Summary
Approximately 50% of patients having metastatic melanoma develop brain metastases during the course of their illness. Evidence exists that melanoma cells have increased aptitude for the repair of sublethal DNA damage caused by ionizing radiation therapy. To address the radio-resistance of melanoma, many groups adopted radiotherapy schedules that deliver larger daily fractions of radiation, but due to the risk of neurotoxicity, these large fractions cannot be delivered to the whole brain for patients with brain metastases. Here, we used orthotopic implanted GRM1 expressing human melanoma cell xenografts in mice, to demonstrate that animals receiving concurrent glutamate signaling blockade (riluzole) and radiation led to a decrease in intracranial tumor growth compared to either modality alone. These preclinical results suggest riluzole may cause radiosensitization that offers enhanced efficacy for a subset of human melanoma patients undergoing radiotherapy for brain metastasis.
Keywords: melanoma, brain metastasis, glutamate signaling
The survival rate for patients with advanced stage melanoma ranges from 2 to 8 months with prolonged survival rates from 15 to 20% after 5 years (American Cancer Society, 2013). Targeted therapies have emerged as a paradigm for the treatment of a variety of cancers including melanoma with inhibition of multiple signaling pathways required to achieve greater successes in preclinical and clinical settings. Aberrant glutamate signaling has been shown to play a role in the transformation and maintenance of various cancer types including melanoma. Furthermore, recent studies have demonstrated that disrupting glutamatergic signaling yields promising therapeutic benefits in melanoma and other cancer types (Khan et al., 2011; Lee et al., 2011; Teh and Chen, 2012; Wall et al., 2014; Wangari-Talbot et al., 2012).
Our group and others have demonstrated that ectopic expression of metabotropic glutamate receptor 1 (GRM1) in melanocytes is sufficient to drive spontaneous melanoma development (Chen et al., 1996; Namkoong et al., 2007; Ohtani et al., 2008; Pollock et al., 2003; Zhu et al., 1998). Despite the lack of specific GRM1 antagonists with clinical applications, our group has successfully used Rilutek® (riluzole), an FDA approved drug for the treatment of amyotrophic lateral sclerosis (ALS), in clinical trials for stage III/IV melanoma patients (Yip et al., 2009). Riluzole functions as an antagonist to GRM1 by decreasing the levels of free ligand available to bind to the receptor resulting in reduced receptor activity (Doble, 1996). We demonstrated that, as in the neuronal system, riluzole suppresses the release of glutamate to the extracellular environment of melanoma cells that express GRM1 resulting in accumulation of cell populations in the G2/M phase of the cell cycle (Namkoong et al., 2007). It is known that cells arrested in the G2 phase are more sensitive to the reagents that induce DNA damage, such as ionizing radiation (IR) (Ballo and Ang, 2004; Ballo et al., 2006; Habermalz and Fischer, 1976). We performed a series of preclinical studies to assess whether riluzole could sensitize melanoma cells and augment the effects of sublethal doses of IR. We demonstrated that when riluzole is combined with IR a further increase in the apoptotic cell population and a decrease in tumor cell proliferation than each one alone was detected in in vitro cultured cells and in in vivo tumorigenicity assays (Khan et al., 2011). We assessed levels of the phosphorylated form of histone variant H2A (γH2AX), a well-known marker of DNA double-stranded break (DSB) and is frequently used to assess damage induced by IR. We found that not only did IR treatment result in DNA DSB, but riluzole treatment alone also yielded substantial levels of positive γH2AX staining. Combining IR with riluzole led to further increase in γH2AX levels. These results suggested that riluzole exposure of GRM1-expressing human melanoma cells contributes to G2/M arrest and sensitizes the cells to IR. Based on these findings and that riluzole crosses the blood–brain barrier, it is relevant that riluzole could be used as a potential therapy with adjuvant irradiation to treat brain metastases. In this report, we use an orthotopic mouse model of brain metastasis to demonstrate that riluzole is able to elicit its antiproliferative effects on human melanoma cells and is enhanced with ionizing radiation.
