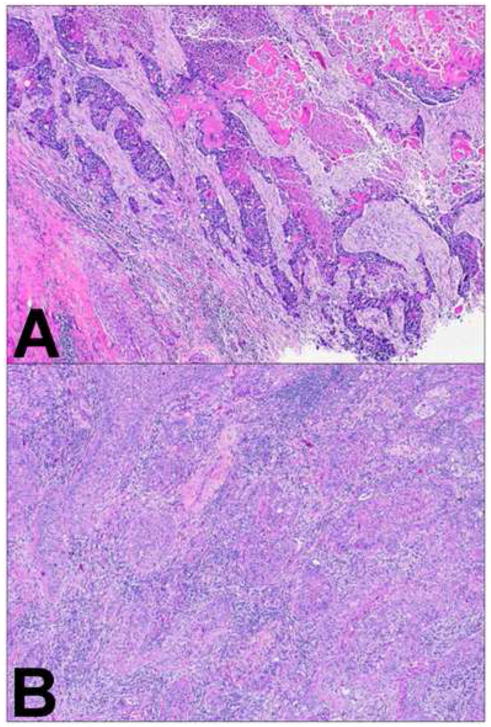
Abstract
The pathological classification of non-small cell lung cancer (NSCLC) is evolving. Lung adenocarcinoma is morphologically heterogeneous, with mixtures of acinar, papillary, bronchioloalveolar and solid patterns in more than 80% of cases. In case of synchronous or metachronous multiple NSCLC, the distinction of intrapulmonary metastases from independent primary tumors is of great clinical importance since it influences staging and potentially the therapeutic strategy. Here we took advantage of a cohort of 20 patients with 42 multiple NSCLC tumors (24 potential pair comparisons) that were annotated molecularly using genomic and mutational profiling to evaluate the value of comprehensive histologic assessment in this setting. Using the Martini-Melamed criteria, paired tumors were characterized as multiple primary NSCLCs in 21 cases and as intra-pulmonary metastases in 3 cases. Genomic and mutational data led to a diagnosis of multiple primaries in 14 cases and of metastases in 8 cases; 2 cases could not be assessed. This molecular characterization contradicted the Martini-Melamed diagnosis in 7 (32%) of the 22 assessable comparisons. Adenocarcinoma was found in 32 (76%) of the 42 tumors. After review in a blinded fashion, semiquantitative comprehensive histologic assessement of paired tumors was different in 16 and similar in 8 paired tumors. We found that comparing adenocarcinomas is a complex issue that requires assessment not only of percentages of the histologic subtypes, but also the recording of additional histologic details such as cytologic features, patterns of stroma, necrosis, discrete nodularity versus miliary growth and variants such as clear cell, signet ring, mucinous, and fetal patterns. We also found that paired squamous cell carcinomas could be compared based on histologic subtyping in addition to cytologic and stromal characteristics. Considering histologically different tumors as multiple primaries, and similar tumors as metastases, comprehensive histologic subtyping was consistent with the molecular characterization in 20 (91%) of the 22 pairs comparisons. In summary, based on a well characterized cohort with detailed clinical, pathologic and molecular data, we found comprehensive histologic assessment is a powerful tool that appears to be a promising way to determine whether multiple lung adenocarcinomas or squamous cell carcinomas are metastatic or multiple primaries. This has great clinical implications for staging and therapeutic management of lung cancer patients with multiple tumors. Given its high correlation with molecular characterization of such tumors, it may provide a much cheaper and faster method to address this problem
Keywords: Non-small cell lung cancer, adenocarcinoma, squamous cell carcinoma histology, classification, subtyping, multiple primary lung cancers, array comparative genomic hybridization, mutational profiling
Introduction
The pathological classification of non-small cell lung cancer (NSCLC) is evolving. Over the past 20 years, lung adenocarcinoma has become the most frequent histological type in the United States and most countries in the world [3], and major advances in the molecular biology of these tumors has led to new molecular strategies in the treatment of lung cancer [2, 9, 18] Lung adenocarcinoma is a highly heterogeneous disease at the morphological level, with the mixed subtype according to the 2004 WHO classification occurring in 80–90% of tumors, with mixtures of acinar, papillary, bronchioloalveolar and solid patterns [20]. Interestingly, recent studies have shown that the main morphological pattern of lung adenocarcinoma may correlate with specific molecular features: EGFR mutations are more frequent in tumors with predominant papillary and bronchioloalveolar patterns [2, 9, 13], KRAS mutations in mucinous bronchioloalveolar carcinomas [9], STK11 and TP53 mutations in solid adenocarcinomas [2], and EML4-ALK genes fusion in acinar tumors [5]. Overall, these data suggest that a more systematic comprehensive histologic assessment of NSCLC may predict specific molecular alterations.
Pathological evaluation of multiple NSCLC plays an important role in distinguishing intrapulmonary metastases versus metachronous or synchronous primaries. This distinction is of great clinical importance since it influences lung cancer staging and therapeutic strategy. Historically this has been achieved using clinicopathologic criteria designed by Martini and Melamed [6] (Table 1). In this classification, there was no distinction of histology beyond major subtype except for adenocarcinoma and bronchiolar carcinoma [6]. Since the 1970’s when these criteria were proposed, a variety of molecular tools have become available such as mutation profiling and array-based comparative hybridization techniques that provide a reliable and powerful way to evaluate the clonal relationships between multiple tumors [19, 12]. Moreover, adenocarcinoma has replaced squamous carcinoma as the most common histologic subtype [16], and we have learned that adenocarcinomas show frequent histologic heterogeneity.
Table 1.
