Abstract
To test the hypothesis that sleep can reverse cognitive impairment during Alzheimer's disease, we enhanced sleep in flies either co-expressing human amyloid precursor protein and Beta-secretase (APP:BACE), or in flies expressing human tau. The ubiquitous expression of APP:BACE or human tau disrupted sleep. The sleep deficits could be reversed and sleep could be enhanced when flies were administered the GABA-A agonist 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol (THIP). Expressing APP:BACE disrupted both Short-term memory (STM) and Long-term memory (LTM) as assessed using Aversive Phototaxic Suppression (APS) and courtship conditioning. Flies expressing APP:BACE also showed reduced levels of the synaptic protein discs large (DLG). Enhancing sleep in memory-impaired APP:BACE flies fully restored both STM and LTM and restored DLG levels. Sleep also restored STM to flies expressing human tau. Using live-brain imaging of individual clock neurons expressing both tau and the cAMP sensor Epac1-camps, we found that tau disrupted cAMP signaling. Importantly, enhancing sleep in flies expressing human tau restored proper cAMP signaling. Thus, we demonstrate that sleep can be used as a therapeutic to reverse deficits that accrue during the expression of toxic peptides associated with Alzheimer's disease.
Highlights
-
•
THIP can be used to enhance sleep in two Drosophila models of Alzheimer's disease.
-
•
Enhanced sleep reverses memory deficits in fly's expressing human APP:BACE and tau.
-
•
Enhanced sleep restores cAMP levels in clock neurons expressing tau.
-
•
Sleep can be used as a therapeutic to reverse Alzheimer's disease related deficits.
1. Introduction
Alzheimer’s disease is a complex disorder that has been linked with altered β-amyloid (Aβ) peptide processing, tau protein hyper-phosphorylation, inflammation, oxidative damage, reduced neurotrophins, an alteration in the balance between excitatory and inhibitory synapses and cognitive impairment leading to dementia (John and Berg, 2015, Li et al., 2016). It has become increasingly clear that abnormal phosphorylation of tau also plays a prominent role in the pathogenesis of Alzheimer's disease (Fernandez-Funez et al., 2015). A crosstalk between Aβ and tau has been demonstrated such that each may not only exert their toxic effects independently but also interact synergistically (Nisbet et al., 2015). As a consequence, therapeutic interventions that target either Aβ or tau separately may not be adequate to fully treat the disorder (Fernandez-Funez et al., 2015, Nisbet et al., 2015). In addition, recent studies suggest that Alzheimer's disease may be a collection of distinct diseases that likely requires separate therapeutic strategies (Ben-Gedalya et al., 2015, Vinters, 2015). Clearly, the complexity of Alzheimer's pathology has hindered the discovery of effective therapeutics.
In recent years, there has been a growing interest in the relationship between sleep and Alzheimer's pathogenesis (Xie et al., 2013, Roh et al., 2014). Importantly, several groups have hypothesized that improving sleep might be beneficial for slowing or attenuating cognitive deficits during Alzheimer's disease (Sperling and Johnson, 2012, Lucey and Holtzman, 2015, Musiek et al., 2015, Mander et al., 2016). Indeed, sleep is well suited for addressing complex diseases that can impact multiple physiological systems (Tononi and Cirelli, 2006, Imeri and Opp, 2009, Stickgold and Walker, 2013). As a consequence, sleep may be useful, even if indirectly, as a co-therapy to restore neuronal-functioning.
We have shown that inducing sleep can reverse age-dependent cognitive declines in Presenilin mutants (Psn), a Drosophila model of Familial Alzheimer's disease (McBride et al., 2010, Dissel et al., 2015a). In addition to restoring memory in Psn mutants, we reported that sleep reversed cognitive deficits in classic memory mutants with opposite neurophysiological deficits (Dissel et al., 2015a). Since sleep can independently improve memory from brains that are disrupted by different underlying mechanisms, our data indicate that sleep may indeed be useful to offset pathology associated with a complex disorder such as Alzheimer's disease. However, a closer inspection of our data indicates that while we have evaluated a diverse set of mutants, they share a common feature. That is, the mutations we have studied prevent a critical gene from carrying out its function (e.g. the adenylyl cyclase mutant rutabaga and the phosphodiesterase mutant dunce) (Dissel et al., 2015a). This stands in contrast to many degenerative diseases in which a mutation leads to the accumulation of toxic compounds that actively disrupt neuronal function over time.
In humans, Alzheimer's disease is associated with the progressive accumulation of β-amyloid plaques, neurofibrillary tangles, and loss of synapses. These pathological hallmarks are due, at least in part, to the accumulation of a toxic form of Aβ (Aβ42). While evidence suggests that Drosophila Psn mutants may generate toxic Aβ fragments (Carmine-Simmen et al., 2009), it is not clear whether these toxic fragments accumulate without co-expressing human amyloid precursor protein (APP) and the β-secretase enzyme involved in the processing of APP (BACE). Thus, the ability of sleep to reverse memory impairments in Psn mutants may not be an adequate test of our hypothesis that sleep can be used as a therapeutic to reverse pathology in the context of Alzheimer's disease. Moreover, the classic memory mutants we have studied are impaired from their first day of life and their memory phenotype does not seem to worsen (Davis, 2011, Kahsai and Zars, 2011). Thus, in some respects, their phenotype is static. In contrast, progressive diseases continuously change neuronal functioning thereby requiring that the brain constantly adapt. If a fundamental role for sleep is to modulate plasticity then sleep may be able to restore adaptive behavior even when neuronal functioning is continuously and progressively disrupted during degeneration. Alternatively, increasing sleep may not be able to overcome pathology when it is due to an active process as is the case for many degenerative diseases.
