Abstract
The definitive diagnosis of spotted fever group (SFG) rickettsioses in humans is challenging due to the retrospective nature and cross reactivity of the serological methods and the absence of reliable and consistent samples for molecular diagnostics. Existing data indicate the transient character of bacteremia in experimentally infected animals. The ability of arthropod vectors to acquire rickettsial infection from the laboratory animals in the absence of systemic infection and known tropism of rickettsial agents to endothelial cells of peripheral blood vessels underline the importance of local infection and consequently the diagnostic potential of skin samples. In order to evaluate the diagnostic sensitivity of rickettsial DNA detection in blood and skin samples, we compared results of PCR testing in parallel samples collected from model laboratory animals infected with Rickettsia rickettsii, Rickettsia parkeri and Rickettsia slovaca-like agent at different time points after infection. Skin samples were collected from ears – away from the site of tick placement and without eschars. Overall, testing of skin samples resulted in a higher proportion of positive results than testing of blood samples. Presented data from model animals demonstrates that testing of skin samples from sites of rickettsial proliferation can provide definitive molecular diagnosis of up to 60–70% of tick-borne SFG rickettsial infections during the acute stage of illness. Detection of pathogen DNA in cutaneous samples is a valuable alternative to blood-PCR at least in model animals.
Keywords: Rocky Mountain spotted fever, Spotted fever rickettsiosis, Diagnosis, Skin biopsies, Guinea pig
1. Introduction
Spotted fever group (SFG) of the genus Rickettsia consists of a large number of diverse arthropod-borne intracellular bacterial species described worldwide and maintained by fleas, ticks, and mites. The list includes endosymbiotic bacteria of the arthropod vectors as well as known pathogens, causing spotted fever group rickettsioses (Walker 1989). At least 9 named SFG Rickettsia species are endemic to the United States (Denison et al., 2014). Rocky Mountain spotted fever (RMSF) caused by Rickettsia rickettsii is the most severe rickettsial illness of humans distributed throughout North, Central, and South America. It is transmitted by tick vectors, including Dermacentor variabilis, Dermacentor andersoni (in North America), several Amblyomma spp. (in Mexico, Central and South America), and Rhipicephalus sanguineus s.l. Rickettsia parkeri transmitted by Amblyomma maculatum is distributed throughout the southeastern and mid-Atlantic United States. It causes moderately severe illness, which shares features with both RMSF and rickettsial pox, including a maculopapular rash and the occurrence of inoculation eschars (Paddock et al., 2008). Rickettsia slovaca-like agent has recently been found in and isolated from D. variabilis ticks (Killmaster et al., 2016). Pathogenicity of this agent in humans has not been established, but it causes mostly subclinical infection in guinea pigs (Zemtsova et al., 2016). Domestic and synanthropic animals are exposed to and infected with rickettsial pathogens as well and may serve as sentinels for assessment of the human risk (Walker 1989; Case et al., 2006; Milagres et al., 2010; McQuiston et al., 2011; Pacheco et al., 2011).
Both diagnosis and surveys of rickettsial infections in domestic animals currently rely primarily on serology (Breitschwerdt et al., 1985; Maggi et al., 2014), just like the clinical diagnosis in humans, where at least 4-fold increase in titers is expected between acute and convalescent serum samples (Chapman et al., 2006). Because it normally takes several weeks from the onset of the disease for the antibody titers to reach diagnostic levels, serological diagnosis is retrospective and provides a confirmation of rickettsial infection only after the patient’s recovery or postmortem. Moreover, pathogen species identification is complicated by serological cross reactivity between SFG Rickettsia spp. Molecular methods are capable of providing better specificity, as well as real-time diagnosis of clinical cases. When molecular diagnosis of RMSF is attempted, it is usually done using acute-stage blood samples. However, SFG rickettsiae primarily target endothelial cells and normally do not infect the circulating blood cells. For example in dogs infected with SFG agents either experimentally or naturally, rickettsial DNA may be detected in the blood only intermittently (Labruna et al., 2009; Levin et al., 2012; Levin et al., 2014). Periods when rickettsial DNA is detectable in the blood of infected in animals are be short in duration, not consistent with the dynamic or severity of clinical symptoms, and is affected by external factors such as initiation of antibiotic treatment (Labruna et al., 2009; Levin et al., 2014). On the other hand, the fact that naïve ticks acquire Rickettsia from infected laboratory animals while the presence of rickettsial DNA cannot be detected by blood-PCR and feeding distantly from the infected ticks, emphasizes the importance of infection in the skin and local mechanisms in rickettsial horizontal transmission (Zemtsova et al., 2010). This corresponds with the previous observation that immunostaining of skin biopsy specimens of rash lesions can identify rickettsiae in 70% of human RMSF patients while blood-PCR was “unacceptably insensitive” (Walker 1995).
