Abstract
Background
Doxorubicin is one of the most commonly used chemotherapeutic drugs for breast cancer; however, its use is limited by drug resistance and side-effects. We hypothesized that adding FTY720, a sphingosine-1- phosphate (S1P) receptor functional antagonist, to doxorubicin would potentiate its effects by suppression of drug-induced inflammation.
Materials and Methods
The Cancer Genome Atlas (TCGA), GEO datasets, and NCI-60 panel were used for gene expressions and gene set enrichment analysis (GSEA). E0771 syngeneic mammary tumor cells were used. OB/OB mice fed with western high fat diet were used as an obesity model.
Results
STAT3 expression was significantly increased after doxorubicin treatment in human breast cancer that implicates that doxorubicin evokes inflammation. Expression of sphingosine kinase 1 (SphK1), the enzyme that produces S1P and links inflammation and cancer, tended to be higher in doxorubicin resistant human cancer and cell lines. In a murine breast cancer model, SphK1, S1P receptor 1 (S1PR1), IL6 and STAT3 were over-expressed in the doxorubicin treated group, whereas all of them were significantly suppressed with addition of FTY720. Combination therapy synergistically suppressed cancer growth both in vitro and in vivo. Furthermore, combination therapy showed higher efficacy in an obesity breast cancer model, where high body mass index (BMI) demonstrated trends toward worse disease free and overall survival, and high serum S1P levels in human patients and volunteers.
Conclusions
We found that FTY720 enhanced the efficacy of doxorubicin by suppression of drug-induced inflammation, and combination therapy showed stronger effect in obesity-related breast cancer.
Keywords: Breast cancer, Doxorubicin, FTY720, Obesity, Sphingosine-1-phosphate, Mouse model
1. Introduction
Doxorubicin, an anthracycline antibiotic, is one of the most commonly used chemotherapeutic agents for breast cancer. It is regarded as one of the most potent chemotherapeutic drugs (1, 2) and its response rate for metastatic lesions is approximately 25–40% (3). Despite its therapeutic effects, there are limitations to its use; one of them is drug resistance, and another is cardiotoxicity, which is a crucial anthracycline specific side-effect (4). Cumulative anthracycline dose results in doxorubicin-induced cardiotoxicity (5). Therefore, doxorubicin dose reduction in combination with another compound is expected to be an important strategy to overcome these limitations.
Sphinigosine-1-phosphate (S1P) is a bioactive lipid mediator generated by sphingosine kinases, SphK1, and SphK2 (6). Activated SphK1 increases intracellular S1P, which is secreted out of the cell (7) and acts extracellularly by binding to and signaling through S1P receptors (S1PRs) in autocrine and/or paracrine manners as “inside-out” signaling (8). This regulates cell proliferation, invasion, and angiogenesis in cancer cells (6, 8, 9) as well as in patients (10). We have established methods to measure the levels of S1P in tumor interstitial fluid (11) and lymphatic fluid (12), and demonstrated that S1P is associated with lymphangiogenesis and lymph node metastasis both in an animal model (13, 14) and in patients (15), which suggests that S1P in the tumor microenvironment worsens cancer progression (9, 13, 16–18). Furthermore, we recently found that S1P signaling plays an even more important role in metastatic triple-negative breast cancers (19). Given that S1P signaling is related to cancer malignant potential and progression, we hypothesized that S1P may contribute to doxorubicin resistance. FTY720, a functional antagonist of S1P receptors, is clinically used for the treatment of multiple sclerosis, thus its phase 1 trials are completed (8). FTY720 also demonstrates proven efficacy in multiple in vitro and in vivo cancer models, suggesting a potential therapeutic role in cancer patients (20).
A feature of obesity-related cancer is low-grade inflammation which increases cancer malignant potential (21, 22). S1P is known to play significant roles in inflammation (23–26) and we recently reported that S1P links inflammation and cancer progression, stimulates tumor-associated inflammation and increases cytokine levels (20, 27). We hypothesized that the addition of FTY720, which blocks S1P signaling and thus suppresses the effect of obesity-mediated inflammation, should enhance the anticancer effects of doxorubicin in this setting. Therefore, we investigated the efficacy of this combination therapy in obesity-related breast cancer.
2. Materials and Methods
2.1. Gene expression before and after doxorubicin treatment
DNA microarray gene expression data of humans before and after doxorubicin treatment were obtained through the GEO database (GSE28844) (28). Out of 33 participants, 17 cases that were not treated with doxorubicin were excluded from our analysis. Of the remaining 16 cases, 12 cases had both pre and post treatment gene expression data. 2 cases were classified as good response, 5 cases were mid response and 5 cases were bad response utilizing Miller and Payne grades (28).
2.2. Human samples
86 cases of The Cancer Genome Atlas (TCGA) contributor from Roswell Park Cancer Institute (RPCI) had body mass index (BMI) data that allowed survival analysis.
For serum S1P level analysis, blood was taken from 11 healthy volunteers at Virginia Commonwealth University Medical Center under the approval from its Institutional Review Board. The power analysis was based on our previous observation that obese patients have a higher serum S1P, with an estimated effect size of about 1.5. Based on this assumption, a total of 12 patients (6 in each of high and low BMI groups) will provide a power of 78% at 0.05 significant levels using one side test. 1, 7 and 4 individuals were found to be in the BMI <20, 20–24.9, and 25–29.9 groups, respectively. Serum was separated by centrifugation, and preserved at −80°C. Lipids were extracted from blood and sphingolipids quantified by liquid chromatography, electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS, 4000 QTRAP, AB Sciex) as previously described (13).
2.3. Gene set enrichment analysis (GSEA) of TCGA database
GSEA was performed on TCGA database using software provided by the Broad Institute (http://software.broadinstitute.org/gsea/index.jsp). We classified the patients into two groups according to BMI; high (BMI > 27) and low (BMI < 27). The enrichment of doxorubicin drug resistance related gene sets were identified within the Molecular Signature Database (MSigDB) curated collection (c2). The genes were preranked based on differentiation gene expression analysis of BMI groups using Bioconductor DESeq2 package.
