Key Points
Question
Are orthostatic hypotension assessments performed within 1 minute of standing as informative for dizziness and long-term outcomes as assessments performed after 1 minute?
Findings
In this cohort study of 11 429 adults with 4 orthostatic hypotension assessments performed 1 to 2 minutes after standing, orthostatic hypotension assessed within 1 minute of standing was associated with higher odds of dizziness and greater risk of falls, fracture, syncope, motor vehicle crash, and mortality than orthostatic hypotension assessed after 1 minute.
Meaning
Contrary to prevailing recommendations to delay orthostatic hypotension assessments by 3 minutes, these findings suggest that orthostatic hypotension should be assessed within 1 minute of standing.
Abstract
Importance
Guidelines recommend assessing orthostatic hypotension (OH) 3 minutes after rising from supine to standing positions. It is not known whether measurements performed immediately after standing predict adverse events as strongly as measurements performed closer to 3 minutes.
Objective
To compare early vs later OH measurements and their association with history of dizziness and longitudinal adverse outcomes.
Design, Setting, and Participants
This was a prospective cohort study of middle-aged (range, 44-66 years) participants in the Atherosclerosis Risk in Communities Study (1987-1989).
Exposures
Orthostatic hypotension, defined as a drop in blood pressure (BP) (systolic BP ≥20 mm Hg or diastolic BP ≥10 mm Hg) from the supine to standing position, was measured up to 5 times at 25-second intervals.
Main Outcomes and Measures
We determined the association of each of the 5 OH measurements with history of dizziness on standing (logistic regression) and risk of fall, fracture, syncope, motor vehicle crashes, and all-cause mortality (Cox regression) over a median of 23 years of follow-up (through December 31, 2013).
Results
In 11 429 participants (mean age, 54 years; 6220 [54%] were women; 2934 [26%] were black) with at least 4 OH measurements after standing, after adjustment OH assessed at measurement 1 (mean [SD], 28 [5.4] seconds; range, 21-62 seconds) was the only measurement associated with higher odds of dizziness (odds ratio [OR], 1.49; 95% CI, 1.18-1.89). Measurement 1 was associated with the highest rates of fracture, syncope, and death at 18.9, 17.0, and 31.4 per 1000 person-years. Measurement 2 was associated with the highest rate of falls and motor vehicle crashes at 13.2 and 2.5 per 1000 person-years. Furthermore, after adjustment measurement 1 was significantly associated with risk of fall (hazard ratio [HR], 1.22; 95% CI, 1.03-1.44), fracture (HR, 1.16; 95% CI, 1.01-1.34), syncope (HR, 1.40; 95% CI, 1.20-1.63), and mortality (HR, 1.36; 95% CI, 1.23-1.51). Measurement 2 (mean [SD], 53 [7.5] seconds; range, 43-83 seconds) was associated with all long-term outcomes, including motor vehicle crashes (HR, 1.43; 95% CI, 1.04-1.96). Measurements obtained after 1 minute were not associated with dizziness and were inconsistently associated with individual long-term outcomes.
Conclusions and Relevance
In contrast with prevailing recommendations, OH measurements performed within 1 minute of standing were the most strongly related to dizziness and individual adverse outcomes, suggesting that OH be assessed within 1 minute of standing.
This cohort study compares early vs later orthostatic hypotension measurements and their association with history of dizziness and long-term adverse outcomes.
Introduction
Orthostatic hypotension (OH) is a common medical condition in older adults that is associated with higher risk of falls, coronary heart disease, stroke, and death. The determination of OH is based on a consensus statement from the American Academy of Neurology, which has since been incorporated into international guidelines. This statement defines OH by a postural reduction in systolic blood pressure (SBP) of 20 mm Hg or greater or a diastolic blood pressure (DBP) of 10 mm Hg or greater, measured 3 minutes after rising from a supine to standing position. However, the 3-minute time delay is often less practical in clinical settings owing to time constraints. It is unclear whether early measurements are as informative as later measurements. Indeed, a number of reports advocate discarding the first blood pressure (BP) measurement owing to presumed changes in physiology, that is, BP “restabilization.” In the recent SPRINT study, investigators measured BP at 1 minute after standing with seemingly paradoxical results, that is, more aggressive antihypertensive treatment was associated with a lower prevalence of OH but higher risk of its sequelae (emergency department visits for OH and syncope) with no increased risk of fall. Whether these counterintuitive findings stem from the shortened time interval is unknown.
To provide evidence for the optimal timing of OH assessment, we compared repeated BP measurement obtained at different times in a large, community-based population of middle-aged adults. Using repeated BP measurements, our objectives were to (1) compare early and later OH measurements with self-reported history of dizziness on standing; and (2) compare the associations between early and later OH measurements relative to long-term, incident clinical outcomes (eg, falls, fractures, syncope, motor vehicle crashes, and mortality). We hypothesized a priori that measurements obtained less than 1 minute after standing would be less informative than measurements obtained 1 to 2 minutes after standing.
Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort of 15 792 adults. Participants, ages 45 to 64 years, were originally enrolled between 1987 and 1989 from 4 US communities and followed for over 2 decades. Physical examinations, medical interviews, and laboratory tests were conducted as part of the original ARIC protocol. We excluded participants who did not have an OH assessment at baseline (n = 2555), who were missing any of the first 4 orthostatic measurements (n = 1210), who were missing dizzy status (n = 15), and who were missing relevant covariate data at baseline (n = 583). This resulted in a study population of 11 429 participants. A comparison of baseline characteristics of participants included and excluded from this study may be found in eTable 1 in the Supplement. Participant health status relative to clinically reported OH symptoms or outcomes was not known at ARIC study entry.
Written informed consent was obtained from all participants, and the study protocol was approved by institutional review boards at all study sites. Participants received compensation.
Orthostatic Hypotension and History of Dizziness
During the baseline visit, supine SBP and DBP were measured with a Dinamap 1846 SX oscillometric device (automatic cuff) after participants had been lying for 20 minutes. An oscillometric device rather than a manual device was used to standardize the timing of repeated measurements between participants. The device was programmed to record up to 5 BP measurements while lying in the 2 minutes preceding the standing phase of the protocol (range, 2-5 supine measurements with at ≥4 measurements obtained for 90% of participants). Prior to standing, each participant was asked if he or she “usually gets dizzy on standing up.” A yes or no response was recorded, and participants responding “yes” were permitted to pause in the seated position at the edge of the examination table until they felt safe to stand. Otherwise, all participants were instructed to stand up quickly, safely, and in 1 smooth motion. If a participant felt dizzy immediately after standing, they were permitted to lean back against the examination table; otherwise, they were instructed to stand away from the table.
After giving the command to stand, the time that both of a participant’s feet were planted on the ground was recorded by ARIC staff, who then proceeded to initiate the automatic cuff, which was programmed to measure BP up to 5 times (range, 2-5 standing measurements with at least 4 measurements recorded for 91% of participants). The end and initiation of each BP measurement was separated by 2 or 3 seconds. The time of each measurement result was recorded and varied based on the time required by the automatic cuff to measure a BP. The OH for each measurement was defined using thresholds similar to the consensus definition, that is, a decrease in either SBP or DBP of at least 20 or 10 mm Hg, respectively.
Long-term Outcomes: Fall, Fracture, Syncope, Motor Vehicle Crash, and Death
Fall, fracture, syncope, and motor vehicle crashes were defined at the first occurrence of any related hospitalization or claim for inpatient or outpatient services after the baseline visit. These outcomes were identified via 2 sources: (1) active surveillance of all hospitalizations for all ARIC participants; and (2) linkage to Centers for Medicare and Medicaid Services (CMS) claims data from 1991 to 2013 (see eMethods 1 and the eReferences in the Supplement).
The annual follow-up rate for the ARIC study is greater than 90%. Participants who were lost to follow-up were administratively censored.
Baseline Covariates of Interest
Covariates of interest were age, sex, race-research center (white, Washington County, Maryland; black, Jackson, Mississippi; white, Minneapolis, Minnesota; black, Forsyth, North Carolina; white, Forsyth, North Carolina) heart rate, body mass index, estimated glomerular filtration rate, diabetes, hypertension, alcohol use, education level, smoking status, physical activity, coronary heart disease, history of stroke, heart failure, hypertension medication use in past 2 weeks, diuretic use, antidepressant use, sedative use, hypnotic use, antipsychotic medication use, anticholinergic medication use, resting SBP, resting DBP, and pulse pressure. Details related to the definitions of these covariates are located in eMethods 2 in the Supplement.
Statistical Analysis
Baseline characteristics were compared using means and proportions. We determined the mean time as well as the mean difference and percentage change in SBP or DBP at each of the 5 measurements.
Using linear splines, we modeled the continuous association between postural change in SBP or DBP and baseline history of dizziness on standing using logistic regression with adjustment for age, sex, and race-research center. Knots were placed at −30, −20, −10, 0, 10, 20, and 30 mm Hg for SBP and −15, −10, −5, 0, 5, 10, and 15 mm Hg for DBP. We examined dizziness given its hypothesized role in causing falls and its relationship to syncope. We also used logistic regression to examine the association of OH, postural change in SBP (per 5–mm Hg increment), or postural change in DBP (per 5–mm Hg increment) with baseline history of dizziness on standing. A 5–mm Hg increment was used for both SBP and DBP to facilitate interpretation and comparison. These models were adjusted for age, sex, race-research center, heart rate, body mass index, estimated glomerular filtration rate, diabetes, hypertension, alcohol use, education level, smoking status, physical activity, coronary heart disease, history of stroke, heart failure, hypertension medication use in past 2 weeks, diuretic use, antidepressant use, sedative use, hypnotic use, antipsychotic medication use, anticholinergic medication use, resting SBP, and resting DBP. To directly compare measurements, we further included measurements 1 to 4 simultaneously in the model described herein, and in a sensitivity analysis included measurements 1 to 5 in the model.
We calculated the incidence rates of fall, fracture, syncope, and motor vehicle crash as well as mortality rates (per 1000 person-years) for participants with and without OH, using Poisson regression with a robust variance estimator, adjusted for age, sex, and race-research center. In addition, we fitted distinct Cox proportional hazard models with each of the 5 measurements separately to examine the independent association of OH, postural change in SBP, and postural change in DBP with risk of falls, fracture, syncope, motor vehicle crash, or death. Schoenfeld residuals were plotted over time to test proportionality assumptions. These were adjusted for all the covariates listed herein.
