Abstract
During typical late-postnatal CNS development, net reductions in dendritic spine densities are associated with activity-dependent learning. Prior results showed agonist exposure in young animals increased spine densities in a subset of song regions while adult exposures did not, suggesting endocannabinoid signaling regulates dendritic spine dynamics important to vocal development. Here we addressed this question using the CB1 receptor-selective antagonist SR141716A (SR) to disrupt endocannabinoid signaling both during and after vocal learning. We hypothesized antagonist exposure during vocal development, but not adulthood, would alter spine densities. Following 25 days of exposure and a 25 day maturation period, 3D reconstructions of Golgi-Cox stained neurons were used to measure spine densities. We found antagonist treatments during both age periods increased densities within Area X (basal ganglia) and following adult treatments within HVC (premotor cortical-like). Results suggest both inappropriate cannabinoid receptor stimulation and inhibition are capable of similar disregulatory effects during establishment of circuits important to vocal learning, with antagonism extending these effects through adulthood. Given clinical evidence of depressant effects of SR, we tested the ability of the antidepressant monoamine oxidase inhibitor (MAOI) phenelzine to mitigate SR-induced spine density increases. This was confirmed implicating interaction between monoamine and endocannabinoid systems. Finally, we evaluated acute effects of these drugs to alter ability of novel song exposure to increase spine densities in auditory NCM and other regions, finding when combined, SR and phenelzine increased densities within Area X. These results contribute to understanding relevance of dendritic spine dynamics in neuronal development, drug abuse, and depression.
Keywords: Dendritic spines, Cannabinoid, SR141716A, MAOI, CNS development, Vocal learning
1. Introduction
Zebra finches are useful for studying vocal development because they learn a complex song, through sensorimotor integration and auditory feedback, in a process that shares features with acquisition of human language (Doupe and Kuhl, 1999). Prior work has shown that CB1 cannabinoid receptor expression is distinctly dense within brain regions that control learning and production of song, implying a role in vocal development (Soderstrom and Johnson, 2001). Developmental treatment with cannabinoid agonist alters zebra finch vocal learning by reducing the stereotypy of song motifs and reducing the number of distinct note types produced (Soderstrom and Johnson, 2003). Reduced note types are associated with fewer notes derived from tutors and increased production of improvised types (Soderstrom and Tian, 2004). Dramatic changes in CB1 receptor expression levels occur over normal zebra finch development: low densities are observed during the auditory learning stage (25 day olds); peak dense expression occurs during sensorimotor learning (50 and 75 days) and; levels wane to approximately that of the auditory stage in adulthood (>100 days, Soderstrom and Tian, 2006). Agonist treatment during this period also results in persistent changes to increase dendritic spine densities and expression of synaptic markers, suggesting that these changes are involved in the mechanism of cannabinoid-altered vocal learning (Gilbert and Soderstrom, 2014, 2011).
Processes important to CNS development during late-postnatal development include activity- and experience-dependent establishment of synaptic networks during brain maturation. In cortical regions of rodents (Blue and Parnavelas, 1983; De Felipe et al., 1997) and primates (Bourgeois and Rakic, 1993; Huttenlocher, 1990) development is associated with a general profusion of synaptic contacts followed by a reduction of spine densities to adulthood. In songbirds, similar processes occur in at least one cortical-like region necessary for zebra finch vocal learning (lMAN, Nixdorf-Bergweiler et al., 1995a). Importantly, these developmental spine density reductions are inhibited by manipulations that alter normal vocal learning, including rearing in social isolation (Wallhäusser-Franke et al., 1995) and exposure to cannabinoid agonists (Gilbert and Soderstrom, 2011).
This ability of cannabinoid agonist to alter learning, behavior and neuronal morphology within song regions suggests that endocannabinoid signaling is an important regulator of vocal development. Such a role is further supported by clear mammalian evidence of endocannabinoid-mediated control over establishment of neural circuits (reviewed by Keimpema et al., 2011; Lee et al., 2016). To the extent that endocannabinoid signaling is important to development of neural circuits related to vocal development, we hypothesized that interfering with this system by antagonizing endocannabinoid receptor activity would alter dendritic spine densities in brain regions important to vocal learning and production. The experiments reported herein test this hypothesis by evaluating effects of the CB1-selective antagonist/inverse agonist SR141716A (SR) to alter dendritic spine densities in brain regions relevant to song learning (Area X, Sohrabji et al., 1990), auditory perception and memory (NCM, Yanagihara and Yazaki-Sugiyama, 2016), and production (HVC, Nottebohm et al., 1976, see Fig. 1).
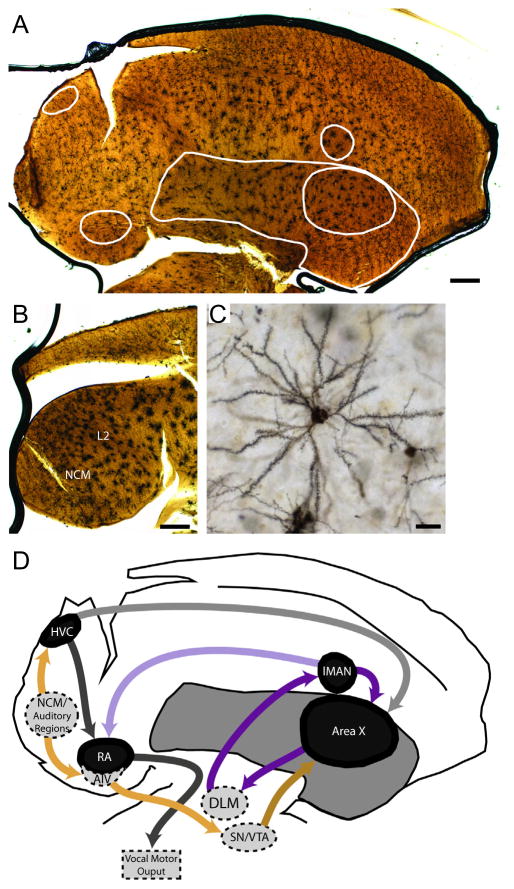
Fig. 1.
