Abstract
The feather is a complex ectodermal organ with hierarchical branching patterns. It provides functions in endothermy, communication, and flight. Studies of feather growth, cycling, and health are of fundamental importance to avian biology and poultry science. In addition, feathers are an excellent model for morphogenesis studies because of their accessibility, and their distinct patterns can be used to assay the roles of specific molecular pathways. Here we review the progress in aspects of development, regeneration, and evolution during the past three decades. We cover the development of feather buds in chicken embryos, regenerative cycling of feather follicle stem cells, formation of barb branching patterns, emergence of intrafeather pigmentation patterns, interplay of hormones and feather growth, and the genetic identification of several feather variants. The discovery of feathered dinosaurs redefines the relationship between feathers and birds. Inspiration from biomaterials and flight research further fuels biomimetic potential of feathers as a multidisciplinary research focal point.
Keywords: Aves, bird, stem cells, poultry science, endocrinology, dinosaurs
There are numerous feathers (usually >20,000) on one bird, and their morphological forms are diverse. One single bird, such as the zebra finch, has feathers with diverse colors and shapes on different parts of the body (Figure 1a). Single follicles can produce feathers with different shapes, sizes, and color patterns for distinct functions at the various life stages of a bird (Figure 1b). How feather diversity from a single follicle is regulated at the molecular level is a wonderful biological question. The basic facts of feathers have been comprehensively described in the two volumes of Avian Anatomy Integuments (1), which are still highly useful. Major genetic variants were covered in Poultry Breeding and Genetics, edited by Crawford (2). Experimental work on feathers, starting from the beginning of the twentieth century, was summarized in a chapter on skin morphogenesis by Sengel (3). On the basis of these seminal works, we overview the progress in development, regeneration, and evolution of feathers during the past three decades.
Figure 1.
Feather diversity: regional specificity and temporal difference. (a) Different types of feathers from different body regions of a finch. Different body regions produce feathers with specific characteristics. In distinct tracts, feathers have unique, region-specific shapes. There also can be size and pigmentation gradients. (b) A bird exhibits different plumage at various ages for different physiological needs. Chicks have downy feathers. At puberty they produce juvenal feathers, and during the reproductive stage they form sexually dimorphic feather types. These different feathers are produced from the same follicle, using similar feather stem cells, in response to different follicle microenvironments. Photo credit: female Silver Laced Wyandotte by Doug and Pete Akers; male Silver Spangled Hamburg by Jim Legendre. (c) An example of Microraptor specimen with bilateral asymmetric remiges attached to both fore and hind limbs (black arrowheads) [from Li et al. (125)].
During this time period, one source of major progress came from cell and molecular biology studies led by the Chuong lab and collaborators. Recent reviews have covered feather bud development (4) and feather follicle regeneration, respectively (5). The other source of major progress came from the inspiring finds of feathered dinosaur fossils by Xu and his colleagues (6) in the Chinese Academy of Sciences. Further, Prum (7) and Chuong et al. (8) presented the Evo-Devo model. We review papers constituting this exciting progression and attempt to bring together these lines of research and highlight the principles they reveal.
DEVELOPMENT OF FEATHER BUDS
Tract Formation
At embryonic day 6.5 (E6.5), chicken skin begins to form as a single epithelial layer overlying a single dermal layer. The first step in feather patterning is the establishment of feather tracts; feathers within different tracts will show different characteristics (1). Which molecules regulate tract formation? Ectopic expression of Noggin, a BMP antagonist, was shown to suppress Msx1 expression and resulted in new tract formation (9). A combination of both Noggin and Sonic hedgehog (Shh) was shown to induce feather-producing skin (10). In contrast, a gradient formed from a bone morphogenetic protein 2 (BMP2) coated bead induced cDermo-1 (Twist 2) and a new feather tract (9). Exogenous cDermo-1 expression led to dense dermis formation and the subsequent induction of ectopic feathers and scales (11). Early Wnt and β-catenin staining patterns suggest their involvement in tract formation (12, 13). Although tract formation is a critical step in feather formation, the roles of only a few molecules have been identified. More work must be done in this area.
Bud Induction
After feather tracts form, periodic patterning takes place within the tracts to divide the originally homogeneous field into feather bud and interbud regions (4). Within the dorsal tract, as the skin begins to mature, epithelial-mesenchymal interactions lead to the formation of feather bud precursors, which appear first along the midline and progress bilaterally over time. During early stages of feather development, dermal condensations form below epithelial placodes.
In an effort to understand the molecular basis for periodic feather patterning, the expression patterns for several genes have been mapped. Some genes initially showed a moderate expression level throughout the tract and then increased in the bud domain or the interbud domain. This expression pattern is called restrictive expression. Molecules with a restrictive expression pattern that progresses to the bud domain include Wnt-7a, β-catenin, L-Fringe, and NCAM (12–15). The expression of β-catenin in the dorsal tract (Figure 2a, left panel) is one example. Also notice the lateral inhibited β-catenin negative zone surrounding the highly β-catenin-positive feather primordia. Molecules demonstrating the restrictive expression pattern that progress to the interbud domain include Gremlin (16, 17) and Wnt-11 (18). These classes of molecules may position the feather buds and later help to define the boundary of feather buds.
Figure 2.
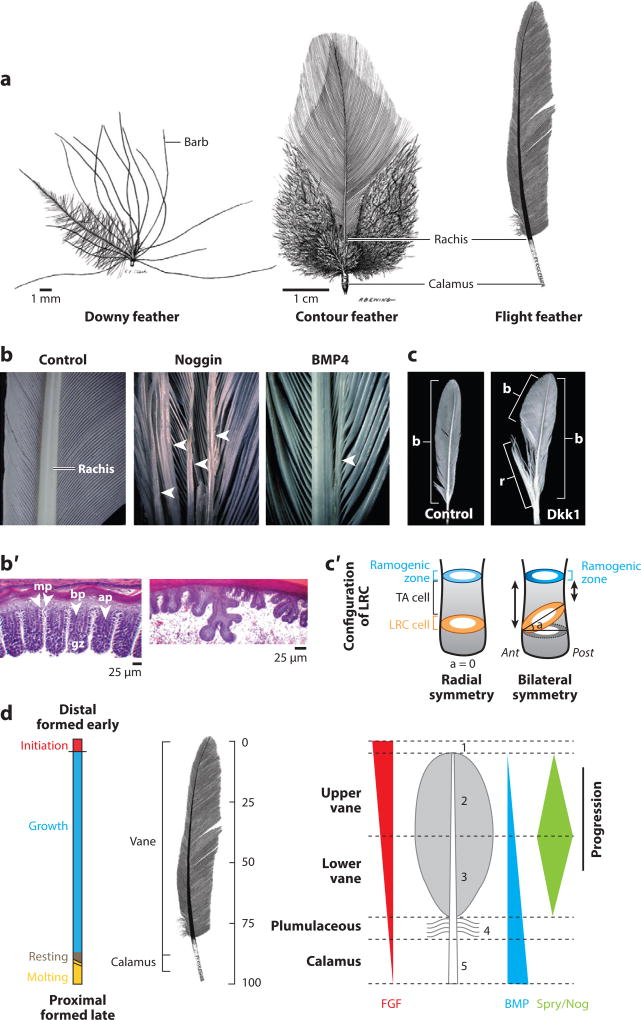
Feather development: formation of buds and follicles. (a) Examples of restrictive (β-catenin, left panel) and de novo (Shh, right panel) expression patterns. (b) Embryonic feather morphogenesis. Molecules that affect major events along the progression to successive stages of feather development are shown. Proliferative cells are indicated in blue, with darker shades indicating areas of higher proliferation. Proliferation starts at the feather tip but recedes down toward the feather base as morphogenesis proceeds. The plane on the rightmost feather shows the height from which the adjacent cross section was taken to show barb ridge (red arrow) and rachis (red arrowhead) formation. (c) Schematic diagram of follicle invagination. (c′) Depiction of three-dimensional structure of a growing feather follicle. (d) Successive stages of feather branching morphogenesis. Cross sections at different levels from proximal to distal are depicted (corresponding levels i–iv in panel c). (d′) Schematic of barb ridge formation. (e) Schematic of adult feather follicle cycling through initiation, growth, rest, and regeneration. Filament epithelia (grey), stem cells (pink), and mesenchymal pulp (tan) are shown. Abbreviations: ap; axial plate; A-P, anterior-posterior; bb, barbules; BGZ, barb generative zone; bp, barb plate; br, barb ridge; GZ, growth zone; mp; marginal plate; P-D, proximal-distal; pe, papillar ectoderm; rm, ramogenic zone. Adapted from Reference 152.
Some regulators for feather bud patterning are the TNF receptors EDAR, TROY, and XEDAR. Genetic lesions in EDA (Ectodysplasin) signaling cause hypohidrotic ectodermal dysplasia in mice and humans, demonstrating its role in skin appendage development (19, 20). All three receptors are expressed during early feather bud morphogenesis. Manipulating their levels with dominant negative or constitutively active forms alters feather bud morphogenesis (21).
A second set of genes are not expressed early but are induced in a pattern after the feather bud primordium is initiated. We call this pattern de novo expression. Genes that show the de novo expression mode include Msx2 and Shh (13, 22–24). Shh is expressed in the distal feather bud, as can be seen in Figure 2a (right panel). Genes expressed with the de novo pattern may be involved in establishing feather boundaries or later events in feather morphogenesis but are not thought to be involved in positioning feather primordia.
Feather Pattern Formation
Feathers are arranged in repeated hexagonal patterns on the avian body surface. How this occurs has drawn the interest of many investigators (25, 26). Some investigators have proposed a pre-patterning mechanism (27). Others have suggested that feather periodic patterning can be generated with combinations of molecules that promote (activators) and suppress (inhibitors) feather bud formation, following the rules of the Turing model (15, 28, 29). Fibroblast growth factors, such as FGF2 and −4, are known activators, and BMPs are known inhibitors in this process. FGF4 bead treatment of E6 chicken skin caused bud fusion (i.e., turning interbud regions into bud regions) (30). Scaleless chickens fail to form feathers, scutate scales, and spurs owing to a genetic defect (see section on Feather Variants for more details). However, FGF2 coated beads were able to induce dermal condensation formation from appendage-free E8 scaleless chicken dermis and were even able to produce buds in the E7 stage (31). FGF4 induces local BMP4 expression, which in turn suppresses FGF4 in a negative feedback mechanism. BMP2 and -4 coated beads inhibit feather bud formation, which demonstrates their inhibitory role in this process (30), possibly by regulating FGF receptor expression (32). Another inhibitor, BMP12, specifically suppresses feather bud formation in the neck tract in birds carrying the Naked neck mutation (see section on Feather Variants for more details).
