Abstract
Brains systems undergo unique and specific dynamic changes at the cellular, circuit, and systems level that underlie the transition to adult-level cognitive control. We integrate literature from these different levels of analyses to propose a novel model of the brain basis of the development of cognitive control. The ability to consistently exert cognitive control improves into adulthood as the flexible integration of component processes, including inhibitory control, performance monitoring, and working memory, increases. Unique maturational changes in brain structure, supported by interactions between dopaminergic and GABAergic systems, contribute to enhanced network synchronization and an improved signal-to-noise ratio. In turn, these factors facilitate the specialization and strengthening of connectivity in networks supporting the transition to adult levels of cognitive control. This model provides a novel understanding of the adolescent period as an adaptive period of heightened experience-seeking necessary for the specialization of brain systems supporting cognitive control.
Keywords: development, cognitive control, networks, dopamine, GABA, inhibition, working memory, performance monitoring
INTRODUCTION
Cognitive control describes the ability to flexibly, voluntarily, and adaptively coordinate behavior in the service of internal goals in a noisy and changing environment (Badre 2011). Cognitive control is underlain by the differential contribution of separable but interacting components, including, but not limited to, task-set switching and maintenance, adaptive gating, working memory, response selection, and response inhibition (Badre 2011, Lenartowicz et al. 2010, Sabb et al. 2008). Here, we propose a model of cognitive control that focuses on the flexible integration of processes supporting the online maintenance of goal-relevant information (working memory); the suppression of competing, goal-irrelevant information (inhibitory control); and the continuous evaluation of the effectiveness of selected actions (performance monitoring).
Although cognitive control is available early in development, the success of its implementation continues to improve into adulthood. As such, the development of cognitive control does not reflect the emergence of a new ability but rather a continued refinement of an existing set of cognitive functions, which is evidenced by increased rates of correct responses into adulthood on tasks requiring cognitive control. Although individuals make dramatic gains in successful cognitive control from childhood to adolescence, adolescent control remains more variable than in adulthood. The transition to adult levels of cognitive control is of particular interest for understanding the dynamics inherent to healthy and impaired cognitive development.
Adolescence begins at the onset of puberty, commonly occurring between the ages of 10–16 years in humans, and spans the second decade of life (Blakemore et al. 2010, Spear 2000). Across cultures and species, the pubertal period is characterized by increases in sensation and novelty seeking underlain by the drive for short-term rewards (Steinberg 2004). Although these behaviors are part of normative development, they can lead to risk-taking that contributes to heightened mortality rates during adolescence (Heron 2012) and have important implications for areas such as juvenile law (Luna 2012). Furthermore, cognitive control is impaired in major psychopathology, which also emerges in the adolescent period (Paus et al. 2008). As such, limitations in readily and flexibly implementing cognitive control in adolescence are thought to play a critical role in the vulnerabilities to adolescent risk-taking and to the emergence of psychopathology.
Cognitive control is supported by the integration of component processes, including inhibitory control, working memory, and performance monitoring as well as modulation by motivational systems. To identify the nature of cognitive control development, researchers have studied components independently. Recent developments in network-based neuroimaging methods have provided a framework to investigate interactions between brain systems, including those supporting the components of cognitive control. In this assessment of the current literature, we present the development of individual component processes of cognitive control as well as the maturation of associated brain networks and their integration. Taken together, these lines of evidence provide support for an interactive component model of cognitive control. This model extends the current developmental literature, which underscores the interplay between cognitive control and motivational systems in adolescence, to mechanisms underlying brain systems–level change. Specifically, we propose that changes in dopaminergic signaling throughout adolescence underlie the enhanced ability of cognitive control components to dynamically integrate and mature throughout adolescence.
DEVELOPMENT OF THE CORE COMPONENTS OF COGNITIVE CONTROL
Inhibitory Control
Inhibitory control describes the ability to suppress a prepotent, goal-incompatible response in favor of a planned, goal-directed response (Bari & Robbins 2013, Luna 2009, Nigg 2000). Although the ability to exert inhibitory control is present even in infancy (Johnson 1995), the rate of inhibitory errors decreases through childhood and adolescence (Bjorklund & Harnishfeger 1995, Dempster 1992, Luna et al. 2004) (Figure 1a). Functional magnetic resonance imaging (fMRI) studies characterizing age-related differences in core inhibitory control circuitry activation have predominantly focused on the role of prefrontal cortex. However, how changes in the integration of prefrontal systems affect the development of inhibitory control is unclear, as studies have found both developmental increases and decreases in the recruitment of these regions (Luna et al. 2001, Marsh et al. 2006, Rubia et al. 2006, Tamm et al. 2002). More recently, studies have demonstrated decreases in prefrontal engagement with age (Alahyane et al. 2014, Ordaz et al. 2013) that parallel improvement in performance (Dwyer et al. 2014). In a longitudinal fMRI study investigating inhibitory control performance, task-related prefrontal engagement decreased from childhood to adolescence, when performance reached adult levels (Ordaz et al. 2013) (Figure 1b). This decreased engagement of prefrontal systems may reflect decreased effort with age in the same way that higher cognitive load conditions require greater effort and greater prefrontal engagement in adults (Carpenter et al. 1999). Additionally, greater synchronization of relevant prefrontal systems may reduce local processing demands that could lower the blood-oxygen-level-dependent (BOLD) response (Ghuman et al. 2008). However, the dorsal anterior cingulate cortex (dACC), which supports performance monitoring (see below), shows increased activation with age and mediated age by performance (Figure 1c). These results suggest that the development of inhibitory control during adolescence is driven, at least in part, by increased engagement of performance monitoring in the dACC. These findings are consistent with cross-sectional fMRI (Adleman et al. 2002, Rubia et al. 2007, Velanova et al. 2008) and extensive EEG studies (Ferdinand & Kray 2014, Santesso & Segalowitz 2008, Segalowitz et al. 2010), which also show increased participation of the ACC with age during inhibitory control tasks, further highlighting the importance of integration among component processes of cognitive control.
Figure 1.
