Abstract
Endocannabinoids (eCBs) are a family of lipid molecules that act as key regulators of synaptic transmission and plasticity. They are synthetized “on demand” following physiological and/or pathological stimuli. Once released from postsynaptic neurons, eCBs typically act as retrograde messengers to activate presynaptic type 1 cannabinoid receptors (CB1) and induce short- or long-term depression of neurotransmitter release. Besides this canonical mechanism of action, recent findings have revealed a number of less conventional mechanisms by which eCBs regulate neural activity and synaptic function, suggesting that eCB-mediated plasticity is mechanistically more diverse than anticipated. These mechanisms include non-retrograde signaling, signaling via astrocytes, participation in long-term potentiation, and the involvement of mitochondrial CB1. Focusing on paradigmatic brain areas, such as hippocampus, striatum, and neocortex, we review typical and novel signaling mechanisms, and discuss the functional implications in normal brain function and brain diseases. In summary, eCB signaling may lead to different forms of synaptic plasticity through activation of a plethora of mechanisms, which provide further complexity to the functional consequences of eCB signaling.
Keywords: Endocannabinoids, synaptic plasticity, hippocampus, striatum, prefrontal cortex, astrocytes
Introduction
Synaptic plasticity is critical to experience-dependent adaptations of neural circuits and normal brain function. From early development to adulthood, changes of synaptic function in response to environmental stimuli and individual experiences are necessary to learn new abilities, form new memories and generate new adaptive behaviors. Key mediators of synaptic plasticity, the endocannabinoids (eCBs) constitute a family of lipid molecules that are typically synthetized “on demand”, following either physiological and/or pathological stimuli (Castillo et al., 2012; Kano et al., 2009; Katona and Freund, 2012) (Piomelli, 2003). 2-AG originates from the metabolism of triacylglycerols by the activity of diacylglycerol (DAG) lipase in response to activation of metabotropic receptors coupled to the PLCβ (e.g. group I metabotropic glutamate receptor-mGluR1/5, muscarinic acetylcoline-mACh-types M1/M3). The biosynthesis of AEA from the precursor N-arachidonoyl-phosphatidylethanolamine (NAPE) requires intracellular Ca2+ elevations upon depolarization and/or activation of ionotropic receptors, and the activity of the enzyme NAPE-PLD. Once released from the postsynaptic neurons, eCBs act primarily as retrograde messengers by activating presynaptic CB1 receptors, one of the most abundant Gi/o protein-coupled receptor in the brain. CB1 activation decreases the probability of neurotransmitter release by diverse mechanisms, including presynaptic inhibition of Ca2+ influx through voltage-gated Ca2+ channels (VGCCs), activation of presynaptic K+ channels and cAMP/PKA signaling (Castillo et al., 2012; Kano et al., 2009). Termination of synaptic eCB signaling is initiated by re-uptake followed by intracellular degradation. 2AG is degraded by the presynaptic enzyme monoacylglycerol lipase (MAGL) and α/β-Hydrolase domain-containing 6 (ABHD6)(Dinh et al., 2002; Marrs et al., 2010), whereas AEA from the fatty acid amide hydrolase (FAAH) (Ahn et al., 2008; Di Marzo, 2009). There is also evidence that both 2-AG and AEA can act in a non-retrograde manner (Castillo et al., 2012), 2-AG by activating postsynaptic CB1 or CB2, and AEA by activating TRPV1.
Furthermore, eCBs released by neurons can modulate presynaptic and postsynaptic circuit elements through the activation of CB1 expressed on astrocytes (Metna-Laurent and Marsicano, 2015; Navarrete et al., 2014). Regulation of synaptic transmission that follows eCB mobilization occurs both on a short and long timescale. eCB-mediated short-term changes in synaptic transmission (tens of seconds) encompass depolarization-induced suppression of excitation (DSE) and inhibition (DSI) depending on whether eCBs target glutamatergic or GABAergic terminals (Castillo et al., 2012; Kano et al., 2009). Long-term synaptic changes (minute to hour) that depend upon eCB signaling can occur in response to diverse patterns of presynaptic and/or postsynaptic activity. Thus, eCBs are powerful regulators of synaptic function through the brain. By modulating both excitatory and inhibitory synaptic strength, eCBs can regulate a number of brain functions, including cognition, motor control, emotion, reward and feeding behaviors. Dysregulation of the eCB system has been implicated in neuropsychiatric conditions, such as depression, autism, schizophrenia, addiction, stress and anxiety (Hillard et al., 2012; Mechoulam and Parker, 2012; Parsons and Hurd, 2015; Volkow et al., 2017). Here, we will discuss recent advances on eCB signaling and synaptic function, emphasizing brain areas where eCBs are thought to regulate learning-, motor- and reward-guided behaviors both in health and disease.
eCB signaling at hippocampal and neocortical synapses
eCBs modulate synaptic function primarily through their effects on presynaptically expressed CB1 receptors in both GABAergic and glutamatergic terminals (Castillo et al., 2012; Kano et al., 2009). In the hippocampus, where CB1 receptors are predominantly expressed at inhibitory terminals (Freund et al., 2003), eCBs exert a profound effect on inhibition by reducing GABA release in a transient (Wilson and Nicoll, 2001) or long-lasting manner by triggering long-term depression (LTD) of inhibition (iLTD) (Chevaleyre and Castillo, 2003) (for a review, see Younts and Castillo, 2014). Disinhibition can robustly shift the excitatory-inhibitory balance in a network and by this means contribute to associative learning (Letzkus et al., 2015). By reducing inhibition, eCBs facilitate the induction of excitatory long-term potentiation (LTP) at Schaffer collateral (SC) to CA1 pyramidal cell synapse (SC-CA1) (Carlson et al., 2002; Chevaleyre and Castillo, 2004). Changes in the inducibility of LTP, a phenomenon known as metaplasticity, could play an important role in learning and memory. Remarkably, eCB-dependent metaplasticity initially described in vitro (Chevaleyre and Castillo, 2004) has recently been reported in vivo and could contribute to the formation of temporal associative memories (Xu et al., 2014). In addition, eCB-iLTD is involved the potentiation of excitatory postsynaptic potential (EPSP)-spike coupling (E-S coupling) (Chevaleyre and Castillo, 2003). Intriguingly, exogenous application of β–amyloid peptide (Aβ1–42), whose accumulation occurs in Alzheimer’s disease, prevents eCB-mediated disinhibition and subsequent ES coupling potentiation presumably by interfering with CB1 signaling (Orr et al., 2014). Although the downstream targets remain to be elucidated, this action of Aβ on eCB signaling represents a mechanism that could underlie the cognitive decline in Alzheimer’s disease.
While eCB-mediated short-term plasticity (i.e. DSI/DSE) relies on the CB1-mediated inhibition of presynaptic calcium influx through voltage-gated calcium channels (Kano et al., 2009) (Castillo et al., 2012). Several effectors downstream CB1 have been identified, including PKA signaling, the active zone protein RIM1α, and voltage-gated calcium channels, although alternative downstream CB1 signaling cascades (Njoo et al., 2015; Roland et al., 2014) leading to structural changes could also be involved. A recent study demonstrated that activation of CB1 receptors stimulates protein synthesis in axon terminals to induce eCB-iLTD in the hippocampus (Younts et al., 2016). In addition, CB1 activation enhances protein translation via mTOR signaling. Moreover, using super-resolution stochastic optical reconstruction microscopy (STORM), this study provided evidence that eukaryotic ribosomal proteins are present in CB1-expressing presynaptic terminals. Newly synthesized proteins may act as a molecular switch to persistently reduce GABA release. Previous work demonstrated a requirement for protein synthesis in striatal eCB-LTD (Adermark et al., 2009; Yin et al., 2006), but not in the nucleus accumbens (Jung et al., 2012). Future work will have to determine the identity of the newly synthesized proteins and how exactly these proteins reduce transmitter release in a long-lasting manner.
In the entorhinal cortex-hippocampus circuit, eCB signaling has been implicated in input-timing-dependent plasticity (Basu et al., 2013; Xu et al., 2012), a form of heterosynaptic plasticity at SC-CA1 synapses that is induced by pairing SC and perforant path (PP) inputs from the entorhinal cortex (EC) (Dudman et al., 2007). This plasticity involves both eCB-iLTD from cholecystokinin (CCK)-positive (CB1-containing) interneuron and SC-CA1 LTP (Basu et al., 2013). Input-timing-dependent plasticity requires precise timing of both SC inputs onto CCK interneurons and PP inputs to postsynaptic CA1 pyramidal cells. Presynaptic eCB-iLTD of feedforward inhibition in CCK interneurons may enable activation of CA1 pyramidal cells to facilitate information salience.
It is well established that in the rodent neocortex eCBs mediate spike timing-dependent LTD (t-LTD) at excitatory synapses, which is typically induced by pairing induction protocols (i.e. postsynaptic firing precedes presynaptic firing) (Caporale and Dan, 2008; Heifets and Castillo, 2009). In the mouse hippocampus, eCB-mediated t-LTD has also been reported at glutamatergic inputs onto principal cells (e.g. SC-CA1 synapses) and interneurons (Andrade-Talavera et al., 2016; Peterfi et al., 2012). Intriguingly, using similar induction protocols, as well as other protocols that typically induce robust eCB-LTD at inhibitory synapses (including chemical induction with CB1 or mGluR agonists), other studies were unable to induce eCB-LTD at SC-CA1 synapses (Rouach and Nicoll, 2003; Younts et al., 2013), or even at the glutamatergic mossy cell to dentate granule cell synapse (Chiu and Castillo, 2008), which expresses uniquely high levels of presynaptic CB1 (Katona et al., 2006; Uchigashima et al., 2011). This discrepancy could be due to slightly different experimental conditions used by these studies.