We developed an in vitro self-organization culture model in which feather progenitor cells were completely dissociated and plated at high cell density. They then were able to reconstitute periodic patterns of approximately uniform size and spacing (15). We used this culture system to assess whether cells within early-stage developing skin were equivalent and able to respond equally to local molecular signals or predestined to form feather buds or interbuds. Prelabeled feather bud dermal cells were dissociated into a single cell suspension and placed beneath intact E7 skin epithelium. If the cells are predestined, cells formerly in the feather bud should once again become feather bud cells. However, we found that the newly formed feather buds and interbud regions contained an equal mix of dermal cells from the previous bud and interbud regions, suggesting their fate was not predestined but occurred in response to local signals derived from tissue interactions (15). Here, the number of feather buds formed was dependent on the number of dermal cells. Low dermal cell numbers decreased the number of feather primordia, whose size remained constant. High dermal cell numbers increased the number of feather buds without affecting their size. When sufficient cells were present, bud spacing reestablished the hexagonal pattern seen in vivo. This pattern forms owing to a maximum packing density of buds. Therefore, feather patterning is not designated by a blueprint but rather occurs through local interactions. The molecules encoded in genes enable cells to interact through physical-chemical properties, some of which invoke Turing reaction diffusion to form these patterns (28).
Bud A-P Axis
In the next step of feather bud morphogenesis, an anterior-posterior polarity emerges from the originally dome-shaped primordia (Figure 2b). Amazingly, feather buds elongate precisely along the body in a rostral-caudal direction. The initial polarity cue for elongation may derive from Wnt-7a emanating from the posterior bud epithelium (12). Wnt-7a induces a nuclear β-catenin positive zone in the underlying mesenchyme, which conducts oriented cell migration using nonmuscle myosin IIB. Meanwhile, the nuclear β-catenin signaling elevates expression of Jag1, which binds to Notch receptors on neighboring cells, generating a local positive feedback to demarcate the boundaries of the nuclear β-catenin zone. This interaction guarantees the precision of feather bud elongation orientation (33). It is interesting that Hex/Prh shows an expression pattern similar to that of Wnt-7a (34). Misexpression of Hex/Prh-induced Wnt-7a and Notch expression suggests that it may lie upstream of this signaling pathway.
Bud Growth
Cell proliferation in the feather bud epithelium is another major factor contributing to the elongation process. During the short bud stage, epithelial cell proliferation localizes mainly to the distal compartment, and as growth progresses, the proliferation zone shifts proximally. Epithelial cell proliferation is likely regulated by WNT6, because (a) the WNT6 expression pattern coincides spatially and temporally with the epithelial proliferation zone and (b) misexpression of WNT6 results in enlarged feather epithelia (35). WNT and myc colocalize at sites of cell proliferation, suggesting these developmental ligands may act through this transcription factor to induce epithelial proliferation (36).
The feather bud epithelium next invaginates into the underlying mesenchyme to form the follicle structure (Figure 2c). This process also involves boundary formation demarcating the inside and outside of a feather follicle. Eph/ephrin-based cell-cell interaction plays a critical role in stabilizing organ boundaries (37–40). Ephrin-B1 and its receptor EphB3 are expressed in the feather epithelium at the invagination stage. Treating reconstituted skin cultures (15) with recombinant ephrin-B1 fused to the human IgG Fc portion (a dominant negative form) resulted in incomplete follicle invagination, blurring the boundary between the follicle and interfollicular regions (41).
Development of Feather Follicles
Feather filaments are made in follicular structures within the skin (Figure 2c′). At the beginning of folliculogenesis, the epithelium wraps around the underlying dermal papilla and then invaginates into the underlying mesenchyme. The mesenchymal compartment consists of the dermal papilla at its proximal end, with loosely packed dermal pulp cells residing above. Epithelial structures include the feather and follicle sheaths. A specialized region within the feather sheath, the collar bulge, houses the epithelial stem cells (1, 42, 43). The feather follicle provides a protected region in which to store these epithelial stem cells. Later feather epithelia differentiate in the ramogenic zone to form epidermal ridges, which produce the feather barbs (Figure 2d).
We wondered how the feather epithelium could invaginate into the underlying mesenchyme. We explored the possible role of matrix metalloproteinase 2 (MMP2) and tissue inhibitor of metalloproteinase 2 (TIMP2), its inhibitor in the process. Both are initially expressed at E9, and their expression patterns are dynamic within the skin. MMP2 is active from E10 through E20. Broad-spectrum MMP inhibitors and TIMP2 each suppressed feather bud development (44). In a separate study, the distribution of ADAMs (adisintegrin and metalloproteases), which bind to integrins and possess metalloprotease activity, was determined (45). In the dorsal tract, ADAM9, 10, 17, and 23 were expressed in the feather bud epidermis, whereas ADAM12 and 22 were expressed in the dermis. At E10, ADAM12 is in the anterior and posterior mesenchyme of midline dorsal tract feathers. In lateral feather buds, it is expressed exclusively in the posterior mesenchyme by E12. In contrast, ADAM22 expression initiates beneath the feather buds and later moves to the anterior mesenchyme. ADAM13 is expressed in both the dermis and epidermis. Although ADAMs are nicely positioned to play a role in invagination, their function has not yet been tested in feather morphogenesis.
FEATHER FOLLICLE: REGENERATION AND MORPHOGENESIS
Regenerative Cycling and Feather Stem Cells
In adult birds, feathers undergo natural cycling through phases of initiation, growth, rest, and molting before beginning a new cycle (Figure 2e). Feathers can regenerate throughout a bird’s life (1). Significant changes in feather morphology take place during the feather cycle (5). Feather cycles largely can be divided into two phases: growth and rest phases (1). During growth phase, the epithelium thickens around the dermal papilla. Proliferation is high in the basal layer of the epithelium. The collar epithelium stem cell niche forms an epithelial ring slightly above the dermal papilla (Figure 3c′). Cells in the ramogenic zone form barb ridges, which will eventually differentiate into the barbs and barbules. The dermal papilla is shaped like a vase, with a narrow region near its center. The pulp mesenchyme lies above the dermal papilla. During rest phase, the width of the feather follicle shrinks in size, as does the dermal papilla. As the pulp mesenchymal cells die and retreat toward the proximal end of the feather, periodically placed epithelial pulp caps are left behind. The stem cells move down to become the papillar ectoderm that surrounds the dermal papilla. Proliferation is greatly reduced in the basal layer of the epithelium. The barbs and barbules keratinize and lack active barb ridge formation. At molting, the feather is shed, leaving the dermal papilla and papilla ectoderm. Some of the stem cells become activated to initiate a new feather cycle during the initiation phase. Cycling can also be induced following feather plucking.
Figure 3.
Feather follicles: regeneration and branching morphogenesis. (a) Feather branching morphogenesis produces different types of chicken feathers. Adapted from Lucas & Stettenheim (1). (b) Splitting and merging the rachis in response to BMPs and their inhibitor, Noggin. Arrowheads indicate rachides. (b′) Effects of Noggin on barb ridge formation. (b, b′) Adapted from Yu et al. (48). (c) Perturbation of the Wnt-3a gradient produces different feather forms. Adapted from Yue et al. (53). (c′) Model for the stem cell niche topology in radially symmetric feathers (left) and bilaterally symmetric feathers. A ring of stem cells lies parallel to the surface of the skin in radially symmetric feathers but is canted at an angle in bilaterally symmetric feathers. Adapted from Yue et al. (52). (d) Time dimension and proximal-distal feather axis during regeneration. Proximal feather is younger than distal region. Gradients of signaling molecules regulate the shape and size of structures that form. Adapted from Lin et al. (5). Abbreviations: Ant, anterior; ap, axial plate; bp, barb plate; gz, growth zone; LRC, label retaining cells; mp, marginal plate; Post, posterior; TA, transient amplifying.
A few molecules have been mapped in adult feather follicles during the feather cycle (5). NCAM is present in the dermal papilla throughout all stages of the feather cycle. Tenascin is present within the papillar ectoderm during initiation phase and then spreads to the dermal papilla and the proximal pulp during growth phase. Its expression retreats to the papillar ectoderm and dermal papilla during rest phase. The expression patterns of these few genes show that molecular expression is very dynamic in the feather follicle during the feather cycle.
Branch Formation
Stem cells divide in the proximal follicle region, and their daughter cells differentiate as they move distally. Branching morphogenesis begins in the ramogenic zone with invagination of the multilayered epithelium (Figure 2d′). Each barb ridge consists of centrally aligned axial plates and centripetally oriented barbule plates. The basal layer flanking each barb ridge becomes the marginal plate epithelia. Barb ridges at the anterior end of the feather fuse to form the rachis, the feather backbone. The posterior end of the feather develops the barb generative zone. As barbule plates keratinize, the marginal plate and axial cells undergo apoptosis (46, 47). Higher up toward the more mature distal end, the feather sheath and pulp epithelium also undergo apoptosis, releasing the feather branches to open (48). In Figure 2d, drawings representing cross sections at four different levels within a follicle show changes in epithelial cylinder morphology as periodically arranged barb ridges form. Because feathers are made in follicles, with an epithelial cylinder enwrapping a mesenchymal core (Figure 2c′) (1), the configuration of their branch patterns allows for many morphological possibilities (49), including radially symmetric down feathers, bilaterally symmetric contour feathers, and bilaterally asymmetric flight feathers (Figure 3a).
Downy feathers are radially symmetric, and the barb ridges lie parallel to one another. These plumulaceous feathers are pliable and provide warmth to the bird. In contrast, bilaterally symmetric contour feathers and bilaterally asymmetric flight feathers form a vane in which tiny hooklets from one barbule attach like Velcro to grooves on a neighboring barbule, producing a more rigid structure (1). The vane structure of these pennaceous feathers allows for the movement of air, which is necessary for flight. The bilaterally asymmetric configuration of flight feathers provides improved aerodynamics.
In chicken and duck embryonic feathers, interactions between Shh and BMP2 were suggested to be involved in feather branching morphogenesis (50). Experiments determined that antagonism between BMP4 and Noggin controls the branching of filament epithelia (48). BMP4 promotes the formation of a giant rachis, whereas Noggin promotes branch formation (Figure 3b) within the barb ridges (Figure 3b′). Shh is expressed by cells of the marginal plate, which will undergo apoptosis (24, 48). Suppression of Shh causes a reduction in apoptosis in the marginal plate, leading to webby feather branches (48). Ephrin B1 is present in both the feather filament and marginal plate epithelium. Ephrin B1–Fc causes barb ridges to form unevenly (41), implying a role of Ephrins in patterning barb ridges. MMP2 is found in the marginal plates. Its inhibitor, TIMP2, is expressed with a complementary pattern in the barb plate epithelium. Inhibition of MMP2 perturbed barb ridge structure (44), implying the involvement of this pathway in barb ridge invagination.
