Abstract
Objectives
This study sought to assess the independent effect of high-density lipoprotein-cholesterol (HDL-C) level on cardiovascular risk in patients with stable ischemic heart disease (SIHD) who were receiving optimal medical therapy (OMT).
Background
Although low HDL-C level is a powerful and independent predictor of cardiovascular risk, recent data suggest that this may not apply when low-density lipoprotein-cholesterol (LDL-C) is reduced to optimal levels using intensive statin therapy.
Methods
We performed a post-hoc analysis in 2,193 men and women with SIHD from the COURAGE trial. The primary outcome measure was the composite of death from any cause or nonfatal myocardial infarction (MI). The independent association between HDL-C levels measured after 6 months on OMT and the rate of cardiovascular events after 4 years was assessed. Similar analyses were performed separately in subjects with LDL-C levels below 70 mg/dl (1.8 mmol/l).
Results
In the overall population, the rate of death/MI was 33% lower in the highest HDL-C quartile as compared with the lowest quartile, with quartile of HDL-C being a significant, independent predictor of death/MI (p = 0.05), but with no interaction for LDL-C category (p = 0.40). Among subjects with LDL-C levels <70 mg/dl, those in the highest quintile of HDL-C had a 65% relative risk reduction in death or MI as compared with the lowest quintile, with HDL-C quintile demonstrating a significant, inverse predictive effect (p = 0.02).
Conclusions
In this post-hoc analysis, patients with SIHD continued to experience incremental cardiovascular risk associated with low HDL-C levels despite OMT during long-term follow-up. This relationship persisted and appeared more prominent even when LDL-C was reduced to optimal levels with intensive dyslipidemic therapy. (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation; NCT00007657)
Keywords: HDL cholesterol, residual risk, stable ischemic heart disease
Population-based studies consistently support high-density lipoprotein cholesterol (HDL-C) as a significant, strong, and independent inverse predictor of cardiovascular risk, noting that for every 1-mg/dl decrease in HDL-C level, risk of future cardiovascular events increases by 2% to 3% (1–5). However, the clinical interaction between low-density lipoprotein cholesterol (LDL-C) and HDL-C level remains unclear, with some analyses supporting a continuing predictive role of HDL-C regardless of achieved LDL-C level, whereas others suggesting that the effect of HDL-C may not be relevant when LDL-C is reduced to very low levels, particularly when potent statin therapy is used (6–10). This is especially important because HDL-C levels are not substantially altered by statin therapy and it can be hypothesized that persistently low levels of HDL-C at baseline could be potentially responsible for some of the residual risk observed in clinical trials among statin-treated patients.
The COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial examined the impact of optimal medical therapy (OMT) with or without percutaneous coronary intervention (PCI) as the initial management strategy in 2,287 patients with stable ischemic heart disease (SIHD) (11). The main trial results revealed no difference in the primary outcome of death or myocardial infarction (MI) during a mean 4.6 years of follow-up. Secondary prevention with OMT was applied equally and intensively to both treatment groups, with excellent adherence and no significant differences in proportion of patients achieving therapeutic goals (12). This post-hoc analysis was conducted to assess the relationship between the rate of adverse cardiovascular events and HDL-C levels in SIHD patients receiving aggressive secondary prevention with lifestyle and pharmacologic interventions, including goal-directed statin therapy. The subset of patients who achieved the optional LDL-C goal of <70 mg/dl established by the Adult Treatment Panel (ATP) III were further investigated to define the effect of HDL-C in presence of optimally achieved and maintained levels of LDL-C on statins, with or without ezetimibe (13).
Methods
The methods of the COURAGE trial (NCT00007657) have been described previously (11–14). The study was sponsored by the Department of Veterans Affairs Cooperative Studies Program, with additional funding from the Canadian Institutes of Health Research and supplemental support from several pharmaceutical companies. An independent data and safety monitoring board monitored the trial. Data management and analyses were performed solely by the data coordinating center and were overseen by the trial’s executive committee, which had full access to the data on completion of the trial and vouched for their accuracy. All patients had significant coronary artery disease with evidence of myocardial ischemia. Detailed inclusion and exclusion criteria have been previously published (11–14). The primary outcome measure was the composite of death from any cause or nonfatal MI. Subjects were followed-up for a median of 4.6 years (range: 2.5 to 7.0 years) after randomization.