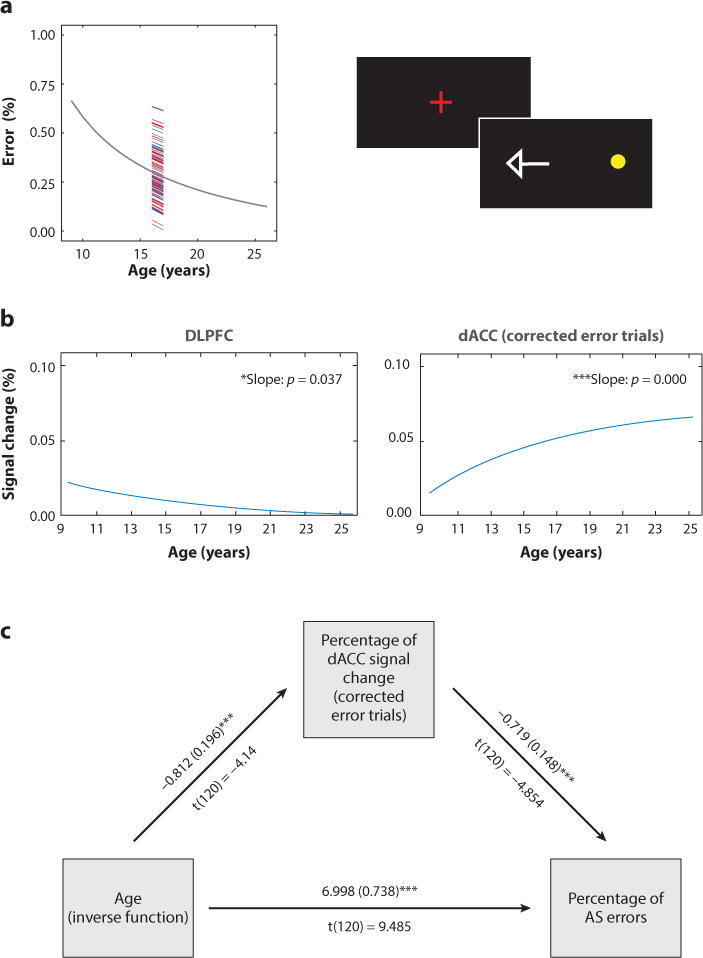
Results from a longitudinal study of inhibitory control. (a) The rate of response inhibition failures in an AS task decreased with age, with intersubject variability remaining constant with age. The vertical bar indicates the rate of decrease for individual subjects (males in blue, females in red). (b) Activation in the DLPFC decreased until adolescence, when it reached adult levels, whereas activation in the dACC increased with age through adolescence (p-value significance of an inverse fit from a mixed model). (c) Furthermore, dACC percent signal change during error trials mediated the effect of age on performance. One asterisk indicates significance at p < 0.05, and three asterisks indicate significance at p < 0.001. Abbreviations: AS, antisaccade; dACC, dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex. Adapted with permission from Ordaz et al. (2013).
Performance Monitoring
Performance monitoring refers to the ability to supervise actions and their consequences. Critical to this process are conflict monitoring and error processing, both of which rely on the ACC (Alexander & Brown 2010, Braver et al. 2001). Specifically, the dACC has been found to support inhibitory control through its supervisory processing (Polli et al. 2005). The ACC, especially its dorsal regions (Brodmann areas 32 and 24), has robust connectivity with many regions, including premotor, supplementary motor, and primary motor regions; insula; nucleus accumbens; dorsal striatum; and the lateral basal nucleus of the amygdala (Shackman et al. 2011, Shenhav et al. 2013). The dACC also receives nociceptive information via the spinothalamic tract, making the dACC an anatomical hub that integrates information regarding pain, negative affect, and performance monitoring (for reviews, see Shackman et al. 2011, Shenhav et al. 2013) (see sidebar, Hubs in Networks). As such, the dACC may serve as a hub for the interconnectivity of networks that may have a protracted development through adolescence.
The increase in the ability to monitor performance is a hallmark of development. As described above, studies have found an increase in dACC recruitment during development (Rubia et al. 2007, Velanova et al. 2008), with compelling longitudinal evidence (Ordaz et al. 2013). Similarly, electrophysiological evidence supports an increased ability throughout adolescence for the anterior cingulate to monitor performance in the form of increased error-related negativity after an error is committed (Santesso & Segalowitz 2008, Wiersema et al. 2007). Given the supervisory role of the performance monitoring system, developmental improvements likely generalize to improvements in other components of cognitive control.
Working Memory
Working memory—the ability to maintain a representation online to guide goal-directed behavior (Baddeley 1986)—is supported by a widely distributed circuitry. Studies have implicated frontoparietal regions, as well as sensory regions, in supporting organizational, supervisory, and mnemonic aspects of working memory (Riggall & Postle 2012). Developmental studies of working memory consistently indicate increasing accuracy from childhood into late adolescence (Conklin et al. 2007, Crone et al. 2006, Luna et al. 2004). In an oculomotor spatial working memory task, overall accuracy appeared adult-like by adolescence; however, precision of subsequent corrective responses continued to improve until young adulthood (Luna et al. 2004). These results suggest interactions between working memory and performance monitoring. fMRI studies indicate that although children and adolescents utilize prefrontal-parietal cortical circuitry similar to that of adults (Geier et al. 2009, Scherf et al. 2006), improvements in working memory performance are paralleled by age-related enhancements in the recruitment of this circuitry (Klingberg et al. 2002, O’Hare et al. 2008, Thomason et al. 2009). Recently, Satterthwaite et al. (2013) investigated an unprecedentedly large cohort (951 8–22 year olds) of subjects and found that developmental improvements in working memory were associated with increased activation of executive network regions and deactivation of default mode network regions. Notably, developmental differences in working memory are greatest when manipulation (Crone et al. 2006) or distraction suppression is required (Olesen et al. 2007), highlighting the importance of integrating inhibitory control processes to support mature working memory.
Influence of Motivational Systems on Cognitive Control
Importantly, cognitive control is influenced by interactions with reward circuitry in the presence of incentives. For example, during adolescence, inhibitory control performance improves to adult-like levels when correct responses are rewarded (Geier & Luna 2012, Padmanabhan et al. 2011). fMRI findings suggest that, in addition to increased activation of motivational systems (e.g., ventral striatum), adolescents recruit control regions to a higher level than adults when presented with a reward contingency (Geier et al. 2010, Padmanabhan et al. 2011). This may reflect a process by which motivational systems enhance behaviors that lead to reward receipt, including cognitive control abilities.
Taken together, these results indicate that core components of cognitive control continue to develop throughout adolescence. Furthermore, age-related improvements in cognitive control ability are related to both the development of component-specific brain systems and their integration. Hence, it is imperative to understand maturational changes in network dynamics supporting the integration of information across distributed brain systems that may affect behavior. Next, we briefly describe the current understanding of network changes through development.