There are more regulatory mechanisms in feather morphogenesis. Endogenous nonprotein-coding microRNAs (miRNAs) of 18–25 nucleotides (nt) have been shown to be involved in a wide variety of cellular processes. miRNAs function as posttranscriptional regulators by binding to the 3′ untranslated region of target mRNAs to repress translation or to cleave mRNAs. Recently, Zhang et al. (51) showed that, based on the bioinformatic data, seven miRNAs collected from the follicles of growing contour and down feathers may target the genes of Wnt/β-catenin, Shh/BMP, and Notch pathways, which are important for feather morphogenesis (33, 48, 52). The differential expression profile between these two feather types was shown, suggesting the presence of a new category of molecular control, although functional data remain to be demonstrated.
Anterior-Posterior Axis
Whereas contour feathers are bilaterally symmetric, the development of feather asymmetries is critical to the acquisition of new feather functions. In radially symmetric feathers, the stem cell ring is placed horizontally. In bilaterally symmetric feathers, the stem cell ring tilts toward the anterior rachis position (Figure 3c′) (43). In bilaterally symmetric feathers, a Wnt-3a gradient formed from anterior to posterior positions. This gradient does not exist in radially symmetrical feathers (52). Ectopic Dkk1 expression flattened the Wnt-3a gradient and converted bilaterally symmetric feathers to radially symmetric feathers (Figure 3c). The release of Wnt-3a from coated beads forms a Wnt gradient and induces the production of a new rachis at the site of highest Wnt-3a concentration (52). These studies demonstrate that skin progenitor cells can generate feathers with diverse forms in response to different molecular signals they may encounter in their environment during development and regeneration.
Proximal-Distal Axis
During feather regeneration, the dermal papilla lies at the base of the feather follicle. Loosely packed dermal pulp cells lie above the dermal papilla (Figure 2c′). This establishes a proximal-distal axis, with the younger cells located near the base of the follicle and the older, more differentiated cells localized at its distal tip. What molecules might regulate the different feather structures that form along the proximal-distal axis? There appears to be a balance between counteracting effects of fibroblast growth factor 10 (FGF10) and its inhibitor, Sprouty 4. Retroviral-mediated misexpression of FGF10 led to short but broad feathers with expanded proximal follicle structures (proliferating collar epithelia and dermal papilla–like mesenchyme). Ectopic expression of Sprouty 4 induces barb ridges from lower feather sheath regions that would not normally branch, forming distal structures in the proximal region (53). This is diagrammed in schematic form in Figure 2d. A general scheme showing how changes in molecular expression can alter feather morphology is also shown in Figure 3d.
Cellular differentiation takes place first in the distal feather bud, but with time it also encompasses the proximal end. Keratins are a hallmark of feather differentiation. There are 111 β-keratin genes that have been identified in chickens (54). To garner a better understanding of feather evolution, the genomic organization of β-keratins was compared between the chicken (order Galliformes) and zebra finch (order Passeriformes). On chromosome 25, in general the feather-like keratins were at the 5′ end, feather keratins were in the middle, and claw keratins were at the 3′ end of the keratin encoding cluster (55,56). However, the number of claw and feather keratin genes differed between species. When compared with those of zebra finch and alligators, the reptile β-keratins formed a monophyletic group with avian scale and feather β-keratin genes (57). Nile crocodile scale β-keratins show 75–90% identity with chicken β-keratins (58). Molecular dating methods suggest that avian β-keratins began to diverge from their archosaurian ancestor approximately 216 million years ago. β-Keratins in extant birds did not begin to diverge until 143 million years ago (59).
Pigmentation Patterns
Birds can be highly decorated with distinct and colorful pigmentation patterns (Figure 1a), which are used to attract a mate or to hide from or frighten a potential predator. These patterns can differ seasonally or at various stages of sexual maturity. During feather regeneration, melanocytes are distributed as a horizontal ring near the base of the feather follicle (Figure 4a). Melanocytes flow from the feather follicle and bulge up to the barb ridges, where they mature and produce pigment (Figure 4a) (60). Pigment patterning is achieved through the regulation of the presence, distribution, and differentiation of these melanocytes (Figure 4a,b). White coloring (apigmentation) is caused by distinct mechanisms in different bird species. Barred Plymouth Rock chickens carry the sex-linked barring allele, and their feathers have stripes of black and white. Melanocytes are present in the black regions but absent from the white regions. However, the melanocytes do not undergo apoptosis; rather, they appear to differentiate prematurely (60). Silver Laced Wyandotte chicken feathers have a central white region surrounded by pigmented peripheral lacing. Here, melanocytes are present in both black and white regions. Agouti signaling protein (ASIP) expressed in the peripheral pulp of white regions prevents the melanocytes from maturing (60). Placing an ASIP coated bead into a black region of a growing feather follicle produces a white region around the bead. The regulation of melanocyte maturation is shown schematically in Figure 4a.
Figure 4.
Feather variations: pigment patterns and texture. (a) Possible steps involved in modulating the homeostasis of melanocyte stem cells, which then affect pigment pattern formation. Modulation can be based on auto-feedback or cross-talk with environmental factors (peripheral pulp, body hormone status). (b) Multidimensional regulation of simple melanocyte behavior allows more temporospatial regulatory freedom. ❶ Pigmentation patterns along the proximal-distal feather axis represent a history of chronological events (60). ❷ The follicle’s cylindrical configuration (62) produces a new medial-lateral plane after the feather vane is opened by apoptosis, which increases patterning diversity. ❸ Signaling factors lying within the peripheral pulp further expand possibilities for pigmentation complexity. ❹ Systemic factors originating from outside of the feather follicle, such as hormones, day length, and temperature variation, contribute to increased diversity (132). The combination of variation from each of these four dimensions enhances the potential to form diverse patterns. (c) One-month-old frizzle chicken. The feathers curl away from rather than toward the body. (c′) Comparison of the wild-type and homozygous frizzle feathers. The wild-type feather image is overlaid with a computer-generated arrow indicating the angle of its rachis. (d) Function θ(s) describes rachis bending, plotted on the length-normalized coordinate. The functions are shifted by arbitrary offsets for clarity. (e) Double immunostaining for K75 (green) and feather keratin (red) in the rachis of normal and frizzle feathers. The yellow dotted line outlines the rachis, and the white dotted line indicates the medulla. (f) Diagram of chicken KRT75 and the cryptic splice site activated by the deletion mutation that covers positions −24 of exon 5 to +59 of intron 5. Boxes represent exon sequences; intron 5 is designated by a line. Carets designating use of the cryptic site (position −69; shown below the pre-RNA diagram) and use of the authentic site (shown above the pre-RNA diagram). (a–c) Adapted from Lin et al. (60), (d–f) adapted from Ng et al. (69). Abbreviations: A, anterior; D, dorsal; ASIP, agouti signaling protein; Dist, distal; LB, lower bulge; LRC, label retaining cell; MB, middle bulge; P, posterior; PE, papillar ectoderm; Prox, proximal; RGZ, ramogenic zone; s, distance from the proximal end of the feather used to determine the rachis angle (θ) at any point; TA, transient amplifying; UB, upper bulge; V, ventral.
Regulation of feather pigment patterns is multidimensional. In a single filament, the melanocyte stem cells lie at the proximal base and mature as they move distally in a one-dimensional fashion (Figure 4b ❶). In a cylinder, this is spread over a cylindrical plane, becoming two-dimensional (Figure 4b ❷). With contributions from the peripheral pulp, regulation occurs within a three-dimensional space, increasing the potential complexity of feather color patterning (Figure 4b ❸). Finally, systemic factors such as hormones can influence melanocyte differentiation in different stages of an individual’s life, thus adding a fourth dimension (Figure 4b ❹).
A theoretical model based on reaction diffusion was proposed to explain the periodic patterning of barb ridges with activators and inhibitors (61). Prum and colleagues (62, 63) have also introduced new concepts in hierarchical parameters that contribute to the formation of diverse feather shapes and pigment patterns. Indeed, several molecules meeting these criteria have been identified that provide a better understanding of how molecular networks can build complex patterned cellular structures.
FEATHER VARIANTS: GENETIC DETERMINANTS OF FEATHERING MORPHOGENESIS
The genetics of feather variants with characteristics selected from various breeds of chickens provided an interesting and efficient pallet to test concepts of Mendelian genetics. Throughout the early twentieth century, the basic genetics of these traits were determined. A summary of some of these variants is shown in Table 1. However, only a few specific genes that underlie the feather variants have been identified. There are several mutants in each category (Table 1). Here we show only some examples with molecular defects that have recently been identified.
Table 1.
Examples of feather variants
| Feather variant | Phenotype | Candidate genes |
Chromosomal regions | References |
|---|---|---|---|---|
| Developmental | ||||
| Scaleless, sc | Feathers are nearly absent except for a few relatively normal feathers in the dorsal and femoral tracks | FGF20 | 64, 65 | |
| Naked neck, Na | Featherless skin extends from the back of the head to the upper portion of the breast and back; incompletely dominant allele | WNT 11, UVRAG, BMP12 | 66–68 | |
| Feather structure | ||||
| Frizzle, Fr | Frizzle feathers have a severe plumage defect that affects not only the rachis but also the barbs and barbules leading to narrow curled fragile feathers that often break off at the calamus; incompletely dominant allele | KRT 75 | E22C19W28_E50C23 linkage group | 69–71 |
| Silkie, h | Hookless feathers lack functioning barbicels and are thus similar to down on the chick; a hairlike appearance exhibited in adult feathers | 134–137 | ||
| Regional specificity | ||||
| Crest, Cr | A tuft of elongated feathers to sprout from the head, the phenotype shows a degree of sexual dimorphism, with males exhibiting more voluminous crests than females; incompletely dominant allele | HOXC8 | E22C19W28 linkage group | 138 |
| Ptilopody | Chickens with this trait have varying amounts of feathers on their shanks, as well as on the surfaces of some of their toes | Pti | rs14999343/rs13505642 on Chromosome 13 | 134, 139 |
| Sex variation | ||||
| Hen feathering, Hf | In male birds with the hen feathering trait, the plumage at these sites is similar to that of females with shorter feathers with rounded ends | 66 | ||
| Growth rate | ||||
| Slow feathering, K | The sex-linked slow-feathering gene is responsible for the speed of feathering in chicks, especially concerning remiges and rectrices; delayed emergence of the primary and secondary feathers | PRLR, SPEF2 | 9966364–10142688 bp on Z chromosome | 140–142 |
| Feather color/pattern | ||||
| Dominant white, I | The Dominant white mutation causes premature melanocyte cell death and thereby inhibits the expression of black eumelanin | REML | 143 | |
| Recessive white, c | White plumage owing to the lack of enzymatic function of tyrosinase, the melanin synthesis pathway is blocked or incomplete; the chickens exhibit an albino phenotype; recessive allele | TYR | 144, 145 | |
| Extended black, E | The mutations causing a constitutively active receptor are dominant and associated with black plumage, whereas loss-of-function mutations are recessive and associated with a red/yellow phenotype | MC1R | 146, 147 | |
| Silver, S | The sex-linked Silver locus controlling Silver and wild-type/gold plumage color | SLC45A2 | 148 | |
| Sex-linked barring, B | The plumage of barring patterns alternates between black and white; sex-linked barring in the white regions is characterized by a complete absence of pigmentation | CDKN2A/B | 149, 150 | |
| Dark-brown, Db | Birds with the dark-brown phenotype have a pattern similar to the black-tailed red phenotype and with a Columbian-like restriction of the expression of eumelanin | SOX10 | 151 | |
Feather Follicle Developmental Defects: Scaleless
In adult chickens that exhibit the scaleless trait, virtually all adult feathers are absent, but there are a few relatively normal feathers in the dorsal and femoral tracks (64). The scaleless trait is controlled by a single autosomal-recessive gene. Early tissue recombination studies indicated that the defect occurred in the epidermis, and the phenotype can be rescued by exogenous FGF2 (31). Most recently, a nonsense mutation in FGF20, a gene expressed in the epidermal placode, was found to be associated with the scaleless phenotype (65).