Illustration of Golgi-Cox staining quality and locations of brain regions studied. A, Parasagittal section (~1 mm lateral of the midline) of a Golgi-Cox stained zebra finch brain (25X magnification) that contains song regions HVC, RA, Area X, and lMAN. Borders of song regions and striatum are traced in white (see labeling in panel D). B, A more medial parasagittal section (~0.15 mm lateral of the midline) imaged at 25X contains auditory regions NCM and L2. C, Higher power image (200X) illustrates a Golgi-Cox impregnated spiny neuron of the type used for dendritic spine density measurements. D, Camera lucida-type tracing of the section presented in panel A illustrates relative locations of, and a subset of relevant interconnections between, regions studied. Black shading corresponds to song regions traced in panel A (HVC, RA, lMAN, Area X) and dark grey shading indicates striatum. Light grey areas with dashed borders indicate relevant regions not present in the section from panel A. Dark purple arrows indicate connections of the anterior forebrain pathway (AFP), a cortico-basal ganglia-thalamic loop critical for sensorimotor vocal learning (reviewed by Perkel, 2004). Light purple arrow illustrates AFP output from lMAN to the vocal motor output region, RA (Bottjer et al., 1989). Dark grey indicates vocal motor pathways, light grey illustrates the output from pre-motor HVC to the basal ganglia region, Area X. Light gold arrows indicate relevant auditory input to the motor system (Kelley and Nottebohm, 1979; Vates et al., 1996) and from the ventral portion of the intermediate arcopallium (AIV) to dopaminergic neurons within substantia nigra (SN)/ventral tegmental area (VTA, Mandelblat-Cerf et al., 2014). Dark gold indicates SN/VTA dopaminergic projections to spiny interneurons within Area X (Ding and Perkel, 2002). In panels A and B rostral is right, dorsal is up and bars = 470 μm. In panel C bar = 30 μm. Abbreviations: DLM (nucleus dorsolateralis anterior, pars medialis), HVC (proper name), lMAN (lateral magnocellular nucleus of the anterior nidopallium), NCM (caudal medial nidopallium), RA (robust nucleus of the arcopallium). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As clinical use of SR to treat obesity revealed that chronic cannabinoid antagonist exposure is associated with increased incidence of depression (Christensen et al., 2007) we also evaluated the ability of the MAOI antidepressant, phenelzine (a monoamine oxidase inhibitor [MAOI] class of antidepressant that enhances dopamine, norepinephrine and serotonin signaling) to block persistent spine density effects of cannabinoid antagonism in our system. In addition to comparing persistent effects of chronic treatments administered during development and in adulthood, we also evaluated acute effects of the drugs to alter responses following novel song exposure. We have used this approach previously to determine that CB1 activation interferes with the ability of novel song to rapidly increase dendritic spine densities within the auditory region, NCM (Gilbert and Soderstrom, 2013). Thus, the novel song paradigm provides a model system within which to study acute drug effects on dendritic spine dynamics.
2. Results
2.1. Chronic exposure experiments during development and adulthood
Prior chronic exposure experiments with the cannabinoid agonist WIN demonstrated that treatments during development, but not in adulthood, significantly increased dendritic spine densities in the vocal motor-associated region HVC and the region of basal ganglia, Area X (Gilbert and Soderstrom, 2011). This led us to test the hypothesis that treatments with the CB1-selective antagonist, SR, during similar treatment periods would alter, and perhaps lower, spine densities in these regions.
As described in the Methods section (4.7 below) we used a mixed-effects modeling statistical approach to analyze percent control spine density data. For chronic experiments, individual animals were treated as random subjects and individual neurons as random factors. Drug treatments (Vehicle, SR, Phenelzine, Phenelzine + SR) were nested at the level of treatment period (Developmental, Adult) which was nested within brain region (Area X, HVC). These nested factors were used as fixed factors in the model. A likelihood ratio test indicated that significant differences in percent control spine densities were observed across individual animals and neurons (LR = 23,668 – 23,243 = χ2 425, 2 d.f., p < 0.001). A second likelihood ratio test indicated that addition of the nested fixed factors significantly improved the model’s fit to the data (LR = 23,243 – 23,167 = χ2 76, 5 d.f., p < 0.001) demonstrating that spine densities varied across treatment groups within brain region and treatment period. Further complicating the model to allow both the intercept and slope of the regression to randomly vary did not converge, suggesting that addition of random slope did not significantly improve fit of the model to the data. Thus, the model used for analysis of percent control spine density data from chronic experiments employed variable intercepts with fixed slopes. Differences across treatment group, brain region and treatment period were determined from resulting estimates of fixed effects. Vehicle-treated groups were used as the redundant parameter to assess ability of other treatments to alter spine densities. The SR-treated group was used as the redundant parameter to determine ability of phenelzine pretreatments to block effects of SR administered alone.
2.1.1. HVC
Within HVC of animals treated during development, the vehicle control intercept was 99.3% +/− 6.6, 83.8 d.f. (Fig. 2A). Neither SR (113.6% +/− 14.1, 104.2 d.f., t = 1.0, p = 0.31) nor phenelzine treatments (110.0% +/− 14.3, 79.2 d.f., t = 0.75, p = 0.46) significantly altered percent control spine densities from vehicle control levels. However, the combination of phenelzine + SR did modestly, but significantly increase spine densities (124.1 +/− 12.1, 63.8 d.f., t = 2.1, /p = 0.045) suggesting a developmental interaction of monoaminergic and endocannabinoid signaling systems within this premotor cortical-like region.
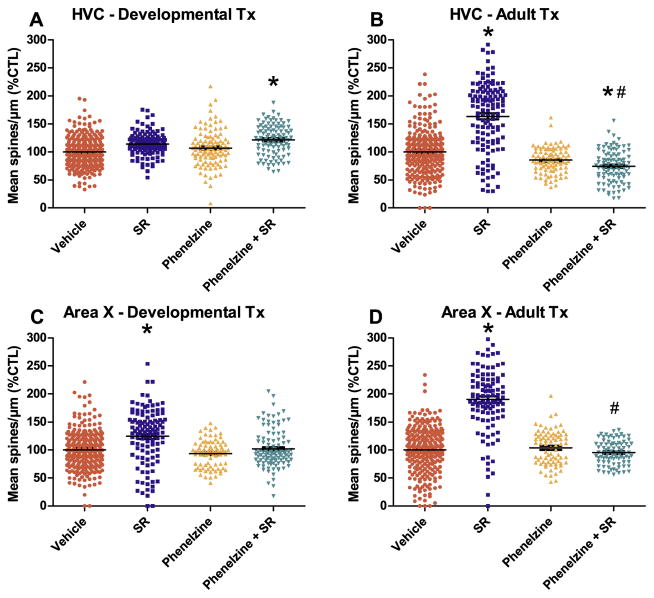
Fig. 2.
Chronic CB1 receptor antagonist (SR141716A [SR]) treatment alters spine densities in an antidepressant sensitive manner. Zebra finches (n = 4) were treated with SR (SR, 6 mg/kg), phenelzine (Phenelzine, 1 mg/kg), SR + phenelzine (Phenelzine + SR), or vehicle (Vehicle) for 25 days followed by 25 days of no treatment in order to allow developing animals to mature, or to simulate this maturation period in adults. Each data point represents mean spine density of an individual dendrite. The MAOI antidepressant phenelzine had no effect by itself (PHE) but when given prior to SR, reversed the CB1 antagonist’s efficacy to increase dendritic spine densities (Phenelzine + SR) within both HVC and Area X following adult treatments, and within Area X following developmental treatments. A, significant changes in percent control spine densities were not observed in motor-related HVC following developmental treatments (50–75 days old). B, however, following antagonist treatments administered during adulthood (>100 days old), HVC percent control spine densities were significantly increased in a manner reversed by pretreatment with the MAOI antidepressant, phenelzine. In Area X following both: C, developmental and; D, adult treatment regimens, SR increased percent control spine densities in a manner prevented by phenelzine. Differences were determined using a mixed-effects ANOVA analysis using animal and neurons as random factors and treatment condition nested within brain region and treatment period as fixed factors, *p < 0.05 vs. Vehicle, #p < 0.05 vs. SR. Points = mean percent control spine density for individual dendrites. Error bars = standard error.
