Abstract
Here we evaluate the potential for local administration of a small molecule FOLH1/GCPII inhibitor 2-phosphonomethyl pentanedioic acid (2-PMPA) as a novel treatment for inflammatory bowel disease (IBD). We found that FOLH1/GCPII enzyme activity was increased in the colorectal tissues of mice with TNBS-induced colitis, and confirmed that 2-PMPA inhibited FOLH1/GCPII enzyme activity ex vivo. In order to maximize local enema delivery of 2-PMPA, we studied the effect of vehicle tonicity on the absorption of 2-PMPA in the colon. Local administration of 2-PMPA in a hypotonic enema vehicle resulted in increased colorectal tissue absorption at 30 min compared to 2-PMPA administered in an isotonic enema vehicle. Furthermore, local delivery of 2-PMPA in hypotonic enema vehicle resulted in prolonged drug concentrations for at least 24 h with minimal systemic exposure. Finally, daily treatment with the hypotonic 2-PMPA enema ameliorated macroscopic and microscopic symptoms of IBD in the TNBS-induced colitis mouse model, indicating the potential of FOLH1/GCPII inhibitors for the local treatment of IBD.
Keywords: 2-PMPA, Colitis, TNBS, Hypotonic, Folate polyglutamate, N-acetyl-aspartyl glutamate
1. Introduction
Inflammatory bowel disease (IBD) is an idiopathic, chronic inflammatory disorder of the gastrointestinal (GI) tract which has two subtypes: Crohn’s disease (CD) and ulcerative colitis (UC), each accounting for approximately 50% of IBD patients [1–3]. It is clear that IBD is a complex multifactorial disease [1–4], yet the precise etiology of the mucosal dysregulation and inflammation remains elusive [4]. Despite various small molecule and biologic therapeutic options available for the management of IBD, approximately one-third of IBD patients do not respond to any given therapy [5]. This emphasizes the significance of exploring and identifying therapies with novel therapeutic targets for patients with IBD.
Recent work found that the expression of the FOLH1 gene is dramatically increased in human IBD [6–8]. FOLH1 encodes a transmembrane glycoprotein that acts as a glutamate carboxypeptidase. In the GI tract, the enzyme is called folate hydrolase and is found on brush border membranes. In the brain and peripheral nervous system, the enzyme is referred to as glutamate carboxypeptidase II (GCPII), and multiple classes of potent and selective small molecule GCPII inhibitors have been synthesized [9–16]. We recently described the use of one of these potent, small molecule FOLH1/GCPII inhibitors, 2-phosphonomethyl pentanedioic acid (2-PMPA), in preclinical animal models of IBD [17]. Based on gene-profiling analyses that showed upregulation of FOLH1 gene in the affected intestinal mucosa of patients with Crohn’s disease, we quantified the activity of FOLH1/GCPII enzyme in normal and diseased mucosa from 20 subjects including healthy controls and patients with IBD [17]. We found significant increase in FOLH1/GCPII activity in these patient samples and similar trend was observed in pre-clinical animal models of IBD such as dextran sodium sulfate (DSS)-induced colitis model as well as IL10−/− model of spontaneous colitis. We also found that FOLH1−/− mice have decreased susceptibility to DSS-induced colitis compared to wild-type mice [17]. We further demonstrated that 2-PMPA inhibited FOLH1/GCPII enzymatic activity, and that daily intraperitoneal (i.p.) administration of 100 mg/kg 2-PMPA ameliorated symptoms of colitis in both the IL10−/− spontaneous colitis model and the DSS-induced mouse model of colitis [6]. However, given that FOLH1/GCPII enzyme is a transmembrane protein, and that staining of diseased patient samples confirmed elevated expression of the protein in the villous epithelium [6], we wanted to explore the effect of local rather than systemic drug dosing.
For two-third of UC patients and for Crohn’s patients that have diffuse disease that also affects the distal colorectum, topical enema therapy is used [18,19]. Enema products are considered more efficacious and associated with lower side effects than oral formulations of other common IBD drugs, such as steroids [20,21]. Further, combining local enema therapy with oral therapy is considered more efficacious than oral therapy alone [18,22]. This is perhaps not surprising, given that enema formulations have the potential to directly access diseased tissues in the distal colorectum and locally deliver high amounts of drug. However, achieving adequate drug distribution in the colorectum is challenging due to the fact that the colorectal epithelium is highly folded and collapsed [23]. To achieve highly uniform drug delivery in the colorectum, we explored the administration of water soluble 2-PMPA in a hypotonic vehicle solution, an approach for modifying the enema vehicle composition that resulted in improved colorectal drug distribution and delivery in our previous study [23].
In order to evaluate the pre-clinical relevance of local FOLH1/GCPII inhibition for treatment of IBD via enema, we chose to use the 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis model. Here, we describe that FOLH1/GCPII activity is also increased in the TNBS model, and this activity is inhibited by 2-PMPA ex vivo. We further evaluate the pharmacokinetics of 2-PMPA upon enema administration, demonstrating that administration in a hypotonic enema vehicle leads to prolonged colorectal tissue levels with minimal systemic exposure. Finally, we demonstrate that local enema administration of 2-PMPA at 1/8th of the dose (12.5 mg/kg) than what was previously tested for i.p. administration (100 mg/kg) provides substantial improvement in disease symptoms in the mouse model of TNBS-induced colitis, further emphasizing the potential for FOLH1/GCPII inhibition as a novel therapeutic option for treating IBD.
