Abstract
The concept of immune privilege of the central nervous system (CNS) has dominated the study of inflammatory processes in the brain. However, clinically relevant models have highlighted that innate pathways limit pathogen invasion of the CNS and adaptive immunity mediates control of many neural infections. As protective responses can result in bystander damage, there are regulatory mechanisms that balance protective and pathological inflammation, but these mechanisms might also allow microbial persistence. The focus of this review is to consider the host-pathogen interactions that influence neurotropic infections and to highlight advances in our understanding of innate and adaptive mechanisms of resistance as key determinants of the outcome of CNS infection. Advances in these areas have broadened our comprehension of how the immune system functions in the brain and can readily overcome immune privilege.
Keywords: Brain, infection, blood brain barrier, astrocyte, neuron, central nervous system, encephalitis, meningitis, T cell, monocyte
The CNS utilizes multiple mechanisms to restrict microbial entry but there are also innate and adaptive mechanisms that control pathogens that access this immune privileged site. Klein and Hunter review the pathogen interactions with the blood brain barrier and resident glial populations that govern the outcome of CNS infection, as well as the immune mechanisms that dictate protective and pathological responses in the brain.
Main Text
Introduction
For nearly 100 years, physicians and scientists have appreciated the unique immunological status of the brain. The notion that the CNS is completely isolated from the periphery and lacks immune responses was supported by early experiments demonstrating a diffusion barrier between the blood and the brain and later by the lack of tissue graft rejection, the latter of which was proven incorrect (Barker and Widner, 2004). The presence of the blood brain barrier (BBB), which isolates the central nervous system (CNS) parenchyma from circulating immune cells and antibodies, and the lack of identifiable lymphatics originally contributed to the designation of the CNS as an immune privileged site (Barker and Widner, 2004, Medawar, 1948, Wilson et al., 2010). The concept of immune privilege has been questioned (Carson et al., 2006, Galea et al., 2007) and has evolved to incorporates the neurovascular unit (NVU), the blood-cerebrospinal fluid (CSF) barrier, and the presence of CNS lymphatics that allow leukocyte egress (Aspelund et al., 2015, Louveau et al., 2015, Weller et al., 2009). The detection of small numbers of leukocytes, particularly memory T cells, within newly identified lymphatics and in the CSF, is in accord with reports that low numbers of T cells in the CNS promote normal neurological function, including processes involved in cognition (Kipnis et al., 2012, Schwartz and Kipnis, 2011). Nevertheless, the most common manifestation of neuroinfectious diseases is the recruitment of inflammatory cells which are critical to mediate local resistance to a variety of viral, bacterial, and parasitic organisms within distinct compartments of the CNS (see Table 1 for a list of CNS compartments targeted by neurotropic pathogens). Multiple studies have highlighted the adverse consequences of immune exclusion and the importance of immune activity to control infections in the brain. Many of these responses are associated with alterations in the biology of resident neural cell populations which not only amplify protective activities but can also impact on neuronal survival and function and lead to life-threatening pathology (Streit et al., 2005). The aim of this article is to consider the host-pathogen interactions that influence neurotropic infections and to review advances in understanding the innate and adaptive mechanisms that balance protective and pathological inflammation, which determine the outcome of CNS infection.
Table 1.
CNS Compartments Targeted by Neurotropic Pathogens
| CNS Compartment | Meninges | Ventricular System | Parenchyma |
|---|---|---|---|
| Bacteria |
S. pneumococcus L. monocytogenes H. influenza N. meningitides T. pallidum |
M. tuberculosis |
L. monocytogenes Nocardia species T. pallidum |
| Fungi | C. neoformans |
Candida albicans Aspergillus niger Zygomycetes Fusarium species Pseudallescheria boydii Pigmented molds Histoplasma capsulatum Coccidioides immitis Penicillium marneffei |
|
| Parasites |
Angiostrongylus cantonensis Trichinella spiralis |
Trypanosoma brucei (sp) Schistosome(sp) |
T. gondii Naegleria fowleri Trypanosoma cruzi Trypanosoma brucei (sp) Taenia solium |
| Viruses | Enteroviruses West Nile virus Japanese encephalitis virus Human Immunodeficiency virus Mumps virus Measles virus |
JC virus | Herpes simplex virus 1 Cytomegalovirus Measles virus West Nile virus Japanese encephalitis virus Zika virus Tick-borne encephalitis virus Equine encephalitis viruses (VEEV, EEEV, WEEV) Enteroviruses Human Immunodeficiency virus Varicella Zoster virus JC virus Influenza A |
Source: The Washington Manual of Infectious Disease Subspecialty Consult, Ed. Nigar Kirmani, Keith Woeltje, and Hilary Babcock; Wolters Kluwer, Lippincott, Williams, and Wiltkins
Primary and Acquired Immune Deficiencies Associated with Susceptibility to CNS Infection
There are numerous clinical approaches such as chemotherapies, bone marrow transplantation, or immunosuppressive drugs that interfere with overall immune function and predispose to neuroinfectious diseases (Britt et al., 1981). The AIDS epidemic revealed that T cells protect the brain from neurotropic opportunistic pathogens such as Cryptococcus neoformans, Toxoplasma gondii, Mycobacterium tuberculosis, cytomegalovirus (CMV), and John Cunnigham (JC) virus (Bowen et al., 2016). Similarly, children with hyper-immunoglobulin M (IgM) syndrome, a group of primary immune deficiency disorders characterized by altered CD40 signaling that results in defects in cell mediated immunity and reduced antibody class switching, exhibit increased susceptibility to encephalitis due to enteric cytopathic human orphan (ECHO) virus, CMV, and T. gondii (Subauste, 2006, Winkelstein et al., 2003). The recent advances in whole-genome sequencing technology have intensified the investigation into the prevalence and causes of neuroinfectious diseases in patients with primary immunodeficiencies (Joshi et al., 2009). In pediatric patients with herpes simplex virus (HSV) encephalitis, these approaches have led to the identification of loss of function mutations in multiple innate immune genes including Unc-93 homolog B1 (UNC93B), Toll-like receptor (TLR)3, TIR-domain-containing adaptor-inducing interferon-β (TRIF), TNF receptor-associated factor (TRAF)3, signal transducer and activator of transcription (STAT)1, NF-κB essential modulator (NEMO), and TRAF family member-associated (TANK)-binding kinase 1 (TBK1), (Casrouge et al., 2006, Guo et al., 2011, Herman et al., 2012, Pérez de Diego et al., 2010, Sancho-Shimizu et al., 2011, Zhang et al., 2007). Advances in the use of human induced pluripotent stem cells (IPSC) to generate different neural cell types means that these populations can be used alone or within three-dimensional models of CNS function and combined with gene editing to dissect the basis for susceptibility. This approach has already been utilized to examine the contribution of TLR3-mediated mechanisms of resistance to HSV in different neuronal populations (Lafaille et al., 2012).
The increased use of biologically based therapies to target inflammatory processes has led to the emergence of a new cohort of patients susceptible to many of the infections described above. An important example is the reactivation of the ubiquitous JC virus, which leads to a potentially fatal CNS infection that causes progressive multifocal leukoencephalopathy (PML) (Berger and Koralnik, 2005). This pathogen has continued to rise in prominence with the advent of the humanized monoclonal antibody therapies natalizumab (anti-α4integrin) (Berger and Koralnik, 2005) and rituximab (anti-CD20) (Jelcic et al., 2015), to treat multiple sclerosis, B cell malignancies, and encephalitis due to autoantibodies against the N-methyl-D-aspartate (NMDA) receptor. The use of another anti-integrin antibody, efalizumab (anti-αLintegrin), to treat psoriasis, is also associated with increased incidence of PML (Molloy and Calabrese, 2009) and has been withdrawn from the market. The broad impact of this type of intervention is illustrated in studies in which blockade of α4 integrin compromises protective immunity in the CNS to T. gondii, simian immunodeficiency virus (SIV), and Bornavirus (Planz et al., 1995, Sasseville et al., 1994, Wilson et al., 2009). There is considerable clinical experience with anti-tumor necrosis factor (TNF) therapies to treat rheumatoid and psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, and Crohn’s disease. These therapies can also lead to infections with pathogens that exhibit tropism for the CNS that include Listeria monocytogenes, Nocardia species, Histoplasma capsulatum, T. gondii, Cryptococcus neoformans, Mycobacterium tuberculosis, and CMV (Ali et al., 2013). These clinical examples illustrate that treatments which negatively impact immune surveillance and effector function can lead to severe infections of the CNS. Moreover, when combined with the experiences with patients with acquired immune deficiencies, they suggest that immune surveillance of the CNS occurs in the absence of overt inflammation and protects from the consequences of reactivation of persistent infections.
The Consequences of Microbial Neurotropism
For many of the micro-organisms that exhibit tropism for the CNS, their presence at this site has little obvious benefit in promoting their life cycle and is frequently catastrophic for the host. For example, the presence of C. neoformans or Listeria monocytogenes in the brain might allow these organisms access to an environment that protects from the peripheral immune system, but without treatment they can cause fatal disease. Under these circumstances there is no obvious evolutionary advantage for these organisms that we can discern. In contrast, a limited subset of pathogens that includes HSV, African trypanosomes, T. gondii and Ebola, the ability to persist in neural tissues can directly aid in transmission or provide a sanctuary from which the pathogen can emerge to continue its infectious life cycle (Jacobs et al., 2016, Swanson and McGavern, 2015). This principle might be relevant to HIV, which persists in microglia and astrocytes and could re-emerge, although this has yet to be proven.
Another consideration is that for many important infections of the CNS, humans are considered dead end hosts and the evolutionary basis for neurotropism in this host is unclear. Thus, West Nile Virus (WNV) and the flavivirus, Zika virus can cause acute and autoimmune encephalitides in adults and severe neurodevelopmental abnormalities in human fetuses. Neither of these viruses appears to spread from mammals back to mosquitoes and it is difficult to make a case that neurotropism in humans is critical for their success. Conversely, WNV is neurotopic in almost all birds (the natural host) and while it is asymptomatic in many species, its spread into new avian hosts indicated that those with CNS involvement generally succumb to infection (Gamino and Höfle, 2013). The underlying molecular basis for these types of events is illustrated by the finding that glycosylation in the E protein of WNV results in increased peripheral viremia and virulence for birds and neuroinvasiveness in rodents (Shirato et al., 2004). It is plausible that strategies that promote neurotropism in a primary hosts provides an evolutionary advantage, which in an aberrant host results in disease.
Pathogen Interactions with Blood and Cerebrospinal Fluid Barriers and Access to the CNS
In the face of evolving (or accidental) neurotropism among pathogens that affect distinct compartments of the CNS (see Table 1), the presence of a barrier that limits microbial entry would have a significant selective advantage (Bundgaard and Abbott, 2008). Indeed, the evolutionary conservation of the chemoprotective properties of the BBB and its role as a physical barrier to pathogen entry might indicate that its role in immune privilege evolved secondarily to these functions. In lower vertebrates and invertebrates this barrier is exclusively formed by glial cells. In higher vertebrates, it is established by polarized endothelial cells and the blood-cerebrospinal barrier (BCFB) and BBB exclude blood borne pathogens from the meninges, CNS parenchyma and ventricular system. The basis for this enhanced barrier function is the formation of specialized inter-epithelial and inter-endothelial tight junctions (Klein et al., 2017). Intercellular tight junctions (TJs) composed of claudins and occludins join meningeal and choroid plexus (CP) epithelial cells, presumably limiting the ability of blood borne pathogens that cross their fenestrated endothelium from gaining access to the CNS. In contrast, parenchymal capillaries and venules exhibit both TJs and adherens junctions (AJs), which prevent permeation of solute, cells, and pathogens through paracellular routes. Mammalian BBB TJs and AJs connect BBB endothelial cells to each other and to cytoskeletal actin filaments. As in peripheral vascular beds and the gut, the lengths of actin fibers, which determine the integrity of TJs and AJs at the BBB, are regulated by Rho GTPases (Cetin et al., 2004, Huveneers et al., 2015, Daniels et al., 2014). Thus, junctional complexes, in addition to barrier-stabilizing proteins expressed by abluminal pericytes and astrocyte endfeet, maintain the BBB (see Figure 1 ).
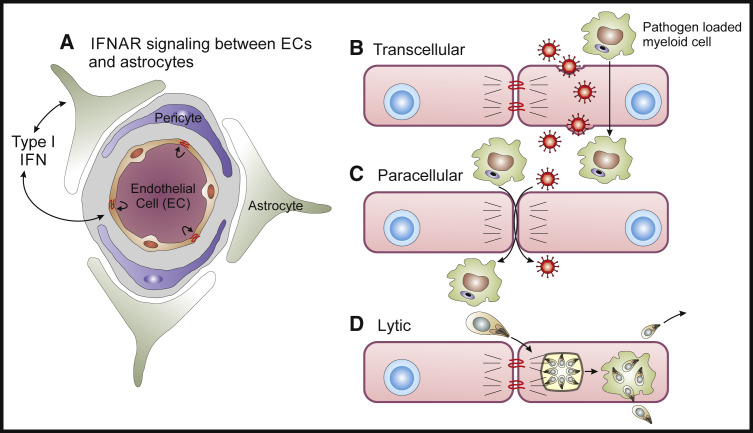
Figure 1.
Pathogen Mechanisms for Crossing the Blood Brain Barrier
(A) The NVU is composed of astrocyte endfeet, pericytes, and endtothelial cells and the local production of type I IFNs has a major role in controlling opening of the BBB (Daniels et al., 2017, Lazear et al., 2015). Micro-organisms utilize three main mechanisms to directly transit the BBB.
(B) Trans-cellular route by which viruses or infected cells directly cross through EC (Dahm et al., 2016).
(C) Para-cellular route whereby the breakdown of the tight junctions allows access of infected cells or extracellular pathogens to access the peri-vascular unit (Santiago-Tirado et al., 2017, Shi et al., 2010, Vu et al., 2013).
(D) Lytic mechanisms in which the ability to infect EC and lyse these cells allows pathogens to access the brain (Konradt et al., 2016).
The meningeal barrier effectively prevents the CNS entry of a majority of bacterial, fungal, and viral pathogens, while only neutrotropic viruses, molds, and certain parasites are able to readily access the CNS parenchyma (see Table 2 and Figure 1). Viruses enter the CNS via multiple routes including retrograde migration along axons, transendothelial entry within the CSF compartment, or transcytosis across an intact BBB, and via delivery by infected mononuclear cells (Dahm et al., 2016). In the setting of certain viral infections TJ proteins, including ZO-1, VE-cadherin, occluding, and claudin 5, might be decreased and so provide a para-cellular route for pathogen or immune cell entry (Bleau et al., 2015, Kim et al., 2015; see Figure 1). This strategy to breach the BBB is relevant to the bacterial organisms L. monocytogenes and N. meningitides, which affect the CNS but use contrasting mechanisms to access this site. The ability of infected monocytes (the Trojan Horse hypothesis) to transport L. monocytogenes across the BBB (Drevets et al., 2004) provides a template for thinking about how other pathogens might access the CNS. For N. meningitides, the association of the meningococci with micro-capillaries with the lowest flow rates is a critical factor that allows access to the CNS (Mairey et al., 2006). In this setting, pilus-mediated adhesion of the meningococci to endothelial cells initiate a signaling cascade which results in the opening of intercellular junctions, allowing meningeal colonization (Bernard et al., 2014, Coureuil et al., 2009, Coureuil et al., 2010).
Table 2.