Determining whether sleep can influence degenerative processes is critical for determining whether sleep can be used as a therapeutic. There are several Drosophila models of Alzheimer's disease that produce neurotoxic misfolded peptides/proteins that result in progressive degeneration (Iijima et al., 2004, Mhatre et al., 2013, Fernandez-Funez et al., 2015, Tabuchi et al., 2015). Several studies have reported that co-expressing human amyloid precursor protein and β-secretase results in the production of abnormal Aβ peptides in flies, defective synapses, and memory impairments (Carmine-Simmen et al., 2009, Chakraborty et al., 2011, Mhatre et al., 2014, Bourdet et al., 2015). In addition, the overexpression of human tau in fly neurons results in age dependent vacuolization, neuronal degeneration and memory impairments (Wittmann et al., 2001, Mershin et al., 2004, Mhatre et al., 2013). Thus, we will evaluate the ability of sleep to reverse pathology and behavioral deficits resulting from either the co-expression of human APP and BACE or the overexpression of human tau.
2. Methods
2.1. Flies
Flies were cultured at 25 °C with 50–60% relative humidity and kept on a diet of yeast, dark corn syrup and agar under a 12-h light:12-h dark cycle. DaGsw-GAL4 were obtained from Marc Tater (Brown University) and UAS-APP:BACE flies were obtained from Daniel Marenda (Drexel University), UAS-Epac1.camps and pdf-GAL4 lines were obtained from Paul Taghert (Washington University in St. Louis), UAS-tau were obtained from the Bloomington stock center.
2.2. Sleep
Sleep was assessed as previously described (Shaw et al., 2000). Briefly, flies were placed into individual 65 mm tubes and all activity was continuously measured through the Trikinetics Drosophila Activity Monitoring System (www.Trikinetics.com, Waltham, Ma). Locomotor activity was measured in 1-minute bins and sleep was defined as periods of quiescence lasting at least 5 min.
2.3. Sleep deprivation
Sleep deprivation was performed as previously described (Shaw et al., 2002, Seugnet et al., 2008). Briefly, flies were placed into individual 65 mm tubes and the sleep-nullifying apparatus (SNAP) was used to sleep deprive these flies for 12 h during the dark phase (lights out to lights on). Sleep homeostasis was calculated for each individual as a ratio of the minutes of sleep gained above baseline during the 48 h of recovery divided by the total min of sleep lost during 12 h of sleep deprivation.
2.4. Short-term memory
Short-term memory (STM) was assessed by Aversive Phototaxic Suppression (APS) as previously described (Seugnet et al., 2008, Seugnet et al., 2009). The experimenters were blinded to conditions. In the APS, flies are individually placed in a T-maze and allowed to choose between a lighted and darkened chamber over 16 trials. Flies that do not display phototaxis during the first block of 4 trials are excluded from further analysis (Le Bourg and Buecher, 2002, Seugnet et al., 2009). During 16 trials, flies learn to avoid the lighted chamber that is paired with an aversive stimulus (quinine/ humidity). The performance index is calculated as the percentage of times the fly chooses the dark vial during the last 4 trials of the 16-trial test. In the absence of quinine, where no learning is possible, it is common to observe flies choosing the dark vial once during the last 4 trials in Block 4 (Seugnet et al., 2009). In contrast, flies never choose the dark vial 2 or more times during Block 4 in the absence of quinine (Seugnet et al., 2009). Thus, STM is defined as two or more photonegative choices in Block 4. For STM experiments following a 12 h sleep deprivation, the deprivation continued until evaluation by the APS. All flies were tested in the morning. Power analysis using G*Power calculates a Cohen’s d of 1.8 and indicates that eight flies/group are needed to obtain statistical differences (Seugnet et al., 2009).
2.5. Photosensitivity
Photosensitivity was evaluated as previously described (Seugnet et al., 2009). Briefly, flies were put in the T-maze over 10 trials in the absence of filter paper. The lightened and darkened chambers appeared equally on both the left and right. The photosensitivity index (PI) is the average of the scores obtained for 5–6 flies ±s.e.m.
2.6. Quinine sensitivity
Quinine sensitivity index (QSI) was evaluated as previously described (Seugnet et al., 2008, Seugnet et al., 2009). Briefly, flies were individually placed at the bottom of a 14 cm transparent cylindrical tube which was uniformly lighted and maintained horizontal after the introduction of the animal. Each half of the apparatus contained separate pieces of filter paper which could be wetted with quinine or kept dry. The QSI was determined by calculating the time in seconds that the fly spent on the dry side of the tube when the other side had been wetted with quinine, during a 5 min period.
2.7. Courtship conditioning
Training for males was based on previously described methods (Donlea et al., 2011). The males were exposed to a mated non-receptive female using a training protocol consisting of three 1-h training sessions, each separated by one hour. Long-term memory was tested forty-eight hours after the beginning of training, when trained and naive males were exposed to virgin females for a 10-min testing period (n=16–30 flies/condition). The Courtship Index (CI) is defined as the percent of time that each subject fly spends in courtship behavior during the testing period. The CIs were subjected to an arcsine square root transformation to approximate normal distribution as described in (Ishimoto et al., 2013). Data are presented as a Performance Index (PI), where PI=((CIaverage-naive−CI individual Trained)/CIaverage×100); PIs were evaluated using the Kruskal-Wallis test. The experimenters were blinded to condition. Vehicle-fed flies were maintained on vehicle throughout the experiment. For the 3-training session experiments with drug (i.e. THIP (T))-induced sleep, naïve males were fed THIP (0.1 mg/mL; 0.1 T) for 4 days prior to training. THIP-fed flies were removed from THIP 1 h prior to and during training (half of the 0.1T-fed flies were trained) and then returned to 0.1 T for 24 h post-training. Vehicle-fed flies were maintained on vehicle throughout the protocol.
