Abstract
Objective
To test the association between sleep duration and telomere length in a pediatric population.
Study design
We analyzed cross-sectional data for 1,567children from the age 9 study wave of the Fragile Families and Child Wellbeing Study, a population-based birth cohort of children born between 1998–2000 in large American cities (population > 200,000). We measured telomere length using quantitative PCR, and children’s typical nightly sleep duration was reported by their primary caregivers. Using linear regression models, we estimated the association between sleep duration and telomere length in both unadjusted models and adjusting for a number of covariates.
Results
We found that children with shorter sleep durations have shorter telomeres than children with longer sleep durations. Each hour less of nightly sleep duration is associated with having telomeres that are 0.015 log-kilobases per chromosome shorter (p < 0.05). We found no difference in this association by race, sex, or socioeconomic status.
Conclusions
We provide preliminary evidence that children with shorter sleep durations have shorter telomeres. This finding is consistent with a broader literature indicating that sub-optimal sleep duration is a risk for increased physiological stress and impaired health. Future research should address the limitations of our study design by using longitudinal study designs and telomere measurements, measuring sleep duration via polysomnography or actigraphy, and assessing the intermediate biological mechanisms of the link between sleep and telomere dynamics.
Keywords: biomarker, birth cohort, Fragile Families and Child Wellbeing Study, 9-year-old
Inadequate nightly sleep duration is linked to morbidities(1–3) and mortality(4–6), as well as a number of physiological sequelae including inflammation, oxidative stress, increased sympathetic tone, and neuroendocrine dysregulation (7). Though little of this research has considered pediatric populations, short sleep duration in childhood is associated with changes in hypothalamic-pituitary- adrenocortical system activity(8), autononomic nervous system activity (9), and metabolic regulation(10). This type of stress-altered HPA activity is known to correlate with telomere length(11,12). Telomeres are repetitive DNA sequences (in humans, TTAGGGn) and associated proteins that cap the end of chromosomes. With each cycle of chromosomal replication and cellular division, the telomere becomes shorter, except in specialized cells expressing telomerase(13). This means that telomere length gradually decreases with age in most cells, though the rate of telomere attrition varies across humans in part because of between-person differences in HPA activation, which is associated with shorter telomere length(11,12). Thus, distal social or experiential predictors of physiological stress – i.e. difficult life experiences(14–16) – may also be associated with an accelerated rate of telomere attrition. Identifying factors that predict telomere length or attrition disparities may indicate opportunities for clinical intervention even if the biological mechanisms by which the experience becomes associated with telomere length are unknown. Telomere length may be a particularly useful biomarker when assessing health in pediatric populations, because disparities in telomere length emerge prior to the manifestation of chronic health conditions in adulthood. In adults, shorter sleep duration has been shown to be one of these adverse life experiences; shorter sleep duration is associated with shorter telomere length in adults(17–21). However, no study has tested whether the same association is found in children. Therefore, we used DNA collected from a diverse, population-based sample of 1,567 9-year-old American children born between 1998– 2000 to assess the association between children’s typical nightly sleep duration (reported by mothers) and telomere length. We hypothesized that children who have shorter nightly sleep durations would have shorter telomeres than their peers who sleep for more hours each night.
Methods
We analyzed cross-sectional data from 1,567 children in the age 9 study wave of the Fragile Families and Child Wellbeing Study, a population-based birth cohort of children born between 1998–2000 in large American cities (population > 200,000). Interviews with mothers and fathers were conducted at child’s birth and again when the child was ages 1, 3, 5, and 9. At age 9, the child’s primary caregiver (a parent or other adult) also provided information about the child. Because only 13 of the children in our analytic sample had primary caregivers who were not their mothers, for brevity we refer to data from these primary caregivers as being mother- reported. DNA from saliva was collected from mothers and children when children were age 9, during an assessment in the home. The Institutional Review Boards of Princeton University and Columbia University approved data collection. Of the 2,482 children for whom telomere length is available, our analysis is based on a complete case analysis of the 1,567 children who also had data on all necessary predictors. Neither telomere length nor sleep duration differed significantly between included and excluded cases.