Besides the classical retrograde eCB signaling, there is growing evidence that eCBs can signal in a non-retrograde manner. An early study in neocortex demonstrated that repetitive activation of a subtype of GABAergic interneuron triggers a CB1-dependent postsynaptic hyperpolarization, which reduced its excitability (Bacci et al., 2004). This slow self-inhibition resulted from activity-dependent rises in intracellular Ca2+, mobilization of 2-AG, and activation of CB1 that couple to a G protein-coupled inwardly rectifying K+ channel. Recent evidence indicates that CB2 can mediate a similar form of self-inhibition both in the hippocampus and neocortex (see below). A provocative recent report has suggested that 2-AG activation of CB1 receptors in dendrites modulates the h-current (Ih), a key regulator of dendritic excitability, in a subset of CA1 pyramidal neurons, i.e. superficial but not deep neurons (Maroso et al., 2016). Activation of this CB1-Ih pathway, which involves a non-canonical signaling cascade, impairs integration of excitatory synaptic inputs, LTP and spatial memory formation. AEA can also act in a non-retrograde manner by activating TRPV1 channels (Castillo et al., 2012). In the dentate gyrus, TRPV1 activation by AEA induces a postsynaptically expressed LTD of medial perforant path inputs onto dentate granule cells (Chavez et al., 2010). Here, activation of type 5 metabotropic glutamate receptors (mGluR5) by glutamate released during repetitive activity, presumably via PLC and Ca2+ release from intracellular stores, promotes the synthesis of AEA that activates TRPV1 channels. A mechanistically similar form of LTD was also reported in the nucleus accumbens (Grueter et al., 2010) and in the bed nucleus of the stria terminalis (Puente et al., 2011).
Recent studies indicate that eCBs can mediate LTP by unconventional mechanisms both in the hippocampus and neocortex. At SC-CA1 synapses, eCBs can trigger LTP of glutamate release through stimulation of astrocyte–neuron signaling (Gomez-Gonzalo et al., 2015) (see below). In the dentate gyrus in vitro, high frequency stimulation of lateral perforant path inputs (LPP) triggers a presynaptically expressed form of LTP whose induction requires postsynaptic NMDAR and mGluR5-dependent calcium rise, 2-AG mobilization from the postsynaptic compartment and activation of presynaptic CB1 receptors (Wang et al., 2016). How exactly CB1 activation leads to a long-lasting increase of glutamate release is unclear but it could involve reorganization of the presynaptic actin cytoskeleton in LPP terminals. In the rodent barrel cortex in vitro, eCBs facilitate the induction of an NMDAR and Brain-Derived Neurotrophic Factor (BDNF)-dependent form LTP at excitatory inputs onto basal dendrites of layer 5 pyramidal neurons (Maglio et al., 2017). In this case, action potential bursts release eCBs, which by reducing inhibitory synaptic transmission, facilitate the generation of calcium spikes and calcium dependent release of BDNF, two critical requirements for the induction of this form of LTP. The notion that eCBs can trigger LTP is not novel, or exclusive to the hippocampus and neocortex. An early study in goldfish reported that eCBs release upon repetitive stimulation from the Mauthner cell lateral dendrite, activates CB1 receptors located on nearby varicosities, which in turn evokes the release of dopamine that activates dopamine D1/D5 receptors to induce LTP of electrical and chemical transmission via a cAMP/PKA-mediated postsynaptic mechanism (Cachope et al., 2007). The mechanism by which CB1 activation evokes the release of dopamine remains unknown. Lastly, eCB-dependent LTP has also been described in the dorsal striatum (see below).
Hippocampal astrocytes have been shown to express functional CB1, which upon stimulation by eCBs elevate the intracellular calcium levels through calcium mobilization from the internal stores (Navarrete and Araque, 2008)(Piomelli, 2003), CB1-evoked calcium elevations in astrocytes are mediated by activation of Gq/11 proteins that stimulate phospholipase C-mediated IP3 production and activation of IP3Rs in the internal calcium stores (Navarrete and Araque, 2008). The eCB-evoked astrocyte calcium signal has been demonstrated in hippocampus (Gomez-Gonzalo et al., 2015; Navarrete and Araque, 2008, 2010), neocortex (Min and Nevian, 2012), dorsal striatum (Martin et al., 2015), as well as in cortical and hippocampal human brain tissue (Navarrete et al., 2013), suggesting that eCB signaling between neurons and astrocytes is a general mechanism.
Calcium elevations in astrocytes have been shown to stimulate the release of gliotransmitters which by acting on neuronal transmitter receptors, regulate synaptic transmission and plasticity (Araque et al., 2014). This functional interaction between astrocytes and neurons have led to the establishment of the tripartite synapse, a functional concept that encapsulates the existence of signaling between astrocytes and neurons and its crucial role in synaptic function (Araque et al., 1999; Perea et al., 2009). In the hippocampus, eCB release from pyramidal neurons activates astrocytes that release the gliotransmitter glutamate (Navarrete and Araque, 2008), which increases neuronal excitability by activating NMDARs and evoking slow inward currents in CA1 pyramidal neurons (Fellin et al., 2004; Navarrete and Araque, 2008; Parri et al., 2001; Perea and Araque, 2005), These results indicate that eCB signaling is involved in reciprocal astrocyte-communication. Remarkably, the glutamate released by astrocytes as a result of eCB release from one neuron may impact adjacent neurons, suggesting that astrocytes responding to eCBs act as a bridge for non-synaptic communication between neurons.
Astrocytic activation by eCBs and the consequent gliotransmitter release may have relevant regulatory consequences on synaptic transmission and plasticity. In contrast to the canonical eCB retrograde signaling between neuronal synaptic elements that leads to synaptic depression though activation of presynaptic CB1, eCB signaling in astrocytes have been shown to transiently potentiate synaptic transmission. This synaptic potentiation was originally reported at CA3-CA1 synapses in the hippocampus, where eCB-induced astrocytic release of glutamate activates presynaptic metabotropic glutamate receptors that enhance neurotransmitter release (Navarrete and Araque, 2010). Here, the eCB-induced astrocyte-mediated synaptic potentiation occurs in synapses contacting adjacent non-stimulated neurons relatively far away from the eCB source. Therefore, eCBs may have opposite and complementary regulatory effects, namely, canonical synaptic depression at local synapses, and synaptic potentiation at distant synapses through activation of astrocytes. The physiological meaning of the latter phenomenon, termed lateral synaptic regulation (Covelo and Araque, 2016), remains unknown, but it could serve a homeostatic mechanism of synaptic transmission to scale synaptic inputs. Indeed, active synapses would induce eCB release from the postsynaptic cell that would depress incoming inputs, while simultaneously enhance adjacent less active synapses through astrocytic activation, thus contributing to maintain a homogeneous level of activity in different synaptic inputs. Recently, lateral synaptic regulation has also been shown in DS (Martin et al., 2015) (see below), suggesting a general mechanism by which eCB signaling in astrocytes can regulate synaptic function.
In addition to the transient synaptic regulation described above, eCBs can mediate long-term synaptic plasticity through different mechanisms that involve CB1 activation in astrocytes. Spike timing-dependent long-term depression (t-LTD) in hippocampal CA3-CA1 synapses has been demonstrated to be mediated by eCBs and to require astrocyte activation and the release of the gliotransmitter D-serine. The proposed mechanism involves D-serine release from astrocytes activated by eCBs, which allows the activation of presynaptic NMDARs by synaptically released glutamate, leading to the hippocampal t-LTD (Andrade-Talavera et al., 2016). A similar mechanism of t-LTD was identified in the neocortex, but rather than D-serine, glutamate was identified as the gliotransmitter involved in this brain area. eCBs released during t-LTD induction protocol elevates astrocytic calcium and stimulates the release of the astrocytic glutamate, which by activating presynaptic NMDA receptors induces the t-LTD (Min and Nevian, 2012). Additionally, using a different stimulation paradigm, it was shown that eCB-mediated release of glutamate from hippocampal astrocytes also leads to long-term plasticity (Gomez-Gonzalo et al., 2015). The coincidence of eCB signaling in astrocytes and postsynaptic activity triggers LTP of transmitter release at single CA3-CA1 synapses. Induction of this astrocyte-mediated LTP requires the concurrent activation of astrocytic and neuronal signaling cascades, including eCB-evoked astrocyte calcium signal that stimulates glutamate release, postsynaptic nitric oxide production, activation of presynaptic protein kinase C and group I metabotropic glutamate receptors. Taken together, these evidences indicate that astrocyte activation by eCBs lead to long-term changes in hippocampal synaptic strength.
Activation of CB1 receptors in astrocytes, by regulating synaptic function, may have important consequences on animal behavior. Taking advantage of transgenic mice lacking CB1 selectively in astrocytes, it has been reported that CB1 activation in hippocampal astrocytes likely mediates the impairment of spatial working memory and LTD at CA3-CA1 synapses induced by THC (Han et al., 2012).
In addition to the classical localization in the presynaptic plasma membrane (Freund et al., 2003), CB1 receptors have also been reported in brain mitochondria, and activation of these receptors alters mitochondrial energetic activity (Benard et al., 2012)(Hebert-Chatelain et al., 2016). Although eCB-mediated plasticity was not formally investigated in this study, the above observations suggest that mitochondrial CB1 is primarily responsible for CB1-mediated suppression of synaptic transmission, an intriguing scenario given that the majority of presynaptic CB1 receptors –which are located in the plasma membrane– remained largely unchanged by removing mitochondrial CB1 (Hebert-Chatelain et al., 2016). Future studies are necessary to determine the specific contribution of CB1 receptors at the plasma membrane and at mitochondrial membranes.