2. Materials and methods
2.1. Materials
2-PMPA was synthesized by our laboratory using methods reported previously [24]. LC-MS grade acetonitrile and water with 0.1% formic acid were obtained from Fisher Scientific (Hanover Park, IL). Sodium hydroxide (AR grade), sodium chloride (AR grade), absolute ethanol and 5% w/v 2,4,6-trinitrobenzenesulfonic acid (TNBS) solution were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. 2-PMPA enema formulation
For the hypotonic formulation, 2-PMPA powder at 2.5 mg/mL concentration was dissolved in water and titrated to pH 7.0 ± 0.1 using 3.5 M sodium hydroxide (NaOH). We previously found that, similar to what was observed in the human colorectum [25], sodium-driven water absorption continued to occur in the mouse colorectum for enema vehicles above the conventional isoosmolar point and up to 400–500 mOsm/kg [23]. Thus, our isotonic vehicle contained 2.5 mg/mL 2-PMPA dissolved in 1.32% w/v sodium chloride and titrated to pH 7.0 ± 0.1 using 3.5 M NaOH (final osmolality of 450 ± 5 mOsm/kg).
2.3. 2,4,6-Trinitrobenzenesulfonic acid (TNBS)-induced colitis model
All experimental procedures were approved by the Johns Hopkins Animal Care and Use Committee. Male 5–6 week old Balb/c mice were purchased from Harlan (Indianapolis, IN) and acclimated in the animal facility for one week. Colitis was induced using our optimized methods for uniform enema administration in order to minimize variability in disease severity. Briefly, the mice were transferred to cages and then food pellets and water bottles were removed from cages to starve the mice for 6 h. The cages used for the starving mice also had stainless steel platform to prevent the mice from eating bedding in the cage. After 6 h of starving, mice received a 200 μL normal saline enema using a flexible feeding tube to clear the remaining pellets in the distal 3–4 cm of the colorectum. Ten minutes after saline enema administration, the mice were placed under anesthesia using an isoflurane vaporizer system to colorectally administer 50 μL of TNBS (2.5% w/v TNBS in 50% ethanol) using a fire-polished capillary tube (Wiretrol). The mice were then held by the tail upside down for 15 s while they recovered from the anesthesia to ensure minimal TNBS expulsion from the anus (Day 0, D0). These procedures resulted in similar average weight reduction in the treatment and control groups within 24 h after TNBS administration (Day 1, D1).
2.4. FOLH1/GCPII enzyme activity assay
For the “healthy” group (n = 4), mice received no pretreatments. For the “IBD” group (n = 4), TNBS induction was performed in mice as described above. Mice with a decrease in body weight of at least 7% on D1 were used for the assay, and the distal 4 cm of the colorectal tissues were obtained on Day 4 (D4). For all tissues, the luminal contents were cleared and the tissues were snap frozen in liquid nitrogen. FOLH1/GCPII activity in the samples was measured using a previously described radioenzymatic assay by our laboratory [26,27]. In brief, colon tissues were weighed and immersed in 0.5 mL of ice-cold 50 mM Tris buffer (pH 7.7 at room temperature). Each tissue was sonicated for 30–60 s using a Kontes ultrasonic cell disrupter. After a 2 min spin at 13,000 g, supernatants were analyzed for protein content (DC Protein Assay Kit; Bio Rad) and GCPII activity.
2.5. Pharmacokinetics (PK) of 2-PMPA following local administration in mice with TNBS-induced colitis
Colitis was induced in mice using TNBS as described above. Mice with a decrease in body weight of at least 7% were used for PK studies. To determine the effect of vehicle tonicity on immediate drug absorption into the colon, mice were then divided into two groups of n = 13 each. Each group received a 100 μL enema containing 2.5 mg/mL 2-PMPA in the hypotonic vehicle or 2.5 mg/mL 2-PMPA in the isotonic sodium-based vehicle. Thirty minutes after enema administration, colorectal tissue was collected and weighed for drug determination. To determine the longer term kinetics of hypotonically administered 2-PMPA in the colorectum and in systemic circulation, mice with TNBS-induced colitis were divided into different groups (n = 3 per group) and 100 μL of hypotonic 2.5 mg/mL 2-PMPA enema was administered. At specified time points after enema administration (30 min, 2 h, 6 h, 24 h), whole blood and colorectal tissue were collected. Blood was collected in EDTA coated tubes and centrifuged at 1300g for 10 min to collect plasma for drug determinations. The distal 2.5 cm of the colorectal tissues was collected and weighed. All samples were frozen and stored at −80 °C until drug concentration determination.
2.6. Bioanalysis of 2-PMPA in plasma and colon samples
Concentrations of 2-PMPA in plasma and colon mucosa were determined via HPLC with tandem mass spectrometry LC-MS/MS as we have previously described [28]. Briefly, 2-PMPA was extracted from plasma and tissue by protein precipitation with 6× methanol containing 2-(phosphonomethyl) succinic acid (2-PMSA; 1 μM) as an internal standard. The samples were vortexed (30 s) and centrifuged (10,000g for 10 min). Supernatants were dried under a gentle stream of nitrogen at 45 °C and the residue reconstituted with 100 μL of acetonitrile. 50 μL of the derivatizing agent N-tert-Butyldimethysilyl-N-methyltrifluoroacetamide (MTBSTFA) was added and samples heated at ~60 °C for 40 min. At the end of 40 min, the derivatized samples were analyzed via LC-MS/MS. Chromatographic analysis was performed using an Accela™ ultra high-performance system consisting of an analytical pump, and an autosampler coupled with TSQ Vantage mass spectrometer (Thermo Fisher Scientific Inc., Waltham MA).
