Abstract
Objective
Seeking consent for minimal risk research in the ICU poses challenges, especially when the research is time-sensitive. Our aim was to determine the extent to which ICU patients or surrogates support a deferred consent process for a minimal risk study without the potential for direct benefits.
Design
Prospective cohort study.
Setting
Five ICUs within a tertiary care hospital.
Patients
Newly admitted ICU patients ≥18 years old.
Interventions
We administered an eight-item verbal survey to patients or surrogates approached for consent to participate in a minimal-risk, ICU based study. The parent study involved non-invasive collection of biosamples and clinical data at the time of ICU admission and again 3 days later. If patients had capacity at the time of ICU admission, or if a surrogate was readily available, consent was sought prior to initial sample collection; otherwise, a waiver of consent was granted and deferred consent was sought 3 days later. Quantitative and qualitative data were analyzed.
Measurements and Main Results
157 individuals were approached for consent to participate in the parent study; none objected to the consent process. 135/157 (86%) competed the survey, including 94 who consented to the parent study and 41 who declined. 44/60 (73%) individuals approached for deferred consent responded positively to the question “Did we make the right choice in waiting until now to ask your consent?” 3/60 (5%) responded negatively, and 13/60 (22%) made a neutral or unrelated response. The most common reason given for endorsing the deferred consent process was the stress of the early ICU experience 25/44 (61%).
Conclusions
Most patients and surrogates accept a deferred consent process for minimal risk research in the ICU. For appropriate ICU-based research, investigators and IRBs should consider a deferred consent process if the subject lacks capacity and an appropriate surrogate is not readily available.
Keywords: deferred consent, critical care, epidemiological research, patient stress, surrogate stress, intensive care unit
INTRODUCTION
The process of seeking consent for clinical research is particularly challenging in the intensive care unit (ICU) setting. Surrogates are often unavailable [1, 2] or, if available, too overwhelmed to participate in research [1, 3]. When surrogates do engage in ICU-based research, there are substantial discrepancies between patient and surrogate preferences regarding clinical research [4, 5].
The requirement for informed consent can introduce clinically meaningful selection bias in minimal risk research [6], and this is especially relevant in the ICU [7–11]. In the ICU, patients frequently lack decisional capacity due to acute illness, and these patients differ meaningfully from those who are able to engage in an informed consent discussion. These challenges can limit the generalizability of results from ICU-based studies and have led to a suggestion that IRBs should consider waiving the requirement for informed consent for some minimal risk research with critically ill patients [4].
Patients and surrogates have generally expressed favorable attitudes towards randomized emergent or ICU-based interventions that are undertaken without consent or using a deferred consent approach [12, 13]. Minimal risk research poses a different set of challenges. United States federal regulations allow for a waiver of the requirement for informed consent when, among other conditions, the research poses no greater than minimal risk and it is not practicable to obtain consent. The determination of practicability is subjective and may be particularly difficult in the ICU setting. This study was conducted to evaluate attitudes towards deferred consent for a minimal risk, no-benefit ICU-based research study involving the collection of biosamples. Our aim was to determine the extent to which patients and their surrogates did or did not endorse deferred consent for minimal risk ICU-based research, and to characterize their opinions.
MATERIALS AND METHODS
Study Design and Eligibility
The goal of the parent study (“the microbiome study”) was to identify patient-related risk factors for ICU-acquired infections. Subjects eligible for the microbiome study were adults admitted to one of five medical or surgical ICUs with an anticipated length of stay of three or more hospital days and no recent history of Clostridium difficile infection. Study procedures included oral and peri-rectal swabs within 4 hours of ICU admission and again 3 days later.
Individuals were eligible for this survey-based study if they were approached for consent either as a subject or a surrogate for the microbiome study. Both studies were approved by the Institutional Review Board (IRB) of Columbia University Medical Center.