Details of risk factor modification applied to both treatment arms have been previously described (12). Lifestyle counseling for diet, smoking cessation, glycemic control, and weight loss was provided. All patients received anti-platelet therapy (low-dose aspirin), anti-ischemic therapy (long-acting metoprolol, amlodipine, and isosorbide mononitrate, alone or in combination) and lisinopril or losartan for hypertension, reduced ejection fraction, or secondary prevention. Patients undergoing PCI also received clopidogrel, in accordance with accepted treatment guidelines. The LDL-C target in COURAGE was 60–85 mg/dl, which during the time the trial was designed in the late 1990s, was more aggressive than the ATP II recommendation of <100 mg/dl (15). In addition, by trial design, there were pre-defined secondary lipid targets for HDL-C (>40 mg/dl) and triglycerides (<150 mg/dl). Ezetimibe, extended-release niacin, and fibrates were used in addition to statins, as needed clinically. Thus, aggressive dyslipidemic therapy was instituted to lower LDL-C as the principal treatment target, and to also target both HDL-C raising and triglyceride lowering with additional agents to achieve secondary lipid targets.
Statistical analysis
For this post-hoc analysis, 2,193 subjects from among the total study population of 2,287 patients who had available HDL-C data were stratified into quartiles based on HDL-C levels on OMT measured at 6 months post-randomization. In order to standardize lipid levels during a time period after enrollment when patients were being switched from other statins prior to randomization to simvastatin, clinical characteristics and lipid levels at month 6 were considered for baseline comparison and endpoint events were left-censored at this time point. Therefore, the average duration of follow-up for this analysis was approximately 4 years, as compared with the overall trial mean follow-up of 4.6 years. Cox regression models were used to determine hazard ratios for endpoints in each HDL-C quartile, adjusting for age, sex, BMI, presence of hypertension, diabetes, current smoking, and triglycerides at 6 months. LDL-C was not included among the variables in the regression model. Instead, the interaction between HDL-C and LDL-C levels on OMT was explored using a stratified regression analysis with pre-determined LDL-C categories (<70, 70 to 100, and >100 mg/dl). Patients in the lowest category of LDL-C (<70 mg/dl) were further analyzed by dividing them into quintiles of HDL-C level (at 6 months) using identical regression models. The independent associations between death or MI and quartiles of the ratio of LDL-C to HDL-C were also evaluated. Additionally, using numeric values of HDL-C at 6 months, similarly adjusted Cox regression models were executed to examine the relationship between continuous HDL-C levels (at 6 months) and risk of death or MI.
Results
Baseline characteristics
Baseline characteristics and lipid levels of the subjects within quartiles of HDL-C are shown in Table 1. Subjects in the lower HDL-C quartiles were younger and more likely to be male with a greater proportion of current smokers than those in higher HDL-C quartiles. Those with low levels of HDL-C had higher baseline BMI, higher triglycerides, and a greater prevalence of hypertension and diabetes, features consistent with the metabolic syndrome. Statin use was similar across HDL-C quartiles at study entry and at 6 months, although overall usage increased universally at the 6-month landmark. As expected, the use of other drugs to treat serum lipids (including niacin and fibrates) was more than double in the lowest HDL-C quartile as compared with the highest HDL-C quartile.
Table 1.
Baseline Characteristics of Patients According to Quartiles of HDL at 6 Months
| Quartile 1 <34.8 mg/dl |
Quartile 2 34.9 to <40.7 mg/dl |