Variations in Feather Follicle Distribution: Naked Neck
Birds that display naked neck are characterized by completely featherless skin that extends from the back of the head to the upper portion of the breast and back. The trait is produced by a single autosomal incompletely dominant allele. Heterozygous birds have a tuft of feathers at the base of the neck above the crop and so can readily be distinguished from homozygotes (66). The mutation has been identified as a ∼73-kb insertion of an intragenic, untranscribed region between the WNT11 and UVRAG gene from chromosome 1 into chromosome 3 (67, 68). This insertion leads to increased skin expression of the neighboring gene encoding BMP12/growth differentiation factor-7 (BMP12) on chromosome 3. Overexpression of BMP12 observed in the naked neck is not sufficient to block feather formation in most regions of the skin. However, a high level of retinoic acid produced in the developing skin of the neck uniquely sensitizes this region to BMP signaling (67). The mechanism by which BMP12 transcripts are increased and the synergy between retinoic acid and BMP remain to be determined.
Variations in Feather Structure: Frizzle
The genes that produce the phenotypes that underlie the frizzle group of traits include building blocks such as keratins. The heterozygous frizzle phenotype becomes apparent when down is replaced with second-generation bilateral contour feathers, which have a rachis. At this stage, the rachis of the body feathers, as well as those of the wings and tails, begins to exhibit a severe twist, causing the feather to be bent backward toward the head of the bird (Figure 4c,c′,d). The homozygote has a severe plumage defect that affects not only the rachis but also the barbs and barbules, leading to narrow, curled, fragile feathers that often break off at the calamus (69). In chickens, the frizzle gene is incompletely autosomal dominant, and the exhibition varieties are typically heterozygous for the mutant allele (70, 71). Birds homozygous for the frizzle allele are viable, but the plumage defect is more profound, with very weak feathers resulting in patches of exposed skin (70).
A recent analysis of a pedigree of exhibition frizzle chickens shows that the phenotype is linked to two small nucleotide polymorphisms in a cluster of keratin genes within the linkage group E22C19W28_E50C23 on chromosome 22 (69). Sequencing of the gene cluster identified a 69– base pair in-frame deletion of the protein coding sequence of the α-keratin-75 gene (69) (Figure 4f). Forced expression of the mutated keratin 75 gene in normal feather follicles produced a twisted rachis (69). The mammalian ortholog of K75 is expressed in the keratinocytes of the companion layer, matrix, and medulla of the hair follicle, and mutations in this gene result in the hair disorder pseudofolliculitis barbae (72).
The feather mainly consists of feather-specific β-keratins, and cellular and biochemical studies have shown that α-keratins play an important role in the early formation of rachides, barbs, and barbules. K75 is an α-keratin, and its transcript is abundantly expressed in the developing ventral rachis, which is destined to become the medulla (Figure 4e) (69). Thus, there is a mechanical defect in the rachis that cannot keep the feather from bending.
SYSTEMIC FACTORS ACTING ON FEATHER GROWTH
Physiological Regeneration
Birds undergo a periodic process to shed worn feathers and replace them with new feather types at different physiological stages (Figure 1b). At least four different plumages appear in avian species from hatching to the end of the first annual molting cycle, including natal down, juvenal, alternate, and basic plumages (1), although the alternate and basic plumages are similar in some species.
Natal down is produced during embryonic development and may cover hatchling chicks densely or sparsely in precocial or altricial chicks, respectively (73). The down is completely replaced by juvenal (juvenile) feathers, which begin to form in the feather follicle during the late embryonic stage. Birds with juvenal feathers usually are not of breeding age and can be easily distinguished from adults. In species that display one molt per year (e.g., American robin, woodpeckers), the prebasic molt follows juvenal plumage in the first year and annually thereafter. The first prebasic molt is not complete, whereas the adult (postnuptial) prebasic molt is usually complete after the breeding season or laying cycle when the bird reaches sexual maturation, which results in adult basic plumage (74). For avian species undergoing two molts a year (such as the red jungle fowl and cedar waxwing), the prenuptial, or so-called prealternate, molt leads to replacement of the basic plumage. Generally, the prealternate molts are partial, but the extent of molting varies among species and between sexes. In adult males, the alternate plumages differ greatly from their basic plumages. However, in females both plumages are similar. After the first year, most birds grow and retain an adult basic plumage that cycles to an alternate, or breeding, plumage for the breeding season.
Regulation by Body Hormones
Birds are subjected to seasonal changes in climate and photoperiod during the year. To ensure abundant food resources to maximize survival of the growing young, most animals constrain their reproductive activity to specific seasons according to the day length and then adapt their physiological and behavioral processes (reproduction, migration, molting, hibernation, or body weight). Old feathers are expelled from the follicle as young new feathers grow in the following cycle. The dermal papilla is induced to grow by exposure to the thyroid substance, as well as progesterone (75–77). Several studies concur that molting is associated with changes in metabolism and neuroendocrine regulation (74, 78). Molting is induced by photostimulation (79, 80), using day length as the possible cue responsible for its initiation. However, because birds also detect light via photoreceptors located in the mediobasal hypothalamus (81), it is unclear whether molting is a direct effect of photoperiod duration or a secondary consequence of hormone levels influenced by photoperiod. Nevertheless, significant data support a role for the regulation of hormones by the hypothalamic-pituitary-gonad axis (HPG axis), hypothalamus-pituitary-adrenal axis (HPA axis), and hypothalamus-pituitary-thyroid axis in avian molting (82, 83; for review, see 84). These pathways link to other physiological events, including breeding, migration, and immunology, to satisfy ecological requirements (85–87).
Several reproductive hormones are suppressed during molting. Most wild and domestic bird species show decreased reproductive activity, i.e., reproductive quiescence, during a natural postnuptial molting. Hence, commercial egg producers induce an artificial molt to resume egg production. At the end of the breeding season or during egg incubation, HPG axis activity is suppressed, as evinced by gonad regression. Luteinizing hormone and gonadal steroid hormone (e.g., testosterone, estradiol, progesterone) levels are reduced in response to natural or artificial molting (82, 88–90). Experimentally increasing plasma testosterone levels with subcutaneous implants postpones the prebasic molt (91, 92), whereas suppressing pituitary gonadotropin-releasing hormone (GnRH) receptors with a GnRH agonist can induce a molt (93). Additionally, birds with high prolactin levels associated with broodiness commence to molt. Lactotroph cells are distributed in the anterior pituitary gland at all stages of molting (94).
The HPA axis helps animals cope with challenges associated with progression through life history stages, e.g., molting, or environmental stress. Plasma glucocorticoid hormones, corticosterone, and cortisol are decreased during molting in birds (83, 95). Although seasonal changes and chronic stress modulate the HPA axis differentially, decreased baseline cortisol and attenuated adrenal sensitivity are modulated at multiple sites along the HPA axis at molting (83, 96). The low level of corticosterone might improve growing feather quality by promoting protein mobilization (97). Recently, Yoshimura (84) reviewed the importance of thyroid hormone for the hypothalamic mechanisms controlling seasonal reproduction in birds. Thyroxine (T4) is known to trigger molting (74, 77), and removal of the thyroid gland will cease photorefractoriness in birds [for review, see Yoshimura (84)] and decrease the level of corticosterone (98). A long photoperiod stimulates the GnRH neurons, leading to subsequent HPG axis activation. This can be mimicked with exogenous thyroid hormone treatment [for review, see Shinomiya et al. (99)]. A significant increase of T4 occurs during induction of molt following food withdrawal (reviewed in Reference 74), or at the end of reproduction (82). However, the trigger for photorefractoriness by thyroid hormones is still unclear. For animals living in the tropical zone (lack of seasonality), circannual rhythmicity may play a role in reproduction activity and in turn regulate molting (for review, see Reference 100).
Molting represents more than a change of plumage. It is a complex physiological process regulated closely by both internal and external signals, which results in significant changes in animal physiological status and phenotype. Future aims will be to link the known physiological hormonal changes with molecular mechanisms of feather cycling control and morphogenesis.
Sexual Dimorphism
Many birds show sexual dimorphism in their plumage. Feather phenotypes can be modulated by sex hormones and can help elucidate mechanisms of sex hormone–dependent growth control. Sexual dimorphism is the systematic difference in phenotype between individuals of different sexes in the same species (101). In birds, the differences include morphology and plumage (102). The differences of plumage in both genders (sexual dichromatism) are believed to result from evolution through sexual selection. These secondary sexual characteristics involve the size, shape, and color of epithelial appendages, forming the basis for sexual selection. Male tail feathers may grow longer and have distinct shapes compared with their female counterparts (Figure 1b) (103). This difference has been attributed to the benefit male birds with longer tails experience during the sexual selection process. Also, it has been shown that increasing testosterone levels affect the extent of white tail feathers (tail white) on the dark-eyed junco (Junco hyemalis carolinensis). Larger tail whites were found to be preferably selected by females, which established a male-male competition (104).
Hormone-dependent and hormone-independent proximate mechanisms have been proposed to be responsible for sexual dichromatism [for review, see Kimball & Ligon (105)]. Hormones believed to be involved in sexual dichromatism are the steroid gonadal hormones, estrogen and testosterone, pituitary peptide hormone, and luteinizing hormone (105). For species with estrogen-dependent dichromatism, bright plumage develops in the absence of estrogen, whereas for testosterone- and LH-dependent birds, the bright coloration develops in the presence of these hormones. A nonhormone-mediated proximate mechanism was supported by the skin graft experiment conducted on Rouen ducks (106) and house sparrows (Passer domesticus) (107). The results showed that the differences in plumage and skin between both sexes remained the same after skin graft exchange or hormone alterations; i.e., the resulting plumage was based on the donor, not the recipient, indicating that a genetic factor may also play a role in sexual dichromatism (106, 107). For example, melanin-based coloration in the barn swallow (Hirundo rustica rustica) was found to differ between genders, plumage regions, and even geographical locations (108). These findings implicate pigmentation patterning as an important and complex issue that requires further investigation.