Martini and Melamed criteria to define multiple primary non-small cell lung cancer (adapted from [6]).
| Synchronous multiple tumors | ||
|---|---|---|
|
| ||
| TUMOR LOCATION | HISTOLOGICAL TYPE | |
| SIMILAR | DIFFERENT | |
| Same segment | metastasis | multiple primary |
| Different segment | - Origin from carcinoma in situ, and no carcinoma in lymphatics common to both, and no systemic metastasis: multiple primary | multiple primary |
| - No carcinoma in situ, or carcinoma in lymphatics common to both, or systemic metastasis: metastasis | multiple primary | |
| Metachronous multiple tumors | ||||
|---|---|---|---|---|
|
| ||||
| TIME INTERVAL | TUMOR LOCATION | HISTOLOGICAL TYPE | FOCI OF CARCINOMA IN SITU | |
| SIMILAR | DIFFERENT | |||
| ≥ 2 years | multiple primary | multiple primary | multiple primary | |
| < 2 years | Same lobe | metastasis | multiple primary | multiple primary |
| Different lobe | - No carcinoma in lymphatics common to both, and no systemic metastasis: multiple primary | multiple primary | multiple primary | |
| - Carcinoma in lymphatics common to both, or systemic metastasis: metastasis | multiple primary | multiple primary | ||
In this study, we took advantage of a cohort of patients with multiple NSCLC tumors that were previously annotated molecularly using genomic and mutational profiling [4], to evaluate the value of comprehensive histologic assessment in differentiating multiple primary tumors from metastases. We also applied the concept of comprehensive histologic assessment to the squamous cell carcinomas in this cohort to see if it provided any useful information.
Materials and Methods
Tumor specimens
Eligible patients for this study were identified using Memorial Sloan-Kettering Cancer Center (MSKCC) Department of Pathology and Thoracic Surgery Service databases. We included all consecutive patients who received multiple operations for NSCLC from 1999 to 2007, and for which tumor frozen specimens from at least two tumors were banked separately. The use of these specimens was approved by the Institutional Review Board of MSKCC. Clinical information was obtained from patients medical records, and paired tumors were characterized as multiple primaries or metastases using the standard Martini-Melamed criteria [6].
Molecular characterization
Detailed molecular analyses have been reported previously [4]. Briefly, we used mutational and genomic profiling techniques to assess the clonal relationships between multiple tumors from individual patients. Using mass spectrometry-based genotyping (Sequenom, San Diego, CA), mutational profiling of selected genes was performed for activating mutations in 9 genes encoding components of the EGFR signaling pathway [2]: EGFR, KRAS, HRAS, NRAS, BRAF, PIK3CA, AKT1, ERBB2, and MEK1. In addition, dideoxynucleotide-based sequencing was performed for EGFR exon 19, and for all exons of TP53. Tumor pairs with similar mutations were diagnosed as metastases, and those with different mutations as multiple primary tumors [1].
For genomic profiling, DNA from each tumor was hybridized to Agilent 244K comparative genomic hybridization (CGH) arrays (Agilent Technologies, Santa Clara, CA). This array identifies copy number alterations on 236,000 coding and noncoding loci across the genome. Gains and losses were compared for paired tumors according to a previously described method that calculates the relative chances that at least one of the observed concordant genomic alterations is of clonal origin and classifies tumor pairs as clonal metastases or as independent multiple primary tumors [12]. Intermediate results were considered “equivocal”.
Comprehensive histologic assessment
Location, number, and size of the tumors were retrieved from pathology reports. Two pathologists (C.D. and W.D.T.) blindly reviewed hematoxylin and eosin (H&E)-stained slides from (1) formalin fixed, paraffin-embedded tumor blocks used for the routine surgical pathology diagnosis, and (2) from cryostat sections of frozen tumor specimens collected for molecular analyses (one slide per sample). Samples used for molecular analyses were selected for tumor content >70%. Histologic classification was done according to the 2004 WHO classification [20], with comprehensive histologic subtyping as previously described by Motoi et al, for mixed subtype adenocarcinomas [6]. Briefly, we semiquantitatively evaluated the relative percentage of each histologic subtype, including the acinar, papillary, bronchioloalveolar, solid, and micropapillary components, in 10% increments. The micropapillary pattern consisted of small papillary tufts observed within alveolar spaces or encased within thin walls of connective tissue, lacking fibrovascular cores [7]. On slides from paraffin-embedded specimens, tumors were also assessed for variants, i.e. mucinous, fetal, colloid, signet ring, or clear cell changes for adenocarcinomas, and papillary, clear cell, basaloid, or sarcomatoid features in squamous cell carcinomas.
The overall method of comprehensive histologic assessment included evaluation of not only the percentages of histologic subtypes, but also additional histologic features such as grade, cytologic features as well as stromal characteristics such as collagen, inflammation, lymphoid hyperplasia and/or necrosis were also considered in comparing the tumors (Figure 1). For squamous cell carcinoma, detailed histologic assessment was made according to the cytologic morphology, amount of keratinization, appearance of the stroma, necrosis, as well the presence of histologic components such as basaloid, clear cell, papillary or sarcomatoid carcinoma. Paired tumors exhibiting similar histological features were considered as metastases, and those showing different histologic features as multiple primaries (Figure 1).
Figure 1.
Comprehensive histologic assessment methodology for multiple non-small cell lung cancer.
Time-to-progression analysis
Time-to-progression was defined as the time interval between the resection of the second (or third) tumor and first recurrence. All patients were included in the statistical calculations. Time-to-progression was assessed using the Kaplan-Meier method. The influence of classifications on time-to-progression was studied using the Log Rank test. Results were considered significant at the 0.05 level. Statistical analyses were performed using the SPSS software program (Chicago, IL), version 17.0. Patient outcome was regarded as another method of evaluation of the clinical relevance of the various approaches to classification of the paired tumors. According to this approach, recurrence versus lack of recurrence was regarded to favor metastases versus synchronous primaries, respectively.
Results
Selection of samples and clinical characteristics
The case selection method and overall clinical features of these cases have been previously reported elsewhere [4]. Forty two NSCLC from twenty patients were studied, including 18 patients with 2 tumors and 2 patients with 3 tumors. This cohort allowed a total of 24 pair comparisons. In cases with two tumors there was one comparison, but in cases with three tumors, there were three comparisons. Tumors were synchronous in 6 patients and metachronous in 14 patients. Median interval between metachronous tumors was 24 months. Using the Martini-Melamed criteria, paired tumors were characterized as multiple primary NSCLCs in 21 cases and as intra-pulmonary metastases in 3 cases. The mean follow-up time for each patient was 4.4 years (range 1.6–10.5 years). Three patients with synchronous tumors and one patient with metachronous tumors received adjuvant chemotherapy.
