Abstract
The unfolded protein response (UPR) is activated in response to impairments of the folding environment in the endoplasmic reticulum (ER). The most conserved arm of the UPR, inositol-requiring ER-to-nucleus signaling protein (IRE1α), has been linked to the regulation of a diverse array of cellular processes including ER-associated degradation, inflammatory signaling, cell proliferation and membrane biogenesis. Recent studies have utilized the selective, small molecule inhibitor, 4μ8c, to examine the role of IRE1α endoribonuclease (RNase) activity in various cell types including multiple myeloma, mouse embryonic fibroblasts and pancreatic beta cells [1–5]. In the present study we utilized this inhibitor to examine the role of IRE1α RNase activity in hepatoma cells (H4IIE), specifically concentrating on cell proliferation and the identification of potential off target effects under both unstressed and stressed conditions. Experiments were performed in H4IIE hepatoma cells in the absence (control conditions (LG)) or presence (LG + Thapsigargin (Thap)) of ER stress. The presence of 4μ8c decreased IRE1α RNase activity, based on reduced splicing of X-box binding protein-1 (XBP1s) and regulated IRE1α-dependent decay of mRNA in both treatments and at concentrations ranging from 10–90 μM. Cell proliferation was significantly reduced at higher concentrations (> 60 μM 4μ8c) in unstressed cells and displayed a dose-response relationship with 4μ8c in both treatments. The presence of 4μ8c did not promote cytoxicity in either of the treatment conditions but higher concentrations of the inhibitor (60 μM) were associated with apparent off-target or compensatory responses that were not observed at 10 μM. Overall, the small-molecule inhibitor, 4μ8c is an effective inhibitor of IRE1α RNase activity in H4IIE cells. Potential off-target effects associated with this inhibitor require the use of multiple inhibitor concentrations in all experiments.
Keywords: endoplasmic reticulum stress, unfolded protein response, hepatocytes
1 Introduction
The endoplasmic reticulum (ER) is critical for maintaining overall cellular homeostasis through its roles in calcium storage, lipid storage and trafficking, and protein folding [6–9]. Perturbations in the ER folding environment, commonly termed ER stress, elicit the unfolded protein response (UPR), an adaptive quality control system that aims to restore ER homeostasis via transcriptional and translational regulation [10–13]. In mammals, the UPR consists of three proximal transmembrane sensors: inositol-requiring ER-to-nucleus signaling protein (IRE1α), RNA-dependent protein kinase-like ER eIF-2α kinase (PERK), and activating transcription factor 6 (ATF6). Upon ER stress, activation of these three sensors elicit distinct but cooperative signaling cascades that ultimately reduce cellular stress or, in severe cases, eliminate stressed cells by inducing apoptosis [14, 15].
Although the three UPR sensors activate distinct signaling cascades, redundancies exist among the cascades in terms of the transcriptional and translational alterations they elicit (e.g. activation of IRE1α and ATF6α upregulate a common set of ER chaperones; ATF6α and XBP1 work cooperatively to regulate genes involved in quality control and degradation, as well as anterograde trafficking in the ER) [14, 15]. These redundancies have obscured our knowledge of the contribution of the individual proximal UPR sensors in maintaining cell health and affecting disease processes. Genetically modified animals and cells that lack particular UPR sensors have provided some insight into the relative importance of each UPR branch, but evidence suggests that these approaches may be limited because of compensatory responses by non-targeted UPR components [14–16]. To overcome these obstacles, considerable attention has been given to the development of small-molecule inhibitors that directly and acutely target specific UPR sensors. Recent high-throughput screens identified 4μ8c, a small-molecule inhibitor of the endoribonuclease (RNase) activity of IRE1α, leaving the kinase activity unaffected [1, 3]. This inhibitor prevented the downstream splicing of the transcription factor X-box binding protein 1 (XBP1) and reduced or prevented IRE1α-mediated mRNA degradation (i.e. regulated IRE1-dependent decay of mRNA or RIDD) [1, 3]. The IRE1α-XBP1 signaling pathway is involved in regulation of chaperone expression, ER-associated degradation, lipogenesis, and ER membrane biogenesis and expansion [17–19]. Although much less is known about the regulation and downstream consequences of RIDD, recent studies have suggested that RIDD can target mRNA encoding proteins that are localized in the cytosol, nucleus or ER and is involved in ER homeostasis under both basal and stressed conditions [20–22]. Recently, studies have demonstrated that 4μ8c attenuated the growth of multiple myeloma and pancreatic beta cells during ER stress, linking IRE1α RNase activity to cell proliferation [3, 4]. This inhibitor reduced IRE1α RNase activity in multiple cell culture models [1–5], but has not been examined in unstressed and stressed hepatoma cells, a cell type often used to identify mechanisms involved in chronic liver diseases and regulation of cell survival. Therefore, the aims of this study were to determine the effectiveness of this inhibitor under basal conditions (unstressed) as well as chemically-induced ER stress and to examine the role of IRE1α RNase activity in regulation of cell proliferation in H4IIE cells.