An unexpected functional connection between eCBs and the synaptic cell-adhesion molecules neuroligin-3 and β-neurexins has recently been identified. Neuroligins are postsynaptic cell-adhesion molecules that interact with presynaptic neurexins. Rare mutations in neuroligins and neurexins predispose to autism, including a neuroligin-3 amino acid substitution (R451C) and a neuroligin-3 deletion. A study on the respective mouse models, a neuroligin-3 (R451C) knockin (KI) and a neuroligin-3 knockout (KO), showed an increase in GABA release probability at CB1-expressing inhibitory synapses onto CA1 pyramidal cells, and this enhancement was due to impaired tonic but not phasic eCB signaling (Foldy et al., 2013). A similar enhancement mediated by impaired eCB-signaling was observed at cortical inhibitory synapses in the neuroligin-3 (R451C) KI mouse (Speed et al., 2015). Together, these studies suggest that disrupted eCB signaling may contribute to autism pathophysiology. Neuroligin-3 could be required to specifically localize the release machinery for tonic eCB release to CB1-containing synapses. In another study that used β-neurexin-specific conditional KO mice, it has been reported that β-neurexins also decrease tonic eCB signaling presumably by suppressing 2-AG synthesis. Intriguingly, this action was observed at hippocampal excitatory but not inhibitory synapses. How exactly β-neurexin transsynaptically modifies 2-AG synthesis remains unsolved.
The eCB system comprises two G protein-coupled receptors, CB1 and CB2, which mediate almost all effects of exogenous and endogenous cannabinoids. The neuronal expression of CB2 receptors has been a matter of debate. Although CB2 receptors are typically found in the immune system and are poorly expressed in the CNS, there is growing evidence for a role of these receptors in the brain (den Boon et al., 2012; Xi et al., 2011). Using CB2 KO mice and selective pharmacology, a recent study demonstrated that CB2 receptors are expressed in hippocampal principal neurons and mediate a cell type-specific self-inhibitory plasticity in CA3 and CA2 pyramidal neurons by modulating the activity of the sodium-bicarbonate co-transporter (Stempel et al., 2016). This CB2-mediated plasticity is induced by repetitive action potential firing that mobilizes 2-AG and is expressed as a long-lasting hyperpolarization that reduces spike probability and alter gamma oscillations in vivo. These findings are reminiscent of a previous study showing that CB2 receptors mediate an activity-induced self-inhibition in medial prefrontal cortical pyramidal neurons, but in this case the receptors were localized to intracellular compartments and coupled to calcium-activated chloride channels (den Boon et al., 2012). Collectively, these findings suggest that CB2 receptors play a complementary role to presynaptic CB1 and may represent a novel therapeutic target for neurological diseases.
Endocannabinoids at striatal circuits
Dorsal striatum
The dorsal striatum (DS) of the basal ganglia plays a critical role in voluntary movement, learning and motivation, and represents the primary site of dysfunction in psychomotor diseases. The DS integrates glutamatergic excitatory inputs from the cortex and the thalamus. These inputs converge on striatal projection neurons (SPNs) of the direct (dSPN) and indirect (iSPN) pathways, which play distinct roles in controlling motor output and hedonic states (Bateup et al., 2010; Cui et al., 2013; Koralek et al., 2012; Kravitz et al., 2010; Kravitz et al., 2012; Tecuapetla et al., 2016; Vicente et al., 2016; Yin et al., 2009).
CB1 are highly expressed at corticostriatal terminals (Hohmann and Herkenham, 2000) (Martin et al., 2008). This is consistent with a wealth of studies demonstrating that the eCB retrograde signaling is a fundamental means by which the activity of SPNs fine tunes the synaptic gain at excitatory cortical afferents to the DS (Castillo et al., 2012; Cerovic et al., 2013; Mathur and Lovinger, 2012). SPNs synthesize and release eCBs upon the synergistic activation of group I mGluRs and dopamine D2 receptors (by inhibition of regulator of G-protein signaling 4, RGS4), and by Ca2+ entry via voltage-gated calcium channels (VGCCs) and NMDA receptors (Lovinger and Mathur, 2012; Uchigashima et al., 2007; Wang et al., 2006; Yin and Lovinger, 2006) (Gerdeman and Lovinger, 2001; Gerdeman and Lovinger, 2003; Gerdeman et al., 2002; Ronesi and Lovinger, 2005; Shen et al., 2008; Trusel et al., 2015; Wang et al., 2006). DSE requires stimulation patterns that depolarize the postsynaptic SPNs and enhance intracellular Ca2+ levels (Uchigashima et al., 2007). eCB-LTD of excitatory synaptic responses occurs either in response to afferent stimulation (10–100 Hz) (Gerdeman et al., 2002; Kreitzer and Malenka, 2007; Ronesi and Lovinger, 2005; Trusel et al., 2015; Wang et al., 2006) or in response to a post-pre paring protocol, in which the postsynaptic spike precedes the presynaptic stimulation (Fino et al., 2010; Nazzaro et al., 2012; Paille et al., 2013; Shen et al., 2008; Shen et al., 2015; Wu et al., 2015). The expression and maintenance of eCB-LTD involves coincident presynaptic mechanisms, which may include protein synthesis (Yin et al., 2006). The consensual view is that eCB-LTD can be reliably induced at glutamatergic synapses on identified iSPNs (Adermark and Lovinger, 2007; Trusel et al., 2015; Wang et al., 2006). In identified dSPNs, eCB-LTD is critically dependent on the relative strength or timing of convergent synaptic inputs (e.g., cortical and thalamic inputs) and on neuromodulation (Bagetta et al., 2011; Nazzaro et al., 2012; Shen et al., 2008; Shen et al., 2015; Wu et al., 2015). Thus, while eCB-LTD may not be cell-type-specific per se, the threshold for the induction of this form of plasticity can be modulated in the two SPN subpopulations in a cell-type-specific manner. In the lateral part of the dorsal striatum, eCB signaling is also required for synaptic depotentiation, the reversal of LTP. eCB-mediated synaptic depotentiation, which occurs at previously potentiated synapses following low-frequency afferent stimulation (2 Hz), does not involve VGCCs and requires activation of mGluR5 (Nazzaro et al., 2012). Contrary to cortical inputs, thalamostriatal synapses are devoid of presynaptic CB1 (Wu et al., 2015), suggesting that eCB signaling by this receptor subtype may not be critical for the thalamic control of striatal function. Consistent with this, LTD at thalamostriatal synapses is reported to be NMDA-mediated and not CB1-mediated. The role of TRPV1 in regulating thalamostriatal synapses remains, however, unexplored.
eCBs also promote LTP via both CB1 and TRPV1 and on 2-AG elevations (Cui et al., 2015; Cui et al., 2016). The emerging picture is that eCBs are bidirectional rather than unidirectional neuromodulators of striatal functions. The proposed model suggests that both the levels and timing of eCB release control the direction (depression vs potentiation) of changes in synaptic strength (Cui et al., 2016). As discussed below, eCBs released from SPNs can also activate astrocytic CB1 and by this means potentiate corticostriatal synaptic transmission.
eCBs modulate GABAergic synapses impinging on SPNs by mediating both depolarization-induced suppression of inhibition (DSI) (Tanimura et al., 2010) and inhibitory LTD (iLTD) (Adermark et al., 2009). CB1 receptors located on GABAergic afferents from local interneurons appear to be more sensitive to eCBs released from SPNs after VGCCs activation in the absence of afferent stimulation (Adermark et al., 2009). CB1 receptors are also localized on local axon collaterals originating from SPNs. These axon collaterals connect to interneurons and other SPNs, and form numerous inhibitory (GABA) synapses onto spines and dendritic shafts of these neurons (Kubota and Kawaguchi, 2000; Van Waes et al., 2012; Wilson, 2007). Electrophysiological findings suggest that CB1 activation inhibits GABAergic synaptic transmission between paired SPNs, and this effect is mediated by presynaptic CB1 receptors (Freiman et al., 2006). As to whether and how this affects synaptic plasticity phenomena in the two connected SPNs remains to be established.
Consistent with a crucial role of eCBs as striatal synaptic neuromodulators, pharmacological or genetic manipulations of the endogenous cannabinoid system profoundly influences DS-dependent behaviors. Acute exposure to the exogenous ligand Δ9-tetrahydrocannabinol (THC), or CB1 deletion, impairs stimulus-response habit formation (Gremel et al., 2016; Hilario et al., 2007; Hilario and Costa, 2008), whilst persistent activation of eCB signaling by chronic THC administration promotes a bias towards habitual behavior (Nazzaro et al., 2012). This latter manipulation results in a reduction of eCB-mediated plasticity (LTD and synaptic depotentiation) secondary to CB1 downregulation and desensitization. Regardless whether habitual behavior is impaired or facilitated by manipulations of the eCB signaling, the emerging view is that affecting CB1 -mediated regulation of glutamatergic and GABAergic function may interfere with the normal acquisition and consolidation of memory processing dependent upon the DS.