Modified Consent Process
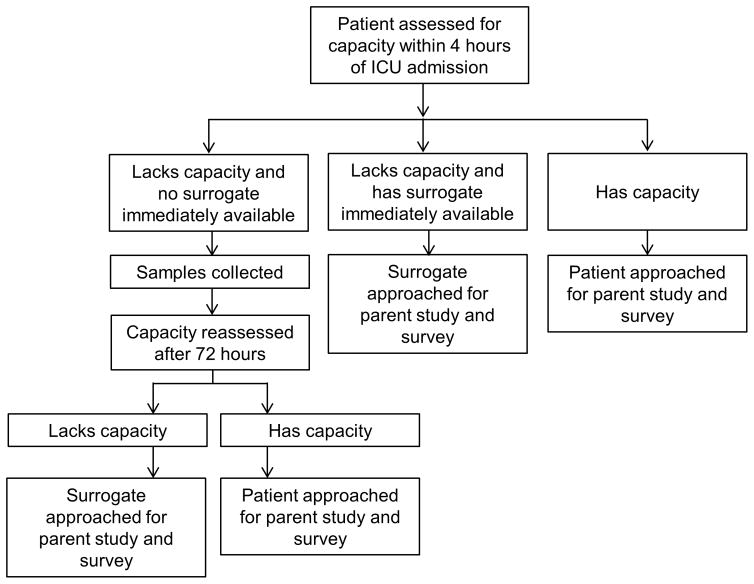
The initial IRB approval of the microbiome study included a traditional requirement for signed written informed consent before enrollment. Nine of the first 14 surrogates approached did not consent, and many of these mentioned that they would prefer that the patient make his or her own decision about participation. Surrogates were not readily available for a high proportion of eligible subjects. At this point, the microbiome study investigators (MAT and DEF) met with the IRB chair (MCM) to discuss these barriers to enrollment. A revised consent process was then developed whereby, if an eligible patient lacked capacity to consent to study participation and if a surrogate was not readily available, or if the acuity of the clinical situation precluded a meaningful informed consent discussion, study procedures began with a waiver of informed consent (i.e., consent was deferred). In these cases, informed consent was sought from the subject or a surrogate 3 days later, at which point it was made clear that samples had been collected at the time of admission but that research participation could be stopped. If the subject or the surrogate declined consent at that point, samples were destroyed, and patient data were removed from the database (Figure 1).
Figure 1.
Approach to the consent process.
Outcomes
The primary outcome was patient or surrogate support of the deferred consent process, ascertained through a verbal survey. Secondary outcomes included patient and surrogate attitudes towards research in the ICU and rates of participation in the parent study.
Survey Development and Implementation
The survey was pre-tested and administered verbally by one of the investigators (MAT or DEF) at the bedside. Patient selection for the survey, survey design and pre-testing, and survey implementation are described in the Supplementary Methods [14, 15]. The survey consisted of 8 multiple choice or open-ended questions; the complete instrument appears in the supplement.
Statistical Analysis
Categorical variables were assessed using a chi-squared test or Fisher’s exact test. Responses to open-ended questions were coded independently by two investigators based on a thematic analysis and a priori hypotheses (see the Supplementary Methods). Logistic regression modeling was used to test the relationship between consent approach (standard versus deferred) and participation in the parent study (participated versus did not participate) after adjusting for potential confounders. To construct the final model, we included demographic variables and testing further variables stepwise, retaining them if they had a significant independent relationship with the outcome or if they changed the β-coefficient representing participation in the parent study by ≥10%. Analyses were performed on STATA 12 at the alpha 0.05 level of significance.
RESULTS
Population
157 patients were invited to complete the consent survey, and 135 (86%) participated. None of the 22 individuals who declined to participate in the survey study expressed any objection to the consent process for the microbiome study. Individuals who consented to the microbiome study were more likely to participate in the survey than were individuals who declined to participate in the microbiome study (94/98, 96% vs. 41/59, 69%; p < 0.01). Deferred compared to standard consent was associated with participation in the parent study (Table 1) and this association persisted after adjusting for other factors (Supplementary Table 1). Those approached using deferred consent were less likely to cite fatigue or discomfort as their reason for not participating compared to those approached using a standard consent process (Table 2).
Table 1.