Measures
Telomere length
We measured telomere length using saliva provided when the child was approximately 9 years old. Prior research using this data has described in detail how absolute telomere length is measured using a quantitative real-time PCR assay(22). In brief, samples were measured in triplicate and averaged. Reference DNA samples derived from cell lines not expressing or expressing telomerase were included in each plate, and to minimize plate-to-plate variation telomere length was normalized using the geometric mean of these reference DNA samples. The coefficient of variation of a standard was 11%. These analyses exclude the 1% tails of shortest and longest telomeres, as extreme values could represent mismeasurement or oral pathology. Telomere length was log-transformed to approximate a normal distribution. Mother’s telomere length, used as a covariate, was measured and logged in the same manner. This approach to measuring telomere length in buccal cells has been compared directly with telomere length measures from peripheral mononuclear blood cells (PMBC). Although buccal cell telomere lengths are longer than PMBC telomere lengths, telomere length in the two cell types is highly correlated (22). Telomeres shorten at equivalent rates across a range of tissues(23).
Sleep duration
The predictor of interest is the child’s average nightly sleep duration, which we treated as a continuous variable. Mothers reported how many hours their child typically slept per night during the week. In the analytic sample, hours of sleep at age 9 ranges from 4 to 14. To adjust for implausible sleep durations, we removed the handful of cases with very short (less than 6 hours, N = 2) or long (more than 12 hours, N = 5) nightly sleep durations using listwise deletion, an approach similar to prior research using this data(24).
Covariates
Our analysis accounted for a wide range of factors known to be associated with either children’s telomere lengths or children’s nightly sleep duration(25). Child characteristics used as covariates included the age in months, low birth weight (< 2,500 grams), and sex (recorded at birth). Pubertal development at age 9 was reported by their mothers in a series of questions on the child’s experience of five physical puberty changes: a height growth spurt, hair growth, skin changes, a deepening voice (boys), facial hair growth (boys), breast growth (girls), or menarche (girls). Mothers indicated the extent to which the child had begun to experience each of these changes with response categories of “No” (coded 1), “Yes, barely” (coded 2), “Yes, definitely” (coded 3), or “Development is completed” (coded 4); for menarche, responses were “No” (coded 1) and “Yes” (coded 4). The responses to all of these questions were averaged to compute a pubertal development score ranging from 1 to 4. The child’s BMI was computed from weight and height measurements taken by the interviewer during the home visit portion of the age 9 interview. Finally, mothers reported if the child had ever been diagnosed with ADHD/ADD or autism.
Additional covariates included characteristics of the child’s parents. Mother’s race/ethnicity, nativity, age, educational attainment, household income, relationship with the child’s biological father, and household composition were assessed at the time of the child’s birth. Mother’s race/ethnicity was self-reported and included categories for white (non-Hispanic), black (non-Hispanic), Hispanic, and some other race/ethnicity. Nativity measures whether or not the mother was born in the United States. Mother’s age and father’s age were self-reported. Maternal education is a categorical variable for less than high school education, high school diploma or GED, some college education, and a college graduate or higher. Household income was reported by the mother. Additionally, mothers were asked at the child’s birth if they were married to or cohabiting with the child’s biological father and how many other children already lived in their home. We also included the mother’s telomere length (measured at the 9-year interview) as a covariate, in order to account for a portion of the sizable heredity in telomere length as well as to capture the effects the environments in which both mothers and children were embedded in the child’s first nine years of life. Finally, to account for the city-based clustering of the baseline sample, we included a series of dummy variables indicating the city in which the child was born and clustered standard errors by sample city.
