Abstract
Archaeal viruses offer exceptional biophysical properties for modification and exploration of their potential in bionanotechnology, bioengineering and nanotherapeutic developments. However, the interaction of archaeal viruses with elements of the innate immune system has not been explored, which is a necessary prerequisite if their potential for biomedical applications to be realized. Here we show complement activation through lectin (via direct binding of MBL/MASPs) and alternative pathways by two extremophilic archaeal viruses (Sulfolobus monocaudavirus 1 and Sulfolobus spindle-shaped virus 2) in human serum. We further show some differences in initiation of complement activation pathways between these viruses. Since, Sulfolobus monocaudavirus 1 was capable of directly triggering the alternative pathway, we also demonstrate that the complement regulator factor H has no affinity for the viral surface, but factor H deposition is purely C3-dependent. This suggests that unlike some virulent pathogens Sulfolobus monocaudavirus 1 does not acquire factor H for protection. Complement activation with Sulfolobus monocaudavirus 1 also proceeds in murine sera through MBL-A/C as well as factor D-dependent manner, but C3 deficiency has no overall effect on viral clearance by organs of the reticuloendothelial system on intravenous injection. However, splenic deposition was significantly higher in C3 knockout animals compared with the corresponding wild type mice. We discuss the potential application of these viruses in biomedicine in relation to their complement activating properties.
Keywords: Complement system, Mannose-binding lectin, Nano-biotechnology, Reticuloendothelial system, Sulfolobus monocaudavirus 1, Sulfolobus spindle-shaped virus 2
1. Introduction
Archaeal viruses constitute a group of morphologically and genetically unique viruses with exceptional life-cycle traits (Prangishvili et al., 2006; Peng et al., 2012). These viruses infect hyperthermophiles belonging to the crenarchaeal genera Sulfolobus and Acidianus or halophiles of the euryarchaeal genera Haloarcula and Halorubrum (Pietila et al., 2014). There are no reports of archaeal virus integration into human or any other eukaryotic genomes (Eckburg et al., 2003). Conceivably, it is unlikely for these viruses to trigger negative downstream effects in mammalian species due to their inability to proliferate.
All isolated archaeal viruses are extremophilic in nature and show adaptations to the extreme environments of their host. For instance, viruses of extreme halophiles show stability in solutions of high salt concentration (3–5 M) (Pina et al., 2011). Similarly, viruses infecting acidophilic hyperthermophiles are stable under very aggressive conditions comprising pH values < 3 and temperatures above 80 °C (Contursi et al., 2006). Archaeal viruses also appear in distinct morphologies to include bottle, spindle and droplet shapes (Pina et al., 2011). These unique features, together with their nanosize ranges, offer attractive traits for exploration of their potential in bionanotechnology and bioengineering arenas as well as for development of nanotherapeutics (Steinmetz et al., 2008; Uldahl et al., 2016b). However, to the best of our knowledge, there are no reports on interaction of archaeal viruses with elements of the mammalian innate immune system, and such studies would be of safety and performance importance for potential new therapeutic developments. It is also conceivable that unlike virulent pathogens and from an evolutionary point of view, archaeal viruses may have not developed strategies to combat the mammalian innate immune system. Here, we have selected two previously characterized spindle-shaped hyperthermostable and acid-resistant archaeal viruses (Sulfolobus monocaudavirus 1, SMV1 and Sulfolobus spindle-shaped virus 2, SSV2) (Uldahl et al., 2016a, 2016b) and studied their interaction with human and mouse complement system. We have further examined viral clearance and biodistribution on intravenous injection into wild type (WT) and C3 knockout (C3 KO) mice in C57/BL6J background.
2. Materials and methods
2.1. Production, purification and characterization of viruses
SSV2 was propagated in S. solfataricus strain 5E6, whereas SMV1 was propagated in S. islandicus CRISPR deletion mutant delta C1C2 as described in detail elsewhere (Gudbergsdottir et al., 2011; Okutan et al., 2013; Uldahl et al., 2016b). Procedures for cultures, ultrafiltration and viral purification by ultracentrifugation through a 10–40% (w/ v) continuous Iodixanol gradient were as before (Uldahl et al., 2016b). The virus preparations were stored at 4 °C until used. Viral morphology was confirmed by transmission electron microscopy, whereas Nano-Sight LM20 Nanoparticle Tracking Analysis (Malvern Instruments, Malvern, UK), equipped with a sample chamber with a 405 nm blue laser and a Viton fluoroelastomer O-ring, was used for determination of the hydrodynamic size distribution and viral particle concentration as described in detail earlier (Uldahl et al., 2016b). The approximate virus titer was determined by a plaque assay as described previously (Uldahl et al., 2016b).
