Abstract
In vitro evaluation of the halogenated pyrrolo[3,2-d]pyrimidines identified antiproliferative activities in compounds 1 and 2 against four different cancer cell lines. Upon screening of a series of pyrrolo[3, 2-d]pyrimidines, the 2,4-Cl compound 1 was found to exhibit antiproliferative activity at low micromolar concentrations. Introduction of iodine at C7 resulted in significant enhancement of potency by reducing the IC50 into sub-micromolar levels, thereby suggesting the importance of a halogen at C7. This finding was further supported by an increased antiproliferative effect for 4 as compared to 3. Cell-cycle and apoptosis studies conducted on the two potent compounds 1 and 2 showed differences in their cytotoxic mechanisms in triple negative breast cancer MDA-MB-231 cells, wherein compound 1 induced cells to accumulate at the G2/M stage with little evidence of apoptotic death. In contrast, compound 2 robustly induced apoptosis with concomitant G2/M cell cycle arrest in this cell model.
Keywords: Thieno[3, 2-d]pyrimidine, Heterocyclic chemistry, Cytostatic, Apoptosis, Cell cycle arrest
1. Introduction
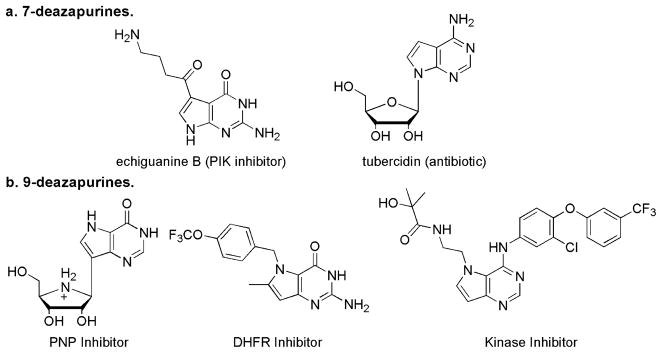
Pyrrolopyrimidines exist as three regioisomers, specifically pyrrolo[2,3-d]pyrimidine, pyrrolo[3,2-d]pyrimidine and pyrrolo [3,4-d]pyrimidine. The former two isomers more commonly referred to as 7-deazapurine and 9-deazapurine, respectively, (Fig. 1) have been of interest for long, due to close resemblance to the purines. Hence these pyrrolopyrimidines have often found use in the pharmacology field as a purine isostere.1–12 The 7-deazapurines (Fig. 1a) are naturally occurring and widely used in drug design primarily due to their propensity to be ribosylated. For example, echiguanine B and tubercidin are two naturally occurring 7-deazapurines exhibiting anticancer properties.
Figure 1.
Deazapurines and their biological properties.
In contrast, the pyrrolo[3,2-d]pyrimidines do not occur naturally and must be synthetically prepared. The 9-deazapurines have also been explored in the past as nucleoside isosteres and these efforts have resulted in the design of purine nucleoside phosphorylase (PNP) inibitors,7,13–22 dihydrofolate reductase (DHFR) inhibitors23,24 and more recently for kinase inhibition (Fig. 1b).25–27
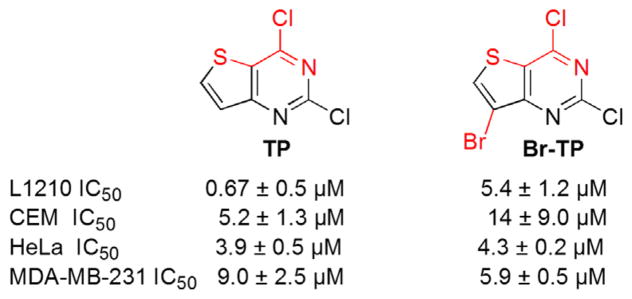
Our laboratory’s interest in exploring the biological properties of fused heterocyclic compounds30–34 has resulted in the identification of antiproliferative properties of halogenated thieno[3,2-d]pyrimidines (TP and Br-TP) as shown in Figure 2. The halogenated thieno[3,2-d]pyrimidines were found to induce cell cycle independent apoptosis in L1210, a mouse lymphocytic leukemia cell line.28 However, they showed remarkable differences in their cytotoxic mechanisms in the aggressive triple negative breast cancer (TNBC) cell line MDA-MB-231, which do not express estrogen or progesterone receptors and do not show HER-2/Neu gene amplification. While both halogenated thieno[3,2-d]pyrimidines shown in Figure 2 were toxic to MDA-MB-231 cells, for the analogue lacking bromine at C7 (TP), cell death was accompanied by dramatic arrest at the G2/M cell cycle transition (Fig. 2), a mechanism not observed when cells were treated with the bromo analogue (Fig. 2, Br-TP).29 Together, these data indicated that 2,4-dichloro thieno[3,2-d]pyrimidines were potently toxic to several tumor cell types, but also that their antiproliferative potency and cytotoxic mechanisms varied as a function of halogenation at C7 in a cell type-specific manner.
Figure 2.
Antiproliferative activities of halogenated thieno[3,2-d]pyrimidines.28,29
In an effort to further expand the repertoire of halogenated fused pyrimidines, our studies were extended to the pyrrolo[3, 2-d]pyrimidine scaffold. Once in hand, the toxicity of halogenated pyrrolo[3,2-d]pyrimidines was tested against four cancer cell lines (L1210, CEM, HeLa and MDA-MB-231). This examination revealed significant antiproliferative activities for compounds 1–4 (Fig. 3) but also indicated that this activity was enhanced by inclusion of iodine at C7. Subsequent testing of compounds 1 and 2 across the NCI-60 cancer cell line panel further supported the impact of iodine at C7 on the cytostatic/cytotoxic activity of the halogenated pyrrolo[3,2-d]pyrimidines and underscore their spectrum of activity. Compounds 1 and 2 were then evaluated for cell cycle distributions and apoptosis of MDA-MB-231 cells to explore their mechanism of antiproliferative activity. The results indicated that both compounds trigger arrest at G2/M, although to varying degrees.
Figure 3.
Halogenated pyrrolo[3,2-d]pyrimidine targets.
2. Results
2.1. Chemistry
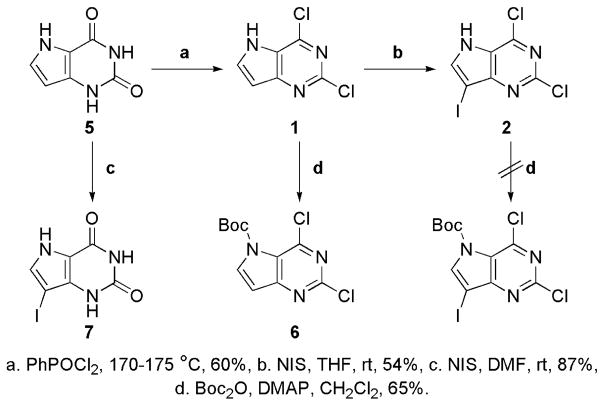
In order to explore the antiproliferative properties of the halogenated pyrrolo[3,2-d]pyrimidines as well as the effect of the halogen at C7, two sets of compounds were synthesized (Schemes 1 and 2). The target compounds in Scheme 1 were designed to explore the anticancer properties of the pyrrolo[3,2-d]pyrimidines possessing a chlorine at C4. A second set of compounds illustrated in Scheme 2 possessing O-benzyl groups on the C2 and C4 positions were synthesized to evaluate the effect of C7 halogen on antiproliferative properties independent of chlorine at C2 and C4. The 2,4-bis-O-benzylated compounds were chosen instead of methoxy analogues primarily because the 2,4-methoxy substitution on thieno[3,2-d]pyrimidines lead to complete loss of activity. Subsequently, we were unable to assess and establish unequivocally the contribution or enhancement of activity by halogen at C7. In order to explore an alternative substituent, 2,4-bis-O-benzylated pyrrolo[3,2-d]pyrimidines were prepared and evaluated for antiproliferative properties.
Scheme 1.
Synthesis of halogenated pyrrolo[3,2-d]pyrimidines.
Scheme 2.
Synthesis of the bis-O-benzyl analogues.