In animal exposed to chronic alcohol administration, increased 2-AG levels are associated with deficits in eCB-LTD in the dorsolateral striatum and with facilitation of reversal learning (Depoy et al., 2013; DePoy et al., 2015). In animal model of Parkinson’s disease, impaired eCB-LTD, secondary to striatal dopamine depletion, is directly related to motor dysfunctions that characterize this pathology. Specifically, it has been shown that the loss of dopaminergic innervation results in deficits of D2-dependent eCB production (Kreitzer and Malenka, 2007; Trusel et al., 2015). Administrating in-vivo D2 receptor agonists (Kreitzer and Malenka, 2007) or activating signaling downstream of D2 receptors (Trusel et al., 2015) restores proper eCB synaptic functions and ameliorated motor dysfunctions, including motor learning.
The eCB-evoked astrocyte calcium signal and consequent synaptic regulation has been recently demonstrated in DS, where eCB-induced lateral synaptic regulation has been shown to be mediated by astrocytic glutamate release and to selectively occur between homotypic, but not heterotypic, dSPN and iSPNs (Martin et al., 2015).
Nucleus Accumbens
The nucleus accumbens (NAc), or ventral striatum, is a component of the mesolimbic reward pathway that enables adaptive behavioral responding to rewards and reward-predictive cues, and plays a key role in the reinforcing properties of addictive drugs (for a recent review, see (Francis and Lobo, 2017; Scofield et al., 2016). The NAc receives glutamatergic excitatory inputs from the prefrontal cortex (PFC), the amygdala, the thalamus and ventral hippocampus, together with dopaminergic neuromodulatory afferents from the ventral tegmental area (VTA). Similar to the DS, GABAergic SPNs in the NAc belong to two neuronal subpopulations, mainly enriched either in dopamine D1 or D2 receptors. The segregation into direct and indirect output pathways is, however, less straight forward compared to the DS. Indeed, D1- and D2- NAc expressing neurons have overlapping projections to ventral pallidum (Smith et al., 2013). The NAc can be divided into two distinct areas: a central core surrounded by an outer shell. Whilst the NAc shell could be conceived as a transitional zone between the striatum and the extended amygdala, the NAc core is more functionally linked to the dorsal striatum, and is involved in the cognitive processing of motor programs related to reward and reinforcement (Sesack and Grace, 2010; Smith et al., 2013).
The key molecular constituents of eCB signaling complex are expressed in the core of the NAc. In this sub region, the enzyme DGLα (Matyas et al., 2007) can be detected in SPN dendrites that face pre-synaptic CB1 receptor located on glutamatergic projections from the medial PFC (mPFC) (Robbe et al., 2001), as well as at the level of local GABAergic interneurons (Manzoni and Bockaert, 2001). Consistent with these anatomical observations, pharmacological activation of NAc CB1 receptors inhibits both inhibitory and excitatory synaptic transmission (Hoffman et al., 2003; Manzoni and Bockaert, 2001; Robbe et al., 2001). Furthermore, moderate frequency stimulation (13 Hz) of cortical afferents to the NAc core induces a form of eCB-LTD, which is dependent upon mGluR5 activation and Ca2+ release from internal stores (Robbe et al., 2002). In NAc D2-SPNs, but not in NAc D1-SPNs, low frequency stimulation (3 Hz) results in LTD of excitatory synaptic responses; this form of synaptic plasticity requires postsynaptic TRPV1 activation, relies on Ca2+-mediated AMPAR endocytosis, and is only partially dependent on CB1 (Grueter et al., 2010). On the one hand, TRPV1-mediated LTD in iSPNs is prevented by in-vivo cocaine administration. On the other hand, genetic deletion of TRPV1 enhanced behavioral sensitization in response to repeated administration of this drug of abuse (Grueter et al., 2010). Thus, these observations support the behavioral relevance of this cell-type specific form of synaptic plasticity due to activation of postsynaptic TRPV1 channels by the eCB AEA. eCBs released by NAc SPNs are also involved in Hebbian spike-timing- dependent LTD (t-LTD) (Bosch-Bouju et al., 2016).
Anatomical and functional observations suggest a cross talk between the NAc eCB system and the serotonergic (5-HT) signaling in regulating NAc synaptic plasticity. In the core NAc core, CB1 and the 5-HT2 receptor subtype appear to co-localize at excitatory terminals (Best and Regehr, 2008). On a functional level, prolonged (20 min) low frequency (4 Hz) stimulation of glutamatergic afferents to this NAc sub region triggers a form of LTD that requires activation of 5-HT2R, CB1 and L-type VGCCs. Recent work revealed that social play, one of the earliest forms of non-mother directed social behavior observed in mammals, implicates a functional interaction between CB1 and Mu Opioid Receptors in the NAc of rats and mice (Manduca et al., 2016). Thus, the regulation of positively valenced social behavior by morphine, synthetic cannabinoids and 2-AG implicates reciprocal interaction between the two cognate presynaptic receptors, perhaps within a heterodimer complex.
Within both the DS and NAc, fast-spiking (FS) interneurons provide tonic inhibition and timing-dependent feedforward inhibition to SPNs upon excitation. Studies performed in the NAc showed that SPNs feed-forward inhibition from CB1-exrpressing FS interneurons is stronger than lateral inhibition from SPNs collaterals (Winters et al., 2012). Given the lack of evidence for CB1 expression on NAc SPN collaterals, it is possible that eCB released upon SPNs activity selectively modulates inhibitory transmission from CB1 positive FS interneurons.
Studies investigating maladaptive alterations of eCB-mediated synaptic plasticity in the NAc have primarily focused on the effects of drugs of abuse, and THC in particular. Both single (Mato et al., 2004) and repeated (Hoffman et al., 2003; Mato et al., 2005) exposure to THC impairs eCB-LTD in the NAc. Additionally, accumbal eCB-LTD is abolished by a single exposure to cocaine (Fourgeaud et al., 2004) or upon cocaine self-administration (McCutcheon et al., 2011a; McCutcheon et al., 2011b). Upon extended withdrawal from cocaine self-administration, there is enhanced sensitivity to CB1 agonists that suggest a persistent attenuation of eCB tone (McCutcheon et al., 2011b). Only very recent work has provided evidence that NAc eCB signaling can be affected by other forms of experience-dependent plasticity, in addition to exposure to addictive drugs (Bosch-Bouju et al., 2016). In mice exposed to chronic social defeat stress, a manipulation that generates robust anxiety-like behavior, NAc eCB-mediated t-LTD is attenuated in non-anxious mice and abolished in anxious mice. Furthermore, anxiety-like behavior in stressed animals is directly associated with their ability to produce t-LTD, supporting the role of NAc synaptic eCB signaling in the adaptive response and resiliency to psychosocial stress (Bosch-Bouju et al., 2016).
eCB signaling in prefrontal cortex
The complex internal circuit organization and extensive connectivity of the mammalian PFC endows this functional network hub in the brain with essential roles in the regulation of our thoughts, actions, and emotions (Goldman-Rakic, 1990; Seamans et al., 1995). Compromised hubs are deleterious to the entire network’s integrity and PFC malfunctions are a common denominator in neuropsychiatric diseases (Goto et al., 2010). Here recent evidences illustrating the participation of dysregulation of the eCB system in PFC-related neuropsychiatric synaptopathies will be reviewed.
Similar to other brain regions (Kano et al., 2009; Katona and Freund, 2012), the highest concentrations of CB1 mRNA in the PFC are found in interneurons. CB1 immunoreactivity is also detected in glutamatergic fibers across the PFC surrounding pyramidal neurons in deep (layers 5/6) and superficial cortical layers (layer 2) (Egertová and Elphick, 2000; Lafourcade et al., 2007). A similar pattern of CB1 immunohistochemistry is found in primate and human PFC tissue (Eggan et al., 2010; Long et al., 2012). Both the AEA and 2-AG synthesis/degradation molecular machinery are found in close quarter with elements of the postsynaptic 2-AG/mGluR5 signaling complex system. Thus, eCBs in the PFC can regulate excitatory and inhibitory synaptic transmission, but also neuromodulatory tone (Katona and Freund, 2012; Lafourcade et al., 2007). Despite the high expression of CB1 in PFC interneurons, eCB-mediated plasticity is more prevalent at excitatory synapses and shows layer specificity. Layer 5 pyramidal rat neurons express both short-term plasticity of glutamatergic synapses (e.g. DSE) (Fortin and Levine, 2007; Heng et al., 2011) and LTD (Lafourcade et al., 2007; Lovelace et al., 2014; Marrs et al., 2010). DSI protocols fail in deep layers PFC neurons (Fortin and Levine, 2007; Marrs et al., 2010). However, a significant portion of inhibitory synapses (>30%) co-express CB1 and D2 receptors and appear sensitive to eCB modulation upon D2 co-activation (Chiu et al., 2010). While eCB-mediated long-term plasticity has not been reported in superficial layers of the PFC, DSI was found in layer 2/3 PFC neurons (Yoshino et al., 2011).
Experience-dependent adaptations of eCB signaling at PFC circuits
Dysfunctional or abnormal activity in the PFC has been linked to control over drug-seeking behavior (George and Koob, 2010; Goldstein and Volkow, 2011). Genetic evidence indicates that eCB signaling in the PFC contributes to ethanol consumption/preference in rats (Hansson et al., 2007) and humans (Hirvonen et al., 2013). Chronic ethanol reduces CB1 density in animals (Vinod et al., 2006) and humans (Ceccarini et al., 2014). Ethanol disrupts PFC mediated cognitive processes and CB1 and eCB-LTD down-regulation is proposed to participate to the shift in the regulation of behavior from PFC to DS (Depoy et al., 2013). Similarly, rodent and human data converge to show that cocaine perturbs several components of the eCB system in the PFC (Bystrowska et al., 2014; Ho et al., 2008). Consistent with this observation, PFC eCB-LTD is abolished in rats self-administering cocaine (Kasanetz et al., 2013). Alterations of eCB signaling in the PFC have been reported with the withdrawal of other drugs of abuse, including morphine (Viganò et al., 2004), the psychostimulant methylphenidate (Burgos et al., 2015), and nicotine (Cippitelli et al., 2011).