2.8. Western blot
Sixteen fly brains per group were dissected and homogenized in 15 µl cell lysis buffer (10 mM Tris, pH 8.5/8 M urea/4% CHAPS /5 mM magnesium acetate) with 1x protease inhibitor cocktail (Roche). Lysates were normalized for proteins (Bradford protein assay-Biorad laboratories) and 1 µg of protein was mixed with sample buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromophenol blue and 0.125 M Tris HCl, pH approx. 6.8 – Sigma Aldrich, St Louis, MO) to a total of 12 µl. The samples were then heated to 100° Celsius for 5 min and then centrifuged for 3 min and loaded on a gradient gel (4–15% TGX (Biorad)). Gel was run at 80 V for 1 h and 100 V until the samples run off the gel and then transferred to PVDF membrane at 4 °C at 100 v for 1.5 h. Blot was probed with Rabbit Anti-Amyloid Precursor Protein (APP) antibody (Sigma Aldrich) 1:4000, mouse anti-TUBULIN antibody (E7-βTUBULIN; - Developmental Studies Hybridoma Bank University of Iowa)) 1:1000 followed by anti-rabbit secondary 1:1000 and anti-mouse secondary 1:1000 (Sigma Aldrich) respectively. Blot was visualized using ECL HRP substrate (Thermoscientific) and a Biorad chemiluminescence detector and quantified using ImageJ software (NIH). After background correction, optical densities were calculated and normalized (by dividing with the within-lane tubulin signal used as loading control). The protein/tubulin ratio of the treated samples was compared to the control lane in the same gel to measure relative changes. Statistical analysis was performed using Student’s t-test for two-group comparisons.
2.9. Pharmacology
THIP was administered at 0.1 mg/mL based upon a previous dose response study (Dissel et al., 2015a). Flies were maintained on the drug for the durations described in the text during which time sleep was monitored. Flies were removed from THIP one hour prior to being tested for short-term memory and one hour prior to being trained for courtship conditioning.
2.10. Live brain imaging
Flies were chilled for approximately 5 min prior to pinning them onto a sylgaard dissection dish. Brains were dissected in calcium-free HL3 (Stewart et al., 1994) and then transferred onto a poly-lysine treated dish (35×10 mm Falcon polystyrene) containing 3 ml of 1.5 mM calcium HL3. Two to four brains were assayed concurrently, typically a mutant line and its genetic controls. Image capture was done using an Olympus BX61 and x,y,z stage movements were set via SLIDEBOOK 5.0 (Intelligent Imaging Innovations), which controlled a Prior H105Plan Power Stage through a Prior ProScanII. Multiple YFP/CFP ratio measurements were recorded in sequence from each brain in the dish. Following baseline measurements, 1 ml of saline containing either PDF or DA, was added to the bath (dilution factor of 1/4). We used synthetic pigment dispersing factor, PDF, (Neo-MPS) and dopamine (Sigma-Aldrich). For further details see (Shafer et al., 2008, Klose et al., 2016).
2.11. Statistics
All comparisons were done using a Student's T-test or, if appropriate, ANOVA and subsequent planned comparisons using modified Bonferroni test unless otherwise stated. Note that a significant omnibus-F is not a requirement for conducting planned comparisons (Keppel, 1982). All statistically different groups are defined as *P<0.05.
3. Results
3.1. Co- expression of human APP and BACE in adult flies disrupts nighttime sleep and short-term memory
When Alzheimer's related genes are expressed in a tissue- and age-dependent manner using the bipartite GAL4-UAS system, flies exhibit a number of pathological hallmarks and behavioral impairments found in human patients with Alzheimer's disease including age dependent neurodegeneration, the accumulation of β-amyloid plaques, synaptic deficits, reduced life-span, and memory deficits (Brand and Perrimon, 1993, Greeve et al., 2004, Chakraborty et al., 2011, Mhatre et al., 2013, Mhatre et al., 2014, Blake et al., 2015). Surprisingly, while sleep disturbances are a common feature of Alzheimer's disease, very few studies have evaluated the impact of Alzheimer's related abnormal proteins on sleep in flies (Dissel et al., 2015a, Tabuchi et al., 2015, Gerstner et al., 2016). With that in mind, we examined sleep parameters in flies co-expressing human amyloid precursor protein (APP) and Beta-secretase (BACE) (UAS-APP:BACE). To avoid potential confounds resulting from the continuous expression of UAS-APP:BACE throughout development, we used the GeneSwitch system to restrict the expression of APP:BACE to adult flies (Osterwalder et al., 2001, Mao et al., 2004). In the GeneSwitch system, flies must be fed a steroid, RU486 (RU), to activate a GAL4-progesterone receptor fusion protein (Osterwalder et al., 2001).
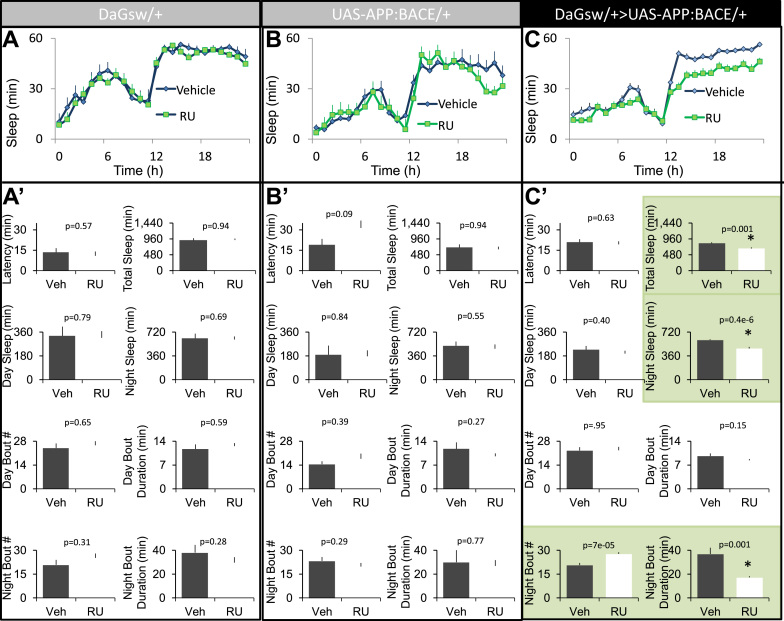
Parental controls, as well as experimental flies, were maintained on food containing either vehicle or RU for 5 days beginning on day 2 post-eclosion. Consistent with previous reports that RU, by itself, has no effect on sleep (Joiner et al., 2006, Seugnet et al., 2008) neither RU-fed DaGsw/+ nor UAS-APP:BACE/+ parental controls showed changes in sleep compared to their vehicle-fed siblings (Fig. 1A and B). A 2(Veh, RU)×Time (24-h) repeated measure ANOVA did not identify any significant ‘drug’ by ‘time’ interactions (ANOVA F[23,529]=0.53; p=0.79 and ANOVA F[23,437]=1.53; p=0.16, respectively). In contrast, RU-fed DaGsw/+>UAS-APP:BACE/+ flies exhibited significant changes in sleep compared to vehicle fed siblings: ANOVA F[23,1334]=3.13; p=8.70E-07(Fig. 1C). Closer examination of several sleep parameters including, sleep latency, daytime sleep, sleep bout duration during the day and night, and the number of sleep bouts revealed that the 5-days of adult expression of UAS-APP:BACE predominantly disrupted sleep parameters during the night (Fig. 1A′–C′; green panels). Thus, as with humans, Alzheimer's related proteins also disrupt sleep in flies.
