Abstract
Our laboratory was one of the first to engineer a live fluorescent tag, enhanced green fluorescent protein (eGFP), that marked the capsid of herpes simplex virus type 1 (HSV-1) and subsequently maturing virus as the particle made its way to the cell surface. In the present study we sought to increase the repertoire of colors available as fusion to the small capsid protein, VP26, so that they can be used alone or in conjunction with other fluorescent tags (fused to other HSV proteins) to follow the virus as it enters and replicates within the cell. We have now generated viruses expressing VP26 fusions with Cerulean, Venus, mOrange, tdTomato, mCherry, and Dronpa3 fluorescent proteins. These fusions were made in a repaired UL35 gene (VP26) background. These fusions do not affect the replication properties of the virus expressing the fusion polypeptide and the fusion tag was stably associated with intranuclear capsids and mature virions. Of note we could not isolate viruses expressing fusions with fluorescent proteins that have a tendency to dimerize.
Keywords: HSV1, VP26, capsid, fluorescent tags, Venus, tdTomato
Visualization of virus particles in cells using light microscopy was significantly advanced by the use of live fluorescent reporters such as green fluorescent protein (GFP) derived from the jellyfish (Aequorea victoria) (Chalfie et al., 1994). For herpes simplex virus (HSV) the ability to generate a fusion polypeptide between a virion protein and GFP allowed one to follow in living cells the virus particle as it assembles in the nucleus, exits this structure and begins on the egress pathway to the cell surface. The first two such recombinant viruses made utilized a tegument protein, VP22 (Elliott and O’Hare, 1999) and the small capsid protein, VP26 (Desai and Person, 1998) to incorporate the fluorescent protein (FP) into the virion. In addition, these and subsequent engineered fusion proteins facilitated the ability to visualize virus entry, cell to cell spread and capsid translocation on the cellular cytoskeleton (Antinone et al., 2006; Bearer et al., 2000; Bohannon et al., 2012; Conway et al., 2010; de Oliveira et al., 2008; del Rio et al., 2005; Desai et al., 2008; Donnelly and Elliott, 2001; Hutchinson et al., 2002; La Boissiere et al., 2004; Maier et al., 2016; Nagel et al., 2012; Nagel et al., 2008; Ogasawara et al., 2001; Radtke et al., 2010; Scherer et al., 2016; Smith, Gross, and Enquist, 2001; Sole et al., 2007; Sugimoto et al., 2008; Taylor et al., 2012; Toropova et al., 2011). In this paper we expand on the repertoire of fluorescent fusions available for the small capsid protein (VP26) of HSV type 1 strain KOS. This VP26 tag is one of the most useful and most used reporters for visualizing all aspects of HSV-1 replication. Although the first engineered version was a GFP fusion we have now engineered several additional colors that can be used individually or in combination for visualizing the virus in living cells.
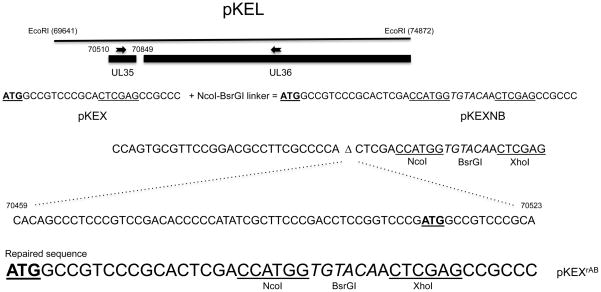
The 5.2-kb EcoRI L fragment of KOS (Fig. 1) cloned into pUC19 and designated pKEL has been previously described (Desai and Person, 1998; Desai et al., 1998). This fragment spans nucleotide 69641 to 74872 of the KOS genome (Macdonald et al., 2012). The plasmid encodes UL35 and the C-terminal portion of UL34 and UL36 genes. When the 5′ end of the UL35 gene encoding VP26 was engineered using overlap PCR methods to introduce an XhoI restriction enzyme site for cloning purposes there was an inadvertent mutation generated that essentially deleted 65 nucleotides upstream of the UL35 ATG as well as the first 5 codons (Fig. 1). This plasmid was designated pKEX (Desai, DeLuca, and Person, 1998). Subsequently a linker sequence comprising the NcoI and BsrGI restriction enzyme sites was introduced into the XhoI site in order to clone and create a translation fusion of GFP at the N-terminus of VP26 (Desai & Person, 1998). It was assumed that the GFP open reading frame (ORF) was fused to the first five codons of VP26 and at the C-terminus to the rest of VP26. The deletion only became apparent after later investigations (Baines, J and Sodeik, B., pers comm.). Fortuitously, the ATG start codon present in the NcoI restriction site enabled translation of GFP fused in frame with VP26 and the virus K26GFP (Desai and Person, 1998) was derived and used for subsequent analysis both by the community and us. This virus replicated with normal wild-type properties in cell culture.