Cannabis has profound consequences on eCB signaling in the PFC. While adults seem resistant to most protracted effects of cannabis exposure (Realini et al., 2011; Schneider and Koch, 2003), adolescent exposure precipitates the development of such aberrant behaviors as addiction and psychosis (for reviews see: Hurd et al., 2014). In animal models, PFC-dependent cognitive functions are impaired in adulthood following cannabis exposure during development (Lovelace et al., 2015; Raver et al., 2013). Enhanced deficits resulting from cannabis use in schizophrenic subjects provide further evidence supporting a role for dysfunction in the eCB system. Schizophrenic individuals show increased responses to THC with regards to multiple symptoms of the disease (D’Souza et al., 2005) including increased frequency and intensity of psychotic episodes (Foti et al., 2010) and adolescent cannabis use positively correlates with increased likelihood severity of schizophrenia (for reviews, see: Casadio et al., 2011).
The PFC is linked genetically, anatomically and functionally to autism spectrum disorders (ASD) (Willsey et al., 2013). Fragile X syndrome (FXS) is an X-chromosome-linked hereditary disorder often linked to autism (Garber et al., 2008) with behavioral signatures linked to PFC directed actions: loss of executive control, deficits in working memory, abnormal social interaction (Cornish et al., 2008). In the Fmr1 KO mouse model, hippocampal synaptic eCB signaling is enhanced at both excitatory and inhibitory synapse (Tang and Alger, 2015; Zhang and Alger, 2010) and CB1 antagonism normalizes altered hippocampal based behavior (Busquets-Garcia et al., 2013). In contrast, current evidence points to age-dependent deficits in eCB plasticity in the PFC of Fmr1 KO mice. The severe deficit in eCB-LTD observed during early adulthood in Fmr1 KO mouse (Jung et al., 2012), self rectifies later in life due to the engagement of TRPV1 (Martin et al., 2016). Pharmacological treatment of Fmr1 KO animals with a drug that enhances eCB signaling, JZL184, has been shown to restore selective behavioral deficits (Jung et al., 2012). The use of cannabis results in acute and chronic deficits in cognition. Consistent with this notion, PFC deficits in eCB signaling have been linked to genetic intellectual disability. In the PFC of the Dyrk1A mice model of Down syndrome (DS), eCB-LTD is impaired as a result reduced 2-AG production but not CB1 function and enhancement of 2-AG levels with the MAGL inhibitor JZL184 effectively restores eCB-LTD (Thomazeau et al., 2014), and promisingly, treatment of mice with JZL184 also restores some of the cognitive deficits found in the Ts65Dn mouse model of DS (Lysenko et al., 2014), suggesting the eCB system may \be a therapeutic target in DS.
Dysfunctional eCB signaling has been linked to emotional disorders in humans (Lutz et al., 2015; McEwen et al., 2015). Genetic deletion of MAGL increased 2-AG levels in the PFC, enhanced the excitatory drive to the PFC and was associated to an anxiogenic phenotype through desensitized CB1 signaling (Imperatore et al., 2015). Increased anxiety-like behaviors were also observed in mice fed with a diet deficient in n-3 polyunsaturated fats, which elevates 2-AG levels (Watanabe et al., 2003) and induces a desensitized or uncoupled state in presynaptic CB1 in the PFC (Lafourcade et al., 2011). While CB1 antagonism enhances stress-induced neuronal responses in the PFC (Patel et al., 2005), direct or indirect CB1 activation in the PFC protects against stress conditions (Rubino et al., 2008) and enhances the extinction of both learned and innate fear responses (Lin et al., 2009). Conversely, stressful experience alters eCB signaling in the PFC. Chronic or repeated stress in rodents decreases and increases AEA and 2-AG signaling, respectively (Bortolato et al., 2007; Rademacher et al., 2008) (Patel et al., 2005) (Hill et al., 2011) and down regulates CB1 mRNA (Campos et al., 2013). Furthermore, chronic stress differentially alters eCB signaling in the PFC in adolescent rats as compared to adult rats. Repeated stress in adult or adolescent rats causes increased CB1 binding in the PFC, however while eCB signaling in adult rats normalizes over a subsequent 40-day recovery period, adolescents exhibit sustained CB1 down regulation following stress-exposure. Indeed, adolescent rats exposed to chronic stress conditions exhibit lasting deficits in PFC function and increased CB1 binding in the PFC (Abush and Akirav, 2013; Lee and Hill, 2013). The emerging picture is that the PFC eCB system integrates multiple stress and anxiety responses.
Conclusions
Research in the last decade continue to show that eCBs are powerful regulators of synaptic function throughout the brain. Significant progress has been made in our understanding of how eCBs signal at neurons and their functional consequences in normal and pathophysiological circuits. While retrograde signaling involving inhibition of transmitter release via presynaptic CB1 remains as the most common mechanism by which eCBs regulate synaptic function, growing evidence indicates that less conventional mechanisms, including non-retrograde signaling and the involvement of astrocytes, may also play a significant role in regulating brain function. Determining the relative contribution of these remarkably diverse mechanisms both in normal and disease states is an important challenge ahead.
Figure 1.
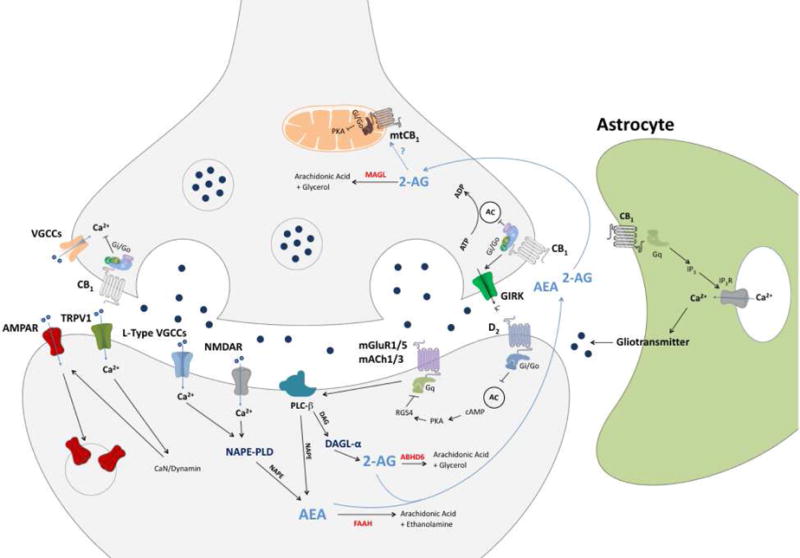
Schematic of eCB signaling at the synapse
Highlights.
Endocannabinoids (eCBs) regulate both synaptic function and neuronal excitability
eCB signaling is complex and may involve astrocytes and non-retrograde signaling
eCBs can mediate short and long-term changes of synaptic transmission
Disrupted eCB signaling may contribute to brain disorders (e.g. autism, dementia)
Acknowledgments
We wish to thank all of the scientists whose studies were reviewed in this paper and apologize to those authors whose work was not cited due to space limitations. We are grateful to Vincent Paget-Blanc for helping with figure preparation.
Funding sources
This work was supported by the Human Frontier Science Program (RGP0036/2014) and NIH-NINDS (R01NS097312-01) to AA, by the NIH (R01-MH081935 and R01-DA17392) to P.E.C, by the Agence Nationale de la Recherche (Cannado & MoodFood) and Fondation pour la Recherche Médicale (Equipe FRM 2015) to OJM, and by the Fondazione Istituto Italiano di Tecnologia and by the Fondazione Cariplo to RT.
List of abbreviations
- 2-AG
2-arachidonoyl glycerol
- AEA
anandamide Δ9-THC: delta-9-tetrahydrocannabinol
- ABHD6
alpha/beta hydrolase domain 6
- AEA
anandamide
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BDNF
brain-derived neurotrphic factor
- CA1
cornu ammonis 1
- cAMP
cyclic adenosine monophosphate
- CB
cannabinoid receptor
- CB1
type 1- cannabinoid receptor
- CB2
type 2- cannabinoid receptor
- CCK
cholecystokinin
- DAG
diacylglycerol
- DAGL
diaciglycerol lipase
- DSI
depolarization-induced suppression of inhibition
- DSE
depolarization-induced suppression excitation
- DS
dorsal striatum
- eCB
endocannabinoid
- eCB-LTD
endocannabinoid-mediated LTD
- EPSP
excitatory postsynaptic potential
- FAAH
fatty acid amide hydrolase
- GABA
gamma-aminobutyric acid
- iLTD
inhibitory LTD
- IP3
inositol-trisphosphate
- KO
knock out
- LFS
low-frequency stimulation
- LPP
lateral performant path inputs
- LTD
long-term depression
- LTP
long-term potentiation
- MAGL
monoacylglycerol lipase
- mGluR 1/5
group I metabotropic glutamate receptor 1 and 5
- mACh M1/M3
muscarinic acetylcoline receptor M1 and M3
- NAc
nucleus accumbens
- NAPE
N-arachidonoylphosphatidylethanolamine
- NAPE-PLD
phospholipase D
- NMDA
N-Methyl-D-aspartate r
- NM II
non-muscle myosin II
- PFC
prefrontal cortex
- PLC
Fphospholipase C beta
- PKA
Protein kinase A
- PP
perforant path
- RGS4
regulator of G-protein signaling 4
- RIM1
Ftype alpha-Rab3 interacting molecule 1
- SPN
striatal projection neuron
- STDP
spike timing-dependent plasticity
- tLTD
spike-time dependent LTD
- TRPV1
transient receptor potential vanilloid-1
- VGCCs
voltage-gated Ca2+ channels
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
The authors declare no competing financial interests.