2.4. Gene expression of doxorubicin sensitive and resistant cell line
Drug sensitivity and gene expression of cell line data were downloaded from NCI-60 panel using Bioconductor package rcellminer version 1.6.0. (29, 30). Based on the NCI-60 drug activity Z-scores derived from the 50% growth-inhibitory levels (GI50) determined by the DTP, we defined the cell line with a Z-score greater than 1.0 as sensitive, less than −1.0 as resistant. The thresholds of Z>1.0 and <−1.0 represent the top/bottom 16% of the cohort. Thus, we consider this cutoff to be more clinically relevant. Out of 60 cell lines, 4 and 6 cell lines were classified as sensitive and resistant, respectively. Expression value of each interested gene was quantified based on downloaded NCI-60 expression z-scores.
2.5. Cell culture and drug sensitive assay
E0771 cells, a mouse mammary adenocarcinoma cell line expressing the estrogen receptor (ER), were purchased from CH3 BioSystem, and were cultured in RPMI 1640 with 10% fetal bovine serum. E0771 cells were treated with either 0.03 μM doxorubicin (Selleck) and/or 2 μM FTY720 (Cayman). 48 hours after drug administration, the number of living cells was measured with Cell Counting Kit-8 (Dojindo), according to the manufacturer’s instructions. Combination index was calculated as (EDox+EFTY)/EDox+FTY (31).
2.6. Animal Models
Approval from the Roswell Park Cancer Institution Animal Care and Use Committee was obtained for all experiments based on our extensive experience in animal models (32–36). Female C57BL/6 mice and B6.cg-Lepob (OB/OB) mice were obtained from Jackson Laboratory. OB/OB mice were fed a high fat diet (Envigo, TD.88137) from 10 days prior to implantation of cancer cells in the obesity model. 1×106 of E0771 breast cancer cells were inoculated into the #2 mammary fat pad under direct vision as described (13, 37–41). Mice were treated with doxorubicin and/or FTY720. Doxorubicin was administrated by i.p. at a dose of 5 mg/kg on Day 0 and 3. FTY720 was administered everyday by gavage at a dose of 1 mg/kg. Anti-SphK1 antibody (Abcam) was used at 1:100 dilutions for immunohistochemistry.
2.7. Statistical Analysis
For continuous variables, statistical analyses were performed using Student t-tests. Categorical variables were compared using chi-square tests or Fisher’s exact tests when appropriate. Survival was estimated using the Kaplan–Meier method with log-rank tests. Differences with P values of less than 0.05 were considered significant. Experiments were repeated at least three times with consistent results. Data analyses were performed using R software version 3.1.2 (The R Foundation) and Bioconductor. All GSEA analyses presented here were performed using the Java GSEA implementation version 2.2.3 and MSigDB version 5.2.
3. Results
3.1. STAT3 was up-regulated after doxorubicin treatment in patient samples
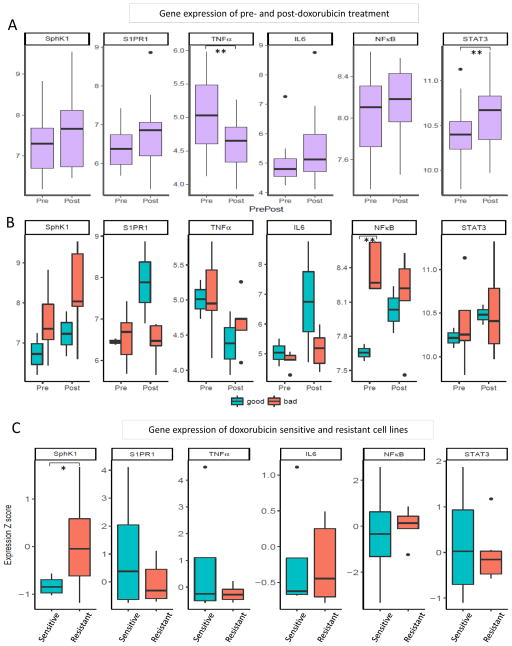
S1P and inflammation related gene expression of doxorubicin treated patients was analyzed using GEO database cohort. First, gene expression was compared between before and after treatments. STAT3 expression was significantly up-regulated after treatment compared to before treatment (p = 0.019). S1P signaling related genes, SphK1, S1PR1, and inflammation related genes, IL6 and NF-κB, all demonstrated the up-regulation trend in patient cancers after doxorubicin treatment (Figure 1A). There was no significant difference between before and after treatment in the expression of these genes; however, this may be due to the limitation of cohort sample size. On the other hand, TNFα expression was significantly reduced post-treatment (p = 0.024), consistent with a previous report (Figure 1A) (42). These patients were subdivided into “good” and “bad” response groups by Miller and Payne grades to doxorubicin by the authors who collected the samples (28). The bad response group expressed higher SphK1 in both pre- and post- treatment compared to the good response group; however, it was statistically not significant which was most likely due to small sample size (Figure 1B). Furthermore, pre-treatment NF-κB expression in the bad response group was significantly higher than in good response group (p = 0.002) (Figure 1B). Both results implicate that SphK1 and inflammation are associated with a bad response to doxorubicin.
Figure 1.
Differences in S1P signaling and inflammation related gene expression with doxorubicin treatment: (A) gene expression in patient tumors pre- and post- doxorubicin treatment, (B) gene expression in good and bad responders pre- and post- doxorubicin treatment, (C) gene expression in doxorubicin sensitive and resistant cell lines. **: p<0.05. *: p<0.1
3.2. Trend towards high expression of SphK1 in doxorubicin resistant cell lines
To evaluate for a difference in gene expression between doxorubicin sensitive and resistant cell lines, we compared gene expression using NCI-60 data. Interestingly, SphK1 expression in resistant cell lines demonstrated a tendency to be higher than sensitive cell lines, although there was no statistical significance (p = 0.096). There were no significant differences in TNFα, S1PR1, NF-κB, IL6 and STAT3 expression (Figure 1C).