Fig. 1.
Engineering of the upstream region of the UL35 gene. Shown in the figure is plasmid pKEL, which encodes the EcoRI L fragment of HSV-1 strain KOS (Macdonald et al., 2012). The UL35 gene and part of the UL36 gene are shown. Below this are shown the predicted nucleotide sequences of the design to engineer the XhoI site in pKEX and the NcoI-BsrGI sites in pKEXNB. The sequence that was deleted during this engineering and the deletion site (Δ) is shown below and spans nucleotides 70459 to 70523 of KOS. The UL35 start codon is underlined and in bold. The repaired sequence is shown below this starting with the UL35 ATG and the engineered NcoI, XhoI (underlined) and BsrGI (italics) restriction enzyme sites. ORFs encoding Cerulean and Venus genes were cloned using NcoI and BsrGI restriction enzyme sites. ORFs encoding Dronpa3, mOrange, tdTomato, mRFP and mCherry were cloned using the BsrGI and XhoI sites in pKEXrAB.
We sought to repair this deletion and then engineer in the different fluorescent genes. PCR methods were used to correct the introduced deletion. To do this we made use of a HpaI restriction site at 70257 and a BstBI site at 70959 (Macdonald et al., 2012). Two PCR products were amplified, one (pcr A) spans the HpaI to the original XhoI site inserted in UL35 and the second (pcr B) from that same XhoI site to the BstBI site (Table 1). Plasmid pKEL-XhoI was first digested with HpaI and XhoI to clone pcr A fragment. This plasmid pKEXrA was sequenced to confirm authentic sequence information and then digested with XhoI and BstBI to clone pcr B. The final plasmid after sequence verification was named pKEXrAB (Fig. 1). This plasmid corrected the mutation but retained the restriction cloning sites that were engineered as part of the original cloning strategy. Thus spanning the first 5–7 codons of UL35 we inserted an NcoI-BsrGI-XhoI engineered sequence encoding restriction sites that were used to clone the different genes expressing fluorescent tags (Fig. 1). The ATG that is part of the NcoI restriction enzyme site is in-frame with that of the UL35 gene. Thus, most of the FP genes can be cloned using Nco1-BsrG1 sites, as these are the sites used to clone the first series of FP genes in the different expression vectors made by Clontech. However, many of the red variants contain an internal NcoI site and so one can use the BrGI-XhoI cloning sites. The fluorescent protein ORFs were amplified by PCR using the following plasmids as templates: pCerulean-VSVG (Cerulean) (Presley et al., 1997), pYFP-N1 (Yellow) (Clontech), pVenus-VSVG (Venus) (Presley et al., 1997), pFBCmCherry (mCherry) (Luitweiler et al., 2013), pCAG2LMKOSimO (mOrange) (Kaji et al., 2009), FUtdTW (tandem dimer tomato) (Rompani and Cepko, 2008), Dronpa3-N1 (Dronpa3) as the template using primers listed in Table 1. The PCR products were cloned into the engineered restriction sites (Fig. 1 and Table 1) in UL35 at codon 5, creating a translational fusion between these two genes. All the fluorescent gene ORFs were cloned as XhoI-BsrGI fragments, except for the Venus and Cerulean genes which were cloned as NcoI-BsrGI fragments into the respective sites in pKEXrAB. The plasmid and virus encoding VP26-mRFP1 fusion was generated in a previous study but is analyzed here together with all the new fluorescent tags (Desai et al., 2008). The YFP ORF in this study was cloned into the original pKEXNB, which contains the deletion and was used to derive a virus expressing this fusion.
Table 1.