Detection of rickettsial DNA has been increasingly reported using eschar tissues or swabs of eschars, although the diagnostic sensitivity of these types of specimens in comparison with traditional blood samples has not been ascertained (Lakos 2002; Wang et al., 2009; Bechah et al., 2011; Mouffok et al., 2011; Myers et al., 2013). Yet, R. rickettsii infections are not usually associated with inoculation eschars, which are rarely observed even in human RMSF patients (Walker et al., 1981; Chen and Sexton 2008), where they would be easier to find than in fur covered animals.
Here we assess whether rickettsial DNA may be identified in skin samples not associated with either eschar, or the site of tick attachment and compare the diagnostic sensitivity of rickettsial DNA detection between blood and skin samples, using guinea pigs as model animals.
2. Materials and methods
Animal studies were conducted at a facility fully-accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. All procedures and husbandry were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (8th edition). Animal protocols were pre-approved by the Centers for Disease Control Institutional Animal Care and Use Committee (IACUC) and monitored by a veterinarian stationed on-site.
Analyses presented here combine PCR results from animals used for multiple studies in vector and reservoir competence of SFG Rickettsia spp. The choice of pathogen isolates and the inoculation dosages had been adjusted so not to cause any mortality in the model animals. Therefore, presented results are from cases of relatively mild, nonlethal, or subclinical rickettsial infections.
One to two month-old specific pathogen free male Hartley guinea pigs weighing 400–500 g were acquired from Charles River Laboratories (http://www.criver.com). Animals were infected with R. rickettsii (isolates BSF-Di6 and AZ3), R. parkeri (isolate Longleaf), or R. slovaca-like agent (D. variabilis isolate (Killmaster et al., 2016). Pathogens were introduced either via a tick-bite or through the needle-inoculation route – intraperitoneally. For tick-borne infections, guinea pigs were exposed to 10–20 nymphal ticks (30–50% prevalence of infection) placed into feeding bags glued onto animals’ backs. Needle-inoculated animals were each injected with spleen/liver tissue homogenate containing a standard infectious dose of 105 DNA copies of a specific Rickettsia sp. Tissue homogenates were prepared by grinding liver and spleen of previously infected guinea pigs in Snider-1 buffer (20% tissue suspension) and cryopreserved in liquid nitrogen as inoculation-ready aliquots until used.
Guinea pigs were observed for the duration of one to three weeks depending on the goal of an individual study. During the observation period, the core temperature and signs of infection of each animal was recorded daily. In guinea pigs infected with R. rick-ettsii, the core temperature usually rose above 39.5 °C at 5–9 days after infection depending on the strain of the pathogen and the mode of infection; and the fever period lasted for 2–7 days. Majority of guinea pigs infected with either R. parkeri or R. slovaca-like agent exhibited only subfebrile temperatures.
Paired blood and skin samples were collected for PCR 2–3 times per week. Skin samples consisted of 2 mm punch biopsies weighing 2 mg taken from an ear, and EDTA-anticoagulated blood samples (100 µl) were collected from a lacerated ear vein. At the end of each individual study, all guinea pigs were euthanized and 5 mg samples of internal organs (liver, spleen, and lung) were also collected for PCR. Blood, skin, and internal tissue samples were immediately stored at −20 °C until DNA extraction. DNA from skin and internal tissues was extracted using the DNeasy Blood & Tissue kit, and DNA from blood samples was extracted with FlexiGene DNA Kit (Qiagen, Gaithersburg, Maryland, USA) according to the manufacturer’s protocols with the final elution volume of 100 µl for all samples. The presence of rickettsial DNA was detected by SYBR green-based PCR assay targeting a 154-bp fragment of the rOmpA gene as described (Eremeeva et al., 2003). All samples were tested in duplicates using 5 µl of the eluted DNA for each reaction. Accordingly, DNA equivalents of 5 µl of whole blood, 0.1 mg of skin tissue, 0.5 mg of internal tissues were represented in each PCR reaction.
In addition, a serum sample was collected from each animal at the end of the study and tested by IFA for the presence of anti-rickettsial IgG antibodies to confirm an infection. IFA was per-formed on guinea pig sera using FITC labeled goat anti-guinea pig IgG (γ) conjugate diluted per manufacturer’s recommendations (KPL, Inc. Gaithersburg, Maryland, USA) and homologous rickettsial antigens.
Proportions of positive skin and blood samples per day post-infection (DPI) were compared using the paired t-test with the hypothesized mean difference = 0 and α = 0.05.