NETWORK DEVELOPMENT
Given that cognitive control is supported by widely distributed circuitries (Mesulam 1990, Bressler & Menon 2010), developmental improvements in cognition are increasingly thought to be driven by enhancements in the functional collaboration among specialized networks (Fair et al. 2009, Luna & Sweeney 2004). The application of graph theoretical approaches to neuroscience has afforded researchers the ability to characterize regional interactions based on the temporal signature of spontaneous brain activity (Biswal et al. 1995) and to understand how these interactions change with development (Bullmore & Sporns 2009). These approaches have been used to identify unique networks that support distinct aspects of behavior (Bressler & Menon 2010). Structural networks are composed of white matter pathways connecting localized brain regions, providing the basis for functional integration. Functional networks refer to the predisposition for brain regions to modulate activation with similar temporal dynamics at rest or during a task, inferring functional collaboration. Structural and functional networks, although uniquely determined, are related, whereby structure constrains function and function, in turn, modulates structure (Adachi et al. 2012, Honey et al. 2007). As detailed below, basic aspects of structural and functional network organization are evident before birth and undergo developmental modifications that support greater systems-level integration among increasingly specialized networks. These developmental changes are influenced by brain, body, and environment interactions (Byrge et al. 2014). These processes support the plastic molding of the brain to its particular environment throughout life. Specifically, adolescence is notable as the stage in which plasticity promotes specialization before processes become intrinsic and relatively stable in adulthood.
Developmental changes in brain networks are assessed using graph theoretical measures, including small-worldness, community structure, hubness, and rich-clubness. Small-world architecture refers to the optimal organization of connections that support highly clustered subnetworks integrating through selected dedicated pathways (Bassett & Bullmore 2006), supporting efficient local and global information processing (Fair et al. 2009, Sporns & Zwi 2004). Network segregation refers to the clustering of densely intraconnected brain regions generating specialized processing, whereas network integration refers to the ability to combine specialized information from distributed brain regions and networks. Both segregation and integration of brain networks are believed to play a pivotal role during development (Fair et al. 2007). Hubs refer to brain regions that are critical for network integration (Buckner et al. 2009, Sporns et al. 2007) (see sidebar, Hubs in Networks). They form an intraconnected network among themselves, referred to as a rich club, supporting global brain communication and interconnectivity across subnetworks (van den Heuvel & Sporns 2011). We next describe known changes in these network features and their implications.
Network Development in Adolescence
Core components of structural network topology, such as small-worldness, are evident by infancy (Batalle et al. 2012). Structural network segregation decreases from 12 to 30 years of age (Dennis et al. 2013a), whereas network integration increases throughout adolescence (Dennis et al. 2013a, Hagmann et al. 2010). Rich clubs in structural brain networks are evident by 30 weeks gestation, with subsequent proliferation of connections until birth (Ball et al. 2014). By childhood, aspects of rich-club organization are stable (Grayson et al. 2014), with additional nodes becoming integrated during adolescence (Dennis et al. 2013b).
Canonical adult functional networks are observable by two years of age (Doria et al. 2010, Fransson et al. 2007, Gao et al. 2014, Thomason et al. 2013). Similar to structural networks, small-worldness is present throughout childhood and adolescence (Hwang et al. 2012). However, the localization of hubs shifts from predominance in the sensory cortices to the association cortices during childhood (Fransson et al. 2011, Menon 2013). By the onset of adolescence, the organization, number, and connectivity of the hub architecture are adult-like (Hwang et al. 2012). Moreover, functional rich-club organization is largely evident in children, with a slight increase in the number of nodes participating in rich-club organization in adulthood (Cao et al. 2014, Grayson et al. 2014). The relatively early stabilization of hub and rich-club organization suggests a foundational architecture in network connectivity that provides a backbone for network specialization and integration. A process of increased integration may be reflected in increases in the strength of connectivity between prefrontal hub and nonhub regions from childhood to adolescence (Hwang et al. 2012). This period of increased integration parallels increased white matter integrity of frontoparietal tracts (Simmonds et al. 2013), engagement of top-down networks supporting cognitive control (Hwang et al. 2010), and performance in cognitive control tasks (Luna et al. 2004).
At a modular level, cognitive control is supported by the effective integration of segregated component processes. Initial studies investigating changes in segregation and integration have found that children have greater strength of short-range connections and weaker long-range connections compared to adults, suggesting that with development, there is a shift in predominance of local to distributed circuit engagement that may reflect increased integration (Cao et al. 2014; Fair et al. 2007, 2009; Supekar et al. 2009). These findings were subsequently undermined by the discovery that head motion, which is greater in children than adults, resulted in spurious, systematic effects that biased stronger short-distance connections in childhood (Hallquist et al. 2013, Power et al. 2012). Furthermore, the modular organization of brain networks should be assessed (van den Heuvel & Sporns 2013) when defining segregation and integration, rather than distance measures alone. Segregation and integration can then be defined by graph theoretical measures sensitive to network structure (Guimerà & Amaral 2005, Power et al. 2013). Although this hypothesis is not yet tested, we predict that segregated components of cognitive control mature relatively early in development, whereas integration of these components should continue to strengthen into adulthood.
Together, functional and structural networks develop along similar trajectories, with organization and efficiency established early in development, whereas integration continues to increase through adolescence. Notably, the ability for networks to integrate is an enduring aspect of network development, suggesting that communication across specialized networks may be a primary feature of age-related improvements in cognitive control.
INTERACTIVE COMPONENT MODEL OF COGNITIVE CONTROL DEVELOPMENT
Thus far, we have provided evidence for the prolonged maturation of the subcomponents of cognitive control ability and their neural correlates. In parallel, we have discussed the development of the brain’s functional and structural network architecture, which support large-scale integration among regions. Recent evidence supports the proposal that the integration of large-scale brain networks underlying cognitive processes is critical for mature cognitive control. For example, integration between the cingulo-opercular network, which subserves task-set maintenance, and the frontoparietal network, which underlies task-set initiation, increases with working memory demands (Cohen et al. 2014). Furthermore, performance in adolescence is associated with greater interaction between the default-mode network and those networks subserving cognitive control, as well as flexible interactions between these and other response-specific networks (Dwyer et al. 2014, Satterthwaite et al. 2013). Next, we synthesize these findings into a new, interactive component model of cognitive control.
We base our model on the proposal that the strengthening of the dynamic interaction of neural systems supporting cognitive control, including working memory, inhibitory control, and performance monitoring, underlies the maturation of cognitive control (Figure 2c,d). This model of control shares features with a recently proposed model of control, relying on context-dependent, cross-component interactions (Cocchi et al. 2013). The components of cognitive control (inhibitory control, performance monitoring, and working memory) are composed of both distinct and overlapping brain regions. Indeed, these three components complement one another, and rarely is one used but not the other (Diamond 2013). Within each component, some regions are more specialized, participating in a singular cognitive control process, while others are shared across processes, playing an integrative role supporting flexible coordinated activity across components (see sidebar, Brain Regions that Support Flexibility). Each pairwise relation between regions carries some connectivity weight, which is modulated by the current task state. As experience accumulates during development, connectivity patterns that facilitate goal achievement would be reinforced, whereas connections leading to unsuccessful or inefficient outcomes would be pruned. This leads to specialization of within- and between-network connectivity in adulthood (Figure 2c). As detailed below, this process is driven by dopamine (DA)-dependent Hebbian plasticity leading to an increase in signal-to-noise ratio and neural synchrony, thereby refining global network structure across the brain (Figure 2a,b). Over the course of development, experience would strengthen connectivity patterns within and, importantly, between components that would support timely and flexible engagement of cognitive control. This proposed model explains decreases in reaction time and response variability in cognitive control tasks, which is a hallmark of cognitive development (Luna et al. 2004, Kail 1991).