Statistical Analyses
Using unadjusted and adjusted linear regression models, we examined to what extent children’s sleep durations were associated with their telomere lengths. Model 1 assessed the unadjusted association between sleep duration and log-telomere length. Model 2 adjusted this estimate for child’s age at the 9-year interview as well as parental ages at the child’s birth. Model 3 added a set of covariates including mother’s log-telomere length, child and family characteristics, and the city in which the child was born. We also conducted sensitivity analyses to assess whether race, sex, or socioeconomic status moderated these associations and whether there was a nonlinear association between sleep duration and telomere length. All analyses were conducted using R(26), and we considered results with p < .05 to be statistically significant.
Results
The characteristics of the analytic sample are described in Table I, and the distribution of hours of sleep is shown in the Figure.
Table 1.
Analytic sample description (N = 1,567)
| Variable | Mean (SD) | N | Percent |
|---|---|---|---|
| Child’s telomere length (logged)a | 2.03 (0.31) | ||
| Hours of sleep – age 9a | 8.97 (1.08) | ||
| Child’s age in monthsa | 112.14 (4.31) | ||
| Child low birth weight (< 2,500 grams)b | 140 | 8.9% | |
| Child maleb | 821 | 52.4% | |
| Child’s puberty progressiona | 1.45 (0.35) | ||
| Child’s BMIa | 19.24 (4.97) | ||
| Child ever diagnosed with ADHD/ADDa | 164 | 10.5% | |
| Child ever diagnosed with autisma | 11 | 0.7% | |
| Mother’s ageb | 25.03 (5.99) | ||
| Father’s ageb | 27.54 (7.17) | ||
| Mother’s telomere length (logged)a | 1.85 (0.30) | ||
| Mother non-Hispanic whiteb | 357 | 22.7% | |
| Mother non-Hispanic blackb | 711 | 45.4% | |
| Mother Hispanicb | 444 | 28.3% | |
| Mother other race/ethnicityb | 55 | 3.5% | |
| Mother foreign bornb | 243 | 15.5% | |
| Mother has less than high school educationb | 487 | 31.1% | |
| Mother has high school diploma or GEDb | 496 | 31.7% | |
| Mother attended some collegeb | 397 | 25.3% | |
| Mother is a college graduateb | 187 | 11.9% | |
| Mother’s household incomeb | $35,276.42 ($34,005.08) | ||
| Biological parents have no relationshipb | 474 | 30.2% | |
| Biological parents cohabitingb | 648 | 41.4% | |
| Biological parents marriedb | 445 | 28.4% | |
| Number of children in homeb | 1.19 (1.25) |
Measured at age 9.
Measured at child’s birth.
Figure 1.
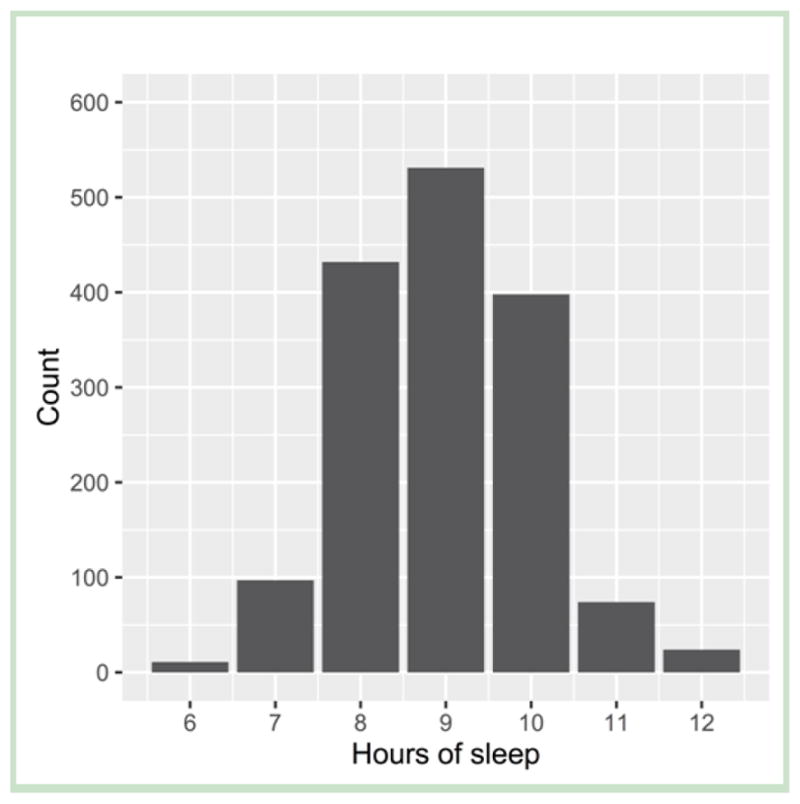
Distribution of children’s hours of sleep at age 9 in the analytic sample (N=1,567)