Fig. 1.
Conditional expression of APP:BACE in adults disrupts nighttime sleep. A–B) Seven-day old DaGsw-GAL4/+ and UAS-APP:BACE/+ parental controls that had been maintained on RU exhibit similar sleep profiles compared to their Vehicle-fed siblings (n=11 flies/condition). C) Seven-day old RU-fed DaGsw-GAL4/+>UAS-APP:BACE/+ flies exhibited significantly disrupted sleep compared to Vehicle-fed siblings (n=28-30/condition). A′–B′) DaGsw-GAL4/+ and UAS-APP:BACE/+ flies fed RU did not exhibit changes in sleep latency, total sleep time, daytime sleep, night time sleep, the number of sleep bouts during the day or night or nighttime sleep compared to their vehicle-fed siblings; t-test. C′) RU fed DaGsw-GAL4/+>UAS-APP:BACE/+ flies exhibited a decrease in total sleep time, and nighttime sleep compared to Vehicle-fed siblings. RU-fed DaGsw-GAL4/+>UAS-APP:BACE/+ flies exhibited a reduction in sleep bout duration at night and an increased number of sleep bouts compared to Vehicle-fed siblings; t-test. Sample size is the same as above; green panels indicate parameters that are significantly different from Vehicle-fed controls. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
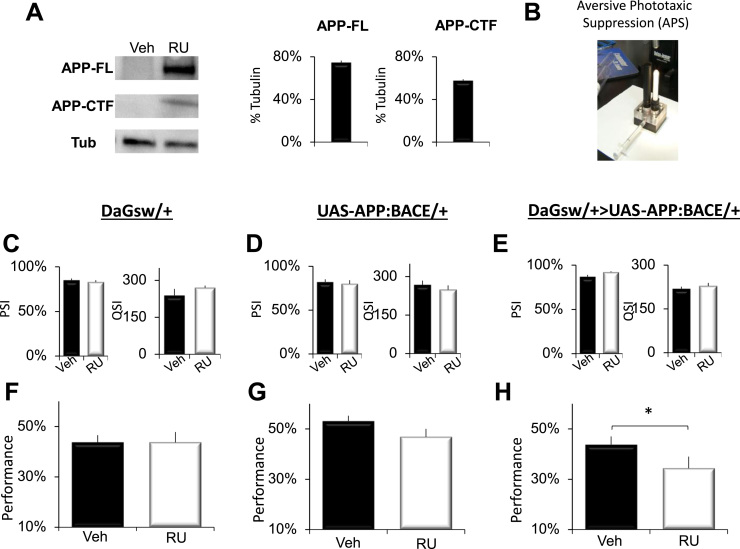
Previous studies have reported that the co-expression of amyloid precursor protein and β-secretase results in the production of Aβ peptides in flies (Carmine-Simmen et al., 2009, Chakraborty et al., 2011, Mhatre et al., 2014). To confirm that we could observe similar results, we performed Western blot analysis to detect both full length APP, ~110 kD (APP-FL), and Aβ C-terminal fragments (CTF),10–12 kD (CTF) in DaGsw-GAL4/+>UAS-APP:BACE flies. As seen in Fig. 2A, extracts from RU-fed DaGsw-GAL4/+>UAS-APP:BACE flies (16 brains/sample) revealed both APP-FL (full length APP, ~110 kD) and CTFs (~10 to 12 kD, using A8717 Sigma). Thus, RU-fed DaGsw-GAL4/+>UAS-APP:BACE flies produce Aβ-CTF peptides similar to that reported by others and that have been shown to give rise to elevated levels of neurotoxic Aβ42 (Chakraborty et al., 2011).
Fig. 2.
Conditional expression of APP:BACE in adults disrupts short-term memory. A) Western blot analysis of human APP and fly tubulin detected in brains (16 brains/sample) of RU-fed DaGsw-GAL4/+>UAS-APP:BACE/+ flies. APP-FL (full length APP, ~110 kD) and APP-CTFs (C-terminal fragments, ~10 to 12 kD) were detected using A8717. B) Image of the APS. C–E) No statistical differences in photosensitivity or quinine sensitivity were found between RU and Vehicle fed siblings of any genotype; t-test; n=6 flies/condition F) 7-day old RU-fed DaGsw-GAL4/+ parental controls perform identically in the APS compared to their Vehicle fed siblings (n=8 flies/group; t-test). G) 7-day old RU and Vehicle-fed UAS-APP:BACE/+ flies obtain statistically similar performance scores in the APS (n=8 flies/group; t-test). H) 7-day old RU-fed DaGsw-GAL4/+>UAS-APP:BACE/+ flies display performance deficits in the APS compared to vehicle-fed siblings (n=8 flies/group; *p<0.05, t-test).