Characteristics of survey participants, stratified according to whether they did or did not participate in the parent study.
| Characteristics | All (n=135) | Participated in parent study n=94 (%) | Did not participate in parent study n=41 (%) | P-value |
|---|---|---|---|---|
| Age of patient eligible for microbiome study | 0.05 | |||
| ≤50 years old | 32 (24%) | 18 (19%) | 14 (34%) | |
| 51 to 65 years old | 48 (36%) | 39 (41%) | 9 (22%) | |
| ≥66 years old | 55 (41%) | 37 (39%) | 18 (44%) | |
|
| ||||
| Sex of patient eligible for microbiome study | 0.91 | |||
| Male | 78 (58%) | 54 (57%) | 24 (59%) | |
| Female | 57 (42%) | 40 (43%) | 17 (41%) | |
|
| ||||
| Reason for admission | 0.70 | |||
| Operative | 69 (51%) | 47 (50%) | 22 (54%) | |
| Non-operative | 66 (49%) | 47 (50%) | 19 (46%) | |
|
| ||||
| Type of approach for consent | <0.01 | |||
| Standard | 66 (49%) | 31 (33%) | 35 (85%) | |
| Deferred | 69 (51%) | 63 (67%) | 6 (15%) | |
|
| ||||
| Person from whom consent was sought | <0.01 | |||
| Patient | 107 (79%) | 82 (87%) | 25 (61%) | |
| Surrogate | 28 (21%) | 12 (13%) | 16 (39%) | |
|
| ||||
| Sex of the surrogate, when consent was obtained from a surrogate* | 0.57 | |||
| Male | 10 (36%%) | 5 (42%) | 5 (31%) | |
| Female | 18 (64%) | 7 (58%) | 11 (69%) | |
Percentages are based on column totals.
N=28 surrogates gave consent.
Table 2.
Reasons cited for participating or not participating in the microbiome study
| Responses to “What made you choose to be part of this research study?” N = the number of responses coded according to each theme.*
| ||||
|---|---|---|---|---|
| Response Characteristics | All (n=94) | Standard Consent N=31 | Deferred Consent N=63 | P-value |
| Participated (n=94) | ||||
| Altruism/belief in research | 72 (77%) | 28 (90%) | 44 (70%) | NS |
| Procedures not a big deal | 13 (14%) | 5 (16%) | 8 (13%) | NS |
| Patient might directly benefit | 7 (8%) | 1 (3%) | 6 (10%) | NS |
| Other | 11 (12%) | 1 (3%) | 10 (16%) | NS |
| Responses to “What made you choose not to be part of this research study?” N = the number of responses coded according to each theme.*
| ||||
|---|---|---|---|---|
| Response Characteristics | All (n=41) | Standard Consent N=35 | Deferred Consent N=6 | P-value |
| Did not participate (n=41) | ||||
| Discomfort or fatigue | 12 (29%) | 11 (31%) | 1 (17%) | <0.01 |
| Rectal swab | 7 (17%) | 7 (20%) | 0 (0%) | NS |
| Stressed/overwhelmed | 5 (12%) | 5 (14%) | 0 (0%) | NS |
| Opposed to research | 3 (7%) | 1 (3%) | 2 (33%) | NS |
| Paperwork | 2 (5%) | 1 (3%) | 1 (17%) | NS |
| Privacy | 2 (5%) | 0 (0%) | 2 (33%) | NS |
| No reason given | 6 (15%) | 6 (17%) | 0 (0%) | NS |
| Other | 5 (12%) | 5 (14%) | 0 (0%) | NS |
Percentages are based on column totals.
Seven respondents cited both altruism and the fact that the research did not substantially affect them, and 3 cited both altruism and the potential that the patient might benefit directly.
Percentages are based on column totals.
One participant cited both feeling overwhelmed and discomfort with the rectal swab as reasons for not participating.
Perspectives on deferred consent
For 69/135 survey participants, initial samples were obtained without a complete informed consent process. There was no association between the type of person giving consent (patient vs surrogate) and approach to consent (standard vs deferred) (p=0.77). 9/69 patients (13%) verbally agreed to participate at the time of ICU admission but were unable to engage in a full consent process or sign the consent document due to critical illness; none of these patients objected when approached for written consent on day 3 of the microbiome study. There were thus 60 patients or surrogates who were first approached for consent on study day 3. These 60 individuals were asked: “Did we make the right choice in waiting until now to ask your consent?” 44/60 (73%) responded positively, 3/60 (5%) responded negatively, and 13/60 (22%) had a neutral or unrelated response. Negative responses included concerns about privacy (n=2) and study interventions (n=1). 5/6 individuals who declined to grant deferred consent nonetheless replied that we were right to delay seeking consent.
