intra-void power spectra. After thoraco-lumbar (Fig. 5B) or mid-thoracic (Fig. 5D) transection, the distributions of Pburst (solid lines) were markedly shifted to the right, with little effect on the distributions of Pburst of the non-void distributions, thereby reducing the % overlap between void and non-void distributions (9 ± 2% and 3 ± 1% for thoraco-lumbar and mid-thoracic transection groups, respectively). Consistent with these shifts in the distribution of Pburst, the % of voids with bursting (where bursting voids were defined as any void with bursting time >0) increased after both transections (Table 1). The degree of increase in the number of voids with bursting after the two transections was comparable (Table 1).
Temporal structure of EUS bursting during voiding
The duration of phasic EUS EMG silence during bursting and bursting frequency have been related to the efficacy of voiding in rats,12,13,27,28 and thus we analyzed the temporal structure of EUS bursting before and after spinal transection. One complication was that EUS bursting was not strongly expressed in five of the 14 rats with intact spinal cords, which precluded performing inter-burst analysis in these cases. Thus, paired (i.e., before and after transection) analysis could only be performed in four mid-thoracic– and five thoraco-lumbar–transected animals. Even with this limited data set, silent period and bursting period were significantly reduced after thoraco-lumbar transection (Table 2). Similar trends were observed after mid-thoracic transection, but the reduction in silent period and bursting period were not statistically significant. Analysis of data from all nine rats with both pre- and post-transection data showed a significant reduction in silent period and bursting period (Table 2). In order to obtain a larger data set, results from an additional eight intact animals reported in a previous study27 and data from all 14 transected rats reported here were included in an ANOVA (Table 2). Silent period and bursting period were significantly reduced after either of the two lesions to the same extent.
Table 2.
Inter-Burst Timing in Intact and Transected Rats
| Paired analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All transections | Mid-thoracic transection only | Thoraco-lumbar transection only | Pooled analysis | ||||||
| Property | Intact | Transected | Intact | Transected | Intact | Transected | Intact | Mid-thoracic | Thoraco-lumbar |
| Active period (msec) | 63 ± 3 | 67 ± 3 | 59 ± 4 | 63 ± 4 | 66 ± 3 | 70 ± 3 | 68 ± 3 | 67 ± 4 | 72 ± 4 |
| Silent period (msec) | 111 ± 13 | 62 ± 8** | 86 ± 4 | 58 ± 13 | 130 ± 20 | 65 ± 11* | 121 ± 10 | 68 ± 10** | 60 ± 9** |
| Bursting period (msec) | 174 ± 15 | 129 ± 10** | 145 ± 3 | 120 ± 16 | 196 ± 23 | 135 ± 13* | 189 ± 12 | 134 ± 13* | 131 ± 11* |
| Number of rats | 9 | 4 | 5 | 17 | 7 | 7 | |||
Active period, time during a single bursting cycle in which the external urethral sphincter electromyography (EUS EMG) was active; silent period, time during a single bursting cycle in which the EUS EMG was not active; bursting period, active period + silent period. Paired analyses show paired t-tests of data from animals with both intact and transected values; pooled analyses show analyses of variance using data from all rats of this study plus eight additional intact rats reported elsewhere.27 Transected significantly different from intact at *p < 0.05, **p < 0.01, and ***p < 0.001.
Relationships between urine output and void and EMG properties
Table 3 reports linear regression analyses (slope, coefficient of determination [r2], and statistical significance of the relationships and changes in slope) of the dependence of urine weight produced per void on other urodynamic and EUS EMG-related factors in pre- and post-transection conditions. Relationships in intact animals were comparable to those previously reported for a larger data set.27 Void size varied most strongly with inter-void interval, void duration, and EUS bursting time (Fig. 6A and 6D for thoraco-lumbar and mid-thoracic lesions, respectively, for the latter variable; regression analysis summarized in Table 3). After transection at either level, significant relationships between these variables were still observed, although some were weaker than in intact animals (particularly after mid-thoracic transection). Significant relationships also were observed in intact and transected animals between void size and Pburst (illustrated in Fig. 6B and 6E for thoraco-lumbar and mid-thoracic lesions, respectively; regression analysis summarized in Table 3). This relationship was primarily due to low-frequency EUS bursting in that void size was correlated with power in the 3- to 5-Hz range, but not in the 8- to 12-Hz range (P3–5 and P8-12, respectively, in Table 3). This is consistent with changes in voiding efficiency varying inversely with bursting frequency.12,13,27,28 That the relationships between void size and other factors were still evident after spinal transection suggests that basic urodynamic mechanisms of urine elimination were still in effect after spinal transection.
Table 3.