Figure 2.
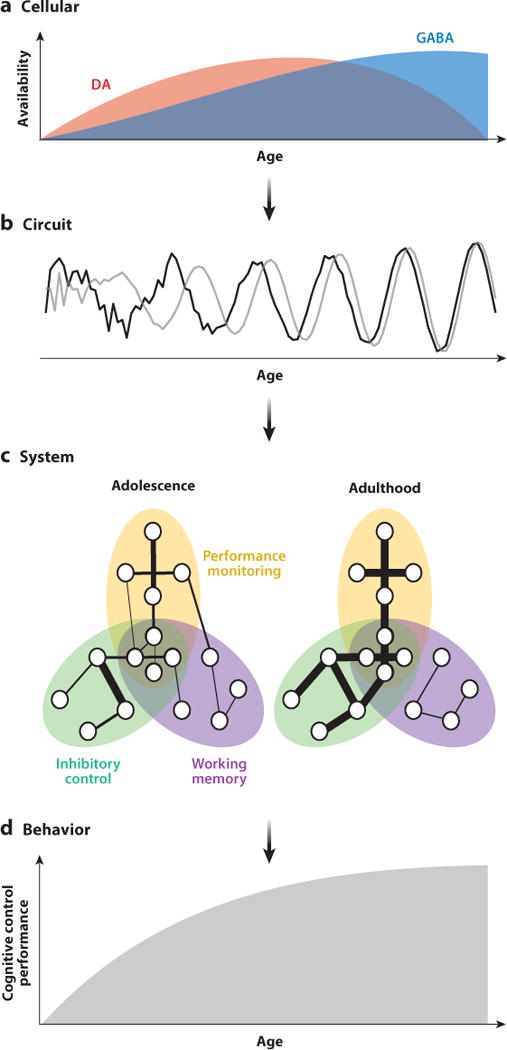
Proposed model of the maturation of cognitive control. At the cellular level (a), DA (red) and GABA (blue) systems undergo dynamic changes throughout adolescence. Maturational neurotransmitter changes lead to increased signal-to-noise ratios, power, and synchrony at the circuit level (b). Gray and black lines represent neural signals from two separate brain regions. These circuit-level changes occur in parallel with systems-level alterations (c) of distributed connectivity patterns (depicted here for successful performance during an inhibitory control task). Ellipses represent components of cognitive control, circles represent brain regions, and lines between them indicate pairwise connections. Line thickness represents connection strength. Circles within overlapping networks represent highly integrative regions (see sidebar, Hubs in Networks). Connections that lead to successful performance are strengthened by adulthood, whereas connections that do not are weakened, pruned, or both. Together, these developmental changes, occurring across multiple levels of brain function, contribute to mature cognitive control behavior (d). Abbreviations: DA, dopamine; GABA, γ-aminobutyric acid.
MECHANISMS SUPPORTING DEVELOPMENTAL SPECIALIZATION OF COGNITIVE CONTROL
Systems-Level Brain Maturation
The brain undergoes a series of progressive and regressive alterations at the systems and molecular levels. Gray matter thins throughout cortical and subcortical regions through adolescence and into adulthood (Gogtay et al. 2004). Synaptic pruning in the prefrontal cortex continues throughout the second decade of life (Petanjek et al. 2011). Gray matter thinning shows protracted development throughout the neocortex and subcortical regions, including the striatum and thalamus (Raznahan et al. 2014, Larsen & Luna 2015) (see sidebar, The Misconception of Prefrontal Systems Maturing Last). The pruning of excess synaptic connections would enhance the signal-to-noise ratio in neural processing within localized regions, supporting complex integration needed for cognitive control. In addition, white matter demonstrates linear increases throughout the brain, with occipital regions preceding parietal, frontal, temporal, and basal ganglia regions, which mature through adolescence (Yakovlev et al. 1967). Myelination significantly increases the integrity of white matter pathways, increasing the speed of neuronal transmission while protecting signal fidelity and enhancing the signal-to-noise ratio. Diffusion tensor imaging (DTI) studies consistently find increases in white matter integrity of corticocortical and corticostriatal tracts, including pathways supporting performance monitoring, socioemotional processing, and cognitive control (Asato et al. 2010, Lebel et al. 2008, Simmonds et al. 2013). Age-related increases in white matter integrity of these pathways are positively correlated with developmental improvements in cognitive performance (Liston et al. 2006, Nagy et al. 2004). Indeed, indices of gray and white matter structure predicted cognitive performance, including working memory, as assessed by a battery of tests (Erus et al. 2014).
Taken together, these findings show that during adolescence, structural development of the brain is starting to reach adult levels, by way of gray matter thinning, synaptic pruning, and myelination. These changes may afford adolescents a newfound ability to employ adult-like circuitry, although less reliably than adults. Specifically, the persistence of excess synapses and relatively slower neuronal signaling may undermine adolescents’ ability to optimally engage brain systems supporting cognitive control.
Cellular Mechanisms of Development
Mechanisms underlying systems-level and behavioral development are driven by cellular-level changes. We propose that interactions between dopaminergic reward circuitry and cortical network functions are central in shaping large-scale control systems. Next, we discuss how age-related changes in dopaminergic and γ-aminobutyric acid (GABA)ergic neurotransmission not only promote neural signaling but also actively shape cortical networks—including component systems of cognitive control—through plasticity (Figure 2a). Although both neurotransmitter systems change throughout life, adolescence may be a critical period for substantive changes within, and between, these systems.