3. Results
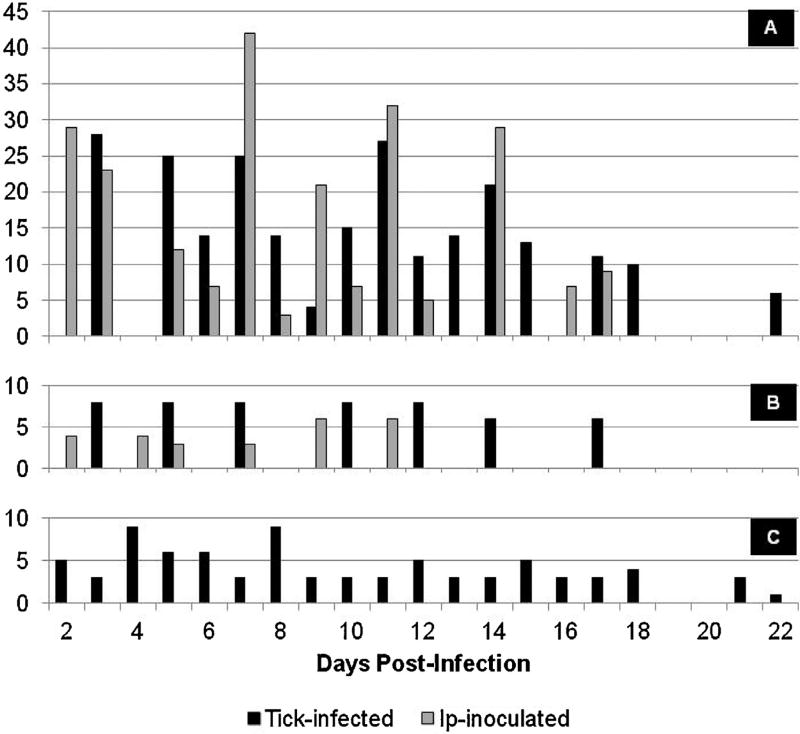
For the purpose of the current comparative analysis, we removed from the data sets any animals that did not become infected as well as all asynchronous samples when both the blood and skin biopsies were not collected at the same time. In total, we compared PCR results of 437 pairs of samples from 161 guinea pigs infected with R. rickettsii, 74 pairs of samples from 31 guinea pigs infected with R. parkeri, and 80 pairs of samples from 24 guinea pigs infected with R. slovaca-like agent. Numbers of paired skin and blood samples tested at different time points post-infection varied from 1 to 42 depending on the pathogen and mode of infection (Fig. 1). Overall, rickettsial DNA was detected in 38.6% of skin samples and in 13.4% of blood samples. Rickettsial DNA was also detected in internal tissues, collected at the time of euthanasia 6–22 DPI, from 109 (67.7%), 24 (77.4%), and 9 (37.5%) of the guinea pigs infected with R. rickettsii, R. parkeri, and R. slovaca-like agent respectively.
Fig. 1.
Total numbers of paired skin and blood samples tested at different time points postinfection: A – from guinea pigs infected with R. rickettsii; B – from guinea pigs infected with R. parkeri; C k from guinea pigs infected with R. slovaca.
3.1. Detection of Rickettsia rickettsii in skin and blood samples
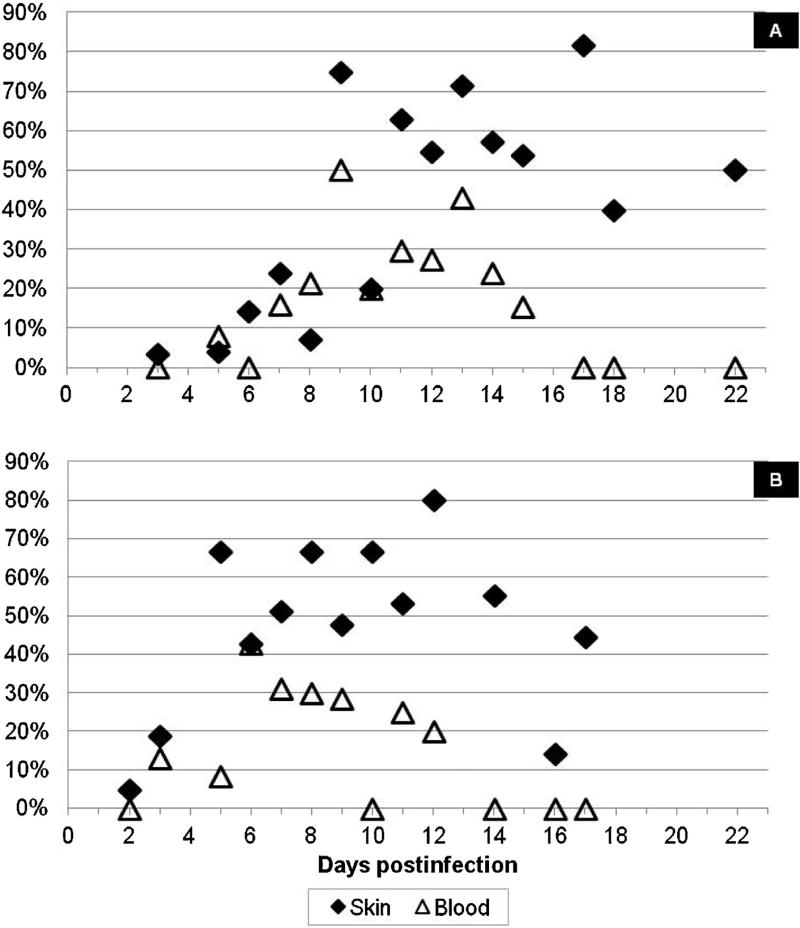
Out of 437 paired samples collected from R. rickettsii-infected guinea pigs for up to three weeks after infection, 173 (39.6%) of skin samples and 74 (16.9%) of blood samples contained rickettsial DNA detectable by PCR (p = 0.00017). The frequency of R. rickettsii detection in either skin or blood samples changed depending on the time post-infection; it was generally higher during the second week of infection (Fig. 2). In guinea pigs infected via tick bite, less than 25% of either blood or skin samples collected from infected animals yielded positive results during the first week post-exposure (Fig. 2A). At 9–17 DPI, rickettsial DNA was detected in 52–82% of skin samples, but only in 15–50% of blood samples. Frequency of detection declined again during the third week of infection. Still, 40–50% of skin samples were PCR-positive during this late period, while all blood samples collected at the same time were negative. Altogether in guinea pigs infected with R. rickettsii via tick bite, 35.7% of skin samples and 16.0% of blood samples collected in three weeks post-infection contained rickettsial DNA (p = 0.0017). Success of pathogen detection in guinea pigs infected via IP inoculation demonstrated similar dynamics (Fig. 2B). Again, the efficiency of pathogen detection was similarly low during the first week of infection, but from 7DPI onward the proportion of skin samples containing rickettsial DNA was consistently higher than that in blood samples. Thus, detection of R. rickettsii DNA in ear-skin was significantly more frequent than in the blood collected from ear vein, even though skin samples were distant from the tick feeding site and not associated with a rash.