Sensitivity analyses were performed using multiple imputation chained equations to impute values of missing standing SBP and DBP measurements as well as missing self-reported dizziness. In addition, we performed sensitivity analyses in strata of baseline hypertension, given its association with OH. We also repeated the analyses with adjustment for pulse pressure as well as proportion with baseline DBP less than 60 mm Hg. All analyses were conducted using Stata 14.0 (StataCorp LP).
Results
Population Characteristics
The study population (n = 11 429) included 6211 women (54%) and 2934 black participants (26%), with a mean (SD) age of 54 (5.7) years at baseline (Table 1). Ten percent of the study population (n = 1138) reported a history of dizziness on standing prior to initiating the OH protocol. The mean (SD) times in seconds of measurements 1 to 5 after standing were 28.0 (5.4), 52.6 (7.5), 76.4 (9.1), 100 (10.4), and 116.0 (4.6), respectively (eTable 2 in the Supplement). The measurement with the largest reduction in SBP from supine to standing positions was measurement 2 at −1.2 (12.4) mm Hg. Similarly, measurement 2 was associated with the smallest increase in DBP at 2.3 (6.3) mm Hg.
Table 1. Baseline Population Characteristics of 11 429 Participants.
| Characteristic | No. (%) |
|---|---|
| Age, mean (SD), y | 54.0 (5.7) |
| Female | 6211 (54) |
| Race-study center | |
| Washington County, Maryland (white) | 2577 (22.5) |
| Jackson, Mississippi (black) | 2582 (22.6) |
| Minneapolis, Minnesota (white) | 3149 (27.5) |
| Forsyth, North Carolina (black) | 352 (3.1) |
| Forsyth, North Carolina (white) | 2789 (24.4) |
| Blood pressure, mean (SD), mm Hg | |
| Systolic | 120.3 (18.4) |
| Diastolic | 73.3 (11.0) |
| Resting heart rate, mean (SD), beats per min | 66.9 (10.1) |
| EGFR, mean (SD), mL/min/1.73 m2 | 102.4 (15.5) |
| BMI, mean (SD) | 27.1 (4.7) |
| Leisure index, mean (SD), U | 2.4 (0.6) |
| Diabetes | 1246 (10.9) |
| Hypertension | 3716 (32.5) |
| Hypertensive medication use in past 2 weeks | 3234 (28.3) |
| History of coronary heart disease | 517 (4.5) |
| History of stroke | 186 (1.6) |
| History of heart failure | 447 (3.9) |
| Dizzy on standing | 1138 (10.0) |
| Diuretic use | 1843 (16.1) |
| Antidepressant use | 320 (2.8) |
| Sedative use | 172 (1.5) |
| Hypnotic medication use | 226 (2.0) |
| Antipsychotic medication use | 78 (0.7) |
| Anticholinergic use | 222 (1.9) |
| Alcohol use | |
| Never | 2743 (24.0) |
| Former | 2062 (18.0) |
| Current | 6624 (58.0) |
| Education attainment | |
| Less than high school | 2440 (21.3) |
| High school or vocational school | 4742 (41.5) |
| At least some college or professional school | 4247 (37.2) |
| Smoking status | |
| Never | 4713 (41.2) |
| Former | 3748 (32.8) |
| Current | 2968 (26.0) |
Abbreviations: BMI (calculated as weight in kilograms divided by height in meters squared), body mass index; EGFR, estimated glomerular filtration rate.
History of Dizziness
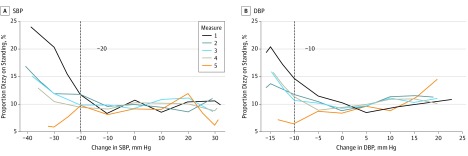
The proportion of participants with a history of dizziness was higher with greater postural drops in SBP or DBP, particularly for measurements 1 to 4 at −10 to −20 mm Hg for SBP and at 0 to −5 mm Hg for DBP (Figure 1). Furthermore, measurement 1 was associated with the highest proportion with dizziness at 13.5% (95% CI, 11.0-16.1) (eTable 3 in the Supplement). Systolic blood pressure or DBP measured during measurement 1 was associated with the greatest proportion of participants who expressed a history of dizziness on standing compared with the other measurements. Moreover, OH identified at measurement 1 was strongly associated with history of dizziness (OR, 1.49; 95% CI, 1.18-1.89) (Table 2). In contrast, OH assessed at measurements 2 to 5 was not associated with history of dizziness. Similarly, there there was not an association between change in SBP and DBP measured at measurement 1 with dizziness alone (not measurements 2-4). Postural change in DBP at measurement 5 was inversely associated with dizziness (OR, 0.92; 95% CI, 0.86-0.99).
Figure 1. Participants Reporting Symptoms of a History of Dizziness on Standing Prior to Initiating the Orthostatic Hypotension Protocol .
Proportions according to a drop in blood pressure or during the orthostatic hypotension protocol adjusted for age, sex, and race-research center. Each line represents 1 of 5 measurements. Changes in systolic (SBP) and diastolic blood pressure (DBP) were modeled as continuous variables. For SBP, knots are places at −30, −20, −10, 0, 10, 20, and 30 mm Hg. For DBP, knots are places at −15, −10, −5, 0, 5, 10, and 15. Note that measurement 5 has fewer participants. Note that there were 11 429 participants for measurements 1 to 4, but only 7385 for measurement 5. Dashed gray vertical lines represent the consensus definition for orthostatic hypotension: a SBP change of −20 mm Hg or a DBP change of −10 mm Hg.