Results of Regression Analysis of the Relationship between Void Size and Inter-Void Interval and between Void Size and EMG Properties
| Thoraco-lumbar | Mid-thoracic | |||||||
|---|---|---|---|---|---|---|---|---|
| Intact | Transected | Intact | Transected | |||||
| Independent Variable (z-scores) | Slope | r2 | Slope | r2 | Slope | r2 | Slope | r2 |
| Inter-void interval | 0.49 | 0.29*** | 0.16¶¶¶ | 0.03*** | 0.56 | 0.29*** | 0.34¶¶¶ | 0.12*** |
| Void duration | 0.54 | 0.30*** | 0.53 | 0.33*** | 0.92 | 0.49*** | 0.43¶¶¶ | 0.21*** |
| Pburst | 0.29 | 0.05*** | 0.37 | 0.11*** | 0.35 | 0.04*** | 0.46¶¶ | 0.09*** |
| P3–5 | 0.21 | 0.05*** | 0.24 | 0.07*** | 0.32 | 0.10*** | 0.17¶¶ | 0.03*** |
| P8–12 | −0.07 | 0.00 | 0.05 | 0.00 | 0.02 | 0.00 | 0.06 | 0.00 |
| Bursting time | 0.41 | 0.13*** | 0.53 | 0.30*** | 0.63 | 0.25*** | 0.52¶ | 0.19*** |
| Silent period | 0.52 | 0.19*** | −0.04¶¶¶ | 0.00 | 0.39 | 0.16** | −0.13¶¶ | 0.02 |
| Active period | 0.31 | 0.13** | −0.05¶ | 0.00 | 0.19 | 0.03 | 0.07 | 0.01 |
| Bursting period | 0.51 | 0.22*** | −0.06¶¶¶ | 0.00 | 0.40 | 0.17** | −0.06¶¶ | 0.00 |
Slope and r2, slope and coefficient of determination for regression of void size on independent variable; void duration, time of urine weight increase; Pburst, power in bursting range of as a percent of total power; P3–5, power at 3–5 Hz (i.e., low bursting frequencies) as a percent of total power; P8–12, power at 8–12 Hz (i.e., high bursting frequencies) as a percent of total power; bursting time, cumulative time during void when power in bursting frequencies exceeds the power in the same range expressed as a percent of total power of amplitude-matched non-voiding epochs by 2 standard deviations; silent period, time during a single bursting cycle in which the external urethral sphincter electromyography (EUS EMG) was not active; active period, time during a single bursting cycle in which the EUS EMG was active; bursting period, duration of a single bursting cycle (i.e., active period + silent period). All variables were z-transformed prior to regression analysis to remove inter-animal differences in mean and variance. For r2, asterisks denote significant relationships between the independent variable and void size. For slope, ¶ denotes significant difference in slope, compared with intact. * or ¶, p ≤ 0.05; ** or ¶¶, p < 0.01,*** or ¶¶¶, p < 0.001.
FIG. 6.
Regression analysis of relationships between urine weight per void and bursting time (A, D), bursting power (Pburst; B, E), and external urethral sphincter (EUS) bursting silent period (C, F) before (circles with thick black regression line) and after (triangles with thin gray regression line) thoraco-lumbar (left panels) or mid-thoracic (right panels) spinal transection. All values are z-transformed to remove inter-animal differences in mean value and variance. Void size is positively correlated with bursting time and with %Pburst both before and after either spinal transection. Void size is positively correlated with silent period duration in spinal-intact animals, but not in spinal-transected animals.
Differences between intact and transected animals were found in the relationships between void size and the temporal structure of EUS bursting. Intact animals exhibited significant positive relationships between void size and both the silent period and the bursting period (Fig. 6C and 6F; regression analysis summarized in Table 3). These relationships were not evident—and if anything, tended towards negative relationships—after either level of spinal transection.
Repetitive non-voiding EUS contractions
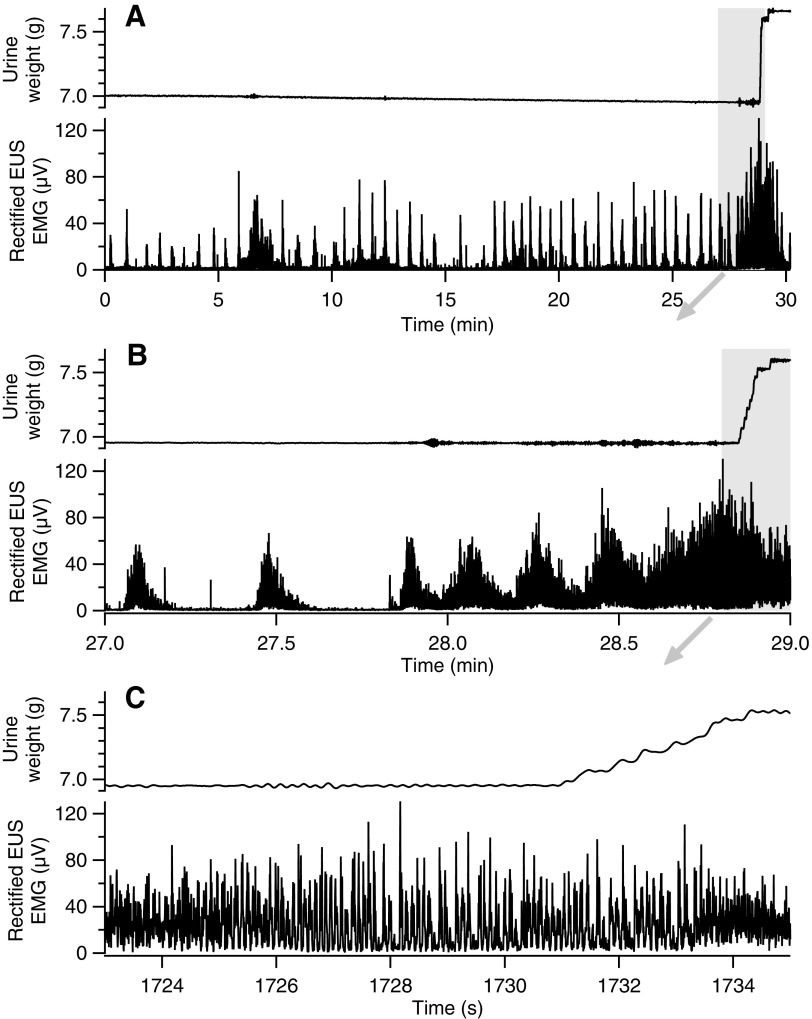
Inter-void EUS EMG was evaluated in 12 of the 14 animals reported here. Ten of 12 animals exhibited highly-patterned EMG during inter-void intervals in the transected, but not the intact, state. This pattern was characterized by series of EMG activations repeated at long, regular intervals (see examples in Fig. 7). These repetitive non-voiding EUS contractions (rNVECs) were roughly uniform in amplitude and duration, and were regularly spaced. The rNVECs occurred over 0.5 to 75 min prior to voiding without any phasic EUS activity or urine accumulation. The features of this repeating pattern of EUS activation are summarized in Table 4. Termination of these slowly-changing EUS contraction cycles typically coincided with voiding associated with EUS bursting. This is consistent with the rhythmic bladder contractions seen in neurogenic detrusor over-activity after spinal transection (although this cannot be stated definitively without simultaneous recording of bladder pressure). This pattern of repeated EUS activation over extended periods suggests that the EUS may receive rhythmic drive from sub-voiding-threshold spinal LUT reflexes. That this pattern of activity manifest, similarly in mid-thoracic–transected and thoraco-lumbar–transected rats suggests that the spinal circuitry between the two transection levels does not affect this cyclic EUS drive.