2.2. Sera preparation and treatment
Human serum was obtained from healthy Caucasian male volunteers (aged 25–40 years) and individuals genetically deficient in MBL according to approved local protocols. Purification and characterization of MBL/MASPs from a healthy human serum was in accordance with previous studies (Moghimi et al., 2006; Andersen et al., 2013). Serum concentration of mannan-binding lectin (MBL) and concentration of purified MBL was determined using MBL-C4 complex ELISA kit (HyCult Biotechnology, The Netherlands) (Andersen et al., 2013). Sera genetically deficient in MBL had MBL levels below 100 ng/mL. C1q-depleted serum was prepared and characterized as detailed previously (Andersen et al., 2013). Mouse sera deficient for C1q, ficolin A (FcnA), MBL-A/C, C1q/MBL-A/C, factor D (fD), MBL-A/C/fD and factor B (fB) were collected from corresponding maintained colonies of C57BL/6 homozygous mice at the University of Colorado Anschutz Medical Campus (Banda et al., 2007, 2010, 2011). Procedures for sera preparation, handling and use for complement activation studies were in accordance with the suggested guidelines of Lachmann (2010).
2.3. Complement activation studies
To measure complement activation in human sera, we determined viral-induced rise of complement activation products C4d, C5a, Bb and sC5b-9 using respective Quidel’s (Quidel, CA, USA) kits according to the manufacturer’s protocols as described previously (Andersen et al., 2013; Wibroe et al., 2017). For measurement of complement activation, the reaction was started by adding the required quantity of viruses to undiluted serum in Eppendorf tubes (in triplicate) in a shaking water bath at 37 °C for 30 min. Reactions were terminated by addition of “sample diluent” provided with assay kit or saline containing 25 mM EDTA. Viral-induced rises of serum complement activation products were then measured following virus removal. Control serum incubations contained saline (the same volume as viruses and other additions) for assessing background levels of complement activation products. In some studies complement activation was monitored following restoration of C1q and MBL/MASPs to depleted or genetically deficient serum. Zymosan, aggregated human IgG and mannan were used as positive control for complement activation. Complement activation by viruses was also monitored in the presence of either 10 mM EGTA/ 2.5 mM Mg2+ or 40 μM compstatin or its control peptide (a gift by Prof. T.E. Mollnes, University of Oslo, Norway). For quantification of complement activation products, standard curves were constructed using the assigned concentration of each respective standard supplied by the manufacturer and validated.
Superparamagnetic iron oxide (SPIO) nanoworms of 110 nm in size was prepared and characterized as described earlier (Chen et al., 2017). In some experiments, viral particles or SPIO nanoworms (1 × 1013 SMV1 or SPIO nanoworms/mL) were incubated with mouse serum or human serum (volume ratio 1:3) for 30 min at 37 °C. At the end of incubation, particles were washed 3 times with 1X phosphate-buffered saline (PBS) by ultracentrifugation at 100,000g for 10 min using Beckman Optima TLX ultracentrifuge. The pellets were re-suspended in 30 μL PBS, pH 7.0. For fB, factor H (fH) and C3 dot blot, 2 μL aliquots were applied in triplicates onto a nitrocellulose membrane. The membranes were blocked using 5% non-fat dry milk in 0.1% Tween® 20 in PBS at room temperature for 1 h, probed with corresponding primary antibodies (anti-human fB antibody and anti-human fH antibody were from Quidel, CA, USA; anti-mouse and anti-human polyclonal C3 antibodies were from MP Biomedicals, OH, USA) at room temperature for 1 h, followed by washing the membranes 3 times with PBS, and finally 1 h incubation with the corresponding IRDye 800CW labelled secondary antibodies (Li-COR Biosciences, NE, USA) against the primary antibody species. The membranes after immunoblotting were scanned using Li-COR Odyssey infrared imager. The integrated dot intensity in the scanned images was determined from 16-bit grayscale images using ImageJ software and plotted using Prism 6 software (GraphPad Software, Inc., CA, USA). The integrated density of fB, fH and C3 was determined by dot blot immunoassay against the standard dilutions of purified fB, fH and iC3b, respectively as described earlier (Chen et al., 2017; Wang et al., 2017).
2.4. Viral biodistribution studies
Wild type and C3−/− mice (Jackson Laboratories, B6;129S4-C3tm1Crr/J) in C57/BL6J background were bred in house according to the approval by University of Colorado Animal Protocol Committee. Viral particles were labelled with 0.1 mM lipophilic near-infrared fluorescent DiIC18(7) (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide) (DiR) (Biotium Inc., CA, USA) for 1 h at 37 °C and washed twice with PBS to remove the unbound dye. DiR labelled viruses (1.7 × 1011) were injected via tail vein into WT and C3KO mice (8 week age, females). Following the injection (1 min and 30 min), the blood was drawn via periorbital plexus using heparin as anti-coagulant. Plasma and washed cells were applied as dots on nitrocellulose membrane and scanned with Li-COR Odyssey scanner (Li-COR Biosciences, NE, USA) at λ = 800 nm. Organs from mice (n = 3 per group) were placed into petri dish and scanned with Li-COR Odyssey. The average fluorescence per organ was determined with ImageJ software by drawing a region of interest around the organ (16-bit gray image; background subtracted) and measuring average intensity with Measure function.