Molecular characterization
Detailed results from molecular characterization of these multiple tumors have been reported separately [4]. Briefly, genomic profiling was informative for 22 tumor pair comparisons, leading to a diagnosis of metastases in 4 cases and of multiple primaries in 14 cases. The conclusion was equivocal in 4 comparisons, mostly because of a high background noise in the genomic profile data.
By mutational profiling, matching point mutations were observed in 8 tumor pairs, including the 4 pairs classified as metastases by genomic profiling and these 4 “equivocal” cases. Discordant mutations were observed in 9 paired tumors. Mutational status was consistent with the genomic profile in all of the 13 cases for which both data were available. This suggests that results from mutational analysis may be a surrogate for genomic profiling in the setting multiple lung tumors [4].
We then integrated the genomic and mutational data to assess clonality between these pairs of multiple primaries. The molecular consensus was first based on genomic profiling, and only if array-CGH data were not conclusive (i.e. in the 4 “equivocal” cases), on mutational analyses. Using this approach, genomic and mutational data led to a diagnosis of multiple primaries in 14 cases and of metastases in 8 cases. In the 2 paired comparisons that could not be assessed by array-CGH, genotyping did not disclose any mutation, and a molecular consensus diagnosis could not be made.
Consensus molecular characterization contradicted the Martini-Melamed diagnosis in 7 (32%) of the 22 comparisons, identifying metastases in 6 cases regarded clinically as multiple primaries, and multiple primaries in 1 case regarded as metastases [4].
Comprehensive histologic assessment
Surgical Pathology Slides
Comprehensive histologic assessment was done on H&E slides from formalin fixed, paraffin embedded tumor specimens (Figures 2–4). The number of slides from each tumor ranged from 2 to 12. Adenocarcinoma was the most frequent histological type found in 32 (76%) of the 42 tumors. Major histologic subtype was papillary in 18 (56%), acinar in 4 (13%), solid in 5 (16%), bronchioloalveolar in 3 (9%), and micropapillary in 2 (6%) tumors. Eight tumors were of squamous cell histology, and 2 tumors were large-cell neuroendocrine carcinoma (LCNEC). The results of comprehensive histologic assessment is shown in Table 2 and Figure 5.
Figure 2.
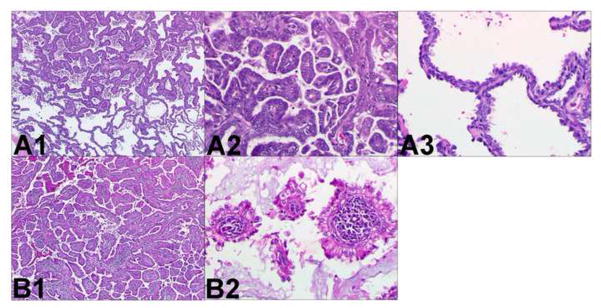
Two different adenocarcinomas. Comprehensive histologic review of case 1 (paraffin-embedded, formalin-fixed tissues, hematoxylin and eosin staining). The following histologic subtypes of adenocarcinoma are identified in tumor A (panel A1, x40): 60% papillary (panel A2, x200) and 40% bronchioloalveolar (panel A3, x200). The following histologic subtypes are identified in tumor B (panel B1, x40): 80% papillary (panel B2, x200), 10% acinar, and 10% bronchioloalveolar. In addition there is prominent lymphoid stroma. These different morphological features suggest these tumors are multiple primary lung cancer.
Figure 4.
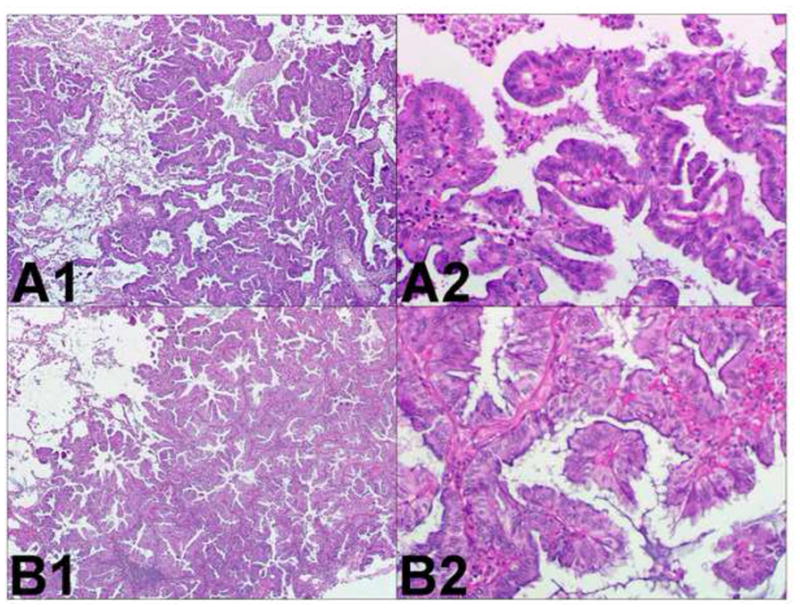
Two similar adenocarcinomas. Comprehensive histologic review of case 7 (paraffin-embedded, formalin-fixed tissues, hematoxylin and eosin staining). The following morphological subtypes are identified in tumor A (panel A1, x40): 80% papillary (panel A2, x200), 10% acinar, and 10% papillary (panel A3, x200). Tumor B (panel B1, H&E staining, x40) is exclusively made of papillary areas (panel B2, x200). These similar morphological features suggest these tumors correspond to metastatic lung cancer.
Table 2.