2.7. Efficacy experiment
TNBS induction was performed in mice as described above. Starting on D1, mice received a 100 μL enema containing either 2.5 mg/mL 2-PMPA in the hypotonic vehicle (n = 12) or an enema containing phosphate buffered saline (PBS) (Untreated, n = 15). On Day 2 (D2) and Day 3 (D3), mice were weighed and given enema treatments for a total of 3 daily treatments. Twenty-four hours after the final treatment (Day 4, D4), mice were weighed and given a 200 μL normal saline enema using a flexible feeding tube to clear the remaining pellets in the distal 3–4 cm of the colorectum prior to excising colorectal tissues. A standard length of 4 cm of colorectal tissue were obtained from each mouse, photographed, weighed, and portioned for subsequent analyses.
2.8. Histology
The distal 2 cm portions of the colorectum (the portion where disease was typically most severe) were cut into 3 smaller segments and fixed in 10% neutral buffered formalin, then processed to paraffin routinely by the JHU Reference Histology lab. The 3 tissue segments were embedded in the same block to ensure obtaining tissue sections throughout the distal colorectum. The tissues were sectioned transversely with 3 step sections cut at approximately 200 μm between sections and stained with hematoxylin and eosin (H&E). A slide representative of the most diseased areas was then scored. Each colorectal tissue sample had 2–3 sections per slide that could be scored (1 mouse from the untreated control group was omitted due to having only 1 tissue section that could be scored). Scoring was performed in a blinded fashion by a board certified veterinary pathologist in the pathology core at Johns Hopkins University using a scoring system previously described for the TNBS model [29]. Briefly, tissues are assigned a score in the range of 0–4, where each grade is a composite measure of inflammatory cell infiltration and mucosal damage: Grade 0 No evidence of inflammation; Grade 1 Low level of leukocyte infiltration with infiltration seen in <10% HPF. No structural changes; Grade 2 Moderate leukocyte infiltration with infiltration seen in 10% to 25% HPF, crypt elongation, bowel wall thickening that does not extend beyond the mucosal layer, and no ulcerations; Grade 3 High level of leukocyte infiltration seen in 25% to 50% HPF, crypt elongation, infiltration beyond the mucosal layer, thickening of the bowel wall and superficial ulcerations; Grade 4 Marked degree of transmural leukocyte infiltration seen in >50% HPF, elongated and distorted crypts, bowel-wall thickening, and extensive ulcerations. Scores for individual tissue slices were averaged for each animal, and then these scores were averaged over the entire group of animals.
2.9. Statistics
The Student’s t-test (two-tailed, unequal variance) was used to compare differences between the two experimental groups. For histological scoring, the non-parametric Mann-Whitney test was used. P values < 0.05 were considered statistically significant for all data.
3. Results
3.1. Increased FOLH1/GCPII enzymatic activity in colorectal tissues from TNBS mice
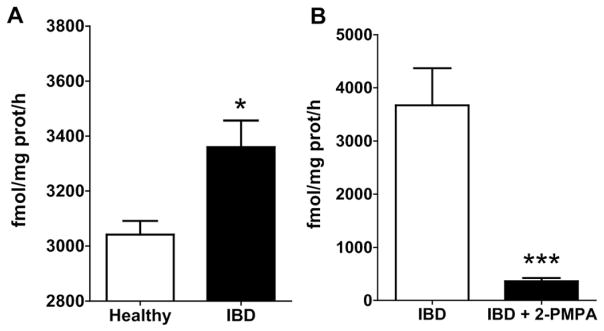
We first examined whether FOLH1/GCPII activity was increased in colorectal tissues from mice with TNBS-induced colitis. We found that FOLH1/GCPII activity was significantly increased (1.1-fold increase in FOLH1/GCPII activity; p < 0.05) in colorectal tissues collected on Day 4 after TNBS induction (Fig. 1A). Further, we found that the enzymatic activity in the colon tissue was >90% inhibited ex vivo by 1 μM 2-PMPA (p < 0.0001) (Fig. 1B).
Fig. 1.
(A) FOLH1/GCPII activity was significantly elevated in colon tissue obtained from mice with TNBS-induced colitis compared to healthy mice (n = 4 mice per group). (B) FOLH1/GCPII activity in colon tissue (n ≥ 8 colon tissues per group) obtained from mice with TNBS-induced colitis was significantly inhibited (>90% inhibition) after ex vivo treatment with 1 μM 2-PMPA. *p < 0.05, ***p < 0.0001).