2.5. Statistical analysis
The results are presented either as mean ± s.d. or mean ± s.e.m, where applicable. Statistical analysis and comparison of different groups in relation to one or two factors were performed with one-way ANOVA or two-way ANOVA as appropriate. The Bonferroni method was subsequently used to correct p values after multiple comparisons to calculate statistical significance.
3. Results and discussion
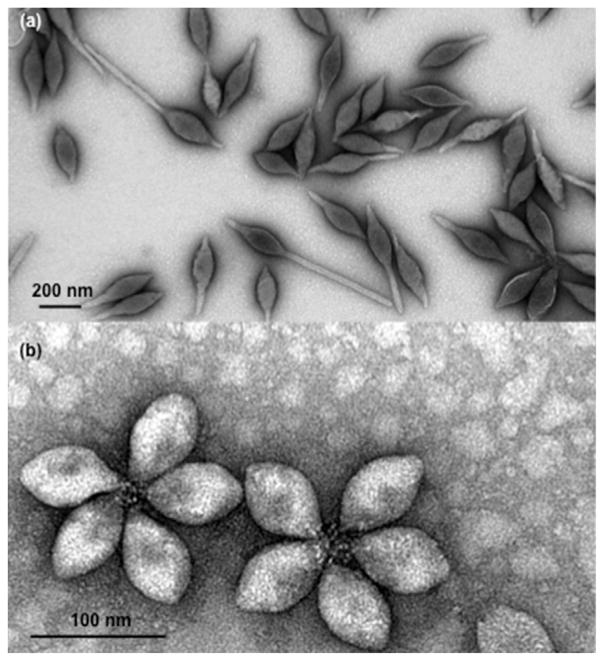
Transmission electron microscopy images of SMV1 and SSV2 are presented in Fig. 1 showing spindle-shape morphology for both virus types. The majority of SMV1 virions either display one-tail or two-tails. We observed rosette-like structures of 3–6 virions for SSV2, but the majority of rosettes were composed of 5 virions. Details of viral size distribution, as determined by NTA, were in accord with our previous study (Uldahl et al., 2016b). However, the mean sphere equivalent hydrodynamic diameter of a typical SSV1 preparation was 115 ± 4 nm (n = 3), whereas for SSV2 the mean sphere equivalent hydrodynamic diameter was 75 ± 3 nm (n = 3). The viral host infectivity (Section 2.1) was further confirmed and the efficiency was similar as described before (Uldahl et al., 2016b).
Fig 1.
Transmission electron micrographs of SMV1 (a) and SSV2 (b). Both viruses are spidle-shape. SMV1 has extending tails of variable lengths. SSV2 predominantly appear in rosette-like arrangement of 3–6 virions, but the electronmicrograph shows rosettes composed of 5 virions. Virus-virus interaction is through their flexous fibres at one pole.
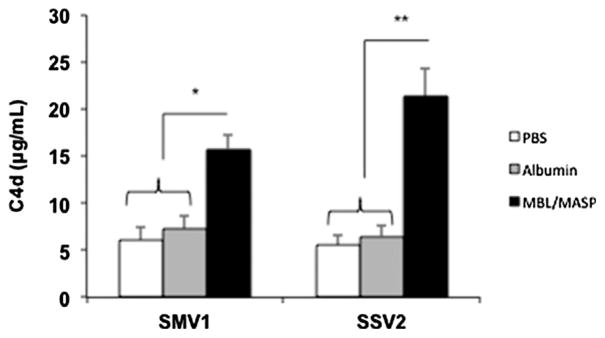
The results in Fig. 2a and b show that both SMV1 and SSV2 in a concentration-dependent manner activate the terminal pathway of human complement system. This is evident from significant rises in the level of soluble complement activation markers C5a and sC5b-9 above background. At a viral concentration of 147 × 108/mL of serum, and regardless of the virus type, the levels of complement activation products were similar to those generated by a zymosan dose of 200 μg/mL (final concentration in serum). Activation of the terminal pathway by both SMV1 and SSV2 is C3-dependent, since compstatin, which binds C3 and interferes with convertase formation and C3 cleavage (Ricklin and Lambris, 2008), totally blocks sC5b-9 generation (Fig. 2c). Next, we studied which pathways of complement activation these viruses trigger. Initially, Ca2+ was excluded from the assay to distinguish alternative pathway operation from classical and lectin pathways, which are Ca2+-dependent (Andersen et al., 2013). Complement activation by SMV1 was partially blocked in the presence of 10 mM EGTA/2.5 mM Mg2+ (Fig. 2c). This suggests a role for both alternative and Ca2+-sensitive pathways in complement activation. In contrast to SMV1, complement activation by SSV1 was dramatically reduced on Ca2+ chelation, thus implying a significant role for Ca2+-sensitive pathways in SSV2-mediated complement activation. To determine which Ca2+-sensitive pathways are involved in complement activation, we monitored C4d generation in sera immunochemically depleted of C1q or genetically deficient in mannose-binding lectin (MBL) (Andersen et al., 2013). Neither C1q depletion, nor addition of physiological levels of C1q (180 μg/mL serum) to the depleted serum had any effect on C4d liberation from C4 by both SMV1 and SSV2 (Fig. 2d). As a positive control we used aggregated human IgG. These observations indicate the inability of C1q to bind to these viruses and