Comprehensive histologic assessment of paraffin-embedded samples
| Case | Presentation (interval) | Tumor | Location | Type | Subtype | Cytology or stroma | Histologic Comparison | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acinar | Papillary | BAC | Solid | Micro-papillary | |||||||
| 1 | synchronous | A | LLL | ADC | 0% | 60% | 40% | 0% | 0% | different | |
| B | RLL | ADC | 10% | 80% | 10% | 0% | 0% | lymphoid hyperplasia | |||
| 2 | synchronous | A | LLL | ADC | 0% | 0% | 100% | 0% | 0% | different | |
| B | RUL | ADC | 20% | 0% | 80% | 0% | 0% | ||||
| 3 | synchronous | A | RUL | ADC | 20% | 20% | 0% | 0% | 60% | different | |
| B | LUL | ADC | 10% | 70% | 0% | 0% | 20% | ||||
| 4 | synchronous | A | RLL | ADC | 10% | 50% | 30% | 0% | 10% | different | |
| B | LUL | ADC | 20% | 40% | 0% | 10% | 30% | ||||
| 5 | synchronous | A | LLL | ADC | 60% | 40% | 0% | 0% | 0% | different* | |
| B | LUL | LCNEC | n/a | n/a | n/a | n/a | n/a | different# | |||
| C | RUL | ADC | 20% | 0% | 0% | 80% | 0% | different ^ | |||
| 6 | synchronous | A | RUL | ADC | 10% | 50% | 30% | 0% | 10% | signet ring | similar |
| B | LLL | ADC | 30% | 50% | 10% | 0% | 10% | signet ring | |||
| 7 | metachronous (14.1 months) | A | LLL | ADC | 10% | 80% | 10% | 0% | 0% | mucinous | similar |
| B | RML | ADC | 0% | 100% | 0% | 0% | 0% | mucinous | |||
| 8 | metachronous (51.1 months) | A | LUL | ADC | 10% | 0% | 0% | 90% | 0% | different | |
| B | RUL | LCNEC | n/a | n/a | n/a | n/a | n/a | ||||
| 9 | metachronous (41.2 months) | A | RLL | ADC | 10% | 80% | 0% | 0% | 10% | similar | |
| B | RUL | ADC | 10% | 80% | 0% | 0% | 10% | ||||
| 10 | metachronous (25.1 months) | A | LUL | SCC | n/a | n/a | n/a | n/a | n/a | desmoplastic stroma necrosis |
different |
| B | RLL | SCC | n/a | n/a | n/a | n/a | n/a | inflammatory stroma | |||
| 11 | metachronous (42.7 months) | A | LUL | ADC | 10% | 0% | 0% | 90% | 0% | different | |
| B | RLL | ADC | 20% | 30% | 0% | 0% | 50% | ||||
| 12 | metachronous (23.0 months) | A | RUL | ADC | 20% | 70% | 0% | 10% | 0% | clear cells | different |
| B | LUL | ADC | 60% | 40% | 0% | 0% | 10% | ||||
| 13 | metachronous (18.9 months) | A | RLL | ADC | 0% | 50% | 40% | 0% | 10% | “miliary-like” extension | similar |
| B | RUL | ADC | 20% | 80% | 0% | 0% | 0% | “miliary-like” extension | |||
| 14 | metachronous (18.6 months) | A | RUL | ADC | 90% | 0% | 10% | 0% | 0% | different | |
| B | LUL | ADC | 80% | 10% | 0% | 10% | 0% | ||||
| 15 | metachronous (50.2 months) | A | LUL | SCC | n/a | n/a | n/a | n/a | n/a | different | |
| B | RUL | SCC | n/a | n/a | n/a | n/a | n/a | necrosis | |||
| 16 | metachronous (53.6 months) | A | LLL | SCC | n/a | n/a | n/a | n/a | n/a | different | |
| B | LUL | ADC | 10% | 90% | 0% | 0% | 0% | ||||
| 17 | metachronous (33.3 months) | A | LUL | SCC | n/a | n/a | n/a | n/a | 0% | different | |
| B | RUL | ADC | 0% | 60% | 40% | 0% | 0% | clear cells | |||
| 18 | metachronous (72.2 months) | A | LUL | ADC | 20% | 20% | 60% | 0% | 0% | mucinous | different |
| B | RLL | ADC | 30% | 50% | 0% | 0% | 0% | fetal (20%) | |||
| 19 | metachronous (13.5 months) | A | LLL | SCC | n/a | n/a | n/a | n/a | n/a | sarcomatoid/basaloid | similar |
| B | LLL | SCC | n/a | n/a | n/a | n/a | n/a | sarcomatoid/basaloid | |||
| 20 | metachronous (6.6 months*) (11.5 months#) (18.1 months^) | A | LLL | ADC | 0% | 30% | 0% | 60% | 10% | clear cells | similar* |
| B | LUL | ADC | 30% | 20% | 0% | 50% | 0% | clear cells | similar# | ||
| C | LUL | ADC | 10% | 10% | 0% | 80% | 0% | clear cells | similar ^ | ||
ADC: adenocarcinoma; SCC: squamous cell carcinoma; LCNEC: large-cell neuroendocrine carcinoma; BAC: bronchiolo-alveolar carcinoma; n/a: not applicable;
tumor A vs. tumor B;
tumor B vs. tumor C;
tumor A vs. tumor C
Figure 5.
Results of the comprehensive histologic assessment of paraffin-embedded samples
Comparison of two adenocarcinomas, however, was complex and required incorporation of multiple pieces of morphologic information. One of the most useful ways to compare two adenocarcinomas to determining whether two tumors were morphologically similar or different was the recording of percentages of histologic subtypes. In all 9 pairs where the adenocarcinomas were regarded as different (cases 1–4, 5 (tumor A vs. tumor C), 11, 12, 14, and 18), the percentages were different between the paired tumors, even if they had the same predominant histologic subtype (Table 2, Figure 5). In addition, in one of the paired tumors from one case each, the presence of lymphoid hyperplasia in the stroma (case 1), or mucinous cells and fetal adenocarcinoma (case 18) in one of the paired tumors, also helped to determine the tumors were morphologically different.