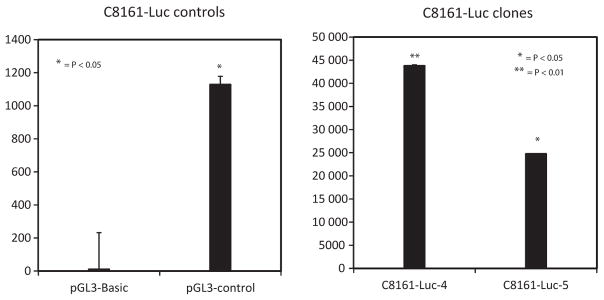
To measure the growth of intracranial lesions without sacrificing the animals at time intervals, we tagged the cells with bioluminescence using the reporter enzyme firefly luciferase (Fluc). We introduced luciferase cDNA into the GRM1-positive human melanoma cell C8161 and produced several stable clones (C8161-luc). IP injection of the substrate luciferin (15 mg/kg) into the animals allows for oxidation of the substrate by the luciferase resulting in photon emission that can be detected by a luminometer within 10–20 minutes after injection. As an initial test to ascertain the expression of functional luciferase in C8161-luc cells, we assessed luciferase activity in vitro using the Dual-Glo luciferase assay system measured using a Veritas Microplate Luminometer. We found that luminescence from the C8161-luc-expressing cells was significantly higher compared to positive control (Figure 1).
Figure 1.
Stable C8161 luciferase containing clones. C8161 cells containing the construct pCI-Neo-Luc+ (C8161-luc+) were assessed for luminescence using a Veritas Microplate Luminometer. The pGL3-basic vector (Promega), which lacks eukaryotic promoter and enhancer sequences, was used as a negative control. The pGL3-control vector contains SV40 promoter and enhancing sequences and was used at a positive control. Each bar represents mean ± SEM *P < 0.05; **P < 0.01.
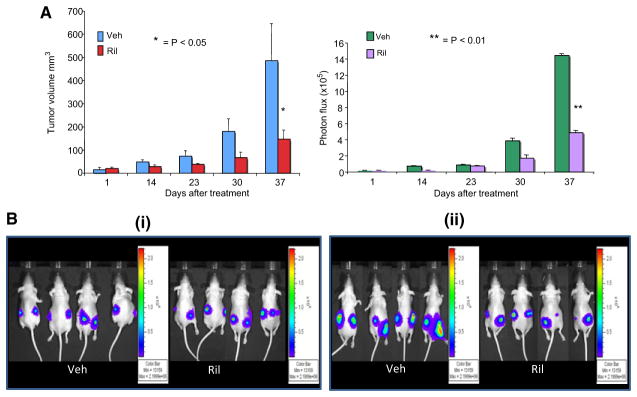
To see that luminescence acquired by an in vivo optical measurement could correlate to physical measurements from subcutaneously inoculated tumor cells, we conducted a pilot xenograft tumor experiment using C8161-luc cells. One-million luciferase expressing cells were injected subcutaneously into the dorsal flanks of athymic nude mice. When the tumor volumes reached 6–10 mm3 as measured using a vernier caliper, the mice were randomized into three treatment groups (n = 5) with similar tumor volume distribution: no treatment (data not shown), vehicle (Veh, DMSO), and riluzole (10 mg/kg b.w.). Treatment was administrated via oral gavage daily for 37 days for vehicle and riluzole groups. Tumor volumes were measured with a vernier caliper and acquisition and quantification of the bioluminescence signal intensities as acquired by IVIS small animal imaging system weekly. The data were expressed as photon flux (photons/s/cm2/steradian, where steradian refers to the photons emitted). The background photon flux was subtracted from signal intensities measured at the same site. Data were normalized to peak signal intensity of each time course. In parallel, tumors were measured using a vernier caliper and volumes calculated using the equation as described (Jacob et al., 2004). This allowed us to compare physical measurements to chemical measurements and conclude that a correlation in tumor size over time could be established (Figure 2). The physical measurement of tumor size correlates well with their luminescence in vivo; however, the correlation coefficient was low due to the small sample size of the experiment. Furthermore, using luminescence, one is able to measure the rate of growth of the same tumor in the same animal rather than sacrificing the animals at various time points throughout the experiment. By doing the experiment this way, one is able to accurately and effectively monitor the efficacy of a given compound on the same tumor throughout a single experiment. This report is simply a proof of principle that it is possible to monitor orthotopically implanted tumors particularly those that do not display obvious tumor progression physically.