3.3. Doxorubicin in combination with S1P receptor functional antagonist FTY720 inhibited breast cancer growth
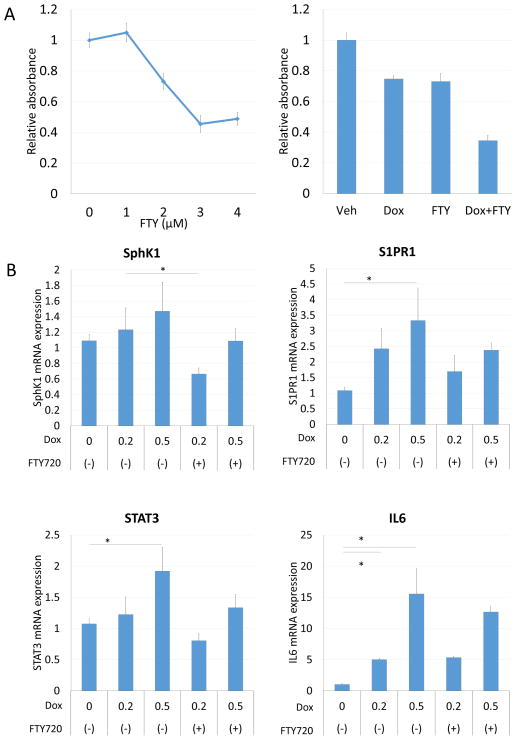
Our results that doxorubicin treatment up-regulate S1P signaling related genes such as SphK1 implies that activation of the SphK1/S1P/S1PR1 axis may play a role in doxorubicin resistance. This led us to hypothesize that antagonizing S1P signaling may enhance anti-cancer activity of doxorubicin. FTY720, a functional antagonist of S1PRs, suppressed E0771 cell proliferation in a dose dependent manner (Figure 2A). We then examined the effect of FTY720 in combination with doxorubicin. Doxorubicin in combination with FTY720 synergistically suppressed E0771 growth in vitro with a combination index of 0.80 (Figure 2A). Doxorubicin treatment induced a dose-dependent increase in SphK1, S1PR1, STAT3 and IL6 expression, whereas these were suppressed by FTY720 in agreement with our notion that FTY720 suppresses inflammation induced by doxorubicin (Figure2B).
Figure 2.
FTY720 enhanced doxorubicin efficacy in vitro. (A) Drug sensitivity assay of FTY720 for E0771. (B) Gene expression of E0771 after doxorubicin with and without FTY720 treatment. *: p<0.05.
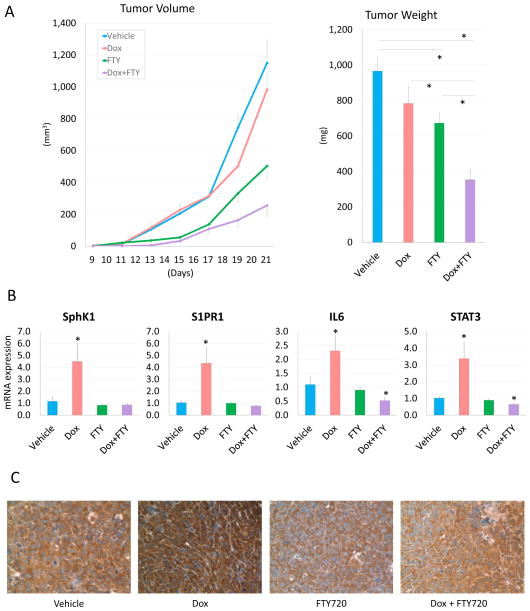
Doxorubicin in combination with FTY720 was then evaluated in a murine model in vivo. Combination therapy significantly reduced tumor burden compared to single drug treatment with either doxorubicin or FTY720 alone evaluated by tumor size measurements (Dox: p = 0.004, FTY720: p = 0.004, respectively) with a combination index of 0.78 (Figure 3A). In agreement with in vitro experiment results, doxorubicin treatment significantly up-regulated mRNA expression levels of S1P signaling related genes, SphK1 and S1PR1, and inflammation related genes, IL6 and STAT3, whereas combination therapy significantly suppressed their expressions (Figure 3B). This result was reproduced in protein level by immunohistochemistry that showed that SphK1 was over-expressed in the doxorubicin treated group, whereas it was decreased in the FTY720 and combination therapy groups (Figure 3C).
Figure 3.
Doxorubicin induced S1P and inflammation related gene overexpression in E0771 syngeneic breast cancer model. (A) Tumor growth with doxorubicin, FTY720 and combination therapy. Tumor weight in the combination therapy group was significantly less than the single drug treatment groups. (B) mRNA expression of SphK1, S1PR1, IL6 and STAT3 in the tumor were determined by qPCR and normalized to levels of GAPDH. (C) Immunohistochemistry of SphK1. SphK1 expression was upregulated in doxorubicin treated tumors. Data are expressed as means ± SEM. *, p < 0.05.