2 Materials and Methods
2.1 Cell Culture
H4IIE cells (American Type Culture Collection, Manassas, VA), a rat liver hepatoma cell line, were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 8 mM glucose and supplemented with 10% fetal bovine serum, penicillin, and streptomycin sulfate. Each experiment was performed in triplicate at 80–100% confluency.
2.2 Experimental agents
Thapsigargin (780nM), a tumor promoting sesquiterpene lactone that inhibits the ER-associated calcium ATPase, was used to induce ER stress [23]. The IRE1α inhibitor, 7-Hydroxy-4-methyl-2-oxo-2H-1-benzopyran-8-carboxaldehyde (4μ8c) was purchased from TOCRIS Bioscience (Minneapolis, MN).
2.3 RNA isolation and analysis
Total RNA was extracted with TRIzol reagent using the manufacturer’s protocol (Invitrogen, Carlsbad, CA). For Real Time PCR, reverse transcription was performed using 0.5 μg of DNase-treated RNA, Superscript II RNase H- and random hexamers. PCR reactions were performed using the Bio-Rad CFX Connect Real Time analyzer and IQ-SYBR green master mix (Bio-Rad, Hercules, CA). PCR efficiency was between 90% and 105% for all primer and probe sets and linear over 5 orders of magnitude. The specificity of products generated for each set of primers was examined for each fragment using a melting curve and gel electrophoresis. Reactions were run in triplicate and data calculated as the change in cycle threshold (DCT) for the target gene relative to the DCT for β2-microglobulin (control gene) according to the procedures of Muller et al. [24]. Target genes (XBP1s, YAP1, MKI-67, TOP2A, CHOP, GADD34, GRP-78) were normalized to β2-microglobulin. Primer sequences are listed in table 1.
Table 1. Primers used in qRT-PCR.
XBP1s, X-box binding protein 1; YAP1, Yes-associated protein 1; MKI-67, antigen identified by monoclonal antibody Ki-67; TOP2A, DNA topoisomerase 2-α; CHOP, C/EBP homologous protein; GADD34, growth arrest and DNA-damage inducible-34; GRP78, glucose-regulated protein-78.
| Gene | Sequence |
|---|---|
| XBP1s | 5′-GTCTGCTGAGTCCGCAGCAGG-3′ 5′-GATTAGCAGACTCTGGGGAAG-3′ |
| YAP1 | 5′-TCAATCCCAGCACAGCAAATG-3′ 5′-CATTCTGAGTCCCTCCATCC-3′ |
| MKI-67 | 5′-CTGCAGAGAAGGTTGGGATAA-3′ 5′-CTGACTTTGCCCAGAGATGAA-3′ |
| TOP2A | 5′-GAAACAGCCAGTAGGGAATAC-3′ 5′-GTGTAATCCTCTCCGCTAAAG-3′ |
| CHOP | 5′-CCAGCAGAGGTCACAAGCAC-3′ 5′-CGCACTGACCACTCTGTTTC-3′ |
| GADD34 | 5′-CTTCCTCTGTCGTCCTCGTCTC-3′ 5′-CCCGCCTTCCTCCCAAGTC-3′ |
| GRP-78 | 5′-AACCCAGATGAGGCTGTAGCA-3′ 5′-ACATCAAGCAGAACCAGGTCAC-3′ |
2.4 Cell proliferation
The Cell Proliferation ELISA 5-bromo-2′-deoxyuridine (BrdU) colorimetric kit (Sigma-Aldrich, St. Louis, MO) was used to measure cell proliferation. This assay is based on BrdU incorporation into DNA in place of thymidine in replicating cells. Briefly, cells were grown in 96-well plates and were labeled with BrdU for 4 hours. The labeling medium was removed and cells were fixed using FixDenat. The anti-BrdU-POD antibody was added which formed a complex with newly synthesized cellular DNA that contained BrdU. The reaction complex was detected via absorbance at 450 nm using a multi-well spectrophotometer (Thermo Labsystems MultiskanEX model #355, Thermo Fisher Scientific, Waltham, MA).