Table 2. Association Between Orthostatic Hypotension and Self-reported Dizziness on Standing According to Measurementa.
| BP Assessment | Measure | Dizziness, OR (95% CI) [n = 1138] |
P Value |
|---|---|---|---|
| Orthostatic hypotension | 1 | 1.49 (1.18-1.89) | .001 |
| 2 | 1.04 (0.83-1.30) | .72 | |
| 3 | 1.11 (0.87-1.42) | .38 | |
| 4 | 1.11 (0.85-1.44) | .45 | |
| 5 | 0.77 (0.49-1.22) | .27 | |
| Postural change in SBP, per −5 mm Hg | 1 | 1.03 (1.00-1.05) | .06 |
| 2 | 1.02 (0.99-1.04) | .21 | |
| 3 | 0.99 (0.97-1.02) | .70 | |
| 4 | 0.99 (0.96-1.02) | .52 | |
| 5 | 0.97 (0.93-1.00) | .07 | |
| Postural change in DBP, per −5 mm Hg | 1 | 1.05 (1.00-1.10) | .05 |
| 2 | 0.96 (0.91-1.01) | .13 | |
| 3 | 0.98 (0.93-1.04) | .52 | |
| 4 | 0.98 (0.93-1.03) | .41 | |
| 5 | 0.92 (0.86-0.99) | .02 |
Abbreviations: BP, blood pressure; DPB, diastolic blood pressure; OR, odds ratio; SBP, systolic blood pressure.
All models adjusted for age, sex, race-study center, heart rate, body mass index, estimated glomerular filtration rate, diabetes, hypertension, alcohol use, education level, smoking status, leisure index, prior coronary heart disease, prior stroke, prior congestive heart failure, hypertension medication use in past 2 weeks, diuretic use, antidepressant use, sedative use, hypnotic use, antipsychotic medication use, anticholinergic medication use, resting SBP, and resting DBP. Unlike eTable 5 in the Supplement, each model includes only 1 measurement of orthostatic hypotension or postural change in SBP or DBP at a time. Measurement 5 has a much smaller sample size. There were 11 429 participants with 1138 reporting dizziness for measurements 1 to 4. There were 7385 participants with 670 reporting dizziness for measurement 5.
Long-term Outcomes
Longitudinal incident clinical outcomes were recorded over a median follow-up of 23 years. These events included 2089 falls (18.3% of events), 3104 fractures (27.2%), 2326 syncopal episodes (20.4%), 426 motor vehicle crashes (3.7%), and 4119 deaths (36.0%). Orthostatic hypotension identified at measurement 1 was associated with the highest incidence and/or mortality rates of fracture, syncope, and death at 18.9, 17.0, and 31.4 per 1000 person-years, respectively (Figure 2; eTable 3 in the Supplement). Orthostatic hypotension identified at measurement 2 was associated with the highest incidence rate of falls and motor vehicle crash at 13.2 and 2.5 per 1000 person-years.
Figure 2. Age-, Sex-, and Race-Research Center–Adjusted Incidence Rates.
Rates were determined by orthostatic hypotension (OH) status using each of measurements 1 through 5. Mean (SD) measurement time in seconds is reported in the key. Error bars indicate 95% CIs. Note that there were 11 429 participants for measurements 1 to 4, but only 7385 for measurement 5.
After adjustment for covariates, OH determined by measurements 1 to 4 were all significantly associated with falls, with measurement 2 demonstrating the strongest association (HR, 1.29; P < .001) (Table 3). Similarly, OH identified in measurements 1 to 2 showed the strongest associations with fracture at HRs of 1.16 (P = .04) and 1.14 (P = .04). Orthostatic hypotension determined at each of measurements 1 to 5 was significantly associated with syncope, while only measurement 2 was significantly associated with motor vehicle crash (HR, 1.43; 95% CI, 1.04-1.96). Finally, regardless of measurement, the presence of OH was associated with mortality.
Table 3. The Association Between Orthostatic Hypotension and Falls, Syncope, Fracture, or Mortality According to Measurement in 11 429 Participantsa.