FIG. 7.
Typical example of rhythmic external urethral sphincter (EUS) activation during inter-void intervals in transected rats. (A) EUS activations (lower trace of pair) occurring at regular intervals for extended periods precede voiding of urine (upper trace of pair) in a rat 4 weeks after mid-thoracic spinal transection. (B) Expansion of the area in panel (A) indicated by the gray rectangle illustrates that the roughly uniform contractions increase in amplitude and occur at shorter intervals during the time leading up to the void. (C) Expansion of the area in panel B indicated by the gray rectangle shows that phasic EUS activity (i.e., bursting) begins and continues for >6 sec before urine accumulation begins. The small amount of drift that is apparent in the urine weight trace (A) has a sufficiently slow time course that it does not affect the measurement of the weight that falls during the void.
Table 4.
Properties of Rhythmic Non-Voiding EUS Contractions (rNVECs) after Spinal Transection
| Transection group | ||
|---|---|---|
| Properties | Thoraco-lumbar | Mid-thoracic |
| # of voids with rNVECs (% of total) | 25 ± 4 | 17 ± 4 |
| rNVEC amplitude (μV) | 15 ± 4 | 22 ± 2 |
| rNVEC duration (sec) | 7 ± 1 | 5 ± 1 |
| Total rNVEC duration (min) | 29 ± 5 | 25 ± 3 |
| Inter-rNVEC interval (sec) | 24 ± 4 | 20 ± 4 |
| % of rats with rNVECs | 100 | 100 |
Values are mean ± standard error of the mean. rNVEC amplitude, peak amplitude of individual rNVEC contraction; rNVEC duration, width of individual rNVEC contraction at one-half-peak amplitude; total rNVEC duration, sum of time of all rNVEC epochs during the inter-void interval; inter-contraction interval, time between peaks of successive contractions.
EUS, external urethral sphincter.
Relationships between bladder size and void and EMG properties
One of the consequences of bladder over-activity after SCI is bladder hypertrophy, which is reflected in an increase in weight measured post-mortem.3,13,29 Bladder weight varied widely in the rats of the present study (see Lesion status and recovery in Results). Given that bladder hypertrophy increases with the severity of spinal contusion,30 the relationships between bladder weight and indices of urinary function were assessed on an animal-by-animal basis. Figure 8 shows that bladder weight exhibited a significant positive relationship with the EUS bursting silent period (p = 0.01 by linear regression using all animals). This relationship was still evident when lesion level (i.e., mid-thoracic vs. thoraco-lumbar) was included as a factor in the regression analysis (p = 0.02 for a significant dependence of bladder weight on silent period alone). Bladder weight also was inversely correlated with the number of voids per 24 h (p = 0.04), but this relationship disappeared after inclusion of lesion level as a factor in the regression analysis (p = 0.2 for the dependence of bladder weight on silent period alone). Bladder weight was not significantly correlated with other urodynamic or EUS EMG-related factors.
FIG. 8.
Relationship between bladder weight and external urethral sphincter (EUS) bursting silent period in rats after thoraco-lumbar (open circles) or mid-thoracic (filled circles) spinal transection. Linear regression analysis shows a significant inverse relationship between these variables (fit shown by dashed line) for all transections. Inclusion of transection level in this analysis did not alter the relationship between bladder weight and silent period.
Discussion
Recordings of EUS EMG along with urine output were performed in awake, freely-behaving rats before and after mid-thoracic or thoraco-lumbar spinal transection. The purpose of this study was to assess the patterns of EUS activity before and after spinal transection in the absence of the confounding effects of anesthesia, restraint, and post-surgical stress and to evaluate the potential influence of the EUS bursting pattern generator on the relationship between EUS behavior and urine output.