2.5 Western blot analysis
Cells were washed with phosphate-buffered saline (PBS) and harvested using a lysis buffer containing 50 mM Tris-HCl pH 8.0, 1% SDS, and previously described protease and phosphatase inhibitors [25]. Equivalent amounts of protein were subjected to SDS-PAGE and transferred to nitrocellulose membranes (EMD Millipore; Billerica, MA). The membranes were incubated with antibodies against total and phosphorylated eukaryotic initiation factor-2α (eIF2α sc-133132; Santa Cruz Biotechnology, Santa Cruz, CA; p-eIF2α NB110-56949; Novus Biologicals, Littleton, CO), or C/EBP homologous protein (CHOP #ab11419; Abcam, Cambridge, UK). The following day, membranes were washed in Tris-buffered saline and Tween 20 (TBST) pH 7.4, incubated for 1 hour with secondary detection antibodies ((IRDye 680 CS-conjugated anti-rabbit, IRDye 800 CS-conjugated anti-rabbit, IRDye 680 CS-conjugated anti-mouse, or IRDye 800 CW-conjugated anti-mouse (LI-COR Biosciences, Lincoln, NE)), washed in TBST, and scanned for infrared signal using the Odyssey Imaging System (LI-COR Biosciences). Density was quantified using Image Studio (LI-COR Biosciences).
2.6 Cell viability
Cell viability was measured using Trypan Blue Exclusion (Thermo Fisher Scientific). Briefly, 0.4% trypan blue solution (pH 7.2–7.3) was added to 1 mL of 90%–100% confluent cells and then examined under a microscope. Using a hemocytometer, at least 1 quadrant of 16 units containing on average 50–100 cells were counted manually and percent viable cells was calculated as:
Trypan blue positive cells (%) = (number of blue cells/number of total cells) × 100.
2.7 Data analysis and statistics
Statistical comparisons were calculated using individual unpaired t tests to compare means between the presence and absence of 4μ8c within treatment groups. Analysis of variance (ANOVA) was used to analyze the group means among a range of concentrations of 4μ8c. Statistical significance was set at P < 0.05. All data are reported as means ± SD.
3 Results
Recently published data have demonstrated that the IRE1α inhibitor, 4μ8c, directly targeted IRE1α endoribonuclease activity and reduced XBP1 splicing (XBP1s) in mouse embryonic fibroblasts (MEFs) [3] and multiple myeloma cells (MM) [1]. To confirm the efficacy of this inhibitor in H4IIE cells, we first examined the ability of 60 μM 4μ8c to inhibit IRE1α-mediated XBP1s. As shown in Figure 1, incubation with 4μ8c significantly decreased XBP1s at 2, 4, and 6 hours in control cells (LG) and in cells treated with the ER stress inducer, thapsigargin (Thap). These time points were chosen because they characterize a period of time in which active cell proliferation and minimal cell death occurs in Thap-treated cells.
Fig 1. Effects of the IRE1α inhibitor 4μ8c on XBP1s.
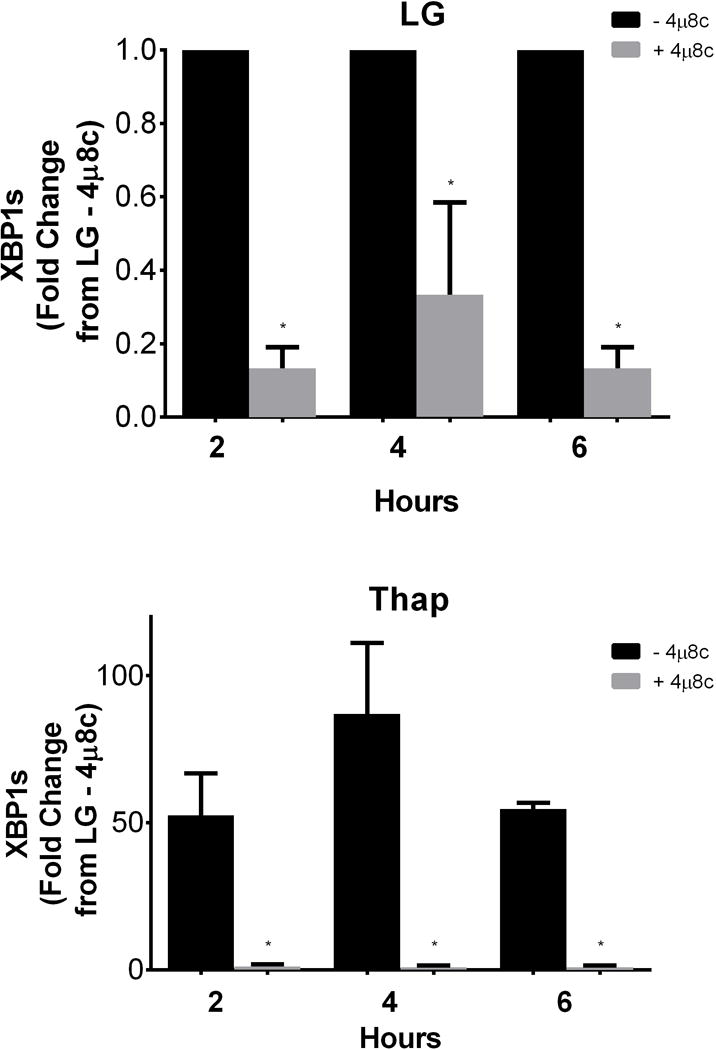
H4IIE cells were incubated for 2, 4, or 6 hours in low glucose (LG) control media or LG supplemented with thapsigargin (Thap; 780 nM), in the absence (−) or presence (+; 60 μM) of 4μ8c. Data represent mean ± SD for 3 independent experiments. *= P < 0.05 from condition minus 4μ8c at corresponding time point.