Sequences of PCR Primers Used.
| Primer name | Primer sequence (5′ to 3′) |
|---|---|
| UL35 A–F | GGGACGGTTTTGGCCCGCTCC |
| UL35 A–R | CCCTCGAGTTGTACACCATGGTCGAGTGCGGGACGGCCATCGGGACC GGAG |
| UL35 B–F | CCCTCGAGCCGCCCCAGCACCGTTACCACC |
| UL35 B–R | GTTACTGGGCTAGACGCGCTCG |
| Venus/Cerulean-Nco1*-F | GGCCATGGTGAGCAAGGGCGAGGAGCTG |
| Venus/Cerulean-BsrG1-R | GCTTTACTTGTACAGCTCGTCCATGCC |
| Dronpa3-Nco1-F | GGCCATGGTGAGTGTGATTAAACCAGAC |
| Dronpa3-BsrG1-R | GTTGTACACCTTGGCCTGCCTCGGCAGCTCAGA |
| mOrange-BsrG1-F | GGGTGTACAACGTGAGCAAGGGCGAGGAGAATAAC |
| mOrange-Xho1-R | GGGCTCGAGTTCTCGTCCATGCCGCCGGTGGAGTG |
| tdTomato-BsrG1-F | GGGTGTACAACGTGAGCAAGGGCGAGGAGGTCATC |
| tdTomato-Xho1-R | GGGCTCGAGTTCTCGTCCATGCCGTACAGGAACAG |
| mRFP-BsrG1-F | CCTGTACACCATGGCCTCCTCCGAGGACGTCATC |
| mRFP-Xho1-R | CCCTCGAGGGGCGCCGGTGGAGTGGCGGCCCTC |
| mCherry-BsrG1-F | GGGTGTACAACGTGAGCAAGGGCGAGGAGGAT |
| mCherry-Xho1-R | GCTCGAGTCGTACAGCTCGTCCATGCCGCC |
restriction enzyme recognition sites are italicized and in bold
YFP was cloned using existing Nco1 and BsrG1 sites in plasmid
The different gene fusions were then recombined into the KOS virus genome using marker-transfer methods. For all transfections and infections we used Vero cells, or a transformed Vero cell line, C32 (Person and Desai, 1998) and for virion preparations we used human embryoninc lung (HEL) cells grown in minimum essential medium- alpha medium supplemented with 10% fetal calf serum (Gibco-Invitrogen) and passaged as described previously (Desai et al., 1998). C32 cells were used because they tend to give rise to larger plaques under methylcellulose and thus were ideal for fluorescence imaging. Virus stocks of the KOS strain of HSV-1 and the recombinant viruses were prepared as described previously (Desai et al., 1998). Subconfluent monolayers of Vero cells in tissue culture dishes were co-transfected with KOS infected cell DNA (Person and Desai, 1998) and HindIII linearized plasmid DNA. Three days post-transfection the cells were collected, freeze-thawed once, sonicated, and progeny viruses were plaqued. Single plaque isolates were visualized under a fluorescent microscope to confirm homologous recombination. Fluorescent plaques were picked and then purified using sequential cycles of limiting dilution. The purified viruses were then amplified to produce high titer working stocks.
We analyzed all the different recombinant viruses for growth properties, expression of the fusion polypeptide, capsid as well as virion incorporation of the VP26-FP. First we imaged all plaques that developed during the course of a 3-day incubation under methylcellulose using a fluorescence microscope (Fig. 2A). These images showed how the different fluorescent tags localize in infected cells. The typical punctate distribution of the VP26FP fluorescence was evident in all the different plaques (inset of Fig. 2A); this is the characteristic distribution of this fluorescent fusion polypeptide (Desai, Akpa, and Person, 2003). Using similar exposure parameters and either a FITC (green) or Rhodamine (red) filters we compared the relative brightness of the green fluorescent proteins and the red fluorescent proteins (Fig. S1) and it was evident that the virus expressing the Venus fluorescent protein fusion was the brightest of the yellow-green fluorescent protein fusions and the tdTomato was the brightest of the red fluorescent protein fusions. We also imaged the virus expressing VP26 Dronpa3 (Fig. 2B). Dronpa3 is a reversible photoswitchable FP (Rego et al., 2012). Imaging in the standard fluorescence mode the fluorescence signal began to weaken (switched off) but following a 2-minute recovery period, the fluorescence was again evident.
Fig. 2.