However, even when two adenocarcinomas were regarded as similar (7 pairs: cases 6, 7, 9, 13, and 20:tumor A vs. tumor C, tumor B vs. tumor C, tumor A vs. tumor B), they did not always have exactly the same percentages of histologic subtypes, and other histologic features were needed to compare the tumors. In all 7 cases, tumors regarded to be similar had the same most prominent subtype. However, in 6 cases, there were differences in the distribution of the other histologic subtypes, but because of similar cytologic features along with the common presence of signet ring (case 6), mucinous cells (case 7), a “miliary”-like extension in the lung parenchyma (case 13) (Figure 6), or clear cells (case 20) (Figure 7), these were considered as being similar (Figure 5).
Figure 6.
Two similar adenocarcinomas. Comprehensive histologic review of case 13 (paraffin-embedded, formalin-fixed tissues, hematoxylin and eosin staining). A “miliary-like” architecture is present in tumor A with small papillary clusters of tumor cell scattered through the lung parenchyma (panel A, x40) and tumor B (panel B, x40). These similar morphological features suggest tumor B is a metastasis from tumor A.
Figure 7.
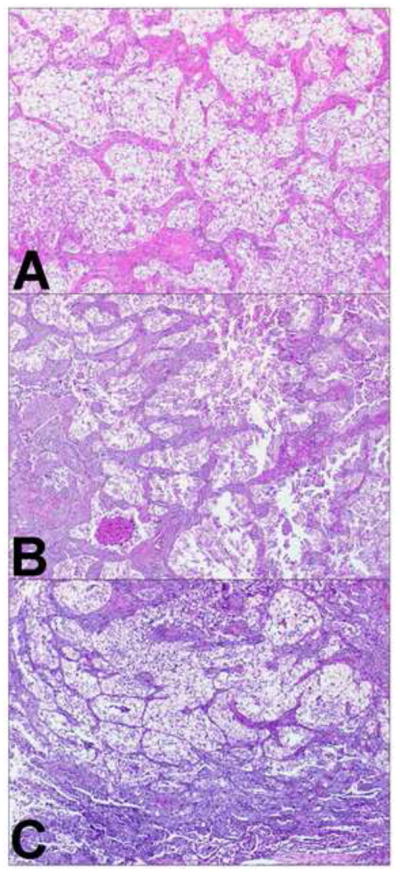
Three similar adenocarcinomas. Comprehensive histologic review of case 20 (paraffin-embedded, formalin-fixed tissues, hematoxylin and eosin staining). Nests of clear tumor cells surrounded by fibrovascular stroma were identified in tumor A (panel A, x40), tumor B (panel B, x40), and tumor C (panel C, x40). These similar morphological features suggest tumor B and C are metastasis from tumor A.
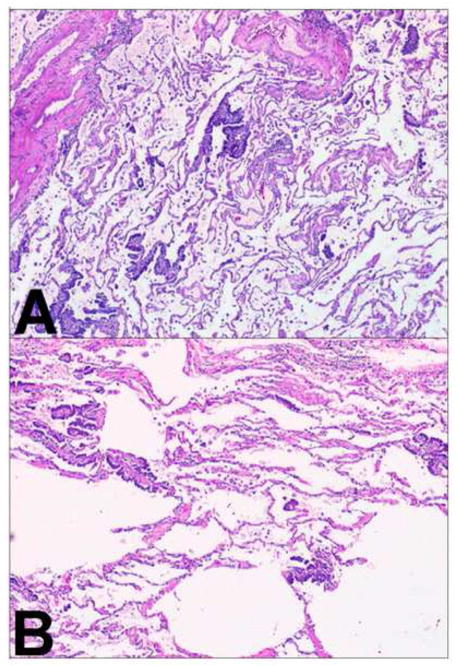
For the three cases of paired squamous cell carcinomas (cases 10, 15, and 19), two pairs were regarded as being morphologically different while one pair was interpreted to be the same. In case 15, the presence of necrosis in only one of the tumors helped to determine they were morphologically different. In case 10, it was the architecture of the stroma and the extent of necrosis that helped to distinguish the two tumors (Figure 8). In case 19, it was the presence of both sarcomatoid and basaloid components in each tumor that helped to determine the tumors were the same histologically (Figure 9). In both of these tumors the sarcomatoid component was less than 10% of the entire tumor, so they were not regarded as a pleomorphic carcinoma.
Figure 8.
Two different squamous cell carcinomas. Comprehensive histologic review of case 10 (paraffin-embedded, formalin-fixed tissues, hematoxylin and eosin staining). Stroma exhibits desmoplastic features in tumor A (panel A, x40) and inflammatory features in tumor B (panel B, x40). These different morphological features suggest these tumors correspond to multiple primary lung cancer.
Figure 9.
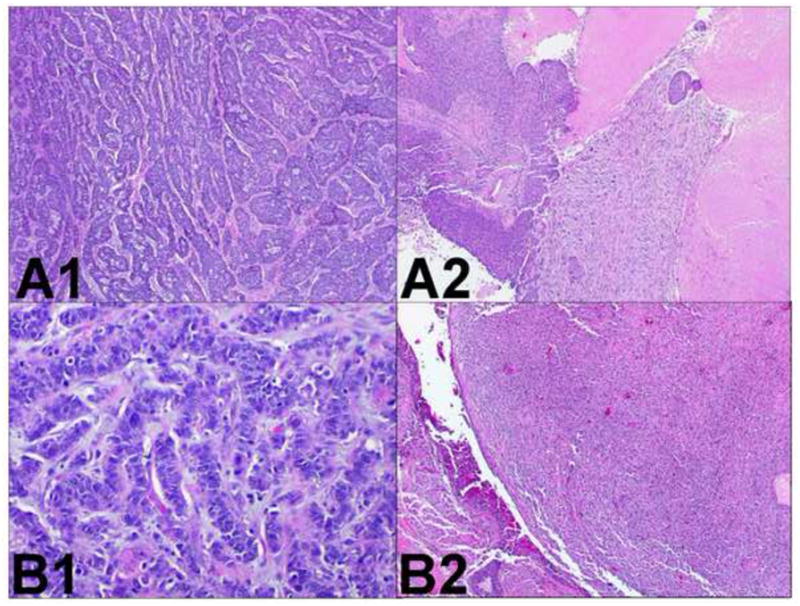
Two similar squamous cell carcinomas. Comprehensive histologic review of case 19 (paraffin-embedded, formalin-fixed tissues, hematoxylin and eosin staining). The sarcomatoid and basaloid variants are identified in tumor A (panel A1, x200 and panel A2, x40) and tumor B (panel B1, x200 and panel B2, x40). These similar morphological features suggest tumor B is a metastasis from tumor A.