Children who sleep for fewer hours each night have shorter telomeres at age 9 than children who sleep for more hours (Tables II and III; Table III available at www.jpeds.com). The unadjusted association between children’s sleep duration and telomere length is statistically significant (p < 0.01) (Model 1). Adjusting for child’s age at the time of telomere assay and parents’ ages at the child’s birth slightly attenuates the magnitude of the association of nightly sleep duration with telomere length, but the association remains statistically significant (p < 0.01) (Model 2). Finally, when covariates for a range of child and family characteristics as well as the child’s city of birth are included in the model, the association of nightly sleep duration with the child’s telomere length remains statistically significant (p < 0.05) (Model 3). As shown by the coefficient for hours of sleep in Model 3, children who sleep for one hour less per night than their comparable peers have, on average, a telomere length that is 0.015 log-kilobases per chromosome (exponentiated, 1.015 kilobases per chromosome) shorter; stated differently, one less hour of sleep per night is associated with a telomere that is shorter by the same magnitude. By comparison, after taking into account sleep duration and other covariates, children in our sample born to parents in no relationship – a known adverse life experience – have telomeres that are 0.059 log-kilobases per chromosome (exponentiated, 1.06 kilobases per chromosome) shorter than children who were born to married parents (Table III).
Table 2.
Selected coefficients and confidence intervals from linear regression models of sleep duration and log-transformed telomere length (N=1,567)
| (1) Unadjusted | (2) Age-adjusted | (3) All covariates | |
|---|---|---|---|
|
|
|||
| Hours of sleep | 0.026** (0.011, 0.042) | 0.022** (0.007, 0.037) | 0.015* (0.002, 0.029) |
| Child’s age in months | ✓ | ✓ | |
| Mother’s age at child’s birth | ✓ | ✓ | |
| Father’s age at child’s birth | ✓ | ✓ | |
| Child and family characteristicsb | ✓ | ||
| City in which the child was born | ✓ | ||
|
| |||
| R2 | 0.008 | 0.020 | 0.141 |
+p<0.10; *p<0.05; **p<0.01; ***p<0.001
These analyses account for the child’s age in months at age 9, whether the child was low birth weight (< 2,500 grams), child’s sex, child’s pubertal progression score, child’s BMI, whether the child was ever diagnosed with ADHD/ADD, whether the child was ever diagnosed with autism, mother’s logged telomere length, mother’s race/ethnicity, mother’s nativity, mother’s educational attainment at the child’s birth, logged maternal household income at the child’s birth, whether biological parents were married or cohabiting at the child’s birth, and the number of children in the home at the child’s birth.
Table 3.