Given 4μ8c’s ability to inhibit cell proliferation in MM cells during ER stress [3], we next investigated whether 60 μM 4μ8c also inhibited cell proliferation in H4IIE cells. The presence of the inhibitor at 60 μM significantly reduced cell proliferation in both LG and Thap conditions (Figure 2). These data suggest that IRE1α regulates cell proliferation in both unstressed and stressed H4IIE cells. We next examined a group of genes markers of cell proliferation: Topoisomerase 2A (TOP2A), ki-67 (MKI67), and yes-associated protein 1 (YAP1). The presence of 4μ8c did not induce changes in any of these markers in LG or Thap (Supplementary Table 1).
Fig 2. Effects of the IRE1α inhibitor 4μ8c on cell proliferation.
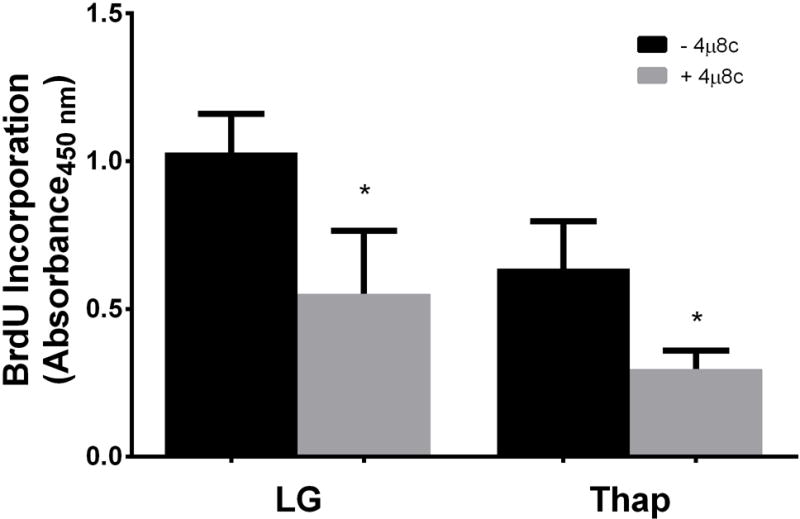
H4IIE liver cells were incubated for 4 hours in low glucose (LG) control media or LG supplemented with thapsigargin (Thap; 780 nM), in the absence (−) or presence (+; 60 μM) of 4μ8c. Data represent mean ± SD for 3 independent experiments. *= P < 0.05 from condition minus 4μ8c. The lack of a SD for BLOC1s1a and BLOC1s1c in the 10 uM condition was do to the fact that the values from the 2 experiments were the same.
Although previous studies used a range of inhibitor concentrations up to 120 μM, the concentration of 4μ8c that produced a 50% reduction in IRE1α RNase activity in other cell types was ~7 μM (1, 3, 4). We therefore performed additional experiments across a range of inhibitor concentrations in H4IIE cells. Equivalent inhibition of XBP1s was observed with concentrations of 4μ8c ranging from 10–90 μM (Figure 3). We also examined 14 putative RIDD-targeted mRNAs (Supplementary Table 2) of which 3 displayed changes consistent with RIDD targets. Carboxylesterase 1 (CES1c) and Biogenesis of Lysosome-Related Organelles Complex 1 Subunit 1a and Subunit 1c (BLOC1s1a, BLOC1s1c) were reduced in the presence of thapsigargin and normalized by 10 μM 4μ8c (Figure 4). No change was observed in the phosphorylation state of IRE1α with low (10μM) and high (60μM) concentrations of 4μ8c (Figure 5). In contrast to the consistent reduction of IRE1α RNase activity observed across all concentrations of 4μ8c, a dose-dependent inhibition of cell proliferation was observed in LG and Thap (Figure 6). Importantly, cell proliferation was not significantly reduced at concentrations of 4μ8c (10–30 μM) that produced maximal inhibition of IRE1 RNase activity in LG (Figure 6). Consistent with past publications [1, 3], all concentrations of 4μ8c significantly decreased cell proliferation in the presence of Thap. In addition, trypan blue positive cells were not significantly increased at any of the concentrations of 4μ8c in LG or Thap (Supplementary Figure 1).