Routes of Pathogen Entry to the CNS
| Mechanism | Bacteria | Fungal | Parasites | Viruses |
|---|---|---|---|---|
| Retrograde transport along axons | None | None | None | Rabies virus (Bauer et al., 2014) Herpes simplex viruses (Kramer and Enquist, 2013) West Nile virus (Maximova et al., 2016) Venezuelan equine encephalitis virus (Trabalza et al., 2013) |
| Transendothelial entry within CSF compartment |
Group B strep (Kim et al., 2015, Schaeffer et al., 2009) M. tuberculosis (Wu et al., 2000) L. monocytogenes (Dinner et al., 2017) N. meningitides (Schwerk et al., 2012) T. pallidum (Castro et al., 2016) |
Angiostrongylus cantonensis (Barratt et al., 2016) | Enteroviruses (Dahm et al., 2016) Measles virus Chikungunya virus Venezuelan/Western equine encephalitis viruses (Phillips et al., 2016) |
|
| Paracellular entry at the BBB |
L. monocytogenes (Dinner et al., 2017) Nocardia species (Beaman and Ogata, 1993) |
Candida albicans (van Sorge and Doran, 2012) Aspergillus niger Zygomycetes Fusarium species Pseudallescheria boydii Pigmented molds Histoplasma capsulatum Coccidioides immitis Penicillium marneffei |
Herpes simplex viruses (Dahm et al., 2016) West Nile virus Japanese encephalitis virus Human immunodeficiency virus Poliovirus |
|
| Transendothelial entry at the BBB | S. pneumococcus (Iovino et al., 2013) |
T. gondii (Konradt et al., 2016) Taenia solium (Marzal et al., 2014) |
Influenza A (Dahm et al., 2016)) West Nile virus Venezuelan/Eastern equine encephalitis viruses Rabies virus |
|
| CNS entry via infected leukocytes (Trojan Horse) | L. monocytogenes (Drevets et al., 2004) | C. neoformans (Santiago-Tirado et al., 2017) | T. gondii (Konradt et al., 2016) |
Of the eukaryotic pathogens that affect the brain, C. neoformans and T. gondii, have the highest recognized incidence, with multiple mechanisms proposed for pathogen entry to the CNS. The ability to dissect these events in vivo is challenging and consequently in vitro studies with primary endothelial cells have been used extensively. Based on this approach, it has been proposed that both pathogens may enter the CNS via direct infection of brain microvascular endothelial cells (BMECs) and transcytosis from the blood to the brain via mechanisms that damage the BBB, either by disruption of TJs with subsequent edema, or via replication and lysis of BMECs (Huang et al., 2011, Jong et al., 2008, Lachenmaier et al., 2011, Maruvada et al., 2012, Tseng et al., 2012). In vitro, C. neoformans leads to alterations in barrier integrity to promote crossing of the BBB via a para-cellular route (Maruvada et al., 2012) or due to transcytosis of infected mononuclear cells (Santiago-Tirado et al., 2017, Shi et al., 2010, Vu et al., 2013). Monocytes infected with T. gondii are present in the blood and have been implicated as Trojan horses that allow this organism to access the brain (Courret et al., 2006). Infection is also accompanied by the presence of numerous free parasites in the blood and the use of parasite mutants and multiphoton imaging indicate that parasite replication in and lysis of endothelial cells precedes invasion of the CNS (Konradt et al., 2016). One common theme that links several of the more recent studies is the ability to combine genetically modified pathogens with imaging techniques to visualize the BBB in vivo. This has provided the opportunity to the dissect the microbial factors that influence interactions with the NVU, and these approaches will be increasingly relevant to understand mechanisms of neurotropism in vivo.
Innate Immunity at the BBB
The NVU is composed of endothelial cells, pericytes, astrocytes, and neurons and it is now recognized that these cells mediate the initial responses to invading micro-organisms and these events are of particular importance to the control of meningeal pathogens (Neal and Gasque, 2013). The local expression of diverse pattern-recognition receptors (PRRs) by the BBB allows detection of pathogen associated molecular patterns (PAMPs) via coordination of interactions between infiltrating immune cells within perivascular spaces. The scavenger receptor MARCO mediates meningeal recognition of N. meningitides and leads to local production of IL-1 (Braun et al., 2011). Similar events might also directly impact on permeability. For example, activation of Toll-like receptor (TLR)3 leads to barrier opening during WNV infection while TLR7 closes it (Daniels et al., 2014, Wang et al., 2004). Expression of cytokines downstream of innate immune signaling such as IL-1β and TNF-α also destabilize the barrier.
The type I IFNs are the hallmark of the innate response to viral infections and have potent anti-viral activities. They also have a context-dependent role in modulating the BBB and signaling via the type I IFN receptor, IFNα/βR (IFNAR) has a prominent role in stabilization of the BBB (Daniels et al., 2014, Lazear et al., 2015). Recent studies have highlighted CNS region-specific roles of IFNAR signaling in other cellular members of the NVU, with astrocyte IFNAR signaling important for BBB integrity within the hindbrain during infection with WNV (Daniels et al., 2017). Both BMECs and astrocytes express the TAM receptors Mertk and Axl, which are receptor tyrosine kinases that bind Growth Arrest Specific 6 (Gas6) and Protein S. TAM signaling is enhanced by ligand interactions with phosphatidylserine displayed by apoptotic cells or enveloped viruses (Bhattacharyya et al., 2013). In animal models of WNV encephalitis MertK and IFNR signaling synergize to augment BBB integrity (Miner et al., 2015). In all of these circumstances, IFNAR-mediated tightening of the barrier limited virus and immune cell entry, which prevented immunopathology within the hindbrain (Daniels et al., 2017). These events might help explain why the majority of patients with WNV encephalitis do not exhibit increased BBB permeability, as assessed via magnetic resonance imaging (MRI) (Chowers et al., 2001, Nash et al., 2001, Olsan et al., 2003).
In contrast to WNV, certain flaviviruses, such as Japanese encephalitis virus, and other arboviruses that include the alphavirus Venezuelan Equine Encephalitis virus (VEEV), do lead to loss of BBB integrity (Salimi et al., 2016). VEEV, however, does not directly disrupt the barrier, because this occurs late in the course of infection and is associated with the infiltration of monocytes (Cain et al., 2017). The related flavivirus, dengue can secrete nonstructural protein (NS)1, which disrupts the endothelial glycocalyx and compromise the integrity of peripheral vascular beds (Puerta-Guardo et al., 2016). This mechanism might contribute to the rare instances when dengue does affect the CNS (Solomon et al., 2000), but it remains to be determined whether proteins secreted by neurotropic flaviviruses contribute to BBB instability during viremic stages of infection. Similarly, the HIV-1 protein Tat might induce disruption of the BBB via increased expression of platelet-derived growth factor subunit (PDGF-β) (Niu et al., 2014). Thus, the effects of viruses on BBB function might occur at both luminal and abluminal sides of the NVU and thereby promote entry of free virions or virally-infected leukocytes to the CNS (see Figure 2 ).
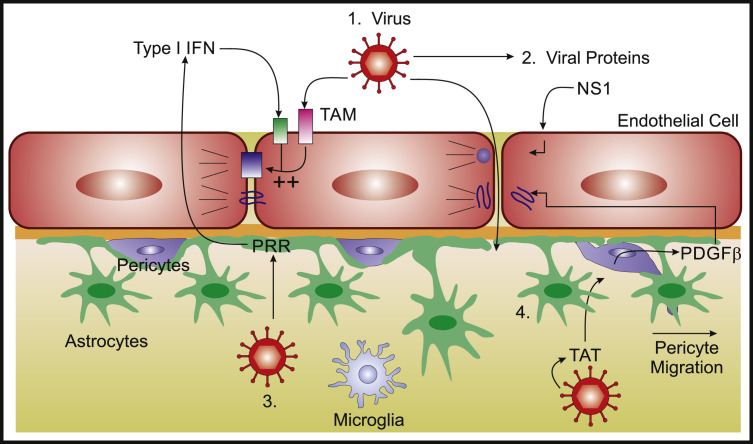
Figure 2.
Viral Modulation of BBB Function
Viruses might impact on TJ integrity via direct and indirect mechanisms: (1) virus detection by brain endothelial TAM receptors in conjunction with type I IFN signaling enhances TJ integrity (Miner et al., 2015); (2) nonstructural protein (NS)1 secretion by flaviviruses might decrease TJ integrity, increasing barrier permeability; (3) PRR signaling during viral infection of astrocytes leads to type I IFN expression which has been shown to improve BBB function via intercellular crosstalk with brain endothelium (Daniels et al., 2014); (Pfefferkorn et al., 2015); and (4) secretion of transactivator protein (TAT) during CNS infection with HIV-1 induces pericyte expression of PDGFβ, which disrupts BBB junctional proteins and promotes pericyte migration away from the vasculature (Niu et al., 2014), (Winkler et al., 2010).
Plasmodium falciparum is a mosquito-transmitted parasite that causes cerebral malaria, a complex syndrome characterized by intravascular sequestration of infected erythrocytes and local inflammation within the microcirculation of the CNS. These events are associated with loss of BBB integrity with subsequent hemorrhage and cerebral edema (reviewed in Hora et al., 2016). While P. falciparum does not directly infect the CNS, studies indicate that the parasite itself, through upregulation of barrier destabilizing inflammatory mediators, or through effects of secreted proteins, might impact on BBB function (Gallego-Delgado et al., 2016, Pal et al., 2016). Thus, P. falciparum-infected erythrocytes induce human brain endothelial cells to express inflammatory molecules including CCL20, CXCL1, CXCL2, IL-6, and IL-8, and intercellular adhesion molecule (ICAM)-1 (Tripathi et al., 2009, Tripathi et al., 2006). In murine models of cerebral malaria that use the P. berghei ANKA strain, blockade of lymphocyte function-associated antigen (LFA) 1, which binds ICAM-1, and treatment with liposomal corticosteroids, which stabilizes TJ integrity, both reduced BBB disruption (Nacer et al., 2012, Waknine-Grinberg et al., 2013). These latter studies illustrate the principal that targeting the effects of pathogens at the BBB might provide an opportunity for adjuvant therapeutic approaches to prevent or manage local immunopathology.
Mechanisms of Innate and Adaptive Protective Immunity in the CNS
Infections of a host with neurotropic pathogens typically occurs at sites such as the skin, mucosa, gut, or lung, via inoculation, ingestion, or inhalation followed by replication and eventual dissemination to the CNS. The temporal nature of these events ensures that the initial activation of innate arms of the immune system occurs in the periphery and influences the generation and expansion of pathogen specific T cells that promote cell intrinsic mechanisms that limit microbial replication. As noted earlier, a wide range of PRRs are expressed by all cells of the NVU and the choroid plexus (CP), and by neuronal and non-neuronal cells within the CNS. These innate sensors direct the local recruitment of leukocytes (most commonly neutrophils, T cells, B cells, monocytes, and dendritic cells), required for microbial control. There are several well-characterized portals of entry for inflammatory cells into the brain, including the fenestrated vasculature within the subarachnoid space and CP, and across epithelial and ependymal cells or astrocyte endfeet of the CP and BBB respectively (Engelhardt et al., 2017). In this section, we will detail the cellular and molecular mechanisms of innate and adaptive immune responses that control pathogen invasion, replication and persistence within the CNS, citing recent reviews for well-established discoveries.
Innate Immunity and Neuro-protection
For many pathogens that are restricted to the NVU, access to the CNS parenchyma is generally prevented by robust innate immune responses within the meninges and ventricular system (Neal and Gasque, 2013). Interactions of infected myeloid cells or free pathogens with the vasculature at these sites lead to expression of adhesion molecules, including vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1 or -2, and chemoattractants CXCL2, leukotriene B4, and complement component C5a, by epithelial or endothelial barriers that recruit neutrophils and monocytes (Neal and Gasque, 2013). There are high constitutive levels of complement proteins present in the CNS, which contribute to normal neuronal function and microglial activity, but less is known about their function during neural infection (Veerhuis et al., 2011). Indirect evidence for their role in the brain is provided by the observation that patients with primary immune deficiencies that affect complement are uniquely susceptible to meningitis caused by N. meningitides (Lewis and Ram, 2014). Further, analysis of human samples of cerebral aspergilosis indicates the highest levels of C3 were associated with fungal control but also that the hyphal forms of this organism were resistant to C3 deposition (Rambach et al., 2008).
The receptor CCR2 and its ligand CCL2 (aka monocyte chemo-attractant protein 1) plays a major role in the mobilization of monocytes and neutrophils. The infiltration of CCR2-expressing Ly6Chi monocytes has been observed in animal models that study the CNS involvement with T. gondii, WNV, or murine hepatitis virus (MHV). In these systems, CCR2 inactivation is associated with decreased leukocyte trafficking and activation of immune cells within the CNS, and a reduced ability to control these pathogens (Benevides et al., 2008, Biswas et al., 2015, Chen et al., 2001). The presence of anti-inflammatory (M2) macrophages or microglia in the CNS is subject of an ongoing debate, but in a murine model of chronic toxoplasmosis, a subset of infiltrating macrophages express canonical M2 markers: CD206 (the mannose receptor), the chitinase AMCase and arginase (Nance et al., 2012). While these cells might be expected to have a role in resolution of inflammation, mice that lack AMCase have a higher parasite burden in the CNS. Based on these results, and those of in vitro studies, it has been proposed that these M2-like populations might be specialized to target the cyst stage of T. gondii (Nance et al., 2012). These reports, in diverse experimental models, highlight the context dependent contribution of chemokine-mediated recruitment of myelomonocytic cells for protective immune responses within the CNS.
Neural Cell Responses to Infection
All neural cell types, including neurons, astrocytes, oligodendrocytes, and microglia, are capable of initiating and responding to inflammatory responses during infection (Nair and Diamond, 2015). Neurons are electrically excitable cells that process and transmit information via electrical and chemical signals. Astrocytes and oligodendrocytes are glial cells that maintain CNS homeostasis, improve neurotransmission via chemical or physical insulation, and support and protect neurons (reviewed in Jäkel and Dimou, 2017). Microglia are ontogenetically distinct members of the reticuloendothelial system that function as tissue macrophages within the CNS (reviewed in Colonna and Butovsky, 2017). Microglia and resident dendritic cells are among the first responders to pathogen invasion, responding to PAMPs via liberation of pro-inflammatory cytokines and clearance of infected cells and debris (Drokhlyansky et al., 2017, Tufail et al., 2017). All of these neural cells express TLRs, Nod-like receptors (NLRs), RIG-like receptors (RLRs), mitochondrial anti-viral signaling (MAVS) (Zhao et al., 2016), AIM2-like receptors (ALRs), C-type lectin receptors, and cytokine receptors (reviewed in Kigerl et al., 2014). Activation of many of these innate immune pathways converge on expression of type I IFNs and mononuclear cell chemoattractants (CCL2, CCL5, and CXCL9-11) and there are many studies that illustrate the relevance to CNS infection. The extended TLR3 signaling pathway is critical in mediating resistance to HSV encephalitis in humans and in mice (Andersen et al., 2015, Casrouge et al., 2006, Dupuis et al., 2003, Herman et al., 2012, Lafaille et al., 2012, Liu et al., 2011, Pérez de Diego et al., 2010, Sancho-Shimizu et al., 2011, Zhang et al., 2007). Other pathways mediate direct anti-pathogen effects, and MAVS sensing of bunyavirus in neurons leads to death of infected cells (Mukherjee et al., 2013). In contrast, sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defense in the CNS (Reinert et al., 2016).
Regional or cellular heterogeneity in innate immune responses of neurons and astrocytes, in particular, might underlie observed differences in pathogen spread, persistence, and immunopathology in different regions of the CNS. Indeed, cortical neurons have low levels of RIG-I, MDA5, ISG54, and ISG56 and consequently are more permissive to WNV than those in the hindbrain (Daffis et al., 2007). Of interest, cerebellar pathology in patients with WNV encephalitis is associated with poorer prognosis (Ali et al., 2005). Further studies are needed to determine whether this subset of patients have underlying defects in hindbrain innate immune function that increase their susceptibility to infection. Differences in baseline expression of genes involved in responsiveness to type I IFN and in susceptibility to viral infections might also help to explain the tropism of viruses for different cell types and regions. The expression of the innate genes Ifnα, Tlr7, Ifit1, Ifit3, Mda5, Ifi44l, Mx1, Rsad2, Stat1, and Oas1 are elevated in murine cerebellar granule cell neurons and astrocytes, compared with cortical neurons and astrocytes (Cho et al., 2013, Daniels et al., 2017). Despite lower basal expression of many IFN stimulated genes in neurons, basal expression of the type I IFN themselves is elevated in primary hippocampal neurons which is important for early control of measles virus (Cavanaugh et al., 2015). While infected neural cells might themselves restrict pathogen replication and dissemination, they can also recruit peripheral myeloid and lymphoid cells to localized sites of infection (Miller et al., 2016). Regional or cellular heterogeneity in cytokine or chemokine expression by neurons and astrocytes might also contribute to different local outcomes. Thus, IL-1 is generally regarded as pro-inflammatory, but during WNV encephalitis IL-1 promotes BBB expression of CXCL12, which limits immunopathology via localization and modulation of T cell fates within peri-vascular spaces (Durrant et al., 2014).