The synthesis of halogenated pyrrolo[3,2-d]pyrimidines (Scheme 1) began with chlorination of the known pyrrolo[3, 2-d]pyrimidin-2,4-dione 5.35–39 Preparation of the sodium salt, followed by heating with phenylphosphonic dichloride (PhPOCl2) at 170–175 °C gave 1.35–39 Next, we decided to introduce a halogen at C7 that would help us evaluate halogen other than chlorine and bromine, thereby expanding the repertoire of halogens on the fused pyrimidine scaffold. Iodine besides being a larger halogen is also easier to displace.40 Thus, the iodo analogue was prepared by using N-iodosuccinimide (NIS) in tetrahydrofuran (THF) at room temperature yielding compound 2.41
Attempts to protect the 5-NH of 2 with tert-butoxycarbonyl (Boc), in order to evaluate the effect of the NH on the biological activity of 2, failed due to the instability of the Boc protecting group during the subsequent silica gel purification. Alternatively, Boc protection of 1 was attempted using Boc2O in the presence of 4-dimethylaminopyridine (DMAP) at room temperature to give 6.42 Iodination of pyrrolo[3,2-d]pyrimidin-2,4-dione 5 by NIS in DMF followed by crystallization from ethanol gave 7 as a yellow solid.43
As shown in Scheme 2, synthesis of the bis-O-benzylated pyrrolo[3,2-d]pyrimidines was initiated by formylation of the 6-methyl group on 9 by heating with DMF-dimethylacetal in DMF at 60–65 °C, to obtain 10 (Scheme 2).37 Subsequently, stirring 10 with zinc (Zn) in acetic acid (AcOH) enabled ring cyclization to give 2,4-OBn pyrrolo[3,2-d]pyrimidine 3 in high yields.37,44–46 Iodination of 3 using NIS in methylene chloride (CH2Cl2) afforded 4 which was followed by the protection of 5-NH using Boc in the presence of DMAP to obtain 10.40
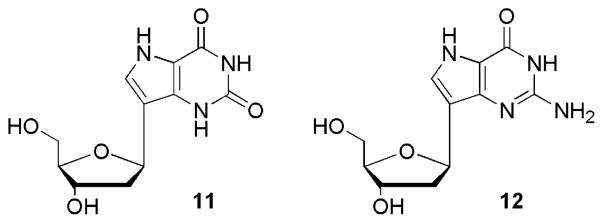
Along with the compounds shown in Schemes 1 and 2, two additional compounds, namely the 9-deaza-2′-deoxynucleosides 11 and 12 were tested owing to past1–7,47–57 and recent58 interest in the pharmacological properties of 9-deazanucleosides. The syntheses of the compounds shown in Figure 4 have been previously reported by our group but their antiproliferative properties had not yet been evaluated.40,59
Figure 4.
9-Deaza-2′-deoxynucleosides 11 and 12.40
2.2. In vitro cell growth inhibition
The cytotoxic activities of the compounds described above were first assessed in three cultured tumor cell models: L1210, a mouse lymphocytic leukemia cell line,60 CCRF-CEM,61 an acute lymphoblastic leukemia cell line, and HeLa, a human cancer cell line derived from a human cervical adenocarcinoma.62,63 Comparisons of resolved IC50 values (Table 1) indicate that the dichloro compounds 1 and 2 show measureable cytostatic activity in all three tumor cell lines. The presence of iodine at C7 on the 2,4-dichloro pyrrolo[3,2-d] (2) increases the antiproliferative activity by a factor of 5 to 20, when compared to 1. An analogous increase in cytotoxicity for bis-O-benzylated pyrrolo[3,2-d]pyrimidine 3 was observed upon introduction of C7 iodine (4). Replacement of the 2,4-chloro moieties with O-benzyl groups led to a loss of activity by a factor of 3–15, however the activity of 4 was comparable to 1. This suggests a compensation for the loss of activity for the O-benzyl groups by the presence of iodine at C7.
Table 1.
Anti-tumor cell activity of test compounds

| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | A | B | R1 | R2 | R3 | X | IC50
*(μM)
|
||
| L1210 | CEM | HeLa | |||||||
| 1 | Cl | Cl | — | — | H | H | 6.8 ± 2.8 | 25 ± 2 | 19 ± 3 |
| 2 | Cl | Cl | — | — | H | I | 0.93 ± 0.0 | 4.9 ± 0.4 | 0.92 ± 0.04 |
| 3 | OBn | OBn | — | — | H | H | 118 ± 8 | 86 ± 22 | 98 ± 14 |
| 4 | OBn | OBn | — | — | H | I | 21 ± 2 | 17 ± 4 | 17 ± 0 |
| 5 | O | O | H | H | H | H | >250 | >250 | >250 |
| 6 | Cl | Cl | — | — | Boc | H | 21 ± 1 | 26 ± 2 | 18 ± 3 |
| 7 | O | O | H | H | H | I | >250 | >250 | >250 |
| 10 | OBn | OBn | — | — | Boc | I | 247 ± 4 | 193 ± 81 | 72 ± 47 |
| 1140 | O | O | H | H | H |

|
>250 | >250 | >250 |
| 1240 | O | NH2 | — | H | H |

|
>250 | >250 | >250 |
| 5FU | — | — | — | — | — | — | 0.33 ± 0.17 | 18 ± 5 | 0.54 ± 0.12 |
50% inhibitory concentration. 5FU—5-fluorouracil.
Introduction of the Boc group on 2,4-dichloro pyrrolo[3, 2-d]pyrimidine 6 did not lead to any loss in activity against CEM and HeLa cells, while activity against L1210 was weakened by a factor of 3. We speculate that these activities arise from the labile nature of Boc protection on 5-NH and release of 1 in the biological environment. On the other hand, the presence of the Boc group on the bis-O-benzyl analogue (10) led to a complete loss in activity. These data support the importance of 7-NH in the cytostatic role of the 2,4-O-benzylated analogues 3 and 4. The amide groups, as found in 5 and 7 do not impart activity against any cancer cell line tested, which is consistent with our previous studies on the thieno[3,2-d]pyrimidines. Similarly, the C-nucleosides 11 and 12 were both inactive and exhibited no cytotoxicity.40
2.3. NCI-60 DTP Human Tumor Cell Line screen
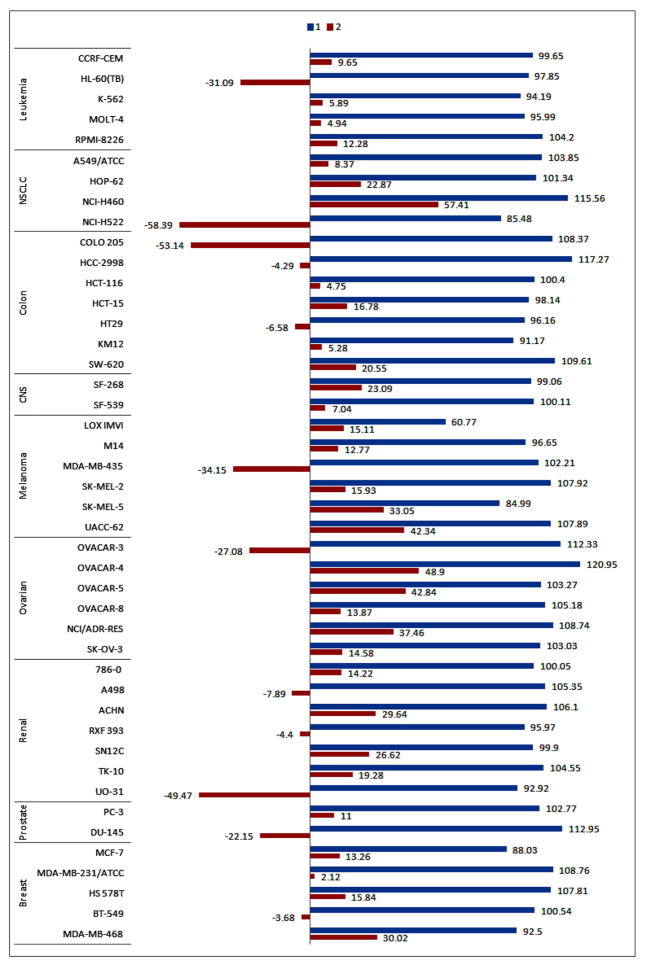
In order to validate the promising activities of compounds 1 and 2 and also the role of C7 iodine, their cytostatic/toxic activities were tested at 10 μM concentrations against the NCI-60 DTP Human Tumor Cell Line screen. These cell lines are derived from many different cancer types including leukemia, non-small cell lung cancer (NSCLC), colon, CNS cancer, melanoma, ovarian, renal, prostate, and breast cancer (Fig. 5). In this screen, 10 μM of each compound is added to cells and incubated for 48 hrs before chemical fixation and measurement of cellular protein content using the sulforhodamine B (SRB) dye.64,65 This assay enables differentiation between cytostatic and cytotoxic activities based on the magnitude and direction of endpoint deviations from initial cell density.66
Figure 5.