(Online). Coefficients and confidence intervals from linear regression models of sleep duration and log-transformed telomere length (N=1,567)
| (1) Unadjusted | (2) Age-adjusted | (3) All covariates | |
|---|---|---|---|
|
|
|||
| Intercept | 1.796*** 1.657, 1.936) | 1.755*** (1.261, 2.250) | 1.569*** (1.108, 2.031) |
| Hours of sleep | 0.026** (0.011, 0.042) | 0.022** (0.007, 0.037) | 0.015* (0.002, 0.029) |
| Child’s age in months | −0.001 (−0.005, 0.004) | −0.003+ (−0.007, 0.0002) | |
| Mother’s age at child’s birth | 0.002 (−0.001, 0.005) | −0.001 (−0.004, 0.003) | |
| Father’s age at child’s birth | 0.004+ (−0.001, 0.008) | 0.003 (−0.001, 0.007) | |
| Child low birth weight (ref: >2,500 grams) | −0.012 (−0.063, 0.039) | ||
| Child male (ref: female) | −0.014 (−0.040, 0.011) | ||
| Child’s puberty score | 0.015 (−0.040, 0.070) | ||
| Child’s BMI | 0.001 (−0.002, 0.004) | ||
| Child ever diagnosed with ADHD/ADD | −0.014 (−0.064, 0.036) | ||
| Child ever diagnosed with autism | 0.062 (−0.121, 0.246) | ||
| Mother’s telomere length (logged) | 0.324*** (0.273, 0.376) | ||
| Mother non-Hispanic black (ref: non-Hispanic white) | 0.001 (−0.058, 0.061) | ||
| Mother Hispanic (ref: non-Hispanic white) | −0.014 (−0.064, 0.035) | ||
| Mother other race/ethnicity (ref: non-Hispanic white) | −0.052 (−0.133, 0.029) | ||
| Mother foreign born | 0.071*** (0.046, 0.096) | ||
| Mother has high school diploma or GED (ref: less than high school education) | 0.002 (−0.032, 0.037) | ||
| Mother attended some college (ref: less than high school education) | 0.025 (−0.024, 0.075) | ||
| Mother is a college graduate (ref: less than high school education) | 0.048 (−0.017, 0.113) | ||
| Log of household income | −0.001 (−0.016, 0.014) | ||
| Biological parents married (ref: no relationship) | 0.059** (0.017, 0.100) | ||
| Biological parents cohabiting (ref: no relationship) | 0.006 (−0.029, 0.042) | ||
| Number of children in home | 0.003 (−0.012, 0.017) | ||
| City fixed effects | ✓ | ||
|
| |||
| R2 | 0.008 | 0.020 | 0.141 |
+p<0.10; *p<0.05; **p<0.01; ***p<0.001
Although we show results from models of log-telomere length because they are a slightly better fit to the data than when telomere length is not logged, shorter sleep durations are also associated with significantly shorter telomere lengths when telomere length is not logged. Our conclusions are not changed by the sensitivity analyses.
Discussion
Building on evidence linking nightly sleep duration to a range of health outcomes across the life course, as well as a nascent literature linking sleep to telomere length in adults, we examined the association between children’s nightly sleep duration (reported by their mothers) and telomere length. We found that children who sleep for fewer hours each night have shorter telomeres than their peers who sleep for more hours each night, both in an unadjusted model and after accounting for a wide range of covariates.
Based on prior research, we also conducted four sensitivity analyses examining whether our results varied by race, sex, or socioeconomic status of the child and if there was a nonlinear association of sleep duration with telomere length. First, several studies of telomere length have found that some factors matter differently for telomere length in blacks and whites(27). Based on this literature, we conducted an additional analysis that included a term that interacted sleep duration with race to test for racial differences in the association of sleep duration with telomere length. We limited this analysis to children in our sample born to either both black parents or both white parents (N = 954). We found no evidence that sleep duration matters differently for telomere length depending on the child’s parents’ racial group. Second, prior studies have also suggested that there may be sex differences in the association of adult sleep with telomere length(7,18,20,28), though all but one of these studies was limited to small samples from particular subpopulations: those with HIV(18), women only(28), or obese people only (81% female)(20). Drawing on these studies, we tested for sex differences in the association of sleep duration with telomere length by including a term that interacted sleep duration with sex. We found that, in this large and diverse sample, there is no evidence of sex differences in the association of sleep duration with telomere length in 9-year-old children. Third, another study using a small number of cases (N = 40) from this dataset found differences in telomere length in boys selected for their extreme levels of advantage or disadvantage(22). Thus, we tested if the association between sleep duration and telomere length was moderated by socioeconomic status by interacting maternal educational attainment and, separately, household income with sleep duration. We found no evidence that associations differ by socioeconomic status. Finally, previous research on the association of sleep duration with a range of health outcomes has indicated that there may be a U-shaped association between sleep duration and health, whereby both excessively short and long nightly sleep durations are risk factors for poor health across the lifespan(1,3,24,29). Therefore, we also evaluated whether this U-shaped association is found in the association of sleep with telomere length by including a quadratic term for sleep duration (sleep duration squared) in our models. There is no evidence of a U- shaped association of sleep duration with telomere length.