Observation of fluorescence of plaques expressing VP26-FP tags. (a) Plaques on C32 cell monolayers were imaged using camera exposure parameters for optimal plaque fluorescence. Inset shows the typical punctate pattern of VP26-FP localization. The fluorescence observed with the Cerulean fusion was not optimal using the standard microscope filters. (b) Observation of the Dronpa3 fluorescent tag fused to VP26. From left-to-right, a plaque expressing VP26-Dronpa3 under normal conditions, switched off, and following a 2-minute recovery period. Scale bar (100 μm) is shown in the left panel.
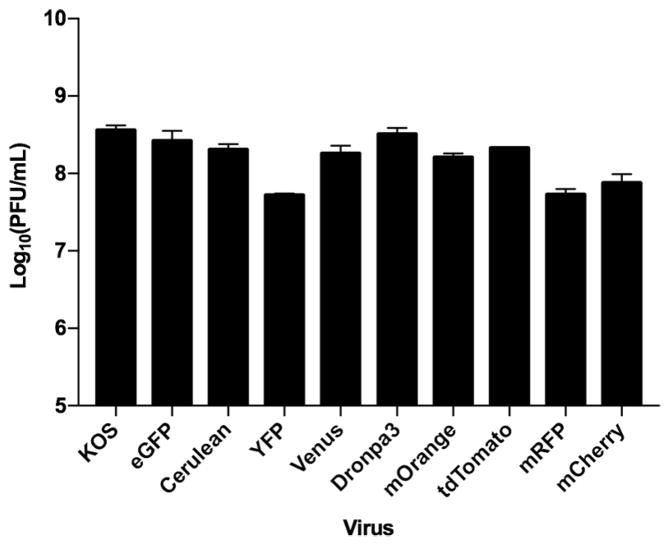
Different types of fluorescent protein fusions can cause differences in the growth ability of the virus expressing those fusions. This was evident when we first isolated the KVP26mRFP1 virus. It displayed a decrease in the virus yields when compared to K26GFP. All the viruses were thus examined in single-step growth assays for the production of progeny virus (Fig. 3). Most viruses replicate and produce virus progeny comparable to wild-type KOS or K26GFP infected cells. The exceptions are VP26 fusions with the red fluorescent proteins and YFP. The yields from infected cells decreased by 5–10 fold relative to the viruses expressing green fluorescent protein fusions. In agreement with data derived by Nagel et al. (Nagel et al., 2012) we could not isolate viruses expressing fluorescent variants that have a tendency to dimerize such as eCFP, eGFP, YFP and mNeptune in the UL35 repaired background. These fusions likely cause the same defects (nuclear aggregation) in growth that Nagel et al. (2012) reported.
Fig. 3.
Replication properties of the VP26 recombinant viruses. All of the different VP26 recombinant viruses were examined for production of progeny following a single cycle of growth. Vero cells were infected with the recombinant viruses at a multiplicity of infection (MOI) of 10 plaque forming units (PFU)/cell. At 24h post-infection, cells were harvested, freeze-thawed one time, sonicated and then titrated on C32 cells to determine the growth properties of the viruses. Data plotted are an average of two independent infections.
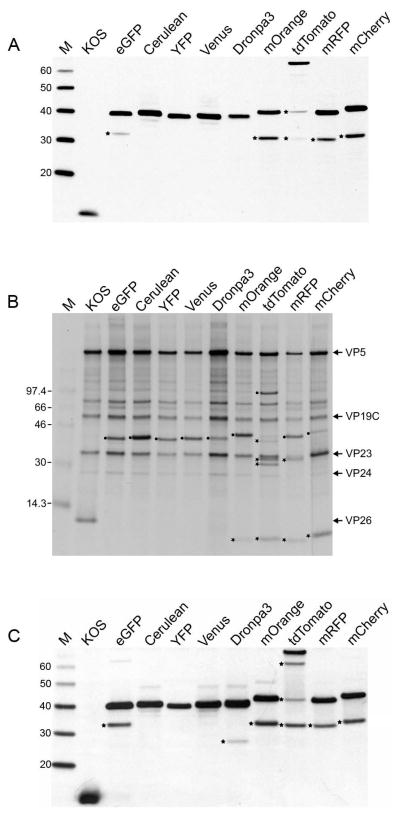
Western blots were also performed to confirm the expression of each fusion polypeptide and stable accumulation (Fig. 4). In most cases the full-length VP26FP polypeptide was detected using anti-VP26 antibody including the tdTomato tandem dimer polypeptide. This analysis too revealed a specific proteolytic cleavage that occurred in the red fluorescent protein fusions. The majority of the fusion protein was still full-length but there was limited proteolysis, which appears to be within the red fluorescent protein and not between the junction of the two protein fusions (Fig. 4A). We next used sedimentation experiments to isolate different capsid and virus particles to examine the incorporation of the VP26-fusions into the different mature particles. Radiolabeled Vero infected cell lysates were sedimented through sucrose gradients and the gradient fractions corresponding to C capsids were analyzed by SDS-PAGE (Fig. 4B). Radioactivity corresponding to the capsid shell proteins was evident in each virus C capsid as well as the wild-type VP26 12kD polypeptide in KOS C capsids. In all the other capsids the fusion polypeptide with the expected molecular weight was evident. As seen in the autoradiograph, we also observed proteolytic fragments of the Red FP fusions. Extracellular virions excreted into the culture medium of HEL cells infected with each virus were also examined by western blot methods following sucrose cushion (20%) purification of the virions (Fig. 4C). All virion particles incorporated the different fluorescent proteins as judged by the detection of the fusion polypeptide.