A different pattern was observed within HVC of animals treated as adults (Fig. 2B) as repeated SR treatments significantly increased spine densities (SR, 164.8% +/− 13.9, 61.9 d.f., t = 4.4, /p < 0.001) when compared to vehicle controls (Vehicle, intercept = 104.0% +/− 6.3, 74.8 d.f.). This indicates an adult sensitivity to effects of cannabinoid antagonism that is not present during the developmental exposure period. Phenelzine by itself trended toward a decrease in spine density, but this effect was not significant (Phenelzine, 81.4% +/− 11.7, 72.5 d.f., t = − 1.63, p = 0.11). However phenelzine administered prior to SR during adulthood reversed the antagonist’s ability to increase spine densities to an extent that they were significantly lower than both vehicle controls (Phenelzine + SR, 71.5% +/− 12.0, 61.9 d.f., t = − 2.7, /p = 0.008) and the group treated with SR alone (− 93.3% +/− 16.0 from SR intercept, 85.3 d.f, t = − 5.8, #p < 0.001). Reversal of the effects of a cannabinoid antagonist with an indirect acting monoaminergic agonist demonstrates an interaction between endocannabinoid and monoaminergic signaling systems in this premotor region during adulthood.
2.1.2. Area X
Within Area X of animals treated during development, in contrast to what was observed in HVC, SR treatments significantly increased spine densities (SR, Fig. 2C, 123.4 +/− 11.4, 51.7 d.f., t = 2.0, /p = 0.047) over vehicle-treated controls (Vehicle intercept = 100.2% +/− 5.9, 58.1 d.f.). Phenelzine administered alone did not alter densities (Phenelzine, 92.7% +/− 12.6, 50.7 d.f., t = − 0.59, p = 0.56). However, phenelzine administered prior to SR resulted in spine densities that did not differ from vehicle controls (Phenelzine + SR, 103.9% +/− 12.9, 78.5 d.f., t = 0.29, p = 0.77). When compared to SR administered alone, phenelzine administered prior to SR treatments did not significantly reduce percent control spine densities (Phenelzine + SR, − 19.5 +/− 15.0 from SR intercept, 67.4 d.f., t = − 1.3, p = 0.20).
Following SR treatments administered to adults, spine densities were also increased (SR, Fig. 2D, 190.8% +/− 10.7, 51.5 d.f., t = 8.6, /p < 0.001) relative to vehicle controls (Vehicle intercept = 98.8% +/− 5.5, 51.7 d.f.). Notably, the magnitude of spine density increases following adult treatments appear greater than those observed following developmental exposures (compare Fig. 2C and D). Similar to the developmental treatment group, adult treatments with phenelzine alone did not significantly alter Area X spine densities (101.8% +/− 11.8, 49.8 d.f., t = 0.25, p = 0.80). Also, phenelzine pretreatments blocked ability of SR to increase spine densities from vehicle control levels (Phenelzine + SR, 94.5% +/− 10.7, 52.8 d.f., t = − 0.40, p = 0.69). When compared to effects of SR administered alone, phenelzine pretreatments resulted in significant reductions (Phenelzine + SR, − 93.2% +/− 14.1 from SR intercept, 53.9 d.f., t = − 6.6, #p < 0.001). Reversal of cannabinoid antagonist effects with an indirect monoaminergic agonist demonstrates an interaction of these signaling systems within Area X of adults.
2.2. Effects on acute responses to novel song exposure
Given similar effects of agonist and antagonist drugs observed following chronic exposures (discussed in 2.1 above) we wished to test the hypothesis that acute efficacies would also be consistent. The acute model that we used was the novel song exposure paradigm that had been used previously to show that novel song rapidly increases spine densities within auditory NCM (Gilbert and Soderstrom, 2013). These novel song-stimulated spine density increases demonstrated that cannabinoid signaling is able to modulate this sensory response, and that endocannabinoids may regulate it. Use of an antagonist to block endocannabinoid activity should demonstrate the extent to which spine densities are dynamically regulated within NCM by this signaling system.
As before for chronic experiments (Section 2.1 above), a mixed-effects model was employed. A likelihood ratio test indicated that significant differences in percent control spine densities were observed across individual animals and neurons (LR = 18,325 − 17,672 = χ2 682, 2 d.f., p < 0.001). Adding the fixed factors of treatment nested within brain region further improved fit of the model (LR = 17,672 − 17,643 = χ2 60, 4 d.f., p < 0.001) indicating spine densities varied across treatment groups within brain regions. As for chronic experiments (discussed in 2.1 above) further complication of the model failed to converge. Differences across treatment group and brain region were determined from resulting estimates of fixed effects setting the redundant parameter to the Vehicle + Silence treatment group for each combination of brain region and treatment period.
2.2.1. Auditory region NCM
Within the auditory region NCM under the control Vehicle + Silence condition the percent control spine density intercept was 96.7% +/− 4.8, 74.2 d.f. (Fig. 3A). Confirming prior work, novel song stimulation rapidly increased these spine densities (Vehicle + Song, 117.8% +/− 6.9, 75.8 d.f., t = 3.1, p = 0.003). SR treatment by itself did not significantly change spine densities from control levels, suggesting that, within this auditory region, spine densities are not normally under an inhibitory endocannabinoid tone (SR + Silence, 88.7% +/− 8.3, 76.5 d.f., t = − 0.97, p = 0.33). In contrast to effects of the cannabinoid agonist WIN reported earlier, the antagonist SR did not block or otherwise significantly alter the effect of novel song stimulation to rapidly increase spine densities (SR + Song, 122.2% +/− 8.0, 93.4 d.f., t = 3.2, p = 0.002). This demonstrates that, unlike spine density effects following chronic exposure, acute effects of the agonist and antagonist are dissimilar. To be consistent with the chronic exposure study (2.1 above) effects of phenelzine on novel song responses were also evaluated. By itself, phenelzine did not alter spine densities from vehicle control levels (Phenelzine + Silence, 104.8% +/− 8.4, d.f. = 60.2, t = 0.96, p = 0.34). Similar to SR, phenelzine did not alter the ability of novel song stimulation to rapidly increase spine densities from Vehicle + Silence controls (Phenelzine + Song, 116.7% +/− 8.4, 60.6 d.f., t = 2.4, p = 0.02).
Fig. 3.