THE ORIGIN AND EVOLUTION OF FEATHERS
Protofeather Forms in Fossils
Recent discoveries of many nonavian dinosaurs with feathers or feather-like integuments have come from the Jehol Biota in Northern China. Although debates about the true identities of these filamentous structures continue (109), the morphologies of these structures are indeed congruent with the transitional stages described in the feather form diversification model proposed by developmental biologists (7, 110). Fossils with major new feather forms are summarized in Figure 5. Unbranched, cylindrical feather-like structures were found on Psittacosaurus (111), Sinosauropteryx (112), and Beipiaosaurus (113). The filamentous structures in Psittacosaurus and Beipiaosaurus are rigid with thick bristles, which are possibly hollow in the center. These bristles and the elongated ribbon-like tail feathers on Epidexipteryx (114) may be specialized decorative appendages that have no direct relationship with modern feathers. Epidexipteryx also has a type of nonshafted feather composed of parallel barbs closely united as an unbranched membranous structure. It may be a transitional form between the singular filaments and downy feathers. Fossils of radially branched, downy feather-like integuments were found on Sinornithosaurus (115), Beipiaosaurus (116), Protarchaeopteryx, Caudipteryx (117), and Dilong (118), and possibly also on Shuvuuia (119) and Yutyrannus (120). Clearly vaned pennaceous feathers (containing rachis) were found on Caudipteryx, Protarchaeopteryx (117), Pedopenna (121), Anchiornis (122), and Similicaudipteryx (6), and possibly also on Xiaotingia (123), as well as on a type of Ornithomimus discovered in Canada (124). Bilaterally asymmetric flight feathers were found on Microraptor (125, 126) and most Avialae, such as Archaeopteryx (127, 128).
Figure 5.
Feather evolution: intermediate protofeathers in feathered dinosaurs and basal avialan (153). Schematic drawings of protofeathers, feather-like appendages or intermediate stages of feathers, in feathered dinosaurs and mesozoic birds. The novel developmental processes that allow the major transition of feather forms during evolution include (a) from a cylindrical filament to radially symmetric branched feathers (e.g., downy feathers); (b) from radially symmetric to bilaterally symmetric feathers (e.g., contour feathers); (c) the number, size, and arrangement of rachis and barbs; and (d) the difference of morphology along the proximal-distal axis of the rachis. Besides these macroscale morphological changes, there are also microscale structural modifications taking place, such as the branching of barbs into barbules and rami, specialization of barbule pennulum and base, and the differential morphogenesis of proximal and distal barbules. Adapted from Wu et al. (154).
One of the well-preserved four-winged dinosaurs is shown in Figure 1c as an example (125). This Microraptor family member had long, narrow, feather-like integuments, which contained rachis-like structures (129). The dromaeosaurid four-winged dinosaur had long and asymmetric veined feathers attached to both the forelimbs and hindlimbs, making extensive airfoils (126). The distribution of feathers across the forelimb also resembles that of modern birds, indicating that this animal may have flown well (130).
Evo-Devo Processes for Feather Evolution
Scientists try to identify the cellular/molecular mechanisms responsible for the diverse protofeather fossil forms or morphotypes (6). With progress in laboratory research, we have learned that feather formation is achieved by multiple evolutionarily novel tissue morphogenetic processes, for instance, follicle formation, tube generation, branching morphogenesis, anterior-posterior polarity formation, and proximal-distal temporal regulation. Each tissue morphogenetic process is based on a molecular module. MMP-dependent tissue invagination allows epithelia to fold the follicle (44). The formation of loose mesenchyme in the follicle core allows for the cylindrical feather filament configuration. Subsequent selective caspase-dependent apoptosis transforms the tube to a plane, allowing feather vane formation (46). The ratio of local activators (e.g., Noggin, Shh) and inhibitors (BMP) regulates the number and size of barbs versus rachidial ridges (48, 61). Barb ridges can be parallel to each other and form radially symmetric feathers or tilt toward the rachis to form bilaterally symmetric feathers, depending on a Wnt-3a gradient (52). During the time course of feather formation, the molecular microenvironment can change, thus shifting the morphology along the distal (formed earlier)–proximal (formed later) axis (131). Selective combinatorial usage of these processes leads to the formation of complex feather forms and diverse functions.
A unique property of feathers is the ability to molt, regenerate, and renew. Through the process of evolution, the stem cells and dermal papilla become clustered at the proximal follicle, poised to generate a new feather (43). Regenerative cycling is based on cyclic activation of Wnt/DKK (131). Upon regeneration, modulated by the body macroenvironment, different feather morphotypes (downy, contour, flight feathers) can form on different body regions and at different physiological stages for optimal adaptation (132, 133).
SUMMARY POINTS AND FUTURE ISSUES
In this review, we have covered many different aspects of the feather organ. On the developmental biology side, we have been using the feather as a Rosetta stone to answer many fundamental biology questions, from how stem cells are regulated under physiological conditions to how organs take shape. On the evolutionary side, the presence of feathers used to be a defining character for birds and the Aves class. Because of the discoveries of different feathered dinosaurs, this concept is no longer true. We learned how keratinocyte progenitors can be molded into different feather forms for diverse functions from the molecular level, tissue/organ level, and organism behavior level with impact on the evolutionary formation of new classes. With functional implications transcending multiscales, the feather is an ideal platform for multidisciplinary research.
One important future direction regards the integration of developmental biology and morphogenesis. We should apply current imaging technology to study the formative processes of embryonic chicken skin. For mice, this process takes place in utero and is hard to reach. The flat and transparent embryonic skin explant culture offers a great opportunity to image and analyze the whole process of how a complex skin is built. Principles learned here will also contribute to tissue engineering of the skin. The second important direction is to take advantage of feather morphological diversity to gain a genomic and epigenetic level of understanding. With progress in epigenetics, the time is ripe to transform our knowledge of morphogen signals in follicle environments to understanding at the genomic level. Genetic variants will help these analyses greatly. The third direction is on the evolutionary front. We should analyze many feathered dinosaurs and correlate them with morphogenetic processes. We should not only study the Evo-Devo of feathers but also take the opportunity to trace the origin of birds. Studies on feather structures provide insight on biomaterials. Birds can be a great biomimetic platform for multidisciplinary research. With these exciting potentials, the biannual International Avian Model system meeting is set up to promote avian research.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers 42177, 47364, and 60306. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. It was also supported by a grant of the Ministry of Science and Technology, Taiwan (grant number NSC101-2311-B-005-008-MY3). A.L. was supported by a CIRM training grant predoctoral fellowship.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Lucas AM, Stettenheim PR. Avian Anatomy Integuments Part I, II. Washington, DC: US Gov. Print. Off; 1972. [Google Scholar]
- 2.Crawford R. Poultry Breeding and Genetics. Amsterdam: Elsevier; 1990. [Google Scholar]
- 3.Sengel P. Morphogenesis of skin. In: Abercrombie M, Newth DR, Torrey JG, editors. Developmental and Cell Biology Series (Book 3) Cambridge: Cambridge Univ. Press; 1976. [Google Scholar]
- 4.Lin CM, Jiang TX, Widelitz RB, Chuong CM. Molecular signaling in feather morphogenesis. Curr. Opin. Cell Biol. 2006;18:730–41. doi: 10.1016/j.ceb.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin SJ, Wideliz RB, Yue Z, Li A, Wu X, et al. Feather regeneration as a model for organogenesis. Dev. Growth Differ. 2013;55:139–48. doi: 10.1111/dgd.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X, Zheng X, You H. Exceptional dinosaur fossils show ontogenetic development of early feathers. Nature. 2010;464:1338–41. doi: 10.1038/nature08965. [DOI] [PubMed] [Google Scholar]
- 7.Prum RO. Development and evolutionary origin of feathers. J. Exp. Zool. 1999;285:291–306. [PubMed] [Google Scholar]
- 8.Chuong CM, Chodankar R, Widelitz RB, Jiang TX. Evo-devo of feathers and scales: building complex epithelial appendages. Curr. Opin. Genet. Dev. 2000;10:449–56. doi: 10.1016/s0959-437x(00)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scaal M, Prols F, Fuchtbauer EM, Patel K, Hornik C, et al. BMPs induce dermal markers and ectopic feather tracts. Mech. Dev. 2002;110:51–60. doi: 10.1016/s0925-4773(01)00552-4. [DOI] [PubMed] [Google Scholar]
- 10.Fliniaux I, Viallet JP, Dhouailly D. Signaling dynamics of feather tract formation from the chick somatopleure. Development. 2004;131:3955–66. doi: 10.1242/dev.01263. [DOI] [PubMed] [Google Scholar]
- 11.Hornik C, Krishan K, Yusuf F, Scaal M, Brand-Saberi B. cDermo-1 misexpression induces dense dermis, feathers, and scales. Dev. Biol. 2005;277:42–50. doi: 10.1016/j.ydbio.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 12.Widelitz RB, Jiang TX, Chen CW, Stott NS, Jung HS, Chuong CM. Wnt-7a in feather morphogenesis: involvement of anterior-posterior asymmetry and proximal-distal elongation demonstrated with an in vitro reconstitution model. Development. 1999;126:2577–87. doi: 10.1242/dev.126.12.2577. [DOI] [PubMed] [Google Scholar]