Quartile 3 40.7 to <48.0 mg/dl |
Quartile 4 48.0 to 94.0 mg/dl |
Overall 12.0 to 94.0 mg/dl |
|
|---|---|---|---|---|---|
| Characteristic | (N = 551) | (N = 545) | (N = 538) | (N = 558) | (N = 2192) |
| Assigned to PCI + OMT, n | 265 | 280 | 273 | 282 | 50.2 (%) |
| Assigned to OMT, n | 286 | 265 | 265 | 276 | 47.8 (%) |
| Male, % | 94.6 | 91.2 | 86.8 | 69.4 | 85.4 |
| White, % | 89.8 | 84.0 | 84.6 | 87.7 | 86.6 |
| Current smoker | 35.6 | 26.7 | 27.8 | 22.6 | 28.1 |
| Body mass index, kg/m2 | 30.8 ± 5.2 | 30.2 ± 5.0 | 29.2 ± 4.6 | 28.1 ± 4.8 | 29.6 ± 5.0 |
| Age, yrs | 60.9 ± 10.1 | 61.4 ± 10.0 | 61.8 ± 9.7 | 64.1 ± 9.4 | 62.0 ± 9.9 |
|
| |||||
| Cardiovascular history, % | |||||
| Myocardial infarction | 42.0 | 37.9 | 37.7 | 37.8 | 38.9 |
| Prior CABG | 14.2 | 9.4 | 10.4 | 10.2 | 11.0 |
| Prior PCI | 21.0 | 13.0 | 13.8 | 14.2 | 15.5 |
| Stroke | 6.0 | 5.9 | 4.1 | 3.6 | 4.9 |
| Peripheral vascular disease | 9.3 | 7.4 | 6.8 | 7.2 | 7.7 |
| Hypertension | 74.2 | 69.1 | 62.3 | 63.2 | 67.3 |
| Heart failure | 4.2 | 5.5 | 3.7 | 4.0 | 4.4 |
| Diabetes | 41.4 | 33.4 | 32.1 | 28.0 | 33.7 |
| Angina | 89.1 | 89.2 | 87.3 | 85.7 | 87.8 |
|
| |||||
| Drugs at study entry, % | |||||
| Statin | 67.0 | 68.6 | 67.1 | 67.4 | 67.5 |
| Other anti-lipid drugs | 9.6 | 7.6 | 4.1 | 5.1 | 6.6 |
| Calcium blocker | 31.9 | 32.7 | 29.2 | 28.8 | 30.7 |
| ACEI or ARB | 45.7 | 45.3 | 48.1 | 49.6 | 47.1 |
| Beta blocker | 73.0 | 72.9 | 69.9 | 68.5 | 71.1 |
| Nitrates | 60.1 | 60.5 | 57.1 | 53.8 | 57.9 |
| Aspirin | 87.3 | 88.6 | 89.0 | 85.7 | 87.6 |
|
| |||||
| Lipid values at 6 months, mg/dl | |||||
| LDL-C | 81.2 ± 27.6 | 85.0 ± 26.2 | 88.5 ± 25.8 | 88.9 ± 28.7 | 85.9 ± 27.3 |
| HDL-C | 30.6 ± 3.3 | 37.7 ± 1.7 | 43.8 ± 2.0 | 57.4 ± 9.1 | 42.4 ± 11.1 |
| Total-C | 148.3 ± 34.5 | 154.0 ± 32.2 | 160.3 ± 32.6 | 170.7 ± 32.8 | 158.4 ± 34.1 |
| Triglycerides | 197.2 ± 128.2 | 160.6 ± 86.8 | 148.6 ± 101.5 | 125.9 ± 67.6 | 158.0 ± 101.7 |
| LDL-C to HDL-C | 2.7 ± 1.0 | 2.3 ± 0.7 | 2.0 ± 0.6 | 1.6 ± 0.6 | 2.1 ± 0.8 |
| Total-C to HDL-C | 4.9 ± .13 | 4.1 ± 0.9 | 3.7 ± 0.8 | 3.0 ± 0.7 | 3.9 ± 1.2 |
|
| |||||
| Anti-lipid drugs at 6 months, % | |||||
| Statin | 92.6 | 94.4 | 95.5 | 94.2 | 94.2 |
| Other lipid lowering | 35.4 | 27.4 | 20.1 | 16.2 | 24.7 |
Values are n, %, or mean ± SD.
ACEI = angiotensin-converting-enzyme inhibitor; ARB = angiotensin receptor blockers; CABG = coronary artery bypass grafting; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; OMT = optimal medical therapy; PCI = percutaneous coronary intervention.
HDL-C quartile and risk of adverse clinical outcomes
At the end of 4 years, the rate of the composite primary endpoint was calculated for each HDL-C quartile in the overall COURAGE population. In univariate analyses, the primary endpoint of death or MI was 36% lower in the highest HDL-C quartile compared with the lowest (p = 0.006). The secondary endpoints of death, MI or stroke and death, MI, or ACS were lower by 33% (p = 0.01) and 34% (p = 0.002), respectively, in the highest versus lowest HDL-C quartiles. After multivariate adjustment, those in the highest HDL-C quartile continued to experience a significant 33% reduction in the risk of the primary endpoint (HR: 0.67; 95% confidence interval [CI]: 0.47 to 0.95; p = 0.02), as well as reductions in both secondary endpoints (HR: 0.72; 95% CI: 0.52 to 1.01; p = 0.06 and HR: 0.72; 95% CI: 0.54 to 0.97; p = 0.03, respectively). Across all quartiles of HDL-C, the risk of death or MI was significantly lower among patients in higher quartiles of HDL-C as compared with those in the lower quartiles (p = 0.02). After adjustment for other variables, the quartile of HDL-C continued to retain nominal statistical significance as a predictor of death or MI (p = 0.05) (Fig. 1).
Figure 1. Multivariate Analysis of the Relationship Between Death or MI and Quartiles of HDL Cholesterol.