Fig. 2.
Percent of PCR-positive skin and blood samples collected from R. rickettsii-infected guinea pigs: A k guinea pigs infected via tick bite; B – guinea pigs infected via IP inoculation.
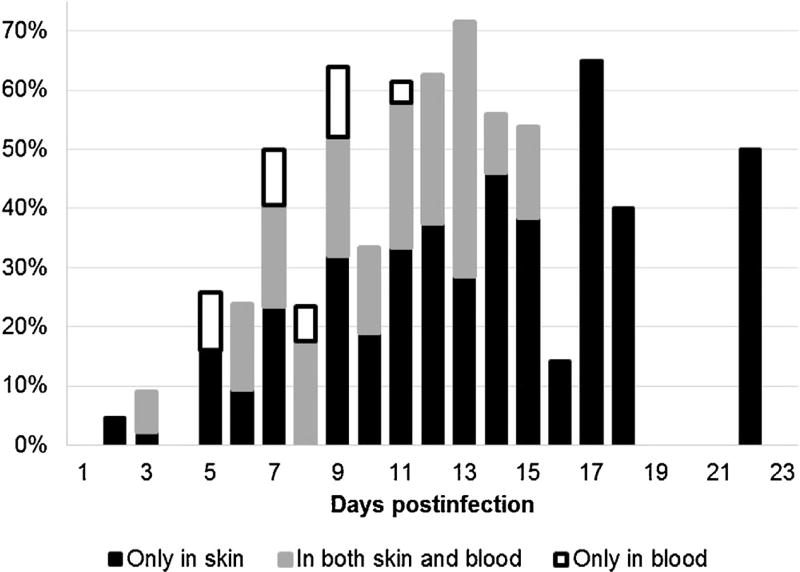
In guinea pigs infected with R. rickettsii, at least one of the samples – either skin or blood – was PCR-positive on 188 occasions out of 437 analyzed sample pairs. Among those 188, rickettsial DNA was detected 15 (8.0%) times in blood samples only, 59 (31.4%) times in both blood and skin samples, and 114 (60.6%) times only in skin samples. Thus, the overall concordance between two types of samples was low – less than one third of positive animals being identified simultaneously by both blood- and skin-PCR. Therefore, we evaluated how the concordance between sample types changes over the 3-wk duration of infection, and whether dual testing of both skin and blood samples may improve the chances for detection of the pathogen (Fig. 3). Both the concordance of results between types of samples and the added value of dual testing varied throughout the observation period. During the first 7–8 DPI, the frequency of pathogen detection in either skin or blood was low, and the dual testing of both was the most beneficial. In fact, 24 (44%) and 10 (18.5%) out of the 54 positive cases identified before 9 DPI would have been missed if we tested only blood or only skin sample respectively. The highest success in pathogen detection was recorded at 9–17 DPI when R. rickettsii DNA was present in up to 60–70% of dual (skin + blood) samples collected from infected animals. During this period, the absolute majority (96.1%) of positive cases would have been successfully identified if just cutaneous samples had been tested. From 16 DPI to the end of the observation period, R. rickettsii DNA was detectable exclusively in skin biopsies, and additional testing of blood samples did not improve the diagnostic sensitivity (Fig. 3).
Fig. 3.
Proportions of skin and blood samples collected simultaneously from guinea pigs infected with R. rickettsii, where rickettsial DNA was identified at different time point after infection – the added value of dual testing.