GABA is the primary inhibitory neurotransmitter in the central nervous system (Farrant & Kaila 2007). Cortical GABA interneurons have unique axonal arborizations that allow a single cell to synapse onto many nearby pyramidal cells (Whittington et al. 1995). This enables GABA interneurons to set the dominant frequency of synchronization, entraining excitatory pyramidal cells in the cortex to support cognitive function (Gonzalez-Burgos & Lewis 2008). Neocortical GABAergic projections undergo substantial modification throughout adolescence. Major GABA synaptic markers continue to increase well into the adolescent period in the prefrontal cortex of macaques, including linear increases in parvalbumin (PV)-positive basket cells (Lewis et al. 2004). PV-basket cells are fast-spiking inhibitory interneurons that primarily synapse onto the soma of postsynaptic pyramidal cells (Gonzalez-Burgos & Lewis 2008). Given that inhibitory-excitatory connections serve to distribute synchronized activity to pyramidal cell populations, the functional maturation of PV-basket cells could serve as a cellular mechanism underlying increased synchrony within and between control networks (Bartos et al. 2007). Furthermore, functional inhibition of pyramidal cells increases into periadolescence in the macaque, enhancing gamma oscillations that support working memory (Roux et al. 2012). Computational models further indicate increases in GABA expression into late adolescence (Gonzalez-Burgos et al. 2014). Increased synchrony would provide a basis for selecting and enhancing the connectivity of relevant brain systems through Hebbian processing, potentially supporting segregation and integration of optimal networks of cognitive control through development. EEG studies show increases in synchronous oscillations throughout adolescence (Uhlhaas et al. 2009). Importantly, maturation of inhibitory neuronal function may facilitate the transition to specialization of cognitive control networks by suppressing spontaneous neural activity in favor of external cues driving cortical activity, as evident in visual system maturation (Katz & Shatz 1996, Toyoizumi et al. 2013).
Similar to GABA, the DA system undergoes dynamic changes throughout the adolescent period. Dopaminergic projections from the ventral tegmental area heavily innervate the striatum, the medial prefrontal cortex, and other areas of the limbic cortex (Björklund & Dunnett 2007, Haber & Knutson 2010), influencing reward and emotion processing (Baik 2013) as well as executive functioning (Haber et al. 1995). Owing to its role in motivational processing underlying learning, memory, cognition, emotion, and reward processing (Cools 2008, Schultz 2002), DA function has been of keen interest regarding adolescence sensation seeking (for reviews, see Padmanabhan & Luna 2013, Spear 2000, Wahlstrom et al. 2010). DA synthesis (Andersen et al. 1997), tone (Andersen 2002), and D1/D2 receptor expression peak during adolescence in the striatum and prefrontal cortex in the rodent (Andersen et al. 2000, Gelbard et al. 1989, Leslie et al. 1991, Tarazi & Baldessarini 2000). However, cortical DA tissue concentrations, DA innervation of the dorsolateral prefrontal cortex (DLPFC) and dACC, and midbrain DA neuron activity increase from childhood through early adulthood in rodents and primates (Berger et al. 1985, Kalsbeek et al. 1988, Rosenberg & Lewis 1994; for reviews, see Padmanabhan & Luna 2013, Spear 2000, Wahlstrom et al. 2010). These findings have been highly influential in the proposal that there is a unique peak in DA availability in the human pubertal period (Chambers et al. 2003, Padmanabhan & Luna 2013, Wahlstrom et al. 2010).
Mesofrontal DA directly regulates firing of GABA interneurons and pyramidal cells, including those in the DLPFC (Lewis 1997), thereby modulating the cortical signal-to-noise ratio through pyramidal excitability and recurrent inhibition (Henze et al. 2000, Winterer & Weinberger 2004). Interactions between inhibitory GABA interneurons and excitatory pyramidal neurons are critical for generating high-frequency oscillations responsible for information processing and cognition across several domains (Bas¸ar et al. 2001, Sohal et al. 2009), highlighting the role of dopaminergic neurotransmission in regulating cognitive function. Given that high-frequency oscillations are hypothesized to be the primary contributor to generating increases in the fMRI BOLD signal (Ojemann et al. 2013), the regulatory effect of DA on cortical signaling should be detectable using fMRI. Indeed, administration of a DA agonist upregulates subcortical integration in resting-state fMRI networks, supporting cognitive control (Cole et al. 2013). DA has also been found to contribute to gating plasticity in the striatum and cortex (Blond et al. 2002) by providing the signaling needed to reinforce successful associations essential for cognitive control (Buschman & Miller 2014). Taken together, these results show that the greater availability of DA during adolescence, paired with increasing GABA function, sets a unique period of specialization during which reward-associated experience may have a particular influence on establishing optimal cognitive control networks and their interaction.
Dopamine-Dependent Neural Plasticity
The properties of the DA system not only lead to enhanced functioning of cortical systems but also actively shape the plasticity of these systems, contributing to the refinement of control processes that rely on cortical networks. Given evidence suggesting heightened functionality of the DA system during adolescence, this mechanism has particular relevance to adolescent development. Several in vitro studies have shown that corticostriatal long-term potentiation (LTP) and long-term depression (LTD) that contribute to Hebbian plasticity are modulated by DA (Blond et al. 2002, Reynolds & Wickens 2002). Computational work suggests that midbrain DA may act as a global reward signal, gating synaptic plasticity, with LTP occurring if a behavior is rewarded and LTD occurring in response to reward omission (Soltani & Wang 2006). This is particularly true when rewards or omissions are unpredicted (Reynolds & Wickens 2002). Computational simulations further indicate that the imposition of this DA-modulated Hebbian reward rule upon nodes of a randomly configured network is sufficient to generate a biologically plausible network structure that can execute a cognitive discrimination task (Bourjaily & Miller 2011). This suggests DA-influenced plasticity at the cellular level can propagate to influence global network structure.
Reward-related LTP and LTD are thought to be driven at the cellular level through midbrain neurons, which fire phasically (15–30 Hz) to presentation and prediction of rewards (Mirenowicz & Schultz 1996). Importantly, recent in vivo work demonstrated that phasic stimulation of the ventral tegmental area induced the formation of mesofrontal axonal terminals in adolescent but not adult mice (Mastwal et al. 2014), suggesting heightened reward-related plasticity of the mesofrontal system during adolescence. As plasticity is associated most with phasic activation, this mechanism is experience dependent. Therefore, environmental contingencies may influence mesofrontal signaling heavily in adolescence. Indeed, Mastwal and colleagues (2014) found that wheel running also promoted mesofrontal terminal formation.
In summary, researchers have demonstrated that interactions between DA and GABA systems enhance synchronized oscillations as well as reward- and experience-dependent plasticity. Small-scale changes evoked by this process then propagate to influence global network structure (Figure 2). Importantly, this process occurs in parallel with global increases in myelination (Simmonds et al. 2013, Yakovlev et al. 1967), increasing the speed and fidelity of the long-range transmission of information, further enhancing the signal-to-noise ratio. The concurrent maturation of these processes during adolescence may underlie the rapid refinement of network architecture supporting the connectivity of components of control, thus leading to its consistently successful instantiation. We conclude by integrating this idea with neuroimaging data to endorse a theory of adolescence as an adaptive period of plasticity that promotes mature cognitive control.