trigger complement through the classical pathway. In contrast to C1q, complement activation by both virus types were MBL-dependent, since significant amounts of C4d were liberated in the MBL-deficient serum on MBL/MASPs (equivalent to 1.5 μg MBL/mL serum) addition. We next studied whether MBL/ MASPs bind directly to the surfaces of the viruses or the binding is secondary to surface deposition of certain serum glycoproteins. Accordingly, viruses were pre-incubated with MBL/MASPs (equivalent to 3.0 μg MBL/mL serum), then washed and added to MBL-deficient serum. For control incubations, viruses were pre-incubated in either phosphate-buffered saline (PBS) or human serum albumin. The results in Fig. 3 show that both SMV1 and SSV2 activate lectin pathway through direct MBL/MASPs deposition. SMV1 has two identified coat proteins; ORF 122 and ORF 153 (Erdmann et al., 2014; Hochstein et al., 2015). Similarly, two coat proteins (ORF 88b and ORF 153) have also been identified on SSV2 (Stedman et al., 2003). However, it is not yet known whether these coat proteins carry glycosylation, but our observations may imply the presence of glycosylated moieties on viral capsid proteins recognizable by MBL. We emphasize that glycosylation of capsid proteins at multiple sites for several other archaeal viruses and, particularly, for a close relative of SSV2 (SSV1) have been described, but the sugar types are not known (Quemin et al., 2015). Accordingly, the precise binding sites for MBL/MASPs on surfaces of SMV1 and SSV2 awaits detailed characterization of the viral capsid glycoproteins and their putative glycosylation composition and architecture.
Fig. 2.
SMV1- and SSV2-mediated complement activation in human serum. Panels (a) and (b) shows the effect of viral concentration on C5a and sC5b-9 generation in a typical normal human serum, respectively. The results represent mean ± s.e.m. *p < 0.05 and **p < 0.01 compared with background. Panel (c) shows the effect of 10 mM EGTA/2.5 mM Mg2+, compstatin and its control peptide (40 μM) on viral-mediated activation of the terminal pathway of the complement system in a normal human serum. Similar patterns were observed in two other tested human sera. *p < 0.05 and **p < 0.01 comparing “no addition” vs. “EGTA/Mg2+”. Compstatin fully inhibited sC5b-9 generation. Panel (d) shows the effect of virus on C4d generation in a serum immunochemically depleted of C1q and a genetically deficient MBL serum. Panel (e) shows the effect of viruses on the alternative pathway turnover (through measurement of Bb) in a genetically deficient MBL serum. Panel (f) represent integrated factor B density on viral surfaces as determined by a dot blot immunoassay following incubation in a normal human serum. In (c–e), incubations contained 588 × 108 viruses, where as in (f) incubations contained 1.7 × 1011 viruses. The results in (c–d) represent mean ± s.d. (n = 3). *p < 0.05 and **p < 0.01 for selected pairs in (d), (e) and (f).
Fig. 3.
The effect of MBL/MASPs binding to SMV1 and SSV2 viruses on C4 activation. Viruses (588 × 108) were pre-incubated with purified human MBL/MASPs (equivalent to 3.0 μg MBL/mL) for 30 min at 37 °C. After washing twice in PBS, viral-mediated C4 cleavage (C4d determination) was followed in a genetically deficient human MBL serum. For control experiments viruses were pre-incubated either in PBS or a protein medium (human serum albumin, 3.0 μg/mL). All incubations were in triplicate and each experiment was repeated 3 times. The results represent mean ± s.d. (n = 3). *p < 0.05 and **p < 0.01.
