Abstract
Little information is available about survival of high-risk pediatric neuroblastoma patients in developing countries. We aimed to assess survival among high-risk pediatric neuroblastoma patients in La Plata, Argentina. Individuals eligible for our cohort were aged <20 years when diagnosed with high-risk neuroblastoma and received cancer-directed therapy including stem cell transplantation at Hospital de Niños Sor Maria Ludovica between February 1999 and February 2015. We estimated overall survival probabilities using an extended Kaplan-Meier approach. Our study population comprised 39 high-risk neuroblastoma patients, of whom 39% were aged >4 years at diagnosis, 54% were male, and 62% had adrenal neuroblastoma. We observed 18 deaths, and the median survival time of our study population was 1.7 years. The 5-year overall survival probability was 24% (95% confidence limits [CL]: 10%, 41%). In contrast, 5-year survival of high-risk neuroblastoma patients ranges between 23% and 76% in developed countries. Survival among high-risk neuroblastoma patients is generally poor regardless of geographic location, but our results illustrate dramatically worse survival for patients in a developing country. We speculate that the observed survival differences could be attenuated or eliminated with improvements in treatment and supportive care, but addressing these issues will require creative solutions because of resource limitations.
Keywords: neuroblastoma, stem cell transplantation, mortality, disparity, developing country
INTRODUCTION
Low- and intermediate-risk neuroblastoma, characterized by favorable stages and age <1 year at time of diagnosis, have 5-year overall survival >90% following chemotherapy and surgical resection. In contrast, 5-year overall survival for high-risk neuroblastoma, characterized by features such as metastasis, age >1 year, and amplified MYCN oncogene, is only 40 – 50% despite intensive treatment protocols that include chemotherapy, surgery, radiation, and stem cell transplantation.(1, 2) The current evidence about survival among high-risk neuroblastoma patients is almost exclusively derived from cohorts in developed countries. Nevertheless, the burden of pediatric neuroblastoma is nearly as high in developing countries such as Argentina as in developed countries such as the United States (age-standardized incidence=7.9(3) and 10 per million children,(4) respectively). Evidence from developing countries may help identify survival disparities among high-risk pediatric neuroblastoma patients compared with developed countries. Therefore, we aimed to describe survival among high-risk pediatric neuroblastoma patients in Argentina and compare survival between our setting and other settings.
METHODS
Study population
Hospital de Niños Sor Maria Ludovica in La Plata, Argentina is a public institution that serves patients regardless of insurance status. The pediatric oncology unit of the hospital includes 17 beds for inpatient care of children with hematologic malignancies and solid tumors. In addition to serving the local catchment area, Hospital de Niños Sor Maria Ludovica is a referral center for high-risk pediatric neuroblastoma patients from hospitals in Argentina and neighboring countries.
Individuals eligible for our cohort were aged <20 years when diagnosed with high-risk neuroblastoma and received cancer-directed therapy including stem cell transplantation at Hospital de Niños Sor Maria Ludovica in La Plata, Argentina between February 1999 and February 2015. Consistent with national guidelines in Argentina, high-risk neuroblastoma was defined as meeting one of the following criteria: 1) age ≥1 year and disseminated disease (stage 4 of International Neuroblastoma Staging System [INSS] or stage M of International Neuroblastoma Risk Group Staging System [INRGSS]); 2) stages 2 or 3 (INSS) or L1, L2, or MS (INRGSS) with MYCN amplification, or 3) age <1 year with MYCN amplification. High-risk neuroblastoma patients were treated according to modified N7(5) or European SIOP Neuroblastoma (SIOPEN HR-NBL1(6)) protocols. The induction therapy backbone included cyclophosphamide, doxorubicin, and vincristine (courses 1,2, and 4), and cisplatin and etoposide (courses 3 and 5). Consolidation therapy involved administration of busulfan (p.o. 4 times per day) and melphalan, with 13-cis retinoic acid administered following autologous stem cell transplantation. Supportive care included prophylaxis with acyclovir and trimethoprim-sulfamethoxazole. The treatment approach remained largely unchanged throughout the study period. This study was considered exempt from institutional review board oversight.
Variables
Medical records containing prospectively documented patient information from time of neuroblastoma diagnosis were abstracted using a standardized data collection form. Baseline information included demographic and clinical characteristics such as neuroblastoma diagnosis and location of primary tumor. We defined malnourishment as a weight for age z-score <−2 using the World Health Organization reference population.(7) Resource limitations in this setting precluded systematic assessments of prognostic factors such as DNA ploidy, MYCN gene amplifications, 1p36 deletions, and serum biomarkers. Follow-up information included basic data about the stem cell transplantation procedure and mortality.