Fig 3. Effects of 10–90 μM 4μ8c on XBP1s.

H4IIE liver cells were incubated for 6 hours in low glucose (LG) control media or LG supplemented with thapsigargin (Thap; 780 nM) in the absence (−) or presence (+; 10 μM–90 μM) of 4μ8c. Data represent mean ± SD for 3 independent experiments. *= P < 0.05 from condition minus 4μ8c.
Fig 4. Effects of 10–90 μM 4μ8c on RIDD-targeted mRNAs.
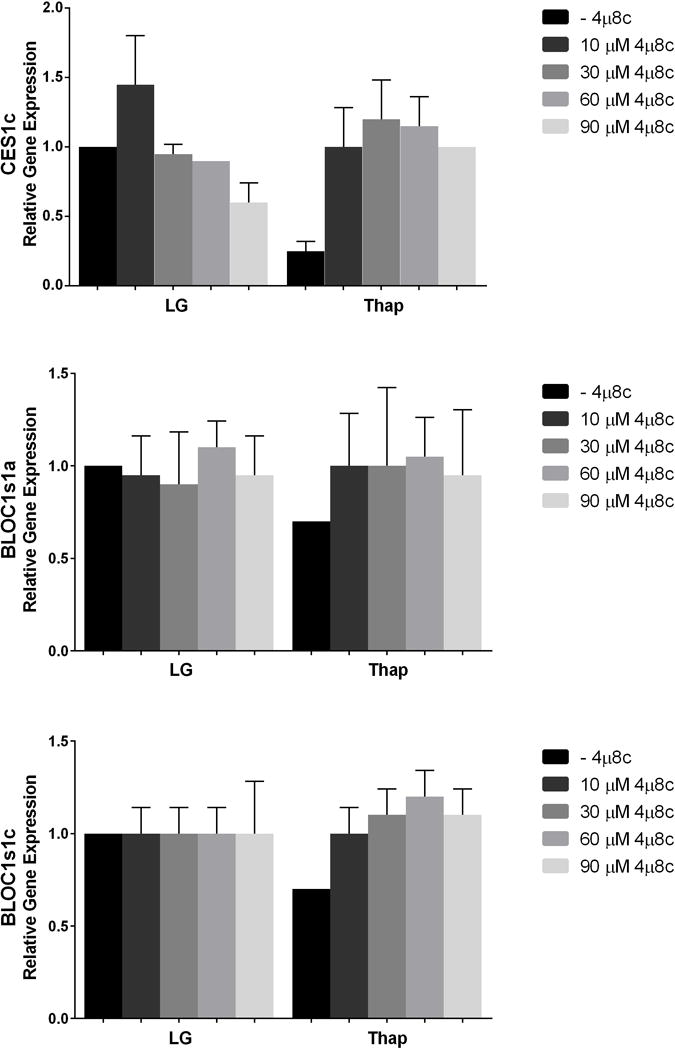
H4IIE liver cells were incubated for 6 hours in low glucose (LG) control media or LG supplemented with thapsigargin (Thap; 780 nM) in the absence (−) or presence (+; 10 μM–90 μM) of 4μ8c. Data represent mean ± SD for 2 independent experiments.
Fig 5. Effects of 10 and 60 μM 4μ8c on IRE1.
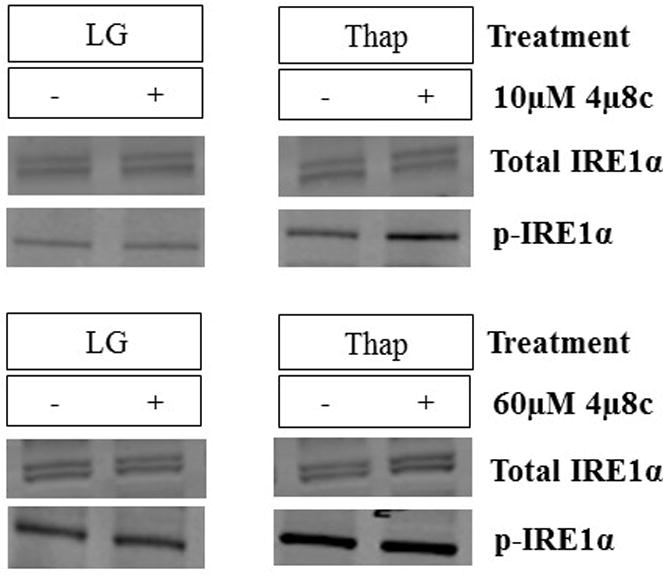
α phosphorylation. H4IIE liver cells were incubated for 6 hours in low glucose (LG) control media or LG supplemented with thapsigargin (Thap; 780 nM) in the absence (−) or presence (+; 10 μM or 60 μM) of 4μ8c. Data represent mean ± SD for 3 independent experiments. *= P < 0.05 from condition minus 4μ8c.