3.2. Detection of Rickettsia parkeri in skin and blood samples
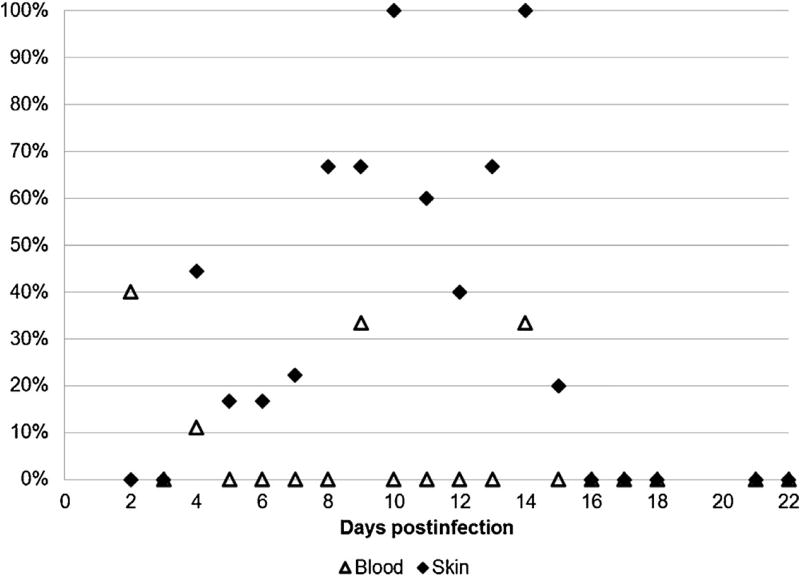
Out of 74 skin biopsies collected from guinea pigs infected with R. parkeri, rickettsial DNA was detected in a total of 20 (27.0%) samples. In particular, 9 of 52 (17.3%) and 11 of 22 (50%) of tested skin samples were PCR-positive in animals infected via tick bite and IP-inoculation respectively. In contrast, all 74 blood samples were PCR-negative (p = 0.0002). The frequency of R. parkeri detection in cutaneous samples fluctuated significantly from one day to another (Fig. 4) due to low numbers of samples tested on any individual day postinfection. Still, in inoculated guinea pigs, the highest percentage of PCR-positive results was recorded at 5–7 DPI; it was highest (3 of 6) on 10 DPI in those exposed to infected ticks. Overall, 27–50% of skin biopsies were PCR-positive if collected between days 4 and 13 after infection.
Fig. 4.
Percent of PCR-positive skin and blood samples collected from R. parkeri-infected guinea pigs.
3.3. Detection of Rickettsia slovaca-like agent in skin and blood samples
A total of 80 paired skin/blood samples were collected from 24 guinea pigs infected with R. slovaca-like agent via infestation with infected D. variabilis ticks. The presence of rickettsial DNA was confirmed in 26 (32.5%) skin samples and 5 (6.3%) blood samples (p = 0.0003). Although numbers of samples tested on each individual day are not sufficient to analyze temporal dynamics of rickettsial growth and distribution, it appears that the frequency of R. slovaca-like agent detection in skin biopsies was higher at 8–14 DPI (Fig. 5). During this period, rickettsial NDA was identified in 60% (3 of 5) to 100% (3 of 3) of samples. During the same period, detection of the same agent in blood was inconsistent and unpredictable – only 1 of 3 samples on days 9 and 14 (Fig. 5).
Fig. 5.
Percent of PCR-positive skin and blood samples collected from guinea pigs infected with R. slovaca-like agent via tick bite.
4. Discussion
Using guinea pigs experimentally infected with R. rickettsii, R. parkeri, and R. slovaca-like agent as model animals, we assessed whether rickettsial DNA can be detected in cutaneous biopsies not associated with inoculation eschars or the site of tick feeding; and compared the frequency of pathogen detection between skin and blood samples. Our results show that DNA of all three pathogens is routinely identifiable in skin biopsies during the acute stage of infection. This is true not only for R. rickettsii and R. parkeri, which engender recognizable clinical signs of illness, but also for the R. slovaca-like agent that consistently produces only subclinical infections in guinea pigs (Zemtsova et al. 2016). Moreover, chances for pathogen detection in skin are significantly higher than in blood, even though both types of samples have been collected simultaneously from the same ear, and despite the fact that the quantity of skin tissue represented in each PCR reaction (0.1 mg) was 50 times lower than that of the blood.
Based on the fact that the guinea pigs core body temperature is higher than that used for propagation of Rickettsia spp. in vitro (32–34 °C), it might be hypothesized that rickettsiae prefer to concentrate in the coolest tissues, namely the scrotum and ears. If this is true, then the ear should also be the most opportune site for detection of rickettsial DNA in other domestic and wild animals. At the same time, SFG rickettsiae are often found in internal organs collected postmortem from guinea pigs (this study), as well as from dogs (Levin et al., 2014) and humans (Walker et al., 1983; Walker and Gear 1985; Favacho et al., 2011). In dogs infected with either R. conorii or R. rickettsii, widespread abundant petechiae on gums and buccal mucosa developed in parallel with macular skin rash (Levin et al., 2012, 2014). Whether the rates of rickettsial growth in the endotelium of live animals is affected by temperature or not, it is logical to expect that chances for detection and identification of rickettsial pathogens in skin biopsies may be maximized by testing samples from finely vasculated sites, which have higher density of endothelial cells available for rickettsial proliferation.