Urine output patterns and individual voids after spinal transection
In the present study, the number of voids per day decreased and void size increased after transection at either level, which was consistent with other reports of urinary function after spinal injury.31,32 The decrease in the number of daily voids could reflect any number of causes, including increased capacity due to bladder enlargement after SCI, enhanced urine retention due to an elevated threshold for voiding, and the inability to release urine due to EUS occlusion of the urethra during attempted voiding (i.e., DSD during non-voiding contraction of the detrusor).3,33–35 Eventually, the threshold for activating the bursting pattern generator is reached and the bladder empties the greater-than-normal accumulation of urine, which could also account, at least in part, for the increased void size and longer void duration after spinal transection.
EUS behavior before and after spinal transection and the influence of anesthesia
The studies described here show that EUS bursting behavior in the absence of anesthesia is markedly different from that in the presence of anesthesia. Urethane-anesthetized spinal-intact rats typically exhibit EUS bursting during voiding.3,8,34,36–38 These results stand in contrast to those of the present study from unanesthetized rats in which EUS bursting was frequently but not exclusively exhibited during voiding in intact rats.22 This anesthesia-induced difference in EUS activity patterns is evident during cystometry in freely moving spinally-intact rats as a lack of high-frequency oscillations in bladder pressure (a manifestation of EUS bursting) and their expression upon administration of anesthestic.39
After mid-thoracic spinal transection, deep urethane anesthesia (typically 1.2 g/kg) is associated with no EUS bursting.3,34,37,38 D'Amico and colleagues37 reported a small amount of EUS bursting in three of 27 mid-thoracic-transected rats that was “not well defined” and of reduced duration (≤ 40% of the duration reported for spinal-intact rats). Another study from the same group using similar methodology reported that none of their 15 spinal-transected rats exhibited any bursting.38 That infrequent and diminished EUS bursting was seen in one study and not in any of the others cited here suggests that deep urethane anesthesia largely suppresses
EUS bursting in rats with mid-thoracic spinal transection
EUS bursting has been reported in rats with mid-thoracic spinal transection under reduced (0.8 g/kg) urethane anesthesia (eight of 18 rats in Cheng and de Groat12; two of six rats in Chang and colleagues21). Additionally, Cheng and de Groat12 reported that after 24 h, when the effect of urethane was still present but markedly reduced, EUS bursting was observed in nearly all rats. Similarly, the reduction in silent period during bursting that was evident under urethane anesthesia was less evident when assessed the next day.12 That EUS bursting only partially recovered under these conditions is likely due to the >24-h duration of effect of urethane.40
The data from transected rats under different levels of urethane anesthesia described above are consistent with a dose-dependent effect of urethane anesthesia on EUS bursting (i.e., little or no bursting at high doses, modest expression of bursting at moderate doses, and frequent expression when anesthetic load is low). Our current results complete this picture, in that animals without anesthesia during recording exhibit bursting in 14 of 14 spinal-transected rats, and the intensity and duration of this bursting is markedly increased compared with that observed in the same animals prior to transection.
The influence of anesthesia on EUS function is not limited to bursting prevalence and intensity. In urethane-anesthetized rats, mid-thoracic transection results in a 2-fold increase in the ratio of the volume of urine voided to the silent period duration,12 suggesting an enhanced effectiveness of bursting in expelling urine. In the unanesthetized freely moving animals reported here, calculation of the same ratio revealed a decrease of 67% and 45% after mid-thoracic and thoraco-lumbar transection, respectively, compared with their pre-transection values. This analysis suggests that anesthesia has a profound effect on the SCI-induced changes in the contribution of the EUS to voiding.
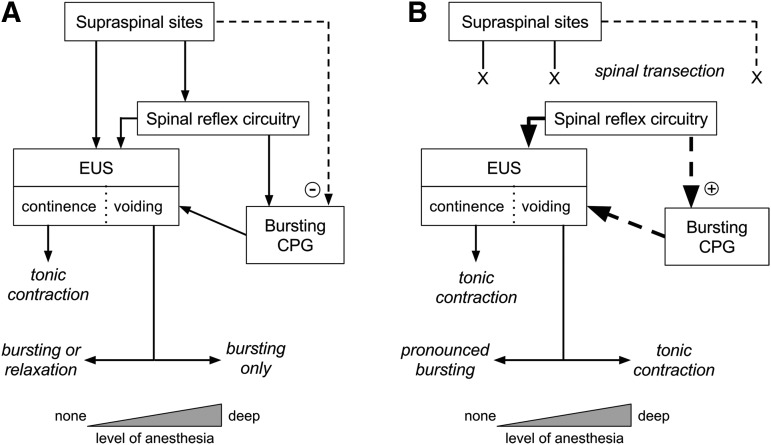
The present data, taken in the context of previous studies, have several implications that are summarized in Figure 9. The variable degree of bursting in unanesthetized rats that changes to nearly obligatory bursting after transection suggests that there is a descending influence of supra-transection origin that inhibits bursting. Given that bursting is predominant in intact anesthetized rats, the present and other39 data suggest that the supraspinal influence on bursting is sensitive to anesthesia in a dose–dependent way. The differential effect of anesthesia on this descending bursting control mechanism and on the brainstem continence/voiding switching mechanism are at least partially independent. In chronic spinal rats, that robust bursting occurs in unanesthetized rats (present data) but not in deeply anesthetized rats3 suggests that post-injury plasticity has enhanced an anesthesia-sensitive spinal pathway that promotes the expression of phasic relaxation (i.e., bursting) over tonic relaxation, despite the appearance of a higher threshold for initiation of bursting than in intact animals.