Fig 6. Effects of 10–90 μM 4μ8c on cell proliferation.
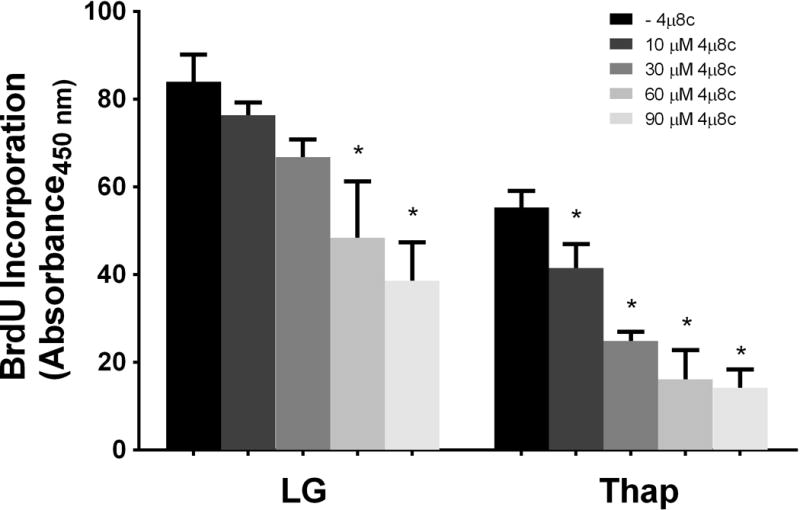
H4IIE liver cells were incubated for 6 hours in low glucose (LG) control media or LG supplemented with thapsigargin (Thap; 780 nM) in the absence (−) or presence (+; 10 μM–90 μM) of 4μ8c. Data represent mean ± SD for 3 independent experiments. *= P < 0.05 from condition minus 4μ8c.
Our data suggests that 4μ8c exerts effects on cell proliferation that are independent of IRE1α-mediated RNase activity. Therefore, we next examined whether 4μ8c influenced other components of the unfolded protein response. Activation of the PERK branch of the UPR results in a global but transient reduction in protein synthesis mediated by the phosphorylation of eIF2α (p-eIF2α) [26]. The presence of 60μM 4μ8c increased p-eIF2α in LG and Thap conditions (Figure 7b). In contrast, 10μM 4μ8c did not result in significant changes of p-eIF2α (Figure 7a).
Fig 7. Effects of 10 μM (a) and 60 μM (b) 4μ8c on eIF2α phosphorylation (p-eIF2α).
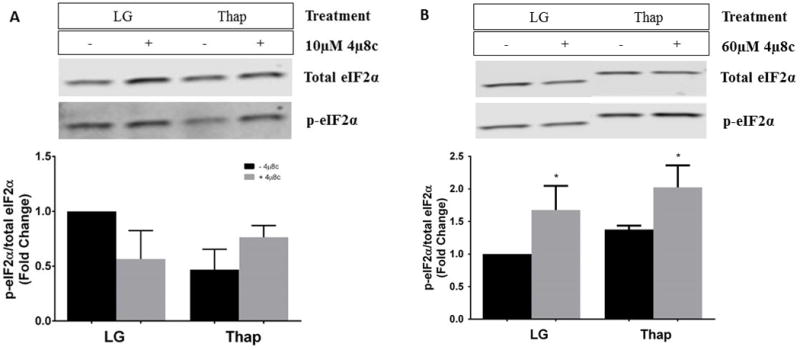
H4IIE liver cells were incubated for 4 hours in low glucose (LG) control media or LG supplemented with thapsigargin (Thap; 780 nM). All conditions represent the fold change of the ratio of p-eIF2ɑ to total eIF2ɑ in the absence (−) or presence (+; 60 μM) of 4μ8c. The representative gel was a single gel that was split, as other samples were run in between LG and Thap samples. Data represent mean ± SD for 3 independent experiments. *= P < 0.05 from condition minus 4μ8c.
Additionally, we examined genes that are typically upregulated in the presence of ER stress. C/EBP homologous protein (CHOP) is a transcription factor involved in the regulation of metabolism and apoptosis and is typically upregulated during ER stress [27]. CHOP mRNA increased ~5 fold with 60μM 4μ8c and ~18 fold with 90μM 4μ8c in LG (Figure 8a). In the presence of Thap, all concentrations of 4μ8c resulted in a significant decrease in CHOP mRNA (Figure 8b). CHOP protein expression was also measured and although it was not significant, a similar trend was observed (Figure 8c). Similar trends were observed for growth arrest and DNA damage-inducible protein (GADD34), a protein that plays a role in ER-mediated cell death via reversal of translation inhibition by dephosphorylation of elF2α [13], and 78 kDa glucose-regulated protein (GRP-78), a molecular chaperone that is induced upon ER stress [13] (Supplementary Table 3).