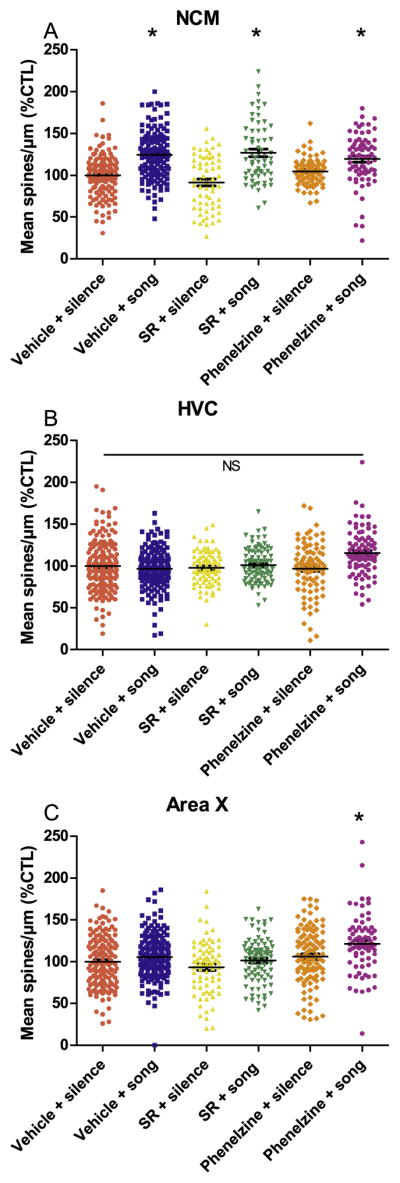
Exposure of zebra finches to an unfamiliar, novel song recording rapidly increases dendritic spine densities in NCM, a region important to auditory perception and memory. Each data point represents mean spine density of an individual dendrite. Prior work demonstrated these novel song-induced increases are prevented by pretreatment with the CB1 agonist, WIN55212-2 (Gilbert and Soderstrom, 2013). Using novel song as a positive control, acute effects of the CB1 receptor antagonist/inverse agonist SR141716A and MAOI phenelzine on spine densities were evaluated within NCM, HVC (premotor), and Area X (basal ganglia). Animals (n = 3–4) were treated with the CB1 receptor antagonist SR141716A (6 mg/kg), MAOI phenelzine (2 mg/kg), or vehicle. 30 min later, a novel song recording was played for 30 min, and animals were euthanized 1 h following the cessation of the song exposure. A, within NCM, novel song exposure increased spine densities as previously reported (compare Vehicle + Silence to Vehicle + Song). Unlike agonist, the CB1 antagonist SR141716A did not reverse song-simulated spine density increases (compare Vehicle + Song to SR + Song). B, No significant effects of song exposure or drug treatments were observed within the premotor region HVC. C, Within Area X, the combination of song-stimulation and phenelzine treatments (Phenelzine + song) modestly increased spine densities, suggesting endocannabinoid/monoaminergic interaction. Data were analyzed by mixed-effects nested ANOVA with individual animals and neurons as random effects and treatment condition nested within brain region as fixed effects, *p < 0.05, vs. Vehicle + silence. Points = mean percent control spine density for individual dendrites. Error bars = standard error.
To test whether effects of novel song exposure are restricted to auditory regions, or perhaps have a more general effect to rapidly stimulate dendritic spine increases in other song regions, we also measured densities within Area X (basal ganglia) and HVC (pre-vocal motor cortex-like).
2.2.2. HVC
Within the premotor cortical-like region HVC neither song nor any combination of song and drug treatments significantly altered spine densities from the Vehicle + Silence control condition (Fig. 3B, intercept = 99.6% +/− 4.6, 69.9 d.f.). Although the combination of phenelzine and song stimulation trended higher than the control, the difference was not significant (Phenelzine + Song, 115.6% +/− 11.5, 145.4 d.f., t = 1.4, p = 0.17). Statistics of the other treatment groups were: Vehicle + Song (96.6% +/− 6.8, 71.6 d.f., t = − 0.44, p = 0.662); SR + Silence (93.6% +/− 8.4, 84.0 d.f., t = − 0.71, p = 0.66); SR + Song (99.4% +/− 7.4, 76.6 d.f., t = − 0.02, p = 0.982); Phenelzine + Silence (97.8% +/− 8.3, 56.8 d.f., t = − 0.21, p = 0.83).
2.2.3. Area X
Within Area X, the Vehicle + Silence percent control spine density intercept was 98.2 +/− 4.5, 64.6 d.f. (Fig. 3C). In contrast to motor-related HVC, within Area X the combination of phenelzine and novel song stimulation resulted in a significant increase in percent control spine density measures when compared to control (Phenelzine + Song, 120.1% +/− 8.3, 57.7 d.f., t = 2.6, p = 0.01). Because neither novel song stimulation alone (Vehicle + Song, 107.0% +/− 6.4, 57.9 d.f., t = − 0.40, p = 0.69) nor phenelzine alone (Phenelzine + Silence, 106.9% +/− 8.2, 54.5 d.f., t = 1.1, p = 0.29) altered spine densities, the efficacy of their combination demonstrates an additive or synergistic effect, and shows that novel song stimulation can influence activity within this basal ganglia region through a mechanism that is modulated by monoaminergic signaling. As was observed within HVC, SR did not alter responses to novel song (SR + Song, 99.7% +/− 7.4, 74.8 d.f., t = 0.20, p = 0.84) and phenelzine alone was also ineffective (Phenelzine + Silence, 106.9% +/− 8.2, 54.5 d.f., t = 1.1, p = 0.29).
3. Discussion
Our interest in the relationship between cannabinoid signaling and dendritic spine densities stems from the clear importance of each to the development and function of the vertebrate CNS. Dendritic spines are post-synaptic structures thought exclusively-associated with excitatory synapses, and are implicated in learning-related long-term potentiation (Bosch and Hayashi, 2012). Spiny neurons are rare outside of vertebrate species, suggesting an important functional role in more complex nervous systems (reviewed by Hering and Sheng, 2001). Perhaps importantly, the classic CB1 and CB2 cannabinoid receptors are absent in species more primitive than chordates (Elphick, 2012) suggesting a similar, possibly related, function in regulating complex CNS signaling.
3.1. Effects of chronic treatments on spine densities
The initial goal of this study was to understand how disrupting endocannabinoid signaling using the CB1-selective antagonist/inverse agonist SR141716A would alter dendritic spine densities in auditory and vocal learning-associated regions of zebra finch telencephalon. Based upon prior work establishing that song regions distinctly and densely express CB1 cannabinoid receptors, and that chronic cannabinoid agonism during sensorimotor vocal learning both alters song (Soderstrom and Johnson, 2003; Soderstrom and Tian, 2004) and increases dendritic spine densities in these regions (Gilbert and Soderstrom, 2013, 2011) we hypothesized that cannabinoid antagonism would produce opposing effects, and that these effects would be restricted to periods of development and not produced following adult treatments. We were surprised not to confirm these hypotheses, finding instead that antagonist exposure during both development and adulthood resulted in spine density increases within Area X, and within HVC following adult treatments (Fig. 2B–D).
Similar effects of cannabinoid agonist and antagonist drugs to increase spine densities within Area X when administered during late-postnatal development suggest that both drug classes are capable of disrupting endocannabinoid signaling important to normal maturational processes that occur during this period. Note that similar cannabinoid agonist and antagonist effects on striatal synaptic morphology have been reported in mammalian models (Lee et al., 2015; Spiga et al., 2011). Such morphological disruption, via either increasing or decreasing cannabinoid receptor activity, appears to interfere with processes regulating spine densities and implies that an appropriate level of endocannabinoid signaling is developmentally important. Exceeding or failing to maintain these levels results in persistently altered spine densities. Inability of SR treatments given during development to raise spine densities within HVC (Fig. 2A) suggests that, during development, this premotor cortical-like region is less sensitive to cannabinoid antagonism than is the region of basal ganglia, Area X (Fig. 2C). Interestingly, within HVC the combination of SR with the MAOI phenelzine during development did significantly increase spine densities over vehicle control levels (Fig. 2A). Distinct monoaminergic sensitivity within HVC of our developing songbirds may be related to high HVC adrenergic receptor levels found in seasonal learners outside of their breeding period (Riters et al., 2002).