In summary, comprehensive histologic comparison of paired tumors was different in 16 cases, and similar in 8 cases. Considering histologically different tumors as multiple primaries, and similar tumors as metastases, comprehensive histologic assessment was consistent with the molecular characterization in 20 (91%) of the 22 pairs comparisons (Table 3). In one pair (case 5, tumor A vs. tumor C), molecular consensus was actually supported only by mutational profiling, as array-CGH interpretation was equivocal. In this case, the molecular consensus was based on the presence of an identical KRAS G12C mutation in both tumors, but comprehensive histologic typing demonstrated striking morphologic differences and furthermore the tumors were contralateral, favoring synchronous primaries. In the second case (case 19), array CGH favored the two tumors were different, but both squamous cell carcinomas had a very distinctive basaloid and focally sarcomatoid pattern, leading the comprehensive histologic interpretation to favor intrapulmonary metastasis. This patient developed recurrent lung cancer 14 months after tumor B. Interestingly, the 2 discrepant cases were also misclassified using the standard Martini-Melamed criteria. Overall, comprehensive histologic assessment contradicted the Martini-Melamed clinical criteria in 5 (21%) cases; in these 5 cases, comprehensive histologic assessment was concordant with the molecular consensus.
Table 3.
Comparison of comprehensive histologic assessment with Martini-Melamed criteria and molecular characterization.
| Case | Martini-Melamed | Molecular consensus | Comprehensive histologic review | |
|---|---|---|---|---|
| frozen | paraffin | |||
| 1 | multiple primary | multiple primary | different | different |
| 2 | multiple primary | multiple primary | similar | different |
| 3 | multiple primary | multiple primary | different | different |
| 4 | multiple primary | multiple primary | similar | different |
| 5 (A vs. B) | multiple primary | multiple primary | different | different |
| 5 (A vs. C)* | multiple primary | metastases | different | different |
| 5 (B vs. C) | multiple primary | multiple primary | different | different |
| 6 | metastases | metastases | similar | similar |
| 7 | multiple primary | metastases | similar | similar |
| 8 | multiple primary | multiple primary | different | different |
| 9 | multiple primary | metastases | similar | similar |
| 10 | multiple primary | multiple primary | n/a | different |
| 11 | multiple primary | unknown | different | different |
| 12 | multiple primary | multiple primary | similar | different |
| 13 | multiple primary | metastases | similar | similar |
| 14 | multiple primary | multiple primary | different | different |
| 15 | multiple primary | multiple primary | n/a | different |
| 16 | multiple primary | multiple primary | different | different |
| 17 | multiple primary | multiple primary | different | different |
| 18 | multiple primary | unknown | different | different |
| 19* | metastases | multiple primary | different | similar |
| 20 (A vs. B) | multiple primary | metastases | similar | similar |
| 20 (B vs. C) | metastases | metastases | similar | similar |
| 20 (A vs. C) | multiple primary | metastases | similar | similar |
indicates discrepancies between the comprehensive histologic assessment and the molecular consensus.
Frozen Section Slides
Evaluation of the frozen section slides from the tissue used for molecular analyses was not as accurate as the interpretation of the formalin fixed, paraffin-embedded sections used for surgical pathology diagnosis (Supplemental Table 1). Especially, adenocarcinoma and squamous cell carcinoma histological subtypes could not be reliably recognized. For adenocarcinomas, generally the major subtypes identified in the permanent sections were seen in the frozen sections 21 cases (66%). In 11 (34%) cases, the major subtype seen on permanent sections was not seen on frozen section. These differences were interpreted to be because of sampling issues or frozen section artifacts.
Overall, the accuracy of comprehensive histologic examination was lower when using H&E slides from frozen tumor tissue specimens, as results were consistent with the molecular characterization in 14 (64%) pairs, discordant in 6 (27%) pairs, and not possible in 2 cases (involving SCCs) (Table 3).
Time-to-progression
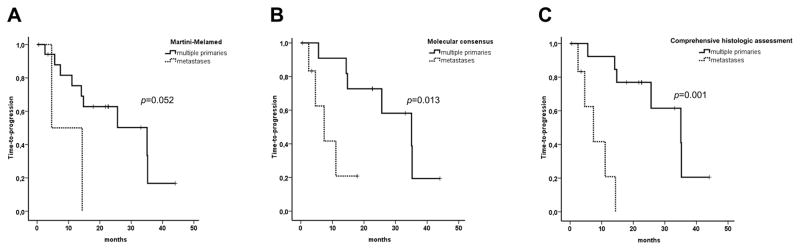
Overall, median time-to-progression from the last resection was 25.6 months (95% CI: 0–51.8 months). According to the Martini-Melamed criteria, molecular characterization, and comprehensive histologic assessment, median time-to-progression was 35.1 months for multiple primaries, and 4.6, 7.4, and 7.4 months for metastases, respectively (p=0.052; p=0.013; p=0.001, respectively) (Figure 10). The reason for the better survival according to comprehensive histologic assessment compared to molecular characterization is because the clinical outcome in cases 5 and 19, was more consistent with the histologic rather than the molecular interpretation. These data suggests that molecular characterization and comprehensive histologic assessment might be more relevant than the Martini-Melamed criteria alone to distinguish multiple primary tumors from metastases.
Figure 10.
Survival analysis. Time to progression of multiple primary and metastatic non-small cell lung tumors, as classified using the Martini-Melamed criteria (A), molecular consensus (B), and comprehensive histologic assessment (C). Time to progression was calculated from the resection of the last tumor. Squamous cell carcinoma was the most common histologic type in the Martini-Melamed study, and adenocarcinoma was most common in the current study.