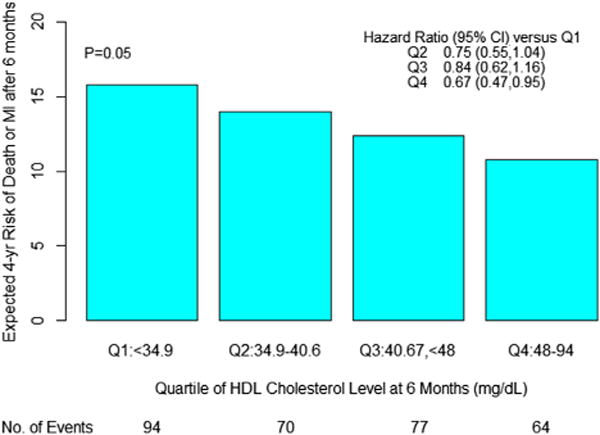
Hazard ratios are adjusted for age, sex, body mass index, hypertension, diabetes, current smoking, and triglycerides, and use lowest quartile (Q1) as referent. CI = confidence interval; HDL = high-density lipoprotein; MI = myocardial infarction.
Effect of LDL-C
A regression analysis was conducted using pre-specified LDL-C categories (<70 mg/dl, n = 634; 70 to 100 mg/dl, n = 979; and >100 mg/dl, n = 573) to determine the interaction between LDL-C and HDL-C. As expected, within each HDL-C quartile, those in the lower LDL-C categories had a lower incidence of death or MI compared with individuals within higher LDL-C categories. Conversely, among patients in the same LDL-C category, those belonging to higher HDL-C quartiles experienced a greater protective effect from death or MI compared with their counterparts in lower HDL-C quartiles (Fig. 2). Overall, the effect of HDL-C quartile on death or MI was independent, with no apparent interaction with LDL-C category (p = 0.40). Further, when using actual values of HDL-C and LDL-C in a continuous regression model, LDL-C level at 6 months did not have any significant effect on the inverse predictive effect of HDL-C (p = 0.37).
Figure 2. Relationship Between HDL-C Quartiles and Death or MI Across Categories of LDL-C.
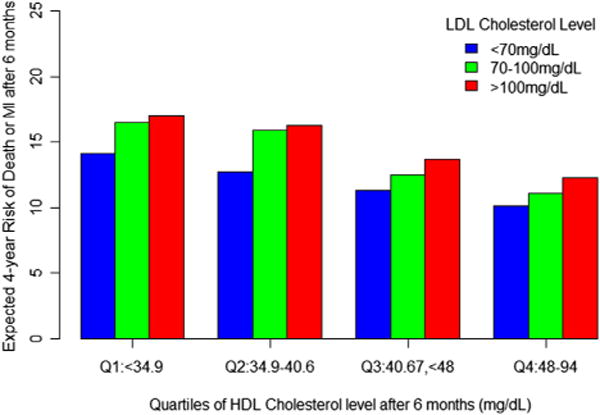
The effect of HDL-C quartile on death or MI was independent, with no apparent interaction with LDL-C category (p = 0.40). LDL = Low-density lipoprotein; other abbreviations as in Figure 1.
In the subgroup of patients who achieved the optimal LDL-C goal of <70 mg/dl, quintiles were created based on HDL-C level at 6 months. In univariate analyses, patients in the highest quintile of HDL-C had a 67% relative risk reduction in the rate of death or MI versus those in the lowest quintile (HR: 0.33; 95% CI: 0.16 to 0.66; p = 0.002), along with a 66% relative risk reduction in the composite of death, MI, or stroke (HR: 0.34; 95% CI: 0.17 to 0.67; p = 0.002) and a 45% relative risk reduction in the composite of death, MI, or ACS (HR: 0.55; 95% CI: 0.31 to 0.99; p = 0.05). Even after adjustment for covariates, those in the highest quintile of HDL-C had a 65% reduction in the rate of the primary endpoint (HR: 0.35; 95% CI: 0.17 to 0.74; p = 0.006) and a 63% decrease in death, MI, or stroke (HR: 0.37; 95% CI: 0.26 to 0.75; p = 0.006). A trend (43% relative risk reduction) towards lower death, MI, or ACS was noted in the top HDL-C quintile compared with the bottom HDL-C quintile (43%, HR: 0.57; 95% CI: 0.30 to 1.08; p = 0.08). Across all HDL-C quintiles in the lowest stratum of LDL-C, the risk of death or MI differed significantly in both unadjusted analyses (p = 0.004), and after adjustment for confounding factors (p = 0.02) (Fig. 3).
Figure 3. Multivariate Analysis of the Relationship Between Death or MI and Quintiles of HDL-C Among Patients With LDL Cholesterol <70 mg/dl.