Figure 2.
Comparison of physical measured tumor volume vs. luminescence intensity (A) C8161-luc+ cells were used to assess the correlation between physical and bioluminescent measurements. The data were expressed as photon flux (photons/s/cm2/steradian, where steradian refers to the photons emitted). (B) Grayscale photograph of the mice was first acquired followed by the acquisition and overlay of the pseudo-color luminescent image from violet (least intense) to red (most intense), representing the distribution of detected photon counts emerging from active luciferase within the animal. Panel (i) contains images of select animals taken on the first day of treatment, vehicle (DMSO) or riluzole (10 mg/kg) was administered by oral gavage daily. Panel (ii) contains images of select animals from day 37 of the experiment. Each bar represents mean ± SEM *P < 0.05; **P < 0.01.
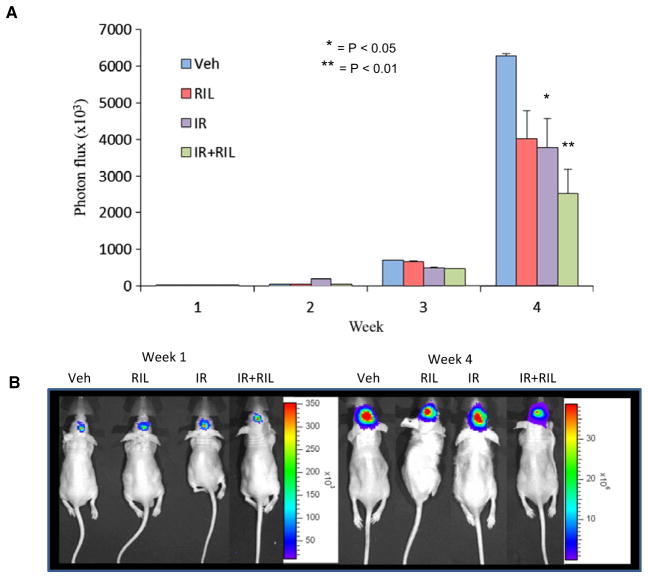
To mimic brain metastases in an animal model, C8161-luc cells were injected intracranially and monitored for tumor establishment using luminescent imaging. Upon initial detection of luminescence signal, mice were randomly divided into the following four groups: Group 1 was treated with dimethyl sulfoxide (DMSO, vehicle); group 2 was treated with vehicle and a weekly dose of 4 gray (Gy) of irradiation (IR); group 3 was treated with 10 mg/kg b.w. of riluzole daily via oral gavage (Ril); and group 4 was treated with 10 mg/kg b.w. of riluzole daily via oral gavage daily as well as a once weekly exposure of 4 Gy of irradiation (Ril + IR). The irradiation was performed in a γ-irradiator (Gammator 50, cesium 137 source; Radiation Machine Corp.) with a sleeve of lead (10 mm) covering the body of the animal leaving only the head exposed. The groups receiving irradiation were exposed to weekly fractions of 4 Gy. Tumors were measured weekly using the IVIS small animal imaging system for the detection of luminescent signal. Our results show that riluzole and irradiation alone resulted in decrease in tumor cell growth over time compared to vehicle treated alone in the intracranial model (Figure 3). The combinatorial treatment led to an even smaller tumor volume as demonstrated by the decrease of luminescent signal detected at week four compared to vehicle control and either modality alone. These results suggest the effectiveness of riluzole in sensitizing GRM1 expressing tumor cells to irradiation in the brain in vivo.
Figure 3.