3.2. Enema administration leads to high local colorectal tissue levels of 2-PMPA with minimal systemic exposure
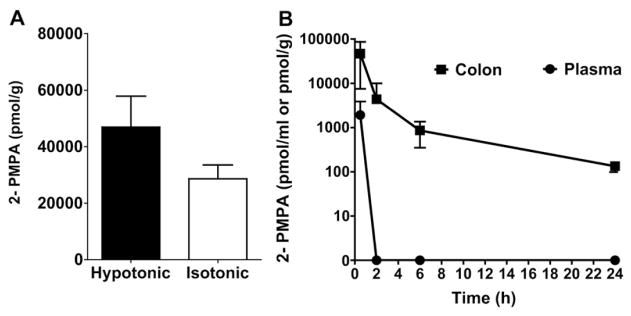
Having demonstrated that FOLH1/GCPII activity is increased in the colorectal tissues of mice with TNBS-induced colitis, we next evaluated the pharmacokinetics of 2-PMPA administered by enema to mice with TNBS-induced colitis. Having previously observed that hypotonic enema solutions provide improved colorectal drug distribution and absorption [23,30], we first tested whether administering 2-PMPA hypotonically would lead to increased colorectal tissue levels in mice with TNBS-induced colitis. Indeed, we found that at 30 mins after enema administration, the hypotonic vehicle provided a 1.6-fold increase in colorectal tissue 2-PMPA levels compared to the isotonic vehicle (Fig. 2A), though the difference was not statistically significant due to the inherent variability in the TNBS model. We then characterized the kinetics of 2-PMPA in the colorectal tissue and the extent of systemic absorption over 24 h after hypotonic enema administration (Fig. 2B). Despite the hypotonically-driven absorption in the colorectum, the systemic exposure of 2-PMPA was minimal, and 2-PMPA was not detectable in the plasma by 2 h (Fig. 2B). However, the colorectal tissue concentrations remained above what was previously shown to be effective after high concentration i.p. dosing [17] at 24 h, which indicated that daily enema dosing was a feasible strategy.
Fig. 2.
(A) Effect of tonicity of 2-PMPA enema on absorption of 2-PMPA in colon tissue of mice with TNBS-induced colitis (n = 13 mice per group). 30 min after enema administration, the hypotonic vehicle provided a 1.6-fold increase in colorectal tissue 2-PMPA levels compared to the isotonic vehicle, though the difference was not statistically significant. (B) Local tissue and systemic absorption of 2-PMPA upon rectal administration of hypotonic 2-PMPA enema (n = 3 mice per time point). 2-PMPA showed prolonged tissue exposure for at least 24 h, whereas systemic exposure was low. 2-PMPA was rapidly cleared from the systemic circulation.
3.3. Daily enema hypotonic administration of 2-PMPA ameliorates disease symptoms in the TNBS-induced colitis model
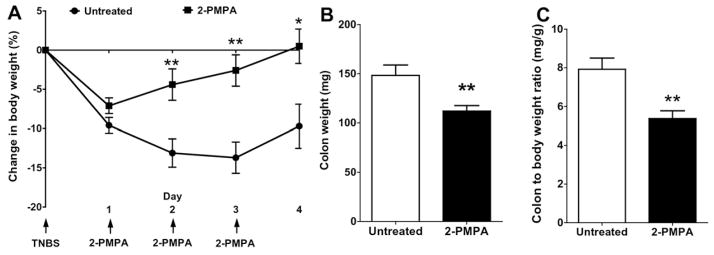
Having demonstrated that 2-PMPA potently inhibits the increased FOLH1 activity observed in the colorectal tissues of mice with TNBS-induced colitis and that hypotonic enema administration leads to high local tissue levels of 2-PMPA for at least 24 h, we evaluated daily 2-PMPA enema dosing for efficacy in the TNBS-induced colitis model. As shown in Fig. 3A, after just 1 enema dose occurring on D1, the 2-PMPA treated animals began to regain weight they had lost after TNBS induction. In contrast, the untreated control mice continued to lose weight, leading to a significant difference in weight loss at D2 (−4.4 ± 2.0% compared to −13.1 ± 1.8%). With the subsequent treatments on D2 and D3, the 2-PMPA treated mice continued to recover lost weight and reached their starting weight by D4 (Fig. 3A). In contrast, the untreated control group maintained an average of ~10% weight loss on D4 (−9.7 ± 2.8%), not showing any recovery compared to D1 (−9.6 ± 1.0%).
Fig. 3.
(A) Mice treated with hypotonic 2-PMPA enema (n = 12 mice) showed a significant recovery in body weight compared to PBS treated controls (Untreated; n = 15 mice) even after a single dose of 2-PMPA. Mice continued to make significant weight gains over the following treatments. Mice treated with 2-PMPA also had significantly reduced (B) colon weight and (C) colon/body weight ratio compared to saline enema treated control mice *p < 0.05, **p < 0.01.
The mucosal damage in the TNBS model is associated with increased colon thickness and weight [31]. As shown in Fig. 3B, the recovery in weight loss from 2-PMPA enema treatment was also associated with a decrease in colon weight (112 ± 5 mg) compared to untreated control mice (148 ± 11 mg), reflecting a reversal of the mucosal inflammation by D4. And thus, the ratio of colon to body weight was also significantly reduced for mice that received hypotonic 2-PMPA enema (5.4 ± 0.4 mg/g) compared to untreated controls (7.9 ± 0.6 mg/g) (Fig. 3C).