3.4. Patients with high BMI have worse overall and disease-free survival
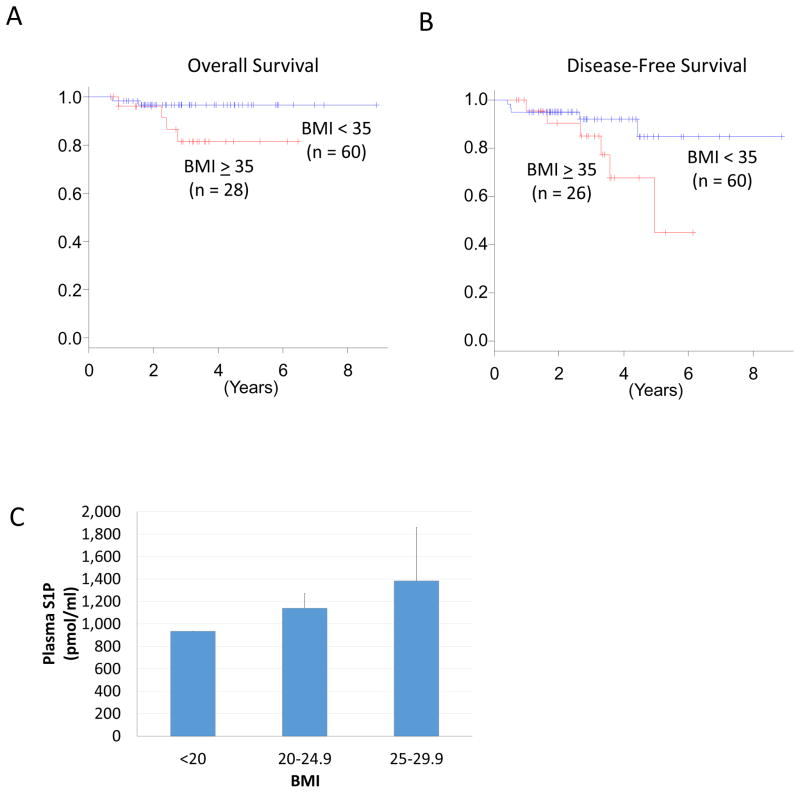
Obesity is an established independent prognostic factor for breast cancer patients (21, 22); however, several reports disagree with this notion (43, 44). In order to clarify whether this is the case in cohort at a national level, we evaluated overall survival (OS) and disease free survival (DFS) in high and low BMI patients in TCGA. We were able to identify only 88 and 86 patients with BMI and survival data for OS and DFS, respectively, in TCGA. We found that high BMI patients trended toward worse OS and DFS; however, statistical significance was not achieved, most likely due to small sample size (p = 0.075 and p = 0.087, respectively) (Figure 4A, B).
Figure 4.
Breast cancer prognosis and serum S1P levels according to BMI. (A, B) Higher BMI trended toward significantly worse OS and DFS. (C) Higher BMI individuals showed significantly higher plasma S1P levels in healthy individuals. Data are expressed as means ± SEM.
3.5. High serum S1P levels in high BMI individuals
In order to explore the relationship between serum S1P level and BMI, serum S1P levels in healthy volunteers were compared based on BMI. Interestingly, serum S1P levels were higher in the higher BMI individuals (Figure 4C). These findings indicated that higher serum S1P level is associated with higher BMI, which is in agreement with previous reports (45).
3.6. Doxorubicin in combination with FTY720 dramatically suppressed tumor growth in an obese syngeneic breast cancer model
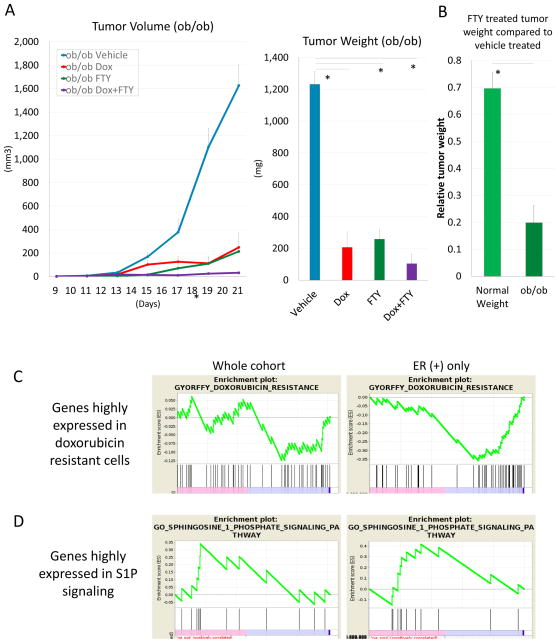
Since S1P links inflammation and cancer progression by stimulating tumor-associated inflammation and increasing cytokine levels, we hypothesized that the combination of doxorubicin with an S1P signaling antagonist has more benefit in obesity-related breast cancer, which is associated with low grade inflammation. Together with the results that S1P level is higher in obesity (Figure 4C) and that doxorubicin treatment induces inflammation by activating S1P signaling (Figure 1, 3), we hypothesized that the addition of FTY720 in combination with doxorubicin in obesity-related breast cancer would show more efficacy than in non-obesity related cancer. We treated genetically obese (OB/OB) mice bearing the E0771 tumor with doxorubicin and/or FTY720. As hypothesized, tumors in vehicle treated obesity mice grew approximately 1.3 times larger compared to normal weight mice (p = 0.024). FTY720 significantly suppressed tumor growth in obesity-related mice compared to vehicle treatment (p < 0.001) (Figure 5A), and the weight of FTY720 treated tumors showed 87% less than vehicle treated tumors, whereas the reduction rate was 48% in the non-obesity model (p = 0.004) (Figure 5B). Doxorubicin also significantly suppressed tumor growth in obesity-related mice (p < 0.001) (Figure 5A). Tumors in the combination therapy group showed smallest tumor size; however, there was no significant difference compared to single drug treatment.
Figure 5.
Doxorubicin and FTY720 suppressed tumor growth in an obesity syngeneic breast cancer model. (A) Tumor growth in doxorubicin, FTY720 and combination therapy groups in OB/OB mice. Tumor weight in each single drug treatment and combination therapy group was significantly less than the vehicle treated group. (B) FTY720 significantly suppressed tumor growth in an obesity model compared to a normal weight model. (C, D) GSEA of TCGA contributor from RPCI whole cohort and ER positive only. Lower BMI was significantly correlated with doxorubicin resistant genes, and higher BMI was significantly correlated with the S1P signaling pathway in the ER positive cohort. Data are expressed as means ± SEM. *, p < 0.05.