Intracranial orthotopic transplants of human melanoma cells. C8161-luc+ melanoma cells were introduced into the brains of 6–7-week-old nude mice. Briefly, C8161-luc+ cells were harvested, counted, and adjusted to 10 000 cells/5 μl in 0.1% agarose in sterile PBS and kept at 37°C. For the procedure, mice were anesthetized using avertin (250–300 μl per 10 g body weight) by way of intraperitoneal injection and maintained throughout the procedure by inhalation of 5% isoflurane. Head was secured into a stereotactic frame using ear bars. Marcaine was injected at the incision site to prevent bleeding. A 1-cm midline scalp incision was made to expose the skull, and a small hole drilled using a #33G drill bit at coordinates 0.5 mm anterior and 2 mm lateral to the bregma. A Hamilton syringe with 10 μl capacity was used to deliver the cells at the rate 0.6 μl/min as to eliminate pressure causing reflux of the suspension. Bone putty was used to seal the drill site and a skin adhesive to close the wound. Once luminescent signal was detected, animals were separated into groups (n = 5) and treated with either DMSO (Veh), riluzole at 10 mg/kg b.w (Ril), weekly fraction of 4 Gy of ionizing radiation (IR), or a combination of the two (IR+Ril). Animals were sacrificed after 3 weeks of treatment due to tumor burden of control animals. (A) Data were expressed as photon flux (photons/s/cm2/steradian, where steradian refers to the photons emitted). (B) Images of animals showing initials images and images at the termination of the experiment. Each bar represents mean ± SEM *P < 0.05; **P < 0.01.
In contrast to most other cancer cell types, melanoma is more resistant to ionizing radiation therapy (Habermalz and Fischer, 1976; McKay and Kefford, 1995). As an attempt to address the perceived radio-resistance of melanoma, many groups have adopted radiotherapy schedules that deliver larger daily fractions of radiation (hypofractionation) to exploit the sensitivity of cells to larger fraction sizes. However, these large daily fractions cannot be delivered to the whole brain for patients with brain metastases due to the risk of late neurotoxicity (McKay and Kefford, 1995).
Our previous preclinical results suggest that riluzole enhances the lethal effects of ionizing irradiation in melanoma cells that ectopically express GRM1 likely through the arrest of melanoma cells in G2/M (Broadbent et al., 2004; Wall et al., 2014). Based on these findings, and the ability of riluzole to cross the blood–brain barrier and only affects cells that express the receptor, GRM1, it is particularly relevant that riluzole could be used as a potential radio-sensitizer enhancing adjuvant focal radiation and spares the rest of the brain and preserves patients’ brains’ function. To identify a compound that is relatively non-toxic for patients with brain metastases and, at the same time, is able to radio-sensitize the tumor cells is a treatment that has been proposed for a long time. Pretreatment with riluzole not only will sensitize brain metastases; the same principle can be applied to breast and prostate as many breast and prostate cancer specimens demonstrate GRM1 expression (Teh and Chen, 2012).
Significance.
Brain metastases represent a common terminal or preterminal event in patients harboring malignancies. Current treatment for patients having brain metastases involves combinations of steroids, surgical resection, stereotactic radiosurgery (SRS), and whole brain radiation therapy (WBRT). In contrast to other forms of cancers, melanoma patients with brain metastases have inferior results, with a 1-year survival rate of < 13%. This finding confirms the known relative radio-resistance of melanoma cell lines in vitro. Based on previous results demonstrating increased radio-sensitivity of human melanoma in vivo, we assessed the radio-sensitizing activity of riluzole, in a mouse model of brain metastasis.