| BP Assessment | Measure | Falls (n = 2089) |
Fracture (n = 3104) |
Syncope (n = 2326) |
Motor Vehicle Crash (n = 426) |
Mortality (n = 4119) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Orthostatic hypotension | 1 | 1.22 (1.03-1.44) | .02 | 1.16 (1.01-1.34) | .04 | 1.40 (1.20-1.63) | <.001 | 1.21 (0.81-1.80) | .35 | 1.36 (1.23-1.51) | <.001 |
| 2 | 1.29 (1.12-1.49) | <.001 | 1.14 (1.01-1.29) | .04 | 1.36 (1.19-1.55) | <.001 | 1.43 (1.04-1.96) | .03 | 1.42 (1.29-1.55) | <.001 | |
| 3 | 1.28 (1.09-1.50) | .002 | 1.09 (0.95-1.26) | .21 | 1.40 (1.21-1.62) | <.001 | 1.39 (0.97-2.00) | .07 | 1.40 (1.27-1.56) | <.001 | |
| 4 | 1.20 (1.01-1.43) | .04 | 1.03 (0.89-1.21) | .67 | 1.32 (1.12-1.54) | .001 | 1.21 (0.81-1.81) | .35 | 1.39 (1.25-1.55) | <.001 | |
| 5 | 0.99 (0.75-1.29) | .93 | 1.02 (0.82-1.26) | .88 | 1.35 (1.07-1.69) | .01 | 1.20 (0.67-2.17) | .54 | 1.28 (1.07-1.51) | .006 | |
| Postural change in SBP, per −5 mm Hg | 1 | 1.07 (1.05-1.09) | <.001 | 1.06 (1.04-1.07) | <.001 | 1.06 (1.04-1.08) | <.001 | 1.03 (0.99-1.07) | .11 | 1.08 (1.07-1.10) | <.001 |
| 2 | 1.09 (1.07-1.11) | <.001 | 1.07 (1.05-1.08) | <.001 | 1.09 (1.07-1.11) | <.001 | 1.04 (1.00-1.09) | .03 | 1.11 (1.10-1.13) | <.001 | |
| 3 | 1.07 (1.05-1.09) | <.001 | 1.05 (1.03-1.06) | <.001 | 1.08 (1.06-1.10) | <.001 | 1.04 (1.00-1.09) | .06 | 1.09 (1.07-1.10) | <.001 | |
| 4 | 1.05 (1.03-1.07) | <.001 | 1.03 (1.01-1.04) | .002 | 1.06 (1.04-1.08) | <.001 | 1.00 (0.96-1.04) | .99 | 1.05 (1.04-1.07) | <.001 | |
| 5 | 1.04 (1.01-1.06) | .008 | 1.02 (1.00-1.04) | .13 | 1.03 (1.00-1.06) | .03 | 1.00 (0.95-1.07) | .89 | 1.02 (1.00-1.04) | .02 | |
| Postural change in DBP, per −5 mm Hg | 1 | 1.14 (1.10-1.18) | <.001 | 1.11 (1.08-1.14) | <.001 | 1.16 (1.12-1.19) | <.001 | 1.01 (0.94-1.09) | .73 | 1.18 (1.15-1.21) | <.001 |
| 2 | 1.16 (1.12-1.20) | <.001 | 1.12 (1.08-1.15) | <.001 | 1.18 (1.14-1.22) | <.001 | 1.05 (0.97-1.14) | .22 | 1.20 (1.17-1.23) | <.001 | |
| 3 | 1.09 (1.05-1.13) | <.001 | 1.07 (1.04-1.10) | <.001 | 1.12 (1.09-1.16) | <.001 | 1.07 (0.99-1.16) | .11 | 1.14 (1.11-1.16) | <.001 | |
| 4 | 1.09 (1.05-1.13) | <.001 | 1.05 (1.02-1.08) | .001 | 1.11 (1.08-1.15) | <.001 | 1.02 (0.95-1.11) | .54 | 1.13 (1.10-1.16) | <.001 | |
| 5 | 1.11 (1.06-1.17) | <.001 | 1.07 (1.03-1.11) | <.001 | 1.09 (1.04-1.14) | <.001 | 0.99 (0.89-1.11) | .91 | 1.10 (1.06-1.14) | <.001 | |
Abbreviations: BP, blood pressure; DPB, diastolic blood pressure; HR, hazard ratio; SBP, systolic blood pressure.
All models adjusted for age, sex, race-study center, heart rate, body mass index, estimated glomerular filtration rate, diabetes, hypertension, alcohol use, education level, smoking status, leisure index, prior coronary heart disease, prior stroke, prior congestive heart failure, hypertension medication use in past 2 weeks, diuretic use, antidepressant use, sedative use, hypnotic use, antipsychotic medication use, anticholinergic medication use, resting SBP, and resting DBP. Unlike eTable 5 in the Supplement, each model includes only 1 measurement of orthostatic hypotension or postural change in SBP or DBP at a time. Measurement 5 has a much smaller sample size (n = 7385).
When postural change in SBP was modeled as a continuous variable, all measurements were associated with falls, syncope, and mortality. Only values from measurements 1 to 4 were associated with fracture, and only measurement 2 was significantly associated with motor vehicle crash (HR, 1.04; 95% CI, 1.00-1.09). Similarly, postural change in DBP as a continuous variable at all measurements was associated with falls, fracture, syncope, and death. However, none of the measurements were associated with motor vehicle crash.
Sensitivity Analyses
Our findings were confirmed in a comparison of OH from measurements 1 to 4 in models that included all measurements simultaneously (see eTable 4 in the Supplement). Measurement 1 was associated with dizziness (OR, 1.59; P = .001), syncope (HR, 1.21; P = .03), and mortality (HR, 1.15; P = .03), and measurement 2 was associated with falls (HR, 1.20; P = .03) and mortality (HR, 1.22; P = .001). Later measurements were not associated with dizziness or outcomes (exception: measurement 3 and mortality; HR, 1.15; P = .04). Similarly, when restricted to the subpopulation with all 5 measurements, only measurements 1 and 2 were associated with either dizziness or long-term outcomes (see eTable 5 in the Supplement).