3.7. Higher BMI negatively correlated with the doxorubicin resistant gene set and positively correlated with the S1P signaling pathway gene sets in ER positive breast cancer
To validate the higher efficacy of FTY720 and doxorubicin in obesity related breast cancer, GSEA was performed. GSEA revealed that there was no correlation between BMI and the doxorubicin resistant gene set in the whole cohort; however, higher BMI was significantly negatively correlated with the doxorubicin resistant gene set in the ER positive patient cohort (ES = −0.359, p < 0.001) (Figure 5C). Similar to the doxorubicin resistant gene set, higher BMI was significantly correlated with the S1P signaling pathway in the ER positive cohort (ES = 0.415, p = 0.021), whereas there was no correlation between BMI and the S1P signaling pathway in the whole cohort (Figure 5D). These results are in agreement with our previous findings that estradiol activates SphK1 via ER in breast cancer cells, which generates S1P that will be secreted out of the cell (6, 7). These results implicate that treatment with doxorubicin in combination with FTY720 may be particularly useful for ER positive obese patients.
4. Discussion
In the current study, we found that doxorubicin treatment increased expression of S1P signaling related genes as well as inflammation related genes in human breast cancer (Figure 1A). In our previous study, we showed higher SphK1 is related to higher S1P levels (15) and we showed that S1P links inflammation and cancer progression, and administration of FTY720 suppressed the SphK1/S1P/S1PR1 axis and up-regulation of NF-κB and STAT3 along with decreasing the inflammatory cytokines of IL-6 suppressed the inflammation-associated colon cancer progression (27). In fact, chemotherapy-related increases in inflammatory cytokines have been observed in preclinical models and in human cancer patients during treatment (46, 47). It was also reported that doxorubicin treatment enhances SphK1 expression in leukemia cells (48). Our current study is in agreement with the notion that S1P signaling links inflammation and cancer.
The effectiveness of the pro-drug FTY720, approved for the treatment of multiple sclerosis, has been extensively used in preclinical studies to demonstrate the therapeutic value of modulating SphK1 and S1P receptor functions in inflammation and cancer. We have demonstrated recently that FTY720 administration interferes with the SphK1/S1P/S1PR1 axis and suppresses the NF-κB/IL-6/STAT3 malicious amplification loop and inflammatory colitis associated colon cancer in mice (20).
We demonstrated that the addition of FTY720, which is an S1P signaling functional antagonist, enhanced doxorubicin efficacy in breast cancer progression in vitro and in a murine syngeneic orthotopic breast cancer model. FTY720 significantly suppressed SphK1 and S1PR1 expression as well as inflammatory cytokine expression, including IL6 and STAT3. We have previously shown that FTY720 suppresses breast cancer progression in a murine model (11, 49). Indeed, it has been shown to induce dose-dependent cell death in human tumor cell lines, including hepatocellular carcinoma and bladder cancer (50, 51), and FTY720 has been shown to reduce tumor growth and metastasis in prostate cancer and melanoma (52, 53). Furthermore, FTY720 enhances the efficacy of cytotoxic anti-cancer therapies (54, 55) as well as agents associated with the S1P signaling for cancer therapy (56, 57). The combination of doxorubicin and SphK1-antisense oligonucleotides has also been shown to be effective against gastric cancer (58).
Initial systemic chemotherapies for breast cancer have been reported to be effective 90% and 50% of the time, in primary and metastatic lesions, respectively (3). However, after a variable period, progression occurs and the rates of response to subsequent agents are lower (3). This drug resistance is one of the major reasons for breast cancer mortality. Recent studies showed that inflammatory cytokines, including IL6, and transcription factors STAT3 and NF-κB, play critical roles in cancer drug resistance (46, 59). S1P is also known to play a role in drug resistance in various types of cancer (60–65). We found that NF-κB levels are predictive of doxorubicin response utilizing doxorubicin treated patients sample data. We provide the first report that showed the difference in NF-κB expression by chemotherapy response in breast cancer patients (Figure 1B). Furthermore, SphK1 expression levels were higher in doxorubicin resistant than sensitive cells (Figure 1C), which is consistent with previous reports (48).
Obesity is one of the most prevalent health issues in the US (66). It has been shown that obesity-related breast cancer is more aggressive with a poor prognosis, which is partly explained by the low-grade inflammation caused by obesity (67–71). Among the molecules involved in obesity-related inflammation, the transcription factors NF-κB and STAT3, and the major inflammatory cytokine IL6, are known key mediators of this process (68, 72, 73). Because we demonstrated that these mediators were suppressed by FTY720 in a normal weight murine model, we hypothesized that doxorubicin in combination with FTY720 would provide more benefit in obesity-related breast cancer. As hypothesized, combination therapy dramatically suppressed tumor growth in a murine obesity breast cancer model. Even single drug treatment with FTY720 demonstrated significantly higher efficacy than in a normal weight murine model (Figure 5B). The GSEA results demonstrated significant enrichment of the doxorubicin resistance gene set and the S1P signaling pathway gene set in ER positive obesity patients, which implied that ER positive obesity related breast cancer is associated with these good responses to doxorubicin as well as FTY720.
There are, however, some limitations in the present study. First, only a single ER positive cell line was used throughout the study. This was because of the limited availability of any other cell lines that are compatible with C57BL/6. Second, the publicly available human data have limitations. It is difficult to obtain drug treated patient sample data, especially pre and post treatment gene expression data, and similarly gene expression data with BMI are also difficult to obtain. Most likely due to small sample size, some of the differences did not reach statistical significance. It would have been ideal to measure serum S1P levels in breast cancer patients before and after doxorubicin treatment and in relationship with BMI; however, such samples were not available to evaluate that question in this study. The third limitation of this study was the inability to preserve tissue for multiple analyses because the mice tumors were so small, and thus SphK1 protein level quantification could not be performed.