In order to be able to disseminate, access, and persist in the CNS, neutrotropic organisms need to be able to evade the innate effector mechanisms that are deployed against them. The principle that the pathways targeted by pathogens are those that matter most is illustrated by the array of mechanisms used by neurotropic organisms that target the type I and II IFNs. This is relevant to certain arboviruses which have evolved mechanisms to inhibit type I IFN signaling, which allows survival of infected neurons (Samuel and Diamond, 2005). The type I IFN receptor, IFNAR, activates STAT1/2 via TYK2 and JAK1 signaling and WNV inhibits TYK2 activity, while the related flavivirus, Zika virus degrades human STAT2 (Brasil et al., 2016). Similarly, VEEV evades activity of the interferon-induced protein with tetratricopeptide repeats 1 (IFIT1) that blocks translation of virion-delivered virus-specific RNAs (Habjan et al., 2013, Hyde et al., 2014). Of relevance to the JAK/STAT pathway, T. gondii secretes an effector molecule into its host cell that recruits a host chromatin repressor complex, which blocks the ability of IFN-γ to mediate transcription through STAT1 (Gay et al., 2016, Olias et al., 2016). That diverse organisms have multiple strategies to target JAK/STAT signaling is consistent with the key role of the IFNs in mediating resistance to these neurotropic pathogens and is relevant to many other innate sensing and effector mechanisms.
Astrocytic Responses to Infection
Astrocytes are considered the most numerous cell type in the CNS and, while frequently defined by the expression of the intermediate filament glial fibrillary acidic protein (GFAP), this is a heterogenous cell type with distinct functions in different anatomical regions of the CNS, associated with neuronal health and maintenance. This topic is covered in depth in this issue by Liddelow and Barres, 2017. The use of Aldh1l1-reporter mice in conjunction with screening for differential expression of surface antigens was used to identify five distinct subpopulations of Aldh1l1+ astrocytes within the olfactory bulb, cortex, brainstem, thalamus, and cerebellum that exhibit unique genetic signatures, including a subpopulation that exhibits higher levels of expression of immune molecules (Yang et al., 2011). Similarly, a comparison of cerebellar with cortical astrocytes revealed that cerebellar cells exhibit higher expression of genes involved in TJ and AJ signaling, supporting their differential effects on BBB stability (Daniels et al., 2017). Whether these subpopulations also exhibit differences in susceptibility to infection, inflammatory responses or effects on BBB function remains to be determined.
The prominent hypertrophy of astrocytes associated with infections of the CNS and the ability to activate these cells in vitro with IFNs to control pathogen replication led to the idea that these cells would have an important role in the host response to infection. The use of lineage targeting approaches to delete the IFNAR on astrocytes increases WNV infection and immunopathology within the hindbrain (Daniels et al., 2017), while the removal of STAT1 from these cells results in increased susceptibility to toxoplasmic encephalitis (TE) (Hidano et al., 2016). Interestingly, a genetic screen to identify host factors that enhance the activation of STAT1 revealed that the orphan nuclear hormone receptor TLX, expressed in neural stem cells and astrocytes, is a modifier of STAT1 activity and is required for resistance to T. gondii (Beiting et al., 2015). Although astrocytes lack the major known IFN-γ-induced pathways that target T. gondii (Halonen and Weiss, 2000, Hunter and Sibley, 2012), two of the IFN-γ-inducible 47 kD Immunity Related GTases (IRGs) are required for murine astrocytes to eliminate T. gondii in vitro (Degrandi et al., 2013, Melzer et al., 2008). Humans lack IRGs but the p67 guanylate-binding proteins (GBPs) have analogous functions (MacMicking, 2012) and GBP2 is required for parasite killing by murine astrocytes in vitro (Degrandi et al., 2013). These proteins are part of a coordinated response that intersects with autophagic processes that lead to parasite control (Degrandi et al., 2013, Niedelman et al., 2013, Van Grol et al., 2013, Yamamoto et al., 2012). From a broader perspective, these findings highlight a major knowledge gap in our understanding of how astrocytes and neurons respond to the IFNs to mediate control of diverse pathogens. To date, almost 2,000 IFN stimulated genes (ISG) have been identified in human and mouse cells but few of these genes have been characterized in the context of infection and improved methodologies to dissect the anti-microbial activities in different neural cell types are needed.
In the context of sterile or microbial-induced CNS injury, astrocytes start to proliferate, increase expression of GFAP and undergo changes in morphology (termed astrogliosis) that allows them to form dense networks or “glial scars” at sites of damage. In mice challenged with Staphylococcus aureus or T. gondii, reactive astrocytes are prominent, but the absence of GFAP limits glial scar formation and the ability to restrict inflammation, which leads to pathogen spread (Stenzel et al., 2004). Similarly, when compared to wild-type controls, mice with astrocyte lineage-specific deletion of the cytokine receptor gp130, exhibited increased astrocyte apoptosis and inflammation. Although these mice control parasite numbers, they exhibited a more extensive inflammatory response and increased susceptibility to the chronic phase of infection (Drögemüller et al., 2008). This phenotype is recapitulated with the loss of TGF-β signaling in astrocytes, which did not affect parasite burden but resulted in increased inflammation and neuronal damage (Cekanaviciute et al., 2014). These studies indicate that the extensive gliosis associated with many CNS infections is part of a conserved response to local injury (analogous to granuloma function) that provides a physical barrier to limit tissue damage and isolate areas of pathogen replication.
Mechanisms of Adaptive Protective Immunity
In the majority of CNS infections discussed above, the initial priming of T cell responses that contribute to pathogen control are generated in the periphery. As a consequence of pathogen replication in the brain, pathogen-specific T cells traffic to the CNS and are able to gain parenchymal access. While there are a limited number of helminth infections that are associated with T helper 2 (Th2) responses that affect the CNS (neurocystocercosis and aberrant migratory nematodes), most clinically relevant infections of the CNS are viral, bacterial, fungal, or protozoan, and it is not surprising that adaptive resistance to these organisms in the brain is mediated by CD4+ and CD8+ T cells that produce IFN-γ. There is a strong dogma that initial entry of pathogen-specific T cells into the CNS is dependent on local antigen presentation which influences their local expansion and retention (Tschen et al., 2006). This is a complex topic, and the reports that activated T cells, regardless of antigen specificity, can traffic to the uninflamed CNS (Wekerle et al., 1986), suggests the presence of a mechanism that allows surveillance of the CNS that might precede and potentially limit pathogen entry. In contrast, during TE the activation and expansion of T cells specific for a non-microbial antigen did not result in their entry to the CNS (Wilson et al., 2009). The observation that activation alone is not sufficient for T cell entry to the inflamed CNS is consistent with the idea that there might be a licensing mechanism that influences T cell entry (Odoardi et al., 2012).
Regardless, once access to the CNS has been achieved, these T cell populations need to find their relevant targets and mediate local pathogen control (see Figure 3 ). Mice infected with T. gondii or LCMV have provided tractable models for intra-vital imaging of how T cells in the CNS interact with infected cells and other antigen presenting cells. Analysis of the movement of parasite specific effector CD8+ T cells in the CNS of mice with TE revealed that these cells did not display a directed migration, rather their behavior was reminiscent of the search strategies utilized in nature to find rare targets (Harris et al., 2012). One unexpected outcome of these studies was the idea that locally produced chemokines did not establish gradients to attract effector T cells, but rather provided motogenic signals to promote T cell movement required for the search strategy (Harris et al., 2012).
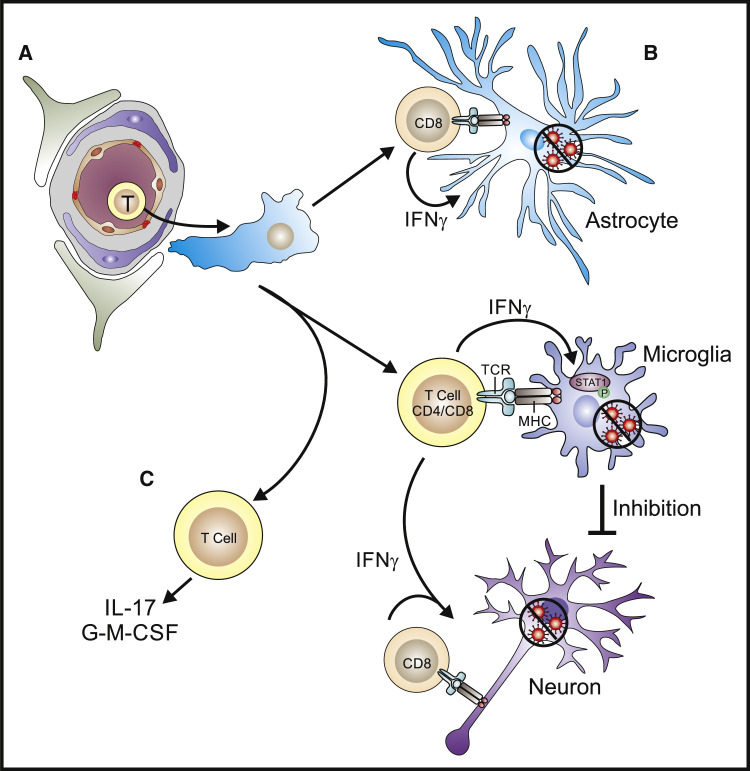
Figure 3.
Effector Mechanisms Used by T Cells to Control CNS Infection
(A) The ability of pathogen-specific CD4+ and CD8+ T cells to exit the circulation, cross the BBB, and access the parenchyma precedes the search for relevant infected CNS-specific targets.
(B) The local production of IFN-γ by CD4+ and CD8+ T cells has a prominent role in the activation of STAT1 and the anti-microbial activities of astrocytes and microglia. While CD8+ T cells can interact with infected astrocytes and microglia to mediate elimination of infected cells or control of pathogen replication it is unclear whether their interactions with neurons is direct through MHC-TCR interactions or indirect through the activation of microglia.
(C) The development of Th17 responses characterized by the production of IL-17 and/or GM-CSF is correlated with the development of immunopathology and the recruitment of inflammatory monocytes.
Based largely on in vitro and correlative studies, there has been a long held belief that microglia are likely to have a prominent role in the local response to infections either as antigen-presenting cells or as phagocytes capable of killing microorganisms. The interactions of pathogen specific T cells with accessory cells (DC and/or microglia has also been visualized using intravital imaging of CD8+ T cells responding to infection with T. gondii (John et al., 2011, Schaeffer et al., 2009). During infection with LCMV, microglia are a major reservoir of infection, and antiviral CD8+ T cells can stably engage microglia in an MHC I-dependent manner. In contrast, CD4+ T cells have less sustained interactions with microglia, likely as consequence of the relative abundance of cognate peptide–MHC I versus MHC II complexes (Herz et al., 2015). A functional role for microglia in modulating CD8 T cell responses was illustrated via pharmacological deletion of microglia during dengue virus infection that resulted in reduced CD8+ T cell responses and increased viral replication (Tsai et al., 2016). The fundamental advances in understanding microglial development combined with ability to visualize how these cells interact with pathogens and other immune cells and the use of genetic approaches to manipulate microglial immune function will provide the opportunity to better understand the contribution of these cells to resistance to infection or resolution of local inflammation.
T cell production of IFN-γ induces a wide range of anti-microbial effector molecules, often in a cell-type-specific fashion (see earlier sections on astrocytes and neurons) and is a key cytokine for neuroinfectious diseases (Griffin, 2003). This principle was established in murine models of toxoplasmosis in which CD4+ and CD8+ T cells in the CNS produce IFN-γ that, in combination with other effectors such as TNF-α and CD40L, mediate local parasite control (Denkers et al., 1997, Gazzinelli et al., 1992, Reichmann et al., 2000, Suzuki et al., 1989, Yap and Sher, 1999). In this system, in addition to effects on astrocytes, the production of IFN-γ drives local macrophage activation, with production of toxic oxygen intermediates such as nitric oxide (NO) required to control intracellular parasites (Deckert-Schlüter et al., 1998, Nansen et al., 1998, Scharton-Kersten et al., 1997).
The neuroimmunology literature has focused on the contribution of T helper 17 (Th17) cells to the development of autoimmune pathology (Korn et al., 2009), but less is known about their function during CNS infection. Perhaps the strongest indication that these cells are protective in the CNS is in the setting of fungal infections where there is a high prevalence of invasive candidiasis of the brain in patients with primary genetic defects in CARD9, an adaptor required for the development of Th17 responses (Alves de Medeiros et al., 2016, Glocker et al., 2009, Herbst et al., 2015). While these patients have reduced Th17 responses, the observation that other genetic deficiencies associated with diminished IL-17 production are not susceptible to Candida suggest that another mechanism might underlie susceptibility. Indeed, these patients also have a reduced ability to produce GM-CSF, a cytokine associated with Th17 responses that promotes macrophage and microglial function. The idea that a reduced ability to make GM-CSF might underlie susceptibility to invasive candidiasis is supported by the success of GM-CSF in these patients as an adjunctive therapy with anti-fungal agents (Gavino et al., 2014). Because T-cell-derived GM-CSF is important in the recruitment of CCR2+ inflammatory monocytes to the CNS during experimental allergic encephalomyelitis (EAE) (Croxford et al., 2015), this pathway might be relevant to models in which monocytes are important for local control of CNS infection (Benevides et al., 2008, Biswas et al., 2015, Chen et al., 2001).
Adaptive Mechanisms to Target Neurons
The neuron, as a long-lived cell with a unique biology that ensures that a single cell can traverses extensive areas of the CNS, seems an ideal refuge for many pathogens. Indeed, several viruses in the CNS are not cytolytic and have evolved strategies to interfere with anti-microbial pathways and to remain latent in neurons. This is illustrated with HSV in which latency associated transcripts generate miRNAs that target immunomodulatory genes that facilitate viral latency (Boss and Renne, 2011). This ability to target neurons is not exclusive to viruses and a select group of apicomplexan parasites (notably T. gondii) have specialized cyst stages that persist in neurons. It has been proposed that the cellular stress associated with the immune response drives cyst formation and extracellular adenosine (likely released from damaged cells) signaling through CD73 promotes cyst formation (Mahamed et al., 2012). It is notable that cysts are typically restricted to neurons, but the loss of STAT1 in astrocytes results in cyst formation in these cells in vivo, a finding that suggests IFN-γ can actively prevent cyst formation (Hidano et al., 2016). The ability to study how neurons respond to infection in vivo remains challenging but there are two examples where the ability to genetically modify pathogens to express the Cre recombinase combined with host reporters has provided novel tools that have advanced this topic. Thus, the use of rabies that express Cre has shown that the majority of RABV-infected neurons survive infection and clearance and these have been transcriptionally profiled (Gomme et al., 2012). Similarly, the use of T. gondii strains that secrete Cre into host cells has been used to visualize neurons infected with Toxoplasma (Cabral et al., 2016).
While there is a role of type I IFNs in limiting viral replication in neurons, there are open questions about the mechanisms used by T cells to target these cells. For example, the non-cytolytic clearance of Measles (the cause of sub-acute sclerosing panencephalitis) and Sindbis from neurons is IFN-γ dependent (Binder and Griffin, 2001, Patterson et al., 2002) and there appears to be a bystander role for CD4+ T cells in some of these events (Solomos et al., 2016). In “carrier mice” that have an established LCMV infection in neurons, there is a literature that identifies “therapeutic” CD8+ T cells as being important in elimination of this virus pool through the production of IFN-γ that licenses microglia to purge virus, present antigen, and amplify the local antiviral T cell response (Herz et al., 2015, Oldstone et al., 1986, Pinschewer et al., 2010). How microglia mediate these effects is uncertain, but is associated with a number of STAT1-dependent processes and the presence of a GM-CSF signature (Herz et al., 2015).