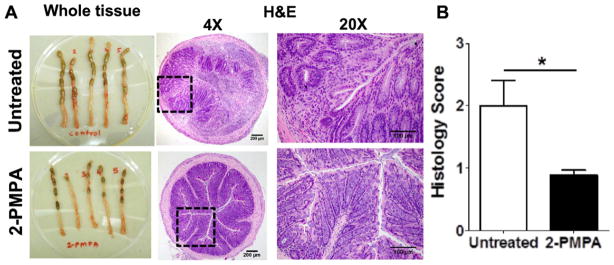
The amelioration of colorectal inflammation provided by 2-PMPA enema administration in the TNBS mice is further characterized in Fig. 4. Fig. 4A shows photographic images of the distal 4 cm of the colorectum of mice dosed daily with PBS (Untreated) or with 2-PMPA hypotonic enema (2-PMPA) and a representative image of a histological tissue section from each group (Fig. 4B). The colorectal tissues from untreated TNBS mice showed hypertrophy/thickening and malformed stools, which was not evident in the 2-PMPA treated mice. The histological images further illustrate the difference in tissue thickness and inflammation (Fig. 4B). Colorectal tissue sections from untreated TNBS mice showed typical signs of disease in this model, including alterations in epithelial structure, infiltration of neutrophils, and increase in tissue cross-sectional thickness. In contrast, the colorectal tissue sections from TNBS mice treated with 2-PMPA enemas showed much less signs of inflammation and tissue thickening. These differences were further confirmed by blinded scoring of the histological sections, showing a significant reduction in the inflammation/mucosal damage score after 2-PMPA treatment (Fig. 4C).
Fig. 4.
(A) In contrast to the colons of mice treated with hypotonic 2-PMPA enema, colons of saline treated control mice had extensive hyperplasia and contained malformed stools. H&E stained colon sections at lower (magnification: 4×; scale bar = 200 μm) and higher (magnification: 20×; scale bar = 100 μm) magnification, further revealed local alterations of cellular structure, neutrophil infiltration, and tissue hyperplasia in untreated control mice. (B) Quantitative histopathological scoring (n = 7 mice per group) based on tissue hyperplasia, destruction of cellular architecture, neutrophil infiltration and inflammation also showed a significant reduction in disease with hypotonic 2-PMPA enema treatment compared to untreated mice. *p < 0.05.
4. Discussion
We previously found that daily i.p. administration of 100 mg/kg 2-PMPA, a FOLH1/GCPII inhibitor, was effective in treating experimental colitis in both the IL10−/− spontaneous colitis model and the DSS-induced mouse model of colitis [17]. However, given the increase in FOLH1/GCPII activity we observed in tissue biopsies from human IBD patients [17] and the protein expression observed in the villous epithelium [6], we wanted to evaluate local delivery and inhibition of FOLH1/GCPII as a therapeutic strategy for treating IBD. Enema administration results in much higher local tissue drug levels and reduced systemic exposure, and thus is more effective for IBD patients with disease affecting the distal colorectum [18,19,32]. Further, reduced systemic exposure was beneficial in the context of our goal of examining the role of local luminal enzymatic inhibition in the GI tract. Thus, we tested daily enema administration of 2-PMPA in the TNBS-induced colitis model, which affects only the distal colorectum that is contacted by enema fluid. Importantly, we found that FOLH1/GCPII enzymatic activity was increased in the colorectal tissue of mice with TNBS-induced colitis, and this activity was potently inhibited by 2-PMPA, further validating the potential of local delivery and also target engagement in the colorectal tissue. Our recent study has also shown that FOLH1−/− mice were less susceptible to DSS-induced colitis compared to wild-type mice [17]. Furthermore, we observed a marked amelioration of macroscopic and microscopic symptoms in murine TNBS-induced colitis model with daily hypotonic 2-PMPA enema administration, suggesting that local FOLH1/GCPII inhibition may be a new target for the treatment of IBD. However, the exact mechanism and downstream effects of FOLH1/GCPII inhibition in colorectal tissue are yet to be elucidated.
The human colon dries the feces, a process driven by active ion transport, absorbing 1.4–1.8 L of water per day [33]. We previously demonstrated that this robust physiological mechanism can be used to provide more effective colorectal drug and nanoparticle delivery in combination with a hypotonic enema vehicle [23]. Similarly, we found here that there was increased colorectal tissue absorption of 2-PMPA in mice with TNBS-induced colitis when dosed with a hypotonic, water absorption-inducing vehicle. However, it may be possible to further improve the absorption and duration of efficacious 2-PMPA tissue concentrations by loading into a muco-inert nanoparticle [30,34], which is the focus of future work. It may also be possible to further tune the ionic composition of the enema vehicle to further maximize local tissue absorption and minimize systemic absorption [23]. Moreover, since oral administration is a preferred method of administration, particularly for maintenance therapy, it is also of interest whether oral administration can lead to effective FOLH1/GCPII inhibition throughout the GI tract. Such an oral therapy may be useful for treating both CD and UC, since FOLH1/GCPII activity was found to be increased in human tissue biopsies from patients with both diseases [17].
Recently, we demonstrated significant upregulation of GCPII in activated microglial cells, and GCPII inhibition led to prevention of neurological damage [35]. Microglial cells belong to the mononuclear phagocyte system and form the resident macrophages in the brain tissue, the spinal cord and the retina. The activated macrophages, key producers of many cytokines (e.g. IL-1β, IL-6, and TNFα) and reactive metabolites of oxygen and nitrogen (e.g. nitric oxide), have also been implicated in the pathogenesis of a variety of chronic and autoimmune diseases including IBD [36]. Our future studies will include assessment of FOLH1/GCPII expression on the intestinal and colorectal macrophages and examination of the downstream effects of FOLH1/GCPII inhibition on macrophage-mediated release of inflammatory cytokines in various pre-clinical models of IBD.