Though this study was not designed to evaluate the biological mechanism by which sleep duration becomes linked to telomere length in children, both telomere erosion (for example, due to oxidative stress) and suppression of telomerase activity are mechanisms that have been studied to explain the link between chronic stress and telomere attrition. Thus, future work should directly test which biological pathways of telomerase activation might explain the association of sleep duration and telomere length. The most likely candidates include a range of physiological consequences of stress, including hypothalamic-pituitary-adrenal axis activation, inflammation, and/or oxidation(13,14,30,31).
Although this study is strengthened by the use of a large and diverse sample of American children and is the first to assess the association between sleep duration and telomere length in children, it also has several limitations. First, our cross-sectional study design does not allow us to rule out a confounding explanation for these findings, whereby shorter sleep duration and shorter telomere length are epiphenomena resulting from a third unmeasured factor, or reverse causation, whereby telomere length may indicate the presence of an unmeasured condition that affects nightly sleep duration. Moreover, this study design means that we only have data on children’s telomere length at one point in time, and thus we cannot test whether sleep duration is linked to different patterns of telomere attrition. Second, children’s nightly sleep duration is reported by their mothers, as opposed to being measured directly (i.e. using polysomnography or actigraphy). Thus, our study provides only preliminary evidence that sleep duration is associated with telomere length in children. However, given that pediatricians generally make recommendations based on a parent’s report of children’s sleep duration rather than directly measured sleep duration, our sleep duration measurement is consistent with pediatric practice. Though parent- reported sleep duration of children consistently overestimates actigraphy-measured sleep duration by about half an hour per night(32,33), the correlation of parent-perceived sleep duration and actigraphy-measured sleep duration is high among 4–9-year-old children(32–34), with parents of older children within this age range making more accurate reports of their children’s sleep(32). Thus, because shorter parent-perceived sleep duration translates to shorter actual sleep duration, this discrepancy is not especially problematic for our study. Third, our study is unable to account for Circadian disruption, which has been linked to altered telomerase activity(35).
Beyond addressing these specific limitations, future research should more generally investigate how the sleep-telomere association in childhood matters for trajectories of health and health disparities across the lifecourse, in order to inform clinical interventions. Our results are consistent with a theoretical model in which shorter telomere length may serve as a mechanism or indicate a biological pathway by which childhood sleep duration is linked to long-term health and wellbeing. It has been speculated that shorter telomeres in childhood may be predictive of adult morbidity and mortality(36), though establishing the biological mechanisms of a life course telomere-disease link is challenging because no large studies have prospectively tracked telomere length from childhood through the end of the life course. Nonetheless, studying telomere length may be promising for understanding how health disparities are developed, maintained, or exacerbated over the life course.
Acknowledgments
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (P2CHD047879, R01HD36916, and 5R01HD076592) as well as a consortium of private foundations.
We would like to thank Sue Rutherford Siegel, PhD (Penn State University), Elizabeth Conroy, MA (Penn State College of Medicine), and Iulia Kotenko, MS (Princeton University) for their insight and technical assistance in the telomere length assays. All were compensated for their work on this project.
Footnotes
Portions of this study were presented during the Population Association of America Meeting, Washington, D.C., March 31, 2016.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah James, Princeton University, Princeton, New Jersey.
Sara McLanahan, Princeton University, Princeton, New Jersey.
Jeanne Brooks-Gunn, Columbia University, New York, New York.
Colter Mitchell, University of Michigan, Ann Arbor, Michigan.
Lisa Schneper, Princeton University, Princeton, New Jersey.
Brandon Wagner, Texas Tech University, Lubbock, Texas.