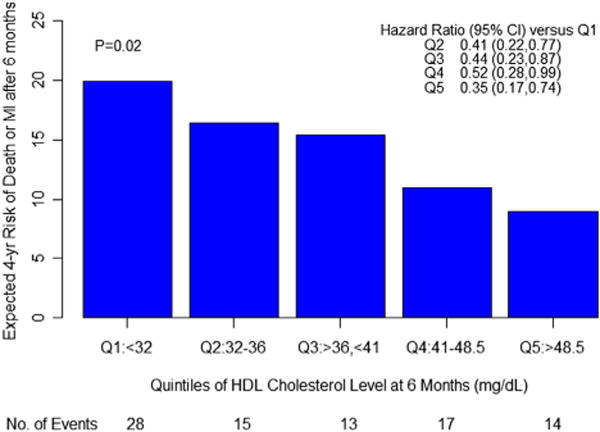
Hazard ratios are adjusted for age, sex, body mass index, hypertension, diabetes, current smoking, and triglycerides, and use lowest quintile (Q1) as referent. Abbreviations as in Figures 1 and 2.
For the primary endpoint of death or MI, the ratio of LDL-C to HDL-C was examined for predictive effect. At 6 months post-randomization, the quartile ratio of LDL-C to HDL-C was inversely related to expected freedom from death or MI. After multifactorial adjustment, although the boundaries for HRs related to individual quartiles could not exclude unity, a significant difference in survival persisted across all quartiles of LDL-C/HDL-C ratio (p = 0.04) (Fig. 4).
Figure 4. Kaplan-Meier Curves for Overall Trial Population.
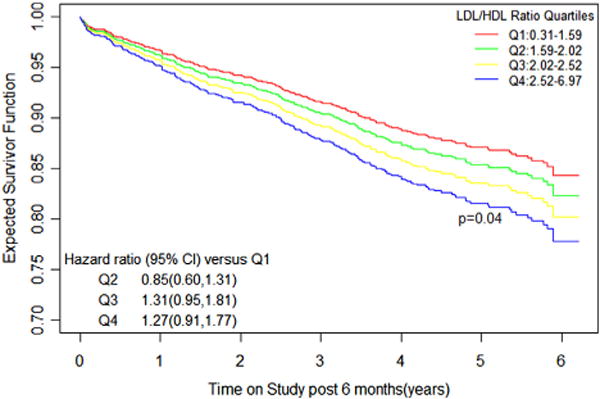
Expected freedom from death or myocardial infarction across quartiles of LDL-C/HDL-C ratio (Q1–Q4) at 6 months. Hazard ratios are adjusted for age, sex, body mass index, hypertension, diabetes, current smoking, and triglycerides, and use the lowest quartile (Q1) as referent. Abbreviations as in Figures 1 and 2.
Continuous HDL-C levels
Using 6-month HDL-C level as a continuous variable, the risk of death or MI was calculated for each 10 mg/dl increase in HDL-C. After adjustment for covariates, a rise of 10 mg/dl in HDL-C was associated with a potential risk reduction of 9.9% (95% CI: 9.8 to 10.0), with a strong trend toward statistical significance (p = 0.08) in death or MI. In particular, among subjects in the lowest LDL-C category of <70 mg/dl, a 10 mg/dl increment in HDL-C was associated with a statistically significant 9.8% (95% CI: 9.5 to 10.0; p = 0.03) reduction in risk of the primary endpoint in adjusted analysis. Age, sex, BMI, diabetes, hypertension, current smoking, LDL-C and triglycerides at 6 months post-randomization did not have any significant interaction with the observed predictive effect of HDL-C.
Discussion
This post-hoc analysis from the COURAGE trial demonstrates that a significant inverse association exists between plasma HDL-C levels and cardiovascular risk that is independent of other confounders, including age, sex, BMI, presence of hypertension, diabetes, current smoking, and triglycerides in patients with SIHD who undergo long-term follow-up. The predictive relationship of low levels of HDL-C remained valid across varying LDL-C levels in the present analysis, and appeared greater in magnitude in patients where the primary guideline-recommended optimal target of lipid-lowering therapy for patients with SIHD had been achieved (LDL-C <70 mg/dl). The results also remained consistent, regardless of whether 6-month HDL-C levels were used as continuous variables or divided into quartiles or quintiles.
Considerable residual risk persists among statin-treated patients, with rates of cardiovascular events being approximately two-thirds to three-quarters that of placebo-treated patients in clinical trials (16–24). Even with maximal statin therapy, over 22% of patients with recent ACS and ~9% patients with SIHD experience endpoint events after 2 years and 5 years of follow-up, respectively, indicating that reducing LDL-C alone may not prevent all prognostically important vascular events (25,26). In addition, patients with metabolic syndrome and diabetes, conditions generally associated with low levels of HDL-C, have approximately twice the level of excess risk compared with those without these comorbidities (27).