The mechanism by which local inhibition of FOLH1/GCPII leads to amelioration of IBD symptoms may involve one or more of its known substrates. One important substrate, N-acetyl-aspartyl glutamate (NAAG), is one of the most-prevalent peptides found in nervous tissues and is involved in NDMA and metabotropic glutamate receptor (mGluR) mediated glutamatergic transmission [37–40]. In the brain and peripheral nervous system, the enzyme GCPII cleaves NAAG to liberate glutamate. Studies have shown that increased glutamate levels are responsible for increased production of inflammatory cytokines in activated T cells in autoimmune diseases [41]. 2-PMPA treatment dampened the function of CD4+ T cells (such as TH1 and TH17), suppressed mGluR1 expression in both periphery and CNS, and reduced the number of mGluR1-positive CD4+ T cells in experimental autoimmune encephalomyelitis [42]. It has been reported that NAAG receptors can be found in the GI tract, though the roles of NAAG or glutamate in the GI tract are not well characterized [43]. However, like other autoimmune disorders, CD4+ T cells (such as TH1, TH2 and TH17) and their production of inflammatory cytokines have a predominant role in the pathogenesis of IBD in human and animal models [3,44]. A second substrate, folate poly-γ-glutamate, is cleaved to liberate folic acid in the GI tract [45]. Increased FOLH1/GCPII activity in IBD could lead to increased folic acid levels in the gut that may augment nucleotide biosynthesis and proliferation of inflammatory cells. Similar to anti-folate drugs that are used for the treatment of IBD, such as methotrexate [46], 2-PMPA may reduce folic acid levels via inhibition of FOLH1/GCPII activity in the GI tract. There may also be some anti-angiogenic effect of FOLH1/GCPII activity in IBD [47].
In summary, we have built upon prior work demonstrating the potential for FOLH1/GCPII inhibition as a novel treatment strategy for IBD. Specifically, we demonstrated that daily local hypotonic enema administration of a potent inhibitor of FOLH1/GCPII enzymatic activity (2-PMPA) ameliorated symptoms of TNBS-induced colitis. Similar to what was observed in human tissue biopsies from IBD patients, FOLH1/GCPII activity was increased in colorectal tissue from mice with TNBS-induced colitis, and the activity was potently inhibited by 2-PMPA. We are currently working to identify relevant substrates and receptors affected by FOLH1/GCPII inhibition in the GI tract, as well as test local inhibition by oral administration. Our findings have important implications toward the development of IBD therapies with a novel mechanism of action.
Acknowledgments
We thank the animal husbandry staff and the Reference Histology lab at Johns Hopkins. We thank Dr. Cory Brayton, Director of the Phenotyping Core at Johns Hopkins, for performing the histological scoring. This work was supported by NIH grants R21/R33AI079740 (LME), R01DK107806 (LME) and R01CA161056 (to BSS) and Sanofi iAWARD (to BSS, RR and LME).
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 4.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton MJ, Snapper SB, Blumberg RS. Update on biologic pathways in inflammatory bowel disease and their therapeutic relevance. J Gastroenterol. 2012;47:1–8. doi: 10.1007/s00535-011-0521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T, Song B, Zhu W, Xu X, Gong QQ, Morando C, Dassopoulos T, Newberry RD, Hunt SR, Li E. An ileal Crohn’s disease gene signature based on whole human genome expression profiles of disease unaffected ileal mucosal biopsies. PLoS One. 2012;7:e37139. doi: 10.1371/journal.pone.0037139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Shachar S, Yanai H, Baram L, Elad H, Meirovithz E, Ofer A, Brazowski E, Tulchinsky H, Pasmanik-Chor M, Dotan I. Gene expression profiles of ileal inflammatory bowel disease correlate with disease phenotype and advance understanding of its immunopathogenesis. Inflamm Bowel Dis. 2013;19:2509–2521. doi: 10.1097/01.MIB.0000437045.26036.00. [DOI] [PubMed] [Google Scholar]
- 8.Noble CL, Abbas AR, Lees CW, Cornelius J, Toy K, Modrusan Z, Clark HF, Arnott ID, Penman ID, Satsangi J, Diehl L. Characterization of intestinal gene expression profiles in Crohn’s disease by genome-wide microarray analysis. Inflamm Bowel Dis. 2010;16:1717–1728. doi: 10.1002/ibd.21263. [DOI] [PubMed] [Google Scholar]
- 9.Nan F, Bzdega T, Pshenichkin S, Wroblewski JT, Wroblewska B, Neale JH, Kozikowski AP. Dual function glutamate-related ligands: discovery of a novel, potent inhibitor of glutamate carboxypeptidase II possessing mGluR3 agonist activity. J Med Chem. 2000;43:772–774. doi: 10.1021/jm9905559. [DOI] [PubMed] [Google Scholar]