References
- American Cancer Society. Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- Ballo MT, Ang KK. Radiotherapy for cutaneous malignant melanoma: rationale and indications. Oncology. 2004;18:99–107. discussion 107–10, 113–4. [PubMed] [Google Scholar]
- Ballo MT, Ross MI, Cormier JN, Myers JN, Lee JE, Gershenwald JE, Hwu P, Zagars GK. Combined-modality therapy for patients with regional nodal metastases from melanoma. Int J Radiat Oncol Biol Phys. 2006;64:106–113. doi: 10.1016/j.ijrobp.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Broadbent AM, Hruby G, Tin MM, Jackson M, Firth I. Survival following whole brain radiation treatment for cerebral metastases: an audit of 474 patients. Radiother Oncol. 2004;71:259–265. doi: 10.1016/j.radonc.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhu H, Wetzel WJ, Philbert MA. Spontaneous melanocytosis in transgenic mice. J Invest Dermatol. 1996;106:1145–1151. doi: 10.1111/1523-1747.ep12340194. [DOI] [PubMed] [Google Scholar]
- Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–S241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- Habermalz HJ, Fischer JJ. Radiation therapy of malignant melanoma: experience with high individual treatment doses. Cancer. 1976;38:2258–2262. doi: 10.1002/1097-0142(197612)38:6<2258::aid-cncr2820380611>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Jacob D, Davis J, Fang B. Xenograftic tumor models in mice for cancer research, a technical review. Gene Ther Mol Biol. 2004;8:213–219. [Google Scholar]
- Khan AJ, Wall B, Ahlawat S, Green C, Schiff D, Mehnert JM, Goydos JS, Chen S, Haffty BG. Riluzole enhances ionizing radiation-induced cytotoxicity in human melanoma cells that ectopically express metabotropic glutamate receptor 1 in vitro and in vivo. Clin Cancer Res. 2011;17:1807–1814. doi: 10.1158/1078-0432.CCR-10-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Wall BA, Wangari-Talbot J, Shin SS, Rosenberg S, Chan JL, Namkoong J, Goydos JS, Chen S. Glutamatergic pathway targeting in melanoma: single-agent and combinatorial therapies. Clin Cancer Res. 2011;17:7080–7092. doi: 10.1158/1078-0432.CCR-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay MJ, Kefford RF. The spectrum of in vitro radiosensitivity in four human melanoma cell lines is not accounted for by differential induction or rejoining of DNA double strand breaks. Int J Radiat Oncol Biol Phys. 1995;31:345. doi: 10.1016/0360-3016(94)e0147-c. [DOI] [PubMed] [Google Scholar]
- Namkoong J, Shin SS, Lee HJ, Marin YE, Wall BA, Goydos JS, Chen S. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67:2298–2305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- Ohtani Y, Harada T, Funasaka Y, Nakao K, Takahara C, Abdel-Daim M, Sakai N, Saito N, Nishigori C, Aiba A. etabotropic glutamate receptor subtype-1 is essential for in vivo growth of melanoma. Oncogene. 2008;27:7162–7170. doi: 10.1038/onc.2008.329. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Cohen-Solal K, Sood R, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- Teh JL, Chen S. Glutamatergic signaling in cellular transformation. Pigment Cell Melanoma Res. 2012;25:331–342. doi: 10.1111/j.1755-148X.2012.00983.x. [DOI] [PubMed] [Google Scholar]
- Wall BA, Wangari-Talbot J, Shin SS, Schiff D, Sierra J, Yu LJ, Khan A, Haffty B, Goydos JS, Chen S. Disruption of GRM1-mediated signalling using riluzole results in DNA damage in melanoma cells. Pigment Cell Melanoma Res. 2014;27:263–274. doi: 10.1111/pcmr.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangari-Talbot J, Wall BA, Goydos JS, Chen S. Functional effects of GRM1 suppression in human melanoma cells. Mol Cancer Res. 2012;10:1440–1450. doi: 10.1158/1541-7786.MCR-12-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip D, Le MN, Chan JL, Lee JH, Mehnert JA, Yudd A, Kempf J, Shih WJ, Chen S, Goydos JS. A phase 0 trial of riluzole in patients with resectable stage III and IV melanoma. Clin Cancer Res. 2009;15:3896–3902. doi: 10.1158/1078-0432.CCR-08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Reuhl K, Zhang X, Botha R, Ryan K, Wei J, Chen S. Development of heritable melanoma in transgenic mice. J Invest Dermatol. 1998;110:247–252. doi: 10.1046/j.1523-1747.1998.00133.x. [DOI] [PubMed] [Google Scholar]