Data analysis
Prior studies of survival among high-risk pediatric neuroblastoma patients used inconsistent measures of survival duration. Some studies measured survival since the time of neuroblastoma diagnosis and others measured survival since the time of stem cell transplantation. Unfortunately, neither of these approaches properly accounted for the time between diagnosis and transplantation, which can result in biased survival probabilities (8) if the goal is to estimate survival after transplantation. Consequently, we used an extended Kaplan-Meier approach for estimating survival probabilities.(8) We used age as the time-scale,(9) where person-time was observed from the age of neuroblastoma diagnosis, but entry into the cohort did not occur until the age at transplantation (i.e. delayed entry). For comparative purposes, we also estimated overall survival probabilities using the age of neuroblastoma diagnosis and the age of transplantation, respectively. Patients contributed follow-up time until death, loss to follow-up, or end of the study period (May 1, 2015).
Comparison of survival with other populations
We systematically searched PubMed/Medline for published reports regarding overall survival among high-risk pediatric neuroblastoma patients who underwent autologous stem cell transplantation. We used combinations of the search terms: pediatric OR paediatric OR child; neuroblastoma; high-risk OR advanced; and overall survival. Eligible reports were published between 2000 and 2016, and reported 5-year overall survival probabilities. Reviews, editorials, commentaries, and phase 1 or phase 2 randomized controlled trials (because of limited follow-up in such studies) were excluded. We abstracted information pertaining to each study, including publication year, study period, region, sample size, consolidation therapy, the measure of survival duration (time since diagnosis or transplantation), and 5-year overall survival probabilities specific to high-risk pediatric neuroblastoma patients who underwent autologous stem cell transplantation.
We estimated 5-year survival differences, which individually compared the 5-year overall survival probabilities reported in eligible studies with the 5-year overall survival probability observed in our study. To estimate 95% confidence limits (CL) for the survival difference, we first estimated the standard errors (SE) for 5-year overall survival probabilities based on the reported 95% CL from eligible studies, where SE = (upper limit of CL – lower limit of CL)/3.92.
These standard errors were then used to compute standard errors for the survival difference (SESD) as follows(10):
where SEA was the standard error of the reported 5-year survival probability and SEB was the standard error of 5-year survival probability observed in our study. The corresponding 95% CL were then computed as: point estimate for survival difference ± 1.96*SESD.
RESULTS
The eligible population comprised 40 high-risk pediatric neuroblastoma patients, but incomplete information for one patient resulted in a study population of 39 patients. Table 1 summarizes the characteristics of our study population. Briefly, the majority of patients (56%) were transplanted between 2000 and 2007. Patients were primarily aged ≤4 years (61%) at diagnosis and the majority were males (54%). The most common site of neuroblastoma was the adrenal gland (62%). The prevalence of malnourishment was low (2.6%) for patients with available evaluable data (average prevalence for hospitalized patients in Argentina is 12% (11)). The median follow-up for surviving patients was 1.7 years (interquartile range = 1.2 – 7.9).
Table 1.
Characteristics of high-risk pediatric neuroblastoma patients treated in La Plata, Argentina between February 1999 and February 2015.
| Characteristic | n (%) |
|---|---|
| Transplant period | |
| 2000 – 2007 | 22 (56) |
| 2008 – 2015 | 17 (44) |
| Age at diagnosis | |
| >4 years | 15 (39) |
| ≤4 years | 24(61) |
| Age at transplantation | |
| >4 years | 23 (59) |
| ≤4 years | 16 (41) |
| Sex | |
| Male | 21 (54) |
| Female | 18 (46) |
| Site of primary tumor | |
| Adrenal | 24 (62) |
| Retroperitoneal | 7 (18) |
| Mediastinal | 3 (7.7) |
| Other | 5 (13) |
| Malnourisheda | |
| Yes | 1 (2.6) |
| No | 29 (97) |
| Vital status at end of study | |
| Alive | 21 (46) |
| Deceased | 18 (54) |
Malnourished status based on weight-for-age z-score at the time of transplantation and the World Health Organization reference standard. Unreliable or missing data precluded z-score estimation for 9 patients.