In this study, we demonstrated that FTY720 significantly enhanced the doxorubicin effects due to suppression of not only S1P signaling, which was shown by decreased mRNA and protein expression of SphK1 and S1PR1, but also inflammatory factors, including IL6 and STAT3. These results provide the first report that SphK1 expression was increased by doxorubicin treatment in breast cancer. Although doxorubicin is one of the most commonly used drugs in breast cancer, its use is limited by drug resistance and cumulative dose associated cardiotoxicity. The findings of our current study propose combination therapy with the addition of FTY720 as a strategy to dose reduction doxorubicin to mitigate toxicity without sacrificing its therapeutic oncologic benefits.
5. Conclusions
In conclusion, our results support further investigation into S1P signaling inhibition with S1PR1 functional antagonist FTY720 as a combination therapy strategy to improve the efficacy and reduce the toxicity of doxorubicin treatment for breast cancer.
Acknowledgments
This work was supported by NIH grant R01CA160688 and Susan G. Komen Foundation Investigator Initiated Research grant (IIR12222224) to K.T, and National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Cancer Institute’s Bioinformatics and Biostatistics Shared Resources.
Footnotes
Authors’ contributions:
E.K. conceptualized and performed experiments and prepared the article.
L.Y. performed bioinformatics analysis
M.N. conceptualized study design
A.R., J.S. and D.L. collected human samples and measured S1P levels.
N.H. and O.R. prepared the article.
K.T. provided supervision of experiments and prepared the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, et al. Doxorubicin: the good, the bad and the ugly effect. Current medicinal chemistry. 2009;16:3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 2.De Laurentiis M, Cancello G, D’Agostino D, Giuliano M, Giordano A, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Advances in experimental medicine and biology. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 4.Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenetics and genomics. 2011;21:440–446. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singhal SS, Singhal J, Sharma R, Singh SV, Zimniak P, et al. Role of RLIP76 in lung cancer doxorubicin resistance: I. The ATPase activity of RLIP76 correlates with doxorubicin and 4-hydroxynonenal resistance in lung cancer cells. International journal of oncology. 2003;22:365–375. [PubMed] [Google Scholar]
- 6.Takabe K, Spiegel S. Export of sphingosine-1-phosphate and cancer progression. Journal of lipid research. 2014;55:1839–1846. doi: 10.1194/jlr.R046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, et al. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. The Journal of biological chemistry. 2010;285:10477–10486. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacological reviews. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takabe K, Yamada A, Rashid OM, Adams BJ, Huang WC, et al. Twofer anti-vascular therapy targeting sphingosine-1-phosphate for breast cancer. Gland surgery. 2012;1:80–83. doi: 10.3978/j.issn.2227-684X.2012.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagahashi M, Tsuchida J, Moro K, Hasegawa M, Tatsuda K, et al. High levels of sphingolipids in human breast cancer. The Journal of surgical research. 2016;204:435–444. doi: 10.1016/j.jss.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagahashi M, Yamada A, Miyazaki H, Allegood JC, Tsuchida J, et al. Interstitial Fluid Sphingosine-1-Phosphate in Murine Mammary Gland and Cancer and Human Breast Tissue and Cancer Determined by Novel Methods. Journal of mammary gland biology and neoplasia. 2016;21:9–17. doi: 10.1007/s10911-016-9354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagahashi M, Yamada A, Aoyagi T, Allegood J, Wakai T, et al. Sphingosine-1-phosphate in the lymphatic fluid determined by novel methods. Heliyon. 2016;2:e00219. doi: 10.1016/j.heliyon.2016.e00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer research. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagahashi M, Kim EY, Yamada A, Ramachandran S, Allegood JC, et al. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2013;27:1001–1011. doi: 10.1096/fj.12-219618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuchida J, Nagahashi M, Nakajima M, Moro K, Tatsuda K, et al. Breast cancer sphingosine-1-phosphate is associated with phospho-sphingosine kinase 1 and lymphatic metastasis. The Journal of surgical research. 2016;205:85–94. doi: 10.1016/j.jss.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoyagi T, Nagahashi M, Yamada A, Takabe K. The role of sphingosine-1-phosphate in breast cancer tumor-induced lymphangiogenesis. Lymphatic research and biology. 2012;10:97–106. doi: 10.1089/lrb.2012.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang WC, Nagahashi M, Terracina KP, Takabe K. Emerging Role of Sphingosine-1-phosphate in Inflammation, Cancer, and Lymphangiogenesis. Biomolecules. 2013:3. doi: 10.3390/biom3030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyay P, Ramanathan R, Takabe K. S1P promotes breast cancer progression by angiogenesis and lymphangiogenesis. Breast cancer management. 2015;4:241–244. doi: 10.2217/bmt.15.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiti A, Takabe K, Hait NC. Metastatic triple-negative breast cancer is dependent on SphKs/S1P signaling for growth and survival. Cellular signalling. 2017;32:85–92. doi: 10.1016/j.cellsig.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagahashi M, Hait NC, Maceyka M, Avni D, Takabe K, et al. Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Advances in biological regulation. 2014;54:112–120. doi: 10.1016/j.jbior.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: weight of the evidence. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:4–7. doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- 22.Ewertz M, Gray KP, Regan MM, Ejlertsen B, Price KN, et al. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the breast international group 1–98 trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:3967–3975. doi: 10.1200/JCO.2011.40.8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediators of inflammation. 