The percentage of animals with identifiable DNA in either skin or blood vary in accordance with the prodromal, acute, and recovery phases of infection. In guinea pigs infected with R. rickettsii, percentage of PCR-positive skin sample was highest at 9–17 DPI, or approximately 4–10 days after the first appearance of fever. During that time, R. rickettsii DNA could be successfully identified in 60% to 80% of infected animals by testing of biopsies. At the same time, chances for detection of R. rickettsii DNA in blood samples did not exceed 35–45% of infected animals. In guinea pigs infected with R. parkeri and R. slovaca-like agent, chances for detection of rickettsial DNA in skin samples during the peak of infection were as high as 33–66% and 60–100% respectively. On the contrary, R. slovaca-like agent was found in only 5 (6.3%) blood samples widely and irregularly distributed throughout the period of observation, and R. parkeri DNA was not present in circulating blood at all. The much greater success of pathogen detection in skin is in agreement with the described predilection of SFG Rickettsia for endothelial cells. The route of infection did not affect the comparative diagnostic value of the two types of samples as the frequency of pathogen detection in blood remained lower than that in skin in animals infected with R. rickettsii or R. parkeri via tick-bite or intraperitoneal route.
Among the three pathogens used in this study, R. rickettsii was detected in blood samples more regularly than either R. parkeri or R. slovaca-like agent. It remains to be studied if this distinction is due to the virulence of different agents or to their preferred routes of dissemination throughout the organism. In infections caused by R. rickettsii, PCR results did not always correspond within blood and skin sample pairs. In approximately 61% of PCR-positive sample pairs, rickettsial DNA was detectable in the skin only, while in 8% of sample pairs it was present only in the blood. Thus, testing of venous blood does add power to identification of R. rickettsii infections, although cutaneous samples provide a significantly higher sensitivity. The degree of incongruence between the sample types changes with the progression of illness. In the studied guinea pigs, this incongruence was the largest at 5–9 DPI when up to 1/5 of the identified infections had positive blood-PCR, but negative skin-PCR. This resulted in diagnosis of infection in approximately 5–10% cases in addition to those 18–52% already identifiable by testing of skin biopsies. The 5–9 DPI time period roughly coincides with the first appearance of fever in guinea pigs infected with the particular isolate of R. rickettsii (DI6) used in this study. This suggests that dual testing of both skin and blood samples may be beneficial for diagnosis of R. rickettsii infections at the time of appearance of the first nonspecific signs of illness. After that, infections can be successfully identified by skin-PCR only, and additional testing of blood samples may not improve detection and identification of R. rickettsii DNA.
Several species of tick-borne Rickettsia cause serious illness in humans. In particular, RMSF caused by R. rickettsii is a severe fast-progressing disease potentially leading to multi-organ failure unless antibiotic treatment is initiated within a week of the first signs of illness. In cases when diagnosis and appropriate treatment are delayed, RMSF can result in significant mortality. Yet, the initial signs and symptoms of rickettsial infections are nonspecific; several doctor visits are often required before the illness is diagnosed and appropriate treatment may be initiated. Molecular biological methods have the potential to expedite specific diagnosis of infection and rickettsial DNA have been successfully detected in eschar tissue or eschar swabs of patients with several SFG rickettsioses (Lakos 2002; Wang et al., 2009; Bechah et al., 2011; Mouffok et al., 2011; Myers et al., 2013) or in biopsies of a skin rash (Kuloglu et al., 2012; Denison et al., 2014). However, R. rickettsii infections are rarely accompanied by an eschar (Walker et al., 1981; Chen and Sexton 2008), and a typical skin rash may not appear until later stages of illness, or not at all (Walker 1989; Sexton and Corey 1992; Favacho et al., 2011). To date, there are no published studies assessing usefulness of skin biopsies not associated with eschars or sites of tick attachment for detection of SFG pathogens in either humans or animals.
Our analysis demonstrates that in model animals, DNA of R. rickettsii, R. parkeri, and R. slovaca-like agent can be identified by PCR in skin biopsies with significantly higher frequency compared to blood samples even if both are taken from the same ear. This confirms earlier suggestion that skin biopsy is more useful for detecting the SFG etiologic agent than an acute blood sample (Chapman et al., 2006). In guinea pigs with relatively mild or subclinical infections, R. rickettsii, R. parkeri, and R. slovaca-like agent DNAs is identified in up to 60–80%, 50–70%, and 60–100% of ear skin samples collected during the acute stage of infection respectively. Interestingly, the percentage of PCR-positive eschar and rash samples (70–73%) reported in human patients with Mediterranean spotted fever in Turkey was comparable to our results (Kuloglu et al., 2012). On the other hand, as the percentage of PCR-positive results appears to rise and fall in accord with the progress of infection, it may be that the frequency of detection of SFG rickettsial DNA in skin will be even higher in more severe cases. Although usefulness of skin biopsies for molecular diagnosis of SFG rickettsioses appear much greater than that of blood samples, the latter may somewhat add in rickettsial diagnosis during the early stages of infection.