To determine whether expressing β-CTF peptides would disrupt short-term memory, we evaluated performance of DaGsw-GAL4/+>UAS-APP:BACE flies and their parental controls using the well-established Aversive Phototaxic Suppression Assay (APS) (Le Bourg and Buecher, 2002, van Swinderen, 2011). In the APS, flies are individually placed in a T-maze and must learn to avoid a lighted chamber that is paired with an aversive stimulus (quinine/humidity) (Seugnet et al., 2008) (Fig. 2B). The performance index is calculated as the percentage of times the fly chooses the dark vial during the last 4 trials of the 16 trial test and STM is defined as selecting the dark vial on 2 or more occasions during Block 4 (Dissel et al., 2015a, Dissel et al., 2015b, Dissel et al., 2015c). Before being tested for STM, flies are first tested to ensure that they exhibit normal photosensitivity and quinine sensitivity (Le Bourg and Buecher, 2002, Seugnet et al., 2008, Seugnet et al., 2009). This step is important since changes to sensory thresholds could confound the ability to detect true changes in associative learning (Kahsai and Zars, 2011, Dubnau and Chiang, 2013, Dissel et al., 2015a). As seen in Fig. 2C and D, both RU-fed and Vehicle-fed DaGsw/+ and UAS-APP:BACE/+ parental controls display similar sensory thresholds. These data are consistent with previous reports demonstrating that RU does not alter a variety of behaviors including performance in the APS, olfactory conditioning, phototaxis, geotaxis, locomotion, the escape response, sleep homeostasis, and quinine sensitivity (Mao et al., 2004, Joiner et al., 2006, Seugnet et al., 2008, Dissel et al., 2015a, Thimgan et al., 2015). Moreover, neither RU-fed nor Vehicle-fed DaGsw-GAL4/+>UAS-APP:BACE siblings show changes in photosensitivity or quinine sensitivity, indicating that the expression of APP:BACE does not disrupt sensory thresholds (Fig. 2E). Despite having normal sensory thresholds, RU-fed DaGsw-GAL4/+>UAS-APP:BACE flies show a significant disruption in STM compared to vehicle-fed siblings(Fig. 2H), while no changes in performance are seen between RU and Vehicle-fed parental controls (Fig. 2F–G). Thus, the adult expression of APP:BACE disrupts STM as measured by the APS.
3.2. Enhancing sleep reverses short-term memory deficits flies expressing APP:BACE
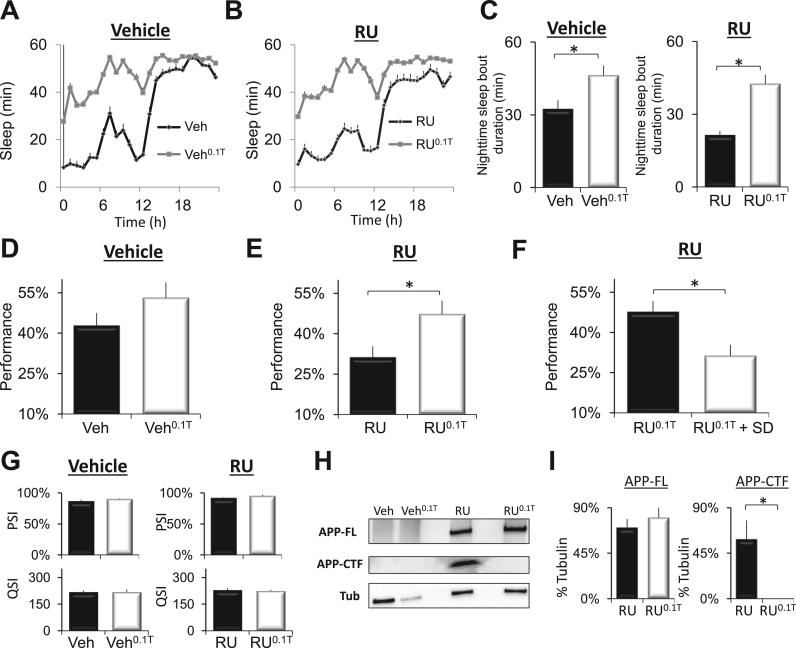
Recent studies in humans suggest that improving sleep might be beneficial for slowing or attenuating deficits associated with Alzheimer's disease in humans (Sperling and Johnson, 2012, Roh et al., 2014). Consistent with this hypothesis, we have shown that 2-days of sleep enhancement can reverse age-dependent cognitive declines in Presenilin mutants (Psn) (Dissel et al., 2015a). Although these latter data are encouraging, it is not clear whether toxic peptide fragments accumulate in Psn mutants without co-expressing APP and BACE (Carmine-Simmen et al., 2009). Thus, we asked whether sleep could reverse STM deficits in DaGsw-GAL4/+>UAS-APP:BACE/+ flies. Flies were maintained on either Vehicle or RU from day-2 post-eclosion until they were 12-days of age. The flies were then switched to food containing 0.1 mg/mL of the GABA-A agonist THIP for two additional days (RU0.1T, veh0.1T). As seen in Fig. 3A and B, THIP retained its ability to increase sleep in RU-fed DaGsw-GAL4/+>UAS-APP:BACE/+ flies compared to Vehicle-fed controls indicating that the sleep regulatory circuits remained accessible at this stage of pathology. A 2(RU, Veh) by 2(Veh, THIP)×24 (time) repeated measures ANOVA revealed a significant interaction; ANOVA F[23,3795]=1.74; p=0.015. Importantly, THIP also increased sleep consolidation at night during the fly's primary sleep period (Fig. 3C). Thus, THIP can be used to enhance sleep in 14 day-old flies over-expressing APP:BACE.
Fig. 3.
THIP-induced sleep restores performance to 14-day old DaGsw/+>UAS-APP:BACE/+ flies A) DaGsw-GAL4/+>UAS-APP:BACE/+ flies were maintained on Vehicle until day 12 and then randomly assigned to two groups for 2 additional days; one group was maintained on Vehicle (Veh), the other was placed on to food containing 0.1 mg/ml THIP (Veh0.1T). Flies maintained on Veh0.1T slept significantly more than their Vehicle-fed siblings (n=42/condition). B) DaGsw-GAL4/+>UAS-APP:BACE/+ flies were placed on to food containing RU for 10 days beginning on day 2 post eclosion. RU fed flies were then randomly assigned to two groups for 2 days; one group was maintained on RU (RU), the other was placed on to RU-food containing 0.1 mg/mL THIP (RU0.1T). Flies maintained on RU0.1T slept significantly more than their RU-fed siblings (n=42/condition). C) THIP administration significantly increased sleep consolidation at night in DaGsw/+>UAS-APP:BACE/+ flies maintained on Veh0.1T and RU0.1T; n=42 / condition. D) STM is not modified in 14-day old DaGsw-GAL4/+>UAS-APP:BACE/+ flies maintained on Veh or Veh0.1T (n=8 flies/group; t-test). E) Two-days of THIP-enhanced sleep restores STM to RU0.1TDaGsw-GAL4/+>UAS-APP:BACE/+ flies compared to RU-fed siblings (n=8 flies/group; *p<0.05, t-test). F) Sleep deprived DaGsw-GAL4/+>UAS-APP:BACE/+ flies maintained on RU0.1T did not show improvements in STM compared to non-sleep deprived RU0.1T controls (n=8 flies/group; *p<0.05, t-test). G) Neither RU, nor THIP significantly modified photosensitivity or quinine sensitivity (n=5–6 flies/group, t-test). H) Western blot analysis of human APP and fly β-actin detected in brains (16 brains/sample) of DaGsw-GAL4/+>UAS-APP:BACE/+ flies. APP-FL (full length APP, ~110 kD) and APP-CTFs (C terminal fragments, ~10 to 12 kD) were detected using A8717 (Sigma) as described in Chakraborty et al. (2011) C). I) Quantification of APP-FL in RU and RU0.1T siblings (n=4 samples/condition).