The contribution of the alternative pathway in viral-mediated complement activation was investigated further through measurement of Bb liberation (an established marker of the alternative pathway) in the MBL-deficient serum. The results in Fig. 2e show that SMV1 directly increases alternative pathway turnover. We did not investigate as to whether this increase in alternative pathway turnover is arising from direct deposition of C3H2O on the viral surface or through covalent binding of nascent C3b. Nevertheless, SMV1 liberated more Bb on restoration of MBL/MASP in MBL-deficient serum, thus suggesting a role for amplification loop of the alternative pathway. In contrast to SMV1, SSV2 was less efficient in increasing alternative pathway turnover and with no apparent involvement of the amplification loop (Fig. 2e). These findings are further corroborated by dot-blot studies, which showed considerably less deposition of fB (and its fragments) on SSV1 compared with SMV1 in a healthy functional serum (Fig. 2f). Collectively, these observations suggest different phenotypes between SMV1 and SSV1 at least with respect to generation of fB fragments.
Since SMV1 was directly capable of increasing the alternative pathway turnover, we further assessed the extent of both C3 and fH (a regulator of the alternative pathway and the amplification loop that binds to C3b and limits assembly of the C3bBb convertase deposition) (Józsi, 2017) deposition from serum on SMV1 and compared this with SPIO nanoworms (of similar size to SMV1). SPIO nanoworms were chosen as an established particulate model that predominantly triggers complement activation in human serum through the alternative pathway (Chen et al., 2017). The results in Fig. 4 shows integrated density (see materials and methods) of both C3 and fH on SMV1 and SPIO nanoworms, on the basis of equivalent particle number, in 3 different sera. Compared with SPIO nanoworms, SMV1 is a more potent activator of the complement system (through assessment of C3 integrated density). Also, far more fH is deposited on SMV1 compared with SPIO. Bearing in mind that SMV1 is as potent as zymosan in activating the human complement system (Fig. 2a and b), this higher level of fH deposition is presumably a reflection of the presence of more C3b on SMV1 surfaces (where fH may bind C3b through its complement control protein domains 1–4 and 19–20; Józsi, 2017) rather than direct fH binding to viruses (C3:fH density of 0.94 ± 0.26 and 3.5 ± 0.23 for SMV1 and SPIO nanoworms, respectively). Indeed, in 10 mM EDTA-treated serum (where complement activation is blocked), C3 deposition on both SMV1 and SPIO nanoworms corresponded to less than 15% of untreated serum. EDTA presence also prevented fH binding to both viruses and SPIO nanoworms. Highly virulent human pathogens are known to acquire complement regulators such as fH, fH-related proteins and C4 binding protein for immune evasion (Stoiber et al., 1997; Zipfel et al., 2002; Józsi, 2017), but the lack of direct fH binding to SMV1 is in line with the notion that this virus is not a virulent human (or eukaryotic) pathogen.
Fig. 4.
Integrated C3 and fH deposition from human serum on SMV1 and super-paramagnetic iron oxide nanoworms (SPIO). C3 fragment and fH deposition were measured by a dot blot immunoassay against the standard dilutions of iC3b and fH. All incubations were in triplicate and contained 1.7 × 1011 particles (SMV1 or SPIO nanoworms). Sera from 3 healthy individuals were used (A, B and C).
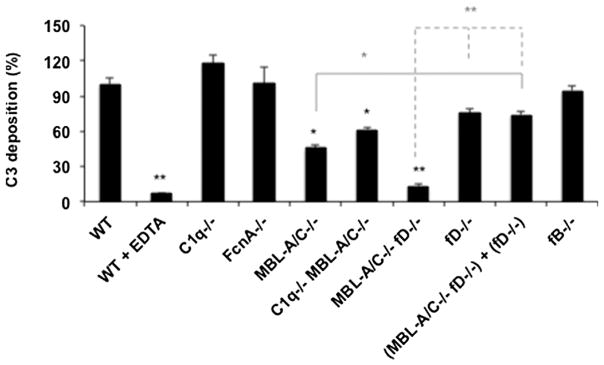
Mice have been widely used in complement activation studies, but there are notable differences between mice and humans on expression of complement components and regulation of cascade activation (Lachmann 2010; Banda et al., 2014; Wang et al., 2017). Accordingly, we selected SMV1 and studied complement activation in mouse serum to reveal similarities and differences with human serum on activation pathways. In order to investigate the pathways of complement activation we examined C3 fragment deposition after viral incubation in sera genetically lacking specific complement components. First, SMV1-mediated complement activation was confirmed in the serum of wild type (WT) mouse and this could be blocked by EDTA (Fig. 5). C3 fragment deposition was comparable in both C1q−/− and ficolin A (FcnA)−/− sera and similar to WT serum, but decreased considerably in both MBL-A/C−/− and double deficient C1q−/−/MBL-A/C−/− sera. This suggested a role for MBL and the lectin pathway in complement activation. Compared with WT serum, C3 deposition was slightly suppressed in fD−/− serum. Furthermore, C3 fragment deposition in double deficient MBL-A/C−/−/fD−/− serum was comparable to that of EDTA-chelated WT serum. On the other hand, adding fD−/− serum into MBL-A/C−/−/fD−/− serum rescued the C3 deposition, thus suggesting the defect of double deficient serum was due to the lectin pathway. Finally, C3 deposition in fB−/− serum proceeded in a comparable manner to WT serum. Collectively, these observations suggest that SMV1 trigger complement activation in mouse serum mainly through the lectin pathway and with a predominant role for MBL-A/C as the sensing molecule. The reason for differences in C3 deposition between fD−/− and fB−/− are not clear, but this may indicate a possible role for MASP3 as a pro-fD activator (Dobo et al., 2016).