FIG. 9.
Interaction of anesthesia and spinal transection on external urethral sphincter (EUS) activity patterns in continence and voiding. Spinal and supraspinal elements are shown in text boxes, and EUS activity outcomes are shown in italics. During voiding, outcomes vary over a continuum (indicated by the horizontal two-headed arrows) depending on the depth of anesthesia (indicated by the width of the triangles at the bottom). (A) In rats with intact spinal cords, the EUS is under descending control from spinal and supraspinal sites.2 It is tonically active during continence to prevent urine flow, while during voiding, it exhibits some form of relaxation that permits urine flow. Under deep anesthesia, the EUS exhibits primarily phasic relaxation (i.e., bursting) that is patterned by intrinsic spinal circuitry (bursting central pattern generator [CPG] reviewed in Fraser47). During voiding in unanesthetized, unstressed rats (i.e., no anesthesia, no restraint), the EUS expresses either bursting (∼75% of time) or tonic relaxation (∼25% of time).22 Voiding during intermediate levels of anesthesia is expected to be associated with an intermediate level of EUS bursting (i.e., between 75 and 100% of voids). Thus, we hypothesize that there is a pathway of supraspinal origin (dashed line) that inhibits (minus sign in circle) the spinal bursting CPG, and that this pathway is itself inhibited by anesthesia or under stressful conditions. (B) In rats with spinal transection, the normal descending control over EUS and bursting CPG function is interrupted, but over time, spontaneous voiding re-emerges as a result of plasticity in spinal reflex pathways (thick solid arrow). The present study has shown that unanesthetized, unrestrained transected rats nearly exclusively exhibit EUS bursting during voiding; anesthetized rats exhibit tonic EUS activity during spontaneous bladder contraction comparable to detrusor-sphincter dyssynergia in humans.3 Lightly anesthetized rats12 or unanesthetized rats shortly after bladder implantation surgery13 exhibit a range of EUS activity patterns that fall between these two extremes (see “EUS behavior before and after spinal transection and the influence of anesthesia” in the Discussion section). Thus, we hypothesize that plasticity in spinal reflex pathways after transection enhances the EUS bursting CPG (down-pointing heavy dashed arrow), which in the absence of anesthesia leads to pronounced bursting (up-pointing heavy dashed arrow) and that anesthesia or stressful conditions suppress this injury-induced enhancement of the bursting CPG, leading to tonic EUS activity during voiding.
Potential influence of stress on EUS activity in unanesthetized rats
Unlike the consistently enhanced EUS bursting after spinal transection reported here, previous studies using unanesthetized rats with mid-thoracic spinal transections reported variable expression of bursting or tonic patterns of EUS activity.13,14 In these studies, rats prepared for cystometric and EUS EMG recordings under gaseous anesthesia were placed in restraining cages, and recordings took place shortly after recovery from anesthesia. In the present study, animals fully recovered from EUS wire implantation surgery before the onset of the experiment, during which they were not restrained (except for the skull-mounted flexible cable that connected the electrodes to the recording equipment). Stress, including that induced by restraint, can have marked effects on LUT function.15–19,41 Thus, post-surgical pain and/or restraint may have contributed to the differences in EUS activity patterns seen between the present and previous studies.
How abnormal is EUS activity during voiding after spinal transection in the rat?
Voiding reflexes and EUS intra- and inter-void activity recover sufficiently after injury such that rats can produce large spontaneous voids. It should be noted, however, that the function that recovers after injury is abnormal in several ways.
First, in freely moving intact rats, the coordination between bladder and EUS during spontaneous voids is either phasic (with robust EUS bursting at low frequencies being closely associated with large voids) or tonic (with decreased EUS activity being associated with larger voids (i.e., similar to the human voiding pattern).22 In transected rats, voiding is almost exclusively associated with EUS bursting.
Second, the bursting that recovers after transection does not appear to be as effective at expelling urine as the bursting in rats with intact neuraxes. For voids in which bursting occurs, bursting productivity (urine weight per second of bursting) provides an indication of how effectively urine is expelled. This quantity was substantially lower in both thoraco-lumbar– and mid-thoracic–transected rats compared to intact rats (Table 1). The transection-induced decrease in bursting productivity may reflect a change in the pattern, intensity, and/or the temporal structure of EUS activity. In spinal-intact rats, void size is correlated on a void-by-void basis with the duration of EUS bursting, EUS EMG power in the lower end of the bursting frequency range, and with the duration of the EUS bursting silent period and bursting period (i.e., the inverse of bursting frequency). After spinal transection, the relationship of void size to bursting duration and power are still evident (albeit reduced in some cases; Table 3), but the relationship between void size and silent period disappeared. In female rats, urine flow during bursting occurs primarily during the periods of EUS EMG silence.42 Studies of drug- and injury-induced change in the temporal structure of EUS bursting have indicated that silent period duration is an important factor in voiding efficiency.12,13,28 Given the relationships described above, EUS bursting is still likely to contribute to voiding after spinal transection, but there may be a minimum duration of EUS silence less than which urine flow is not enhanced by EUS relaxation. The spinal transection–induced shift towards shorter silent and bursting periods (Table 2) may have crossed below this threshold, resulting in the observed lack of correlation between silent period duration and urine elimination.