Growth inhibition of cancer cell lines by compounds 1 and 2 at 10 μM. Compounds 1 and 2 tested at 10 μM concentrations against the NCI-60 DTP Human Tumor Cell Line panel. The blue bars indicate percent changes in cell densities following incubation with 1 at 10 μM for 48 h, while the red bars indicate percent changes in cell densities following 48 h incubation with 10 μM 2. The number on each bar indicates the measured % growth.
Compound 1 did not show meaningful inhibition of growth of the cancer cell lines at 10 μM concentration (Fig. 5) however introduction of iodine at C7 (compound 2) led to an increase in the ability of the halogenated pyrrolo[3,2-d]pyrimidine to inhibit the growth of all cancer cell lines tested. The results of single-dose testing suggests that compound 2 predominately exhibits cytostatic activity (growth percent 0–50%), but in some instances led to a cytotoxic effect (growth percent <0%). The latter was observed in HL-60(TB), NCI-H522, COLO 205, MDA-MB-435, OVACAR-3, UO-31 and DU-145 (Fig. 5). These results, in addition to the earlier testing, verify that the iodine at C7 plays a key role in increasing the potential anticancer properties of the C7-iodinated compound 2. The screening of compound 1 highlights the cytotoxic activities against specific cell lines in various cancer subsets, which may be due to a common or similar mechanism of action. The study of common characteristics of these cell lines is of our interest to elucidate the biological target and mechanism of action.
2.4. Cell cycle and apoptosis studies
Previously, 2,4-dichloro thieno[3,2-d]pyrimidines (see Fig. 2) were shown to induce apoptosis independent of cell cycle arrest in L1210 cells.28 Our recent findings show that a 2,4-dichloro thieno[3,2-d]pyrimidine can also induce cell cycle arrest at G2/M in the MDA-MB-231 breast cancer cell line.29 Subsequently, we chose to study the effects of 2,4-dichloro pyrrolo[3,2-d]pyrimidines 1 and 2 on cell cycle progression and apoptosis in MDA-MB-231 cells for two principal reasons. First, the MDA-MB-231 line is a well-characterized cell model of TBNC, an aggressive breast cancer type for which no biomarker-targeted therapies are currently available.67 Second, constitutive overexpression of anti-apoptotic factors generally prevents chemotherapeutic induction of apoptosis in breast cancer cells including MDA-MB-231,68–70 so alternative triggering mechanisms such as mitotic arrest may represent appealing therapeutic modalities for these tumors. MTT cytotoxicity assays resolved IC50 values of 6.0 ± 1.3 μM and 0.51 ± 0.10 μM for compounds 1 and 2, respectively, in MDA-MB-231 cell cultures (Fig. 6). These data provided dosage information critical for subsequent cell cycle and apoptosis assays, but also further confirm that introduction of iodine at C7 increases the potency by at least a factor of 10.
Figure 6.
MTT assays for cytotoxicity by 1 and 2 in MDA-MB-231 cells. Cells were treated with select concentrations of compounds 1 (a) and 2 (b). After 48 h, cell viability was measured using MTT assays. Compound cytotoxicity was calculated by non-linear regression to a sigmoidal dose response function. Quoted IC50 values represent the mean ± SD of three independent experiments.
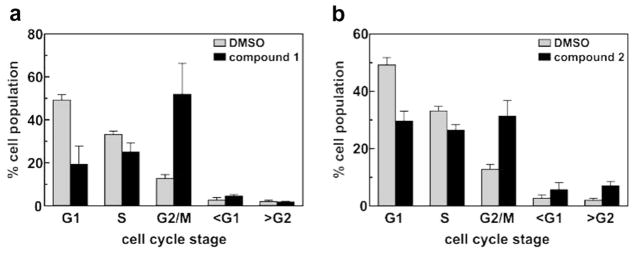
To determine whether the anticancer activities of pyrrolo[3,2-d]pyrimidine compounds 1 and 2 are associated with perturbation of the cell cycle, we monitored the effects of each compound on cell cycle distributions using propidium iodide staining and flow cytometry. At the IC80 concentration of 15 μM, compound 1 induced profound accumulation of the tumor cells at the G2/M stage after 48 hours (Fig. 7a), consistent with mitotic arrest and similar to the effect of the non-brominated 2,4-dichloro thieno[3,2-d]pyrimidine (Fig. 2, TP) on MDA-MB-231 cells reported previously.29 By contrast, treatment with an equitoxic concentration (1.75 μM) of compound 2 yielded a much smaller but still statistically significant enrichment in the G2/M fraction (Fig. 7b, P = 0.0006 vs vehicle control). The similarities in IC50 values and the accumulation of the tumor cells in the G2/M phase observed for compound 1 and the non-brominated thieno[3,2-d]pyrimidine described previously29 suggests that they may induce MDA-MB-231 tumor cell death via similar mechanisms. However, the dramatic enhancement in IC50 observed with compound 2 concomitant with its decreased impact on the cell cycle suggests that introduction of iodine at C7 might induce cytotoxicity by a distinct molecular mechanism. That such an impact might be directed by the iodine group on C7 is also supported by the abrogation of G2/M accumulation that was observed when 2,4-dichloro thieno[3,2-d]pyrimidine was brominated at the same site (Fig. 2, Br-TP).29
Figure 7.
Cell cycle analyses of MDA-MB-231 cells following treatment with 1 and 2. Cells were treated with IC80 concentrations of compound 1 (a, 15 μM) or compound 2 (b, 1.75 μM) for 48 h. The cell cycle distributions of drug-treated cells and vehicle (DMSO) controls were analyzed by flow cytometry of fixed, propidium iodide-stained cells. A minimum of 3000 cells were analyzed per cell population. Each bar represents the mean ± SD across 4 independent cell samples.
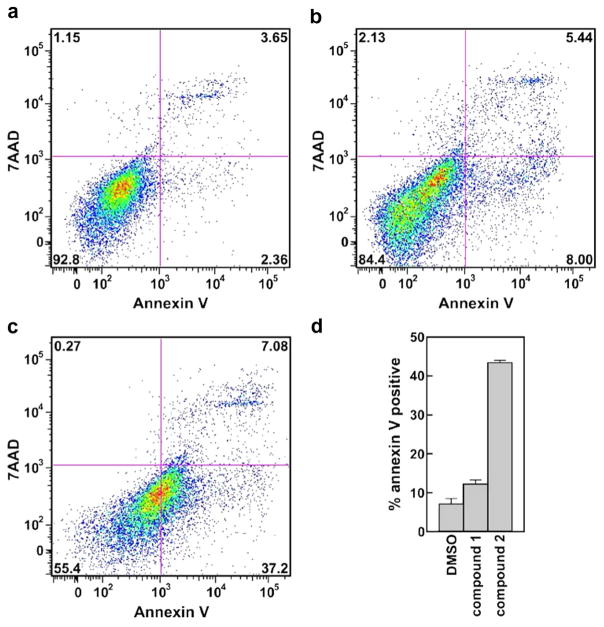
Next, we analyzed unfixed MDA-MB-231 cell samples by staining with annexin V and 7-amino-actinomycin D (7-AAD) to determine whether pyrrolo[3,2-d]pyrimidine compounds 1 and 2 direct cell death using classical apoptotic or necrotic mechanisms. Vehicle (DMSO)-treated cells yielded very low proportions of apoptotic or necrotic cells after 48 h (Fig. 8a). Curiously, treating cells with the IC80 concentration (15 μM) of compound 1 only slightly increased the proportion of cells recovered in early/late apoptosis (Fig. 8b and d). By contrast, over 40% of MDA-MB-231 tumor cells treated with compound 2 at its IC80 (1.75 μM) were annexin V-positive after 48 h, with the larger proportion of these 7-AAD negative consistent with early apoptosis (Fig. 8c and d).