Fig. 5.
C3 fragment deposition on SMV1 in mice sera deficient from selected complement proteins. C3 fragment deposition was measured by a dot blot immunoassay against the standard dilutions of iC3b. The extent of C3 fragment deposition is compared with that of the wild-type (WT) serum. All incubations were in triplicate and contained 1.7 × 1011 viruses. *p < 0.05 and **p < 0.01.
Next, we evaluated SMV1 biodistibution in both WT and C3 knockout (C3 KO) mice on bolus intravenous injection. The results in Fig. 6a show that although the majority of virus disappears from the blood within 30 min of injection, the clearance is slower in C3 KO mice. Within the blood, viruses were associated with the cell fraction in WT, but not C3 KO, mice. This may suggest a role for C3 opsonization in viral recognition by blood leukocytes. Organ distribution was evaluated at 24 h post injection, where liver and spleen played a dominant role in viral clearance (Fig. 6b). Although the extent of viral localization to the liver in C3 KO mice was lower than that of WT mice, the differences were not statistically significant. However, splenic deposition was significantly higher in C3 KO compared with the WT mice. Since viruses were circulating for longer periods of time in the blood of C3 KO than WT mice, this presented more viruses to the spleen for filtration (Moghimi, 1995). Accordingly, we can disregard a predominant role for C3 opsonization in viral clearance by Kupffer cells and splenic marginal zone macrophages. The efficient removal of viruses by liver and spleen of the C3 KO mice also suggest involvement of other receptors, such as various classes of scavenger receptors and Fcγ receptors (Peiser et al., 2002; Xu et al., 2008) as well as Toll-like receptors (Medzhitov, 2001) in viral recognition.
Fig. 6.
Biodistribution of DiR labelled SMV1 in wild type (WT) and C3KO mice after intravenous injection. Animals (n = 3) were injected with 1.7 × 1011 viruses. Panel (a) represent plasma level of viruses at 1 and 30 min post injection. Association of viruses with the blood cell fraction was also measured. Two representative cell-associated fluorescent dots on a nitrocellulose membrane, scanned with Li-COR Odyssey scanner, are shown. Panel (b) shows organ distribution of labelled viruses at 24 h post injection. The right portion shows organs scans with Li-COR Odyssey. The results represent mean ± s.d. K = kidneys; L = liver; Lu/H = lungs/heart; S = spleen.
In summary, we have demonstrated differences in complement activation by two extremophilic archaeal viruses (SMV1 and SSV2) in human serum as well as some differences in activation pathways between human and mouse sera with respct to SMV1. Mechanistic aspects of complement activation by these viruses, however, await detailed characterisation of their capsid proteins. Nevertheless, strong complement activation by these viruses together with their unique physicochemical features and the inability to integrate into human or any other eukaryotic genomes may provide new opportunities for studying diseases processes as well as development of new therapeutics. For instance, these viruses may be used to induce compartmental complement activation (e.g., brain, eye, solid tumours) for assessing the role of complement on disease processes (e.g., Alzheimer’s disease, tumour progression, macular degeneration). Alternatively, these viruses (or their coat proteins) may be used as adjuvants in vaccine formulations, since the complement activation product C3d can induce B lymphocyte activation, which is necessary for maintenance of long-term B cell memory (Dempsey et al., 1996). Finally, the capsid proteins of both SMV1 and SSV2 contain multiple units of accessible lysine, aspartic acid and glutamate (Supplementary Fig. S1.). These amino acids could chemoselectively be modified with antigens. Surface chemical modifications may further be extended to synthetic polymers (as well as complement inhibitors/regulators) for modulating viral circulation half-lives and biodistribution in relation to engineering and development of multifunctional biosensors (Moghimi et al., 2012).