References
- Abush H, Akirav I. Cannabinoids ameliorate impairments induced by chronic stress to synaptic plasticity and short-term memory. Neuropsychopharmacology. 2013;38:1521–1534. doi: 10.1038/npp.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. J Neurosci. 2007;27:6781–6787. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci. 2009;29:32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Talavera Y, Duque-Feria P, Paulsen O, Rodriguez-Moreno A. Presynaptic Spike Timing-Dependent Long-Term Depression in the Mouse Hippocampus. Cereb Cortex. 2016;26:3637–3654. doi: 10.1093/cercor/bhw172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Bagetta V, Picconi B, Marinucci S, Sgobio C, Pendolino V, Ghiglieri V, Fusco FR, Giampa C, Calabresi P. Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: implications for Parkinson’s disease. J Neurosci. 2011;31:12513–12522. doi: 10.1523/JNEUROSCI.2236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron. 2013;79:1208–1221. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci U S A. 2010;107:14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard G, Massa F, Puente N, Lourenco J, Bellocchio L, Soria-Gomez E, Matias I, Delamarre A, Metna-Laurent M, Cannich A, Hebert-Chatelain E, Mulle C, Ortega-Gutierrez S, Martin-Fontecha M, Klugmann M, Guggenhuber S, Lutz B, Gertsch J, Chaouloff F, Lopez-Rodriguez ML, Grandes P, Rossignol R, Marsicano G. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat Neurosci. 2012;15:558–564. doi: 10.1038/nn.3053. [DOI] [PubMed] [Google Scholar]
- Best AR, Regehr WG. Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses. J Neurosci. 2008;28:6508–6515. doi: 10.1523/JNEUROSCI.0678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Bosch-Bouju C, Larrieu T, Linders L, Manzoni OJ, Laye S. Endocannabinoid-Mediated Plasticity in Nucleus Accumbens Controls Vulnerability to Anxiety after Social Defeat Stress. Cell Rep. 2016;16:1237–1242. doi: 10.1016/j.celrep.2016.06.082. [DOI] [PubMed] [Google Scholar]
- Burgos H, Cofre C, Hernandez A, Saez-Briones P, Agurto R, Castillo A, Morales B, Zeise ML. Methylphenidate has long-lasting metaplastic effects in the prefrontal cortex of adolescent rats. Behav Brain Res. 2015;291:112–117. doi: 10.1016/j.bbr.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Gomis-Gonzalez M, Guegan T, Agustin-Pavon C, Pastor A, Mato S, Perez-Samartin A, Matute C, de la Torre R, Dierssen M, Maldonado R, Ozaita A. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 2013;19:603–607. doi: 10.1038/nm.3127. [DOI] [PubMed] [Google Scholar]
- Bystrowska B, Smaga I, Frankowska M, Filip M. Changes in endocannabinoid and N-acylethanolamine levels in rat brain structures following cocaine self-administration and extinction training. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:1–10. doi: 10.1016/j.pnpbp.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Cachope R, Mackie K, Triller A, O’Brien J, Pereda AE. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron. 2007;56:1034–1047. doi: 10.1016/j.neuron.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Ferreira FR, da Silva WA, Jr, Guimaraes FS. Predator threat stress promotes long lasting anxiety-like behaviors and modulates synaptophysin and CB1 receptors expression in brain areas associated with PTSD symptoms. Neurosci Lett. 2013;533:34–38. doi: 10.1016/j.neulet.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike Timing-Dependent Plasticity: A Hebbian Learning Rule. Annu Rev Neurosci. 2008 doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci. 2002;5:723–724. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis use in young people: the risk for schizophrenia. Neurosci Biobehav Rev. 2011;35:1779–1787. doi: 10.1016/j.neubiorev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini J, Hompes T, Verhaeghen A, Casteels C, Peuskens H, Bormans G, Claes S, Van Laere K. Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J Neurosci. 2014;34:2822–2831. doi: 10.1523/JNEUROSCI.0849-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerovic M, d’Isa R, Tonini R, Brambilla R. Molecular and cellular mechanisms of dopamine-mediated behavioral plasticity in the striatum. Neurobiol Learn Mem. 2013;105:63–80. doi: 10.1016/j.nlm.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chiu CQ, Castillo PE. Input-specific plasticity at excitatory synapses mediated by endocannabinoids in the dentate gyrus. Neuropharmacology. 2008;54:68–78. doi: 10.1016/j.neuropharm.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Puente N, Grandes P, Castillo PE. Dopaminergic modulation of endocannabinoid-mediated plasticity at GABAergic synapses in the prefrontal cortex. J Neurosci. 2010;30:7236–7248. doi: 10.1523/JNEUROSCI.0736-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Astarita G, Duranti A, Caprioli G, Ubaldi M, Stopponi S, Kallupi M, Sagratini G, Rodriguez de Fonseca F, Piomelli D, Ciccocioppo R. Endocannabinoid regulation of acute and protracted nicotine withdrawal: effect of FAAH inhibition. PLoS One. 2011;6:e28142. doi: 10.1371/journal.pone.0028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Li L, Kogan CS, Jacquemont S, Turk J, Dalton A, Hagerman RJ, Hagerman PJ. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008;44:628–636. doi: 10.1016/j.cortex.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covelo A, Araque A. Lateral regulation of synaptic transmission by astrocytes. Neuroscience. 2016;323:62–66. doi: 10.1016/j.neuroscience.2015.02.036. [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Paille V, Xu H, Genet S, Delord B, Fino E, Berry H, Venance L. Endocannabinoids mediate bidirectional striatal spike-timing-dependent plasticity. J Physiol. 2015;593:2833–2849. doi: 10.1113/JP270324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Prokin I, Xu H, Delord B, Genet S, Venance L, Berry H. Endocannabinoid dynamics gate spike-timing dependent depression and potentiation. Elife. 2016;5:e13185. doi: 10.7554/eLife.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- den Boon FS, Chameau P, Schaafsma-Zhao Q, van Aken W, Bari M, Oddi S, Kruse CG, Maccarrone M, Wadman WJ, Werkman TR. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc Natl Acad Sci U S A. 2012;109:3534–3539. doi: 10.1073/pnas.1118167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoy L, Daut R, Brigman JL, Macpherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci U S A. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Wright T, Camp M, Crowley N, Noronha B, Lovinger D, Holmes A. Chronic alcohol alters rewarded behaviors and striatal plasticity. Addict Biol. 2015;20:345–348. doi: 10.1111/adb.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacological research : the official journal of the Italian Pharmacological Society. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman JT, Tsay D, Siegelbaum SA. A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron. 2007;56:866–879. doi: 10.1016/j.neuron.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertová M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Mizoguchi Y, Stoyak SR, Lewis DA. Development of cannabinoid 1 receptor protein and messenger RNA in monkey dorsolateral prefrontal cortex. Cereb Cortex. 2010;20:1164–1174. doi: 10.1093/cercor/bhp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fino E, Paille V, Cui Y, Morera-Herreras T, Deniau JM, Venance L. Distinct coincidence detectors govern the corticostriatal spike timing-dependent plasticity. J Physiol. 2010;588:3045–3062. doi: 10.1113/jphysiol.2010.188466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldy C, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron. 2013;78:498–509. doi: 10.1016/j.neuron.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb Cortex. 2007;17:163–174. doi: 10.1093/cercor/bhj133. [DOI] [PubMed] [Google Scholar]
- Foti DJ, Kotov R, Guey LT, Bromet EJ. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167:987–993. doi: 10.1176/appi.ajp.2010.09020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Lobo MK. Emerging Role for Nucleus Accumbens Medium Spiny Neuron Subtypes in Depression. Biol Psychiatry. 2017;81:645–653. doi: 10.1016/j.biopsych.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman I, Anton A, Monyer H, Urbanski MJ, Szabo B. Analysis of the effects of cannabinoids on identified synaptic connections in the caudate-putamen by paired recordings in transgenic mice. J Physiol. 2006;575:789–806. doi: 10.1113/jphysiol.2006.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur J Hum Genet. 2008;16:666–672. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM. Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol. 2003;140:781–789. doi: 10.1038/sj.bjp.0705466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325–335. doi: 10.1016/s0079-6123(08)62688-6. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gonzalo M, Navarrete M, Perea G, Covelo A, Martin-Fernandez M, Shigemoto R, Lujan R, Araque A. Endocannabinoids Induce Lateral Long-Term Potentiation of Transmitter Release by Stimulation of Gliotransmission. Cereb Cortex. 2015;25:3699–3712. doi: 10.1093/cercor/bhu231. [DOI] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, Deisseroth K, Lovinger DM, Costa RM. Endocannabinoid Modulation of Orbitostriatal Circuits Gates Habit Formation. Neuron. 2016;90:1312–1324. doi: 10.1016/j.neuron.2016.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous DN, Mendizabal-Zubiaga J, Grandes P, Liu Q, Bai G, Wang W, Xiong L, Ren W, Marsicano G, Zhang X. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, Rodriguez de Fonseca F, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–126. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- Hebert-Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria-Gomez E, Busquets-Garcia A, Pagano Zottola AC, Delamarre A, Cannich A, Vincent P, Varilh M, Robin LM, Terral G, Garcia-Fernandez MD, Colavita M, Mazier W, Drago F, Puente N, Reguero L, Elezgarai I, Dupuy JW, Cota D, Lopez-Rodriguez ML, Barreda-Gomez G, Massa F, Grandes P, Benard G, Marsicano G. A cannabinoid link between mitochondria and memory. Nature. 2016;539:555–559. doi: 10.1038/nature20127. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65:278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario MR, Clouse E, Yin HH, Costa RM. Endocannabinoid signaling is critical for habit formation. Front Integr Neurosci. 2007;1:6. doi: 10.3389/neuro.07.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario MR, Costa RM. High on habits. Front Neurosci. 2008;2:208–217. doi: 10.3389/neuro.01.030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT, Karatsoreos IN, Mackie K, Viau V, Pickel VM, McEwen BS, Liu QS, Gorzalka BB, Hillard CJ. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Weinlander KM, Stuhr KL. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. 2012;204:207–229. doi: 10.1016/j.neuroscience.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Zanotti-Fregonara P, Umhau JC, George DT, Rallis-Frutos D, Lyoo CH, Li CT, Hines CS, Sun H, Terry GE, Morse C, Zoghbi SS, Pike VW, Innis RB, Heilig M. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry. 2013;18:916–921. doi: 10.1038/mp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Barrett DA, Randall MD. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci. 2003;23:4815–4820. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76(Pt B):416–424. doi: 10.1016/j.neuropharm.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperatore R, Morello G, Luongo L, Taschler U, Romano R, De Gregorio D, Belardo C, Maione S, Di Marzo V, Cristino L. Genetic deletion of monoacylglycerol lipase leads to impaired cannabinoid receptor CB1 R signaling and anxiety-like behavior. J Neurochem. 2015;135:799–813. doi: 10.1111/jnc.13267. [DOI] [PubMed] [Google Scholar]
- Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, Katona I, Piomelli D, Manzoni OJ. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, Fiancette JF, Renault P, Piazza PV, Manzoni OJ. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry. 2013;18:729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralek JD, Meier D, Hinton JP, Bauer A, Parameswaran SA, Vishwanath A, Ramesh R, Schoenlein RW, Pfleiderer C, Orenstein J. Observation of coherent helimagnons and gilbert damping in an itinerant magnet. Phys Rev Lett. 2012;109:247204. doi: 10.1103/PhysRevLett.109.247204. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci. 2000;20:375–386. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS One. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, Matias I, De Smedt-Peyrusse V, Labrousse VF, Bretillon L, Matute C, Rodriguez-Puertas R, Laye S, Manzoni OJ. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci. 2011;14:345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- Lee TT, Hill MN. Age of stress exposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB(1) receptor binding in male rats. Neuroscience. 2013;249:106–114. doi: 10.1016/j.neuroscience.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Luthi A. Disinhibition, a Circuit Mechanism for Associative Learning and Memory. Neuron. 2015;88:264–276. doi: 10.1016/j.neuron.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex. 2009;19:165–175. doi: 10.1093/cercor/bhn075. [DOI] [PubMed] [Google Scholar]
- Long LE, Lind J, Webster M, Weickert CS. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci. 2012;13 doi: 10.1186/1471-2202-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace JW, Corches A, Vieira PA, Hiroto AS, Mackie K, Korzus E. An animal model of female adolescent cannabinoid exposure elicits a long-lasting deficit in presynaptic long-term plasticity. Neuropharmacology. 2015;99:242–255. doi: 10.1016/j.neuropharm.2015.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace JW, Vieira PA, Corches A, Mackie K, Korzus E. Impaired fear memory specificity associated with deficient endocannabinoid-dependent long-term plasticity. Neuropsychopharmacology. 2014;39:1685–1693. doi: 10.1038/npp.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Mathur BN. Endocannabinoids in striatal plasticity. Parkinsonism Relat Disord. 2012;18(Suppl 1):S132–134. doi: 10.1016/S1353-8020(11)70041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B, Marsicano G, Maldonado R, Hillard CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci. 2015;16:705–718. doi: 10.1038/nrn4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysenko LV, Kim J, Henry C, Tyrtyshnaia A, Kohnz RA, Madamba F, Simon GM, Kleschevnikova NE, Nomura DK, Ezekowitz RA, Kleschevnikov AM. Monoacylglycerol lipase inhibitor JZL184 improves behavior and neural properties in Ts65Dn mice, a model of down syndrome. PLoS One. 2014;9:e114521. doi: 10.1371/journal.pone.0114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglio LE, Noriega-Prieto JA, Maraver MJ, Fernandez de Sevilla D. Endocannabinoid-Dependent Long-Term Potentiation of Synaptic Transmission at Rat Barrel Cortex. Cereb Cortex. 2017:1–14. doi: 10.1093/cercor/bhx053. [DOI] [PubMed] [Google Scholar]
- Manduca A, Lassalle O, Sepers M, Campolongo P, Cuomo V, Marsicano G, Kieffer B, Vanderschuren LJ, Trezza V, Manzoni OJ. Interacting Cannabinoid and Opioid Receptors in the Nucleus Accumbens Core Control Adolescent Social Play. Front Behav Neurosci. 2016;10:211. doi: 10.3389/fnbeh.2016.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni OJ, Bockaert J. Cannabinoids inhibit GABAergic synaptic transmission in mice nucleus accumbens. Eur J Pharmacol. 2001;412:R3–5. doi: 10.1016/s0014-2999(01)00723-3. [DOI] [PubMed] [Google Scholar]
- Maroso M, Szabo GG, Kim HK, Alexander A, Bui AD, Lee SH, Lutz B, Soltesz I. Cannabinoid Control of Learning and Memory through HCN Channels. Neuron. 2016;89:1059–1073. doi: 10.1016/j.neuron.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS, Woodruff G, Fung S, Lafourcade M, Alexander JP, Long JZ, Li W, Xu C, Moller T, Mackie K, Manzoni OJ, Cravatt BF, Stella N. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AB, Fernandez-Espejo E, Ferrer B, Gorriti MA, Bilbao A, Navarro M, Rodriguez de Fonseca F, Moratalla R. Expression and function of CB1 receptor in the rat striatum: localization and effects on D1 and D2 dopamine receptor-mediated motor behaviors. Neuropsychopharmacology. 2008;33:1667–1679. doi: 10.1038/sj.npp.1301558. [DOI] [PubMed] [Google Scholar]
- Martin HG, Lassalle O, Manzoni OJ. Differential Adulthood Onset mGlu5 Signaling Saves Prefrontal Function in the Fragile X Mouse. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw328. [DOI] [PubMed] [Google Scholar]
- Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science. 2015;349:730–734. doi: 10.1126/science.aaa7945. [DOI] [PubMed] [Google Scholar]
- Mathur BN, Lovinger DM. Endocannabinoid-dopamine interactions in striatal synaptic plasticity. Front Pharmacol. 2012;3:66. doi: 10.3389/fphar.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci. 2004;7:585–586. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- Mato S, Robbe D, Puente N, Grandes P, Manzoni OJ. Presynaptic homeostatic plasticity rescues long-term depression after chronic Delta 9-tetrahydrocannabinol exposure. J Neurosci. 2005;25:11619–11627. doi: 10.1523/JNEUROSCI.2294-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Watanabe M, Mackie K, Katona I, Freund TF. Molecular architecture of the cannabinoid signaling system in the core of the nucleus accumbens. Ideggyogy Sz. 2007;60:187–191. [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011a;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011b;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Mechanisms of stress in the brain. Nat Neurosci. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA. The Endocannabinoid System and the Brain. Annu Rev Psychol. 2012 doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- Metna-Laurent M, Marsicano G. Rising stars: modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia. 2015;63:353–364. doi: 10.1002/glia.22773. [DOI] [PubMed] [Google Scholar]
- Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci. 2012;15:746–753. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68:113–126. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Diez A, Araque A. Astrocytes in endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130599. doi: 10.1098/rstb.2013.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Perea G, Maglio L, Pastor J, Garcia de Sola R, Araque A. Astrocyte calcium signal and gliotransmission in human brain tissue. Cereb Cortex. 2013;23:1240–1246. doi: 10.1093/cercor/bhs122. [DOI] [PubMed] [Google Scholar]
- Nazzaro C, Greco B, Cerovic M, Baxter P, Rubino T, Trusel M, Parolaro D, Tkatch T, Benfenati F, Pedarzani P, Tonini R. SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nat Neurosci. 2012 doi: 10.1038/nn.3022. [DOI] [PubMed] [Google Scholar]