Figure 8.
Apoptosis analyses of MDA-MB-231 cells following treatment with IC80 concentrations of compounds 1 and 2. Cells were treated with (a) an equal volume of vehicle (DMSO), (b) 15 μMcompound 1, or (c) 1.75 μMcompound 2. After 48 h, cells were stained using annexin V and 7-AAD and analyzed by flow cytometry. A minimum of 10,000 cells were analyzed per condition, and subdivided into four categories: non-apoptotic/non-necrotic (bottom left), early apoptotic (bottom right), late apoptotic/necrotic (top right), and necrotic (top left). Percentages of total cells detected in each quadrant are indicated. (d) Total apoptotic (early + late) cell fractions detected in each treatment group. Bars show the mean ± SD values compiled across four independent experiments.
While compound 1 is clearly toxic to MDA-MB-231 cells at the tested concentration, only a modest (although statistically significant) increase in annexin V-positive cells was detected. There are several possibilities that could account for this, including: (i) that cell lysis occurs rapidly following induction of apoptosis by compound 1, preventing significant accumulation of annexin V-positive bodies, or (ii) that a distinct (i.e., non-apoptotic/necrotic) cell death pathway is triggered by 1. On the other hand, the robust accumulation of annexin V-positive cells observed following treatment with compound 2 is consistent with apoptotic cell death, similar to that previously observed for the thieno[3,2-d]pyrimidine compounds shown in Figure 2a in mouse lymphocytic leukemia L1210 cells.28
3. Discussion
Halogenated pyrrolo[3,2-d]pyrimidines have been commonly used as key intermediates to install various types of functional groups on a pyrrolo[3,2-d]pyrimidine scaffold in order to evaluate the effect of substituents for a variety of medicinal properties. 26,27,39,41,43 However, the potential of halogenated pyrrolo[3,2-d]pyrimidines themselves as a therapeutically relevant scaffold has not previously been evaluated. Prompted by our earlier findings with the thieno[3,2-d]pyrimidines,28 2,4-dichloropyrrolo[3,2-d]pyrimidines 1 and 2 were evaluated for their antiproliferative properties against a number of cancer cell lines (Table 1 and Fig. 2). Since the importance of the C4 Cl group was already established,28 we studied the effect of pyrrole on the antiproliferative properties of a halogenated fused-pyrimidine scaffold. The activity of 2,4-dichloro pyrrolo[3,2-d]pyrimidine 1 is slightly weaker than its thieno counterpart, however, the presence of a 7-iodo enhanced the cytotoxicity to an extent that the activity was comparable to the halogenated thieno[3,2-d]pyrimidines shown in Figure 2. This notable increase in cytotoxic activity for 2 led to evaluation of bis-O-benzylated pyrrolo[3,2-d]pyrimidines 3 and 4 to assess the effect of C7 iodine independent of the C2 and C4 chlorines. The enhancement of cytotoxic activity for both 2 and 4 compared with the C7-unsubstituted parent compounds highlights the ability of the C7 iodine in imparting cytotoxic properties to the pyrrolo[3,2-d]pyrimidine scaffold independently.
Our recent studies on the cytotoxicity of 2,4-dichlorothieno[3,2-d]pyrimidines in MDA-MB-231 cells have shown that an analogue lacking a functional group at C7 arrests the cell cycle at the G2/M checkpoint in this cell model. By contrast, the presence of a bromine at C7 induced cell death via a distinct, cell cycle-independent mechanism.29 Consistent with these findings, cell cycle distribution experiments conducted on MDA-MB-231 cells treated with pyrrolo[3,2-d]pyrimidines 1 and 2 indicated that while both induce G2/M cell cycle arrest, the presence of the C7 iodine substantially diminished the accumulation of cells at the G2/M stage (Fig. 7). Furthermore, the accumulation of annexin V-positive bodies among cells treated with compound 2 (Fig. 8) indicated contributions from apoptotic pathways to cytotoxicity, an observation that was not evident in cells treated with compound 1, which lacks the C7 iodine. This observation is particularly intriguing in the TNBC MDA-MB-231 cell model since elevated levels of the apoptosis inhibitor Bcl-xL and a mutation in the tumor suppressor p53 significantly weaken its responsiveness to many apoptotic stimuli.68 Similar apoptotic resistance mechanisms are common in breast cancers;69,70 this complication is a major motivator for the development of novel therapeutic strategies.
In summary, the antiproliferative activities of the halogenatedfused pyrimidines highlight the potential of reactive species as therapeutically useful scaffolds. Previously chemically labile moieties as electrophilic covalent inhibitors have been considered undesirable71–78 due to their off-target interactions. However in the recent past interest in chemically labile molecules has revived owing to their utility to act as covalent irreversible inhibitors.79–81 The biological activity exhibited by halogenated pyrrolo[3,2-d]pyrimidines underscores their utility as potential lead molecules that may be exploited to treat cancer in a novel and effective manner.
4. Conclusion
We have identified interesting antiproliferative properties for several 2,4-dichloro pyrrolo[3,2-d]pyrimidines. Further, the observation that the presence of iodine at C7 markedly enhances cytotoxic properties is notable. These studies highlight their pharmacological relevance in addition to expanding the domain of the halogenated fused [3,2-d]pyrimidine scaffold. These halogenated compounds exhibited potent activity against TNBC MDA-MB-231 cells involving arrest of the cell cycle in G2/M. We have previously established the importance of the chlorine at C4, however herein we have demonstrated that inclusion of an additional halogen at C7 dramatically increases cytotoxicity, which, in the case of MDA-MB-231 cells, includes characteristics of apoptotic cell death. Studies are underway to further expand the repertoire of these molecules with varied substituents on the scaffold to improve the antiproliferative activities of these halogenated pyrrolo[3,2-d]pyrimidines. Those results will help expand the chemical space of halogenated heterocyclic compounds with an ability to induce death in cancer cells.
5. Experimental section
5.1. Synthetic methods
5.1.1. General
All chemicals and reagents listed in this section were purchased through commercially available sources unless otherwise noted. Anhydrous CH2Cl2, CH3CN, and THF were obtained from a solvent purification system (SPS, Model: mBraun Labmaster 130). Anhydrous DMF, MeOH and pyridine were obtained from Sigma–Aldrich or Acros Organics. All 1H and 13C NMR spectra were obtained from a JEOL ECX 400 MHz NMR. All 1H and 13C NMR spectra were referenced to internal tetramethylsilane (TMS) at 0.0 ppm. The spin multiplicities are indicated by the symbols s (singlet), d (doublet), dd (doublet of doublets), t (triplet), q (quartet), m (multiplet), and br (broad). All NMR solvents were obtained from Cambridge Isotope Laboratories. All reactions were monitored by thin layer chromatography (TLC) on 0.25 mm precoated glass plates. All column chromatography was run on 32–63 μ silica gel obtained from Dynamic Adsorbents Inc. (Norcross, GA, USA). Melting points are uncorrected. Yields refer to chromatographically and spectroscopically (1H and 13C NMR) homogeneous materials. All mass spectra (MS) were recorded and obtained from the University of Maryland Baltimore County Mass Spectrometry Facility and Johns Hopkins Mass Spectrometry Facility. The FAB mass spectra were obtained using double focusing magnetic sector mass spectrometer equipped with a Cs ion gun and Fourier transform ion cyclotron resonance equipped with ESI source.
5.1.1.1. Pyrrolo[3,2-d]pyrimidin-2,4-dione (5)
To a stirred slurry of (E)-6-(2-(dimethylamino)vinyl)-5-nitropyrimidin-2, 4-dione 435,38 (1 g, 4.4 mol) in fresh glacial AcOH (50 mL), Zinc dust (stabilized) (2 g) was added in two lots of 1 g with an interval of 1 h. Upon overnight stirring the yellow slurry changed to a pale yellow to off-white slurry which was filtered and the filtrate concentrated in vacuo to obtain brown syrup. The product was precipitated from the brown syrup using ethanol to obtain 4 as white solid (0.6 g, 89.8%). The spectral data agrees with reported data.38 1H NMR (400 MHz, DMSO-d6): δ 5.82–5.83 (t, 1H, J = 2.28 Hz), 7.12–7.13 (t, 1H, J = 2.72 Hz, J = 2.96 Hz), 10.57 (s, 1H), 10.74 (s, 1H), 11.82 (s, 1H). 13C NMR (400 MHz, DMSO-d6): δ 96.5, 110.9, 127.4, 135.1, 152.0, 156.3.