Fig. 4.
Expression and Incorporation of VP26-FP into capsids and mature virions. (a) Western Blot analysis of infected cells to demonstrate VP26-FP expression. Vero cells in 12 well trays (5 × 105 cells/well) were infected at a MOI of 10 PFU/cell. Cells were harvested 24h after infection and protein lysates were solubilized in 2X Laemmli buffer. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using Nu-Page 4–12% Bis-Tris gels (Invitrogen) and transferred to nitrocellulose membranes and processed as described by (Luitweiler et al., 2013). Membranes were incubated with a rabbit peptide antibody to the C-terminus of VP26 (95RRTYSPFVVREPSTPGTP112) at a 1:500 dilution, horseradish peroxidase-linked secondary antibodies (GE Healthcare) were used and detection was done by enhanced chemiluminescence (ECL) reagent (GE Healthcare). Magic marker protein standards (Invitrogen) are in lane M and correspond to mass standards (kD) indicated on the left. Proteolytic products of the VP26-FP are indicated by a star on the left of each lane. (b). Metabolic radiolabeling of C capsids. Radiolabeled infected cell lysates were sedimented through 20–50% sucrose gradients (Walters et al., 2003). Infections and labeling were performed as described in (Desai, 2000). The proteins in the C capsid fraction were separated by SDS-PAGE using a 4–12% gel and the autoradiograph is shown. Positions of the capsids proteins are indicated on the right of the figure, and the 14C labeled protein standards (kD) are in lane M. The fusion polypeptides corresponding to VP26-FP are marked with a black circle and the proteolytic products of this polypeptide are labeled with a star on the left side of each lane. (c) Extracellular virions incorporate all VP26-FP tags. HEL cells in tissue culture dishes (8.6 × 106 cells) were infected at an MOI of 10 PFU/cell. Virions released into the culture medium of the infected HEL cells were collected at 48h post-infection and sedimented through a sucrose cushion (20%) and the pellet material was analyzed by immunoblot using anti-VP26 antibody. Protein standards (kD) are in lane M and the observed proteolytic products are indicated by a star on the left of each lane where visible.
In summary, we have now generated several additional fluorescent tags that decorate HSV-1 capsids and are thus incorporated into mature virions. Many of these new viruses have properties that maybe more useful than the original K26GFP virus, such as the superior brightness of the Venus protein and the Cerulean protein which is brighter than the original Cyan fluorescent protein and folds more efficiently under physiological conditions. The mCherry red variant is the brightest in its class and surprisingly the larger tdTomato protein was tolerated well by the virus and thus could be useful for small animal imaging. Finally, the VP26-Dronpa3 photoswitchable fluorescent protein fusion is an important reagent and a means to visualize dynamic localization of this protein as well as the virus in super resolution microscopy applications. These viruses as well as the previously described viruses express proteins that emit fluorescence in the cyan, green, yellow-green, orange and red classes and thus should be useful for the community of scientists working on HSV-1 biology.
Supplementary Material
Highlights.