Daniel Notterman, Princeton University, Princeton, New Jersey and Rutgers – Robert Wood Johnson Medical School, New Brunswick, New Jersey.
References
- 1.Buxton OM, Marcelli E. Soc Sci Med. 5. Vol. 71. Elsevier Ltd; 2010. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States; pp. 1027–36. [DOI] [PubMed] [Google Scholar]
- 2.Magee L, Hale L. Sleep Med Rev. 3. Vol. 16. Elsevier Ltd; 2012. Jun, Longitudinal associations between sleep duration and subsequent weight gain: A systematic review; pp. 231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taheri S, Lin L, Austin D, Young T, Mignot E. Short Sleep Duration is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandner MA, Hale L, Moore M, Patel NP. Sleep Med Rev. 3. Vol. 14. Elsevier Ltd; 2010. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future; pp. 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen X, Wu Y, Zhang D. Sci Rep. Vol. 6. Nature Publishing Group; 2016. Nighttime sleep duration, 24-hour sleep duration and risk of all- cause mortality among adults: a meta-analysis of prospective cohort studies; p. 21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves da Silva A, Gorga Bandiera de Mello R, Schaan CW, Fuchs FD, Redline S, Fuchs SC. Sleep duration and mortality in the elderly: a systematic review with meta-analysis. BMJ Open. 2016;6:e008119. doi: 10.1136/bmjopen-2015-008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackowska M, Hamer M, Carvalho LA, Erusalimsky JD, Butcher L, Steptoe A. Short Sleep Duration Is Associated with Shorter Telomere Length in Healthy Men: Findings from the Whitehall II Cohort Study. PLoS One. 2012;7(10):1–4. doi: 10.1371/journal.pone.0047292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Räikkönen K, Matthews KA, Pesonen A-K, Pyhälä R, Paavonen EJ, Feldt K, et al. Poor Sleep and Altered Hypothalamic-Pituitary-Adrenocortical and Sympatho-Adrenal- Medullary System Activity in Children. J Clin Endocrinol Metab. 2010;95(5):2254–61. doi: 10.1210/jc.2009-0943. [DOI] [PubMed] [Google Scholar]
- 9.El-Sheikh M, Erath SA, Bagley EJ. Parasympathetic nervous system activity and children’s sleep. J Sleep Res. 2013;22(3):282–8. doi: 10.1111/jsr.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spruyt K, Molfese DL, Gozal D. Sleep Duration, Sleep Regularity, Body Weight, and Metabolic Homeostasis in School-Aged Children. Pediatrics. 2011;127(2):e345–52. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomiyama AJ, O’Donovan A, Lin J, Puterman E, Lazaro A, Chan J, et al. Physiol Behav. 1. Vol. 106. Elsevier Inc; 2012. Does cellular aging relate to patterns of allostasis?. An examination of basal and stress reactive HPA axis activity and telomere length; pp. 40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J, et al. MolPsychiatry. 5. Vol. 20. Nature Publishing Group; 2015. Telomere length and cortisol reactivity in children of depressed mothers; pp. 615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Notterman DA, Mitchell C. Epigenetics and Understanding the Impact of Social Determinants of Health. Pediatr Clin North Am. 2015;62(5):1227–40. doi: 10.1016/j.pcl.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, et al. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013;38:1835–42. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathur MB, Epel E, Kind S, Desai M, Parks CG, Sandler DP, et al. Brain Behav Immun. Vol. 54. Elsevier Inc; 2016. Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field; pp. 158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci. 2004 Dec 7;101(49):17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cribbet MR, Carlisle M, Cawthon RM, Uchino BN, Williams PG, Smith TW, et al. Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep. 2014;37:65–70. doi: 10.5665/sleep.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KA, Gay C, Humphreys J, Portillo CJ, Pullinger CR, Aouizerat BE. Telomere length is associated with sleep duration but not sleep quality in adults with human immunodeficiency virus. Sleep. 