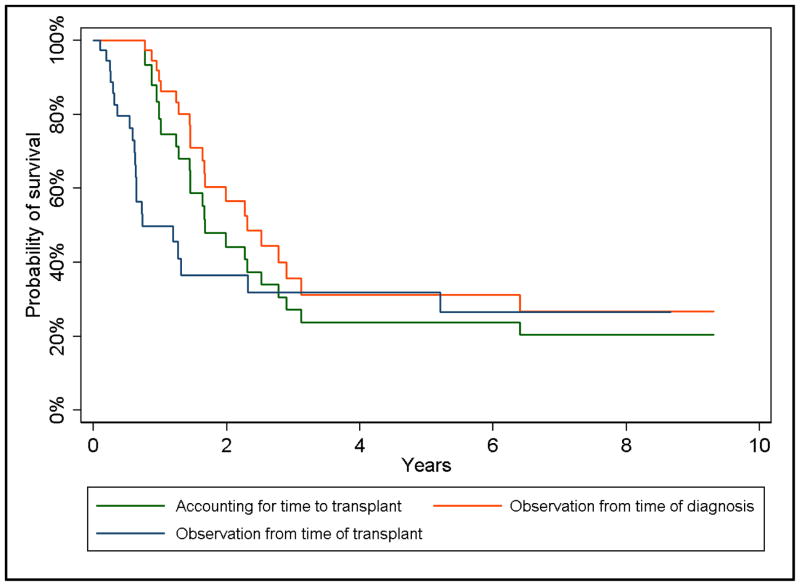
Figure 1 illustrates the survival curves for our study population based on approaches used in prior studies and the approach that accounts for time to transplantation. We observed 18 observed deaths, of which 15 were attributable to disease progression, 1 was attributable to pulmonary aspergillosis, 1 was attributable to engraftment failure, and 1 was attributable to sepsis. The median survival for this cohort was 1.7 years and the 5-year overall survival probability was 24% (95% CL: 10%, 41%) when accounting for time to transplant. The median survival was 2.4 years and 0.74 years if age at neuroblastoma diagnosis and age at transplant, respectively, were designated as the start of observation.
Figure 1.
Survival curves for high-risk pediatric neuroblastoma patients in La Plata, Argentina based on the measure of survival duration.
Table 2 describes 13 published reports,(12–24) and the current study, of 5-year overall survival among high-risk pediatric neuroblastoma and summarizes survival differences compared with our population. Half of the reports pertained to high-risk pediatric neuroblastoma cohorts in North America. The median sample size was 43 (interquartile range=26 – 89). Consolidation regimens were heterogeneous across studies, but 57% included either total body or localized radiation. Survival duration was measured as time since transplantation in 46% of studies. Except for one study,(24) the 5-year overall survival estimate in our population was lower than other populations, with the largest 5-year absolute survival difference being 52% (95% CL: 33%, 71%).
Table 2.
Comparison of 5-year overall survival in studies of high-risk pediatric neuroblastoma patients treated with autologous stem cell transplantation.
| Study (year) | Study period | Region | Sample size | Consolidation regimen | Survival duration | 5-year survival (95% CL) | 5-year survival difference (95% CL) |
|---|---|---|---|---|---|---|---|
| Kushner et al. (2015) (12) | 2003 – 2013 | North America | 60 | Localized radiation, 3F8, isotretinoin | Time since transplantation | 76% (66%, 88%) | 52% (33%, 71%) |
| Englum et al. (2015) (13) | 1990 – 2012 | North America | 87 | Total body irradiation, carboplatin, etoposide, cyclophosphamide, melphalan | Time since diagnosis | 47% (37%, 59%) | 23% (3.9%, 42%) |
| Mazloom et al. (2014) (14) | 2006 – 2011 | North America | 30 | Localized radiation, carboplatin, etoposide, melphalan | Time since diagnosis | 59% (45%, 78%) | 35% (12%, 58%) |
| Tan et al. (2012) (15) | 1987 – 2008 | Asia | 18 | Localized radiation, busulfan, melphalan | Time since diagnosis | 28% (4.0%, 53%) | 4.2% (−25%, 33%) |
| Granger et al. (2012) (16) | 1998 – 2000 | North America | 22 | Localized radiation, carboplatin, etoposide, cyclophosphamide, thiotepa | Time since transplantation | 46% (35%, 57%) | 22% (2.9%, 41%) |
| Monnereau-Laborde et al (2011) (17) | 1995 – 1996 | Europe | 20 | Busulfan, melphalan | Time since diagnosis | 35% (24%, 46%) | 11% (−8.1%, 30%) |
| Matthay et al. (2009) (18) | 1991 – 1996 | North America | 189 | Total body irradiation, carboplatin, etoposide, melphalan | Time since transplantation | 39% (35%, 43%) | 15% (−1.2%, 32%) |
| Trahair et al. (2007) (19) | 1985 – 2003 | Australia | 40 | Total body irradiation, cisplatin, teniposide, adriamycin, melphalan; thiotepa, etoposide, cyclophosphamide; carboplatin, etoposide, melphalan | Time since diagnosis | 60% (52%, 68%) | 36% (18%, 54%) |