To determine whether THIP-induced sleep could reverse cognitive impairments, we also examined the performance of 14-day old DaGsw-GAL4/+>UAS-APP:BACE/+ flies in the APS. Consistent with previous reports that flies do not show impairments in the APS during natural aging (Le Bourg, 2004), neither vehicle nor veh0.1T fed DaGsw-GAL4/+>UAS-APP:BACE/+ flies showed performance decrements (Fig. 3D). In contrast to their vehicle-fed siblings, 14 day-old DaGsw-GAL4/+>UAS-APP:BACE/+ flies maintained on RU exhibited impaired STM (Fig. 3E, black). Importantly, two days of THIP-induced sleep (RU0.1T) beginning on day 12 reversed STM deficits (Fig. 3E, white). To determine whether the improvements in performance were due to increases in sleep per se or due to non-specific effects of the drug, flies were sleep deprived while on THIP. In the absence of sleep, THIP did not restore STM (Fig. 3F). Neither RU, nor THIP altered photosensitivity or quinine sensitivity indicating that the improved performance was due to specific changes in STM and not to changes in sensory thresholds (Fig. 3G). To determine what impact THIP might have on the processing of APP, we used Western blot analysis to evaluate the expression of APP-FL and APP-CTF. As seen in Fig. 3H and I, following sleep enhancement by THIP treatment, the levels of APP-CTF were reduced while full length APP levels remained unchanged. Thus, enhancing sleep can reverse cognitive deficits in flies co-expressing human APP and BACE and reduced Aβ C-terminal fragments which are known to be further cleaved in the toxic Aβ fragments.
3.3. Enhancing sleep reverses long-term memory deficits flies expressing APP:BACE
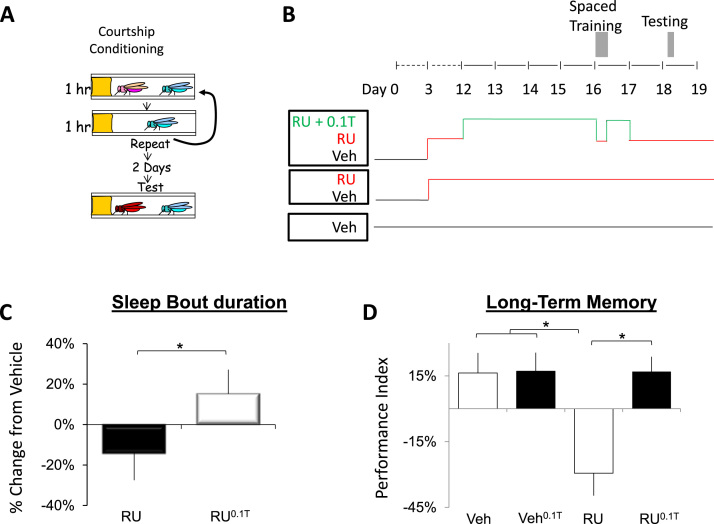
Although it is clear that independent memory assays cannot be used to validate each other, not all memory tasks are likely to benefit from sleep. Thus, we investigated whether inducing sleep may be beneficial for an additional and ethologically relevant memory assay (McBride et al., 2005, McBride et al., 2010). Previous studies have found that the pan-neuronal expression of APP:BACE resulted in deficits in short-term memory as assessed using courtship conditioning but the effects on long-term memory (LTM) using courtship memory remain unknown (Chakraborty et al., 2011). In courtship conditioning, a naïve male fly is exposed to a mated, non-receptive female who rejects his advances; associative memories are detected by a subsequent reduction in courtship when exposed to a receptive female (Fig. 4A) (Donlea et al., 2011, Donlea et al., 2012, Dissel et al., 2015a). To evaluate LTM, flies are exposed to three one-hour training sessions, each separated by one hour; reductions in courtship (e.g., LTM) can be found several days later. To evaluate the effects of sleep on LTM in DaGsw-GAL4/+>UAS-APP:BACE/+ flies, naïve males were maintained on RU until they were 12 days of age as above (Fig. 4B). On day 12, flies were placed onto food containing both RU and 0.1 mg/mL of THIP (RU0.1T) for 4 days. Flies were removed from THIP during training to ensure that they wouldn’t be too somnolent to exhibit normal courtship (Dissel et al., 2015a) (Fig. 4B). A negative-rebound is observed immediately following removal from THIP (Dissel et al., 2015a). As a consequence, flies were placed back onto RU0.1T food for 24 h following training to prevent the negative rebound from occurring during memory consolidation (Dissel et al., 2015a). After 24 h, flies were placed onto food containing only RU for 24 h before being tested for LTM (Fig. 4B).
Fig. 4.