Subjects were then asked to explain why they did or did not think we were right to defer consent. The most common response among those who supported deferred consent was that families or patients were too stressed or distracted to consider research participation at the time of ICU admission (n=25, for example: “Because of the stress, we wouldn’t have been able to think clearly about this”). Three respondents commented that deferred consent made the research more efficient, and one endorsed deferred consent but then offered an apparent contradiction: “Well, you know, I have a little problem with the swabs before my permission.” The negative responses were: “You shouldn’t do anything without permission, it’s an invasion of patients’ privacy,” and “Because I don’t want him to go through the same thing again.” Four participants offered a suggestion for an alternative consent process, for example suggesting that researchers seek consent during the hospital admission or pre-operative consent process.
Finally, survey participants were asked what consent approach we should use for a hypothetical future study that would involve taking one extra blood sample when patients are admitted to the ICU. Of the 75 patients/surrogates who had provided prospective consent, 40 (53%) replied that for the future hypothetical study consent should be sought first, 23 (31%) that consent should be deferred, and 12 (16%) gave other replies. Of the 60 patients/surrogates who had provided deferred consent, the replies were 30 (50%) consent first, 21 (35%) deferred consent, and 9 (15%) other.
Perspectives on ICU research
Survey participants were asked to briefly describe what happens to people who choose to be part of the microbiome study. 60/135 (44%) made responses that were categorized as correct, 17 (13%) that were partially correct, and 7 (5%) that were incorrect. 39/135 responses (29%) were too vague to categorize, and 12 individuals (9%) responded, “Nothing happens.” There was no difference in these responses based on participation in the parent study or on use of standard versus deferred consent (Supplementary Table 2).
97% of survey participants strongly agreed or agreed with the statement “Doctors should do research in the ICU to learn how to better help patients in the future,” and 62% strongly agreed or agreed with the statement “It is stressful to be asked about medical research in the ICU” (Table 3). Those who did not consent to the parent study were more likely to agree that it is stressful to be asked about medical research in the ICU.
Table 3.
Responses to Likert Scale questions
| Response Characteristics | All (n=135) | Participated n=94 (%) | Did Not Participate n=41 (%) | p-value |
|---|---|---|---|---|
| “Doctors should do research in the ICU to try to learn how to better help patients in the future.” | NS | |||
| Strongly agree | 105 (78%) | 78 (83%) | 27 (66%) | |
| Somewhat agree | 26 (19%) | 13 (14%) | 13 (32%) | |
| Somewhat disagree | 2 (2%) | 2 (2%) | 0 (0%) | |
| Strongly disagree | 2 (2%) | 1 (1%) | 1 (1%) | |
|
| ||||
| “It is stressful to be asked about medical research in the ICU.”* | 0.04** | |||
| Strongly agree | 27 (20%) | 17 (18%) | 10 (24%) | |
| Somewhat agree | 57 (42%) | 36 (38%) | 21 (51%) | |
| Somewhat disagree | 26 (19%) | 17 (18%) | 9 (22%) | |
| Strongly disagree | 25 (19%) | 24 (26%) | 1 (2%) | |
Percentages are based on column totals.
One patient declined to choose between somewhat agree and somewhat disagree.
Strongly/somewhat agree vs. strongly/somewhat disagree.
Reasons for Participating or Not Participating
Survey participants were all asked why they chose to participate or not to participate in the parent study. The most common theme for participating in the parent study was altruism (Table 2). Other common themes included that the study was “not a big deal,” and the incorrect belief that there might be direct personal benefit derived from participation. The most common reasons given for not participating in the parent study were general discomfort, aversion to a rectal swab, and feeling overwhelmed or stressed. Characteristic responses are given in Supplementary Table 3.
Enrollment in the Parent Study
Prior to the modified consent process, 38 individuals (14 patients and 24 surrogates) were approached for consent to participate in the parent study, and 19/38 (50%) consented. Additionally, an unrecorded number of subjects were not approached for consent during the pre-modified consent period because the patient lacked capacity and no surrogate was available. After the modified consent process, 168 individuals (132 patients and 36 surrogates) were approached for consent to participate in the parent study, and 108/160 (65%) consented (p=0.14 compared to before the modified consent process). Among those who completed the survey, participation in the microbiome study was more likely when a patient was asked compared to a surrogate (87% versus 61% respectively, p<0.01) and when consent was deferred (47% participation with standard consent versus 91% deferred, p<0.01).