Fig 8. Effects of 10–90 μM 4μ8c on gene and protein expression of CHOP.

H4IIE liver cells were incubated for 6 hours in low glucose (LG) control media (a) or LG supplemented with thapsigargin (Thap; 780 nM) (b) in the absence (−) or presence (+; 10 μM–90 μM) of 4μ8c. A representative blot of protein expression is shown in c. Data represent mean ± SD for 2 independent experiments. *= P < 0.05 from condition minus 4μ8c.
4 Discussion
In the present study we investigated the role of IRE1α RNase activity in the regulation of cell proliferation under control and ER stress conditions in H4IIE cells using a small-molecule inhibitor, 4μ8c. Three independent experiments were performed in the absence and presence of chemically-induced ER stress. The results demonstrate that 4μ8c successfully inhibited IRE1α RNase activity in H4IIE cells under control conditions as well as during ER stress. Further, we demonstrate that while IRE1α RNase activity is involved in the regulation of cell proliferation during Thap-induced ER stress it does not play a significant role in the regulation of cell proliferation in unstressed cells. Lastly, we demonstrate that higher concentrations of 4μ8c result in effects on both cell proliferation and the UPR that appear to be independent of IRE1α RNase activity.
Cross et al., characterized 4μ8c as an effective inhibitor of IRE1α RNase activity base on reduced splicing of the downstream effector XBP1 and reduced RIDD in MM and/or MEF cell lines [1]. Our first goal was to determine the effectiveness of this inhibitor in H4IIE cells. Our results indicate that 4μ8c reduced XBP1s across a wide range of concentrations (10–90 μM) and time points (2–6 hours) in both unstressed and ER stressed H4IIE cells. In addition, 4μ8c prevented the thapsigargin-induced reduction of three putative RIDD-targeted mRNAs [20, 28]. This extends previous reports by demonstrating that 4μ8c is a potent inhibitor of IRE1α RNase activity in a hepatoma cell line [1, 3, 4]. Previous studies have observed a Ki for 4μ8c of ~7μM, which approximates the lowest concentration used in the present study (1).
The regulation of IRE1α RNase activity and the downstream outputs that arise as a result of IRE1α RNase activity involve complex protein-protein interactions, as well as post-translational modifications to IRE1α [29–31]. IRE1α has been linked to the regulation of cell proliferation and ER membrane expansion in both unstressed and ER stressed cells [12, 32]. However, whereas IRE1α-mediated XBP1 splicing has been linked to most prosurvival outputs, RIDD appears to be associated with apoptosis [20]. Although the present study was not designed to examine the relative roles of XBP1s and RIDD in cell fate decisions, our results provide new evidence for the regulation of cell proliferation in H4IIE cells by the small molecule inhibitor 4μ8c. In the present study, 4μ8c inhibited cell proliferation in H4IIE cells treated with thapsigargin, a finding consistent with previous work in MM and MEF cells [1]. In contrast, the concentration of 4μ8c that resulted in inhibition IRE1α RNase activity (10μM) had no effect on cell proliferation in unstressed (control) H4IIE cells. Higher concentrations of 4μ8c (60μM) reduced cell proliferation in unstressed (control) H4IIE cells, suggesting that this inhibition is likely independent of IRE1α RNase activity, and perhaps the result of off-target effects.
Given that we observed effects of 4μ8c on cell proliferation that appeared to be independent of IRE1α-RNase activity, we also assessed the effects of 4μ8c on additional markers of UPR activation: p-eIF2α, CHOP, GADD34, and GRP-78 [13, 33]. Under control conditions, we observed a significant increase in CHOP mRNA and protein expression and GADD34 mRNA with 90 μM 4μ8c in H4IIE cells. Since IRE1α-mediated XBP1s and RIDD were equivalently reduced with 10 μM and 90 μM 4μ8c, these data suggest that changes in CHOP and GADD34 involve off-target effects of 4μ8c. In contrast to control conditions, these same UPR markers were reduced by 10 μM 4μ8c in cells treated with thapsigargin. It is possible that IRE1α RNase activity contributes to the upregulation of CHOP and GADD34 in response to chemical induction of ER stress. However, genetic deletion of IRE1α in the liver has typically resulted in increased expression levels of CHOP in response to chemical induction of ER stress [14]. It is also possible that the restoration of RIDD targets following exposure to 4μ8c result in suppression of CHOP and GADD34.