CONCLUSIONS
The adolescent period is a unique period of development critical to establishing adult-level stability of cognitive control processing. The unique neurobiology of the mesofrontal DA system during adolescence has two important consequences for the development of control systems. First, heightened functionality of the mesofrontal DA system during adolescence enhances subcortical sensitivity to reward stimuli. As discussed above, fMRI studies provide evidence for adolescent striatal hyperreactivity to reward contingencies (Ernst et al. 2005, Galvan et al. 2006, Geier et al. 2010, Padmanabhan et al. 2011, Van Leijenhorst et al. 2010), which correspond to enhanced phasic mesofrontal DA signaling (Mirenowicz & Schultz 1996). As reward-related phasic DA firing is associated with LTD- and LTP-related neural plasticity (Reynolds & Wickens 2002) as well as the proliferation of mesofrontal dopaminergic axonal terminals (Mastwal et al. 2014), DA-related plasticity mechanisms may be particularly accelerated during adolescence. Secondly, peaks in striatal DA neurobiology promote exploratory behavior and novelty seeking during adolescence (Chambers et al. 2003, Spear 2000, Steinberg 2004), creating a drive to accumulate experience. Importantly, during the same period, social autonomy and exploratory behavior typically increase across cultures and mammalian species (Blakemore 2008, Spear 2000). Although the negative effects of adolescent novelty seeking have been widely discussed (e.g., drug use, reckless behavior), the confluence of establishing new social structures and the enhanced drive for exploration results in a vast accumulation of experience under novel contexts, which translates to increased opportunities for experience-dependent plasticity of cortical systems. Ultimately, adolescence can be seen as a period during which the development of motivational circuitry, in combination with increasing social autonomy, both drives adolescents to explore novel contexts and enhances reward sensitivity, leading to accelerated cortical plasticity, enhanced neural synchrony (and thus the refinement of cortical network architecture), and the emergence of mature cognitive control.
HUBS IN NETWORKS.
Hubs are highly influential nodes within networks, as they often have high degree, i.e., a large number of connections (Bullmore & Sporns 2009). This is the case for social networks, airports, and anatomical brain networks. However, because functional brain networks are based on statistical relations, hub definition in this traditional sense does not carry straightforward interpretability (van den Heuvel & Sporns 2013). Thus, hub status based solely on degree-based network (number of connections) measures has recently been called into question, as the node degree in fMRI correlation networks can be largely explained by subnetwork size (Power et al. 2013). As such, for hub status, equally if not more important than degree is the distribution of a node’s connections to other subnetworks. A node with distributed connections to many subnetworks is intuitively more critical for global brain function than a node with all its connections within its own subnetwork. Therefore, future studies using functional connectivity in fMRI should first consider the subnetwork architecture and use graph analytical measures sensitive to that architecture (Guimerà & Amaral 2005, Power et al. 2013, van den Heuvel & Sporns 2013).
BRAIN REGIONS THAT SUPPORT FLEXIBILITY.
Brain regions that support flexibility need to (a) contain a relatively large number of connections to multiple brain systems subserving disparate functions; (b) variably coactivate in a context-dependent manner to multiple regions during cognition; and (c) be able to update representations but maintain goal-relevant signals despite competing stimuli. Examples of core regions enabling flexible cognition between these systems include the DLPFC and the dACC. DLPFC function is for the most part at adult levels by adolescence, affording the ability to integrate executive processes in a flexible manner; however, dACC function continues to increase through adolescence, suggesting that the ability to flexibly engage processes that differ with demands is still sluggish. Connectivity to flexible regions should increase in number and strength with development. The overall ability to engage appropriate, intrinsically stable networks and integrate flexibly in a timely fashion may be a property inherent across networks that, with use, become specialized by strengthening effective pathways.
THE MISCONCEPTION OF PREFRONTAL SYSTEMS MATURING LAST.
Early histological studies led to a common misconception that the brain develops in a hierarchical fashion with prefrontal systems maturing last in comparison to other brain systems. Some studies have shown that the number of synapses reaches adult levels later in the middle frontal gyrus compared to Brodmann area 17 and Heschl’s gyrus (Huttenlocher & Dabholkar 1997). However, other cortical association areas have not been comprehensively assessed. MRI studies of gray matter thinning indicate that the prefrontal cortex has a protracted trajectory, but other cortical regions, such as temporal areas, and subcortical regions, such as the striatum and thalamus, show even longer maturational trajectories (Gogtay et al. 2004, Raznahan et al. 2014). Histological studies also show that whereas myelination in the visual cortex precedes myelination in the prefrontal cortex, parietal and temporal regions display a similar prolonged trajectory (Yakovlev et al. 1967). Likewise, DTI studies indicate that the white matter integrity of tracts that provide connectivity to dorsal prefrontal regions are at adult levels by adolescence, whereas those supporting connectivity of the ventral and medial prefrontal cortex to limbic and temporal regions continue to change into young adulthood (Lebel et al. 2008, Simmonds et al. 2013). Hence, the prefrontal cortex does not mature last.
Acknowledgments
The work was supported by the National Institutes of Health grants MH080243, MH067924, and DA022761 and by the Staunton Farm Foundation. We also thank Dr. Guillermo González-Burgos for helpful discussions.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adachi Y, Osada T, Sporns O, Watanabe T, Matsui T, et al. Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex. 2012;22(7):1586–92. doi: 10.1093/cercor/bhr234. [DOI] [PubMed] [Google Scholar]
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, et al. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Alahyane N, Brien DC, Coe BC, Stroman PW, Munoz DP. Developmental improvements in voluntary control of behavior: effect of preparation in the fronto-parietal network? Neuroimage. 2014;98:103–17. doi: 10.1016/j.neuroimage.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Computational models of performance monitoring and cognitive control. Top Cogn Sci. 2010;2(4):658–77. doi: 10.1111/j.1756-8765.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL. Changes in the second messenger cyclic AMP during development may underlie motoric symptoms in attention deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2002;130(1–2):197–201. doi: 10.1016/s0166-4328(01)00417-x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (±)-7-OH-DPAT. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;356(2):173–81. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37(2):167–69. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescents: a DTI study. Cereb Cortex. 2010;20(9):2122–31. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working Memory. New York: Oxford Univ. Press; 1986. [Google Scholar]
- Badre D. Defining an ontology of cognitive control requires attention to component interactions. Top Cogn Sci. 2011;3(2):217–21. doi: 10.1111/j.1756-8765.2011.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik J-H. Dopamine signaling in reward-related behaviors. Front Neural Circuits. 2013;7:152. doi: 10.3389/fncir.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Aljabar P, Zebari S, Tusor N, Arichi T, et al. Rich-club organization of the newborn human brain. PNAS. 2014;111(20):7456–61. doi: 10.1073/pnas.1324118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakaş S, Schürmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39(2–3):241–48. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12(6):512–23. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Batalle D, Eixarch E, Figueras F, Muñoz-Moreno E, Bargallo N, et al. Altered small-world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. Neuroimage. 2012;60(2):1352–66. doi: 10.1016/j.neuroimage.2012.01.059. [DOI] [PubMed] [Google Scholar]