2014;37(1):157–66. doi: 10.5665/sleep.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang G, Schernhammer E, Qi L, Gao X, de Vivo I, Han J. Associations between rotating night shifts, sleep duration, and telomere length in women. PLoS One. 2011;6(8):4–8. doi: 10.1371/journal.pone.0023462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prather AA, Gurfein B, Moran P, Daubenmier J, Acree M, Bacchetti P, et al. Brain Behav Immun. Vol. 47. Elsevier Inc; 2015. Tired telomeres: Poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women; pp. 155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tempaku PF, Mazzotti DR, Tufik S. Telomere length as a marker of sleep loss and sleep disturbances: a potential link between sleep and cellular senescence. Sleep Med. 2015;16(5):559–63. doi: 10.1016/j.sleep.2015.02.519. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, et al. Social disadvantage, genetic sensitivity, and children’s telomere length. Proc Natl Acad Sci. 2014 Apr 22;111(16):5944–9. doi: 10.1073/pnas.1404293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James S, Hale L. Sleep Duration and Child Well-Being: A Nonlinear Association. J Clin Child Adolesc Psychol. 2016;0(0):1–11. doi: 10.1080/15374416.2016.1204920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hale L, Parente V, Phillips GK. Social Determinants of Children’s Sleep. In: Wolfson AR, Montgomery-Downs HE, editors. The Oxford Handbook of Infant, Child, and Adolescent Sleep and Behavior. 1. Oxford: Oxford University Press; 2013. pp. 99–112. [Google Scholar]
- 26.Team RC. R. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 27.Oliveira BS, Zunzunegui MV, Quinlan J, Fahmi H, Tu MT, Guerra RO. Ageing Res Rev. Vol. 26. Elsevier B.V; 2016. Systematic review of the association between chronic social stress and telomere length: A life course perspective; pp. 37–52. [DOI] [PubMed] [Google Scholar]
- 28.Prather AA, Puterman E, Lin J, Donovan AO, Krauss J, Tomiyama AJ, et al. Shorter Leukocyte Telomere Length in Midlife Women with Poor Sleep Quality. 2011 doi: 10.4061/2011/721390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams NJ, Grandner MA, Snipes SA, Rogers A, Williams O, Airhihenbuwa C, et al. Sleep Heal. 1. Vol. 1. National Sleep Foundation; 2015. Racial/ethnic disparities in sleep health and health care: importance of the sociocultural context; pp. 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fyhrquist F, Saijonmaa O, Strandberg T. NatRevCardiol. 5. Vol. 10. Nature Publishing Group; 2013. The roles of senescence and telomere shortening in cardiovascular disease; pp. 274–83. [DOI] [PubMed] [Google Scholar]
- 31.von Zglinicki T. Oxidative stress shortens telomeres. TRENDS Biochem Sci. 2002;27(7):339–44. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 32.Nelson TD, Lundahl A, Molfese D. Estimating Child Sleep From Parent Report of Time in Bed: Development and Evaluation of Adjustment Approaches. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tikotzky L, Sadeh A. Sleep Patterns and Sleep Disruptions in Kindergarten Children. 2001;30(4):581–91. doi: 10.1207/S15374424JCCP3004_13. [DOI] [PubMed] [Google Scholar]
- 34.Kushnir J, Ph D, Sadeh A, Sc D, Child T, Unit P, et al. Correspondence between Reported and Actigraphic Sleep Measures in Preschool Children. The Role of a Clinical Context. 2013;9(11) doi: 10.5664/jcsm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W-D, Wen M-S, Shie S-S, Lo Y-L, Wo H-T, Wang C-C, et al. Biochem Biophys Res Commun. 3. Vol. 451. Elsevier Inc; 2014. The circadian rhythm controls telomeres and telomerase activity; pp. 408–14. [DOI] [PubMed] [Google Scholar]
- 36.Shalev I. Early life stress and telomere length: Investigating the connection and possible mechanisms. Bioessays. 2012 Nov;34(11):943–52. doi: 10.1002/bies.201200084. [DOI] [PMC free article] [PubMed] [Google Scholar]