Imputation of missing standing SBP and DBP measurements as well as dizziness did not meaningfully change our findings (see eTable 6 and eTable 7 in the Supplement). In a similar fashion, repeating analyses in strata of hypertension (see eTable 8 and eTable 9 in the Supplement) or adjusting for pulse pressure and proportion with DBP less than 60 mm Hg at baseline (see eTable 10 in the Supplement) yielded virtually similar results.
Discussion
In this middle-aged, community-based population, these data demonstrate that OH assessments collected within the first 30 seconds after standing were most closely associated with a self-reported history of orthostatic dizziness. Furthermore, the earliest measurement was informative as to risk of future fall, fracture, syncope, and mortality. Orthostatic assessments that were delayed by more than 60 seconds after standing were less informative as to long-term outcomes and were not associated with dizziness. These findings suggest that the practice of assessing OH within 1 minute of standing provides clinically useful information that may be missed when only later measurements are recorded.
Orthostatic hypotension is an important clinical sign. It is associated with a number of adverse outcomes, including falls, coronary heart disease, stroke, and death. Short-term consequences of OH include lightheadedness, vision problems, weakness, fatigue, and trouble concentrating. It is thought that these symptoms mediate health outcomes, but in some circumstances, such as death, it is unclear whether OH is the underlying cause or whether it reflects autonomic dysfunction or another primary disease (eg, heart failure, diabetes) or neurodegenerative conditions (eg, Parkinson disease). The identification of clinically important OH has potential treatment implications, as behavioral and pharmacologic interventions might be instituted if OH is identified early. Interventions include physical maneveurs; compression stockings; volume status optimization; pharmacologic agents, such as fludricortisone or midodrine; pacemakers; or simply avoiding medications known to exacerbate fall risk. Thus, early identification of OH is important for preventing adverse events.
There is substantial debate over the optimal timing of OH measurements. In clinical practice, convenience often dictates initiating assessment of orthostatic BP right after standing, reflecting a scenario similar to measurement 1 in the ARIC study protocol. However, there have been concerns about assessments occurring too early based on short-term physiologic changes in BP on standing. For example, beat-to-beat BP assessments show short-term increases in BP immediately after rising. As a result, a number of studies have advocated against using BP measurements obtained soon after standing to assess OH; however, it is unclear how long one should wait prior to performing the standing measurement. The original American Autonomic Society and the American Academy of Neurology consensus definition called for a 3-minute delay. In contrast, some studies have advocated for at least 5 minutes prior to assessment of OH. Our study showed that the largest mean reduction in SBP occurred at measurement 2 for SBP and the lowest mean increase in DBP occurred at measurement 2 (mean changes in DBP were greater than those in SBP). Furthermore, we show that earlier measurements, particularly those within 1 minute (measurements 1 or 2), were the most informative for both dizziness and long-term outcomes in this population.
We showed that for the prospective outcomes of interest as well as for history of dizziness, earlier measurements, that is, intended for collection within the first minute of standing, were more informative than later measurements. Both measurements 1 (mean, 28 seconds after standing) and measurement 2 (mean, 53 seconds) were informative as to risk of falls, fracture, syncope, and mortality. Furthermore, measurement 1 was the only measurement associated with history of dizziness, and measurement 2 was the only measurement associated with future risk of a motor vehicle crash. This suggests that the clinical practice of early OH assessments may be more informative than the consensus recommendation in terms of both characterizing concurrent symptoms and identifying long-term risk. As dizziness reflects the causal pathway for variable consciousness, possible presyncope or syncope, falls, fractures, and perhaps motor vehicle crashes, clinical measurements modeling measurement 1, that is, performed immediately after standing, may ultimately be the most clinically informative time measurement.
Limitations
This study has a number of limitations that should be considered if these data are to be considered for use in clinical populations. First, falls, fracture, syncope, and motor vehicle crashes were derived from International Classification of Diseases, Ninth Revision (ICD-9), codes of hospital records and CMS claims and were not adjudicated. Prior studies have shown that while these codes are specific, the use of these codes is known to be insensitive (clearly demonstrated in the case of falls). As a result, associations are likely conservative owing to underascertainment. Second, there was variability as to the length of time required by participants to stand as well as when time assessments were obtained. Thus, we compared measurement number rather than time of measurement. We believe this is more reflective of real-world scenarios in which there are a number of factors that contribute to variability in the timing of BP assessments. Third, the measurement protocol was designed to terminate with symptoms of discomfort or dizziness. As a result, ARIC participants missing 1 of the first 4 measurements likely had a higher prevalence of concurrent dizziness with standing. Fourth, driver status or driver eligibility for ARIC participants was not ascertained and is unknown. As a result, some members of the study population were less likely to be drivers of motor vehicle crashes, which would result in lower event rates for motor vehicle crashes. Fifth, other relevant variables (eg, fainting spells, cardiac failure, seizures) were not available. Sixth, the study lacked follow-up OH measurements precluding examination of change over time.