In summary, 4μ8c is an effective inhibitor of IRE1α RNase activity in H4IIE cells. Inhibition of IRE1α RNase activity by 4μ8c confirms that this UPR pathway regulates cell proliferation during ER stress. However, regulation of cell proliferation in unstressed cells appears to not involve IRE1α RNase activity. Importantly, higher concentrations of 4μ8c appear to have effects on both cell proliferation and UPR-related genes that are independent of IRE1α RNase activity. These effects do not involve cytotoxicity. Therefore, to understand IRE1α regulation in cellular processes using 4μ8c requires the use of multiple concentrations and assessment of a broad range of UPR markers.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant DK072017 and the Lillian Fountain Smith Endowment (MJP), as well as The Boettcher Foundation’s Webb-Waring Biomedical Research Program (CLG).
Footnotes
Conflict of Interest: The authors state no conflict of interest.
References
- 1.Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci U S A. 2012;109(15):E869–78. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kemp KL, Lin Z, Zhao F, Gao B, Song J, Zhang K, et al. The serine-threonine kinase inositol-requiring enzyme 1alpha (IRE1alpha) promotes IL-4 production in T helper cells. J Biol Chem. 2013;288(46):33272–82. doi: 10.1074/jbc.M113.493171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117(4):1311–4. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma RB, O‘Donnell AC, Stamateris RE, Ha B, McCloskey KM, Reynolds PR, et al. Insulin demand regulates beta cell number via the unfolded protein response. J Clin Invest. 2015;125(10):3831–46. doi: 10.1172/JCI79264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storniolo A, Raciti M, Cucina A, Bizzarri M, Di Renzo L. Quercetin affects Hsp70/IRE1alpha mediated protection from death induced by endoplasmic reticulum stress. Oxid Med Cell Longev. 2015;2015:645157. doi: 10.1155/2015/645157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch GL. The endoplasmic reticulum and calcium storage. Bioessays. 1990;12(11):527–31. doi: 10.1002/bies.950121105. [DOI] [PubMed] [Google Scholar]
- 7.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86(4):1133–49. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 8.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569(1–2):29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 9.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13(10):1211–33. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110(10):1389–98. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 13.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14(1):20–8. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15(6):829–40. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoulders MD, Ryno LM, Genereux JC, Moresco JJ, Tu PG, Wu C, et al. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 2013;3(4):1279–92. doi: 10.1016/j.celrep.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13(3):351–64. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Sha H, He Y, Chen H, Wang C, Zenno A, Shi H, et al. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009;9(6):556–64. doi: 10.1016/j.cmet.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sriburi R, Bommiasamy H, Buldak GL, Robbins GR, Frank M, Jackowski S, et al. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J Biol Chem. 2007;282(10):7024–34. doi: 10.1074/jbc.M609490200. [DOI] [PubMed] [Google Scholar]
- 19.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167(1):35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurel M, Chevet E, Tavernier J, Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem Sci. 2014;39(5):245–54. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Moore K, Hollien J. Ire1-mediated decay in mammalian cells relies on mRNA sequence, structure, and translational status. Mol Biol Cell. 2015;26(16):2873–84. doi: 10.1091/mbc.E15-02-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tam AB, Koong AC, Niwa M. Ire1 has distinct catalytic mechanisms for XBP1/HAC1 splicing and RIDD. Cell Rep. 2014;9(3):850–8. doi: 10.1016/j.celrep.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990;87(7):2466–70. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT PCR (vol 32, pg 1378, 2002) Biotechniques. 2002;33(3):514. [PubMed] [Google Scholar]
- 25.Wei Y, Wang D, Pagliassotti MJ. Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol Cell Biochem. 2007;303(1–2):105–13. doi: 10.1007/s11010-007-9461-2. [DOI] [PubMed] [Google Scholar]
- 26.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34(Pt 1):7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 27.Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. J Biochem. 2012;151(3):217–9. doi: 10.1093/jb/mvr143. [DOI] [PubMed] [Google Scholar]
- 28.Bright MD, Itzhak DN, Wardell CP, Morgan GJ, Davies FE. Cleavage of BLOC1S1 mRNA by IRE1 Is Sequence Specific, Temporally Separate from XBP1 Splicing, and Dispensable for Cell Viability under Acute Endoplasmic Reticulum Stress. Mol Cell Biol. 2015;35(12):2186–202. doi: 10.1128/MCB.00013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312(5773):572–6. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 30.Hetz C, Glimcher LH. Fine-tuning of the unfolded protein response: Assembling the IRE1alpha interactome. Mol Cell. 2009;35(5):551–61. doi: 10.1016/j.molcel.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, Calay ES, Fan J, Arduini A, Kunz RC, Gygi SP, et al. METABOLISM. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science. 2015;349(6247):500–6. doi: 10.1126/science.aaa0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I, et al. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity. 2003;18(2):243–53. doi: 10.1016/s1074-7613(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 33.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11(4):381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