While there has been a long-held belief that neurons express no MHC class I (which would make them an ideal cell type to evade CD8+ T cells), the application of more sensitive technologies has led to the recognition that different subsets of these cells can express MHC class I in the developing and adult brain and that these molecules have a role in neurological function (Cullheim and Thams, 2010, Shatz, 2009). There are reports of the induction of neuronal class I during infection (Neumann et al., 1995), but there remains a major knowledge gap in our understanding of whether neuronal antigen presentation influences resistance or susceptibility to infection in vivo. There is in vitro and in vivo evidence for CD8+ T cells interacting directly with infected neurons during Theiler’s virus, LCMV, Borna and picornavirus infection (Chevalier et al., 2011, Huseby Kelcher et al., 2017, McDole et al., 2010, Rall et al., 1995) and there is evidence that the ability of HSV to inhibit class I expression in neurons in basal ganglia is key to avoid CD8+ T cell responses (Orr et al., 2007). Unexpectedly, imaging studies indicate that CD11c+ cells (likely microglia or DC), but not parasite-specific CD8+ T cells, frequently interact with cysts of T. gondii (John et al., 2011, Schaeffer et al., 2009). The ability to generate parasites with altered expression of stage-specific antigens (cyst versus non-cyst), has demonstrated that antigens associated with latency do not trigger the same immune responses as those produced during active infection (Kim and Boothroyd, 2005). These results highlight an immune evasion strategy to promote parasite persistence that would likely be relevant to many pathogens that have latent stages.
Tissue-Resident Memory Populations in the CNS
For many of the pathogens discussed here, the control of the acute phase of the disease is mediated by T cells and as antigen load is reduced the number of pathogen-specific T cells contract. One consequence of these events is the generation of a long-lived pool of tissue-resident memory T (Trm) cells, which reside in barrier tissues like the lung, skin reproductive tract, and gut where they maintain post-infection homeostatic surveillance (Ariotti et al., 2012, Schenkel et al., 2014, Steinert et al., 2015). Identification of these populations has been based on the expression of CD103 (the αE integrin) and CD69 (a proximal marker of TCR activation and tissue retention). In several models, viral-specific CD8+ Trm are maintained in the CNS in the absence of detectable virus. Thus, in mice challenged with neurotopic influenza virus, CD8+ T cells were present at this site 320 days after infection (Hawke et al., 1998). Similarly, following clearance of VSV from the brains of mice, clusters of CD103+ CD8+ T cells persist at likely sites of previous infection (Wakim et al., 2010). LCMV-specific brain Trm are also found close to epithelial cells of the ventricular lining, within the choroid plexus, and in the meninges (Steinbach et al., 2016). The ability to generate CNS-specific Trm is not specific to viral infection or dependent on antigen clearance and increasing chronicity of toxoplasma infection is associated with an enrichment of cells that co-express CD103 and CD69 and which are transcriptionally similar to VSV-specific Trm (Landrith et al., 2017).
With the recognition that pathogen-specific Trm populations are present in the CNS there has been interest in understanding the processes that influence their formation. Following WNV infection, the production of TGF-β results in increased expression of CD103 by CD8+ T cells, which allows a large pool of resident memory T cells to be maintained (Graham et al., 2014). During acute infection with the mouse polyoma virus the high-affinity effector cells give rise to high-affinity Trm. Functional studies have shown that virus-specific Trm have a TCR affinity that is approximately 20-fold higher in CD8+ T cells derived from the brain versus those of the spleen (Frost et al., 2015). This selection for high TCR affinity could improve the ability of Trm to detect infected cells that express low levels of pathogen derived MHC-peptide complexes. Concrete evidence that Trm are of functional significance in the CNS is provided by the use of mice that had cleared LCMV. In this setting, intracerebral inoculation of virus resulted in LCMV specific CD8+ Trm entering cell cycle and mediating viral control independently of peripheral CD8+ T cells via production of IFN-γ and perforin (Steinbach et al., 2016). These types of experimental systems will help understand the rules that govern these populations and aid with the development of strategies to promote the generation of Trm that could limit pathogen reactivation within the CNS.
Limiting Inflammation in the CNS: Immunomodulatory Cytokines, Inhibitory Receptors, and Treg Cells
While the presence of inflammatory cells in the CNS is critical for local control of infection, this immune response is often pathological and regardless of the initiating factor, will frequently converge on pathways that induce neurotoxicity. A variety of models can be used to distinguish the immune processes required for resistance to infections from those that induce bystander damage or post-infectious sequelae. Indeed, there are numerous examples in which altered chemokine signaling or reduction of T cell numbers in the setting of CNS infection results in unexpected but improved outcome (Campanella et al., 2008, Christensen et al., 2006, Reichmann et al., 1999).
During murine infections with WNV, CCL-dependent accumulation of monocytes is associated with development of pathology (Bardina et al., 2015, Getts et al., 2008, Lim et al., 2011), whereas in mice infected with HSV, innate cell infiltration is associated with fatal disease (Lundberg et al., 2008). Similarly, the CCCR2-dependent monocytes associated with Theiler’s murine encephalitis virus (TMEV) mediate the demyelination that characterizes this infection (Bennett et al., 2007). The intra-cerebral challenge with LCMV results in immunopathologic CD8+ T cell responses in the meningeal compartment that is characterized by the recruitment of neutrophils and monocytes that mediate the vascular injury and the onset of seizures (Kim et al., 2009). In the latter model, the type I IFN have a key role in priming the pathological monocyte response (Nayak et al., 2013) and can lead to edema, neuronal death, and herniation (Matullo et al., 2011, Matullo et al., 2010). A similar phenotype is apparent in mice infected with picornovirus (Huseby Kelcher et al., 2017) and in murine models of cerebral malaria. In the latter system, CD8+ T cell responses are associated with alterations in TJs and vascular breakdown in the meninges and parenchyma, resulting in edema, neuronal cell death within the brainstem, and cerebral herniation (Huggins et al., 2017, Swanson et al., 2016), reminiscent of the disease process observed in humans (Seydel et al., 2015). Thus, inflammation that results in bystander neuronal death or damage to regions of the brain that control autonomic functions can significantly impact host survival.
Another possible consequence of local inflammation is the release of autoantigens and breakdown of tolerance that might lead to a bona fide autoimmune process mediated by T or B cells. For example, after HSV encephalitis the development of auto-antibodies specific for N-methyl-D-aspartate receptor (NMDAR) can lead to a post-viral encephalitic syndrome characterized by seizures and psychosis (Höftberger et al., 2013, Westman et al., 2016). Similarly, damaged oligodendrocytes during MHV infection promote the recognition of Myelin Basic Protein by auto-reactive CD4+ T cells, leading to epitope spreading and the development of demyelinating disease that is reminiscent of MS (McMahon et al., 2005). It seems likely that the true prevalence and consequences of these types of post-infectious sequlae in patients is not fully appreciated (Klein et al., 2017).
While the development of neuroinflammation is important to respond to local infections, there are also conserved regulatory processes that help with the resolution of CNS inflammation or which exist to limit the development of overwhelming immune pathology (Galea et al., 2007). The cytokine IL-10 is important negative regulator of inflammation, which acts directly on DCs, microglia, and macrophages to downregulate expression of costimulatory molecules and the ability to present antigen and produce inflammatory cytokines. While there are multiple sources of IL-10, in the setting of many infections, the ability of T cells to produce IL-10 provides a critical negative regulatory loop that prevents T cell hyper-activity (Couper et al., 2008). In mice chronically infected with T. gondii the absence of IL-10, leads to lethal CNS inflammation mediated by CD4+ T cells and characterized by elevated IL-17 production (Wilson et al., 2005). For LCMV, the presence of IL-10 promotes viral persistence in the CNS (Brooks et al., 2006), while IL-10 deficient mice infected with Sindbis develop an aberrant Th17 response associated with neuronal cell death (Kulcsar et al., 2014) and IL-10 derived from CD8+ T cells protect from pathology during coronavirus infection (Trandem et al., 2011).
IL-27 is a cytokine produced by microglia and other macrophage populations in the inflamed CNS that has emerged as having an important role in limiting neuroinflammation. Thus, in the absence of IL-27 signaling, mice infected with T. gondii, Coronaviruses, or Sindbis are characterized by elevated production of IL-17 and GM-CSF in the CNS, which correlate with the development of T cell-mediated immune pathology (de Aquino et al., 2014, Kulcsar et al., 2014, Stumhofer et al., 2006, Tirotta et al., 2012, Young et al., 2012). While there is good evidence that IL-27 can directly limit T cell production of IL-17 and GM-CSF, it can also promote T cell production of IL-10, Treg cell responses, and T cell expression of inhibitory receptors (Do et al., 2016, Hall et al., 2012, Hirahara et al., 2012). The enhanced CNS pathology observed in the absence of IL-10 or IL-27 suggests that while they might target different cell populations and have fundamentally different mechanisms of action, these cytokines are shared components of an immunoregulatory cassette that acts to temper T cell mediated CNS inflammation.
Many of the overarching principles that have guided the study of “inhibitory receptors” (PD-1, PD-L1, CTLA-4, TIGIT, Tim-3, LAG-3) that provide cell-intrinsic signals that limit T cell responses emerged from studies of peripheral responses to LCMV (Jin et al., 2010, Wherry, 2011). One model suggests that serial engagement of T cells in the context of persistent antigen results in a hierarchical acquisition of inhibitory receptors that results in “exhaustion” but allows détente between viral persistence and the pathological consequences of T cell responses (Wherry, 2011). During neural involvement of LCMV there is increased T cell expression of PD-1 in the CNS (Blackburn et al., 2010), but there is a paucity of studies that have dissected the impact of inhibitory receptors on local responses in the brain. Similarly, during HSV infection in mice, viral-specific CD8+ T cells express increased levels of LAG3, TIM3, and PD-1, which is associated with an inability to control viral replication in the CNS (Menendez et al., 2016). During toxoplasmosis, parasite-specific CD8+ T cells recruited to the brain downregulate their ability to produce IFN-γ and granzyme over time, which correlates with increased expression of PD-1, LAG3, and TIGIT (Hidano et al., 2016, Wilson et al., 2009). The potential significance of these observations is emphasized by the report that the blockade of PD-1 during the chronic phase of this infection results in enhanced parasite clearance (Bhadra et al., 2011). This is clearly a topic where additional studies are required to determine the relative impact of diverse inhibitory receptors on anti-microbial effector activities and whether they promote microbial persistence.
Treg cells are defined by the expression of the transcription factor Foxp3 and maintain homeostasis within the T cell compartment through direct antagonism of T cell function, as well as effects on DC populations (Josefowicz et al., 2012). These suppressive activities are attributed to multiple mechanisms that include the production of IL-10 as well as expression of inhibitory receptors. Treg cells are absent from the uninflamed CNS, but during infection are co-recruited with effector T cells. Given their prominent role in other inflammatory diseases settings, there have been questions about whether these regulatory populations contribute to the outcome of CNS infection. In mice infected with measles virus, expansion of Treg cells resulted in enhanced viral levels in neurons while the transient depletion of Foxp3+ Treg cells led to expanded populations of virus-specific CD8+ T cells and improved viral control (Reuter et al., 2012). Following challenge with MHV, virus-specific Treg cells are present in the CNS and have a role in limiting immune-pathology (Anghelina et al., 2009). These MHV-specific Treg cells inhibit the expansion of viral specific CD4+ T cell effector responses, which is associated with reduced microglial activation and decreased mortality, without affecting virus clearance (Zhao et al., 2014). Intravital imaging of Treg cells has shown that they are highly migratory in secondary lymphoid tissues but during TE they appear restricted to perivascular regions where they form sustained interactions with DC (O’Brien et al., 2017). While it is possible that Treg cells might lose expression of Foxp3 as they migrate into the CNS, this exclusion from the parenchyma might allow enhanced effector cell function within the brain. This observation also supports the idea that perivascular compartments represent a site where regulation of T and B cell effector functions occurs (Durrant et al., 2014, Durrant et al., 2013, Phares et al., 2013).
Future Directions
Can CNS Infection Be Beneficial through Innate Immune Activation?
While persistent immune activation in the brain is associated with a number of infectious, autoimmune, and neurodegenerative conditions, it is unclear whether all inflammatory pathways are detrimental for brain function. There is evidence that many of the innate immune molecules discussed above in the context of infection, are required for normal CNS development, function, and repair. Ontology studies have shown that microglia are derived from erythromyeloid precursors in the yolk sac, migrating, and proliferating within the CNS early during embryonic development, via processes that requires CSF-1R, its ligand IL-34 and TGF-β. Mice deficient in these molecules exhibit severely decreased numbers of microglia in many brain regions (Colonna and Butovsky, 2017). Activated, CD68+ microglia are normally present in the developing forebrain subventricular zone, where neural precursors reside. Treatment of mice with minocycline, a broad-spectrum antibiotic that decreases microglia activation, or genetic deficiency in CSF-1R, is associated with disruption of periventricular cellular architecture, decreased neurogenesis, and oligogenesis, the latter of which produces myelinating cells of the CNS (Erblich et al., 2011, Farmer et al., 2013). Similarly, TLRs and TAM receptor signaling is critical for adult neurogenesis within the dentate gyrus, a process that is essential for learning and memory (Ji et al., 2013, Matsuda et al., 2015, Moraga et al., 2014). There are also numerous reports that link the microbiota with neural development that is covered in depth in this issue by Yoo and Mazmanian (2017) but is exemplified by the link between microbiome-driven TLR4 signaling in EC that leads to cerebral cavernous malformations (Tang et al., 2017). Neuronal differentiation is further associated with expression of IRF9, IFNAR, and STAT1 (Farmer et al., 2013). Moreover, mice deficient in IFNAR signaling develop defects in neuronal autophagy, suggesting a critical role for this molecule in CNS homeostasis (Ejlerskov et al., 2015). TAM signaling within neural cells might also exhibit synergistic effects on type I IFN-mediated cellular processes (Miner et al., 2015), supporting the notion that baseline activation of IFNAR supports normal brain function.
Previous sections have emphasized the development of protective inflammatory response and the mechanisms that limit pathology but there are also regenerative processes that serve to restore neural function. Given the role of immune pathways in normal neuronal development, it is not surprising that innate responses triggered during infection are also involved in CNS homeostasis, repair, and recovery. Chemokines can also promote expression of anti-inflammatory molecules and growth factors, such as transforming growth factor (TGF)-β, insulin growth factor (IGF)-1, fibroblast growth factor (FGF)2, and platelet derived growth factor (PDGF)-A (Church et al., 2016, Wattananit et al., 2016). Myeloid cells are instrumental in CNS repair by virtue of their phagocytic and cytokine expression functions (London et al., 2013) (Herz et al., 2017). Activation of PRRs on myeloid cells, including TLRs 1 and 2, CR3, Dectin-1 and 2, promotes phagocytosis, oxidative burst, and cytokine production. Phagocytic receptors CD14, CD36, TREM2, and TLR4 are critical for pathogen clearance while complement opsonins C1q, C3 and iC3b and PRRs, TAMs, and TREM2 facilitate removal of apoptotic cells (reviewed in Colonna and Butovsky, 2017). Studies using mice deficient in TREM2 or TLR4 demonstrate that lack of debris elimination results in defects in repair of demyelination and axonal regeneration, respectively (Cantoni et al., 2015, Rajbhandari et al., 2014). CNS region-specific effects of macrophage-specific PRRs have also been observed; activation of dectin-1, a C-type lectin receptor, promotes optic nerve, but not spinal cord, axon regeneration via Erk1/2 MAP-kinase signaling and cAMP response element-binding protein (CREB) activation, while TLR2 activation has the reverse effects during injury of these two CNS regions (Baldwin et al., 2015, Gensel et al., 2015). Cooperative signaling between CD14, TLRs, and dectin-1 in activated myeloid cells also leads to expression of type I IFN, which can limit glial scar formation, remove degenerating axons and promote their regrowth (Hosmane et al., 2012, Nishimura et al., 2013, Zanon et al., 2010). The identification of innate immune pathways that promote recovery from pathogen-mediated brain injury could provide new targets for the development of therapies to treat non-infectious CNS disorders.