Conclusions
Our study demonstrates that early assessments of OH (within 1 minute) may be not only time-saving but also most clinically relevant and highly informative for long-term prognosis. While our data were derived from a relatively healthy community population, they imply that OH measurement protocols might achieve their greatest prognostic value by including early poststanding BP measurement time points. These results represent compelling evidence for earlier time measurements in the assessment of OH in middle-aged adults.
eMethods 1. Long-term Outcome Ascertainment
eMethods 2. Covariate Definitions
eTable 1. Comparison of baseline population characteristics between participants included in analytic sample (N = 11,429) and those excluded due to missing a standing blood pressure (N = 1,210) or missing data regarding self-reported dizziness (N = 15), mean (SD) or N (%)
eTable 2. Sequential Blood Pressure measurements in the ARIC study: Timing and Associated Blood Pressure Changes
eTable 3. Proportion (%) dizzy and incidence rates (per 1,000 person-years) adjusted for age, sex, and race.
eTable 4. Independent association of orthostatic hypotension assessed at measures 1-4 with dizziness or longitudinal outcomes
eTable 5. Independent association of orthostatic hypotension with dizziness or longitudinal outcomes adjusted for each of measures 1-5
eTable 6. The Association between Orthostatic Hypotension and Self-Reported Dizziness Upon Standing (Odds Ratio, 95% CI) according to Measurement, using imputed values for missing data, N = 11,654
eTable 7. The Association between Orthostatic Hypotension and Falls, Syncope, Fracture, or Mortality (Hazard Ratio [HR], 95% CI) according to measurement using imputed values for missing blood pressure measurements or dizziness, N = 11,654
eTable 8. The Association between Orthostatic Hypotension and Dizziness, Falls, Syncope, Fracture, Motor Vehicle Accident, or Mortality (Hazard Ratio, 95% CI) according to Measurement among Participants with Hypertension at Baseline
eTable 9. The Association between Orthostatic Hypotension and Dizziness, Falls, Syncope, Fracture, Motor Vehicle Accident, or Mortality (Hazard Ratio, 95% CI) according to Measurement among Participants without Hypertension at Baseline
eTable 10. The Association between Orthostatic Hypotension and Dizziness, Falls, Syncope, Fracture, Motor Vehicle Accident, or Mortality (Hazard Ratio, 95% CI) adjusted for Pulse Pressure & Proportion with Baseline Diastolic Blood Pressure less than 60 mm Hg
eReferences
References
- 1.Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS; CHS Collaborative Research Group . Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6, pt 1):508-519. [DOI] [PubMed] [Google Scholar]
- 2.Yatsuya H, Folsom AR, Alonso A, Gottesman RF, Rose KM; ARIC Study Investigators . Postural changes in blood pressure and incidence of ischemic stroke subtypes: the ARIC study. Hypertension. 2011;57(2):167-173. doi: 10.1161/HYPERTENSIONAHA.110.161844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (the Malmo Preventive Project). Eur Heart J. 2010;31(1):85-91. doi: 10.1093/eurheartj/ehp329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Consensus Committee of the American Autonomic Society and the American Academy of Neurology Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46(5):1470. [DOI] [PubMed] [Google Scholar]
- 5.Lahrmann H, Cortelli P, Hilz M, Mathias CJ, Struhal W, Tassinari M. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13(9):930-936. doi: 10.1111/j.1468-1331.2006.01512.x [DOI] [PubMed] [Google Scholar]
- 6.Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386. [DOI] [PubMed] [Google Scholar]
- 7.Rose KM, Tyroler HA, Nardo CJ, et al. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens. 2000;13(6, pt 1):571-578. [DOI] [PubMed] [Google Scholar]
- 8.SPRINT Research Group A randomized trial of intensive vs standard blood-pressure control. N Engl J Med. 2015;373:2103-2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. [PubMed] [Google Scholar]
- 10.Jackson R, Chambless LE, Yang K, et al. ; Atherosclerosis Risk in Communities (ARIC) Study Investigators . Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. J Clin Epidemiol. 1996;49(12):1441-1446. [DOI] [PubMed] [Google Scholar]
- 11.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223-233. [DOI] [PubMed] [Google Scholar]
- 12.Mundt KA, Chambless LE, Burnham CB, Heiss G. Measuring ankle systolic blood pressure: validation of the Dinamap 1846 SX. Angiology. 1992;43(7):555-566. [DOI] [PubMed] [Google Scholar]
- 13.National Heart, Lung, and Blood Institute. ARIC Manual 11. Sitting blood pressure. http://www.cscc.unc.edu/aric/visit/Sitting_Blood_Pressure_and_Postural_Changes_in_Blood_Pressure_and_Heart_Rate.1_11.pdf. Accessed April 4, 2016.