- Njoo C, Agarwal N, Lutz B, Kuner R. The Cannabinoid Receptor CB1 Interacts with the WAVE1 Complex and Plays a Role in Actin Dynamics and Structural Plasticity in Neurons. PLoS Biol. 2015;13:e1002286. doi: 10.1371/journal.pbio.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AL, Hanson JE, Li D, Klotz A, Wright S, Schenk D, Seubert P, Madison DV. beta-Amyloid inhibits E-S potentiation through suppression of cannabinoid receptor 1-dependent synaptic disinhibition. Neuron. 2014;82:1334–1345. doi: 10.1016/j.neuron.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paille V, Fino E, Du K, Morera-Herreras T, Perez S, Kotaleski JH, Venance L. GABAergic circuits control spike-timing-dependent plasticity. J Neurosci. 2013;33:9353–9363. doi: 10.1523/JNEUROSCI.5796-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16:579–594. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci. 2005;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Peterfi Z, Urban GM, Papp OI, Nemeth B, Monyer H, Szabo G, Erdelyi F, Mackie K, Freund TF, Hajos N, Katona I. Endocannabinoid-mediated long-term depression of afferent excitatory synapses in hippocampal pyramidal cells and GABAergic interneurons. J Neurosci. 2012;32:14448–14463. doi: 10.1523/JNEUROSCI.1676-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L, Grandes P, Manzoni OJ. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci. 2011;14:1542–1547. doi: 10.1038/nn.2974. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Raver SM, Haughwout SP, Keller A. Adolescent cannabinoid exposure permanently suppresses cortical oscillations in adult mice. Neuropsychopharmacology. 2013;38:2338–2347. doi: 10.1038/npp.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini N, Vigano D, Guidali C, Zamberletti E, Rubino T, Parolaro D. Chronic URB597 treatment at adulthood reverted most depressive-like symptoms induced by adolescent exposure to THC in female rats. Neuropharmacology. 2011;60:235–243. doi: 10.1016/j.neuropharm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland AB, Ricobaraza A, Carrel D, Jordan BM, Rico F, Simon A, Humbert-Claude M, Ferrier J, McFadden MH, Scheuring S, Lenkei Z. Cannabinoid-induced actomyosin contractility shapes neuronal morphology and growth. Elife. 2014;3:e03159. doi: 10.7554/eLife.03159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Lovinger DM. Induction of striatal long-term synaptic depression by moderate frequency activation of cortical afferents in rat. J Physiol. 2005;562:245–256. doi: 10.1113/jphysiol.2004.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Nicoll RA. Endocannabinoids contribute to short-term but not long-term mGluR-induced depression in the hippocampus. Eur J Neurosci. 2003;18:1017–1020. doi: 10.1046/j.1460-9568.2003.02823.x. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Vigano D, Marras E, Petrosino S, Perletti G, Maccarrone M, Di Marzo V, Parolaro D. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex. 2008;18:1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev. 2016;68:816–871. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Plotkin JL, Francardo V, Ko WK, Xie Z, Li Q, Fieblinger T, Wess J, Neubig RR, Lindsley CW, Conn PJ, Greengard P, Bezard E, Cenci MA, Surmeier DJ. M4 Muscarinic Receptor Signaling Ameliorates Striatal Plasticity Deficits in Models of L-DOPA-Induced Dyskinesia. Neuron. 2015;88:762–773. doi: 10.1016/j.neuron.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr Opin Neurobiol. 2013;23:546–552. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed HE, Masiulis I, Gibson JR, Powell CM. Increased Cortical Inhibition in Autism-Linked Neuroligin-3R451C Mice Is Due in Part to Loss of Endocannabinoid Signaling. PLoS One. 2015;10:e0140638. doi: 10.1371/journal.pone.0140638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, Otte DM, Wojtalla A, Racz I, Ponomarenko A, Xi ZX, Zimmer A, Schmitz D. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron. 2016;90:795–809. doi: 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Alger BE. Homer protein-metabotropic glutamate receptor binding regulates endocannabinoid signaling and affects hyperexcitability in a mouse model of fragile X syndrome. J Neurosci. 2015;35:3938–3945. doi: 10.1523/JNEUROSCI.4499-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, Sakimura K, Kano M. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Jin X, Lima SQ, Costa RM. Complementary Contributions of Striatal Projection Pathways to Action Initiation and Execution. Cell. 2016;166:703–715. doi: 10.1016/j.cell.2016.06.032. [DOI] [PubMed] [Google Scholar]
- Thomazeau A, Lassalle O, Iafrati J, Souchet B, Guedj F, Janel N, Chavis P, Delabar J, Manzoni OJ. Prefrontal deficits in a murine model overexpressing the down syndrome candidate gene dyrk1a. J Neurosci. 2014;34:1138–1147. doi: 10.1523/JNEUROSCI.2852-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusel M, Cavaccini A, Gritti M, Greco B, Saintot PP, Nazzaro C, Cerovic M, Morella I, Brambilla R, Tonini R. Coordinated Regulation of Synaptic Plasticity at Striatopallidal and Striatonigral Neurons Orchestrates Motor Control. Cell Rep. 2015;13:1353–1365. doi: 10.1016/j.celrep.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M, Yamazaki M, Yamasaki M, Tanimura A, Sakimura K, Kano M, Watanabe M. Molecular and morphological configuration for 2-arachidonoylglycerol-mediated retrograde signaling at mossy cell-granule cell synapses in the dentate gyrus. J Neurosci. 2011;31:7700–7714. doi: 10.1523/JNEUROSCI.5665-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Beverley JA, Siman H, Tseng KY, Steiner H. CB1 Cannabinoid Receptor Expression in the Striatum: Association with Corticostriatal Circuits and Developmental Regulation. Front Pharmacol. 2012;3:21. doi: 10.3389/fphar.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente AM, Galvao-Ferreira P, Tecuapetla F, Costa RM. Direct and indirect dorsolateral striatum pathways reinforce different action strategies. Curr Biol. 2016;26:R267–269. doi: 10.1016/j.cub.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viganò D, Valenti M, Cascio MG, Di Marzo V, Parolaro D, Rubino T. Changes in endocannabinoid levels in a rat model of behavioural sensitization to morphine. Eur J Neurosci. 2004;20:1849–1857. doi: 10.1111/j.1460-9568.2004.03645.x. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hampson AJ, Baler RD. Don’t Worry, Be Happy: Endocannabinoids and Cannabis at the Intersection of Stress and Reward. Annu Rev Pharmacol Toxicol. 2017;57:285–308. doi: 10.1146/annurev-pharmtox-010716-104615. [DOI] [PubMed] [Google Scholar]
- Wang W, Trieu BH, Palmer LC, Jia Y, Pham DT, Jung KM, Karsten CA, Merrill CB, Mackie K, Gall CM, Piomelli D, Lynch G. A Primary Cortical Input to Hippocampus Expresses a Pathway-Specific and Endocannabinoid-Dependent Form of Long-Term Potentiation. eNeuro. 2016;3 doi: 10.1523/ENEURO.0160-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Doshi M, Hamazaki T. n-3 Polyunsaturated fatty acid (PUFA) deficiency elevates and n-3 PUFA enrichment reduces brain 2-arachidonoylglycerol level in mice. Prostaglandins Leukot Essent Fatty Acids. 2003;69:51–59. doi: 10.1016/s0952-3278(03)00056-5. [DOI] [PubMed] [Google Scholar]
- Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA, Reilly SK, Lin L, Fertuzinhos S, Miller JA, Murtha MT, Bichsel C, Niu W, Cotney J, Ercan-Sencicek AG, Gockley J, Gupta AR, Han W, He X, Hoffman EJ, Klei L, Lei J, Liu W, Liu L, Lu C, Xu X, Zhu Y, Mane SM, Lein ES, Wei L, Noonan JP, Roeder K, Devlin B, Sestan N, State MW. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell. 2013;155:997–1007. doi: 10.1016/j.cell.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. GABAergic inhibition in the neostriatum. Prog Brain Res. 2007;160:91–110. doi: 10.1016/S0079-6123(06)60006-X. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Winters BD, Kruger JM, Huang X, Gallaher ZR, Ishikawa M, Czaja K, Krueger JM, Huang YH, Schluter OM, Dong Y. Cannabinoid receptor 1-expressing neurons in the nucleus accumbens. Proc Natl Acad Sci U S A. 2012;109:E2717–2725. doi: 10.1073/pnas.1206303109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, Kim JI, Tawfik VL, Lalchandani RR, Scherrer G, Ding JB. Input- and cell-type-specific endocannabinoid-dependent LTD in the striatum. Cell Rep. 2015;10:75–87. doi: 10.1016/j.celrep.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Antion MD, Nomura T, Kraniotis S, Zhu Y, Contractor A. Hippocampal metaplasticity is required for the formation of temporal associative memories. J Neurosci. 2014;34:16762–16773. doi: 10.1523/JNEUROSCI.2869-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JY, Zhang J, Chen C. Long-Lasting Potentiation of Hippocampal Synaptic Transmission by Direct Cortical Input Is Mediated via Endocannabinoids. The Journal of physiology. 2012 doi: 10.1113/jphysiol.2011.223511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Davis MI, Ronesi JA, Lovinger DM. The role of protein synthesis in striatal long-term depression. J Neurosci. 2006;26:11811–11820. doi: 10.1523/JNEUROSCI.3196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci U S A. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H, Miyamae T, Hansen G, Zambrowicz B, Flynn M, Pedicord D, Blat Y, Westphal RS, Zaczek R, Lewis DA, Gonzalez-Burgos G. Postsynaptic diacylglycerol lipase mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. The Journal of physiology. 2011;589:4857–4884. doi: 10.1113/jphysiol.2011.212225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younts TJ, Castillo PE. Endogenous cannabinoid signaling at inhibitory interneurons. Curr Opin Neurobiol. 2014;26:42–50. doi: 10.1016/j.conb.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younts TJ, Chevaleyre V, Castillo PE. CA1 pyramidal cell theta-burst firing triggers endocannabinoid-mediated long-term depression at both somatic and dendritic inhibitory synapses. J Neurosci. 2013;33:13743–13757. doi: 10.1523/JNEUROSCI.0817-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younts TJ, Monday HR, Dudok B, Klein ME, Jordan BA, Katona I, Castillo PE. Presynaptic Protein Synthesis Is Required for Long-Term Plasticity of GABA Release. Neuron. 2016;92:479–492. doi: 10.1016/j.neuron.2016.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci. 2010;30:5724–5729. doi: 10.1523/JNEUROSCI.0795-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