DISCUSSION
We found that a deferred consent process for minimal risk research in the ICU was well accepted by most patients or their surrogates. Our data also suggest that a deferred consent process may increase study enrollment (47% with a standard consent approach vs 91% with deferred consent). Our study addresses the question of whether subjects who lack capacity and who do not have a readily available surrogate will object if they are enrolled in minimal risk research, then later given an opportunity to withdraw. Previous studies have found that patients and surrogates generally accept deferred consent for randomized interventions that must be undertaken rapidly, in situations where patients frequently lack capacity [12, 13]. This study suggests that patients and surrogates may hold similar views for ICU-based minimal risk research.
Participation in the parent study was more likely when consent was sought from the patient rather than a surrogate, a finding consistent with previously published literature [4]. Intuitively, it seems plausible that people are more willing to be altruistic on their own than to impose no-benefit research on a family member whose interests one is charged with protecting. A major potential advantage to the deferred consent approach is that it permits some patients to participate in the consent process who would not have been able to participate using a standard approach.
Patients and surrogates who were approached for deferred consent largely supported the deferred consent process. While some individuals raised concerns about deferred consent, many more (25/60, 42%) cited the stress of the ICU admission day as a reason they preferred deferred consent. However, support for deferred consent for a future, hypothetical, ICU study that involved taking a blood sample was much less robust. Among subjects who were approached using deferred consent, there was an apparent discrepancy in that 73% responded that we were right to defer consent for the actual study but only 50% responded that we should defer consent for a future study. There are several factors that may have contributed to this discrepancy. First, participants likely had a better understanding of the concrete details of the parent study and understood that it was minimal risk. With only a one-line description of a future, hypothetical study, participants may have been less comfortable supporting deferred consent. Previous studies have also found discrepancies between support for specific versus open-ended or hypothetical research studies [16, 17]. Second, in the description of the future, hypothetical study, we did not specify that the option for deferred consent would apply only to subjects who lack capacity. Third, the hypothetical study involved blood samples taken purely for research purposes, while the microbiome study involved research-driven swabs but used only residual blood. Many patients have strong beliefs regarding additional blood draws and the use of blood for medical research, which may have limited support for the hypothetical study [18]. The comparatively small number of survey participants who broadly endorsed a deferred consent approach suggests that investigators and IRBs should continue to take care to maximize subjects’ ability to engage in the informed consent process. Further work is needed to determine whether subjects and their surrogates endorse deferred consent for studies that involve drawing additional blood.
The requirement for informed consent may be waived for a variety of reasons for ICU-based research. Even for high-risk interventional studies, an exception to the requirement for informed consent is allowable in certain circumstances [19]. Scales et al. found that most survivors of critical illness say they would prefer involvement of a surrogate to a deferred consent process when presented with a hypothetical, interventional ICU study [20]. However, our study differs significantly in that the study procedures posed minimal risk, and subjects or their surrogates were given the option of having any previously collected samples destroyed if they chose not to consent to participate (i.e., the ability to “opt out” was fully preserved).
An IRB may waive or modify the requirement for informed consent when the risk posed by research participation is no more than minimal and it is not practicable to obtain informed consent [19]. The Secretary’s Advisory Committee on Human Research Protections (SACHRP) has commented that “These waivers are already permitted in the existing regulations, but nuances in the language have deterred IRBs from exercising the flexibility that the regulations were intended to provide” [21]. For SACHRP, the “impracticability” standard should not be interpreted to mean that a waiver should only be granted when it is impossible to seek informed consent: “Once the IRB has determined that the waiver or alteration does not adversely impact the ethical nature or scientific rigor of the research, logistical issues (e.g. cost, convenience, speed) may be considered” [21]. We believe the impracticability standard does apply to many time-sensitive, minimal-risk ICU-based research studies, such as ours.
It is also important to maximize the extent to which patients and surrogates have the opportunity to decide whether to participate in research. Critically ill patients constitute a vulnerable population, and a rigorous informed consent processes can minimize the potential for exploitation [22]. On the other hand, the high-stress circumstances surrounding ICU admission often render informed consent for research inadequate no matter how good the intentions of investigators. Strong research oversight may be more important than informed consent in protecting the rights and interests of ICU patients, but involving patients or their surrogates (to the extent possible) in the decision of whether they will participate in research promotes respect for persons [23]. A deferred consent process, rather than a complete waiver of informed consent, may help maximize patient and surrogate autonomy without impeding valuable research in many circumstances. When a deferred consent approach is considered, it is important to distinguish whether there are risks involved in sample acquisition or whether patients/surrogates are truly able to decline without having faced any potential risks.