Can Immune-Mediated Strategies Be Developed to Better Manage CNS Infections?
From a clinical perspective, the management of patients with neuroinfectious disease is complicated by the lack of treatment options available to suppress inflammation without compromising protective responses. Moreover, the concept that boosting anti-pathogen responses in the CNS is a clinically viable strategy runs counter to the perception that inflammation of the CNS should be avoided. The use of clinically relevant models described throughout this review have already provided insights into the balance between stringent regulation of immune cell access to the CNS and the need for anti-pathogen immunity. The natural history of human infections and the use of antibody-based therapies to treat inflammatory diseases illustrates the importance of surveillance and effector mechanism that are relevant to immunity in the CNS but also the ability to balance therapeutic dose to allow clinical benefit versus compromise of CNS surveillance (Ali et al., 2013, Berger and Koralnik, 2005). One area where there might be an opportunity to augment anti-pathogen responses in the CNS relates to the function of inhibitory receptors. The clinical success of targeting PD-1, PD-L1, and CTLA-4 to reinvigorate pre-existing anti-tumor T cell responses has the potential to determine whether these pathways operate constitutively in the CNS. There is an expanding cancer patient base that are treated with these therapies, but it is not yet clear whether these treatments will impact the immune response to latent infections of the CNS. In one scenario, treatments that target inhibitory pathways might lead to silent clearance of pre-existing chronic infections of the CNS and/or result in overt or transient immune pathology, perhaps associated with pathogen clearance. The latter findings would argue that immunotherapy is a viable approach to target latent of persistent infections of the CNS. The utility of this type of approach is illustrated by the success of CAR T cell therapies to target cancer and which might be applicable to infectious diseases (Parida et al., 2015). The willingness to target CNS infections would need to be based on the belief that T cells are part of the normal surveillance in the brain, and that these populations could be engineered to traffic into the CNS and function there with specificity and minimal bystander damage.
Acknowledgments
The authors would like to acknowledge Deb Argento for outstanding help with the figures. Grant support from the National Institutes of Health: DTRA1-11-16-BRCWMD-BAA,NIH/NINDS P01 NS059560, NIH/NIAID U19 AI083019, and NIH/NINDS R01NS052632 (all to R.S.K.). NIAID R01 AI110201, NIAID R01 AI 41158, Commonwealth of Pennsylvania (all to C.A.H.).
References
- Ali M., Safriel Y., Sohi J., Llave A., Weathers S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am. J. Neuroradiol. 2005;26:289–297. [PMC free article] [PubMed] [Google Scholar]
- Ali T., Kaitha S., Mahmood S., Ftesi A., Stone J., Bronze M.S. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc. Patient Saf. 2013;5:79–99. doi: 10.2147/DHPS.S28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves de Medeiros A.K., Lodewick E., Bogaert D.J., Haerynck F., Van Daele S., Lambrecht B., Bosma S., Vanderdonckt L., Lortholary O., Migaud M., et al. Chronic and Invasive Fungal Infections in a Family with CARD9 Deficiency. J. Clin. Immunol. 2016;36:204–209. doi: 10.1007/s10875-016-0255-8. [DOI] [PubMed] [Google Scholar]
- Andersen L.L., Mørk N., Reinert L.S., Kofod-Olsen E., Narita R., Jørgensen S.E., Skipper K.A., Höning K., Gad H.H., Østergaard L., et al. Functional IRF3 deficiency in a patient with herpes simplex encephalitis. J. Exp. Med. 2015;212:1371–1379. doi: 10.1084/jem.20142274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anghelina D., Zhao J., Trandem K., Perlman S. Role of regulatory T cells in coronavirus-induced acute encephalitis. Virology. 2009;385:358–367. doi: 10.1016/j.virol.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti S., Beltman J.B., Chodaczek G., Hoekstra M.E., van Beek A.E., Gomez-Eerland R., Ritsma L., van Rheenen J., Marée A.F., Zal T., et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc. Natl. Acad. Sci. USA. 2012;109:19739–19744. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A., Antila S., Proulx S.T., Karlsen T.V., Karaman S., Detmar M., Wiig H., Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin K.T., Carbajal K.S., Segal B.M., Giger R.J. Neuroinflammation triggered by β-glucan/dectin-1 signaling enables CNS axon regeneration. Proc. Natl. Acad. Sci. USA. 2015;112:2581–2586. doi: 10.1073/pnas.1423221112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardina S.V., Michlmayr D., Hoffman K.W., Obara C.J., Sum J., Charo I.F., Lu W., Pletnev A.G., Lim J.K. Differential Roles of Chemokines CCL2 and CCL7 in Monocytosis and Leukocyte Migration during West Nile Virus Infection. J. Immunol. 2015;195:4306–4318. doi: 10.4049/jimmunol.1500352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R.A., Widner H. Immune problems in central nervous system cell therapy. NeuroRx. 2004;1:472–481. doi: 10.1602/neurorx.1.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt J., Chan D., Sandaradura I., Malik R., Spielman D., Lee R., Marriott D., Harkness J., Ellis J., Stark D. Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology. 2016;143:1087–1118. doi: 10.1017/S0031182016000652. [DOI] [PubMed] [Google Scholar]
- Bauer A., Nolden T., Schröter J., Römer-Oberdörfer A., Gluska S., Perlson E., Finke S. Anterograde glycoprotein-dependent transport of newly generated rabies virus in dorsal root ganglion neurons. J. Virol. 2014;88:14172–14183. doi: 10.1128/JVI.02254-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B.L., Ogata S.A. Ultrastructural analysis of attachment to and penetration of capillaries in the murine pons, midbrain, thalamus, and hypothalamus by Nocardia asteroides. Infect. Immun. 1993;61:955–965. doi: 10.1128/iai.61.3.955-965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiting D.P., Hidano S., Baggs J.E., Geskes J.M., Fang Q., Wherry E.J., Hunter C.A., Roos D.S., Cherry S. The Orphan Nuclear Receptor TLX Is an Enhancer of STAT1-Mediated Transcription and Immunity to Toxoplasma gondii. PLoS Biol. 2015;13:e1002200. doi: 10.1371/journal.pbio.1002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevides L., Milanezi C.M., Yamauchi L.M., Benjamim C.F., Silva J.S., Silva N.M. CCR2 receptor is essential to activate microbicidal mechanisms to control Toxoplasma gondii infection in the central nervous system. Am. J. Pathol. 2008;173:741–751. doi: 10.2353/ajpath.2008.080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J.L., Elhofy A., Charo I., Miller S.D., Dal Canto M.C., Karpus W.J. CCR2 regulates development of Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Viral Immunol. 2007;20:19–33. doi: 10.1089/vim.2006.0068. [DOI] [PubMed] [Google Scholar]
- Berger J.R., Koralnik I.J. Progressive multifocal leukoencephalopathy and natalizumab--unforeseen consequences. N. Engl. J. Med. 2005;353:414–416. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- Bernard S.C., Simpson N., Join-Lambert O., Federici C., Laran-Chich M.P., Maïssa N., Bouzinba-Ségard H., Morand P.C., Chretien F., Taouji S., et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat. Med. 2014;20:725–731. doi: 10.1038/nm.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra R., Gigley J.P., Weiss L.M., Khan I.A. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc. Natl. Acad. Sci. USA. 2011;108:9196–9201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Zagórska A., Lew E.D., Shrestha B., Rothlin C.V., Naughton J., Diamond M.S., Lemke G., Young J.A. Enveloped viruses disable innate immune responses in dendritic cells by direct activation of TAM receptors. Cell Host Microbe. 2013;14:136–147. doi: 10.1016/j.chom.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder G.K., Griffin D.E. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science. 2001;293:303–306. doi: 10.1126/science.1059742. [DOI] [PubMed] [Google Scholar]
- Biswas A., Bruder D., Wolf S.A., Jeron A., Mack M., Heimesaat M.M., Dunay I.R. Ly6C(high) monocytes control cerebral toxoplasmosis. J. Immunol. 2015;194:3223–3235. doi: 10.4049/jimmunol.1402037. [DOI] [PubMed] [Google Scholar]
- Blackburn S.D., Crawford A., Shin H., Polley A., Freeman G.J., Wherry E.J. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: implications for CD8 T-cell exhaustion. J. Virol. 2010;84:2078–2089. doi: 10.1128/JVI.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleau C., Filliol A., Samson M., Lamontagne L. Brain Invasion by Mouse Hepatitis Virus Depends on Impairment of Tight Junctions and Beta Interferon Production in Brain Microvascular Endothelial Cells. J. Virol. 2015;89:9896–9908. doi: 10.1128/JVI.01501-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss I.W., Renne R. Viral miRNAs and immune evasion. Biochim. Biophys. Acta. 2011;1809:708–714. doi: 10.1016/j.bbagrm.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen L.N., Smith B., Reich D., Quezado M., Nath A. HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2016;12:662–674. doi: 10.1038/nrneurol.2016.149. [DOI] [PubMed] [Google Scholar]
- Brasil P., Pereira J.P., Jr., Moreira M.E., Ribeiro Nogueira R.M., Damasceno L., Wakimoto M., Rabello R.S., Valderramos S.G., Halai U.A., Salles T.S., et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun B.J., Slowik A., Leib S.L., Lucius R., Varoga D., Wruck C.J., Jansen S., Podschun R., Pufe T., Brandenburg L.O. The formyl peptide receptor like-1 and scavenger receptor MARCO are involved in glial cell activation in bacterial meningitis. J. Neuroinflammation. 2011;8:11. doi: 10.1186/1742-2094-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt R.H., Enzmann D.R., Remington J.S. Intracranial infection in cardiac transplant recipients. Ann. Neurol. 1981;9:107–119. doi: 10.1002/ana.410090203. [DOI] [PubMed] [Google Scholar]
- Brooks D.G., Trifilo M.J., Edelmann K.H., Teyton L., McGavern D.B., Oldstone M.B. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard M., Abbott N.J. All vertebrates started out with a glial blood-brain barrier 4-500 million years ago. Glia. 2008;56:699–708. doi: 10.1002/glia.20642. [DOI] [PubMed] [Google Scholar]
- Cabral C.M., Tuladhar S., Dietrich H.K., Nguyen E., MacDonald W.R., Trivedi T., Devineni A., Koshy A.A. Neurons are the Primary Target Cell for the Brain-Tropic Intracellular Parasite Toxoplasma gondii. PLoS Pathog. 2016;12:e1005447. doi: 10.1371/journal.ppat.1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain M.D., Salimi H., Gong Y., Yang L., Hamilton S.L., Heffernan J.R., Hou J., Miller M.J., Klein R.S. Virus entry and replication in the brain precedes blood-brain barrier disruption during intranasal alphavirus infection. J. Neuroimmunol. 2017 doi: 10.1016/j.jneuroim.2017.04.008. Published May 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella G.S., Tager A.M., El Khoury J.K., Thomas S.Y., Abrazinski T.A., Manice L.A., Colvin R.A., Luster A.D. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc. Natl. Acad. Sci. USA. 2008;105:4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantoni C., Bollman B., Licastro D., Xie M., Mikesell R., Schmidt R., Yuede C.M., Galimberti D., Olivecrona G., Klein R.S., et al. TREM2 regulates microglial cell activation in response to demyelination in vivo. Acta Neuropathol. 2015;129:429–447. doi: 10.1007/s00401-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M.J., Doose J.M., Melchior B., Schmid C.D., Ploix C.C. CNS immune privilege: hiding in plain sight. Immunol. Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casrouge A., Zhang S.Y., Eidenschenk C., Jouanguy E., Puel A., Yang K., Alcais A., Picard C., Mahfoufi N., Nicolas N., et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- Castro R., Águas M.J., Batista T., Araújo C., Mansinho K., Pereira Fda.L. Detection of Treponema pallidum Sp. Pallidum DNA in Cerebrospinal Fluid (CSF) by Two PCR Techniques. J. Clin. Lab. Anal. 2016;30:628–632. doi: 10.1002/jcla.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh S.E., Holmgren A.M., Rall G.F. Homeostatic interferon expression in neurons is sufficient for early control of viral infection. J. Neuroimmunol. 2015;279:11–19. doi: 10.1016/j.jneuroim.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekanaviciute E., Dietrich H.K., Axtell R.C., Williams A.M., Egusquiza R., Wai K.M., Koshy A.A., Buckwalter M.S. Astrocytic TGF-β signaling limits inflammation and reduces neuronal damage during central nervous system Toxoplasma infection. J. Immunol. 2014;193:139–149. doi: 10.4049/jimmunol.1303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin S., Ford H.R., Sysko L.R., Agarwal C., Wang J., Neal M.D., Baty C., Apodaca G., Hackam D.J. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J. Biol. Chem. 2004;279:24592–24600. doi: 10.1074/jbc.M313620200. [DOI] [PubMed] [Google Scholar]
- Chen B.P., Kuziel W.A., Lane T.E. Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. J. Immunol. 2001;167:4585–4592. doi: 10.4049/jimmunol.167.8.4585. [DOI] [PubMed] [Google Scholar]
- Chevalier G., Suberbielle E., Monnet C., Duplan V., Martin-Blondel G., Farrugia F., Le Masson G., Liblau R., Gonzalez-Dunia D. Neurons are MHC class I-dependent targets for CD8 T cells upon neurotropic viral infection. PLoS Pathog. 2011;7:e1002393. doi: 10.1371/journal.ppat.1002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H., Proll S.C., Szretter K.J., Katze M.G., Gale M., Jr., Diamond M.S. Differential innate immune response programs in neuronal subtypes determine susceptibility to infection in the brain by positive-stranded RNA viruses. Nat. Med. 2013;19:458–464. doi: 10.1038/nm.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers M.Y., Lang R., Nassar F., Ben-David D., Giladi M., Rubinshtein E., Itzhaki A., Mishal J., Siegman-Igra Y., Kitzes R., et al. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg. Infect. Dis. 2001;7:675–678. doi: 10.3201/eid0704.010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J.E., de Lemos C., Moos T., Christensen J.P., Thomsen A.R. CXCL10 is the key ligand for CXCR3 on CD8+ effector T cells involved in immune surveillance of the lymphocytic choriomeningitis virus-infected central nervous system. J. Immunol. 2006;176:4235–4243. doi: 10.4049/jimmunol.176.7.4235. [DOI] [PubMed] [Google Scholar]
- Church J.S., Kigerl K.A., Lerch J.K., Popovich P.G., McTigue D.M. TLR4 Deficiency Impairs Oligodendrocyte Formation in the Injured Spinal Cord. J. Neurosci. 2016;36:6352–6364. doi: 10.1523/JNEUROSCI.0353-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M., Butovsky O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper K.N., Blount D.G., Riley E.M. IL-10: the master regulator of immunity to infection. J. Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Coureuil M., Mikaty G., Miller F., Lécuyer H., Bernard C., Bourdoulous S., Duménil G., Mège R.M., Weksler B.B., Romero I.A., et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–87. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureuil M., Lécuyer H., Scott M.G., Boularan C., Enslen H., Soyer M., Mikaty G., Bourdoulous S., Nassif X., Marullo S. Meningococcus Hijacks a β2-adrenoceptor/β-Arrestin pathway to cross brain microvasculature endothelium. Cell. 2010;143:1149–1160. doi: 10.1016/j.cell.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Courret N., Darche S., Sonigo P., Milon G., Buzoni-Gâtel D., Tardieux I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxford A.L., Lanzinger M., Hartmann F.J., Schreiner B., Mair F., Pelczar P., Clausen B.E., Jung S., Greter M., Becher B. The Cytokine GM-CSF Drives the Inflammatory Signature of CCR2+ Monocytes and Licenses Autoimmunity. Immunity. 2015;43:502–514. doi: 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Thams S. Classic major histocompatibility complex class I molecules: new actors at the neuromuscular junction. Neuroscientist. 2010;16:600–607. doi: 10.1177/1073858410381534. [DOI] [PubMed] [Google Scholar]