5.1.1.2. 2,4-Dichloropyrrolo[3,2-d]pyrimidine (1)
To pyrrolo[3,2-d]pyrimidin-2,4-dione 5 (2.00 g, 13.2 mmol), 1 N NaOH (15 mL), and 0.60 g NaOH in 15 mL H2O was added and the mixture stirred at 40 °C until a clear solution was obtained. The solution was cooled to room temperature (21–25 °C) and then placed in an ice bath to obtain thick slurry. The slurry was then filtered to obtain a pale yellow solid. The solid was dissolved in 1 N NaOH (15 mL), and heated to 40 °C to obtain a clear solution that upon cooling provided white crystals. The crystals were washed with MeOH (20 mL) and acetone (20 mL), and then dried under vacuum. The dry solids were taken in phenylphosphonic dichloride (10 mL) and heated to 170–175 °C for 5 h during which the reaction mixture became a brown-black solution. After 5 h the hot reaction mixture was poured onto ice, extracted with EtOAc (200 mL) and the organic layer washed with satd NaHCO3 solution (3× 100 mL) till all effervescence subsided. The organic layer was then washed with brine and dried over MgSO4. The organic layer was concentrated in vacuo and loaded onto silica. The product was purified using column chromatography eluting with 9:1 then 3:1 hexanes/EtOAc to obtain 1 as an off-white solid (1.50 g, 7.9 mmol, 60%). Rf 0.5 in 3:1 hexanes/EtOAc. Mp 228.3–232.0 °C. 1H NMR (400 MHz, DMSO-d6): δ 6.71 (d, 1H, J = 3.2 Hz), 8.09 (d, 1H, J = 2.8 Hz), 12.75 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δ 103.2, 124.3, 138.0, 143.5, 149.6, 153.9. ESI-MS m/z for C6H3Cl2N3 calculated [M+H]+ 187.9776, found 187.9777.
5.1.1.3. 7-Iodo-2,4-dichloro pyrrolo[3,2-d]pyrimidine (2)
To a solution of 2,4-dichloro pyrrolo[3,2-d]pyrimidine 1 (100 mg, 0.53mmol) in anhydrous THF (5 mL), NIS (144 mg, 0.64mmol) was added under N2 atmosphere and stirred for 2 h after which TLC indicated consumption of 1. The solvent was removed in vacuo and the residue dissolved in EtOAc. The organic phase was washed with aq. solution of Na2S2O3 followed by water, brine and then dried over MgSO4. The organic layer was concentrated in vacuo and loaded on silica. The product was purified using column chromatography eluting with 9:1 hexanes/EtOAc to obtain product as off-white solid (90 mg, 54%). Rf 0.55 in 3:1 hexanes/EtOAc. Mp decomposed from 160 to 230 °C. Spectral data agrees reported data.41 1H NMR (400MHz, DMSO-d6): δ 8.29 (s, 1H), 13.19 (s, 1H, NH). 13C NMR (400MHz, DMSO-d6): δ 58.2, 124.4, 140.9, 143.5, 149.8, 153.5. ESI-MS m/z for calculated [M+H]+ 313.8743, found 313.8740.
5.1.1.4. N-tert-Butyloxycarbonyl-2,4-dichloro pyrrolo[3,2-d]pyrimidine (6)
To a mixture of 2,4-dichloro pyrrolo[3,2-d]pyrimidine 1 (50 mg, 0.26 mmol), di-tert-butyl carbonate (116 mg, 0.53 mmol) and DMAP (6.5 mg, 0.053 mmol), anhydrous THF (5 mL) was added under N2 atmosphere and stirred overnight upon which TLC indicated consumption of 1. The reaction mixture was concentrated and loaded on silica. The product was purified using column chromatography eluting with 49:1 hexanes/EtOAc to obtain product as white solid (50 mg, 65%). Rf 0.5 in 19:1 hexanes/EtOAc. Mp 101.0–103.2 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.66 (s, 9H), 6.71–6.72 (d, 1H, J = 3.68 Hz), 8.01–8.02 (d, 1H, J = 3.68 Hz). 13C NMR (400 MHz, DMSO-d6): δ 28.0, 87.2, 106.6, 123.4, 137.3, 146.5, 147.0, 153.1, 158.1. ESI-MS m/z for C11H11Cl2N3O2 calculated [M+H]+ 288.0301, found 288.0301 (2× 35Cl), 289.0335 (2× 35Cl, 13C), 290.0272 (35Cl37Cl).
5.1.1.5. 7-Iodo-pyrrolo[3,2-d]pyrimidine-2,4-dione (7)
Pyrrolo[3,2-d]pyrimidine-2,4-dione 5 (500 mg, 3.31 mmol) was suspended in anhydrous DMF (10 mL) under N2 atmosphere and cooled to −10 to −5 °C. To this slurry, N-iodosuccinimide (894 mg, 3.97 mmol) was added and the mixture stirred for 2 h at −10 to −5 °C, at which point the DMF was removed in vacuo to provide a brown sticky solid. The product was crystallized from the residue using 50% EtOH (6 mL) to give 7 (800 mg, 87.2%) as a yellow solid. Mp >300 °C. 1H NMR (400 MHz, DMSO-d6): 7.31 (s, 1H), 10.64 (br s, 1H), 10.76 (br s, 1H), 11.07 (br s, 1H). 13C NMR: 93.5, 111.8, 131.7, 136.9, 151.8, 155.7. FAB-MS for C6H4IN3O2 calculated M+ 276.9348, found 276.9345, calculated [M+H]+ 277.9421, found 277.9419.
5.1.1.6. 2,4-Bis-benzyloxy-5-nitro-6-dimethylaminovinyl pyrimidine (9)
To a solution of 2,4-bis-O-benzyl-6-methyl-5-nitro pyrimidine 937 (2.3 g, 6.5 mmol) in DMF (20 mL), DMF-dimethyl acetal (1.74 mL, 13 mmol) was added at room temperature under N2 atmosphere. The reaction was lowered in a preheated oil bath at 60–65 °C and stirred overnight upon which TLC indicated absence of starting material. The solvents were removed and the residue loaded on silica. The product was purified using column chromatography eluting with 9:1 hexanes/EtOAc to obtain product 10 as orange-yellow solid (2 g, 75%). Rf 0.5 in 3:1 hexanes/EtOAc. Spectral data agrees with reported data.37 1H NMR (400 MHz, CDCl3): δ 2.87–2.94 (br d, 6H), 5.33–5.36 (d, 1H, J = 12.36 Hz), 5.38 (s, 2H), 5.44 (s, 2H), 7.31–7.41 (m, 10H), 7.98–8.01 (d, 1H, J = 12.36 Hz). 13C NMR (400 MHz, CDCl3): δ 68.9, 69.5, 87.9, 127.5, 128.0, 128.1, 128.3 128.5, 135.8, 136.6, 151.8, 160.7, 161.6, 163.5. FAB-MS for C22H22N4O4 calculated [M+H]+ 407.1714, found 407.1717.