The ability of cannabinoid agonists to alter dendritic morphology and spine densities in developing rodent species is well-established (Kolb et al., 2006; Rubino et al., 2009). Less well-established are developmental effects following chronic antagonism, and thus our experiments are among the first to explore this area. Others have recently reported distinct effects of agonists vs. antagonists in cultured mouse hippocampal neurons, finding differential effects on morphology and distinct effects at different developmental stages (Tapia et al., 2017). A study with a design similar to ours, but employing rats and the CB1-selective antagonist AM-251, demonstrated both distinct effects following peri-adolescent vs. adult treatments and similar efficacies of agonist and antagonist on stress coping behaviors and expression levels of endocannabinoid signaling elements (Lee et al., 2015). Although a monoamine-altering antidepressant was not employed in an attempt to reverse cannabinoid antagonist effects, this rat study also implicated CB1 antagonist interaction with monoaminergic signaling in norepinephrine-dependent stress coping behaviors. One explanation offered for similar efficacies of cannabinoid agonists and antagonists is a significant and persistent increase in CB1 receptor expression levels within prefrontal cortex following chronic antagonist treatments that may make the system more sensitive to endocannabinoid release.
3.2. Adult sensitivity to CB1 antagonism
Distinct roles for endocannabinoid signaling during different stages of mammalian neuronal development are well-documented (reviewed by Gaffuri et al., 2012) and we have previously seen these types of differential effects in our avian model (Gilbert and Soderstrom, 2013, 2011; Soderstrom et al., 2011). The present experiments demonstrate antagonist efficacy within Area X to increase spine density that is not restricted to development (Fig. 2C and D). This finding of antagonist effects in adults suggests that activity of the endocannabinoid system, either through constitutive receptor activation or basal release of 2-AG, is important not only to development of neuronal morphology, but also to its maintenance through adulthood. Antagonist-induced increases in spine densities following adult treatments suggests presence of a tonic inhibitory endocannabinoid tone that when relieved results in increased spine densities in both basal ganglia (Area X, Fig. 2D) and premotor cortical-like regions (HVC, Fig. 2B). Distinct adult sensitivity to disruption of endocannabinoid signaling is further supported by an apparently greater magnitude of spine density increases in adults compared to developing animals (compare panels A,B and C,D, Fig. 2). Thus, adults appear more sensitive to antagonist exposure than do developing animals, which may have implications for the use of these drugs in treating obesity.
3.3. Evidence for interaction between endocannabinoid and monoaminergic signaling systems
Endocannabinoid signaling has a clear role in modulation of stress responses and depression (reviewed by Morena et al., 2016). Rodent models of stress and depression have revealed that distinct changes in dendritic spine densities are associated with anxious and depressed states (Qiao et al., 2016). Because the CB1 antagonist we used (SR, once marketed under the brand Rimonabant for the treatment of obesity) was withdrawn from the market due to depression of mood (Beyer et al., 2010; Gamble-George et al., 2013; Horder et al., 2009; Stuart et al., 2013) we hypothesized that its effects could be reversed by an antidepressant. We found that when administered prior to SR, chronic treatments with the MAOI, phenelzine (a drug that enhances monoaminergic dopamine, norepinephrine and serotonin signaling) reversed ability of SR to increase spine densities (compare SR to PHE + SR, Fig. 2). On their own, chronic phenelzine treatments had no persistent effect on spine densities (compare VEH to PHE, Fig. 2B and D). This demonstrates ability of an antidepressant treatment to prevent morphological changes caused by the cannabinoid antagonist that may be related to changes in mood, particularly during adulthood (Norrholm and Ouimet, 2001).
The ability to reverse effects of a cannabinoid antagonist with a MAOI suggests that increased spine densities are related to decreased monoaminergic signaling following CB1 antagonism. This is supported by evidence from a rat model demonstrating CB1 antagonist mitigation of striatal monoamine release following administration of several classes of abused drugs (Cheer et al., 2007). In mammalian striatum, a region with similarities to avian Area X, a weight of evidence suggests that CB1 receptors are not present on terminals of midbrain dopaminergic afferents (Fernández-Ruiz et al., 2010; Oleson and Cheer, 2012) and therefore cannabinoid agonist-induced striatal monoamine release is thought to be indirect and attributable to disinhibition of dopamine neurons within substantia nigra/VTA (Lupica and Riegel, 2005). Given similarities of avian and mammalian dopaminergic circuits (Ding and Perkel, 2014) a similar mechanism may function within avian striatum.
3.4. Effects of acute exposures on responsiveness to novel song
Both monoaminergic and cannabinoid signaling systems are known to mediate responsiveness of zebra finches to novel conspecific song. Novel song exposure rapidly increases expression of the immediate early gene Zenk within the auditory region, NCM (Mello et al., 1992). This response is both modulated by monoaminergic signaling (Sasaki et al., 2006; Velho et al., 2012) and the resulting protein expression blocked by cannabinoid agonist exposure (Whitney et al., 2003).
We reported earlier that novel song exposure also rapidly increases dendritic spine densities within NCM, and that this acute effect is blocked by a cannabinoid agonist (Gilbert and Soderstrom, 2013). We therefore hypothesized that the antagonist may also acutely alter spine densities in the song stimulation model. We found that the cannabinoid antagonist SR and MAOI phenelzine did not alter song-stimulated spine density increases in NCM; that is, novel song increased spine densities within NCM, and neither SR nor phenelzine changed this responsiveness (Fig. 3A). This is notable given dependence of novel song responsiveness within NCM on the monoamine, norepinephrine (Velho et al., 2012) and implies that auditory-related monoamine signaling is maximally effective in rapidly increasing spine densities, and not subject to further enhancement by inhibiting monoamine inactivation.
Within the regions HVC and Area X neither novel song stimulation, SR nor phenelzine treatments alone were able to alter dendritic spine densities (Fig. 3B and C). However, in the case of phenelzine when combined with novel song stimulation, spine densities were modestly, though significantly, increased within Area X (compare Vehicle + silence to Phenelzine + song, Fig. 3C). This demonstrates an additive effect that may involve novel song-stimulated monoamine release. This hypothesis is supported by modest effects of song-stimulus treatments to alter monoamine metabolism in Area X, HVC and auditory brain regions of European starlings (Salvante et al., 2010). Additive effects of phenelzine and song stimulation suggest a coincidence detection or gating mechanism, capable of rapidly modifying synaptic structure within this motor learning region in response to convergent monoaminergic input.