- Daffis S., Samuel M.A., Keller B.C., Gale M., Jr., Diamond M.S. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-dependent and -independent mechanisms. PLoS Pathog. 2007;3:e106. doi: 10.1371/journal.ppat.0030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm T., Rudolph H., Schwerk C., Schroten H., Tenenbaum T. Neuroinvasion and Inflammation in Viral Central Nervous System Infections. Mediators Inflamm. 2016;2016:8562805. doi: 10.1155/2016/8562805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels B.P., Holman D.W., Cruz-Orengo L., Jujjavarapu H., Durrant D.M., Klein R.S. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. MBio. 2014;5 doi: 10.1128/mBio.01476-14. e01476–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels B.P., Jujjavarapu H., Durrant D.M., Williams J.L., Green R.R., White J.P., Lazear H.M., Gale M., Jr., Diamond M.S., Klein R.S. Regional astrocyte IFN signaling restricts pathogenesis during neurotropic viral infection. J. Clin. Invest. 2017;127:843–856. doi: 10.1172/JCI88720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aquino M.T., Kapil P., Hinton D.R., Phares T.W., Puntambekar S.S., Savarin C., Bergmann C.C., Stohlman S.A. IL-27 limits central nervous system viral clearance by promoting IL-10 and enhances demyelination. J. Immunol. 2014;193:285–294. doi: 10.4049/jimmunol.1400058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert-Schlüter M., Bluethmann H., Rang A., Hof H., Schlüter D. Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75), in murine toxoplasmosis. J. Immunol. 1998;160:3427–3436. [PubMed] [Google Scholar]
- Degrandi D., Kravets E., Konermann C., Beuter-Gunia C., Klümpers V., Lahme S., Wischmann E., Mausberg A.K., Beer-Hammer S., Pfeffer K. Murine guanylate binding protein 2 (mGBP2) controls Toxoplasma gondii replication. Proc. Natl. Acad. Sci. USA. 2013;110:294–299. doi: 10.1073/pnas.1205635110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkers E.Y., Yap G., Scharton-Kersten T., Charest H., Butcher B.A., Caspar P., Heiny S., Sher A. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 1997;159:1903–1908. [PubMed] [Google Scholar]
- Dinner S., Kaltschmidt J., Stump-Guthier C., Hetjens S., Ishikawa H., Tenenbaum T., Schroten H., Schwerk C. Mitogen-activated protein kinases are required for effective infection of human choroid plexus epithelial cells by Listeria monocytogenes. Microbes Infect. 2017;19:18–33. doi: 10.1016/j.micinf.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Do J.S., Visperas A., Sanogo Y.O., Bechtel J.J., Dvorina N., Kim S., Jang E., Stohlman S.A., Shen B., Fairchild R.L., et al. An IL-27/Lag3 axis enhances Foxp3+ regulatory T cell-suppressive function and therapeutic efficacy. Mucosal Immunol. 2016;9:137–145. doi: 10.1038/mi.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets D.A., Dillon M.J., Schawang J.S., Van Rooijen N., Ehrchen J., Sunderkötter C., Leenen P.J. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J. Immunol. 2004;172:4418–4424. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- Drögemüller K., Helmuth U., Brunn A., Sakowicz-Burkiewicz M., Gutmann D.H., Mueller W., Deckert M., Schlüter D. Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J. Immunol. 2008;181:2683–2693. doi: 10.4049/jimmunol.181.4.2683. [DOI] [PubMed] [Google Scholar]
- Drokhlyansky E., Göz Aytürk D., Soh T.K., Chrenek R., O’Loughlin E., Madore C., Butovsky O., Cepko C.L. The brain parenchyma has a type I interferon response that can limit virus spread. Proc. Natl. Acad. Sci. USA. 2017;114:E95–E104. doi: 10.1073/pnas.1618157114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis S., Jouanguy E., Al-Hajjar S., Fieschi C., Al-Mohsen I.Z., Al-Jumaah S., Yang K., Chapgier A., Eidenschenk C., Eid P., et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- Durrant D.M., Robinette M.L., Klein R.S. IL-1R1 is required for dendritic cell-mediated T cell reactivation within the CNS during West Nile virus encephalitis. J. Exp. Med. 2013;210:503–516. doi: 10.1084/jem.20121897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant D.M., Daniels B.P., Klein R.S. IL-1R1 signaling regulates CXCL12-mediated T cell localization and fate within the central nervous system during West Nile Virus encephalitis. J. Immunol. 2014;193:4095–4106. doi: 10.4049/jimmunol.1401192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejlerskov P., Hultberg J.G., Wang J., Carlsson R., Ambjørn M., Kuss M., Liu Y., Porcu G., Kolkova K., Friis Rundsten C., et al. Lack of Neuronal IFN-β-IFNAR Causes Lewy Body- and Parkinson’s Disease-like Dementia. Cell. 2015;163:324–339. doi: 10.1016/j.cell.2015.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B., Vajkoczy P., Weller R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- Erblich B., Zhu L., Etgen A.M., Dobrenis K., Pollard J.W. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS ONE. 2011;6:e26317. doi: 10.1371/journal.pone.0026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J.R., Altschaefl K.M., O’Shea K.S., Miller D.J. Activation of the type I interferon pathway is enhanced in response to human neuronal differentiation. PLoS ONE. 2013;8:e58813. doi: 10.1371/journal.pone.0058813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E.L., Kersh A.E., Evavold B.D., Lukacher A.E. Cutting Edge: Resident Memory CD8 T Cells Express High-Affinity TCRs. J. Immunol. 2015;195:3520–3524. doi: 10.4049/jimmunol.1501521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea I., Bechmann I., Perry V.H. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Gallego-Delgado J., Basu-Roy U., Ty M., Alique M., Fernandez-Arias C., Movila A., Gomes P., Weinstock A., Xu W., Edagha I., et al. Angiotensin receptors and β-catenin regulate brain endothelial integrity in malaria. J. Clin. Invest. 2016;126:4016–4029. doi: 10.1172/JCI87306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamino V., Höfle U. Pathology and tissue tropism of natural West Nile virus infection in birds: a review. Vet. Res. (Faisalabad) 2013;44:39. doi: 10.1186/1297-9716-44-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavino C., Cotter A., Lichtenstein D., Lejtenyi D., Fortin C., Legault C., Alirezaie N., Majewski J., Sheppard D.C., Behr M.A., et al. CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy. Clin. Infect. Dis. 2014;59:81–84. doi: 10.1093/cid/ciu215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay G., Braun L., Brenier-Pinchart M.P., Vollaire J., Josserand V., Bertini R.L., Varesano A., Touquet B., De Bock P.J., Coute Y., et al. Toxoplasma gondii TgIST co-opts host chromatin repressors dampening STAT1-dependent gene regulation and IFN-γ-mediated host defenses. J. Exp. Med. 2016;213:1779–1798. doi: 10.1084/jem.20160340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R., Xu Y., Hieny S., Cheever A., Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- Gensel J.C., Wang Y., Guan Z., Beckwith K.A., Braun K.J., Wei P., McTigue D.M., Popovich P.G. Toll-Like Receptors and Dectin-1, a C-Type Lectin Receptor, Trigger Divergent Functions in CNS Macrophages. J. Neurosci. 2015;35:9966–9976. doi: 10.1523/JNEUROSCI.0337-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts D.R., Terry R.L., Getts M.T., Müller M., Rana S., Shrestha B., Radford J., Van Rooijen N., Campbell I.L., King N.J. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J. Exp. Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker E.O., Hennigs A., Nabavi M., Schäffer A.A., Woellner C., Salzer U., Pfeifer D., Veelken H., Warnatz K., Tahami F., et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomme E.A., Wirblich C., Addya S., Rall G.F., Schnell M.J. Immune clearance of attenuated rabies virus results in neuronal survival with altered gene expression. PLoS Pathog. 2012;8:e1002971. doi: 10.1371/journal.ppat.1002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J.B., Da Costa A., Lund J.M. Regulatory T cells shape the resident memory T cell response to virus infection in the tissues. J. Immunol. 2014;192:683–690. doi: 10.4049/jimmunol.1202153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D.E. Immune responses to RNA-virus infections of the CNS. Nat. Rev. Immunol. 2003;3:493–502. doi: 10.1038/nri1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Audry M., Ciancanelli M., Alsina L., Azevedo J., Herman M., Anguiano E., Sancho-Shimizu V., Lorenzo L., Pauwels E., et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J. Exp. Med. 2011;208:2083–2098. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habjan M., Hubel P., Lacerda L., Benda C., Holze C., Eberl C.H., Mann A., Kindler E., Gil-Cruz C., Ziebuhr J., et al. Sequestration by IFIT1 impairs translation of 2'O-unmethylated capped RNA. PLoS Pathog. 2013;9:e1003663. doi: 10.1371/journal.ppat.1003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A.O., Beiting D.P., Tato C., John B., Oldenhove G., Lombana C.G., Pritchard G.H., Silver J.S., Bouladoux N., Stumhofer J.S., et al. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen S.K., Weiss L.M. Investigation into the mechanism of gamma interferon-mediated inhibition of Toxoplasma gondii in murine astrocytes. Infect. Immun. 2000;68:3426–3430. doi: 10.1128/iai.68.6.3426-3430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.H., Banigan E.J., Christian D.A., Konradt C., Tait Wojno E.D., Norose K., Wilson E.H., John B., Weninger W., Luster A.D., et al. Generalized Lévy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486:545–548. doi: 10.1038/nature11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke S., Stevenson P.G., Freeman S., Bangham C.R. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J. Exp. Med. 1998;187:1575–1582. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst M., Gazendam R., Reimnitz D., Sawalle-Belohradsky J., Groll A., Schlegel P.G., Belohradsky B., Renner E., Klepper J., Grimbacher B., et al. Chronic Candida albicans Meningitis in a 4-Year-Old Girl with a Homozygous Mutation in the CARD9 Gene (Q295X) Pediatr. Infect. Dis. J. 2015;34:999–1002. doi: 10.1097/INF.0000000000000736. [DOI] [PubMed] [Google Scholar]
- Herman M., Ciancanelli M., Ou Y.H., Lorenzo L., Klaudel-Dreszler M., Pauwels E., Sancho-Shimizu V., Pérez de Diego R., Abhyankar A., Israelsson E., et al. Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. J. Exp. Med. 2012;209:1567–1582. doi: 10.1084/jem.20111316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Filiano A.J., Smith A., Yogev N., Kipnis J. Myeloid Cells in the Central Nervous System. Immunity. 2017;46:943–956. doi: 10.1016/j.immuni.2017.06.007. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Johnson K.R., McGavern D.B. Therapeutic antiviral T cells noncytopathically clear persistently infected microglia after conversion into antigen-presenting cells. J. Exp. Med. 2015;212:1153–1169. doi: 10.1084/jem.20142047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidano S., Randall L.M., Dawson L., Dietrich H.K., Konradt C., Klover P.J., John B., Harris T.H., Fang Q., Turek B., et al. STAT1 Signaling in Astrocytes Is Essential for Control of Infection in the Central Nervous System. MBio. 2016;7:7. doi: 10.1128/mBio.01881-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K., Ghoreschi K., Yang X.P., Takahashi H., Laurence A., Vahedi G., Sciumè G., Hall A.O., Dupont C.D., Francisco L.M., et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höftberger R., Armangue T., Leypoldt F., Graus F., Dalmau J. Clinical Neuropathology practice guide 4-2013: post-herpes simplex encephalitis: N-methyl-Daspartate receptor antibodies are part of the problem. Clin. Neuropathol. 2013;32:251–254. doi: 10.5414/NP300666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hora R., Kapoor P., Thind K.K., Mishra P.C. Cerebral malaria--clinical manifestations and pathogenesis. Metab. Brain Dis. 2016;31:225–237. doi: 10.1007/s11011-015-9787-5. [DOI] [PubMed] [Google Scholar]
- Hosmane S., Tegenge M.A., Rajbhandari L., Uapinyoying P., Kumar N.G., Thakor N., Venkatesan A. Toll/interleukin-1 receptor domain-containing adapter inducing interferon-β mediates microglial phagocytosis of degenerating axons. J. Neurosci. 2012;32:7745–7757. doi: 10.1523/JNEUROSCI.0203-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.H., Long M., Wu C.H., Kwon-Chung K.J., Chang Y.C., Chi F., Lee S., Jong A. Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells is mediated through the lipid rafts-endocytic pathway via the dual specificity tyrosine phosphorylation-regulated kinase 3 (DYRK3) J. Biol. Chem. 2011;286:34761–34769. doi: 10.1074/jbc.M111.219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins M.A., Johnson H.L., Jin F., N Songo A., Hanson L.M., LaFrance S.J., Butler N.S., Harty J.T., Johnson A.J. Perforin Expression by CD8 T Cells Is Sufficient To Cause Fatal Brain Edema during Experimental Cerebral Malaria. Infect. Immun. 2017;85:85. doi: 10.1128/IAI.00985-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C.A., Sibley L.D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 2012;10:766–778. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseby Kelcher A.M., Atanga P.A., Gamez J.D., Cumba Garcia L.M., Teclaw S.J., Pavelko K.D., Macura S.I., Johnson A.J. Brain atrophy in picornavirus-infected FVB mice is dependent on the H-2D(b) class I molecule. FASEB J. 2017;31:2267–2275. doi: 10.1096/fj.201601055R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S., Daemen M.J., Hordijk P.L. Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ. Res. 2015;116:895–908. doi: 10.1161/CIRCRESAHA.116.305720. [DOI] [PubMed] [Google Scholar]
- Hyde J.L., Gardner C.L., Kimura T., White J.P., Liu G., Trobaugh D.W., Huang C., Tonelli M., Paessler S., Takeda K., et al. A viral RNA structural element alters host recognition of nonself RNA. Science. 2014;343:783–787. doi: 10.1126/science.1248465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovino F., Orihuela C.J., Moorlag H.E., Molema G., Bijlsma J.J. Interactions between blood-borne Streptococcus pneumoniae and the blood-brain barrier preceding meningitis. PLoS ONE. 2013;8:e68408. doi: 10.1371/journal.pone.0068408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Rodger A., Bell D.J., Bhagani S., Cropley I., Filipe A., Gifford R.J., Hopkins S., Hughes J., Jabeen F., et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet. 2016;388:498–503. doi: 10.1016/S0140-6736(16)30386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäkel S., Dimou L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell. Neurosci. 2017;11:24. doi: 10.3389/fncel.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelcic I., Jelcic I., Faigle W., Sospedra M., Martin R. Immunology of progressive multifocal leukoencephalopathy. J. Neurovirol. 2015;21:614–622. doi: 10.1007/s13365-014-0294-y. [DOI] [PubMed] [Google Scholar]
- Ji R., Tian S., Lu H.J., Lu Q., Zheng Y., Wang X., Ding J., Li Q., Lu Q. TAM receptors affect adult brain neurogenesis by negative regulation of microglial cell activation. J. Immunol. 2013;191:6165–6177. doi: 10.4049/jimmunol.1302229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H.T., Anderson A.C., Tan W.G., West E.E., Ha S.J., Araki K., Freeman G.J., Kuchroo V.K., Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B., Ricart B., Tait Wojno E.D., Harris T.H., Randall L.M., Christian D.A., Gregg B., De Almeida D.M., Weninger W., Hammer D.A., Hunter C.A. Analysis of behavior and trafficking of dendritic cells within the brain during toxoplasmic encephalitis. PLoS Pathog. 2011;7:e1002246. doi: 10.1371/journal.ppat.1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong A., Wu C.H., Shackleford G.M., Kwon-Chung K.J., Chang Y.C., Chen H.M., Ouyang Y., Huang S.H. Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell. Microbiol. 2008;10:1313–1326. doi: 10.1111/j.1462-5822.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- Josefowicz S.Z., Lu L.F., Rudensky A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A.Y., Iyer V.N., Hagan J.B., St Sauver J.L., Boyce T.G. Incidence and temporal trends of primary immunodeficiency: a population-based cohort study. Mayo Clin. Proc. 2009;84:16–22. doi: 10.4065/84.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl K.A., de Rivero Vaccari J.P., Dietrich W.D., Popovich P.G., Keane R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.K., Boothroyd J.C. Stage-specific expression of surface antigens by Toxoplasma gondii as a mechanism to facilitate parasite persistence. J. Immunol. 2005;174:8038–8048. doi: 10.4049/jimmunol.174.12.8038. [DOI] [PubMed] [Google Scholar]