Supplementary Material
Acknowledgments
S.M.M. acknowledges financial support by International Science and Technology Cooperation of Guangdong Province (reference 2015A050502002) and Guangzhou City (reference 2016201604030050) with RiboBio Co, Ltd., China. L.W. acknowledges financial support from Drug Discovery Pipeline of Guangzhou Institutes of Biomedicine and Health. X.P. acknowledges financial support by the Danish Council for Independent Research/Technology and Production (grant number DFF-7017-00060). D.S. acknowledges financial support by the University of Colorado Denver start-up fund and NIH (IR01EB022040).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.molimm.2017.08.009.
Footnotes
Competing interests
The authors declare no competing financial interests.
References
- Andersen AJ, Robinson JT, Dai H, Hunter AC, Andresen TL, Moghimi SM. Single-walled carbon nanotube surface control of complement recognition and activation. ACS Nano. 2013;7:1108–1119. doi: 10.1021/nn3055175. [DOI] [PubMed] [Google Scholar]
- Banda NK, Takahashi K, Wood AK, Holers VM, Arend WP. Pathogenic complement activation in collagen antibody-induced arthritis in mice requires amplification by the alternative pathway. J Immunol. 2007;179:4101–4109. doi: 10.4049/jimmunol.179.6.4101. [DOI] [PubMed] [Google Scholar]
- Banda NK, Levitt B, Wood AK, Takahashi K, Stahl GL, Holers VM, Arend WP. Complement activation pathways in murine immune complex-induced arthritis and in C3a and C5a generation in vitro. Clin Exp Immunol. 2010;159:100–108. doi: 10.1111/j.1365-2249.2009.04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda NK, Takahashi M, Takahashi K, Stahl GL, Hyatt S, Glogowska M, Wiles TA, Endo Y, Fujita T, Holers VM, Arend WP. Mechanisms of mannose-binding lectin-associated serine proteases-1/3 activation of the alternative pathway of complement. Mol Immunol. 2011;49:281–289. doi: 10.1016/j.molimm.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda NK, Mehta G, Chao Y, Wang G, Inturi S, Fossati-Jimack L, Botto M, Wu L, Moghimi SM, Simberg D. Mechanisms of complement activation by dextran-coated supermagnetic iron oxide (SPIO) nanoworms in mouse versus human serum. Part Fibre Toxicol. 2014;11:64. doi: 10.1186/s12989-014-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Wang G, Griffin JI, Brenneman B, Banda NK, Holers VM, Backos DS, Wu LP, Moghimi SM, Simberg D. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat Nanotechnol. 2017;12:387–393. doi: 10.1038/nnano.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contursi P, Jensen S, Aucelli T, Rossi M, Bartolucci S, She Q. Characterization of the Sulfolobus host- SSV2 virus interaction. Extremophiles. 2006;10:615–627. doi: 10.1007/s00792-006-0017-2. [DOI] [PubMed] [Google Scholar]
- Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- Dobo J, Szakács D, Oroszlán G, Kortvely E, Kiss B, Boros E, Szász R, Závodszky P, Gál P, Pál G. MASP-3 is the exclusive pro-factor D activator in resting blood: the lectin and the alternative complement pathways are fundamentally linked. Sci Rep. 2016;6:31877. doi: 10.1038/srep31877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Lepp PW, Relman DA. Archaea and their potential role in human disease. Infect Immun. 2003;71:591–596. doi: 10.1128/IAI.71.2.591-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann S, Le Moine Bauer S, Garrett RA. Interviral conflicts that exploit host CRISPR immune systems of Sulfolobus. Mol Microbiol. 2014;91:900–917. doi: 10.1111/mmi.12503. [DOI] [PubMed] [Google Scholar]
- Gudbergsdottir S, Deng L, Chen ZJ, Jensen JVK, Jensen LR, She Q, Garrett RA. Dynamic properties of the Sulfolobus CRISPR/Cas and CRISPR/Cmr systems when challenged with vector-borne viral and plasmid genes and protospacers. Mol Microbiol. 2011;79:35–49. doi: 10.1111/j.1365-2958.2010.07452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein R, Bollschweiler D, Engelhardt H, Lawrence CM, Young M. Large tailed spindle viruses of Archaea: a new way of doing viral business. J Virol. 2015;89:9146–9149. doi: 10.1128/JVI.00612-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józsi M. Factor H family proteins in complement evasion of microorganisms. Front Immunol. 2017;8:571. doi: 10.3389/fimmu.2017.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann PJ. Preparing serum for functional complement assays. J Immunol Methods. 2010;352:195–197. doi: 10.1016/j.jim.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Hamad I, Andresen TL, Jørgensen K, Szebeni J. Methylation of the phosphate oxygen moiety of phospholipid-methoxy(polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production. FASEB J. 2006;20:2591–2593. doi: 10.1096/fj.06-6186fje. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Hunter AC, Andresen TL. Factors controlling nanoparticle pharmacokinetics: an integrated analysis and perspective. Annu Rev Pharmacol Toxicol. 2012;52:481–503. doi: 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- Moghimi SM. Mechanisms of splenic clearance of blood cells and particles: towards development of splenotropic agents. Adv Drug Deliv Rev. 1995;17:103–115. [Google Scholar]