The importance of the transection-induced change in the temporal structure of EUS bursting is underscored by the relationship between bladder weight and silent period (Fig. 8). SCI typically results in bladder hypertrophy.3,13,29,30 Bladder weight varies with contusion injury severity.30 Thus, the transection-induced decrease in silent period during EUS bursting and concomitant decrease in urine expulsion rate may contribute to elevated bladder pressure that leads to bladder hypertrophy.
Third, in addition to abnormal intra-void EMG in rats, inter-void EMG exhibits cyclic fluctuation in EUS EMG activation (Fig. 7). Spinal transection is known to induce bladder over-activity consisting of multiple non-voiding bladder contractions that arise as a result of sensitization of bladder C-fiber afferents.3,12,35,43–45 Although bladder over-activity cannot be definitively identified in the present results due to the lack bladder pressure recording, the observed patterns of inter-void EUS activation are consistent with a spinal guarding reflex occurring in response to cyclic bladder over-activity. The lack of voiding during these cyclic episodes indicates a DSD-like phenomenon that contributes to the reduced voiding frequency and increased void size observed after transection. Even if bursting is triggered, it may be of sufficient high frequency that it does not permit efficient voiding. In the example shown in Figure 7C, high-frequency bursting occurs for several seconds without urine production, which only occurs once longer silent periods occur. The cycle of rhythmic non-voiding EUS (and presumably bladder) contraction is eventually interrupted when reflex and/or other central mechanisms trigger low-frequency EUS bursting that allows the bladder to be emptied.
Implications of findings for bursting pattern generator
The findings of these transection studies have several implications concerning the putative bursting pattern generator. First, that the EUS almost exclusively exhibits bursting during voiding reflexes in transected rats but not intact rats suggests that supraspinal inputs control the pattern of EUS activity in intact rats. A model for an EUS bursting central pattern generator has been proposed where dorsal gray commissure interneurons exert inhibitory influence on EUS motoneurons in which auto-inhibition of the proposed interneurons would elicit oscillatory inhibition of EUS motoneurons.46 Pre-synaptic inhibition of these collaterals could result in tonic inhibition of the dorsal gray commissure interneurons, leading to tonic inhibition of the EUS.47 If such pre-synaptic inhibition existed, it would likely be exerted through pathways descending from supraspinal sites, given the present finding that voiding is almost exclusively associated with EUS bursting in rats with chronic spinal transections.
Second, strong EUS bursting in thoraco-lumbar–transected rats indicates that the spinal circuitry controlling EUS bursting—the putative bursting pattern generator—lies caudal to the most caudal thoraco-lumbar lesion level (i.e. the L1-L2 junction). A previous study proposed that the bursting pattern generator was located between the T8-T9 and the L3-L4 junctions because EUS bursting was evident after the former, but not the latter, lesion.21 Given the difficulty in making viable lesions caudal to L1 due to the anatomy of the lumbar vasculature (see “Spinal transections” in the Methods section), it is possible that compromised vascular supply could have contributed to the failure to detect EUS bursting after the L3-L4 lesion. Thus, the caudal limit of the location of the bursting pattern generator remains unclear.
Third, bursting-related parameters were largely similar in mid-thoracic– and thoraco-lumbar–lesioned rats. This demonstrates that the circuitry remaining below the thoraco-lumbar lesion can produce EUS bursting comparable to that of mid-thoracic lesion (i.e., without the circuitry between the T8-T9 and L1-L2 junctions). The one transection level-dependent difference observed was that bursting power at 10 Hz was significantly larger after mid-thoracic than thoraco-lumbar transection (Fig. 4). This suggests that there may be spinal circuitry between the two transection levels that contributes to the control of bursting frequency that is separate from the circuitry responsible for producing the pattern of bursting. These data also suggest that while local segmental and autonomic inputs between these levels may affect LUT function, they do not appear to affect the circuitry that controls EUS bursting.
Conclusions
The present study evaluated the behavior of the EUS during voiding and continence in unanesthetized freely moving female rats before and after mid-thoracic or thoraco-lumbar spinal transection. Our previous studies showed that the spinal-intact rat exhibits phasic EUS activation (bursting) during the majority of voids, but also exhibits non-phasic relaxation (consistent with human-like EUS relaxation during voids) during micturition. Spinal transection at either level abolished the human-like EUS voiding activity and increased the prevalence, intensity, and duration of EUS bursting. Both transections also decreased the EUS bursting silent period and increased the bursting frequency, resulting in a decrease in the rate of urine expulsion during voiding. These findings, along with previous studies in anesthetized or restrained rats, suggest that in spinal-intact rats, the spinal bursting pattern generator is under inhibitory control from anesthesia- and/or stress-sensitive descending (presumably supraspinal) influences, and in spinal-transected rats, the bursting pattern generator is facilitated by plasticity in anesthesia- and/or stress-sensitive spinal reflex pathways.