- Kim J.V., Kang S.S., Dustin M.L., McGavern D.B. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.J., Hancock B.M., Bermudez A., Del Cid N., Reyes E., van Sorge N.M., Lauth X., Smurthwaite C.A., Hilton B.J., Stotland A., et al. Bacterial induction of Snail1 contributes to blood-brain barrier disruption. J. Clin. Invest. 2015;125:2473–2483. doi: 10.1172/JCI74159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J., Gadani S., Derecki N.C. Pro-cognitive properties of T cells. Nat. Rev. Immunol. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R.S., Garber C., Howard N. Infectious immunity in the central nervous system and brain function. Nat. Immunol. 2017;18:132–141. doi: 10.1038/ni.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradt C., Ueno N., Christian D.A., Delong J.H., Pritchard G.H., Herz J., Bzik D.J., Koshy A.A., McGavern D.B., Lodoen M.B., Hunter C.A. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat. Microbiol. 2016;1:16001. doi: 10.1038/nmicrobiol.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kramer T., Enquist L.W. Directional spread of alphaherpesviruses in the nervous system. Viruses. 2013;5:678–707. doi: 10.3390/v5020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcsar K.A., Baxter V.K., Greene I.P., Griffin D.E. Interleukin 10 modulation of pathogenic Th17 cells during fatal alphavirus encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2014;111:16053–16058. doi: 10.1073/pnas.1418966111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachenmaier S.M., Deli M.A., Meissner M., Liesenfeld O. Intracellular transport of Toxoplasma gondii through the blood-brain barrier. J. Neuroimmunol. 2011;232:119–130. doi: 10.1016/j.jneuroim.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille F.G., Pessach I.M., Zhang S.Y., Ciancanelli M.J., Herman M., Abhyankar A., Ying S.W., Keros S., Goldstein P.A., Mostoslavsky G., et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature. 2012;491:769–773. doi: 10.1038/nature11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrith T.A., Sureshchandra S., Rivera A., Jang J.C., Rais M., Nair M.G., Messaoudi I., Wilson E.H. CD103(+) CD8 T Cells in the Toxoplasma-Infected Brain Exhibit a Tissue-Resident Memory Transcriptional Profile. Front. Immunol. 2017;8:335. doi: 10.3389/fimmu.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Daniels B.P., Pinto A.K., Huang A.C., Vick S.C., Doyle S.E., Gale M., Jr., Klein R.S., Diamond M.S. Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci. Transl. Med. 2015;7:284ra59. doi: 10.1126/scitranslmed.aaa4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L.A., Ram S. Meningococcal disease and the complement system. Virulence. 2014;5:98–126. doi: 10.4161/viru.26515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S.A., Barres B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. this issue. [DOI] [PubMed] [Google Scholar]
- Lim J.K., Obara C.J., Rivollier A., Pletnev A.G., Kelsall B.L., Murphy P.M. Chemokine receptor Ccr2 is critical for monocyte accumulation and survival in West Nile virus encephalitis. J. Immunol. 2011;186:471–478. doi: 10.4049/jimmunol.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Okada S., Kong X.F., Kreins A.Y., Cypowyj S., Abhyankar A., Toubiana J., Itan Y., Audry M., Nitschke P., et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London A., Cohen M., Schwartz M. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front. Cell. Neurosci. 2013;7:34. doi: 10.3389/fncel.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D., Derecki N.C., Castle D., Mandell J.W., Lee K.S., et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg P., Ramakrishna C., Brown J., Tyszka J.M., Hamamura M., Hinton D.R., Kovats S., Nalcioglu O., Weinberg K., Openshaw H., Cantin E.M. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. J. Virol. 2008;82:7078–7088. doi: 10.1128/JVI.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J.D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 2012;12:367–382. doi: 10.1038/nri3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed D.A., Mills J.H., Egan C.E., Denkers E.Y., Bynoe M.S. CD73-generated adenosine facilitates Toxoplasma gondii differentiation to long-lived tissue cysts in the central nervous system. Proc. Natl. Acad. Sci. USA. 2012;109:16312–16317. doi: 10.1073/pnas.1205589109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairey E., Genovesio A., Donnadieu E., Bernard C., Jaubert F., Pinard E., Seylaz J., Olivo-Marin J.C., Nassif X., Duménil G. Cerebral microcirculation shear stress levels determine Neisseria meningitidis attachment sites along the blood-brain barrier. J. Exp. Med. 2006;203:1939–1950. doi: 10.1084/jem.20060482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruvada R., Zhu L., Pearce D., Zheng Y., Perfect J., Kwon-Chung K.J., Kim K.S. Cryptococcus neoformans phospholipase B1 activates host cell Rac1 for traversal across the blood-brain barrier. Cell. Microbiol. 2012;14:1544–1553. doi: 10.1111/j.1462-5822.2012.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzal M., Guerra-Giraldez C., Paredes A., Cangalaya C., Rivera A., Gonzalez A.E., Mahanty S., Garcia H.H., Nash T.E., Cysticercosis Working Group in Peru Evans blue staining reveals vascular leakage associated with focal areas of host-parasite interaction in brains of pigs infected with Taenia solium. PLoS ONE. 2014;9:e97321. doi: 10.1371/journal.pone.0097321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T., Murao N., Katano Y., Juliandi B., Kohyama J., Akira S., Kawai T., Nakashima K. TLR9 signalling in microglia attenuates seizure-induced aberrant neurogenesis in the adult hippocampus. Nat. Commun. 2015;6:6514. doi: 10.1038/ncomms7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matullo C.M., O’Regan K.J., Hensley H., Curtis M., Rall G.F. Lymphocytic choriomeningitis virus-induced mortality in mice is triggered by edema and brain herniation. J. Virol. 2010;84:312–320. doi: 10.1128/JVI.00727-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matullo C.M., O’Regan K.J., Curtis M., Rall G.F. CNS recruitment of CD8+ T lymphocytes specific for a peripheral virus infection triggers neuropathogenesis during polymicrobial challenge. PLoS Pathog. 2011;7:e1002462. doi: 10.1371/journal.ppat.1002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximova O.A., Bernbaum J.G., Pletnev A.G. West Nile Virus Spreads Transsynaptically within the Pathways of Motor Control: Anatomical and Ultrastructural Mapping of Neuronal Virus Infection in the Primate Central Nervous System. PLoS Negl. Trop. Dis. 2016;10:e0004980. doi: 10.1371/journal.pntd.0004980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDole J.R., Danzer S.C., Pun R.Y., Chen Y., Johnson H.L., Pirko I., Johnson A.J. Rapid formation of extended processes and engagement of Theiler’s virus-infected neurons by CNS-infiltrating CD8 T cells. Am. J. Pathol. 2010;177:1823–1833. doi: 10.2353/ajpath.2010.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon E.J., Bailey S.L., Castenada C.V., Waldner H., Miller S.D. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- Medawar P. Immunity to homologous grafted skin. III. Br. J. Exp. Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- Melzer T., Duffy A., Weiss L.M., Halonen S.K. The gamma interferon (IFN-gamma)-inducible GTP-binding protein IGTP is necessary for toxoplasma vacuolar disruption and induces parasite egression in IFN-gamma-stimulated astrocytes. Infect. Immun. 2008;76:4883–4894. doi: 10.1128/IAI.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez C.M., Jinkins J.K., Carr D.J. Resident T Cells Are Unable To Control Herpes Simplex Virus-1 Activity in the Brain Ependymal Region during Latency. J. Immunol. 2016;197:1262–1275. doi: 10.4049/jimmunol.1600207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.D., Schnell M.J., Rall G.F. Keeping it in check: chronic viral infection and antiviral immunity in the brain. Nat. Rev. Neurosci. 2016;17:766–776. doi: 10.1038/nrn.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J.J., Daniels B.P., Shrestha B., Proenca-Modena J.L., Lew E.D., Lazear H.M., Gorman M.J., Lemke G., Klein R.S., Diamond M.S. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat. Med. 2015;21:1464–1472. doi: 10.1038/nm.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy E.S., Calabrese L.H. Therapy: Targeted but not trouble-free: efalizumab and PML. Nat. Rev. Rheumatol. 2009;5:418–419. doi: 10.1038/nrrheum.2009.142. [DOI] [PubMed] [Google Scholar]
- Moraga A., Pradillo J.M., Cuartero M.I., Hernández-Jiménez M., Oses M., Moro M.A., Lizasoain I. Toll-like receptor 4 modulates cell migration and cortical neurogenesis after focal cerebral ischemia. FASEB J. 2014;28:4710–4718. doi: 10.1096/fj.14-252452. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Woods T.A., Moore R.A., Peterson K.E. Activation of the innate signaling molecule MAVS by bunyavirus infection upregulates the adaptor protein SARM1, leading to neuronal death. Immunity. 2013;38:705–716. doi: 10.1016/j.immuni.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacer A., Movila A., Baer K., Mikolajczak S.A., Kappe S.H., Frevert U. Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathog. 2012;8:e1002982. doi: 10.1371/journal.ppat.1002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Diamond M.S. Innate immune interactions within the central nervous system modulate pathogenesis of viral infections. Curr. Opin. Immunol. 2015;36:47–53. doi: 10.1016/j.coi.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J.P., Vannella K.M., Worth D., David C., Carter D., Noor S., Hubeau C., Fitz L., Lane T.E., Wynn T.A., Wilson E.H. Chitinase dependent control of protozoan cyst burden in the brain. PLoS Pathog. 2012;8:e1002990. doi: 10.1371/journal.ppat.1002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansen A., Christensen J.P., Röpke C., Marker O., Scheynius A., Thomsen A.R. Role of interferon-gamma in the pathogenesis of LCMV-induced meningitis: unimpaired leucocyte recruitment, but deficient macrophage activation in interferon-gamma knock-out mice. J. Neuroimmunol. 1998;86:202–212. doi: 10.1016/s0165-5728(98)00055-1. [DOI] [PubMed] [Google Scholar]
- Nash D., Mostashari F., Fine A., Miller J., O’Leary D., Murray K., Huang A., Rosenberg A., Greenberg A., Sherman M., et al. 1999 West Nile Outbreak Response Working Group The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- Nayak D., Johnson K.R., Heydari S., Roth T.L., Zinselmeyer B.H., McGavern D.B. Type I interferon programs innate myeloid dynamics and gene expression in the virally infected nervous system. PLoS Pathog. 2013;9:e1003395. doi: 10.1371/journal.ppat.1003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal J.W., Gasque P. How does the brain limit the severity of inflammation and tissue injury during bacterial meningitis? J. Neuropathol. Exp. Neurol. 2013;72:370–385. doi: 10.1097/NEN.0b013e3182909f2f. [DOI] [PubMed] [Google Scholar]
- Neumann H., Cavalié A., Jenne D.E., Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- Niedelman W., Sprokholt J.K., Clough B., Frickel E.M., Saeij J.P. Cell death of gamma interferon-stimulated human fibroblasts upon Toxoplasma gondii infection induces early parasite egress and limits parasite replication. Infect. Immun. 2013;81:4341–4349. doi: 10.1128/IAI.00416-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Natsume A., Ito M., Hara M., Motomura K., Fukuyama R., Sumiyoshi N., Aoki I., Saga T., Lee H.J., et al. Interferon-β delivery via human neural stem cell abates glial scar formation in spinal cord injury. Cell Transplant. 2013;22:2187–2201. doi: 10.3727/096368912X657882. [DOI] [PubMed] [Google Scholar]
- Niu F., Yao H., Zhang W., Sutliff R.L., Buch S. Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J. Neurosci. 2014;34:11812–11825. doi: 10.1523/JNEUROSCI.1139-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien C.A., Overall C., Konradt C., O’Hara Hall A.C., Hayes N.W., Wagage S., John B., Christian D.A., Hunter C.A., Harris T.H. CD11c-Expressing Cells Affect Regulatory T Cell Behavior in the Meninges during Central Nervous System Infection. J. Immunol. 2017;198:4054–4061. doi: 10.4049/jimmunol.1601581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odoardi F., Sie C., Streyl K., Ulaganathan V.K., Schläger C., Lodygin D., Heckelsmiller K., Nietfeld W., Ellwart J., Klinkert W.E., et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- Oldstone M.B., Blount P., Southern P.J., Lampert P.W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986;321:239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Olias P., Etheridge R.D., Zhang Y., Holtzman M.J., Sibley L.D. Toxoplasma Effector Recruits the Mi-2/NuRD Complex to Repress STAT1 Transcription and Block IFN-γ-Dependent Gene Expression. Cell Host Microbe. 2016;20:72–82. doi: 10.1016/j.chom.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsan A.D., Milburn J.M., Baumgarten K.L., Durham H.L. Leptomeningeal enhancement in a patient with proven West Nile virus infection. AJR Am. J. Roentgenol. 2003;181:591–592. doi: 10.2214/ajr.181.2.1810591. [DOI] [PubMed] [Google Scholar]
- Orr M.T., Mathis M.A., Lagunoff M., Sacks J.A., Wilson C.B. CD8 T cell control of HSV reactivation from latency is abrogated by viral inhibition of MHC class I. Cell Host Microbe. 2007;2:172–180. doi: 10.1016/j.chom.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal P., Daniels B.P., Oskman A., Diamond M.S., Klein R.S., Goldberg D.E. Plasmodium falciparum Histidine-Rich Protein II Compromises Brain Endothelial Barriers and May Promote Cerebral Malaria Pathogenesis. MBio. 2016;7:7. doi: 10.1128/mBio.00617-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida S.K., Poiret T., Zhenjiang L., Meng Q., Heyckendorf J., Lange C., Ambati A.S., Rao M.V., Valentini D., Ferrara G., et al. T-Cell Therapy: Options for Infectious Diseases. Clin. Infect. Dis. 2015;(61, Suppl 3):S217–S224. doi: 10.1093/cid/civ615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C.E., Lawrence D.M., Echols L.A., Rall G.F. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J. Virol. 2002;76:4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez de Diego R., Sancho-Shimizu V., Lorenzo L., Puel A., Plancoulaine S., Picard C., Herman M., Cardon A., Durandy A., Bustamante J., et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity. 2010;33:400–411. doi: 10.1016/j.immuni.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn C., Kallfass C., Lienenklaus S., Spanier J., Kalinke U., Rieder M., Conzelmann K.K., Michiels T., Staeheli P. Abortively Infected Astrocytes Appear To Represent the Main Source of Interferon Beta in the Virus-Infected Brain. J. Virol. 2015;90:2031–2038. doi: 10.1128/JVI.02979-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares T.W., Stohlman S.A., Hinton D.R., Bergmann C.C. Astrocyte-derived CXCL10 drives accumulation of antibody-secreting cells in the central nervous system during viral encephalomyelitis. J. Virol. 2013;87:3382–3392. doi: 10.1128/JVI.03307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A.T., Rico A.B., Stauft C.B., Hammond S.L., Aboellail T.A., Tjalkens R.B., Olson K.E. Entry Sites of Venezuelan and Western Equine Encephalitis Viruses in the Mouse Central Nervous System following Peripheral Infection. J. Virol. 2016;90:5785–5796. doi: 10.1128/JVI.03219-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinschewer D.D., Schedensack M., Bergthaler A., Horvath E., Brück W., Löhning M., Merkler D. T cells can mediate viral clearance from ependyma but not from brain parenchyma in a major histocompatibility class I- and perforin-independent manner. Brain. 2010;133:1054–1066. doi: 10.1093/brain/awq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planz O., Bilzer T., Stitz L. Immunopathogenic role of T-cell subsets in Borna disease virus-induced progressive encephalitis. J. Virol. 1995;69:896–903. doi: 10.1128/jvi.69.2.896-903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerta-Guardo H., Glasner D.R., Harris E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016;12:e1005738. doi: 10.1371/journal.ppat.1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajbhandari L., Tegenge M.A., Shrestha S., Ganesh Kumar N., Malik A., Mithal A., Hosmane S., Venkatesan A. Toll-like receptor 4 deficiency impairs microglial phagocytosis of degenerating axons. Glia. 2014;62:1982–1991. doi: 10.1002/glia.22719. [DOI] [PubMed] [Google Scholar]