Unlike the consistent inverse epidemiologic association between HDL-C and cardiovascular risk in patients with normal or elevated LDL-C levels, conflicting results have been noted in the setting of low LDL-C levels. Among high-risk patients with recent ACS treated with high-dose statins, 1 trial found no incremental predictive value of HDL-C (7), whereas the other showed that HDL-C, but not LDL-C, measured in the initial stage of ACS predicted the risk of short-term recurrent cardiovascular events (28). In intermediate-risk patients with SIHD, low HDL-C levels were independently predictive of higher cardiovascular risk, even when LDL-C levels were reduced to <70 mg/dl (8). The difference in risk between the highest and the lowest HDL-C quintile was diminished and failed to reach statistical significance among those on maximal dose atorvastatin. Finally, in a lower-risk primary prevention population, HDL-C levels were inversely related to vascular risk only in patients receiving placebo, but not in patients assigned to receive potent statin therapy with resultant very low on-treatment LDL-C levels (6). A meta-analysis of 20 large trials confirmed the significant and independent inverse association between low HDL-C levels and cardiovascular risk among statin-treated patients, with no evidence of any modification or attenuation by statin therapy (10). The present findings complement the results of this meta-analysis, extending the concept of incremental cardiovascular risk associated with low levels of HDL-C in SIHD patients treated with OMT, particularly among subjects in the lowest quintile of HDL-C. Instead of just focusing on fixed-dose high-potency statin treatment, the COURAGE trial used goal-directed LDL-C lowering within the framework of comprehensive and aggressive risk factor modification, an ideal approach closer to what published clinical practice guidelines support therapeutically. Regardless, those with lower levels of HDL-C continued to experience greater cardiovascular events, even when LDL-C was reduced to <70 mg/dl.
Clinical trial evidence to support the benefits of HDL-C raising has been limited. The VA-HDL Intervention Trial studied 2,531 male veterans with established coronary heart disease, LDL-C ≤140 mg/dl, and HDL-C ≤40 mg/dl, treated with gemfibrozil or placebo in the pre-statin era (29). At 5 years, despite no change in LDL-C levels as compared with baseline, a modest 6% relative increase in HDL-C and a 31% relative decrease in triglycerides was associated with a significant 22% reduction in the primary endpoint of cardiovascular mortality or MI. However, in the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes) trial, in similar SIHD patients pre-selected for low baseline levels of HDL-C, there was no incremental clinical benefit associated with the co-administration of high-dose (1,500 to 2,000 mg/day) extended-release niacin and simvastatin as compared with simvastatin monotherapy during an abbreviated 3-year follow-up for the trial primary endpoint of major cardiovascular events or any of the component secondary or tertiary endpoints (30). More recently, the HPS-2 (Heart Protection Study-2) investigators have presented study findings in more than 25,000 subjects treated with simvastatin with or without niacin (and laropiprant) during a long-term 5-year follow-up, and likewise failed to show incremental benefit over statin monotherapy (31). Importantly, in both AIM-HIGH and HPS-2, patients had very low baseline LDL-C levels (ranging from the low 60s to low 70s), and thus it remains uncertain whether the potential clinical benefits of HDL-C raising therapies may be mitigated in this very low range of LDL-C levels. It is also unclear if the results would have been different in patients whose LDL-C values were not as well-controlled or in those naïve to statin therapy. Cholesteryl ester transfer protein (CETP) inhibitors anacetrapib and evacetrapib, perhaps the most promising investigational HDL-C raising therapies remain under study in large-scale phase III clinical trials (32,33). However, the CETP inhibitor torcetrapib was shown previously to increase mortality and, more recently, dalcetrapib was found to have no incremental benefit when added to statin therapy in ACS, despite significant HDL-C raising (34,35). These disappointing results to date suggest that CETP inhibition as a therapeutic strategy may not confer clinical benefit, despite significant HDL-C raising. Alternatively, the negative results in these 4 clinical trials raise the very real possibility that, although low levels of HDL-C may be an important epidemiologic risk marker, the concentration or content of HDL in plasma alone may not be a reliable therapeutic target for pharmacologic intervention to reduce clinical events. Indeed, there are data to support HDL particle size and number as a potentially better measure of cardiovascular risk (36), though no clinical trials to date have enrolled patients based on particle size determinants alone, nor have they targeted changes in particle size/number as a measure of treatment efficacy. Lastly, it is possible that investigators have not targeted patients with the lowest levels of HDL-C (e.g., <30 mg/dl), an important subgroup of patients who may be at the highest risk for cardiovascular events and in whom the potential exists to demonstrate clinical benefit with a non-statin intervention.