2016;2016:8606878. doi: 10.1155/2016/8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K. Corrigendum to “Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential”. Mediators of inflammation. 2016;2016:2856829. doi: 10.1155/2016/2856829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang WC, Liang J, Nagahashi M, Avni D, Yamada A, et al. Sphingosine-1-phosphate phosphatase 2 promotes disruption of mucosal integrity, and contributes to ulcerative colitis in mice and humans. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2016;30:2945–2958. doi: 10.1096/fj.201600394R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donoviel MS, Hait NC, Ramachandran S, Maceyka M, Takabe K, et al. Spinster 2, a sphingosine-1-phosphate transporter, plays a critical role in inflammatory and autoimmune diseases. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2015;29:5018–5028. doi: 10.1096/fj.15-274936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer cell. 2013;23:107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vera-Ramirez L, Sanchez-Rovira P, Ramirez-Tortosa CL, Quiles JL, Ramirez-Tortosa M, et al. Transcriptional shift identifies a set of genes driving breast cancer chemoresistance. PloS one. 2013;8:e53983. doi: 10.1371/journal.pone.0053983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinhold WC, Sunshine M, Liu H, Varma S, Kohn KW, et al. CellMiner: a web-based suite of genomic and pharmacologic tools to explore transcript and drug patterns in the NCI-60 cell line set. Cancer research. 2012;72:3499–3511. doi: 10.1158/0008-5472.CAN-12-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luna A, Rajapakse VN, Sousa FG, Gao J, Schultz N, et al. rcellminer: exploring molecular profiles and drug response of the NCI-60 cell lines in R. Bioinformatics (Oxford, England) 2016;32:1272–1274. doi: 10.1093/bioinformatics/btv701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacology research & perspectives. 2015;3:e00149. doi: 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aoki H, Aoki M, Yang J, Katsuta E, Mukhopadhyay P, et al. Murine model of long-term obstructive jaundice. The Journal of surgical research. 2016;206:118–125. doi: 10.1016/j.jss.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoki H, Aoki M, Katsuta E, Ramanathan R, Idowu MO, et al. Host sphingosine kinase 1 worsens pancreatic cancer peritoneal carcinomatosis. The Journal of surgical research. 2016;205:510–517. doi: 10.1016/j.jss.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terracina KP, Aoyagi T, Huang WC, Nagahashi M, Yamada A, et al. Development of a metastatic murine colon cancer model. The Journal of surgical research. 2015;199:106–114. doi: 10.1016/j.jss.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rashid OM, Nagahashi M, Ramachandran S, Dumur CI, Schaum JC, et al. Is tail vein injection a relevant breast cancer lung metastasis model? Journal of thoracic disease. 2013;5:385–392. doi: 10.3978/j.issn.2072-1439.2013.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demasi SC, Katsuta E, Takabe K. Live animals for preclinical medical student surgical training. Edorium J Surg. 2016;5:24–31. [PMC free article] [PubMed] [Google Scholar]
- 37.Katsuta E, DeMasi SC, Terracina KP, Spiegel S, Phan GQ, et al. Modified breast cancer model for preclinical immunotherapy studies. The Journal of surgical research. 2016;204:467–474. doi: 10.1016/j.jss.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashid OM, Nagahashi M, Ramachandran S, Dumur C, Schaum J, et al. An improved syngeneic orthotopic murine model of human breast cancer progression. Breast cancer research and treatment. 2014;147:501–512. doi: 10.1007/s10549-014-3118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rashid OM, Nagahashi M, Ramachandran S, Graham L, Yamada A, et al. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surgery. 2013;153:771–778. doi: 10.1016/j.surg.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rashid OM, Takabe K. Animal models for exploring the pharmacokinetics of breast cancer therapies. Expert opinion on drug metabolism & toxicology. 2015;11:221–230. doi: 10.1517/17425255.2015.983073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rashid OM, Maurente D, Takabe K. A Systematic Approach to Preclinical Trials in Metastatic Breast Cancer. Chemotherapy. 2016:5. doi: 10.4172/2167-7700.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao W, Ma SL, Tang J, Shi J, Lu Y. A combined treatment TNF-alpha/doxorubicin alleviates the resistance of MCF-7/Adr cells to cytotoxic treatment. Biochimica et biophysica acta. 2006;1763:182–187. doi: 10.1016/j.bbamcr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Mowad R, Chu QD, Li BD, Burton GV, Ampil FL, et al. Does obesity have an effect on outcomes in triple-negative breast cancer? The Journal of surgical research. 2013;184:253–259. doi: 10.1016/j.jss.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 44.Herlevic VC, Mowad R, Miller JK, Darensburg NA, Li BD, et al. Breast cancer outcomes in a population with high prevalence of obesity. The Journal of surgical research. 2015;198:371–376. doi: 10.1016/j.jss.2015.03.088. [DOI] [PubMed] [Google Scholar]
- 45.Kowalski GM, Carey AL, Selathurai A, Kingwell BA, Bruce CR. Plasma sphingosine-1-phosphate is elevated in obesity. PloS one. 2013;8:e72449. doi: 10.1371/journal.pone.0072449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saha S, Mukherjee S, Khan P, Kajal K, Mazumdar M, et al. Aspirin Suppresses the Acquisition of Chemoresistance in Breast Cancer by Disrupting an NFkappaB-IL6 Signaling Axis Responsible for the Generation of Cancer Stem Cells. Cancer research. 2016;76:2000–2012. doi: 10.1158/0008-5472.CAN-15-1360. [DOI] [PubMed] [Google Scholar]