5.1.1.7. 2,4-Bis-benzyloxy-5H-pyrrolo[3,2-d]pyrimidine40 (3)
To a suspension of 2,4-bis-O-benzyl-5-nitro-6-β-dimethylaminovinyl pyrimidine 10 (2 g, 4.9 mmol) in AcOH (40 ml), Zn (4 g) was added in lot of 2 g with an interval of 4hrs. The reaction mixture was stirred overnight at room temperature during which a dark yellow suspension became pale yellow suspension. The reaction mixture was filtered and the filtrate concentrated in vacuo to obtain syrup which was dissolved in CH2Cl2 then washed with saturated aq. NaHCO3 followed by brine. The organic phase was dried over MgSO4 and loaded on silica. The product was purified using column chromatography eluting with 4:1 and 1:1 hexanes/EtOAc to obtain product as pale-yellow solid (1.45 g, 90%). Rf 0.3 in 1:3 hexanes/EtOAc. Spectral data agrees with reported data.37 1H NMR (400 MHz, CDCl3): δ 5.47 (s, 2H), 5.54 (s, 2H), 6.50–6.51 (dd, 1H, J = 1.84, 2.28), 7.29–7.38 (m, 7H), 7.43–7.45 (m, 2H), 7.52–7.53 (m, 2H), 8.41 (br s, 1H, NH). 13C NMR (400 MHz, CDCl3): δ 68.3, 69.0, 102.6, 111.9, 127.8, 128.3, 128.4, 128.5, 128.6, 128.7, 128.8, 136.1, 137.3, 151.8, 156.79, 159.7. FAB-MS for C20H17N3O2 calculated [M+H]+ 332.1394, found 332.1398.
5.1.1.8. 7-Iodo-2,4-bis-benzyloxy-5H-pyrrolo[3,2-d]pyrimidine40 (4)
To a stirred solution of 2,4-bis-O-benzyl-5H-pyrrolo[3,2-d]pyrimidine 3 (1.43 g, 4.3 mmol) in anhydrous CH2Cl2 (15 mL) under N2, NIS (1.069 g, 4.7 mmol) was added at which point the reaction mixture turned from pink to orange. The mixture was stirred overnight until the TLC indicated the absence of starting material. The reaction mixture was washed with aqueous Na2S2O3 (15 mL) followed by brine (15 mL). The organic layer was dried over MgSO4, loaded onto silica and purified using column chromatography eluting with 4:1 then 1:1 hexanes/EtOAc to obtain 4 as a pale-yellow solid (1.77 g, 3.88 mmol, 90%).40 Rf 0.4 in 1:3 hexanes/EtOAc. Mp 157.8–158.4 °C. 1H NMR (400 MHz, CDCl3): δ 5.53 (s, 4H), 7.32–7.41 (m, 9H), 7.55–7.57 (m, 2H), 8.71 (br s, 1H, NH). 13C NMR: 57.3, 68.7, 69.3, 111.9, 127.9, 128.3, 128.6, 128.66, 128.7, 128.9, 132.4, 135.8, 137.2, 152.0, 156.9, 160.1. FAB-MS m/z for C20H16IN3O2 calculated [M+H]+ 458.0360, found 458.0357.
5.1.1.9. 2,4-Bis-benzyloxy-5-N-Boc-7-iodopyrrolo[3,2-d]pyrimidine (10)
To a solution of 7-iodo-2,4-bis-benzyloxy-5H-pyrrolo[3,2-d]pyrimidine 4 (100 mg, 0.218mmol) in dry THF (5 mL), Boc2O (96 mg, 0.437 mmol) and DMAP (145.33mg, 0.0437mmol) were added and the mixture stirred for 1 h until TLC indicated the absence of starting material. The solvent was removed in vacuo and the residue purified by column chromatography eluting with 49:1 hexanes/EtOAc to obtain 11 as a white solid (65.1 mg, 116mmol, 53.4%).40 Rf 0.5 in 19:1 hexanes/EtOAc. Mp 118.5–122.5 °C. 1H NMR (400 MHz, CDCl3): δ 1.51 (d, 9H), 5.59 (s, 2H), 5.50 (s, 2H), 7.38–7.30 (m, 6H), 7.47 (d, 2H), 7.56 (d, 2H), 7.91 (s, 1H). 13C NMR: δ 27.8, 64.3, 68.9, 69.5, 85.3, 110.9, 127.9, 128.4, 128.5, 128.8, 136.2, 136.5, 136.9, 147.2, 156.6, 157.9, 161.1. FAB-MS m/z for C25H24IN3O4 calculated [M+H]+ 558.0884, found MH+ 558.0885.
5.1.1.10. 9-Deaza-2′-deoxyxanthosine (11)
White solid Mp 185.3–186.8 °C. 1H NMR (400 MHz, DMSO d6): δ 1.84–1.95 (m, 2H), 3.49–3.57 (m, 2H), 3.75 (br s, 1H), 4.19 (br s, 1H), 4.96 (d, 1H, J = 3.24 Hz), 5.05 (dd, 1H, J = 5.92 Hz, J = 10.08 Hz), 5.62 (br s, 1H), 7.11 (s, 1H) 10.56 (br s, 1H, NH), 10.58 (br s, 1H, NH), 11.67 (br s, 1H, NH). 13C NMR (400 MHz, DMSO d6): δ 43.0, 62.7, 73.6, 73.7, 87.7, 111.2, 111.5, 125.2, 132.7, 151.8, 156.3. FAB-MS for C11H13N3O5 calculated M+ 267.0855, found 267.0853; calculated [M+H+] 268.0928 found 268.0930.
5.1.1.11. 9-Deaza-2′-deoxyguanosine (12)
White solid Mp >300 °C. Rf 0.5 in 4:1 CH2Cl2: MeOH. 1H NMR (400 MHz, DMSO d6): δ 1.86 (dd, 1H, J = 5.26 Hz, J = 12.62 Hz), 2.13 (td, 1H, J = 5.04 Hz, J = 11.69 Hz), 3.37 (ddd, 2H, J = 4.12 Hz, J = 11.66 Hz, J = 11.44 Hz), 3.69 (t, 1H, J = 4.12 Hz), 4.15 (br s, 1H), 4.85 (d, 1H, J = 3.64 Hz), 5.05 (dd, 1H, J=5.28 Hz, J = 10.76 Hz), 5.66 (br s, 2H, NH2), 5.81 (br s, 1H), 7.09 (d, 1H, J = 3.2 Hz), 10.41 (br s, 1H, NH), 11.33 (br s, 1H, NH). 13C NMR (400 MHz, DMSO d6): δ 42.6, 63.8, 73.6, 73.9, 88.3, 113.8, 115.7, 126.1, 151.3, 155.4, 164.0. ESI-MS m/z for C11H14N4O4 calculated [M+H+] 267.1087, found 267.1089.
5.2. Cell proliferation assays
All assays were performed in 96-well microtiter plates. To each well were added (5–7.5) × 104 tumor cells and a given amount of the test compound. The cells were allowed to proliferate for 48 h (murine leukemia L1210 cells) or 72 h (human lymphocytic CEM and human cervix carcinoma HeLa cells) at 37 °C in a humidified CO2-controlled atmosphere. At the end of the incubation period, the cells were counted in a Coulter counter. The IC50 (50% inhibitory concentration) was defined as the concentration of the compound that inhibited cell proliferation by 50%.
5.3. MTT assays
MDA-MB-231 cells (ATCC) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) plus 10% fetal bovine serum (FBS). Cells were seeded in 96 well plates at 5 × 103 cells per well and treated with compounds 1 and 2 across a range of concentrations. After 48 h, relative numbers of viable cells were quantified using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Cell Proliferation Assay kit (ATCC) according to the manufacturer’s instructions. The fraction of viable cells relative to wells treated with vehicle alone was analyzed as a function of drug concentration using a sigmoidal dose response model with PRISM v3.03 software (GraphPad) to resolve IC50.
5.4. Cell cycle distribution and apoptosis assays
The cell cycle distributions of MDA-MB-231 cells treated with compounds 1 or 2 were analyzed using propidium iodide staining and flow cytometry. 2 × 106 cells were seeded in 100 mm dishes and treated with vehicle alone or compounds 1 or 2 at the IC80 values determined by cell viability assays. 48 h following treatment the cells were collected, fixed, and stained with propidium iodide (Sigma Aldrich) as described82 immediately before analysis by flow cytometry. To analyze apoptotic cell death, MDA-MB-231 cells were seeded as described above but then treated with vehicle alone or compounds 1 or 2 at their IC80 concentrations of 15 μM and 1.75 μM, respectively. After 48 h, the cells were then collected and the cellular fractions undergoing early apoptotic, late apoptotic/necrotic, and necrotic cell death were measured by flow cytometry following staining with annexin V and 7-amino-actinomycin D (7-AAD) using the BD Pharmingen PE Annexin V Apoptosis Detection Kit I (BD Pharmingen) as described.83
Supplementary Material
Acknowledgments
We are grateful to Lizette van Berckelaer for technical assistance with the cytostatic activity evaluations. The research of J.B. was supported by the GOA 15/19 TMB. The authors would like to acknowledge the National Institutes of Health for funding (NIH T32GM066706-12, K.S.R. and C.R.R., and NIH R01CA102428, G.M.W.). The Flow Cytometry Shared Service facility of the University of Maryland Greenebaum Cancer Center is supported in part by P30 CA134274.