Study limitations
The COURAGE trial was not designed specifically to study the residual cardiovascular risk associated with low levels of HDL-C, resulting in some limitations inherent in this post-hoc analysis. It is possible that using 6-month levels of HDL-C and LDL-C rather than baseline levels obtained prior to randomization might have resulted in different outcomes. However, because there was no effect of PCI versus OMT on clinical outcomes, and the potential contribution of cardiac events occurring within the first 6 months of follow-up to overall long-term trial outcomes was likely minimal, it is doubtful that censoring events within the initial 6 months would have altered our findings. Although we attempted to adjust for known confounders, the presence of unmeasured differences could account, in part, for the additional cardiovascular risk noted in patients on OMT, and therefore, could potentially influence the predictive value of HDL-C levels. The role of the metabolic syndrome was not separately assessed, although adjustments were made for BMI, triglycerides, diabetes, and hypertension. Additionally, no attempts were made to distinguish or measure HDL-C subfractions, particle size, or functionality, all of which may have effects independent of total plasma HDL-C levels. Although our findings should be considered hypothesis-generating and exploratory in nature, they may have important therapeutic implications, in that this is one of the largest prospective trials of SIHD patients in whom long-term clinical outcomes have been assessed as a function of both low levels of HDL-C and LDL-C.
Conclusions
Our analysis suggests that patients with SIHD continue to experience significant, long-term cardiovascular risk associated with low HDL-C levels despite optimal medical therapy with proven secondary prevention modalities, including aggressive lifestyle modification and intensive goal-directed statin treatment. The adverse clinical effect of low baseline levels of HDL-C we observed persisted despite adjustment for other baseline risk predictors, and was demonstrable across the full range of LDL-C levels. When LDL-C was reduced to optimally low levels (<70 mg/dl) using intensive lipid-lowering therapy, the risk for subsequent cardiovascular events among those with low baseline levels of HDL-C remained statistically and clinically significant and, in fact, appeared to be magnified. Thus, further prospective study is needed to more precisely identify those patients with either very low levels of HDL-C or abnormal HDL particle composition who may be considered appropriate candidates for future therapeutic interventions to improve clinical outcomes.
Acknowledgments
The COURAGE study was supported by the Cooperative Studies Program of the U.S. Department of Veterans Affairs Office of Research and Development, in collaboration with the Canadian Institutes of Health Research; and by unrestricted research grants from the following companies: Merck, Pfizer, Bristol-Myers Squibb, Fujisawa, Kos Pharmaceuticals, Datascope, AstraZeneca, Key Pharmaceutical, sanofi-aventis, First Horizon, and GE Healthcare, including in-kind support with Food and Drug Administration-approved drugs used by study participants. All industrial funding in support of the trial was directed through the U.S. Department of Veterans Affairs. Dr. Mancini has received grants, honoraria, and is on the speakers’ bureau of Amgen Merck CanaSanofi, and AstraZeneca; and has received honoraria from Merck, Amgen, Roche, Miraculins, Pfizer, sanofi-aventis, Servier, GlaxoSmithKline, and Valeant. Dr. Spertus owns the copyright to the Seattle Angina Questionnaire; is a consultant for United Healthcare, St. Jude Medical, Abbott Vascular, and Genentech; has received research grant support from Gilead, Genentech, Amgen, and Eli Lilly & Company; has received support from the American Heart Association, American College of Cardiology Foundation, United Healthcare, Health Outcomes Science; and owns the copyright to Seattle Angina Questionnaire; Kansas City Cardiomyopathy Questionnaire; and Position Analysis Questionnaire. Dr. Chaitman is a consultant to Pfizer, Forest, Merck, Roche, Sanofi, and Takeda; and is a member of the speakers’ bureau for Gilead.