- Berger B, Verney C, Febvret A, Vigny A, Helle KB. Postnatal ontogenesis of the dopaminergic innervation in the rat anterior cingulate cortex (area 24). Immunocytochemical and catecholamine fluorescence histochemical analysis. Brain Res. 1985;353(1):31–47. doi: 10.1016/0165-3806(85)90021-5. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30(5):194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF, Harnishfeger KK. The evolution of inhibition mechanisms and their role in human cognition and behavior. In: Dempster FN, Brainerd CJ, editors. Interference and Inhibition in Cognition. San Diego, CA: Academic; 1995. pp. 141–73. [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9(4):267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31(6):926–33. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond O, Crépel F, Otani S. Long-term potentiation in rat prefrontal slices facilitated by phased application of dopamine. Eur J Pharmacol. 2002;438(1–2):115–16. doi: 10.1016/s0014-2999(02)01291-8. [DOI] [PubMed] [Google Scholar]
- Bourjaily MA, Miller P. Synaptic plasticity and connectivity requirements to produce stimulus-pair specific responses in recurrent networks of spiking neurons. PLOS Comput Biol. 2011;7(2):e1001091. doi: 10.1371/journal.pcbi.1001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–36. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–90. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Goal-direction and top-down control. Philos Trans R Soc B Biol Sci. 2014;369(1655):20130471. doi: 10.1098/rstb.2013.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrge L, Sporns O, Smith LB. Developmental process emerges from extended brain-body-behavior networks. Trends Cogn Sci. 2014;18(8):395–403. doi: 10.1016/j.tics.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Wang JH, Dai ZJ, Cao XY, Jiang LL, et al. Topological organization of the human brain functional connectome across the lifespan. Dev Cogn Neurosci. 2014;7:76–93. doi: 10.1016/j.dcn.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Keller TA, Eddy W, Thulborn K. Graded functional activation in the visuospatial system with the amount of task demand. J Cogn Neurosci. 1999;11(1):9–24. doi: 10.1162/089892999563210. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L, Zalesky A, Fornito A, Mattingley JB. Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn Sci. 2013;17(10):493–501. doi: 10.1016/j.tics.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Gallen CL, Jacobs EG, Lee TG, D’Esposito M. Quantifying the reconfiguration of intrinsic networks during working memory. PLOS ONE. 2014;9(9):e106636. doi: 10.1371/journal.pone.0106636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Oei NYL, Soeter RP, Both S, van Gerven JMA, et al. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex. 2013;23(7):1509–16. doi: 10.1093/cercor/bhs136. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: behavioral evidence of protracted frontal lobe development. Dev Neuropsychol. 2007;31(1):103–28. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Cools R. Role of dopamine in the motivational and cognitive control of behavior. Neuroscientist. 2008;14(4):381–95. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. PNAS. 2006;103(24):9315–20. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: toward a unified theory of cognitive development and aging. Dev Rev. 1992;12:45–75. [Google Scholar]
- Dennis EL, Jahanshad N, McMahon KL, de Zubicaray GI, Martin NG, et al. Development of brain structural connectivity between ages 12 and 30: a 4-Tesla diffusion imaging study in 439 adolescents and adults. Neuroimage. 2013a;64:671–84. doi: 10.1016/j.neuroimage.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Jahanshad N, Toga AW, McMahon KL, de Zubicaray GI, et al. Proc IEEE 10th Int Symp Biomed Imaging: From Nano to Macro. San Francisco, CA: 2013b. Apr 7–11, Development of the “rich club” in brain connectivity networks from 438 adolescents & adults aged 12 to 30; pp. 624–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–68. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, et al. Emergence of resting state networks in the preterm human brain. PNAS. 2010;107(46):20015–20. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DB, Harrison BJ, Yücel M, Whittle S, Zalesky A, et al. Large-scale brain network dynamics supporting adolescent cognitive control. J Neurosci. 2014;34(42):14096–107. doi: 10.1523/JNEUROSCI.1634-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Erus G, Battapady H, Satterthwaite TD, Hakonarson H, Gur RE, et al. Imaging patterns of brain development and their relationship to cognition. Cereb Cortex. 2014 doi: 10.1093/cercor/bht425. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, et al. Functional brain networks develop from a “local to distributed” organization. PLOS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, et al. Development of distinct control networks through segregation and integration. PNAS. 2007;104(33):13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Kaila K. The cellular, molecular and ionic basis of GABAA receptor signalling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- Ferdinand NK, Kray J. Developmental changes in performance monitoring: how electrophysiological data can enhance our understanding of error and feedback processing in childhood and adolescence. Behav Brain Res. 2014;263:122–32. doi: 10.1016/j.bbr.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 2011;21(1):145–54. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, et al. Resting-state networks in the infant brain. PNAS. 2007;104(39):15531–36. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Elton A, Zhu H, Alcauter S, Smith JK, et al. Intersubject variability of and genetic effects on the brain’s functional connectivity during infancy. J Neurosci. 2014;34(34):11288–96. doi: 10.1523/JNEUROSCI.5072-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Garver K, Terwilliger R, Luna B. Development of working memory maintenance. J Neurophysiol. 2009;101(1):84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Luna B. Developmental effects of incentives on response inhibition. Child Dev. 2012;83(4):1262–74. doi: 10.1111/j.1467-8624.2012.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex. 2010;20(7):1613–29. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard HA, Teicher MH, Faedda G, Baldessarini RJ. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Dev Brain Res. 1989;49(1):123–30. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- Ghuman AS, Bar M, Dobbins IG, Schnyer DM. The effects of priming on frontal-temporal communication. PNAS. 2008;105(24):8405–9. doi: 10.1073/pnas.0710674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, et al. Dynamic mapping of human cortical development during childhood through early adulthood. PNAS. 2004;101(21):8174–79. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34(5):944–61. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Miyamae T, Pafundo DE, Yoshino H, Rotaru DC, et al. Functional maturation of GABA synapses during postnatal development of the monkey dorsolateral prefrontal cortex. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu122. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, Ray S, Carpenter S, Iyer S, Dias TG, et al. Structural and functional rich club organization of the brain in children and adults. PLOS ONE. 2014;9(2):e88297. doi: 10.1371/journal.pone.0088297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimerà R, Amaral LAN. Cartography of complex networks: modules and universal roles. J Stat Mech. 2005;2005(P02001):P02001-1–P02001-13. doi: 10.1088/1742-5468/2005/02/P02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15(7):4851–67. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, et al. White matter maturation reshapes structural connectivity in the late developing human brain. PNAS. 2010;107(44):19067–72. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: Spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82:208–25. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, González-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol. 2000;84(6):2799–807. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- Heron M. Deaths: leading causes for 2008. Natl Vital Stat Rep. 2012;60(6):1–90. [PubMed] [Google Scholar]
- Honey CJ, Kötter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. PNAS. 2007;104(24):10240–45. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hwang K, Hallquist MN, Luna B. The development of hub architecture in the human functional brain network. Cereb Cortex. 2012;23(10):2380–93. doi: 10.1093/cercor/bhs227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J Neurosci. 2010;30(46):15535–45. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. The inhibition of automatic saccades in early infancy. Dev Psychobiol. 1995;28(5):281–91. doi: 10.1002/dev.420280504. [DOI] [PubMed] [Google Scholar]