| Kim et al. (2007) (20) | 1996 – 2004 | Asia | 46 | Carboplatinum, etoposide, melphalan; carboplatin, topotecan, thiotepa; etoposide, melphalan; carboplatin, topotecan, melphalan | Time since transplantation | 69% (60%, 78%) | 45% (27%, 93%) |
| George et al. (2006) (21) | 1994 – 2002 | North America | 89 | Total body irradiation, carboplatin, etoposide, melphalan, cyclophosphamide | Time since diagnosis or transplantation | 62% (51%, 72%) | 38% (19%, 57%) |
| Pritchard et al. (2005) (22) | 1982 – 1985 | Europe | 90 | Melphalan | Time since transplantation | 47% (30%, 64%) | 23% (−0.05%, 46%) |
| Berthold et al. (2005) (23) | 1997 – 2002 | Europe | 295 | Carboplatin, etoposide, melphalan | Time since diagnosis | 45% (39%, 52%) | 21% (4.1%, 38%) |
| Klaassen et al. (2003)a (24) | 1989 – 1995 | North America | 26 | Cyclophosphamide, carboplatinum, etoposide; cyclophosphamide, carboplatin, melphalan | Time since diagnosis | 23%b | −1%** |
| Current | 2000 – 2015 | South America | 39 | Busulfan, melphalan | Time since transplantation accounting for time since diagnosis | 24% (10%, 41%) | Reference |
Presented results pertain to stage 4 neuroblastoma
Inadequate data reported to estimate confidence limits
DISCUSSION
We analyzed data from 39 patients observed over 16 years in La Plata, Argentina to address a gap in the literature about survival of high-risk pediatric neuroblastoma patients treated with stem cell transplantation in developing countries. Our results suggest markedly worse survival in this setting compared with high-risk pediatric neuroblastoma patients in North America, Europe, and Asia. Specifically, 5-year overall survival in our setting was 24% (95% CL: 10%, 41), whereas the highest reported 5-year overall survival in North America was 76% (95% CL: 66%, 88%) in a highly select group of high-risk pediatric neuroblastoma patients.(12) In addition, our review of prior studies suggests considerable variation in survival even between developed countries. Nevertheless, several issues warrant consideration when interpreting the survival estimate for our population and the survival differences that compare our estimates with published estimates.
Our overall survival estimate may be sensitive to bias from diverse mechanisms. For example, neuroblastoma may be underdiagnosed in developing countries.(25) If children with high-risk pediatric neuroblastoma, who would have been eligible for inclusion in our study, died before being diagnosed and these children had a rapid course of disease, then the survival probabilities in our study may be overestimated. Even if neuroblastoma were diagnosed, the standard criteria for high-risk neuroblastoma evolved over the course of our study period. Although all patients in our study population met current criteria, certain neuroblastoma patients might have been high-risk but unidentified, particularly considering the lack of systematic cytogenetic assessment in our setting. We speculate that if any high-risk neuroblastoma patients were excluded from our study population, our survival probabilities are overestimated because survival of unidentified but eligible high-risk patients would have been poor without appropriate therapy. Lastly, similar to other studies of pediatric cancer populations in developing countries,(26) loss to follow-up was common in our study. Loss to follow-up was censored in our analysis, but the evidence does not suggest that patients would have survived without completing therapy. Consequently, this mechanism of bias would also result in overestimated survival probabilities in our study.