Abbreviations and Acronyms
- ACS
acute coronary syndrome(s)
- ATP
Adult Treatment Panel
- BMI
body mass index
- CETP
cholesteryl ester transfer protein
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- MI
myocardial infarction
- OMT
optimal medical therapy
- PCI
percutaneous coronary intervention
- SIHD
stable ischemic heart disease
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–14. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, Garrison RJ, Castelli WP, Feinleib M, McNamara PM, Kannel WB. Prevalence of coronary heart disease in the Framingham Offspring Study: role of lipoprotein cholesterols. Am J Cardiol. 1980;46:649–54. doi: 10.1016/0002-9149(80)90516-0. [DOI] [PubMed] [Google Scholar]
- 3.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256:2835–8. [PubMed] [Google Scholar]
- 4.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124(Suppl):S11–20. doi: 10.1016/0021-9150(96)05852-2. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Genest J, Boekholdt SM, et al. for the JUPITER Trial Study Group HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet. 2010;376:333–9. doi: 10.1016/S0140-6736(10)60713-1. [DOI] [PubMed] [Google Scholar]
- 7.Ray KK, Cannon CP, Cairns R, Morrow DA, Ridker PM, Braunwald E, for the TIMI Study Group Prognostic utility of apoB/AI, total cholesterol/HDL, non-HDL cholesterol, or hs-CRP as predictors of clinical risk in patients receiving statin therapy after acute coronary syndromes: results from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol. 2009;29:424–30. [Google Scholar]
- 8.Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–10. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 9.deGoma EM, Leeper NJ, Heidenreich PA. Clinical significance of high-density lipoprotein cholesterol in patients with low low-density lipoprotein cholesterol. J Am Coll Cardiol. 2008;51:49–55. doi: 10.1016/j.jacc.2007.07.086. [DOI] [PubMed] [Google Scholar]
- 10.Jafri H, Alsheikh-Ali AA, Karas RH. Meta-analysis: statin therapy does not alter the association between low levels of high-density lipo-protein cholesterol and increased cardiovascular risk. Ann Intern Med. 2010;153:800–8. doi: 10.7326/0003-4819-153-12-201012210-00006. [DOI] [PubMed] [Google Scholar]
- 11.Boden WE, O’Rourke RA, Teo KK, et al. for the COURAGE Trial Research Group Optimal Medical Therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 12.Maron DJ, Boden WE, O’Rourke RA, et al. for the COURAGE Trial Research Group Intensive multifactorial intervention for stable coronary artery disease: optimal medical therapy in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial. J Am Coll Cardiol. 2010;55:1348–58. doi: 10.1016/j.jacc.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Merz CN, et al. for the Coordinating Committee of the National Cholesterol Education Program Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–32. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Boden WE, O’Rourke RA, Teo KK, et al. Design and rationale of the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial Veterans Affairs Cooperative Studies Program no. 424. Am Heart J. 2006;151:1173–9. doi: 10.1016/j.ahj.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 15.National Cholesterol Education Program. Second Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) Circulation. 1994;89:1333–445. doi: 10.1161/01.cir.89.3.1333. [DOI] [PubMed] [Google Scholar]
- 16.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 17.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 19.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 20.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 21.The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–64. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 22.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 23.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libby PJ. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46:1225–8. doi: 10.1016/j.jacc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 25.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 26.Cannon CP, Braunwald E, McCabe CH, et al. for the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 27.Deedwania P, Barter P, Carmena R, et al. for the Treating to New Targets Investigators Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet. 2006;368:919–28. doi: 10.1016/S0140-6736(06)69292-1. [DOI] [PubMed] [Google Scholar]
- 28.Olsson AG, Schwartz GG, Szarek M, et al. High-density lipoprotein, but not low-density lipoprotein cholesterol levels influence short-term prognosis after acute coronary syndrome: results from the MIRACL Trial. Eur Heart J. 2005;26:890–6. doi: 10.1093/eurheartj/ehi186. [DOI] [PubMed] [Google Scholar]
- 29.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, VA-HIT Study Group Veterans Affairs High-Density Lipoprotein Intervention Trial. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285:1585–91. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 30.AIM-HIGH Investigators. Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 31.Armitage J, for the HPS2-THRIVE Collaborative Group HPS2-THRIVE: Randomized placebo-controlled trial of ER Niacin and laropriprant in 25, 673 patients with pre-existing cardiovascular disease; Abstract presented at: American College of Cardiology Scientific Session 2013; March 9, 2013; San Francisco, CA. [Google Scholar]
- 32.Cannon CP, Dansky HM, Davidson M, et al. for the DEFINE Investigators Design of the DEFINE trial: determining the EFficacy and tolerability of CETP INhibition with AnacEtrapib. Am Heart J. 2009;158:513–9. doi: 10.1016/j.ahj.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Eli Lilly and Company. A Study of Evacetrapib in High-Risk Vascular Disease (ACCELERATE) Available at: http://clinicaltrials.gov/show/NCT01687998. Accessed March 24, 2013.
- 34.Barter PJ, Caulfield M, Eriksson M, et al. for the ILLUMINATE Investigators Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz GG, Olsson AG, Abt M, et al. for the dal-OUTCOMES Investigators Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 36.deGoma EM, Rader DJ. High-density lipoprotein particle number: a better measure to quantify high-density lipoprotein? J Am Coll Cardiol. 2012;60:517–20. doi: 10.1016/j.jacc.2012.03.058. [DOI] [PubMed] [Google Scholar]