- Kail R. Processing time declines exponentially during childhood and adolescence. Dev Psychol. 1991;27(2):259–66. [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269(1):58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–38. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Larsen B, Luna B. In vivo evidence of neurophysiological maturation of the human adolescent striatum. Dev Cogn Neurosci. 2015;12:74–85. doi: 10.1016/j.dcn.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;140(3):1044–55. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, Kalar DJ, Congdon E, Poldrack RA. Towards an ontology of cognitive control. Top Cogn Sci. 2010;2(4):678–92. doi: 10.1111/j.1756-8765.2010.01100.x. [DOI] [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Cutler AJ, Bennett JP., Jr Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: a quantitative autoradiographic analysis. Dev Brain Res. 1991;62(1):109–14. doi: 10.1016/0165-3806(91)90195-o. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16(6):385–98. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann NY Acad Sci. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16(4):553–60. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Luna B. Developmental changes in cognitive control through adolescence. Adv Child Dev Behav. 2009;37:233–78. doi: 10.1016/s0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B. The relevance of immaturities in the juvenile brain to culpability and rehabilitation. Hastings Law Rev. 2012;63:1469–86. [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–72. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Ann NY Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–93. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27(11):848–63. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastwal S, Ye Y, Ren M, Jimenez DV, Martinowich K, et al. Phasic dopamine neuron activity elicits unique mesofrontal plasticity in adolescence. J Neurosci. 2014;34(29):9484–96. doi: 10.1523/JNEUROSCI.1114-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Developmental pathways to functional brain networks: emerging principles. Trends Cogn Sci. 2013;17(12):627–40. doi: 10.1016/j.tics.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379(6564):449–51. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16(7):1227–33. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull. 2000;126(2):220–46. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. Neuroimage. 2008;42(4):1678–85. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA, Ramsey NF, Ojemann J. Relation between functional magnetic resonance imaging (fMRI) and single neuron, local field potential (LFP) and electrocorticography (ECoG) activity in human cortex. Front Hum Neurosci. 2013;7:34. doi: 10.3389/fnhum.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Macoveanu J, Tegnér J, Klingberg T. Brain activity related to working memory and distraction in children and adults. Cereb Cortex. 2007;17(5):1047–54. doi: 10.1093/cercor/bhl014. [DOI] [PubMed] [Google Scholar]
- Ordaz SJ, Foran W, Velanova K, Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci. 2013;33(46):18109–24. doi: 10.1523/JNEUROSCI.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Geier CF, Ordaz SJ, Teslovich T, Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Dev Cogn Neurosci. 2011;1(4):517–29. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Luna B. Developmental imaging genetics: linking dopamine function to adolescent behavior. Brain Cogn. 2013;89:27–38. doi: 10.1016/j.bandc.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HB, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. PNAS. 2011;108(32):13281–86. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. PNAS. 2005;102(43):15700–5. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79(4):798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. PNAS. 2014;111(4):1592–97. doi: 10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JNJ, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15(4–6):507–21. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Riggall AC, Postle BR. The relationship between working memory storage and elevated activity as measured with functional magnetic resonance imaging. J Neurosci. 2012;32(38):12990–98. doi: 10.1523/JNEUROSCI.1892-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry. 1994;36:272–77. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci. 2012;32(36):12411–20. doi: 10.1523/JNEUROSCI.0421-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–77. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27(12):973–93. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabb FW, Bearden CE, Glahn DC, Parker DS, Freimer N, Bilder RM. A collaborative knowledge base for cognitive phenomics. Mol Psychiatry. 2008;13(4):350–60. doi: 10.1038/sj.mp.4002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ. Developmental differences in error-related ERPs in middle- to late-adolescent males. Dev Psychol. 2008;44(1):205–17. doi: 10.1037/0012-1649.44.1.205. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Erus G, Ruparel K, Elliott MA, et al. Functional maturation of the executive system during adolescence. J Neurosci. 2013;33(41):16249–61. doi: 10.1523/JNEUROSCI.2345-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. J Cogn Neurosci. 2006;18:1045–58. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Jetha MK. Electrophysiological changes during adolescence: a review. Brain Cogn. 2010;72(1):86–100. doi: 10.1016/j.bandc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–40. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds D, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2013;92:356–68. doi: 10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani A, Wang X-J. A biophysically based neural model of matching law behavior: melioration by stochastic synapses. J Neurosci. 2006;26(14):3731–44. doi: 10.1523/JNEUROSCI.5159-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Neurobehavioral changes in adolescence. Curr Dir Psychol Sci. 2000;9:111–14. [Google Scholar]
- Sporns O, Honey CJ, Kötter R. Identification and classification of hubs in brain networks. PLOS ONE. 2007;2(10):e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics. 2004;2(2):145–62. doi: 10.1385/NI:2:2:145. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann NY Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLOS Biol. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1231–38. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2 and D4 receptors in rat forebrain. Int J Dev Neurosci. 2000;18(1):29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Dassanayake MT, Shen S, Katkuri Y, Alexis M, et al. Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med. 2013;5(173):173ra24. doi: 10.1126/scitranslmed.3004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Race E, Burrows B, Whitfield-Gabrieli S, Glover GH, Gabrieli JD. Development of spatial and verbal working memory capacity in the human brain. J Cogn Neurosci. 2009;21(2):316–32. doi: 10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoizumi T, Miyamoto H, Yazaki-Sugiyama Y, Atapour N, Hensch TK, Miller KD. A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron. 2013;80(1):51–63. doi: 10.1016/j.neuron.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. PNAS. 2009;106:9866–71. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31(44):15775–86. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. An anatomical substrate for integration among functional networks in human cortex. J Neurosci. 2013;33(36):14489–500. doi: 10.1523/JNEUROSCI.2128-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51(1):345–55. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex. 2008;18(11):2505–22. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010;72(1):146–59. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373(6515):612–15. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Wiersema JR, van der Meere JJ, Roeyers H. Developmental changes in error monitoring: an event-related potential study. Neuropsychologia. 2007;45(8):1649–57. doi: 10.1016/j.neuropsychologia.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27(11):683–90. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR, Minkowski A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford, UK: Blackwell Sci; 1967. pp. 3–70. [Google Scholar]