Generation of new HSV-1 recombinant viruses expressing VP26 fluorescent protein fusions
New fluorescent colors include Cerulean, Venus, yellow fluorescent protein, mOrange, tdTomato, mCherry and mRFP
VP26-fusion with Dronpa3 produces photoswitchable fluorescence signal
Acknowledgments
This work was supported by PHS grants from the National Institutes of Health (R21AI107537, R21AI107530 and R01AI061382). We want to thank Brandon Henson for technical assistance. Plasmids pCerulean-VSVG (Addgene plasmid # 11913) and pVenus-VSVG (Addgene plasmid # 11914) were gifts from Jennifer Lippincott-Schwartz. Dronpa3-N1 was a gift from Michael Davidson (Addgene plasmid # 54682). FUtdTW was a gift from Connie Cepko (Addgene plasmid # 22478) and pCAG2LMKOSimO was a gift from Keisuke Kaji (Addgene plasmid # 20866).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antinone SE, Shubeita GT, Coller KE, Lee JI, Haverlock-Moyns S, Gross SP, Smith GA. The Herpesvirus capsid surface protein, VP26, and the majority of the tegument proteins are dispensable for capsid transport toward the nucleus. J Virol. 2006;80:5494–8. doi: 10.1128/JVI.00026-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer EL, Breakefield XO, Schuback D, Reese TS, LaVail JH. Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument. Proc Natl Acad Sci U S A. 2000;97:8146–50. doi: 10.1073/pnas.97.14.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon KP, Sollars PJ, Pickard GE, Smith GA. Fusion of a fluorescent protein to the pUL25 minor capsid protein of pseudorabies virus allows live-cell capsid imaging with negligible impact on infection. J Gen Virol. 2012;93:124–9. doi: 10.1099/vir.0.036145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–5. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Conway JF, Cockrell SK, Copeland AM, Newcomb WW, Brown JC, Homa FL. Labeling and localization of the herpes simplex virus capsid protein UL25 and its interaction with the two triplexes closest to the penton. J Mol Biol. 2010;397:575–86. doi: 10.1016/j.jmb.2010.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira AP, Glauser DL, Laimbacher AS, Strasser R, Schraner EM, Wild P, Ziegler U, Breakefield XO, Ackermann M, Fraefel C. Live visualization of herpes simplex virus type 1 compartment dynamics. J Virol. 2008;82:4974–90. doi: 10.1128/JVI.02431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio T, Ch’ng TH, Flood EA, Gross SP, Enquist LW. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J Virol. 2005;79:3903–19. doi: 10.1128/JVI.79.7.3903-3919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P, Akpa JC, Person S. Residues of VP26 of herpes simplex virus type 1 that are required for its interaction with capsids. J Virol. 2003;77:391–404. doi: 10.1128/JVI.77.1.391-404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P, DeLuca NA, Person S. Herpes simplex virus type 1 VP26 is not essential for replication in cell culture but influences production of infectious virus in the nervous system of infected mice. Virology. 1998;247:115–24. doi: 10.1006/viro.1998.9230. [DOI] [PubMed] [Google Scholar]
- Desai P, Person S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol. 1998;72:7563–8. doi: 10.1128/jvi.72.9.7563-7568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P, Sexton GL, Huang E, Person S. Localization of herpes simplex virus type 1 UL37 in the Golgi complex requires UL36 but not capsid structures. J Virol. 2008;82:11354–61. doi: 10.1128/JVI.00956-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai PJ. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J Virol. 2000;74:11608–18. doi: 10.1128/jvi.74.24.11608-11618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M, Elliott G. Fluorescent tagging of herpes simplex virus tegument protein VP13/14 in virus infection. J Virol. 2001;75:2575–83. doi: 10.1128/JVI.75.6.2575-2583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G, O’Hare P. Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection. J Virol. 1999;73:4110–9. doi: 10.1128/jvi.73.5.4110-4119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson I, Whiteley A, Browne H, Elliott G. Sequential localization of two herpes simplex virus tegument proteins to punctate nuclear dots adjacent to ICP0 domains. J Virol. 2002;76:10365–73. doi: 10.1128/JVI.76.20.10365-10373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–5. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Boissiere S, Izeta A, Malcomber S, O’Hare P. Compartmentalization of VP16 in cells infected with recombinant herpes simplex virus expressing VP16-green fluorescent protein fusion proteins. J Virol. 