The estimated 5-year survival differences between our setting and other settings may be largely attributable to differences in treatment protocols and supportive care.(26, 27) The 5-year survival difference was greatly attenuated (4.2%, 95% CL: −25%, 33%; 11%, 95% CL: −8.1%, 30%) when our setting was compared with settings that used a similar consolidation regimen.(15, 17) In addition, treatment delays, host prognostic factors, and environmental factors may contribute to variation in survival between settings.(26, 28)
In summary, survival among high-risk neuroblastoma patients is generally poor regardless of geographic location, but our results illustrate dramatically worse survival for high-risk neuroblastoma patients in a developing country. We speculate that the observed survival differences could be attenuated or eliminated with improvements in treatment and supportive care, but addressing these issues will require creative solutions because of resource limitations. These resource limitations may be addressed with a focus on capacity building through collaborative efforts.(28) For example, twinning programs, which are based on partnering and interaction between hospitals in developing countries and cancer centers in developed countries, have improved survival and outcomes for pediatric cancer patients in developing countries.(29, 30) Despite the success of twinning programs, this approach has not been specifically applied in the context of high-risk neuroblastoma. Future studies are needed to assess the effectiveness of twinning programs and other approaches for improving survival among high-risk neuroblastoma patients in developing countries.
Acknowledgments
The authors are grateful to Wanda Goldman, MD for assistance with clinical care of this study population. This study was partially supported by grant 5R25CA23944 from the United States National Cancer Institute and the American Lebanese Syrian Associated Charities. The funding agencies were not involved in the study design, analysis, interpretation, or decision to submit this manuscript.
Footnotes
CONFLICT OF INTEREST
The authors declare no financial or non-financial conflicts of interest.
AUTHOR CONTRIBUTIONS
JCE contributed to data analysis, interpretation of results, and drafted the manuscript. SG contributed to data collection and interpretation of results. PHA contributed to data analysis and interpretation of the results. JMC contributed to data analysis. ABF contributed to data collection CR contributed to data collection. RPO developed the study design, and contributed to data analysis and interpretation of results. All authors contributed to critical revision of the manuscript and approved the final version.
References
- 1.Irwin MS, Park JR. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am. 2015;62:225–256. doi: 10.1016/j.pcl.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Park JR, Bagatell R, London WB, et al. Children’s Oncology Group’s 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer. 2013;60:985–993. doi: 10.1002/pbc.24433. [DOI] [PubMed] [Google Scholar]
- 3.Moreno F, Loria D, Abriata G, Terracini B network R. Childhood cancer: incidence and early deaths in Argentina, 2000–2008. Eur J Cancer. 2013;49:465–473. doi: 10.1016/j.ejca.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 4.NH, AMN, MK, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; 2012. [Google Scholar]
- 5.Valteau-Couanet D, Le Deley MC, Bergeron C, et al. Long-term results of the combination of the N7 induction chemotherapy and the busulfan-melphalan high dose chemotherapy. Pediatric blood & cancer. 2014;61:977–981. doi: 10.1002/pbc.24713. [DOI] [PubMed] [Google Scholar]
- 6.Ladenstein R, Valteau-Couanet D, Brock P, et al. Randomized Trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: the European HR-NBL1/SIOPEN study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3516–3524. doi: 10.1200/JCO.2009.27.3524. [DOI] [PubMed] [Google Scholar]
- 7.Group WMGRS. WHO Child Growth Standards: Methods and development: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. World Health Organization; 2006. [Google Scholar]
- 8.Cole SR, Hudgens MG. Survival analysis in infectious disease research: describing events in time. Aids. 2010;24:2423–2431. doi: 10.1097/QAD.0b013e32833dd0ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cologne J, Hsu WL, Abbott RD, et al. Proportional Hazards Regression in Epidemiologic Follow-up Studies: An Intuitive Consideration of Primary Time Scale. Epidemiology. 2012;23:565–573. doi: 10.1097/EDE.0b013e318253e418. [DOI] [PubMed] [Google Scholar]
- 10.Parner ET, Andersen PK. Regression analysis of censored data using pseudo-observations. Stata Journal. 2010;10:408. [Google Scholar]