Footnotes
Supplementary data (the 1H, 13C NMR spectra, High Resolution Mass Spectrometry (HRMS) and single dose response date from NCI-60 DTP Human Tumor Cell Line Screen) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2015.06.025.
References and notes
- 1.Lim MI, Klein RS, Fox JJ. J Org Chem. 1979;44:3826. [Google Scholar]
- 2.Lim MI, Klein RS. Tetrahedron Lett. 1981;22:25. [Google Scholar]
- 3.Lim MI, Ren WY, Otter BA, Klein RS. J Org Chem. 1983;48:780. [Google Scholar]
- 4.Zimmerman TP, Deeprose RD, Wolberg G, Stopford CR, Duncan GS, Miller WH, Miller RL, Lim MI, Ren WY, Klein RS. Biochem Pharmacol. 1983;32:1211. doi: 10.1016/0006-2952(83)90274-5. [DOI] [PubMed] [Google Scholar]
- 5.Chu MY, Zuckerman LB, Sato S, Crabtree GW, Bogden AE, Lim MI, Klein RS. Biochem Pharmacol. 1984;33:1229. doi: 10.1016/0006-2952(84)90174-6. [DOI] [PubMed] [Google Scholar]
- 6.Rao KVB, Ren WY, Burchenal JH, Klein RS. Nucleosides Nucleotides. 1986;5:539. [Google Scholar]
- 7.Stoeckler JD, Ryden JB, Parks RE, Jr, Chu MY, Lim MI, Ren WY, Klein RS. Cancer Res. 1986;46:1774. [PubMed] [Google Scholar]
- 8.Seley KL, O’Daniel PI, Salim S. Nucleosides, Nucleotides Nucleic Acids. 2003;22:2133. doi: 10.1081/ncn-120026635. [DOI] [PubMed] [Google Scholar]
- 9.McCarty RM, Bandarian V. Chem Biol (Cambridge, MA, US) 2008;15:790. doi: 10.1016/j.chembiol.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naus P, Pohl R, Votruba I, Dzubak P, Hajduch M, Ameral R, Birkus G, Wang T, Ray AS, Mackman R, Cihlar T, Hocek M. J Med Chem. 2010;53:460. doi: 10.1021/jm901428k. [DOI] [PubMed] [Google Scholar]
- 11.Wu R, Smidansky ED, Oh HS, Takhampunya R, Padmanabhan R, Cameron CE, Peterson BR. J Med Chem. 2010;53:7958. doi: 10.1021/jm100593s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourderioux A, Naus P, Perlikova P, Pohl R, Pichova I, Votruba I, Dzubak P, Konecny P, Hajduch M, Stray KM, Wang T, Ray AS, Feng JY, Birkus G, Cihlar T, Hocek M. J Med Chem. 2011;54:5498. doi: 10.1021/jm2005173. [DOI] [PubMed] [Google Scholar]
- 13.Sircar JC, Kostlan CR, Gilbertsen RB, Bennett MK, Dong MK, Cetenko WJ. J Med Chem. 1992;35:1605. doi: 10.1021/jm00087a015. [DOI] [PubMed] [Google Scholar]
- 14.Erion MD, Niwas S, Rose JD, Ananthan S, Allen M, Secrist JA, III, Babu YS, Bugg CE, Guida WC, et al. J Med Chem. 1993;36:3771. doi: 10.1021/jm00076a004. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery JA, Niwas S, Rose JD, Secrist JA, III, Babu YS, Bugg CE, Erion MD, Guida WC, Ealick SE. J Med Chem. 1993;36:55. doi: 10.1021/jm00053a008. [DOI] [PubMed] [Google Scholar]
- 16.Secrist JA, III, Niwas S, Rose JD, Babu YS, Bugg CE, Erion MD, Guida WC, Ealick SE, Montgomery JA. J Med Chem. 1993;36:1847. doi: 10.1021/jm00065a007. [DOI] [PubMed] [Google Scholar]
- 17.Guida WC, Elliott RD, Thomas HJ, Secrist JA, III, Babu YS, Bugg CE, Erion MD, Ealick SE, Montgomery JA. J Med Chem. 1994;37:1109. doi: 10.1021/jm00034a008. [DOI] [PubMed] [Google Scholar]
- 18.Niwas S, Chand P, Pathak VP, Montgomery JA. J Med Chem. 1994;37:2477. doi: 10.1021/jm00041a027. [DOI] [PubMed] [Google Scholar]
- 19.Farutin V, Masterson L, Andricopulo AD, Cheng J, Riley B, Hakimi R, Frazer JW, Cordes EH. J Med Chem. 1999;42:2422. doi: 10.1021/jm990037y. [DOI] [PubMed] [Google Scholar]
- 20.Shi W, Li CM, Tyler PC, Furneaux RH, Grubmeyer C, Schramm VL, Almo SC. Nat Struct Biol. 1999;6:588. doi: 10.1038/9376. [DOI] [PubMed] [Google Scholar]
- 21.Clinch K, Evans GB, Frohlich RFG, Furneaux RH, Kelly PM, Legentil L, Murkin AS, Li L, Schramm VL, Tyler PC, Woolhouse AD. J Med Chem. 2009;52:1126. doi: 10.1021/jm801421q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norman MH, Chen N, Chen Z, Fotsch C, Hale C, Han N, Hurt R, Jenkins T, Kincaid J, Liu L, Lu Y, Moreno O, Santora VJ, Sonnenberg JD, Karbon W. J Med Chem. 2000;43:4288. doi: 10.1021/jm000269t. [DOI] [PubMed] [Google Scholar]
- 23.Taylor EC, Young WB, Ward CC. Tetrahedron Lett. 1993;34:4595. [Google Scholar]
- 24.Gangjee A, Li W, Yang J, Kisliuk RL. J Med Chem. 2008;51:68. doi: 10.1021/jm701052u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Venkatesan AM, Dehnhardt CM, Ayral-Kaloustian S, Brooijmans N, Mallon R, Feldberg L, Hollander I, Lucas J, Yu K, Kong F, Mansour TS. J Med Chem. 2010;53:3169. doi: 10.1021/jm901783v. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa T, Seto M, Banno H, Kawakita Y, Oorui M, Taniguchi T, Ohta Y, Tamura T, Nakayama A, Miki H, Kamiguchi H, Tanaka T, Habuka N, Sogabe S, Yano J, Aertgeerts K, Kamiyama K. J Med Chem. 2011;54:8030. doi: 10.1021/jm2008634. [DOI] [PubMed] [Google Scholar]
- 27.Kawakita Y, Banno H, Ohashi T, Tamura T, Yusa T, Nakayama A, Miki H, Iwata H, Kamiguchi H, Tanaka T, Habuka N, Sogabe S, Ohta Y, Ishikawa T. J Med Chem. 2012;55:3975. doi: 10.1021/jm300185p. [DOI] [PubMed] [Google Scholar]
- 28.Temburnikar KW, Zimmermann SC, Kim NT, Ross CR, Gelbmann C, Salomon CE, Wilson GM, Balzarini J, Seley-Radtke KL. Bioorg Med Chem. 2014;22:2113. doi: 10.1016/j.bmc.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross CR, Temburnikar KW, Wilson GM, Seley-Radtke KL. Bioorg Med Chem Lett. 2015;25:1715. doi: 10.1016/j.bmcl.2015.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seley KL, Januszczyk P, Hagos A, Zhang L, Dransfield DT. J Med Chem. 2000;43:4877. doi: 10.1021/jm000326i. [DOI] [PubMed] [Google Scholar]
- 31.Seley-Radtke KL, Zhang Z, Wauchope OR, Zimmermann SC, Ivanov A, Korba B. Nucleic Acids Symp Ser. 2008;52:635. doi: 10.1093/nass/nrn321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Wauchope OR, Seley-Radtke KL. Tetrahedron. 2008;64:10791. doi: 10.1016/j.tet.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wauchope OR, Tomney MJ, Pepper JL, Korba BE, Seley-Radtke KL. Org Lett. 2010;12:4466. doi: 10.1021/ol101482h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace LJM, Candlish D, Hagos A, Seley KL, De Koning HP. Nucleosides, Nucleotides Nucleic Acids. 2004;23:1441. doi: 10.1081/NCN-200027660. [DOI] [PubMed] [Google Scholar]
- 35.Bourke DG, Burns CJ, Cuzzupe AN, Feutrill JT, Kling MR, Nero TL. 2009062258 WO.