The least controversial interpretation of our data is that deferring consent for minimal risk research is appropriate when the subject clearly lacks capacity and no surrogate is available. Our also data suggest that it may sometimes be appropriate to defer consent for minimal risk research when the subject or a surrogate could engage in a consent discussion but conditions such as pain or stress limit their ability to engage fully. Further data are needed to develop a best practices model for minimal risk research that is initiated at the time of ICU admission or at the time of acute medical deteriorations.
A complete waiver of consent may still be appropriate for many types of minimal risk research, such as research that involves use of de-identified residual biospecimens [24, 25]. The issue of residual samples is important, but was not directly addressed by this study.
Our study has some major strengths. Unlike prior studies, this was not a hypothetical question: these were real ICU patients and surrogates, asked regarding preferences about a real study. Our study assessed a heterogeneous group of ICU patients and elicited the reasons behind preferences rather than preferences alone.
There are limitations to the study. Survey response effects are possible, and survey respondents may also have altered their true responses in order to please the interviewers. Specifically, survey respondents were asked: “Did we make the right choice in waiting?” for a deferred consent. This question may be particularly susceptible to social desirability bias, as respondents may have been reluctant to respond that we were wrong. There is the possibility of selection bias because not all of the patients or surrogates who were approached were willing to complete the survey. Last, the data represents experience from a single, academic medical center.
CONCLUSIONS
In this single center study, most critically ill patients or their surrogates accepted a deferred consent process for minimal risk ICU research, and a deferred consent process appeared to increase overall participation in research. Future studies should examine the potential impact of deferred consent on minimal risk research in the ICU.
Supplementary Material
Footnotes
Conflicts of Interest and Source of Funding: Dr. Freedberg was supported by a Research Scholar Award from the American Gastroenterological Association (AGA). The views expressed in this article represent those of the authors and do not necessarily represent the views of the AGA. The remaining authors have disclosed that they do not have any conflicts of interest.
References
- 1.Larkin MEBC, Magyar K, Macey L, et al. Obtaining surrogate consent for a minimal-risk research study in the intensive care unit setting. Clinical Trials. 2013;10:93–6. doi: 10.1177/1740774512464727. [DOI] [PubMed] [Google Scholar]
- 2.Burns KE, Zubrinich C, Tan W, et al. Research Recruitment Practices and Critically Ill Patients. A Multicenter, Cross-Sectional Study (The Consent Study) American Journal of Respiratory and Critical Care Medicine. 2013;187:1212–8. doi: 10.1164/rccm.201208-1537OC. [DOI] [PubMed] [Google Scholar]
- 3.Pochard F, Azoulay E, Chevret S, et al. Symptoms of anxiety and depression in family members of intensive care unit patients: ethical hypothesis regarding decision-making capacity. Critical Care Medicine. 2001;29:1893–7. doi: 10.1097/00003246-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Ciroldi M, Cariou A, Adrie C, et al. Ability of family members to predict patient’s consent to critical care research. Intensive Care Medicine. 2008;33:807–13. doi: 10.1007/s00134-007-0582-6. [DOI] [PubMed] [Google Scholar]
- 5.Freeman BD, Bolcic-Jankovic D, Kennedy CR, et al. Perspectives of Decisional Surrogates and Patients Regarding Critical Illness Genetic ResearchPerspectives of Decisional Surrogates and Patients Regarding Critical Illness Genetic Research. AJOB Emperical Bioethics. 2015;7:39–47. doi: 10.1080/23294515.2015.1039148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox EE, Bulger EM, Dickerson AS, et al. Waiver of consent in noninterventional, observational emergency research: the PROMMTT experience. J Trauma Acute Care Surg. 2013;75:S3–8. doi: 10.1097/TA.0b013e31828fa3a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yawn BP, Yawn RA, Geier GA, et al. The impact of requiring patient authorization for use of data in medical records research. Journal of Family Practice. 1998;47:361–5. [PubMed] [Google Scholar]