3.5. Summary
The work reported herein demonstrates that chronic cannabinoid antagonism is able to alter morphology of excitatory synapses in an antidepressant-sensitive manner. These changes occur within regions of basal ganglia and motor cortical-like regions important for sensorimotor learning that have functional analogs within mammalian brain. Unlike cannabinoid agonism, these effects are not restricted to a sensitive period of development, and also occur, perhaps with greater sensitivity, in adults. In contrast to effects of a cannabinoid agonist reported previously, the antagonist did not alter song-stimulated spine density increases in the auditory region, NCM. However, combined song stimulation and MAOI treatments were effective in increasing spine densities in Area X suggesting a convergent monoaminergic gating mechanism capable of rapidly altering synaptic morphology in this learning-related region of basal ganglia. Our results provide insight to monoaminergic involvement in mechanisms underlying persistent effects of CB1 antagonists, and contribute to evidence that altered synaptic morphology underlies effects on mood that limit the clinical utility of these drugs. The experiments also contribute to a convincing weight of evidence that the songbird model is relevant for understanding learning- and development-related neurophysiology in a manner that translates to mammalian systems. Because this learning in songbirds is training-independent, it may model development-dependent sensorimotor maturation more accurately than is possible with conventional rodent species.
4. Experimental procedure
4.1. Animals
Male zebra finches were hatched and raised to about 25 days of age in a large flight aviary with other birds of both sexes and varying age. Following initial rearing birds were group housed with an adult male tutor until 50 days of age (+/− five days) after which they were either raised to adulthood (>100 days of age) in a group cage for acute treatment experiments, or housed singly for the developmental experiment. A 14:10 light:dark cycle was maintained at 30 C. Birds were provided ad libitum birdseed, water, cuttlebone and grit. All husbandry and experimental procedures were approved by East Carolina University’s Institutional Animal Care and Use Committee.
4.2. Drugs
The CB1 receptor antagonist/inverse agonist SR141716A was provided by the NIDA drug supply program. Stocks were dissolved in dimethyl sulfoxide (DMSO) and suspended in Alkamuls EL-620 (castor oil ethoxylate) and phosphate-buffered saline (PBS; pH = 7.4) in a ratio of DMSO:Alkamuls EL-620:PBS, 1:1:18. The MAOI phenelzine was a gift from Professor Brian McMillen (ECU Brody School of Medicine, Department of Pharmacology and Toxicology) and dissolved in PBS. Treatments were administered via intramuscular injection of 50 μL into the pectoral muscle using a 30 gauge needle.
4.3. Chronic exposure experiments during development and adulthood
Injections were made daily, at 30 min before lights on, with SR141716A (6 mg/kg), phenelzine (1 mg/kg), SR141716A + phenelzine (6 mg/kg, 1 mg/kg, respectively) or vehicle in separate experiments. In the developmental group, the animals began experiments at 50 days of age (+/− five days), and in the adult group, they started at >100 days of age. Following the 25 day treatment regimen of daily injections, animals were allowed to mature 25 additional days to allow developing birds to reach adulthood and to simulate this maturation period in adults. Following treatment and maturation, animals were euthanized and brains prepared for Golgi-Cox staining. At time of euthanasia, all animals were adults. Each group contained 4 animals.
4.4. Acute effects on responses to novel song exposure
The novel song paradigm was adapted from a previously described method (Whitney et al., 2003). Animals were treated with vehicle, SR141716A (6 mg/kg), phenelzine (2 mg/kg) or a combination of SR141716A (6 mg/kg) and phenelzine (2 mg/kg). Thirty minutes following injections, a novel song recording was played for 30 min (15 s song + 45 s silence, repeated 30×). During the playback session, the animal was in visual and acoustic isolation from other birds and had access to feed and water. One hour following cessation of novel song exposures, animals were euthanized. Each group contained 3–4 animals.
4.5. Golgi-Cox staining
Golgi-Cox staining was done according to a previously described protocol for zebra finches (Gilbert and Soderstrom, 2011). For euthanasia, animals were overdosed with Equithesin and transcardially perfused with ice-cold PBS. Brains were placed in Golgi-Cox solution (5% potassium chloride, 5% mercuric chloride, 5% potassium chromate) for 5 days in the dark at room temperature and then moved to 30% sucrose at 4 °C for 7 days. Brains were sectioned into 200 μm parasagittal sections using a vibrating microtome. Sections were kept on ice and placed in cold 80, 60, 40, 20% glycerol for 2 min each, placed in cold distilled water (dH2O) for 2 min, rinsed in cold dH2O three times, placed in cold 0.05% gold chloride for 80 min, rinsed in cold dH2O three times, placed in cold 0.2% oxalic acid for one to two minutes, and rinsed in cold dH2O three times. Next, the sections were removed from ice and placed in room temperature 1% sodium thiosulfate for 30 min, placed in a second portion of room temperature 1% sodium thiosulfate for 30 min, rinsed in cold dH2O once, placed in room temperature 7% ammonium hydroxide for 30 min in the dark, rinsed in cold water dH2O, placed in Kodak Fixer solution for 30 min in the dark, and placed in cold dH2O. Sections were mounted to slides using 0.3% gelatin. Slides were hydrated with dH2O, dehydrated with graded ethanol solutions (50, 70, 95, 100%), and cleared with xylene. All solutions were freshly made. Staining was done in batches that included each group to ensure that conditions across groups were spread consistently. An example of the quality of staining produced by this method is illustrated in Fig. 1.
4.6. 3D neuronal reconstruction
At 25X magnification, regions of interest (HVC, Area X, NCM) were identified and their borders traced. At 100X magnification, markers were placed over 20 pyramidal-like spiny neurons suitable for analysis within each region, and five of these 20 were randomly selected for analysis. At 1000X magnification, 3D reconstructions were created using Neurolucida software (MBF Bioscience, Williston, VT) by tracing each cell body and dendrite and placing markers for each spine on all dendrites. The mean number of dendrites counted per neuron = 4.9 (range = 3–11). Data were exported using Neuroexplorer software (MBF Bioscience, Williston, VT) for statistical analysis.
14.7. Statistical analysis
Three researchers contributed to manual dendritic spine labeling with Neurolucida software and were blinded to treatment group. Neuron reconstruction procedures were conducted in increments of 5 neurons per 4 animals (20 neurons total) with all experimental groups represented (e.g., Vehicle-Silence, Vehicle-Song, Drug-Silence, Drug-Song for acute novel song stimulation experiments and Vehicle, SR, Phenelzine, Phenelzine+SR administered during either development or adulthood for chronic experiments). Spines along each dendrite of each neuron selected for analysis were identified with virtual marks using the Neurolucida software. Dendrites were then traced to determine length, and spine density for each dendrite calculated. Due to the variable number of dendrites per neuron, a variable number of neuron-dependent spine density measures were collected per the five neurons studied per brain region per animal. To account for subjectivity differences across researchers in the manual labeling of spines, raw dendritic spine density data were transformed to percent control for each researcher. This transformation also served to normalize the data.
To assess spine density differences across treatment groups we used a mixed-effects modeling approach and the mixed models procedure using SPSS software (version 22). This method allows problems with lack of independence of multiple measures derived from single animals and neurons to be controlled (Aarts et al., 2014). Thus, this approach is particularly well suited to analyses of neuronal morphological data (Wilson et al., 2017).