- 13.Widelitz RB, Jiang TX, Lu J, Chuong CM. β-Catenin in epithelial morphogenesis: conversion of part of avian foot scales into feather buds with a mutated β-catenin. Dev. Biol. 2000;219:98–114. doi: 10.1006/dbio.1999.9580. [DOI] [PubMed] [Google Scholar]
- 14.Chen CW, Chuong CM. Dynamic expression of lunatic fringe during feather morphogenesis: a switch from medial-lateral to anterior-posterior asymmetry. Mech. Dev. 2000;1–2:351–54. doi: 10.1016/s0925-4773(99)00285-3. [DOI] [PubMed] [Google Scholar]
- 15.Jiang TX, Jung HS, Widelitz RB, Chuong CM. Self-organization of periodic patterns by dissociated feather mesenchymal cells and the regulation of size, number and spacing of primordia. Development. 1999;126:4997–5009. doi: 10.1242/dev.126.22.4997. [DOI] [PubMed] [Google Scholar]
- 16.Bardot B, Lecoin L, Fliniaux I, Huillard E, Marx M, Viallet JP. Drm/Gremlin, a BMP antagonist, defines the interbud region during feather development. Int. J. Dev. Biol. 2004;2–3:149–56. [PubMed] [Google Scholar]
- 17.Ohyama A, Saito F, Ohuchi H, Noji S. Differential expression of two BMP antagonists, gremlin and Follistatin, during development of the chick feather bud. Mech. Dev. 2001;100:331–33. doi: 10.1016/s0925-4773(00)00525-6. [DOI] [PubMed] [Google Scholar]
- 18.Chang CH, Jiang TX, Lin CM, Burrus LW, Chuong CM, Widelitz R. Distinct Wnt members regulate the hierarchical morphogenesis of skin regions (spinal tract) and individual feathers. Mech. Dev. 2004;2:157–71. doi: 10.1016/j.mod.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kere J, Srivastava AK, Montonen O, Zonana J, Thomas N, et al. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat. Genet. 1996;13:409–16. doi: 10.1038/ng0895-409. [DOI] [PubMed] [Google Scholar]
- 20.Thesleff I, Mikkola ML. Death receptor signaling giving life to ectodermal organs. Sci. STKE. 2002;131:pe22. doi: 10.1126/stke.2002.131.pe22. [DOI] [PubMed] [Google Scholar]
- 21.Drew CF, Lin CM, Jiang TX, Blunt G, Mou C, et al. The Edar subfamily in feather placode formation. Dev. Biol. 2007;305:232–45. doi: 10.1016/j.ydbio.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noramly S, Freeman A, Morgan BA. β-Catenin signaling can initiate feather bud development. Development. 1999;126:3509–21. doi: 10.1242/dev.126.16.3509. [DOI] [PubMed] [Google Scholar]
- 23.Noveen A, Jiang TX, Ting-Berreth SA, Chuong CM. Homeobox genes Msx-1 and Msx-2 are associated with induction and growth of skin appendages. J. Investig. Dermatol. 1995;104:711–19. doi: 10.1111/1523-1747.ep12606960. [DOI] [PubMed] [Google Scholar]
- 24.Ting-Berreth SA, Chuong CM. Sonic Hedgehog in feather morphogenesis: induction of mesenchymal condensation and association with cell death. Dev. Dyn. 1996;207:157–70. doi: 10.1002/(SICI)1097-0177(199610)207:2<157::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Jiang TX, Widelitz RB, Shen WM, Will P, Wu DY, et al. Integument pattern formation involves genetic and epigenetic controls: feather arrays simulated by digital hormone models. Int. J. Dev. Biol. 2004;48:117–35. doi: 10.1387/ijdb.041788tj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengel P. The determinism of the differentiation of the skin and the cutaneous appendages of the chick embryo. In: Montagna W, Lobitz WC, editors. The Epidermis. New York: Academic; 1964. pp. 15–33. [Google Scholar]
- 27.Oster GF, Murray JD, Harris AK. Mechanical aspects of mesenchymal morphogenesis. J. Embryol. Exp. Morphol. 1983;78:83–125. [PubMed] [Google Scholar]
- 28.Maini PK, Baker RE, Chuong CM. Developmental biology. The Turing model comes of molecular age. Science. 2006;314:1397–98. doi: 10.1126/science.1136396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turing AM. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1952;237:37–72. doi: 10.1098/rstb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung HS, Francis-West PH, Widelitz RB, Jiang TX, Ting-Berreth S, et al. Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev. Biol. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- 31.Song HK, Lee SH, Goetinck PF. FGF-2 signaling is sufficient to induce dermal condensations during feather development. Dev. Dyn. 2004;231:741–49. doi: 10.1002/dvdy.20243. [DOI] [PubMed] [Google Scholar]
- 32.Noramly S, Morgan BA. BMPs mediate lateral inhibition at successive stages in feather tract development. Development. 1998;125:3775–87. doi: 10.1242/dev.125.19.3775. [DOI] [PubMed] [Google Scholar]
- 33.Li A, Chen M, Jiang TX, Wu P, Nie Q, et al. Shaping organs by a wingless-int/Notch/nonmuscle myosin module which orients feather bud elongation. PNAS. 2013;110:E1452–61. doi: 10.1073/pnas.1219813110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obinata A, Akimoto Y. Expression of Hex during feather bud development. Int. J. Dev. Biol. 2005;49:885–90. doi: 10.1387/ijdb.052037ao. [DOI] [PubMed] [Google Scholar]
- 35.Chodankar R, Chang CH, Yue Z, Jiang TX, Suksaweang S, et al. Shift of localized growth zones contributes to skin appendage morphogenesis: role of the Wnt/β-catenin pathway. J. Investig. Dermatol. 2003;1:20–26. doi: 10.1046/j.1523-1747.2003.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desbiens X, Quéva C, Jaffredo T, Stéhelin D, Vandenbunder B. The relationship between cell proliferation and the transcription of the nuclear oncogenes c-myc, c-myb and c-ets-1 during feather morphogenesis in the chick embryo. Development. 1991;111:699–713. doi: 10.1242/dev.111.3.699. [DOI] [PubMed] [Google Scholar]
- 37.Batlle E, Wilkinson DG. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb. Perspect. Biol. 2012;4:a008227. doi: 10.1101/cshperspect.a008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahmann C, Oates AC, Brand M. Boundary formation and maintenance in tissue development. Nat. Rev. Genet. 2011;12:43–55. doi: 10.1038/nrg2902. [DOI] [PubMed] [Google Scholar]
- 39.Mellitzer G, Xu Q, Wilkinson DG. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. [DOI] [PubMed] [Google Scholar]
- 40.Xu Q, Mellitzer G, Robinson V, Wilkinson DG. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–71. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- 41.Suksaweang S, Jiang TX, Roybal P, Chuong CM, Widelitz R. Roles of EphB3/ephrin-B1 in feather morphogenesis. Int. J. Dev. Biol. 2012;56:719–28. doi: 10.1387/ijdb.120021rw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lillie FR, Wang H. Physiology of development of the feather V. Experimental morphogenesis. Physiol. Zool. 1941;14:103–35. [Google Scholar]
- 43.Yue Z, Jiang TX, Widelitz RB, Chuong CM. Mapping stem cell activities in the feather follicle. Nature. 2005;438:1026–29. doi: 10.1038/nature04222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang TX, Tuan TL, Wu P, Widelitz RB, Chuong CM. From buds to follicles: matrix metal-loproteinases in developmental tissue remodeling during feather morphogenesis. Differentiation. 2011;81:307–14. doi: 10.1016/j.diff.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin J, Luo J, Redies C. Differential regional expression of multiple ADAMs during feather bud formation. Dev. Dyn. 2011;240:2142–52. doi: 10.1002/dvdy.22703. [DOI] [PubMed] [Google Scholar]
- 46.Chang CH, Yu M, Wu P, Jiang TX, Yu HS, et al. Sculpting skin appendages out of epidermal layers via temporally and spatially regulated apoptotic events. J. Investig. Dermatol. 2004;6:1348–55. doi: 10.1111/j.0022-202X.2004.22611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alibardi L, Knapp LW, Sawyer RH. β-Keratin localization in developing alligator scales and feathers in relation to the development and evolution of feathers. J. Submicrosc. Cytol. Pathol. 2006;2–3:175–92. [PubMed] [Google Scholar]
- 48.Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–12. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prum RO. Evolution of the morphological innovations of feathers. J. Exp. Zool. B Mol. Dev. Evol. 2005;304:570–79. doi: 10.1002/jez.b.21073. [DOI] [PubMed] [Google Scholar]
- 50.Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J. Exp. Zool. 2002;294:160–76. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Nie Q, Su Y, Xie X, Luo W, et al. MicroRNA profile analysis on duck feather follicle and skin with high-throughput sequencing technology. Gene. 2013;519:77–81. doi: 10.1016/j.gene.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 52.Yue Z, Jiang TX, Widelitz RB, Chuong CM. Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. PNAS. 2006;103:951–55. doi: 10.1073/pnas.0506894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yue Z, Jiang TX, Wu P, Widelitz RB, Chuong CM. Sprouty/FGF signaling regulates the proximal-distal feather morphology and the size of dermal papillae. Dev. Biol. 2012;372:45–54. doi: 10.1016/j.ydbio.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Int. Chick. Genome Seq. Consort. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- 55.Greenwold MJ, Sawyer RH. Genomic organization and molecular phylogenies of the beta (β) keratin multigene family in the chicken (Gallus gallus) and zebra finch (Taeniopygia guttata): implications for feather evolution. BMC Evol. Biol. 2010;10:148. doi: 10.1186/1471-2148-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Presland RB, Whitbread LA, Rogers GE. Avian keratin genes. II. Chromosomal arrangement and close linkage of three gene families. J. Mol. Biol. 1989;209:561–76. doi: 10.1016/0022-2836(89)90594-9. [DOI] [PubMed] [Google Scholar]
- 57.Greenwold MJ, Sawyer RH. Molecular evolution and expression of archosaurian β-keratins: diversification and expansion of archosaurian β-keratins and the origin of feather β-keratins. J. Exp. Zool. B Mol. Dev. Evol. 2013;320:393–405. doi: 10.1002/jez.b.22514. [DOI] [PubMed] [Google Scholar]
- 58.Dalla Valle L, Nardi A, Gelmi C, Toni M, Emera D, Alibardi L. β-Keratins of the crocodilian epidermis: composition, structure, and phylogenetic relationships. J. Exp. Zool. B Mol. Dev. Evol. 2009;312:42–57. doi: 10.1002/jez.b.21241. [DOI] [PubMed] [Google Scholar]
- 59.Greenwold MJ, Sawyer RH. Linking the molecular evolution of avian beta (β) keratins to the evolution of feathers. J. Exp. Zool. B Mol. Dev. Evol. 2011;316:609–16. doi: 10.1002/jez.b.21436. [DOI] [PubMed] [Google Scholar]