THIP-induced sleep restores Long-Term Memory (LTM) to 14-day old DaGsw/+>UAS-APP:BACE/+ flies A) Schematic of courtship conditioning (see text for details). B) Protocol for the timing of administration of RU, THIP, and training. Vehicle-fed flies were maintained on Vehicle throughout the experiment (black); RU fed flies were placed onto RU beginning on Day 2 post-eclosion (red). Flies treated with 0.1 mg/mL THIP were placed onto RU on Day 2 post-eclosion and switched to food containing both RU and THIP (RU0.1T) for 4 days beginning on day 12 and again for 24 h after training (Red). C) Sleep bout duration measured between the end of training and lights out (Zeitgeber time 12) for each group of trained flies (Vehicle, RU and RU0.1T) expressed as a percentage change from trained Vehicle-fed siblings. RU0.1TDaGsw/+>UAS-APP:BACE/+ flies initiated significantly longer sleep bouts following training than RU fed siblings; n=18-19 flies/ condition; *p<0.05, t-test. D) RU-fed DaGsw/+>UAS-APP:BACE/+ flies did not show evidence of LTM as indicated by a significantly reduced performance index (PI) compared to both Vehicle-fed flies, vehicle-fed flies maintained on 0.1 mg/mL of THIP (veh0.1T), or RU0.1T siblings; a 2 (veh, RU)×2 (veh, THIP) two-way ANOVA, n=17-18 flies/group, *p<0.05 modified Bonferroni test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
An increase in sleep is frequently observed directly after training in flies that subsequently exhibit an LTM 48-h later (Ganguly-Fitzgerald et al., 2006, Donlea et al., 2011). Thus, we asked whether RU or RU0.1T flies would show an increase in sleep consolidation in the hours following training. Interestingly, RU-fed DaGsw-GAL4/+>UAS-APP:BACE/+ flies displayed a deficit in their ability to consolidate sleep following training compared to their THIP fed siblings (Fig. 4C). Not surprisingly then, RU-fed DaGsw-GAL4/+>UAS-APP:BACE/+ flies also displayed deficits in LTM compared to their vehicle-fed siblings (Fig. 4D). In contrast, RU0.1T flies exhibited courtship memory that was not significantly different from healthy, vehicle fed siblings (Fig. 4D); a 2 (veh, RU)×2 (veh, THIP) two-way ANOVA revealed a significant interaction ANOVA F[1,67]=6.84; p=0.011. Krustal-Wallis, p=0.001. Thus, increasing sleep restores cognitive behavior when assessed using two independent memory assays that utilize different sensory modalities and neuronal circuits.
3.4. Sleep reverses synaptic deficits in flies expressing APP:BACE
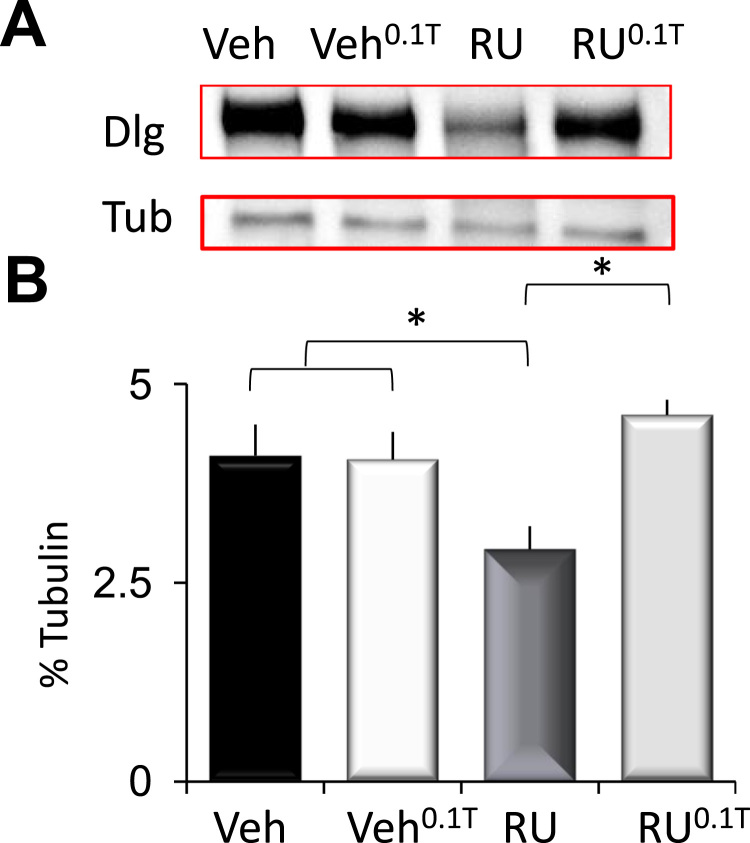
The co-expression of APP and BACE has been reported to damage synapses (Mhatre et al., 2014). Thus we examined whether THIP-induced sleep would impact the expression of the synaptic protein discs large (DLG). As seen in Fig. 5, levels of DLG protein were reduced in RU-fed 14-d old DaGsw-GAL4/+>UAS-APP:BACE/+ flies compared to vehicle-fed siblings. Importantly, DLG levels return to baseline following THIP-induced sleep (Fig. 5A–B). A 2(Veh, RU)×2 (Veh, 0.1 T) ANOVA revealed a significant drug (Veh, Ru)×drug (Veh, 0.1 T) interaction; ANOVA F[1,4]=78.67; p=0.05. Together these data suggest that sleep can benefits both physiology and behavior in flies co-expressing human APP and BACE.
Fig. 5.
Sleep restores the levels of the synaptic protein discs large (DLG) in DaGsw/+> APP:BACE/+ flies. A) Western blot of DaGsw/+> APP:BACE/+ flies maintained on Vehicle or RU, and Vehicle and RU with 0.1 mg/mL of THIP (Veh0.1T, RU0.1T); (6 brains/sample). RU-fed flies show reduced DLG protein compared to their vehicle fed siblings. B) Quantification of DLG. Following THIP induced sleep, DLG levels return to baseline (RU0.1T); *p<0.05, modified Bonferroni test.