- 14.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69-72. doi: 10.1007/s10286-011-0119-5 [DOI] [PubMed] [Google Scholar]
- 15.Juraschek SP, Daya N, Appel LJ, et al. Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens. 2017;30(2):188-195. doi: 10.1093/ajh/hpw108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucharska-Newton AM, Heiss G, Ni H, et al. Identification of heart failure events in Medicare claims: the Atherosclerosis Risk in Communities (ARIC) Study. J Card Fail. 2016;22(1):48-55. doi: 10.1016/j.cardfail.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR Jr, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24(4):685-693. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea: validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8(9):1007-1014. [DOI] [PubMed] [Google Scholar]
- 20.Gangavati A, Hajjar I, Quach L, et al. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59(3):383-389. doi: 10.1111/j.1532-5415.2011.03317.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22(2):79-90. doi: 10.1007/s10286-011-0146-2 [DOI] [PubMed] [Google Scholar]
- 22.Gorelik O, Feldman L, Cohen N. Heart failure and orthostatic hypotension. Heart Fail Rev. 2016;21(5):529-538. doi: 10.1007/s10741-016-9541-z [DOI] [PubMed] [Google Scholar]
- 23.van Hateren KJJ, Kleefstra N, Blanker MH, et al. Orthostatic hypotension, diabetes, and falling in older patients: a cross-sectional study. Br J Gen Pract. 2012;62(603):e696-e702. doi: 10.3399/bjgp12X656838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senard JM, Raï S, Lapeyre-Mestre M, et al. Prevalence of orthostatic hypotension in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;63(5):584-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Lieshout JJ, ten Harkel AD, Wieling W. Physical manoeuvres for combating orthostatic dizziness in autonomic failure. Lancet. 1992;339(8798):897-898. [DOI] [PubMed] [Google Scholar]
- 26.Podoleanu C, Maggi R, Brignole M, et al. Lower limb and abdominal compression bandages prevent progressive orthostatic hypotension in elderly persons: a randomized single-blind controlled study. J Am Coll Cardiol. 2006;48(7):1425-1432. doi: 10.1016/j.jacc.2006.06.052 [DOI] [PubMed] [Google Scholar]
- 27.Shannon JR, Diedrich A, Biaggioni I, et al. Water drinking as a treatment for orthostatic syndromes. Am J Med. 2002;112(5):355-360. [DOI] [PubMed] [Google Scholar]
- 28.Campbell IW, Ewing DJ, Clarke BF. 9-Alpha-fluorohydrocortisone in the treatment of postural hypotension in diabetic autonomic neuropathy. Diabetes. 1975;24(4):381-384. [DOI] [PubMed] [Google Scholar]
- 29.Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA; Midodrine Study Group . Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension: a randomized, double-blind multicenter study. JAMA. 1997;277(13):1046-1051. [PubMed] [Google Scholar]
- 30.Abe H, Numata T, Hanada H, Kohshi K, Nakashima Y. Successful treatment of severe orthostatic hypotension with cardiac tachypacing in dual chamber pacemakers. Pacing Clin Electrophysiol. 2000;23(1):137-139. [DOI] [PubMed] [Google Scholar]
- 31.de Jong MR, Van der Elst M, Hartholt KA. Drug-related falls in older patients: implicated drugs, consequences, and possible prevention strategies. Ther Adv Drug Saf. 2013;4(4):147-154. doi: 10.1177/2042098613486829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finucane C, O’Connell MDL, Fan CW, et al. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation. 2014;130(20):1780-1789. doi: 10.1161/CIRCULATIONAHA.114.009831 [DOI] [PubMed] [Google Scholar]
- 33.Streeten DH, Anderson GH Jr. Delayed orthostatic intolerance. Arch Intern Med. 1992;152(5):1066-1072. [PubMed] [Google Scholar]
- 34.Naschitz JE, Rosner I. Orthostatic hypotension: framework of the syndrome. Postgrad Med J. 2007;83(983):568-574. doi: 10.1136/pgmj.2007.058198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Numé A-K, Gislason G, Christiansen CB, et al. Syncope and motor vehicle crash risk: a Danish nationwide study. JAMA Intern Med. 2016;176(4):503-510. doi: 10.1001/jamainternmed.2015.8606 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Long-term Outcome Ascertainment
eMethods 2. Covariate Definitions
eTable 1. Comparison of baseline population characteristics between participants included in analytic sample (N = 11,429) and those excluded due to missing a standing blood pressure (N = 1,210) or missing data regarding self-reported dizziness (N = 15), mean (SD) or N (%)
eTable 2. Sequential Blood Pressure measurements in the ARIC study: Timing and Associated Blood Pressure Changes
eTable 3. Proportion (%) dizzy and incidence rates (per 1,000 person-years) adjusted for age, sex, and race.
eTable 4. Independent association of orthostatic hypotension assessed at measures 1-4 with dizziness or longitudinal outcomes
eTable 5. Independent association of orthostatic hypotension with dizziness or longitudinal outcomes adjusted for each of measures 1-5
eTable 6. The Association between Orthostatic Hypotension and Self-Reported Dizziness Upon Standing (Odds Ratio, 95% CI) according to Measurement, using imputed values for missing data, N = 11,654
eTable 7. The Association between Orthostatic Hypotension and Falls, Syncope, Fracture, or Mortality (Hazard Ratio [HR], 95% CI) according to measurement using imputed values for missing blood pressure measurements or dizziness, N = 11,654
eTable 8. The Association between Orthostatic Hypotension and Dizziness, Falls, Syncope, Fracture, Motor Vehicle Accident, or Mortality (Hazard Ratio, 95% CI) according to Measurement among Participants with Hypertension at Baseline
eTable 9. The Association between Orthostatic Hypotension and Dizziness, Falls, Syncope, Fracture, Motor Vehicle Accident, or Mortality (Hazard Ratio, 95% CI) according to Measurement among Participants without Hypertension at Baseline
eTable 10. The Association between Orthostatic Hypotension and Dizziness, Falls, Syncope, Fracture, Motor Vehicle Accident, or Mortality (Hazard Ratio, 95% CI) adjusted for Pulse Pressure & Proportion with Baseline Diastolic Blood Pressure less than 60 mm Hg
eReferences