Discussion
The major purpose of this study was to investigate whether detailed surgical pathology assessment of multiple NSCLC could predict whether the tumors were intrapulmonary metastases vs. synchronous primaries. In this study, using routine light microscopic pathologic assessment of multiple lung carcinomas with comprehensive histologic subtyping in addition to assessment of other detailed tumor histologic characteristics, we showed that the accuracy of pathology to differentiate multiple primary tumors from metastases, as defined by molecular characterization, was as high as 91%. Furthermore, the significantly worse outcome for patients with tumors regarded as metastases compared to synchronous tumors, according to both surgical pathology as well as molecular assessment supports that these assessments were clinically relevant. Both molecular and comprehensive histologic assessment appeared to more accurately classify multiple tumors than the Martini-Melamed criteria.
In our cohort, the standard Martini-Melamed criteria correlated with molecular characterization only in 68% of cases. Not only is adenocarcinoma currently the most common histologic subtype of lung cancer, in contrast to the time when Martini and Melamed proposed their criteria and squamous cell carcinoma was most common, but recent surgical series report that most multiple lung cancers are adenocarcinomas [11, 14, 15, 17]. In the Martini Melamed criteria, tumors were distinguished if they were in separate WHO major histologic subtypes such as adenocarcinoma, or squamous cell carcinoma but at that time, the 1967 WHO classification was in effect and there were only two types of lung adenocarcinoma recognized: bronchiolar and adenocarcinoma [21]. In addition, the current 2004 WHO classification provides limited information when comparing multiple lung adenocarcinomas, since over 90% of all tumors are classified as the mixed subtype [20]. Our results suggest that comprehensive histologic assessment supersedes the Martini-Melamed clinical criteria to differentiate multiple primary tumors from metastases [9]. However, this is a complex issue that requires assessment not only of percentages of the histologic subtypes in lung adenocarcinomas [10], but also the recording of additional histologic details (Figure 1). It is expected that there would be some variation in percentages even in identical tumors based on differences in sampling of the gross specimen. Furthermore in lung carcinomas with a poorly differentiated component, one might expect to see a greater proportion of the poorly differentiated histology in the metastatic lesion.
The clinical significance of comprehensive histologic assessment has not been tested in squamous cell carcinomas. Although these tumors do not typically show the same degree of heterogeneity as lung adenocarcinomas, there can be distinctive histologic characteristics that allow for comparison between tumor pairs such as degree of keratinization, amount of necrosis, quality of stroma including pattern of desmoplasia or inflammation as well as the presence of currently recognized variants including clear cell, papillary, basaloid or sarcomatoid patterns [8]. Although we only had three patients with paired squamous cell carcinomas in this study, there were distinctive histologic features in each of these patients that allowed for recognition that tumors were different in 2 cases or similar in one. Despite this small number of cases, these findings suggest that attention to details of morphologic characteristics even for some squamous cell carcinomas may allow pathologists to distinguish whether tumors are similar or different.
We had more difficulty in accurately classifying multiple lung carcinomas as synchronous primaries versus metastases when reviewing cryostat sections from frozen specimens, as compared with the slides from complete surgical pathology specimens based on formalin-fixed, paraffin-embedded samples. Although the frozen sections examined were from the tissue used for molecular analysis, these findings are applicable to the intraoperative consultation setting where pathologists may be asked to address the problem of similar versus different morphology on frozen sections. It is understandable that the data would be less accurate, due to limited histologic sampling of a single piece of tissue, making it more difficult to assess adenocarcinoma percentages of histologic subtypes. This is especially true in this study, because most of the frozen specimens were smaller than 0.5 cm. In addition, poorly differentiated tumors such as adenosquamous carcinoma and large-cell neuroendocrine carcinoma as well as some morphological variants of adenocarcinoma, including clear cell or signet-ring patterns, are difficult to identify in non-fixed tissues. Therefore, we would recommend caution in the frozen section setting for comparing separate lung cancers.
Comprehensive histologic assessment may also have advantages over molecular analyses, because it is a more rapid and inexpensive method which is feasible in a routine pathology practice setting. Compared to array-CGH, comprehensive histologic assessment led to a more reliable diagnosis, since array-CGH was uninformative in some cases based on poor DNA quality. Moreover, compared to the mutational profiling performed in this study, comprehensive histologic assessment was also more informative, since many tumors were wildtype for all mutations tested, making clonality difficult to establish. Although one could also consider applying immunohistochemistry and mucin stains to this problem, we found light microscopy alone was sufficient in the cases we studied.
Comprehensive histologic assessment is a relatively new concept that needs further study, not only in the setting of multiple lung tumors, but also in the context of histologic and molecular classification of lung adenocarcinomas and squamous cell carcinomas [9]. Its reproducibility among pathologists remains to be established, although for the problem of multiple lung cancers, reproducibility between different pathologists should not be an issue if in a single patient the same pathologist is able to review all the available tumors. This also underscores the importance of obtaining the histologic slides of all resected tumors for comparison when assessing the question of metastatic versus synchronous or metachronous primaries. If slides are not available from a lung cancer that has been previously diagnosed with comprehensive histologic assessment, it is possible in some cases from the report alone for a pathologist to make an educated assessment whether two tumors might be similar or different morphologically. Since at the moment, there are no known specific molecular correlations with histologic patterns, comprehensive histologic subtyping cannot be used to predict the underlying genetic differences/similarities among tumors from different patients. Finally, if only small biopsies are available rather than surgical resection specimens, mutational profiling may represent the only way to characterize multiple lung tumors.
In summary, based on a well characterized dataset with clinical and detailed molecular data, we have identified a way to turn the often frustrating problem of histologic heterogeneity of lung adenocarcinoma into a powerful tool to accurately classify multiple lung cancers as synchronous primaries versus metastasis. To our surprise, we also found there may be a way to apply this approach to squamous cell carcinomas in addition to adenocarcinomas. This has great clinical implications for staging and therapeutic management of lung cancer patients with multiple tumors. Overall, we recommend that comprehensive histologic assessment could be considered as a promising way to determine whether multiple adenocarcinomas and squamous cell carcinomas are metastatic versus synchronous or metachronous primaries. Given its high correlation with molecular characterization of such tumors, it may provide a much cheaper and faster method to address this problem. A limitation of this study is the small number of patients, but our findings in a highly characterized clinical and molecular dataset are compelling enough to warrant testing in larger datasets.