- 10.Kozikowski AP, Nan F, Conti P, Zhang J, Ramadan E, Bzdega T, Wroblewska B, Neale JH, Pshenichkin S, Wroblewski JT. Design of remarkably simple, yet potent urea-based inhibitors of glutamate carboxypeptidase II (NAALADase) J Med Chem. 2001;44:298–301. doi: 10.1021/jm000406m. [DOI] [PubMed] [Google Scholar]
- 11.Rong SB, Zhang J, Neale JH, Wroblewski JT, Wang S, Kozikowski AP. Molecular modeling of the interactions of glutamate carboxypeptidase II with its potent NAAG-based inhibitors. J Med Chem. 2002;45:4140–4152. doi: 10.1021/jm010561g. [DOI] [PubMed] [Google Scholar]
- 12.Kozikowski AP, Zhang J, Nan F, Petukhov PA, Grajkowska E, Wroblewski JT, Yamamoto T, Bzdega T, Wroblewska B, Neale JH. Synthesis of urea-based inhibitors as active site probes of glutamate carboxypeptidase II: efficacy as analgesic agents. J Med Chem. 2004;47:1729–1738. doi: 10.1021/jm0306226. [DOI] [PubMed] [Google Scholar]
- 13.Ferraris DV, Shukla K, Tsukamoto T. Structure-activity relationships of glutamate carboxypeptidase II (GCPII) inhibitors. Curr Med Chem. 2012;19:1282–1294. doi: 10.2174/092986712799462658. [DOI] [PubMed] [Google Scholar]
- 14.Stoermer D, Vitharana D, Hin N, Delahanty G, Duvall B, Ferraris DV, Grella BS, Hoover R, Rojas C, Shanholtz MK, Smith KP, Stathis M, Wu Y, Wozniak KM, Slusher BS, Tsukamoto T. Design, synthesis, and pharmacological evaluation of glutamate carboxypeptidase II (GCPII) inhibitors based on thioalkylbenzoic acid scaffolds. J Med Chem. 2012;55:5922–5932. doi: 10.1021/jm300488m. [DOI] [PubMed] [Google Scholar]
- 15.Majer P, Jančařík A, Krečmerová M, Tichý T, Tenora L, Wozniak K, Wu Y, Pommier E, Ferraris D, Rais R, Slusher BS. Discovery of orally available prodrugs of the glutamate carboxypeptidase II (GCPII) inhibitor 2-phosphonomethylpentanedioic acid (2-pmpa) J Med Chem. 2016;59:2810–2819. doi: 10.1021/acs.jmedchem.6b00062. [DOI] [PubMed] [Google Scholar]
- 16.Novakova Z, Wozniak KM, Jancarik A, Rais R, Wu Y, Pavlicek J, Ferraris D, Havlinova B, Ptacek J, Vavra J, Hin N, Rojas C, Majer P, Slusher BS, Tsukamoto T, Barinka C. Unprecedented binding mode of hydroxamate-based inhibitors of glutamate carboxypeptidase II: structural characterization and biological activity. J Med Chem. 2016;59:4539–4550. doi: 10.1021/acs.jmedchem.5b01806. [DOI] [PubMed] [Google Scholar]
- 17.Rais R, Jiang W, Zhai H, Wozniak KM, Stathis M, Hollinger KR, Thomas AG, Rojas C, Vornov JJ, Marohn M, Li X, Slusher BS. FOLH1/GCPII is elevated in IBD patients, and its inhibition ameliorates murine IBD abnormalities. JCI Insight. 2016;1:e88634. doi: 10.1172/jci.insight.88634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen R. Advantages in IBD: current developments in the treatment of inflammatory bowel diseases. Gastroenterol Hepatol. 2010;6:309–316. [PMC free article] [PubMed] [Google Scholar]
- 19.Ham M, Moss AC. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev Clin Pharmacol. 2012;5:113–123. doi: 10.1586/ecp.12.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bautzova T, Rabiskova M, Lamprecht A. Multiparticulate systems containing 5-aminosalicylic acid for the treatment of inflammatory bowel disease. Drug Dev Ind Pharm. 2011;37:1100–1109. doi: 10.3109/03639045.2011.560156. [DOI] [PubMed] [Google Scholar]
- 21.Sherlock ME, Seow CH, Steinhart AH, Griffiths AM. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2010:CD007698. doi: 10.1002/14651858.CD007698.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Probert CS, Dignass AU, Lindgren S, Oudkerk Pool M, Marteau P. Combined oral and rectal mesalazine for the treatment of mild-to-moderately active ulcerative colitis: rapid symptom resolution and improvements in quality of life. J Crohns Colitis. 2014;8:200–207. doi: 10.1016/j.crohns.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Maisel K, Chattopadhyay S, Moench T, Hendrix C, Cone R, Ensign LM, Hanes J. Enema ion compositions for enhancing colorectal drug delivery. J Control Release. 2015;209:280–287. doi: 10.1016/j.jconrel.2015.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson PF, Cole DC, Slusher BS, Stetz SL, Ross LE, Donzanti BA, Trainor DA. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated alpha-linked acidic dipeptidase. J Med Chem. 1996;39:619–622. doi: 10.1021/jm950801q. [DOI] [PubMed] [Google Scholar]
- 25.Billich CO, Levitan R. Effects of sodium concentration and osmolality on water and electrolyte absorption from the intact human colon. J Clin Invest. 1969;48:1336–1347. doi: 10.1172/JCI106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojas C, Frazier ST, Flanary J, Slusher BS. Kinetics and inhibition of glutamate carboxypeptidase II using a microplate assay. Anal Biochem. 2002;310:50–54. doi: 10.1016/s0003-2697(02)00286-5. [DOI] [PubMed] [Google Scholar]
- 27.Robinson MB, Blakely RD, Couto R, Coyle JT. Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate. Identification and characterization of a novel N-acetylated alpha-linked acidic dipeptidase activity from rat brain. J Biol Chem. 1987;262:14498–14506. [PubMed] [Google Scholar]