- 47.Weymann KB, Wood LJ, Zhu X, Marks DL. A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain, behavior, and immunity. 2014;37:84–94. doi: 10.1016/j.bbi.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu J, et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hait NC, Avni D, Yamada A, Nagahashi M, Aoyagi T, et al. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERalpha expression and enhances hormonal therapy for breast cancer. Oncogenesis. 2015;4:e156. doi: 10.1038/oncsis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho JW, Man K, Sun CK, Lee TK, Poon RT, et al. Effects of a novel immunomodulating agent, FTY720, on tumor growth and angiogenesis in hepatocellular carcinoma. Molecular cancer therapeutics. 2005;4:1430–1438. doi: 10.1158/1535-7163.MCT-05-0021. [DOI] [PubMed] [Google Scholar]
- 51.Azuma H, Takahara S, Horie S, Muto S, Otsuki Y, et al. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. The Journal of urology. 2003;169:2372–2377. doi: 10.1097/01.ju.0000064938.32318.91. [DOI] [PubMed] [Google Scholar]
- 52.Pchejetski D, Bohler T, Brizuela L, Sauer L, Doumerc N, et al. FTY720 (fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer research. 2010;70:8651–8661. doi: 10.1158/0008-5472.CAN-10-1388. [DOI] [PubMed] [Google Scholar]
- 53.Pereira FV, Arruda DC, Figueiredo CR, Massaoka MH, Matsuo AL, et al. FTY720 induces apoptosis in B16F10-NEX2 murine melanoma cells, limits metastatic development in vivo, and modulates the immune system. Clinics (Sao Paulo, Brazil) 2013;68:1018–1027. doi: 10.6061/clinics/2013(07)21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishitsuka A, Fujine E, Mizutani Y, Tawada C, Kanoh H, et al. FTY720 and cisplatin synergistically induce the death of cisplatin-resistant melanoma cells through the downregulation of the PI3K pathway and the decrease in epidermal growth factor receptor expression. International journal of molecular medicine. 2014;34:1169–1174. doi: 10.3892/ijmm.2014.1882. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed D, de Verdier PJ, Ryk C, Lunqe O, Stal P, et al. FTY720 (Fingolimod) sensitizes hepatocellular carcinoma cells to sorafenib-mediated cytotoxicity. Pharmacology research & perspectives. 2015;3:e00171. doi: 10.1002/prp2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nemoto S, Nakamura M, Osawa Y, Kono S, Itoh Y, et al. Sphingosine kinase isoforms regulate oxaliplatin sensitivity of human colon cancer cells through ceramide accumulation and Akt activation. The Journal of biological chemistry. 2009;284:10422–10432. doi: 10.1074/jbc.M900735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guillermet-Guibert J, Davenne L, Pchejetski D, Saint-Laurent N, Brizuela L, et al. Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Molecular cancer therapeutics. 2009;8:809–820. doi: 10.1158/1535-7163.MCT-08-1096. [DOI] [PubMed] [Google Scholar]
- 58.Fuereder T, Hoeflmayer D, Jaeger-Lansky A, Rasin-Streden D, Strommer S, et al. Sphingosine kinase 1 is a relevant molecular target in gastric cancer. Anti-cancer drugs. 2011;22:245–252. doi: 10.1097/cad.0b013e328340bd95. [DOI] [PubMed] [Google Scholar]
- 59.Ghandadi M, Sahebkar A. Interleukin-6: A Critical Cytokine in Cancer Multidrug Resistance. Current pharmaceutical design. 2016;22:518–526. doi: 10.2174/1381612822666151124234417. [DOI] [PubMed] [Google Scholar]
- 60.Alshaker H, Wang Q, Kawano Y, Arafat T, Bohler T, et al. Everolimus (RAD001) sensitizes prostate cancer cells to docetaxel by down-regulation of HIF-1alpha and sphingosine kinase 1. Oncotarget. 2016 doi: 10.18632/oncotarget.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J, Wu H, Li W, Yin L, Guo S, et al. Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHK1/Akt/NF-kappaB signaling. Oncogene. 2016;35:5501–5514. doi: 10.1038/onc.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cilloni D, Messa F, Arruga F, Defilippi I, Morotti A, et al. The NF-kappaB pathway blockade by the IKK inhibitor PS1145 can overcome imatinib resistance. Leukemia. 2006;20:61–67. doi: 10.1038/sj.leu.2403998. [DOI] [PubMed] [Google Scholar]
- 63.Ciucci A, Gianferretti P, Piva R, Guyot T, Snape TJ, et al. Induction of apoptosis in estrogen receptor-negative breast cancer cells by natural and synthetic cyclopentenones: role of the IkappaB kinase/nuclear factor-kappaB pathway. Molecular pharmacology. 2006;70:1812–1821. doi: 10.1124/mol.106.025759. [DOI] [PubMed] [Google Scholar]
- 64.Fu D, Li Y, Li J, Shi X, Yang R, et al. The effect of S1P receptor signaling pathway on the survival and drug resistance in multiple myeloma cells. Molecular and cellular biochemistry. 2017;424:185–193. doi: 10.1007/s11010-016-2854-3. [DOI] [PubMed] [Google Scholar]
- 65.Matula K, Collie-Duguid E, Murray G, Parikh K, Grabsch H, et al. Regulation of cellular sphingosine-1-phosphate by sphingosine kinase 1 and sphingosine-1-phopshate lyase determines chemotherapy resistance in gastroesophageal cancer. BMC cancer. 2015;15:762. doi: 10.1186/s12885-015-1718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. Jama. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 67.Ligibel JA, Cirrincione CT, Liu M, Citron M, Ingle JN, et al. Body Mass Index, PAM50 Subtype, and Outcomes in Node-Positive Breast Cancer: CALGB 9741 (Alliance) Journal of the National Cancer Institute. 2015:107. doi: 10.1093/jnci/djv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nature reviews Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 69.Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: Links to cancer. Journal of carcinogenesis. 2013;12:19. doi: 10.4103/1477-3163.119606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goodwin PJ, Chlebowski RT. Obesity and Cancer: Insights for Clinicians. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34:4197–4202. doi: 10.1200/JCO.2016.70.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiralerspong S, Goodwin PJ. Obesity and Breast Cancer Prognosis: Evidence, Challenges, and Opportunities. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34:4203–4216. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 72.Alshaker H, Wang Q, Frampton AE, Krell J, Waxman J, et al. Sphingosine kinase 1 contributes to leptin-induced STAT3 phosphorylation through IL-6/gp130 transactivation in oestrogen receptor-negative breast cancer. Breast cancer research and treatment. 2015;149:59–67. doi: 10.1007/s10549-014-3228-8. [DOI] [PubMed] [Google Scholar]
- 73.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34:4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]