- 36.Cupps TL, Wise DS, Townsend LB. J Org Chem. 1983;48:1060. [Google Scholar]
- 37.Evans GB, Furneaux RH, Hutchison TL, Kezar HS, Morris PE, Jr, Schramm VL, Tyler PC. J Org Chem. 2001;66:5723. doi: 10.1021/jo0155613. [DOI] [PubMed] [Google Scholar]
- 38.Guimaraes CRW, Kopecky DJ, Mihalic J, Shen S, Jeffries S, Thibault ST, Chen X, Walker N, Cardozo M. J Am Chem Soc. 2009;131:18139. doi: 10.1021/ja9064359. [DOI] [PubMed] [Google Scholar]
- 39.Girgis NS, Cottam HB, Larson SB, Robins RK. J Heterocycl Chem. 1987;24:821. [Google Scholar]
- 40.Temburnikar K, Brace K, Seley-Radtke KL. J Org Chem. 2013;78:7305. doi: 10.1021/jo400913k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bambuch V, Otmar M, Pohl R, Masojidkova M, Holy A. Tetrahedron. 2007;63:1589. [Google Scholar]
- 42.Dey S, Garner P. J Org Chem. 2000;65:7697. doi: 10.1021/jo000983i. [DOI] [PubMed] [Google Scholar]
- 43.Cassidy F, Olsen RK, Robins RK. J Heterocycl Chem. 1968;5:461. [Google Scholar]
- 44.Furneaux RH, Tyler PC. J Org Chem. 1999;64:8411. doi: 10.1021/jo990903e. [DOI] [PubMed] [Google Scholar]
- 45.Li JJ, Corey EJ. Name Reactions in Heterocyclic Chemistry. Wiley-Interscience; 2011. [Google Scholar]
- 46.Batcho AD, Leimgruber W. 3976639 US.
- 47.Bhattacharya BK, Lim MI, Otter BA, Klein RS. Tetrahedron Lett. 1986;27:815. [Google Scholar]
- 48.Fox JJ, Watanabe KA, Klein RS, Chu CK, Tam SYK, Reichman U, Hirota K, Hwang JS, De las Heras FG, et al. Chem Biol Nucleosides Nucleotides [Pap Symp] 1978:415. [Google Scholar]
- 49.Fox JJ, Watanabe KA, Klein RS, Chu CK, Tam SYK, Reichman U, Hirota K, Wempen I, Lopez C, Burchenal JH. Colloq INSERM. 1979;81:241. [Google Scholar]
- 50.Klein RS, Lim MI, Ren W, Burchenal JH. 71227 EP.
- 51.Lim MI, Klein RS, Fox JJ. Tetrahedron Lett. 1980;21:1013. [Google Scholar]
- 52.Marr JJ, Berens RL, Cohn NK, Nelson DJ, Klein RS. Antimicrob Agents Chemother. 1984;25:292. doi: 10.1128/aac.25.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren WY, Lim MI, Otter BA, Klein RS. J Org Chem. 1982;47:4633. [Google Scholar]
- 54.Ren WY, Rao KVB, Klein RS. J Heterocycl Chem. 1986;23:1757. [Google Scholar]
- 55.Tam SYK, Hwang JS, De las Heras FG, Klein RS, Fox JJ. J Heterocycl Chem. 1976;13:1305. [Google Scholar]
- 56.Tam SYK, Klein RS, De las Heras FG, Fox JJ. J Org Chem. 1979;44:4854. [Google Scholar]
- 57.Tam SYK, Klein RS, Wempen I, Fox JJ. J Org Chem. 1979;44:4547. [Google Scholar]
- 58.Metobo SE, Xu J, Saunders OL, Butler T, Aktoudianakis E, Cho A, Kim CU. Tetrahedron Lett. 2012;53:484. [Google Scholar]
- 59.Temburnikar K, Zhang Z, Seley-Radtke K. Nucleosides, Nucleotides Nucleic Acids. 2012;31:319. doi: 10.1080/15257770.2012.656212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Law LW, Dunn TB, et al. J Natl Cancer Inst. 1949;10:179. [PubMed] [Google Scholar]
- 61.Foley GE, Lazarus H, Farber S, Uzman BG, Boone BA, McCarthy RE. Cancer. 1965;18:522. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 62.Scherer WF, Syverton JT, Gey GO. J Exp Med. 1953;97:695. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahbari R, Sheahan T, Modes V, Collier P, Macfarlane C, Badge RM. Biotechniques. 2009;46:277. doi: 10.2144/000113089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Cancer Res. 1988;48:589. [PubMed] [Google Scholar]
- 65.Boyd MR, Paull KD. Drug Dev Res. 1995;34:91. [Google Scholar]
- 66.Shoemaker RH. Nat Rev Cancer. 2006;6:813. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 67.Chavez KJ, Garimella SV, Lipkowitz S. Breast Dis. 2010;32:35. doi: 10.3233/BD-2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nieves-Neira W, Pommier Y. Int J Cancer. 1999;82:396. doi: 10.1002/(sici)1097-0215(19990730)82:3<396::aid-ijc13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 69.Gewirtz DA. Breast Cancer Res Treat. 2000;62:223. doi: 10.1023/a:1006414422919. [DOI] [PubMed] [Google Scholar]
- 70.Fan Y, Borowsky Alexander D, WeissRobert H. Mol Cancer Ther. 2003;2:773. [PubMed] [Google Scholar]
- 71.Singh J, Petter RC, Baillie TA, Whitty A. Nat Rev Drug Disc. 2011;10:307. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- 72.Singh J, Dobrusin EM, Fry DW, Haske T, Whitty A, McNamara DJ. J Med Chem. 1997;40:1130. doi: 10.1021/jm960380s. [DOI] [PubMed] [Google Scholar]
- 73.Barf T, Kaptein A. J Med Chem. 2012;55:6243. doi: 10.1021/jm3003203. [DOI] [PubMed] [Google Scholar]
- 74.Fry DW, Bridges AJ, Denny WA, Doherty A, Greis KD, Hicks JL, Hook KE, Keller PR, Leopold WR, Loo JA, McNamara DJ, Nelson JM, Sherwood V, Smaill JB, Trumpp-Kallmeyer S, Dobrusin EM. Proc Natl Acad Sci USA. 1998;95:12022. doi: 10.1073/pnas.95.20.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fry DW. Pharmacol Ther. 1999;82:207. doi: 10.1016/s0163-7258(98)00050-3. [DOI] [PubMed] [Google Scholar]
- 76.Johnson CM, Linsky TW, Yoon DW, Person MD, Fast W. J Am Chem Soc. 2011;133:1553. doi: 10.1021/ja109207m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson CM, Monzingo AF, Ke Z, Yoon DW, Linsky TW, Guo H, Robertus JD, Fast W. J Am Chem Soc. 2011;133:10951. doi: 10.1021/ja2033684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gersch M, Kreuzer J, Sieber SA. Nat Prod Rep. 2012;29:659. doi: 10.1039/c2np20012k. [DOI] [PubMed] [Google Scholar]
- 79.Silverman RB. The Organic Chemistry of Drug Design and Drug Action. 2. Elsevier Academic Press; USA: 2004. [Google Scholar]
- 80.Cravatt BF, Wright AT, Kozarich JW. Annu Rev Biochem. 2008;77:383. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 81.Heal WP, Dang THT, Tate EW. Chem Soc Rev. 2011;40:246. doi: 10.1039/c0cs00004c. [DOI] [PubMed] [Google Scholar]
- 82.Riccardi C, Nicoletti I. Nat Protoc. 2006;1:1458. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 83.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. J Immunol Methods. 1995;184:39. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.