Acknowledgments
This work was supported by grants from the National Institutes of Health 1P41 EB-018783 (J.R.W.), HD-36020 (X.Y.C.), NS-22189 (J.R.W.); the Craig H. Neilsen Foundation 124953 (J.S.C.); and the New York State Spinal Cord Injury Research Board C30601GG (J.S.C.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fowler C.J., Griffiths D., and de Groat W.C. (2008). The neural control of micturition. Nat. Rev. Neurosci. 9, 453–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Groat W.C. and Wickens C. (2013). Organization of the neural switching circuitry underlying reflex micturition. Acta Physiol. (Oxf.) 207, 66–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruse M.N., Belton A.L., and de Groat W.C. (1993). Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am. J. Physiol. 264, R1157–1163 [DOI] [PubMed] [Google Scholar]
- 4.Weld K.J., Graney M.J., and Dmochowski R.R. (2000). Clinical significance of detrusor sphincter dyssynergia type in patients with post-traumatic spinal cord injury. Urology 56, 565–568 [DOI] [PubMed] [Google Scholar]
- 5.Swartz D. (1946). The neurogenic bladder in spinal cord injury. Can. Med. Assoc. J. 54, 333–339 [PubMed] [Google Scholar]
- 6.Benevento B.T. and Sipski M.L. (2002). Neurogenic bladder, neurogenic bowel, and sexual dysfunction in people with spinal cord injury. Phys. Ther. 82, 601–612 [PubMed] [Google Scholar]
- 7.Castro-Diaz D. and Taracena Lafuente J.M. (2006). Detrusor-sphincter dyssynergia. Int. J. Clin. Practice Suppl., 17–21 [DOI] [PubMed] [Google Scholar]
- 8.Maggi C.A., Giuliani S., Santicioli P., and Meli A. (1986). Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. Am. J. Physiol. 251, R250–R257 [DOI] [PubMed] [Google Scholar]
- 9.Conte B., Maggi C.A., Parlani M., Lopez G., Manzini S., and Giachetti A. (1991). Simultaneous recording of vesical and urethral pressure in urethane-anesthetized rats: effect of neuromuscular blocking agents on the activity of the external urethral sphincter. J. Pharmacol. Methods 26, 161–171 [DOI] [PubMed] [Google Scholar]
- 10.Yoshiyama M., deGroat W.C., and Fraser M.O. (2000). Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urology 55, 956–960 [DOI] [PubMed] [Google Scholar]
- 11.Kruse M.N. and de Groat W.C. (1990). Micturition reflexes in decerebrate and spinalized neonatal rats. Am. J. Physiol. 258, R1508–R1511 [DOI] [PubMed] [Google Scholar]
- 12.Cheng C.L. and de Groat W.C. (2004). The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp. Neurol. 187, 445–454 [DOI] [PubMed] [Google Scholar]
- 13.Leung P.Y., Johnson C.S., and Wrathall J.R. (2007). Comparison of the effects of complete and incomplete spinal cord injury on lower urinary tract function as evaluated in unanesthetized rats. Exp. Neurol. 208, 80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Douglas K., Jin H., Eldaif B., Nassar R., Fraser M., and Dolber P. (2008). Sprouting of substance P-expressing primary afferent central terminals and spinal micturition reflex NK1 receptor dependence after spinal cord injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R2084–R2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morikawa K., Kakiuchi M., Fukuoka M., Kato H., Ito Y., and Gomi Y. (1990). Effects of various drugs on bladder function in conscious restrained-denervated rats placed in a restraining cage and produced by transection of the hypogastric nerve. Jpn. J. Pharmacol. 52, 405–411 [DOI] [PubMed] [Google Scholar]
- 16.Spanos C., Pang X., Ligris K., Letourneau R., Alferes L., Alexacos N., Sant G.R., and Theoharides T.C. (1997). Stress-induced bladder mast cell activation: implications for interstitial cystitis. J. Urol. 157, 669–672 [PubMed] [Google Scholar]
- 17.Robbins M.T., DeBerry J., and Ness T.J. (2007). Chronic psychological stress enhances nociceptive processing in the urinary bladder in high-anxiety rats. Physiol. Behav. 91, 544–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith A.L., Leung J., Kun S., Zhang R., Karagiannides I., Raz S., Lee U., Glovatscka V., Pothoulakis C., Bradesi S., Mayer E.A., and Rodríguez L.V. (2011). The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology 78, 967.e1–e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang A., Butler S., Sliwoski J., Valentino R., Canning D., and Zderic S. (2009). Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am. J. Physiol. Renal. Physiol. 297, F1101–F1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiddoo D.A., Valentino R.J., Zderic S., Ganesh A., Leiser S.C., Hale L., and Grigoriadis D.E. (2006). Impact of state of arousal and stress neuropeptides on urodynamic function in freely moving rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1697–R1706 [DOI] [PubMed] [Google Scholar]
- 21.Chang H.Y., Cheng C.L., Chen J.J., and de Groat W.C. (2007). Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am. J. Physiol. Renal Physiol. 292, F1044–F1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaPallo B.K., Wolpaw J.R., Chen X.Y., and Carp J.S. (2014). Long-term recording of external urethral sphincter EMG activity in unanesthetized unrestrained rats. Am. J. Physiol. Renal Physiol. 307, F485–F497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tveten L. (1976). Spinal cord vascularity. IV. The spinal cord arteries in the rat. Acta Radiol. Diagn. (Stockh.) 17, 385–398 [DOI] [PubMed] [Google Scholar]