- 8.Jacobsen SJ, Xia Z, Campion ME, et al. Potential effect of authorization bias on medical record research. Mayo Clinic Proceedings. 1999;75:330–8. doi: 10.4065/74.4.330. [DOI] [PubMed] [Google Scholar]
- 9.Woolf SH, Rothemich SF, Johnson RE, et al. Selection bias from requiring patients to give consent to examine data for health services research. Archives of Family Medicine. 2000;9:1111–8. doi: 10.1001/archfami.9.10.1111. [DOI] [PubMed] [Google Scholar]
- 10.Tu JV, Willison DJ, Silver FL, et al. Impracticability of informed consent in the Registry of the Canadian Stroke Network. NEJM. 2004;350:1414–21. doi: 10.1056/NEJMsa031697. [DOI] [PubMed] [Google Scholar]
- 11.Buckley B, Murphy AW, Byrne M, et al. Selection Bias Resulting from the Requirement for Prior Consent in Observational Research: A Community Cohort of People with Ischaemic Heart Disease. Heart. 2007;93:1116–20. doi: 10.1136/hrt.2006.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potter JE, McKinley S, Delaney A. Research participants’ opinions of delayed consent for a randomised controlled trial of glucose control in intensive care. Intensive Care Med. 2013;39:472–80. doi: 10.1007/s00134-012-2732-8. [DOI] [PubMed] [Google Scholar]
- 13.Dickert NW, Mah VA, Baren JM, et al. Enrollment in research under exception from informed consent: the Patients’ Experiences in Emergency Research (PEER) study. Resuscitation. 2013;84:1416–21. doi: 10.1016/j.resuscitation.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns KE, Duffett M, Kho ME, et al. A guide for the design and conduct of self-administered surveys of clinicians. CMAJ. 2008;179:245–52. doi: 10.1503/cmaj.080372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett C, Khangura S, Brehaut JC, et al. Reporting guidelines for survey research: an analysis of published guidance and reporting practices. PLoS Med. 2010;8:e1001069. doi: 10.1371/journal.pmed.1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biros MH, Sargent C, Miller K. Community attitudes towards emergency research and exception from informed consent. Resuscitation. 2009;80:1382–7. doi: 10.1016/j.resuscitation.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickert NW, Scicluna VM, Baren JM, et al. Patients’ perspectives of enrollment in research without consent: the patients’ experiences in emergency research-progesterone for the treatment of traumatic brain injury study. Crit Care Med. 2015;43:603–12. doi: 10.1097/CCM.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moodley K, Sibanda N, February K, et al. “It’s my blood”: ethical complexities in the use, storage and export of biological samples: perspectives from South African research participants. BMC Med Ethics. 2014;15:4. doi: 10.1186/1472-6939-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Code of Federal Regulations. 45 CFR 46.116 General Requirements for Informed Consent. Accessible at: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/
- 20.Scales DC, Smith OM, Pinto R, et al. Patients’ preferences for enrolment into critical-care trials. Intensive Care Med. 2009;35:1703–12. doi: 10.1007/s00134-009-1552-y. [DOI] [PubMed] [Google Scholar]
- 21.SACHRP. SACHRP Letter to the HHS Secretary; Attachment D: Informed Consent and Waiver of Consent. 2013 Jan 10;2013 Accessible at: https://www.hhs.gov/ohrp/sachrp-committee/recommendations/2013-january-10-letter/index.html. [Google Scholar]
- 22.Silverman H. Protecting Vulnerable Research Subjects in Critical Care Trials: Enhancing the Informed Consent Process and Recommendations for Safeguards. Annals of Intensive Care. 2011;1:1–7. doi: 10.1186/2110-5820-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luce J. Is the concept of informed consent applicable to clinical research involving critically ill patients? Critical Care Medicine. 2003;31:S153–60. doi: 10.1097/01.CCM.0000054901.80339.01. [DOI] [PubMed] [Google Scholar]
- 24.Federal Register. 2015 Sep 8;80(173) Accessible at: https://www.gpo.gov/fdsys/pkg/FR-2015-09-08/pdf/2015-21756.pdf. [Google Scholar]
- 25.Federal Register. 2017 Jan 19;82(12) Accessible at: https://www.gpo.gov/fdsys/pkg/FR-2017-01-19/pdf/2017-01058.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.