- Okutan E, Deng L, Mirlashari S, Uldahl K, Halim M, Liu C, Garrett RA, She Q, Peng X. Novel insights into gene regulation of the rudivirus SIRV2 infecting Sulfolobus cells. RNA Biol. 2013;10:875–885. doi: 10.4161/rna.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser L, Mukhapahyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123–128. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- Peng X, Garrett RA, She Q. Archaeal viruses: nove, diverse and enigmatic. Sci China Life Sci. 2012;55:422–433. doi: 10.1007/s11427-012-4325-8. [DOI] [PubMed] [Google Scholar]
- Pietila MK, Demina TA, Atanasova NS, Oksanen HM, Bamford DH. Archaeal viruses and bacteriophages: comparisons and contrasts. Trends Microbiol. 2014;22:334–344. doi: 10.1016/j.tim.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Pina M, Bize A, Forterre P, Prangishvili D. The archeoviruses. FEMS Microbiol Rev. 2011;35:1035–1054. doi: 10.1111/j.1574-6976.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- Prangishvili D, Forterre P, Garrett RA. Viruses of the Archaea: a unifying view. Nat Rev Microbiol. 2006;4:837–848. doi: 10.1038/nrmicro1527. [DOI] [PubMed] [Google Scholar]
- Quemin ERJ, Pietilä MK, Oksanen HM, Forterre P, Rijpstra WIC, Schouten S, Bamford DH, Prangishvili D, Krupovic M. Sulfolobus spindle-shaped virus 1 contains glycosylated capsid proteins, a cellular chromatin protein, and host-derived lipids. J Virol. 2015;89:11681–11691. doi: 10.1128/JVI.02270-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Compstatin: a complement inhibitor on its way to clinical application. Adv Exp Med Biol. 2008;632:273–292. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman KM, She Q, Phan H, Arnold HP, Holz I, Garrett RA, Zilig W. Relationships between fuselloviruses infecting the extremely thermophilic archaeon Sulfolobus: SSV1 and SSV2. Res Microbiol. 2003;154:295–302. doi: 10.1016/S0923-2508(03)00074-3. [DOI] [PubMed] [Google Scholar]
- Steinmetz NF, Bize A, Findlay KC, Lomonossoff GP, Manchester M, Evans DJ, Prangishvili D. Site-specific and spatially controlled addressability of a new viral nanobuilding block: sulfolobus islandicus rod-shaped virus 2. Adv Funct Mater. 2008;18:3478–3486. [Google Scholar]
- Stoiber H, Clivio A, Dierich MP. Role of complement in HIV infection. Annu Rev Immunol. 1997;15:649–674. doi: 10.1146/annurev.immunol.15.1.649. [DOI] [PubMed] [Google Scholar]
- Uldahl KB, Jensen SB, Bhoobalan-Chitty Y, Martínez-Álvarez L, Papathanasiou P, Peng X. Life cycle characterization of Sulfolobus monocaudavirus 1, an extremophilic spindle-shaped virus with extracellular tail development. J Virol. 2016a;90:5693–5699. doi: 10.1128/JVI.00075-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldahl KB, Wu L, Hall A, Papathanasiou P, Peng X, Moghimi SM. Recognition of extremophilic archaeal viruses by eukaryotic cells: a promising nanoplatform from the third domain of life. Sci Rep. 2016b;6:37966. doi: 10.1038/srep37966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Griffin JI, Inturi S, Brenneman B, Banda NK, Holers VM, Moghimi SM, Simberg D. In vitro and in vivo differences in murine third complement component (C3) opsonization and macrophage/leukocyte responses to antibody-functionalized iron oxide nanoworms. Front Immunol. 2017;8:151. doi: 10.3389/fimmu.2017.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibroe PP, Anselmo AC, Nilsson PH, Sarode A, Gupta V, Urbanic R, Szebeni J, Hunter AC, Mitragotri S, Mollness TE, Moghimi SM. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat Nanotechnol. 2017;12:589–594. doi: 10.1038/nnano.2017.47. [DOI] [PubMed] [Google Scholar]
- Xu Z, Tian J, Smith JS, Byrnes AP. Clearance of adenoviruses by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol. 2008;82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Sherka C, Hellwage J, Jokiranta ST, Meri S, Brade V, Kraiczy P, Noris M, Remuzzi G. Factor H family proteins: on complement, microbes and human diseases. Biochem Soc Trans. 2002;30:971–978. doi: 10.1042/bst0300971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.