- Rall G.F., Mucke L., Oldstone M.B. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J. Exp. Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambach G., Maier H., Vago G., Mohsenipour I., Lass-Flörl C., Defant A., Würzner R., Dierich M.P., Speth C. Complement induction and complement evasion in patients with cerebral aspergillosis. Microbes Infect. 2008;10:1567–1576. doi: 10.1016/j.micinf.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Reichmann G., Villegas E.N., Craig L., Peach R., Hunter C.A. The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. J. Immunol. 1999;163:3354–3362. [PubMed] [Google Scholar]
- Reichmann G., Walker W., Villegas E.N., Craig L., Cai G., Alexander J., Hunter C.A. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect. Immun. 2000;68:1312–1318. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert L.S., Lopušná K., Winther H., Sun C., Thomsen M.K., Nandakumar R., Mogensen T.H., Meyer M., Vægter C., Nyengaard J.R., et al. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat. Commun. 2016;7:13348. doi: 10.1038/ncomms13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter D., Sparwasser T., Hünig T., Schneider-Schaulies J. Foxp3+ regulatory T cells control persistence of viral CNS infection. PLoS ONE. 2012;7:e33989. doi: 10.1371/journal.pone.0033989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi H., Cain M.D., Klein R.S. Encephalitic Arboviruses: Emergence, Clinical Presentation, and Neuropathogenesis. Neurotherapeutics. 2016;13:514–534. doi: 10.1007/s13311-016-0443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M.A., Diamond M.S. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Shimizu V., Pérez de Diego R., Lorenzo L., Halwani R., Alangari A., Israelsson E., Fabrega S., Cardon A., Maluenda J., Tatematsu M., et al. Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. J. Clin. Invest. 2011;121:4889–4902. doi: 10.1172/JCI59259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Tirado F.H., Onken M.D., Cooper J.A., Klein R.S., Doering T.L. Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen. MBio. 2017;8:8. doi: 10.1128/mBio.02183-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasseville V.G., Newman W., Brodie S.J., Hesterberg P., Pauley D., Ringler D.J. Monocyte adhesion to endothelium in simian immunodeficiency virus-induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/alpha 4 beta 1 integrin interactions. Am. J. Pathol. 1994;144:27–40. [PMC free article] [PubMed] [Google Scholar]
- Schaeffer M., Han S.J., Chtanova T., van Dooren G.G., Herzmark P., Chen Y., Roysam B., Striepen B., Robey E.A. Dynamic imaging of T cell-parasite interactions in the brains of mice chronically infected with Toxoplasma gondii. J. Immunol. 2009;182:6379–6393. doi: 10.4049/jimmunol.0804307. [DOI] [PubMed] [Google Scholar]
- Scharton-Kersten T.M., Yap G., Magram J., Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel J.M., Fraser K.A., Beura L.K., Pauken K.E., Vezys V., Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Kipnis J. A conceptual revolution in the relationships between the brain and immunity. Brain Behav. Immun. 2011;25:817–819. doi: 10.1016/j.bbi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerk C., Papandreou T., Schuhmann D., Nickol L., Borkowski J., Steinmann U., Quednau N., Stump C., Weiss C., Berger J., et al. Polar invasion and translocation of Neisseria meningitidis and Streptococcus suis in a novel human model of the blood-cerebrospinal fluid barrier. PLoS ONE. 2012;7:e30069. doi: 10.1371/journal.pone.0030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydel K.B., Kampondeni S.D., Valim C., Potchen M.J., Milner D.A., Muwalo F.W., Birbeck G.L., Bradley W.G., Fox L.L., Glover S.J., et al. Brain swelling and death in children with cerebral malaria. N. Engl. J. Med. 2015;372:1126–1137. doi: 10.1056/NEJMoa1400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C.J. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Li S.S., Zheng C., Jones G.J., Kim K.S., Zhou H., Kubes P., Mody C.H. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J. Clin. Invest. 2010;120:1683–1693. doi: 10.1172/JCI41963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Miyoshi H., Goto A., Ako Y., Ueki T., Kariwa H., Takashima I. Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J. Gen. Virol. 2004;85:3637–3645. doi: 10.1099/vir.0.80247-0. [DOI] [PubMed] [Google Scholar]
- Solomon T., Dung N.M., Vaughn D.W., Kneen R., Thao L.T., Raengsakulrach B., Loan H.T., Day N.P., Farrar J., Myint K.S., et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053–1059. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- Solomos A.C., O’Regan K.J., Rall G.F. CD4(+) T cells require either B cells or CD8(+) T cells to control spread and pathogenesis of a neurotropic infection. Virology. 2016;499:196–202. doi: 10.1016/j.virol.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach K., Vincenti I., Kreutzfeldt M., Page N., Muschaweckh A., Wagner I., Drexler I., Pinschewer D., Korn T., Merkler D. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J. Exp. Med. 2016;213:1571–1587. doi: 10.1084/jem.20151916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert E.M., Schenkel J.M., Fraser K.A., Beura L.K., Manlove L.S., Igyártó B.Z., Southern P.J., Masopust D. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel W., Soltek S., Schlüter D., Deckert M. The intermediate filament GFAP is important for the control of experimental murine Staphylococcus aureus-induced brain abscess and Toxoplasma encephalitis. J. Neuropathol. Exp. Neurol. 2004;63:631–640. doi: 10.1093/jnen/63.6.631. [DOI] [PubMed] [Google Scholar]
- Streit W.J., Conde J.R., Fendrick S.E., Flanary B.E., Mariani C.L. Role of microglia in the central nervous system’s immune response. Neurol. Res. 2005;27:685–691. doi: 10.1179/016164105X49463a. [DOI] [PubMed] [Google Scholar]
- Stumhofer J.S., Laurence A., Wilson E.H., Huang E., Tato C.M., Johnson L.M., Villarino A.V., Huang Q., Yoshimura A., Sehy D., et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Subauste C.S. Primary immunodeficiencies and susceptibility to parasitic infections. Parasite Immunol. 2006;28:567–575. doi: 10.1111/j.1365-3024.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Conley F.K., Remington J.S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J. Immunol. 1989;143:2045–2050. [PubMed] [Google Scholar]
- Swanson P.A., 2nd, McGavern D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015;11:44–54. doi: 10.1016/j.coviro.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson P.A., 2nd, Hart G.T., Russo M.V., Nayak D., Yazew T., Peña M., Khan S.M., Janse C.J., Pierce S.K., McGavern D.B. CD8+ T Cells Induce Fatal Brainstem Pathology during Cerebral Malaria via Luminal Antigen-Specific Engagement of Brain Vasculature. PLoS Pathog. 2016;12:e1006022. doi: 10.1371/journal.ppat.1006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A.T., Choi J.P., Kotzin J.J., Yang Y., Hong C.C., Hobson N., Girard R., Zeineddine H.A., Lightle R., Moore T., et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature. 2017;545:305–310. doi: 10.1038/nature22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirotta E., Duncker P., Oak J., Klaus S., Tsukamoto M.R., Gov L., Lane T.E. Epstein-Barr virus-induced gene 3 negatively regulates neuroinflammation and T cell activation following coronavirus-induced encephalomyelitis. J. Neuroimmunol. 2012;254:110–116. doi: 10.1016/j.jneuroim.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabalza A., Georgiadis C., Eleftheriadou I., Hislop J.N., Ellison S.M., Karavassilis M.E., Mazarakis N.D. Venezuelan equine encephalitis virus glycoprotein pseudotyping confers neurotropism to lentiviral vectors. Gene Ther. 2013;20:723–732. doi: 10.1038/gt.2012.85. [DOI] [PubMed] [Google Scholar]
- Trandem K., Zhao J., Fleming E., Perlman S. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J. Immunol. 2011;186:3642–3652. doi: 10.4049/jimmunol.1003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A.K., Sullivan D.J., Stins M.F. Plasmodium falciparum-infected erythrocytes increase intercellular adhesion molecule 1 expression on brain endothelium through NF-kappaB. Infect. Immun. 2006;74:3262–3270. doi: 10.1128/IAI.01625-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A.K., Sha W., Shulaev V., Stins M.F., Sullivan D.J., Jr. Plasmodium falciparum-infected erythrocytes induce NF-kappaB regulated inflammatory pathways in human cerebral endothelium. Blood. 2009;114:4243–4252. doi: 10.1182/blood-2009-06-226415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T.T., Chen C.L., Lin Y.S., Chang C.P., Tsai C.C., Cheng Y.L., Huang C.C., Ho C.J., Lee Y.C., Lin L.T., et al. Microglia retard dengue virus-induced acute viral encephalitis. Sci. Rep. 2016;6:27670. doi: 10.1038/srep27670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschen S.I., Stohlman S.A., Ramakrishna C., Hinton D.R., Atkinson R.D., Bergmann C.C. CNS viral infection diverts homing of antibody-secreting cells from lymphoid organs to the CNS. Eur. J. Immunol. 2006;36:603–612. doi: 10.1002/eji.200535123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng H.K., Liu C.P., Price M.S., Jong A.Y., Chang J.C., Toffaletti D.L., Betancourt-Quiroz M., Frazzitta A.E., Cho W.L., Perfect J.R. Identification of genes from the fungal pathogen Cryptococcus neoformans related to transmigration into the central nervous system. PLoS ONE. 2012;7:e45083. doi: 10.1371/journal.pone.0045083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail Y., Cook D., Fourgeaud L., Powers C.J., Merten K., Clark C.L., Hoffman E., Ngo A., Sekiguchi K.J., O’Shea C.C., et al. Phosphatidylserine Exposure Controls Viral Innate Immune Responses by Microglia. Neuron. 2017;93:574–586 e578. doi: 10.1016/j.neuron.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Grol J., Muniz-Feliciano L., Portillo J.A., Bonilha V.L., Subauste C.S. CD40 induces anti-Toxoplasma gondii activity in nonhematopoietic cells dependent on autophagy proteins. Infect. Immun. 2013;81:2002–2011. doi: 10.1128/IAI.01145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sorge N.M., Doran K.S. Defense at the border: the blood-brain barrier versus bacterial foreigners. Future Microbiol. 2012;7:383–394. doi: 10.2217/fmb.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerhuis R., Nielsen H.M., Tenner A.J. Complement in the brain. Mol. Immunol. 2011;48:1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu K., Eigenheer R.A., Phinney B.S., Gelli A. Cryptococcus neoformans promotes its transmigration into the central nervous system by inducing molecular and cellular changes in brain endothelial cells. Infect. Immun. 2013;81:3139–3147. doi: 10.1128/IAI.00554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim L.M., Woodward-Davis A., Bevan M.J. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waknine-Grinberg J.H., Even-Chen S., Avichzer J., Turjeman K., Bentura-Marciano A., Haynes R.K., Weiss L., Allon N., Ovadia H., Golenser J., Barenholz Y. Glucocorticosteroids in nano-sterically stabilized liposomes are efficacious for elimination of the acute symptoms of experimental cerebral malaria. PLoS ONE. 2013;8:e72722. doi: 10.1371/journal.pone.0072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Town T., Alexopoulou L., Anderson J.F., Fikrig E., Flavell R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Wattananit S., Tornero D., Graubardt N., Memanishvili T., Monni E., Tatarishvili J., Miskinyte G., Ge R., Ahlenius H., Lindvall O., et al. Monocyte-Derived Macrophages Contribute to Spontaneous Long-Term Functional Recovery after Stroke in Mice. J. Neurosci. 2016;36:4182–4195. doi: 10.1523/JNEUROSCI.4317-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H., Linington C., Lassmann H., Meyermann R. Cellular immune reactivity within the CNS. Trends Neurosci. 1986;9:271–277. [Google Scholar]
- Weller R.O., Djuanda E., Yow H.Y., Carare R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- Westman G., Studahl M., Ahlm C., Eriksson B.M., Persson B., Rönnelid J., Schliamser S., Aurelius E. N-methyl-d-aspartate receptor autoimmunity affects cognitive performance in herpes simplex encephalitis. Clin. Microbiol. Infect. 2016;22:934–940. doi: 10.1016/j.cmi.2016.07.028. [DOI] [PubMed] [Google Scholar]
- Wherry E.J. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wilson E.H., Wille-Reece U., Dzierszinski F., Hunter C.A. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J. Neuroimmunol. 2005;165:63–74. doi: 10.1016/j.jneuroim.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Wilson E.H., Harris T.H., Mrass P., John B., Tait E.D., Wu G.F., Pepper M., Wherry E.J., Dzierzinski F., Roos D., et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30:300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E.H., Weninger W., Hunter C.A. Trafficking of immune cells in the central nervous system. J. Clin. Invest. 2010;120:1368–1379. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J.A., Marino M.C., Ochs H., Fuleihan R., Scholl P.R., Geha R., Stiehm E.R., Conley M.E. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine (Baltimore) 2003;82:373–384. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- Winkler E.A., Bell R.D., Zlokovic B.V. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol. Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.S., Kolonoski P., Chang Y.Y., Bermudez L.E. Invasion of the brain and chronic central nervous system infection after systemic Mycobacterium avium complex infection in mice. Infect. Immun. 2000;68:2979–2984. doi: 10.1128/iai.68.5.2979-2984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Okuyama M., Ma J.S., Kimura T., Kamiyama N., Saiga H., Ohshima J., Sasai M., Kayama H., Okamoto T., et al. A cluster of interferon-γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity. 2012;37:302–313. doi: 10.1016/j.immuni.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Yang Y., Vidensky S., Jin L., Jie C., Lorenzini I., Frankl M., Rothstein J.D. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia. 2011;59:200–207. doi: 10.1002/glia.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap G.S., Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med. 1999;189:1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo B.B., Mazmanian S.K. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity. 2017;46:910–926. doi: 10.1016/j.immuni.2017.05.011. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A., Linehan E., Hams E., O’Hara Hall A.C., McClurg A., Johnston J.A., Hunter C.A., Fallon P.G., Fitzgerald D.C. Cutting edge: suppression of GM-CSF expression in murine and human T cells by IL-27. J. Immunol. 2012;189:2079–2083. doi: 10.4049/jimmunol.1200131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanon R.G., Cartarozzi L.P., Victório S.C., Moraes J.C., Morari J., Velloso L.A., Oliveira A.L. Interferon (IFN) beta treatment induces major histocompatibility complex (MHC) class I expression in the spinal cord and enhances axonal growth and motor function recovery following sciatic nerve crush in mice. Neuropathol. Appl. Neurobiol. 2010;36:515–534. doi: 10.1111/j.1365-2990.2010.01095.x. [DOI] [PubMed] [Google Scholar]
- Zhang S.Y., Jouanguy E., Ugolini S., Smahi A., Elain G., Romero P., Segal D., Sancho-Shimizu V., Lorenzo L., Puel A., et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhao J., Perlman S. Virus-specific regulatory T cells ameliorate encephalitis by repressing effector T cell functions from priming to effector stages. PLoS Pathog. 2014;10:e1004279. doi: 10.1371/journal.ppat.1004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Vijay R., Zhao J., Gale M., Jr., Diamond M.S., Perlman S. MAVS Expressed by Hematopoietic Cells Is Critical for Control of West Nile Virus Infection and Pathogenesis. J. Virol. 2016;90:7098–7108. doi: 10.1128/JVI.00707-16. [DOI] [PMC free article] [PubMed] [Google Scholar]