For these analyses each animal was used as a random subject, and individual neurons were included as random factors. A variance components covariance structure was used with the maximum likelihood method. Fixed explanatory variables were added to models as described in the results section for each experiment. Improvements to more complicated models gained by addition of successive explanatory variables were determined through likelihood ratio tests of differences between 2/log likelihood values calculated by the SPSS linear mixed model procedure from the fit of each model to spine density data. Through this process, the fit of mixed models to percent control spine density data from each study were optimized. Differences across treatment groups were determined by comparing fixed effects intercepts. Data are reported as the regression intercept +/− standard error with t value and degrees of freedom. Note that the mixed model procedure calculates fractional degrees of freedom. Group differences in degrees of freedom are due to the variable numbers of dendrites counted per neuron.
Each mixed-model analysis used percent control dendritic spine density calculated for each dendrite studied as the dependent variable. For the chronic treatment study, treatment groups (Vehicle, SR, Phenelzine, Phenelzine + SR) were replicated within treatment period (Development vs. Adult) and brain region (Area X vs. HVC). However, each subject only contributed to one combination of these factors. Therefore, treatment groups were added to the mixed model as a fixed factor nested within the fixed factors of treatment period and brain region. For the novel song stimulation study, because treatment groups (Vehicle + silence, Vehicle + song, SR + silence, SR + song, Phenelzine + silence, Phenelzine + song) were replicated within each brain region (Area X, HVC, NCM) and subjects only contributed to only one combination, treatment group was nested within brain region and these were used as fixed factors.
Acknowledgments
This work was supported in part by the National Institutes of Health [Grant DA020109] and the ECU Brody School of Medicine Department of Pharmacology and Toxicology. SR141716A was provided by the NIDA drug supply program. The authors are grateful to Marcoita T. Gilbert for help with the Golgi staining procedure and Julien Dodu and Abigail Taylor for excellent technical assistance with the Neurolucida system.
Abbreviations
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- SR
SR141716A
- MAOI
monoamine oxidase inhibitor
- HVC
used as a proper name
- Area X
Area X of striatum
- DLM
nucleus dorsolateralis anterior, pars medialis
- IMAN
lateral magnocellular nucleus of the anterior nidopallium
- NCM
caudal medial nidopallium
- RA
robust nucleus of the arcopallium
References
- Aarts E, Verhage M, Veenvliet JV, Dolan CV, van der Sluis S. A solution to dependency: using multilevel analysis to accommodate nested data. Nat Neurosci. 2014;17:491–496. doi: 10.1038/nn.3648. http://dx.doi.org/10.1038/nn.3648. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Dwyer JM, Piesla MJ, Platt BJ, Shen R, Rahman Z, Chan K, Manners MT, Samad TA, Kennedy JD, Bingham B, Whiteside GT. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol Dis. 2010;39:148–155. doi: 10.1016/j.nbd.2010.03.020. http://dx.doi.org/10.1016/j.nbd.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat. I Qualitative analysis. J Neurocytol. 1983;12:599–616. doi: 10.1007/BF01181526. [DOI] [PubMed] [Google Scholar]
- Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. 2012;22:383–388. doi: 10.1016/j.conb.2011.09.002. http://dx.doi.org/10.1016/j.conb.2011.09.002. Synaptic structure and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. http://dx.doi.org/10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien MLAV, Ariansen JL, Aragona BJ, Phillips PEM, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lu J, Zuo Y. Spatiotemporal dynamics of dendritic spines in the living brain. Front Neuroanat. 2014;8:28. doi: 10.3389/fnana.2014.00028. http://dx.doi.org/10.3389/fnana.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet Lond Engl. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. http://dx.doi.org/10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- De Felipe J, Marco P, Fairén A, Jones EG. Inhibitory synaptogenesis in mouse somatosensory cortex. Cereb Cortex N Y N. 1997;1991(7):619–634. doi: 10.1093/cercor/7.7.619. [DOI] [PubMed] [Google Scholar]
- Ding L, Perkel DJ. Dopamine modulates excitability of spiny neurons in the avian basal ganglia. J Neurosci. 2002;22:5210–5218. doi: 10.1523/JNEUROSCI.22-12-05210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Perkel DJ. Two tales of how expectation of reward modulates behavior. Curr Opin Neurobiol. 2014;29:142–147. doi: 10.1016/j.conb.2014.07.011. http://dx.doi.org/10.1016/j.conb.2014.07.011. SI. Neuromodulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. http://dx.doi.org/10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Elphick MR. The evolution and comparative neurobiology of endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2012;367:3201–3215. doi: 10.1098/rstb.2011.0394. http://dx.doi.org/10.1098/rstb.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Hernández M, Ramos JA. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:e72–e91. doi: 10.1111/j.1755-5949.2010.00144.x. http://dx.doi.org/10.1111/j.1755-5949.2010.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffuri AL, Ladarre D, Lenkei Z. Type-1 cannabinoid receptor signaling in neuronal development. Pharmacology. 2012;90:19–39. doi: 10.1159/000339075. http://dx.doi.org/10.1159/000339075. [DOI] [PubMed] [Google Scholar]
- Gamble-George JC, Conger JR, Hartley ND, Gupta P, Sumislawski JJ, Patel S. Dissociable effects of CB1 receptor blockade on anxiety-like and consummatory behaviors in the novelty-induced hypophagia test in mice. Psychopharmacology. 2013;228:401–409. doi: 10.1007/s00213-013-3042-8. http://dx.doi.org/10.1007/s00213-013-3042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MT, Soderstrom K. Late-postnatal cannabinoid exposure persistently elevates dendritic spine densities in area X and HVC song regions of zebra finch telencephalon. Brain Res. 2011;1405:23–30. doi: 10.1016/j.brainres.2011.06.019. http://dx.doi.org/10.1016/j.brainres.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MT, Soderstrom K. Novel song-stimulated dendritic spine formation and Arc/Arg3.1 expression in zebra finch auditory telencephalon are disrupted by cannabinoid agonism. Brain Res. 2013;1541:9–21. doi: 10.1016/j.brainres.2013.10.012. http://dx.doi.org/10.1016/j.brainres.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MT, Soderstrom K. Developmental but not adult cannabinoid treatments persistently alter axonal and dendritic morphology within brain regions important for zebra finch vocal learning. Brain Res. 2014;1558:57–73. doi: 10.1016/j.brainres.2014.02.039. http://dx.doi.org/10.1016/j.brainres.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dentritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. http://dx.doi.org/10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Horder J, Cowen PJ, Di Simplicio M, Browning M, Harmer CJ. Acute administration of the cannabinoid CB1 antagonist rimonabant impairs positive affective memory in healthy volunteers. Psychopharmacology. 2009;205:85–91. doi: 10.1007/s00213-009-1517-4. http://dx.doi.org/10.1007/s00213-009-1517-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Keimpema E, Mackie K, Harkany T. Molecular model of cannabis sensitivity in developing neuronal circuits. Trends Pharmacol Sci. 2011;32:551–561. doi: 10.1016/j.tips.2011.05.004. http://dx.doi.org/10.1016/j.tips.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DB, Nottebohm F. Projections of a telencephalic auditory nucleus–field L–in the canary. J Comp Neurol. 1979;183:455–469. doi: 10.1002/cne.901830302. http://dx.doi.org/10.1002/cne.901830302. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with Delta-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse N Y N. 2006;60:429–436. doi: 10.1002/syn.20313. http://dx.doi.org/10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- Lee TTY, Hill MN, Hillard CJ, Gorzalka BB. Disruption of periadolescent endocannabinoid signaling modulates adult neuroendocrine and behavioral responses to stress in male rats. Neuropharmacology. 2015;99:89–97. doi: 10.1016/j.neuropharm.2015.07.021. http://dx.doi.org/10.1016/j.neuropharm.2015.07.021. [DOI] [PubMed] [Google Scholar]