- 60.Lin SJ, Foley J, Jiang TX, Yeh CY, Wu P, et al. Topology of feather melanocyte progenitor niche allows complex pigment patterns to emerge. Science. 2013;340:1442–45. doi: 10.1126/science.1230374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris MP, Williamson S, Fallon JF, Meinhardt H, Prum RO. Molecular evidence foran activator-inhibitor mechanism in development of embryonic feather branching. PNAS. 2005;102:11734–39. doi: 10.1073/pnas.0500781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prum RO, Williamson S. Reaction-diffusion models of within-feather pigmentation patterning. Proc. Biol. Sci. 2002;269:781–92. doi: 10.1098/rspb.2001.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prum RO, Dyck J. A hierarchical model of plumage: morphology, development, and evolution. J. Exp. Zool. B Mol. Dev. Evol. 2003;298:73–90. doi: 10.1002/jez.b.27. [DOI] [PubMed] [Google Scholar]
- 64.Abbott UK, Asmundson VS. Scaleless, an inherited ectodermal defect in the domestic fowl. J. Hered. 1957;48:63–70. [Google Scholar]
- 65.Wells KL, Hadad Y, Ben-Avraham D, Hillel J, Cahaner A, Headon DJ. Genome-wide SNP scan of pooled DNA reveals nonsense mutation in FGF20 in the scaleless line of featherless chickens. BMC Genomics. 2012;13:257. doi: 10.1186/1471-2164-13-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Somes RG., Jr . Mutations and major variant of plumage and skin in chickens. In: Crawford R, editor. Poultry Breeding and Genetics. Amsterdam: Elsevier; 1990. pp. 169–208. [Google Scholar]
- 67.Mou C, Pitel F, Gourichon D, Vignoles F, Tzika A, et al. Cryptic patterning of avian skin confers a developmental facility for loss of neck feathering. PLOS Biol. 2011;9:e1001028. doi: 10.1371/journal.pbio.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pitel F, Bergé R, Coquerelle G, Crooijmans RP, Groenen MA, et al. Mapping the naked neck (NA) and polydactyly (PO) mutants of the chicken with microsatellite molecular markers. Genet. Sel. Evol. 2000;32:73–86. doi: 10.1186/1297-9686-32-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng CS, Wu P, Foley J, Foley A, McDonald ML, et al. The chicken frizzle feather is due to an a-keratin (KRT75) mutation that causes a defective rachis. PLOS Genet. 2012;8:e1002748. doi: 10.1371/journal.pgen.1002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Landauer W, Dunn LC. The frizzle character of fowls. J. Hered. 1930;21:291–305. [Google Scholar]
- 71.Hutt F. The inheritance of frizzled plumage. J. Genet. 1930;22:128–29. [Google Scholar]
- 72.Winter H, Schissel D, Parry DA, Smith TA, Liovic M, et al. An unusual Ala12Thr polymorphism in the 1A a-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J. Investig. Dermatol. 2004;122:652–57. doi: 10.1111/j.0022-202X.2004.22309.x. [DOI] [PubMed] [Google Scholar]
- 73.Starck JM, Ricklefs RE. Avian Growth and Development: Evolution Within the Altricial-Precocial Spectrum. Oxford, UK: Oxford Univ. Press; 1998. [Google Scholar]
- 74.Kuenzel WJ. Neurobiology of molt in avian species. Poult. Sci. 2003;82:981–91. doi: 10.1093/ps/82.6.981. [DOI] [PubMed] [Google Scholar]
- 75.Shaffner CS. Feather papilla stimulation by progesterone. Science. 1954;120:345. doi: 10.1126/science.120.3113.345. [DOI] [PubMed] [Google Scholar]
- 76.Shaffner CS. Progesterone induced molt. Poult. Sci. 1955;34:840–42. [Google Scholar]
- 77.Juhn M, Harris PC. Local effects on the feather papilla of thyroxine and of progesterone. Proc. Soc. Exp. Biol. Med. 1955;90:202–4. doi: 10.3181/00379727-90-21982. [DOI] [PubMed] [Google Scholar]
- 78.Vézina F, Gustowska A, Jalvingh KM, Chastel O, Piersma T. Hormonal correlates and thermoregulatory consequences of molting on metabolic rate in a Northerly Wintering Shorebird. Physiol. Biochem. Zool. 2009;82:129–42. doi: 10.1086/596512. [DOI] [PubMed] [Google Scholar]
- 79.Dawson A, Goldsmith AR. Plasma prolactin and gonadotrophins during gonadal development and the onset of photorefractoriness in male and female starlings (Sturnus vulgaris) on artificial photoperiods. J. Endocrinol. 1983;97:253–60. doi: 10.1677/joe.0.0970253. [DOI] [PubMed] [Google Scholar]
- 80.Nolan V, Jr, Ketterson ED. Effect of long days on molt and autumn migratory state of site-faithful juncos held at their winter sites. Wilson Bull. 1990;102:469–79. [Google Scholar]
- 81.Ikegami K, Yoshimura T. Circadian clocks and the measurement of daylength in seasonal reproduction. Mol. Cell. Endocrinol. 2012;349:76–81. doi: 10.1016/j.mce.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 82.Péczely P, Bogenfürst F, Kulcsár M, Polgár B. Role of gonadal and adrenal steroids and thyroid hormones in the regulation of molting in domestic goose. Acta Biol. Hung. 2011;62:1–21. doi: 10.1556/ABiol.61.2011.1.1. [DOI] [PubMed] [Google Scholar]
- 83.Romero LM, Soma KK, Wingfield JC. Hypothalamic-pituitary-adrenal axis changes allow seasonal modulation of corticosterone in a bird. Am. J. Physiol. 1998;274:R1338–44. doi: 10.1152/ajpregu.1998.274.5.R1338. [DOI] [PubMed] [Google Scholar]
- 84.Yoshimura T. Thyroid hormone and seasonal regulation of reproduction. Front. Neuroendocrinol. 2013;34:157–66. doi: 10.1016/j.yfrne.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 85.Done T, Gow EA, Stutchbury BJM. Corticosterone stress response and plasma metabolite levels during breeding and molt in a free-living migratory songbird, the wood thrush (Hylocichla mustelina) Gen. Comp. Endocrinol. 2011;171:176–82. doi: 10.1016/j.ygcen.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Hurley LL, Wallace AM, Sartor JJ, Ball GF. Photoperiodic induced changes in reproductive state of border canaries (Serinus canaria) are associated with marked variation in hypothalamic gonadotropin-releasing hormone immunoreactivity and the volume of song control regions. Gen. Comp. Endocrinol. 2008;158:10–19. doi: 10.1016/j.ygcen.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin LB. Trade-offs between molt and immune activity in two populations of house sparrows (Passer domesticus) Can. J. Zool. 2005;83:780–87. [Google Scholar]
- 88.Bacon WL, Long DW. Secretion of luteinizing hormone during a forced molt in turkey hens. Poult. Sci. 1996;75:1579–86. doi: 10.3382/ps.0751579. [DOI] [PubMed] [Google Scholar]
- 89.Braw-Tal R, Yossefi S, Pen S, Shinder D, Bar A. Hormonal changes associated with ageing and induced moulting of domestic hens. Br. Poult. Sci. 2004;45:815–22. doi: 10.1080/00071660400012782. [DOI] [PubMed] [Google Scholar]
- 90.Jacquet JM, Seigneurin F, De Reviers M. Induced moulting in cockerels: effects on sperm production, plasma concentrations of luteinising hormone, testosterone and thyroxine, and on pituitary sensitivity to luteinising hormone-releasing hormone. Br. Poult. Sci. 1993;34:765–75. doi: 10.1080/00071669308417635. [DOI] [PubMed] [Google Scholar]
- 91.Nolan VJ, Ketterson ED, Ziegenfus C, Cullen DP, Chandler CR. Testosterone and avian life histories: effects of experimentally elevated testosterone on prebasic molt and survival in male dark-eyed juncos. Condor. 1992;94:364–70. [Google Scholar]
- 92.Schleussner G, Dittami JP, Gwinner E. Testosterone implants affect molt in male European starlings, Sturnus vulgaris. Physiol. Zool. 1985;585:597–604. [Google Scholar]
- 93.Dickerman RW, Bahr JM. Molt induced by gonadotropin-releasing hormone agonist as model for studying endocrine mechanisms of molting in laying hens. Poult. Sci. 1989;68:1402–8. doi: 10.3382/ps.0681402. [DOI] [PubMed] [Google Scholar]
- 94.Sandhu MA, Rahman ZU, Riaz A, Rahman SU, Javed I, Ullah N. Somatotrophs and lactotrophs: an immunohistochemical study of Gallus domesticus pituitary gland at different stages of induced moult. Eur. J. Histochem. 2010;54:e25. doi: 10.4081/ejh.2010.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Romero LM. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. [DOI] [PubMed] [Google Scholar]
- 96.Lattin CR, Bauer CM, de Bruijn R, Romero LM. Hypothalamus-pituitary-adrenal axis activity and the subsequent response to chronic stress differ depending upon life history stage. Gen. Comp. Endocrinol. 2012;178:494–501. doi: 10.1016/j.ygcen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 97.DesRochers DW, Reed JM, Awerman J, Kluge JA, Wilkinson J, et al. Exogenous and endogenous corticosterone alter feather quality. Comp. Biochem. Physiol. A. 2009;152:46–52. doi: 10.1016/j.cbpa.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 98.Péczely P, Pethes G. Effect of ovariectomy, thyroidectomy and of thyroxine treatment on the plasma level of corticosterone of the female Japanese quail. Acta Biol. Acad. Sci. Hung. 1981;32:1–6. [PubMed] [Google Scholar]
- 99.Shinomiya A, Shimmura T, Nishiwaki-Ohkawa T, Yoshimura T. Regulation of seasonal reproduction by hypothalamic activation of thyroid hormone. Front. Endocrinol. 2014;5:12. doi: 10.3389/fendo.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J. Biol. Rhythms. 2001;16:365–80. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- 101.Mayer JA, Chuong CM, Widelitz R. Rooster feathering, androgenic alopecia, and hormone-dependent tumor growth: What is in common? Differentiation. 2004;72:474–88. doi: 10.1111/j.1432-0436.2004.07209003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andersson MB. Sexual Selection. Princeton, NJ: Princeton Univ. Press; 2010. [Google Scholar]
- 103.Aparicio JM, Bonal R, Cordero PJ. Evolution of the structure of tail feathers: implications for the theory of sexual selection. Evolution. 2003;57:397–405. doi: 10.1111/j.0014-3820.2003.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 104.McGlothlin JW, Jawor JM, Greives TJ, Casto JM, Phillips JL, Ketterson ED. Hormones and honest signals: Males with larger ornaments elevate testosterone more when challenged. J. Evol. Biol. 2008;21:39–48. doi: 10.1111/j.1420-9101.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- 105.Kimball RT, Ligon JD. Evolution of avian plumage dichromatism from a proximate perspective. Am. Nat. 1999;154:182–93. [Google Scholar]
- 106.Mueller NS. An experimental study of sexual dichromatism in the duck Anas platyrhynchos. J. Exp. Zool. 1970;173:263–68. [Google Scholar]
- 107.Keck WN. The control of the secondary sex characters in the English sparrow Passer domesticus (Linnaeus) J. Exp. Zool. 1934;67:315–41. [Google Scholar]