- 24.Schievink W.I., Luyendijk W., and Los J.A. (1988). Does the artery of Adamkiewicz exist in the albino rat? J. Anat. 161, 95–101 [PMC free article] [PubMed] [Google Scholar]
- 25.Ward P.J. and Hubscher C.H. (2012). Persistent polyuria in a rat spinal contusion model. J. Neurotrauma 29, 2490–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medeiros B.A., Santos, dos C.L., Palheta R.C., de Queiroz D.A.F., da Graça J.R.V., Santos, dos A.A., Rola F.H., Lima A.A.M., andGondim F., de A.A. (2008). Spinal cord transection modifies ileal fluid and electrolyte transport in rats. Autonomic Neurosci. 139, 24–29 [DOI] [PubMed] [Google Scholar]
- 27.LaPallo B.K., Wolpaw J.R., Chen X.Y., and Carp J.S. (2016). Contribution of the external urethral sphincter to urinary void size in unanesthetized unrestrained rats. Neurourol. Urodyn. 35, 696–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng C.L. and de Groat W.C. (2010). The role of 5-HT1A receptors in the control of lower urinary tract function in anesthetized rats. Am. J. Physiol. Renal Physiol. 298, F771–F778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagatomi J., Gloeckner D.C., Chancellor M.B., DeGroat W.C., and Sacks M.S. (2004). Changes in the biaxial viscoelastic response of the urinary bladder following spinal cord injury. Ann. Biomed. Eng. 32, 1409–1419 [DOI] [PubMed] [Google Scholar]
- 30.Pikov V. and Wrathall J.R. (2001). Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. J. Neurosci. 21, 559–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitsui T., Murray M., and Nonomura K. (2014). Lower urinary tract function in spinal cord-injured rats: midthoracic contusion versus transection. Spinal Cord 52, 658–661 [DOI] [PubMed] [Google Scholar]
- 32.Lee Y.S., Lin C.Y., Jiang H.H., Depaul M., Lin V.W., and Silver J. (2013). Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J. Neurosci. 33, 10591–10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mimata H., Satoh F., Tanigawa T., Nomura Y., and Ogata J. (1993). Changes of Rat Urinary Bladder during Acute Phase of Spinal Cord Injury. Urol. Int. 51, 89–93 [DOI] [PubMed] [Google Scholar]
- 34.Kruse M.N., Bennett B., and de Groat W.C. (1994). Effect of urinary diversion on the recovery of micturition reflexes after spinal cord injury in the rat. J. Urol. 151, 1088–1091 [DOI] [PubMed] [Google Scholar]
- 35.Yoshiyama M., Nezu F.M., Yokoyama O., de Groat W.C., and Chancellor M.B. (1999). Changes in micturition after spinal cord injury in conscious rats. Urol. 54, 929–933 [DOI] [PubMed] [Google Scholar]
- 36.Kakizaki H., Fraser M.O., and de Groat W.C. (1997). Reflex pathways controlling urethral striated and smooth muscle function in the male rat. Am. J. Physiol. 272, R1647–R1656 [DOI] [PubMed] [Google Scholar]
- 37.D'Amico S.C., Schuster I.P., and Collins W.F. (2011). Quantification of external urethral sphincter and bladder activity during micturition in the intact and spinally transected adult rat. Exp. Neurol. 228, 59–68 [DOI] [PubMed] [Google Scholar]
- 38.D'Amico S.C. and Collins W.F. (2012). External urethral sphincter motor unit recruitment patterns during micturition in the spinally intact and transected adult rat. J. Neurophysiol. 108, 2554–2567 [DOI] [PubMed] [Google Scholar]
- 39.Matsuura S. and Downie J.W. (2000). Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol. Urodyn. 19, 87–99 [DOI] [PubMed] [Google Scholar]
- 40.Field K.J., White W.J., and Lang C.M. (1993). Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab. Anim. 27, 258–269 [DOI] [PubMed] [Google Scholar]
- 41.Valentino R.J., Wood S.K., Wein A.J., and Zderic S.A. (2011). The bladder-brain connection: putative role of corticotropin-releasing factor. Nat. Rev. Urol. 8, 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Streng T., Santti R., Andersson K.-E., and Talo A. (2004). The role of the rhabdosphincter in female rat voiding. B.J.U. Int. 94, 138–142 [DOI] [PubMed] [Google Scholar]
- 43.Cheng C.L., Ma C.P., and de Groat W.C. (1995). Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 678, 40–48 [DOI] [PubMed] [Google Scholar]
- 44.Kakizaki H. and de Groat W.C. (1997). Reorganization of somato-urethral reflexes following spinal cord injury in the rat. J. Urol. 158, 1562–1567 [PubMed] [Google Scholar]
- 45.Pikov V., Gillis R.A., Jasmin L., and Wrathall J.R. (1998). Assessment of lower urinary tract functional deficit in rats with contusive spinal cord injury. J. Neurotrauma 15, 375–386 [DOI] [PubMed] [Google Scholar]
- 46.Dolber P.C., Gu B., Zhang X., Fraser M.O., Thor K.B., and Reiter J.P. (2007). Activation of the external urethral sphincter central pattern generator by a 5-HT(1A) receptor agonist in rats with chronic spinal cord injury. Am. J. Physiol. Regul. Integr. Comp Physiol. 292, R1699–R1706 [DOI] [PubMed] [Google Scholar]
- 47.Fraser M. (2011). New insights into the pathophysiology of detrusor-sphincter dyssynergia. Curr. Bladder Dysfunct. Rep. 6, 93–99 [Google Scholar]