- Lee TTY, Hill MN, Lee FS. Developmental regulation of fear learning and anxiety behavior by endocannabinoids. Genes Brain Behav. 2016;15:108–124. doi: 10.1111/gbb.12253. http://dx.doi.org/10.1111/gbb.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105–1116. doi: 10.1016/j.neuropharm.2005.03.016. http://dx.doi.org/10.1016/j.neuropharm.2005.03.016. Future Directions in Cannabinoid Therapeutics: “From the Bench to the Clinic”. [DOI] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y, Las L, Denisenko N, Fee MS. A role for descending auditory cortical projections in songbird vocal learning. eLife. 2014:3. doi: 10.7554/eLife.02152. http://dx.doi.org/10.7554/eLife.02152. [DOI] [PMC free article] [PubMed]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, Hill MN. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41:80–102. doi: 10.1038/npp.2015.166. http://dx.doi.org/10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler BE, Wallhäusser-Franke E, DeVoogd TJ. Regressive development in neuronal structure during song learning in birds. J Neurobiol. 1995;27:204–215. doi: 10.1002/neu.480270207. http://dx.doi.org/10.1002/neu.480270207. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Altered dendritic spine density in animal models of depression and in response to antidepressant treatment. Synapse N Y N. 2001;42:151–163. doi: 10.1002/syn.10006. http://dx.doi.org/10.1002/syn.10006. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. http://dx.doi.org/10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Cheer JF. A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harbor Perspect Med. 2012:2. doi: 10.1101/cshperspect.a012229. http://dx.doi.org/10.1101/cshperspect.a012229. [DOI] [PMC free article] [PubMed]
- Perkel DJ. Origin of the anterior forebrain pathway. Ann N Y Acad Sci. 2004;1016:736–748. doi: 10.1196/annals.1298.039. http://dx.doi.org/10.1196/annals.1298.039. [DOI] [PubMed] [Google Scholar]
- Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM. Dendritic spines in depression: what we learned from animal models. Neural Plast. 2016;2016:e8056370. doi: 10.1155/2016/8056370. http://dx.doi.org/10.1155/2016/8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Ball GF. Seasonal changes in the densities of alpha(2) noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. J Comp Neurol. 2002;444:63–74. doi: 10.1002/cne.10131. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, Guidali C, Pinter M, Sala M, Bartesaghi R, Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. http://dx.doi.org/10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Salvante KG, Racke DM, Campbell CR, Sockman KW. Plasticity in singing effort and its relationship with monoamine metabolism in the songbird telencephalon. Dev Neurobiol. 2010;70:41–57. doi: 10.1002/dneu.20752. http://dx.doi.org/10.1002/dneu.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. http://dx.doi.org/10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Johnson F. Zebra finch CB1 cannabinoid receptor: pharmacology and in vivo and in vitro effects of activation. J Pharmacol Exp Ther. 2001;297:189–197. [PubMed] [Google Scholar]
- Soderstrom K, Johnson F. Cannabinoid exposure alters learning of zebra finch vocal patterns. Dev Brain Res. 2003;142:215–217. doi: 10.1016/s0165-3806(03)00061-0. http://dx.doi.org/10.1016/S0165-3806(03)00061-0. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q. Distinct periods of cannabinoid sensitivity during zebra finch vocal development. Brain Res Dev Brain Res. 2004;153:225–232. doi: 10.1016/j.devbrainres.2004.09.002. http://dx.doi.org/10.1016/j.devbrainres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q. Developmental pattern of CB1 cannabinoid receptor immunoreactivity in brain regions important to zebra finch (Taeniopygia guttata) song learning and control. J Comp Neurol. 2006;496:739–758. doi: 10.1002/cne.20963. http://dx.doi.org/10.1002/cne.20963. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Poklis JL, Lichtman AH. Cannabinoid exposure during zebra finch sensorimotor vocal learning persistently alters expression of endocannabinoid signaling elements and acute agonist responsiveness. BMC Neurosci. 2011;12:3. doi: 10.1186/1471-2202-12-3. http://dx.doi.org/10.1186/1471-2202-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Spiga S, Lintas A, Diana M. Altered mesolimbic dopamine system in THC dependence. Curr Neuropharmacol. 2011;9:200–204. doi: 10.2174/157015911795017083. http://dx.doi.org/10.2174/157015911795017083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SA, Butler P, Munafò MR, Nutt DJ, Robinson ES. A translational rodent assay of affective biases in depression and antidepressant therapy. Neuropsychopharmacology. 2013;38:1625–1635. doi: 10.1038/npp.2013.69. http://dx.doi.org/10.1038/npp.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia M, Dominguez A, Zhang W, Del Puerto A, Ciorraga M, Benitez MJ, Guaza C, Garrido JJ. Cannabinoid receptors modulate neuronal morphology and AnkyrinG density at the axon initial segment. Front Cell Neurosci. 2017;11:5. doi: 10.3389/fncel.2017.00005. http://dx.doi.org/10.3389/fncel.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata) J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. http://dx.doi.org/10.1002/(SICI)1096-9861(19960318)366:4//613::AID-CNE5//3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Velho TAF, Lu K, Ribeiro S, Pinaud R, Vicario D, Mello CV. Noradrenergic control of gene expression and long-term neuronal adaptation evoked by learned vocalizations in songbirds. PLoS One. 2012:7. doi: 10.1371/journal.pone.0036276. http://dx.doi.org/10.1371/journal.pone.0036276. [DOI] [PMC free article] [PubMed]
- Wallhäusser-Franke E, Nixdorf-Bergweiler BE, DeVoogd TJ. Song isolation is associated with maintaining high spine frequencies on zebra finch 1MAN neurons. Neurobiol Learn Mem. 1995;64:25–35. doi: 10.1006/nlme.1995.1041. [DOI] [PubMed] [Google Scholar]
- Whitney O, Soderstrom K, Johnson F. CB1 cannabinoid receptor activation inhibits a neural correlate of song recognition in an auditory/perceptual region of the zebra finch telencephalon. J Neurobiol. 2003;56:266–274. doi: 10.1002/neu.10233. http://dx.doi.org/10.1002/neu.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MD, Sethi S, Lein PJ, Keil KP. Valid statistical approaches for analyzing sholl data: mixed effects versus simple linear models. J Neurosci Methods. 2017;279:33–43. doi: 10.1016/j.jneumeth.2017.01.003. http://dx.doi.org/10.1016/j.jneumeth.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara S, Yazaki-Sugiyama Y. Auditory experience-dependent cortical circuit shaping for memory formation in bird song learning. Nat Commun. 2016;7:11946. doi: 10.1038/ncomms11946. http://dx.doi.org/10.1038/ncomms11946. [DOI] [PMC free article] [PubMed] [Google Scholar]