- 108.Saino N, Romano M, Rubolini D, Teplitsky C, Ambrosini R, et al. Sexual dimorphism in melanin pigmentation, feather coloration and its heritability in the barn swallow (Hirundo rustica) PLOS ONE. 2013;8:e58024. doi: 10.1371/journal.pone.0058024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lingham-Soliar T, Feduccia A, Wang X. A new Chinese specimen indicates that ‘protofeathers’ in the Early Cretaceous theropod dinosaur Sinosauropteryx are degraded collagen fibres. Proc. Biol. Sci. 2007;274:1823–29. doi: 10.1098/rspb.2007.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chuong CM, Wu P, Zhang FC, Xu X, Yu M, et al. Adaptation to the sky: defining the feather with integument fossils from mesozoic China and experimental evidence from molecular laboratories. J. Exp. Zool. B Mol. Dev. Evol. 2003;298:42–56. doi: 10.1002/jez.b.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mayr G, Peters DS, Plodowski G, Vogel O. Bristle-like integumentary structures at the tail of the horned dinosaur. Psittacosaurus Naturwissenschaften. 2002;89:361–65. doi: 10.1007/s00114-002-0339-6. [DOI] [PubMed] [Google Scholar]
- 112.Chen P, Dong Z, Zhen S. An exceptionally well-preserved theropod dinosaur from the Yixian Formation of China. Nature. 1998;391:147–52. [Google Scholar]
- 113.Xu X, Zheng X, You H. A new feather type in a nonavian theropod and the early evolution of feathers. PNAS. 2009;106:832–34. doi: 10.1073/pnas.0810055106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang F, Zhou Z, Xu X, Wang X, Sullivan C. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature. 2008;455:1105–8. doi: 10.1038/nature07447. [DOI] [PubMed] [Google Scholar]
- 115.Xu X, Zhou Z, Prum RO. Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature. 2001;410:200–4. doi: 10.1038/35065589. [DOI] [PubMed] [Google Scholar]
- 116.Xu X, Tang Z, Wang X. A therizinosauroid dinosaur with integumentary structures from China. Nature. 1999;399:350–54. [Google Scholar]
- 117.Qiang J, Currie PJ, Norell MA, Shu-An J. Two feathered dinosaurs from northeastern China. Nature. 1998;393:753–61. [Google Scholar]
- 118.Xu X, Norell MA, Kuang X, Wang X, Zhao Q, Jia C. Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids. Nature. 2004;431:680–84. doi: 10.1038/nature02855. [DOI] [PubMed] [Google Scholar]
- 119.Schweitzer MH, Watt JA, Avci R, Knapp L, Chiappe L, et al. Beta-keratin specific immunological reactivity in feather-like structures of the Cretaceous Alvarezsaurid, Shuvuuia deserti. J. Exp. Zool. 1999;285:146–57. [PubMed] [Google Scholar]
- 120.Xu X, Wang K, Zhang K, Ma Q, Xing L, et al. A gigantic feathered dinosaur from the lower cretaceous of China. Nature. 2012;484:92–95. doi: 10.1038/nature10906. [DOI] [PubMed] [Google Scholar]
- 121.Xu X, Zhang F. A new maniraptoran dinosaur from China with long feathers on the metatarsus. Naturwissenschaften. 2005;92:173–77. doi: 10.1007/s00114-004-0604-y. [DOI] [PubMed] [Google Scholar]
- 122.Hu D, Hou L, Zhang L, Xu X. A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature. 2009;461:640–43. doi: 10.1038/nature08322. [DOI] [PubMed] [Google Scholar]
- 123.Xu X, You H, Du K, Han F. An Archaeopteryx-like theropod from China and the origin of Avialae. Nature. 2011;475:465–70. doi: 10.1038/nature10288. [DOI] [PubMed] [Google Scholar]
- 124.Zelenitsky DK, Therrien F, Erickson GM, DeBuhr CL, Kobayashi Y, et al. Feathered non-avian dinosaurs from North America provide insight into wing origins. Science. 2012;338:510–14. doi: 10.1126/science.1225376. [DOI] [PubMed] [Google Scholar]
- 125.Li Q, Gao KQ, Meng Q, Clarke JA, Shawkey MD, et al. Reconstruction of Microraptor and the evolution of iridescent plumage. Science. 2012;335:1215–19. doi: 10.1126/science.1213780. [DOI] [PubMed] [Google Scholar]
- 126.Xu X, Zhou Z, Wang X, Kuang X, Zhang F, Du X. Four-winged dinosaurs from China. Nature. 2003;421:335–40. doi: 10.1038/nature01342. [DOI] [PubMed] [Google Scholar]
- 127.Carney RM, Vinther J, Shawkey MD, D’Alba L, Ackermann J. New evidence on the colour and nature of the isolated Archaeopteryx feather. Nat. Commun. 2012;3:637. doi: 10.1038/ncomms1642. [DOI] [PubMed] [Google Scholar]
- 128.Feduccia A, Tordoff HB. Feathers of Archaeopteryx: Asymmetric vanes indicate aerodynamic function. Science. 1979;4384:1021–22. doi: 10.1126/science.203.4384.1021. [DOI] [PubMed] [Google Scholar]
- 129.Xu X, Zhou Z, Wang X. The smallest known non-avian theropod dinosaur. Nature. 2000;408:705–8. doi: 10.1038/35047056. [DOI] [PubMed] [Google Scholar]
- 130.Chiappe LM. Glorified Dinosaurs: The Origin and Early Evolution of Birds. Hoboken, NJ: John Wiley; 2007. [Google Scholar]
- 131.Chu Q, Cai L, Fu Y, Chen X, Yan Z, et al. Dkk2/Frzb in the dermal papillae regulates feather regeneration. Dev. Biol. 2014;2:167–78. doi: 10.1016/j.ydbio.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chuong CM, Randall VA, Widelitz RB, Wu P, Jiang TX. Physiological regeneration of skin appendages and implications for regenerative medicine. Physiology. 2012;27:61–72. doi: 10.1152/physiol.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chuong CM, Yeh CY, Jiang TX, Widelitz R. Module-based complexity formation: periodic patterning in feathers and hairs. Wiley Interdiscip. Rev. Dev. Biol. 2013;2:97–112. doi: 10.1002/wdev.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dorshorst B, Okimoto R, Ashwell C. Genomic regions associated with dermal hyperpigmentation, polydactyly and other morphological traits in the Silkie chicken. J. Hered. 2010;101:339–50. doi: 10.1093/jhered/esp120. [DOI] [PubMed] [Google Scholar]
- 135.Bitgood JJ, Dochnahl J, Schlafly P, Briles RW, Briles WE. A study of linkage relationships of blood group P with naked neck, silkie feathering, and recessive white in chicken. Poult. Sci. 1984;63:592–94. doi: 10.3382/ps.0630592. [DOI] [PubMed] [Google Scholar]
- 136.Fan WL, Ng CS, Chen CF, Lu MY, Chen YH, et al. Genome-wide patterns of genetic variation in two domestic chickens. Genome Biol. Evol. 2013;5:1376–92. doi: 10.1093/gbe/evt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Feng C, Gao Y, Dorshorst B, Song C, Gu X, et al. A cis-regulatory mutation of PDSS2 causes silky-feather in chickens. PLOS Genet. 2014;10(8):e1004576. doi: 10.1371/journal.pgen.1004576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y, Gao Y, Imsland F, Gu X, Feng C, et al. The crest phenotype in chicken is associated with ectopic expression of HOXC8 in cranial skin. PLOS ONE. 2012;7:e34012. doi: 10.1371/journal.pone.0034012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Somes RG., Jr Identifying the ptilopody (feathered shank) loci of the chicken. J. Hered. 1992;83:230–34. doi: 10.1093/oxfordjournals.jhered.a111200. [DOI] [PubMed] [Google Scholar]
- 140.Bacon LD, Smith E, Crittenden LB, Havenstein GB. Association of the slow feathering (K) and an endogenous viral (ev21) gene on the Z chromosome of chickens. Poult. Sci. 1988;67:191–97. doi: 10.3382/ps.0670191. [DOI] [PubMed] [Google Scholar]
- 141.Elferink MG, Vallee AA, Jungerius AP, Crooijmans RP, Groenen MA. Partial duplication of the PRLR and SPEF2 genes at the late feathering locus in chicken. BMC Genomics. 2008;9:391. doi: 10.1186/1471-2164-9-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Luo C, Shen X, Rao Y, Xu H, Tang J, et al. Differences of Z chromosome and genomic expression between early- and late-feathering chickens. Mol. Biol. Rep. 2012;39:6283–88. doi: 10.1007/s11033-012-1449-7. [DOI] [PubMed] [Google Scholar]
- 143.Kerje S, Sharma P, Gunnarsson U, Kim H, Bagchi S, et al. The Dominant white, Dun and Smoky color variants in chicken are associated with insertion/deletion polymorphisms in the PMEL17 gene. Genetics. 2004;168:1507–18. doi: 10.1534/genetics.104.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chang CM, Coville JL, Coquerelle G, Gourichon D, Oulmouden A, Tixier-Boichard M. Complete association between a retroviral insertion in the tyrosinase gene and the recessive white mutation in chickens. BMC Genomics. 2006;7:19. doi: 10.1186/1471-2164-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chang CM, Furet JP, Coville JL, Coquerelle G, Gourichon D, Tixier-Boichard M. Quantitative effects of an intronic retroviral insertion on the transcription of the tyrosinase gene in recessive white chickens. Anim. Genet. 2007;38:162–67. doi: 10.1111/j.1365-2052.2007.01581.x. [DOI] [PubMed] [Google Scholar]
- 146.Kerje S, Lind J, Schutz K, Jensen P, Andersson L. Melanocortin 1-receptor (MC1R) mutations are associated with plumage colour in chicken. Anim. Genet. 2003;34:241–48. doi: 10.1046/j.1365-2052.2003.00991.x. [DOI] [PubMed] [Google Scholar]
- 147.Smyth JR. In: Genetics of plumage, skin and pigmentation in chickens. In Poultry Breeding and Genetics. Crawford RD, editor. New York: Elsevier Sci; 1996. pp. 109–67. [Google Scholar]
- 148.Gunnarsson U, Hellström AR, Tixier-Boichard M, Minvielle F, Bed’hom B, et al. Mutations in SLC45A2 cause plumage color variation in chicken and Japanese quail. Genetics. 2007;175:867–77. doi: 10.1534/genetics.106.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hellström AR, Sundström E, Gunnarsson U, Bed’Hom B, Tixier-Boichard M, et al. Sex-linked barring in chickens is controlled by the CDKN2A/B tumour suppressor locus. Pigment Cell Melanoma Res. 2010;23:521–30. doi: 10.1111/j.1755-148X.2010.00700.x. [DOI] [PubMed] [Google Scholar]
- 150.Moore JW, Smyth JR. Genetic factors associated with the plumage pattern of the barred Fayoumi. Poult. Sci. 1972;51:1149–56. doi: 10.3382/ps.0511149. [DOI] [PubMed] [Google Scholar]
- 151.Gunnarsson U, Kerje S, Bed’hom B, Sahlqvist AS, Ekwall O, et al. The Dark brown plumage color in chickens is caused by an 8.3-kb deletion upstream of SOX10. Pigment Cell Melanoma Res. 2011;24:268–74. doi: 10.1111/j.1755-148X.2011.00825.x. [DOI] [PubMed] [Google Scholar]
- 152.Widelitz RB, Veltmaat JM, Mayer JA, Foley J, Chuong CM. Mammary glands and feathers: comparing two skin appendages which help define novel classes during vertebrate evolution. Semin. Cell Dev. Biol. 2007;18:255–66. doi: 10.1016/j.semcdb.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zheng XT, You HL, Xu X, Dong ZM. An Early Cretaceous heterodontosaurid dinosaur with filamentous integumentary structures. Nature. 2009;458:333–36. doi: 10.1038/nature07856. [DOI] [PubMed] [Google Scholar]
- 154.Wu P, Hou L, Plikus M, Hughes M, Scehnet J, et al. Evo-Devo of amniote integuments and appendages. Int. J. Dev. Biol. 2004;48:249–70. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]