- 11.Correia MI, Campos AC, Study EC. Prevalence of hospital malnutrition in Latin America: the multicenter ELAN study. Nutrition. 2003;19:823–825. doi: 10.1016/s0899-9007(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 12.Kushner BH, Ostrovnaya I, Cheung IY, et al. Lack of survival advantage with autologous stem-cell transplantation in high-risk neuroblastoma consolidated by anti-GD2 immunotherapy and isotretinoin. Oncotarget. 2015 doi: 10.18632/oncotarget.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Englum BR, Rialon KL, Speicher PJ, et al. Value of surgical resection in children with high-risk neuroblastoma. Pediatric blood & cancer. 2015;62:1529–1535. doi: 10.1002/pbc.25504. [DOI] [PubMed] [Google Scholar]
- 14.Mazloom A, Louis CU, Nuchtern J, et al. Radiation therapy to the primary and postinduction chemotherapy MIBG-avid sites in high-risk neuroblastoma. International journal of radiation oncology, biology, physics. 2014;90:858–862. doi: 10.1016/j.ijrobp.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan C, Sabai SM, Tin AS, Quah TC, Aung L. Neuroblastoma: experience from National University Health System, Singapore (1987–2008) Singapore Med J. 2012;53:19–25. [PubMed] [Google Scholar]
- 16.Granger M, Grupp SA, Kletzel M, et al. Feasibility of a tandem autologous peripheral blood stem cell transplant regimen for high risk neuroblastoma in a cooperative group setting: a Pediatric Oncology Group study: a report from the Children’s Oncology Group. Pediatric blood & cancer. 2012;59:902–907. doi: 10.1002/pbc.24207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monnereau-Laborde S, Munzer C, Valteau-Couanet D, et al. A dose-intensive approach (NB96) for induction therapy utilizing sequential high-dose chemotherapy and stem cell rescue in high-risk neuroblastoma in children over 1 year of age. Pediatric blood & cancer. 2011;57:965–971. doi: 10.1002/pbc.23232. [DOI] [PubMed] [Google Scholar]
- 18.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trahair TN, Vowels MR, Johnston K, et al. Long-term outcomes in children with high-risk neuroblastoma treated with autologous stem cell transplantation. Bone marrow transplantation. 2007;40:741–746. doi: 10.1038/sj.bmt.1705809. [DOI] [PubMed] [Google Scholar]
- 20.Kim EK, Kang HJ, Park JA, Choi HS, Shin HY, Ahn HS. Retrospective analysis of peripheral blood stem cell transplantation for the treatment of high-risk neuroblastoma. J Korean Med Sci. 2007;22(Suppl):S66–72. doi: 10.3346/jkms.2007.22.S.S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George RE, Li S, Medeiros-Nancarrow C, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:2891–2896. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 22.Pritchard J, Cotterill SJ, Germond SM, Imeson J, de Kraker J, Jones DR. High dose melphalan in the treatment of advanced neuroblastoma: results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatric blood & cancer. 2005;44:348–357. doi: 10.1002/pbc.20219. [DOI] [PubMed] [Google Scholar]
- 23.Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. The lancet oncology. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 24.Klaassen RJ, Trebo MM, Koplewitz BZ, Weitzman SS, Calderwood S. High-risk neuroblastoma in Ontario: a report of experience from 1989 to 1995. Journal of pediatric hematology/oncology. 2003;25:8–13. doi: 10.1097/00043426-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Stiller CA, Parkin DM. International variations in the incidence of neuroblastoma. International journal of cancer Journal international du cancer. 1992;52:538–543. doi: 10.1002/ijc.2910520407. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Galindo C, Friedrich P, Morrissey L, Frazier L. Global challenges in pediatric oncology. Current opinion in pediatrics. 2013;25:3–15. doi: 10.1097/MOP.0b013e32835c1cbe. [DOI] [PubMed] [Google Scholar]
- 27.Kirby J, Ojha RP, Johnson KM, Bittner EC, Caniza MA. Challenges in managing infections among pediatric cancer patients: Suboptimal national essential medicines lists for low and middle income countries. Pediatric blood & cancer. 2014 doi: 10.1002/pbc.25273. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Galindo C, Friedrich P, Alcasabas P, et al. Toward the Cure of All Children With Cancer Through Collaborative Efforts: Pediatric Oncology As a Global Challenge. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:3065–3073. doi: 10.1200/JCO.2014.60.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pui CH, Ribeiro RC. International collaboration on childhood leukemia. Int J Hematol. 2003;78:383–389. doi: 10.1007/BF02983810. [DOI] [PubMed] [Google Scholar]
- 30.Abboud MR, Ghanem K, Muwakkit S. Acute lymphoblastic leukemia in low and middle-income countries: disease characteristics and treatment results. Current opinion in oncology. 2014;26:650–655. doi: 10.1097/CCO.0000000000000125. [DOI] [PubMed] [Google Scholar]