- 28.Rais R, Rojas C, Wozniak K, Wu Y, Zhao M, Tsukamoto T, Rudek MA, Slusher BS. Bioanalytical method for evaluating the pharmacokinetics of the GCP-II inhibitor 2-phosphonomethyl pentanedioic acid (2-PMPA) J Pharm Biomed Anal. 2013;88:162–169. doi: 10.1016/j.jpba.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheiffele F, Fuss IJ. Induction of TNBS colitis in mice. In: Coligan JE, Bierer B, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. Wiley-VCH Verlag GmbH & Co; KGaA, Weinheim: 2002. (Unit 15.19, 15.19.1–15.19.14) [DOI] [PubMed] [Google Scholar]
- 30.Maisel K, Ensign L, Reddy M, Cone R, Hanes J. Effect of surface chemistry on nano-particle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. J Control Release. 2015;197:48–57. doi: 10.1016/j.jconrel.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 32.Christophi GP, Rengarajan A, Ciorba MA. Rectal budesonide and mesalamine formulations in active ulcerative proctosigmoiditis: efficacy, tolerance, and treatment approach. Clin Exp Gastroenterol. 2016;9:125–130. doi: 10.2147/CEG.S80237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandle GI. Salt and water absorption in the human colon: a modern appraisal. Gut. 1998;43:294–299. doi: 10.1136/gut.43.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ensign LM, Hoen TE, Maisel K, Cone RA, Hanes JS. Enhanced vaginal drug delivery through the use of hypotonic formulations that induce fluid uptake. Biomaterials. 2013;34:6922–6929. doi: 10.1016/j.biomaterials.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Bassam B, Thomas AG, Williams M, Liu J, Nance E, Rojas C, Slusher BS, Kannan S. Maternal inflammation leads to impaired glutamate homeostasis and up-regulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116–128. doi: 10.1016/j.nbd.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slusher BS, Vornov JJ, Thomas AG, Hurn PD, Harukuni I, Bhardwaj A, Traystman RJ, Robinson MB, Britton P, Lu XC, Tortella FC, Wozniak KM, Yudkoff M, Potter BM, Jackson PF. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med. 1999;5:1396–1402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- 38.Khacho P, Wang B, Ahlskog N, Hristova E, Bergeron R. Differential effects of N-acetyl-aspartyl-glutamate on synaptic and extrasynaptic NMDA receptors are subunit-and pH-dependent in the CA1 region of the mouse hippocampus. Neurobiol Dis. 2015;82:580–592. doi: 10.1016/j.nbd.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Neale JH, Pomper MG, Kozikowski AP. NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov. 2005;4:1015–1026. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]
- 40.Neale JH, Olszewski RT, Zuo D, Janczura KJ, Profaci CP, Lavin KM, Madore JC, Bzdega T. Advances in understanding the peptide neurotransmitter NAAG and appearance of a new member of the NAAG neuropeptide family. J Neurochem. 2011;118:490–498. doi: 10.1111/j.1471-4159.2011.07338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganor Y, Levite M. The neurotransmitter glutamate and human T cells: glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. J Neural Transm. 2014;121:983–1006. doi: 10.1007/s00702-014-1167-5. [DOI] [PubMed] [Google Scholar]
- 42.Ha D, Bing SJ, Ahn G, Kim J, Cho J, Kim A, Herath KH, Yu HS, Jo SA, Cho IH, Jee Y. Blocking glutamate carboxypeptidase II inhibits glutamate excitotoxicity and regulates immune responses in experimental autoimmune encephalomyelitis. FEBS J. 2016;283:3438–3456. doi: 10.1111/febs.13816. [DOI] [PubMed] [Google Scholar]
- 43.Julio-Pieper M, Flor PJ, Dinan TG, Cryan JF. Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev. 2011;63:35–58. doi: 10.1124/pr.110.004036. [DOI] [PubMed] [Google Scholar]
- 44.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 45.Navrátil M, Ptáček J, Šácha P, Starková J, Lubkowski J, Bařinka C, Konvalinka J. Structural and biochemical characterization of the folyl-poly-gamma-l-glutamate hydrolyzing activity of human glutamate carboxypeptidase II. FEBS J. 2014;281:3228–3242. doi: 10.1111/febs.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ardizzone S, Cassinotti A, de Franchis R. Immunosuppressive and biologic therapy for ulcerative colitis. Expert Opin Emerg Drugs. 2012;17:449–467. doi: 10.1517/14728214.2012.744820. [DOI] [PubMed] [Google Scholar]
- 47.Conway RE, Rojas C, Alt J, Nováková Z, Richardson SM, Rodrick TC, Fuentes JL, Richardson NH, Attalla J, Stewart S, Fahmy B, Barinka C, Ghosh M, Shapiro LH, Slusher BS. Prostate-specific membrane antigen (PSMA)-mediated laminin proteolysis generates a pro-angiogenic peptide. Angiogenesis. 2016;19:487–500. doi: 10.1007/s10456-016-9521-x. [DOI] [PubMed] [Google Scholar]