Supplementary Material
Figure 3.
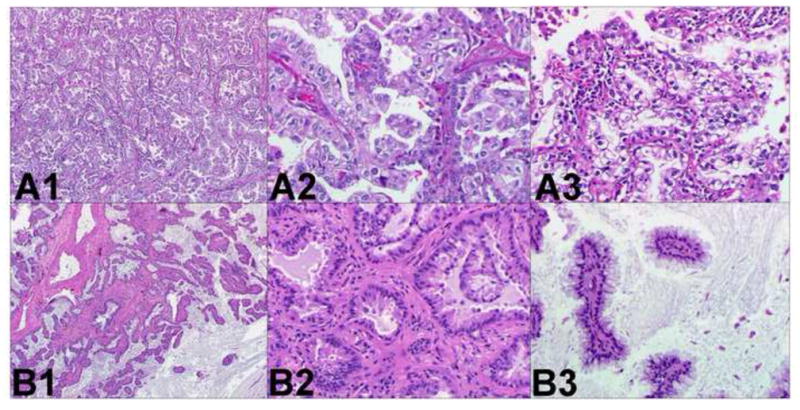
Two different adenocarcinomas. Comprehensive histologic review of case 12 (paraffin-embedded, formalin-fixed tissues, hematoxylin and eosin staining). The following morphological subtypes are identified in tumor A (panel A1, x40): 70% papillary (panel A2, x200) and 30% acinar with clear cell features (panel A3, x200). The following morphological subtypes are identified in tumor B (panel B1, x40): 60% acinar (panel B2, x200) and 40% papillary with abundant extracellular mucin. These different morphological features suggest these tumors are multiple primary lung cancer.
Acknowledgments
We thank the Tumor Procurement Service of the MSKCC Department of Pathology, Bernard Park from the Department of Surgery for his helpful assistance in the identification and the clinical characterization of tumor samples; Marc Ladanyi for his assistance for mass-spectrometry sequencing; and Colin B. Begg and Irina Ostrovmaya for analyzing the CGH data and providing helpful comments.
This study was supported by the National Cancer Institute (CA124504; CBB), the HOPP Lung Cancer Research Fund (WP), the Rosalind Warren Memorial Fund (WP), and the MSKCC Geoffrey Beene Cancer Research Center (WP).
Nicolas Girard is a recipient of travel grants from the College des Professeurs de Pneumologie (CEP)/AstraZeneca, the National Federation of French Comprehensive Cancer Centers/Fondation de France, and the Philippe Foundation.
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- 1.Braakhuis BJ, Tabor MP, Leemans CR, et al. Second primary tumors and field cancerization in oral and oropharyngeal cancer: molecular techniques provide new insights and definitions. Head Neck. 2002;24:198–206. doi: 10.1002/hed.10042. [DOI] [PubMed] [Google Scholar]
- 2.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabrielson E. Worldwide trends in lung cancer pathology. Respirology. 2006;11:533–538. doi: 10.1111/j.1440-1843.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 4.Girard N, Ostrovnaya I, Lau C, et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-09-0594. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3:13–17. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 6.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606–612. [PubMed] [Google Scholar]
- 7.Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol. 2003;27:101–109. doi: 10.1097/00000478-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Moro-Sibilot D, Lantuejoul S, Diab S, et al. Lung carcinomas with a basaloid pattern: a study of 90 cases focusing on their poor prognosis. Eur Respir J. 2008;31:854–859. doi: 10.1183/09031936.00058507. [DOI] [PubMed] [Google Scholar]
- 9.Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810–827. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 10.Nakano H, Soda H, Nakamura Y, et al. Different epidermal growth factor receptor gene mutations in a patient with 2 synchronous lung cancers. Clin Lung Cancer. 2007;8:562–564. doi: 10.3816/CLC.2007.n.043. [DOI] [PubMed] [Google Scholar]
- 11.Oliaro A, Filosso PL, Cavallo A, et al. The significance of intrapulmonary metastasis in non-small cell lung cancer: upstaging or downstaging? A re-appraisal for the next TNM staging system. Eur J Cardiothorac Surg. 2008;34:438–443. doi: 10.1016/j.ejcts.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 12.Ostrovnaya I, Olshen AB, Seshan VE, et al. Metastasis or a Second Independent Cancer? Evaluating the Clonal Origin of Tumors Using Array Comparative Genomic Hybridization Data. doi: 10.1002/sim.3866. Available at http://www.bepress.com/mskccbiostat/paper15/ [DOI] [PMC free article] [PubMed]
- 13.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice D, Kim HW, Sabichi A, et al. The risk of second primary tumors after resection of stage I non-small cell lung cancer. Ann Thorac Surg. 2003;76:1001–1007. doi: 10.1016/s0003-4975(03)00821-x. [DOI] [PubMed] [Google Scholar]
- 15.Riquet M, Cazes A, Pfeuty K, Ngabou UD, Foucault C, Dujon A, Banu E. Multiple lung cancers prognosis: what about histology? Ann Thorac Surg. 2008 Sep;86(3):921–6. doi: 10.1016/j.athoracsur.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 16.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75:191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::aid-cncr2820751307>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Trousse D, Barlesi F, Loundou A, et al. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J Thorac Cardiovasc Surg. 2007;133:1193–1200. doi: 10.1016/j.jtcvs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Weir BA, Woo MS, Getz G, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–898. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss MM, Kuipers EJ, Meuwissen SG, et al. Comparative genomic hybridisation as a supportive tool in diagnostic pathology. J Clin Pathol. 2003;56:522–527. doi: 10.1136/jcp.56.7.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Travis WB, Brambilla A, Muller-Hermelinck HK, Harris CC, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. WHO histological classification of tumours of the lung; p. 10. [Google Scholar]
- 21.World Health Organization. Histological typing of lung tumours. Geneva, Switzerland: World Health Organization; 1967. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.