Here, we analyzed the available data to compare sensitivity of rickettsial detection between blood and skin samples in guinea pigs only. To the best of our knowledge, comparable volumes of data characterizing either domestic and wild animal species or human patients are not available. Further studies in both animals and humans are needed to determine the best sites for specimen collection, improve the sensitivity of acute-stage diagnosis of rickettsial infections, as well as to find least invasive sampling techniques.
Acknowledgments
This project was supported in part by an appointment of Alyssa N. Snellgrove to the Internship/Research Participation Program at the Centers for Disease Control and Prevention, administered by the Oak Ridge Institute for Science and Education through an inter-agency agreement between the U.S. Department of Energy and CDC.
Footnotes
Disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Centers for Disease Control and Prevention or the U.S. Government. The authors, as employees of the U.S. Government, conducted the work as part of their official duties.
References
- Bechah Y, Socolovschi C, Raoult D. Identification of rickettsial infections by using cutaneous swab specimens and PCR. Emerg. Infect. Dis. 2011;17:83–86. doi: 10.3201/eid1701.100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Meuten DJ, Walker DH, Levy M, Kennedy K, King M, Curtis B. Canine Rocky Mountain spotted fever: a kennel epizootic. Am. J. Vet. Res. 1985;46:2124–2128. [PubMed] [Google Scholar]
- Case JB, Chomel B, Nicholson W, Foley JE. Serological survey of vector-borne zoonotic pathogens in pet cats and cats from animal shelters and feral colonies. J. Feline Med. Surg. 2006;8:111–117. doi: 10.1016/j.jfms.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, Sexton DJ, Buckingham SC, Marshall GS, Storch GA, Dasch GA, McQuiston JH, Swerdlow DL, Dumler SJ, Nicholson WL, Walker DH, Eremeeva ME, Ohl CA Tickborne Rickettsial Diseases Working Group, CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis? United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm. Rep. 2006;55:1–27. [PubMed] [Google Scholar]
- Chen LF, Sexton DJ. What’s new in Rocky Mountain spotted fever? Infect. Dis. Clin. North Am. 2008;22:415–432. doi: 10.1016/j.idc.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Denison AM, Amin BD, Nicholson WL, Paddock CD. Detection of Rickettsia rickettsii Rickettsia parkeri, and Rickettsia akari in skin biopsy specimens using a multiplex real-time polymerase chain reaction assay. Clin. Infect. Dis. 2014;59:635–642. doi: 10.1093/cid/ciu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremeeva ME, Dasch GA, Silverman DJ. Evaluation of a PCR assay for quantitation of Rickettsia rickettsii and closely related spotted fever group rickettsiae. J. Clin. Microbiol. 2003;41:5466–5472. doi: 10.1128/JCM.41.12.5466-5472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favacho AR, Rozental T, Calic SB, Scofield MA, Lemos ER. Fatal Brazilian spotless fever caused by Rickettsia rickettsii in a dark-skinned patient. Rev. Soc. Bras. Med. Trop. 2011;44:395–396. doi: 10.1590/s0037-86822011000300028. [DOI] [PubMed] [Google Scholar]
- Killmaster LF, Zemtsova GE, Montgomery M, Schumacher L, Burrows M, Levin ML. Isolation of a Rickettsia slovaca-like agent from Dermacentor variabilis ticks in Vero cell culture. Vector Borne Zoonotic Dis. 2016;16:61–62. doi: 10.1089/vbz.2015.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuloglu F, Rolain JM, Akata F, Eroglu C, Celik AD, Parola P. Mediterranean spotted fever in the Trakya region of Turkey. Ticks Tick-Borne Dis. 2012;3:298–304. doi: 10.1016/j.ttbdis.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Labruna MB, Kamakura O, Moraes J, Horta MC, Pacheco RC. Rocky Mountain spotted fever in dogs, Brazil. Emerg. Infect. Dis. 2009;15:458–460. doi: 10.3201/eid1503.081227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakos A. Tick-borne lymphadenopathy (TIBOLA) Wien.K lin. Wochenschr. 2002;114:648–654. [PubMed] [Google Scholar]
- Levin ML, Killmaster LF, Zemtsova GE. Domestic dogs (Canis familiaris) as reservoir hosts for Rickettsia conorii. Vector Borne Zoonotic Dis. 2012;12:28–33. doi: 10.1089/vbz.2011.0684. [DOI] [PubMed] [Google Scholar]