2004;78:8002–14. doi: 10.1128/JVI.78.15.8002-8014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luitweiler EM, Henson BW, Pryce EN, Patel V, Coombs G, McCaffery JM, Desai PJ. Interactions of the Kaposi’s Sarcoma-associated herpesvirus nuclear egress complex: ORF69 is a potent factor for remodeling cellular membranes. J Virol. 2013;87:3915–29. doi: 10.1128/JVI.03418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald SJ, Mostafa HH, Morrison LA, Davido DJ. Genome sequence of herpes simplex virus 1 strain KOS. J Virol. 2012;86:6371–2. doi: 10.1128/JVI.00646-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier O, Sollars PJ, Pickard GE, Smith GA. Visualizing herpesvirus procapsids in living cells. J Virol. 2016 doi: 10.1128/JVI.01437-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel CH, Dohner K, Binz A, Bauerfeind R, Sodeik B. Improper tagging of the non-essential small capsid protein VP26 impairs nuclear capsid egress of herpes simplex virus. PLoS One. 2012;7:e44177. doi: 10.1371/journal.pone.0044177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel CH, Dohner K, Fathollahy M, Strive T, Borst EM, Messerle M, Sodeik B. Nuclear egress and envelopment of herpes simplex virus capsids analyzed with dual-color fluorescence HSV1(17+) J Virol. 2008;82:3109–24. doi: 10.1128/JVI.02124-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara M, Suzutani T, Yoshida I, Azuma M. Role of the UL25 gene product in packaging DNA into the herpes simplex virus capsid: location of UL25 product in the capsid and demonstration that it binds DNA. J Virol. 2001;75:1427–36. doi: 10.1128/JVI.75.3.1427-1436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person S, Desai P. Capsids are formed in a mutant virus blocked at the maturation site of the UL26 and UL26.5 open reading frames of herpes simplex virus type 1 but are not formed in a null mutant of UL38 (VP19C) Virology. 1998;242:193–203. doi: 10.1006/viro.1997.9005. [DOI] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–5. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Radtke K, Kieneke D, Wolfstein A, Michael K, Steffen W, Scholz T, Karger A, Sodeik B. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog. 2010;6:e1000991. doi: 10.1371/journal.ppat.1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego EH, Shao L, Macklin JJ, Winoto L, Johansson GA, Kamps-Hughes N, Davidson MW, Gustafsson MG. Nonlinear structured-illumination microscopy with a photoswitchable protein reveals cellular structures at 50-nm resolution. Proc Natl Acad Sci U S A. 2012;109:E135–43. doi: 10.1073/pnas.1107547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompani SB, Cepko CL. Retinal progenitor cells can produce restricted subsets of horizontal cells. Proc Natl Acad Sci U S A. 2008;105:192–7. doi: 10.1073/pnas.0709979104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer J, Yaffe ZA, Vershinin M, Enquist LW. Dual-color Herpesvirus Capsids Discriminate Inoculum from Progeny and Reveal Axonal Transport Dynamics. J Virol. 2016 doi: 10.1128/JVI.01122-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Gross SP, Enquist LW. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci U S A. 2001;98:3466–70. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole M, Perkins EM, Frisancho A, Huang E, Desai P. The N terminus of the herpes simplex virus type 1 triplex protein, VP19C, cannot be detected on the surface of the capsid shell by using an antibody (hemagglutinin) epitope tag. J Virol. 2007;81:8367–70. doi: 10.1128/JVI.00819-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Uema M, Sagara H, Tanaka M, Sata T, Hashimoto Y, Kawaguchi Y. Simultaneous tracking of capsid, tegument, and envelope protein localization in living cells infected with triply fluorescent herpes simplex virus 1. J Virol. 2008;82:5198–211. doi: 10.1128/JVI.02681-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MP, Kramer T, Lyman MG, Kratchmarov R, Enquist LW. Visualization of an alphaherpesvirus membrane protein that is essential for anterograde axonal spread of infection in neurons. MBio. 2012:3. doi: 10.1128/mBio.00063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toropova K, Huffman JB, Homa FL, Conway JF. The herpes simplex virus 1 UL17 protein is the second constituent of the capsid vertex-specific component required for DNA packaging and retention. J Virol. 2011;85:7513–22. doi: 10.1128/JVI.00837-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JN, Sexton GL, McCaffery JM, Desai P. Mutation of single hydrophobic residue I27, L35, F39, L58, L65, L67, or L71 in the N terminus of VP5 abolishes interaction with the scaffold protein and prevents closure of herpes simplex virus type 1 capsid shells. J Virol. 2003;77:4043–59. doi: 10.1128/JVI.77.7.4043-4059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.