- Levin ML, Killmaster LF, Zemtsova GE, Ritter JM, Langham G. Clinical presentation, convalescence, and relapse of Rocky Mountain spotted fever in dogs experimentally infected via tick bite. PLoS One. 2014;9:e115105. doi: 10.1371/journal.pone.0115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi RG, Birkenheuer AJ, Hegarty BC, Bradley JM, Levy MG, Breitschwerdt EB. Comparison of serological and molecular panels for diagnosis of vector-borne diseases in dogs. Parasites Vectors. 2014;7:127. doi: 10.1186/1756-3305-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston JH, Guerra MA, Watts MR, Lawaczeck E, Levy C, Nicholson WL, Adjemian J, Swerdlow DL. Evidence of exposure to spotted fever group rickettsiae among Arizona dogs outside a previously documented outbreak area. Zoonoses Public Health. 2011;58:85–92. doi: 10.1111/j.1863-2378.2009.01300.x. [DOI] [PubMed] [Google Scholar]
- Milagres BS, Padilha AF, Barcelos RM, Gomes GG, Montandon CE, Pena DC, Nieri Bastos FA, Silveira I, Pacheco R, Labruna MB, Bouyer DH, Freitas RN, Walker DH, Mafra CL, Galvao MA. Rickettsia in synanthropic and domestic animals and their hosts from two areas of low endemicity for Brazilian spotted fever in the eastern region of Minas Gerais, Brazil. Am. J. Trop. Med. Hyg. 2010;83:1305–1307. doi: 10.4269/ajtmh.2010.10-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouffok N, Socolovschi C, Benabdellah A, Renvoise A, Parola P, Raoult D. Diagnosis of rickettsioses from eschar swab samples, Algeria. Emerg. Infect. Dis. 2011;17:1968–1969. doi: 10.3201/eid1710.110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers T, Lalani T, Dent M, Jiang J, Daly PL, Maguire JD, Richards AL. Detecting Rickettsia parkeri infection from eschar swab specimens. Emerg. Infect. Dis. 2013;19:778–780. doi: 10.3201/eid1905.120622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco RC, Moraes-Filho J, Guedes E, Silveira I, Richtzenhain LJ, Leite RC, Labruna MB. Rickettsial infections of dogs, horses and ticks in Juiz de Fora southeastern Brazil, and isolation of Rickettsia rickettsii from Rhipicephalus sanguineus ticks. Med. Vet. Entomol. 2011;25:148–155. doi: 10.1111/j.1365-2915.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, McLellan SL, Goddard J, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremeeva ME. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin. Infect. Dis. 2008;47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- Sexton DJ, Corey GR. Rocky Mountain spotless and almost spotless fever: a wolf in sheep’s clothing. Clin. Infect. Dis. 1992;15:439–448. doi: 10.1093/clind/15.3.439. [DOI] [PubMed] [Google Scholar]
- Walker DH, Gear JH. Correlation of the distribution of Rickettsia conorii, microscopic lesions, and clinical features in South African tick bite fever. Am. J. Trop. Med. Hyg. 1985;34:361–371. doi: 10.4269/ajtmh.1985.34.361. [DOI] [PubMed] [Google Scholar]
- Walker DH, Gay RM, Valdes-Dapena M. The occurrence of eschars in Rocky Mountain spotted fever. J. Am. Acad. Dermatol. 1981;4:571–576. doi: 10.1016/s0190-9622(81)70059-8. [DOI] [PubMed] [Google Scholar]
- Walker DH, Hawkins HK, Hudson P. Fulminant Rocky Mountain spotted fever: its pathologic characteristics associated with glucose-6-phosphate dehydrogenase deficiency. Arch. Pathol. Lab. Med. 1983;107:121–125. [PubMed] [Google Scholar]
- Walker DH. Rickettsioses of the spotted fever group around the world. J. Dermatol. 1989;16:169–177. doi: 10.1111/j.1346-8138.1989.tb01244.x. [DOI] [PubMed] [Google Scholar]
- Walker DH. Rocky Mountain spotted fever: a seasonal alert. Clin. Infect. Dis. 1995;20:1111–1117. doi: 10.1093/clinids/20.5.1111. [DOI] [PubMed] [Google Scholar]
- Wang JM, Hudson BJ, Watts MR, Karagiannis T, Fisher NJ, Anderson C, Roffey P. Diagnosis of Queensland tick typhus and African tick bite fever by PCR of lesion swabs. Emerg. Infect. Dis. 2009;15:963–965. doi: 10.3201/eid1506.080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemtsova G, Killmaster LF, Mumcuoglu KY, Levin ML. Co-feeding as a route for transmission of Rickettsia conorii israelensis between Rhipicephalus sanguineus ticks. Exp. Appl. Acarol. 2010;52:383–392. doi: 10.1007/s10493-010-9375-7. [DOI] [PubMed] [Google Scholar]
- Zemtsova GE, Killmaster LF, Montgomery M, Burrows M, Schumacher L, Levin ML. First report of Rickettsia identical to R. slovaca in colony-originated D. variabilis in the US: detection, laboratory animal model and vector competence of ticks. Vector Borne Zoonotic Dis. 2016;16:77–84. doi: 10.1089/vbz.2015.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]