3.5. Enhancing sleep reverses memory deficits flies expressing human Tau
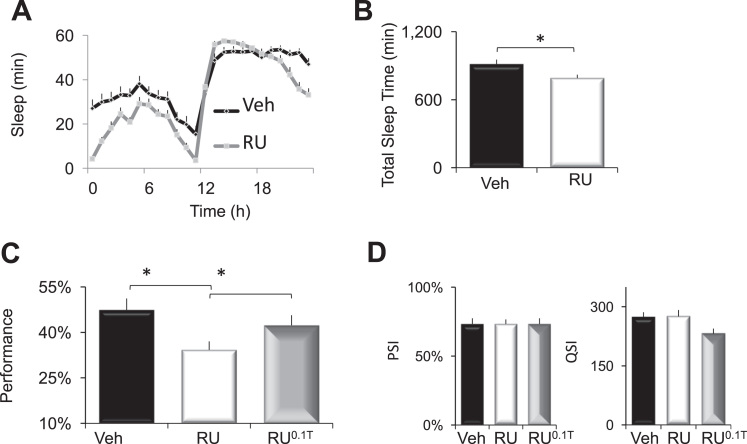
As mentioned above, it has become increasingly clear that tau also plays a prominent role in the pathogenesis of Alzheimer's disease (Fernandez-Funez et al., 2015). We have previously reported age-dependent cognitive decline in flies expressing UAS-tau under the control of the MBGeneSwitch-GAL4 driver (Seugnet et al., 2009). Thus, we asked whether sleep might also be beneficial to flies expressing UAS-tau. To allow comparisons with the APP:BACE data presented above, we used the Daughterless-GeneSwitch-GAL4 (DaGsw) driver to express UAS-tau in adult flies after 5 days of RU administration. As seen in Fig. 6A, sleep in RU-fed DaGsw/+>UAS-tau/+ flies was significantly different than Vehicle-fed siblings; A 2(Vehicle, RU) X 24 (time) ANOVA revealed a significant Drug X Time interaction ANOVA F[23,1357]=5.86; p=9.99E-16. RU-fed DaGsw/+>UAS-tau/+ flies showed significant reductions in total sleep time (Fig. 6B). Surprisingly, and in contrast to APP:BACE expression, sleep was significantly reduced during the day but not the night and other sleep parameters (e.g., sleep latency, sleep bout duration) were not significantly altered (data not shown). These data suggest that APP:BACE and tau differentially modify sleep circuitry. Identifying how these proteins differ will be an interesting topic for future investigations.
Fig. 6.
THIP-induced sleep restores Short -Term Memory (STM) to DaGsw/+>UAS-tau/+ flies. A) Sleep in RU-fed DaGsw/+>UAS-tau/+ flies was significantly different from Vehicle-fed siblings; n=27–32 flies/condition. B) Total Sleep Time was significantly reduced in RU-fed DaGsw/+>UAS-tau/+ flies compared to Vehicle-fed siblings; n=27–32 flies/condition; p<0.05, t-test. C) RU-fed DaGsw/+>UAS-tau/+ flies exhibited disrupted STM compared to Vehicle-fed siblings and siblings maintained on RU and THIP for 2 days (n=13–19 flies/group); *p<0.05, modified Bonferroni test. D) Neither RU, nor THIP significantly modified altered photosensitivity or quinine sensitivity ANOVA F[2,15]=0; p=1 and ANOVA F[2,14]=3.6; p=0.058, respectively (n=5–6 flies/group).
Although our data suggest that sleep can be beneficial for flies mutant for Presenilin or flies expressing APP:BACE, the pathophysiology of tau may be difficult to reverse. Thus, we examined STM in DaGsw/+>UAS-tau/+ after 5 days of RU administration. As seen in Fig. 6C, RU-fed DaGsw/+>UAS-tau/+ flies showed significantly impaired STM in the APS compared to vehicle-fed siblings and these deficits were reversed following two days of THIP-induced sleep; a One-way ANOVA revealed a main effect for treatment (Veh, RU, RU0.T) F[2,48]=4.2; p=0.02. Importantly, photosensitivity and quinine sensitivity were statistically similar in DaGsw/+>UAS-tau/+ siblings maintained on Vehicle, RU, or RU0.T (Fig. 6D) indicating that the changes in performance were due to associative learning and not confounding alterations in sensory thresholds. Thus, sleep can reverse cognitive impairments in Psn mutants, as well as flies co-expressing human APP and BACE and flies overexpressing tau.
3.6. Enhancing sleep reverses cAMP signaling deficits in clock neurons expressing tau
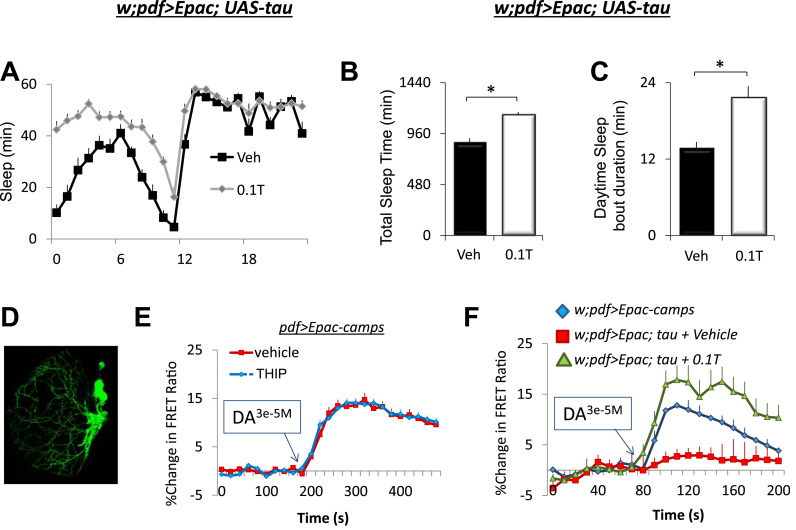
Several elegant studies have recently shown that expressing transgenic Alzheimer's related genes in clock neurons can alter morphology, physiology and behavior (Chen et al., 2014; Blake et al., 2015; Bouleau and Tricoire, 2015). Thus, we wished to examine the anatomical and physiological consequences of expressing UAS-tau in clock neurons. Given the important role that the large-and small ventral lateral neurons (lLNvs, sLNvs) play in regulating sleep and waking, as well as the circadian clock (Parisky et al., 2008; Shang et al., 2008; Sheeba et al., 2008; Chung et al., 2009), we determined if expressing UAS-tau altered the physiological properties of lLNvs and whether any such changes could be modulated by sleep. Several studies have used live-brain imaging to define cAMP response properties in individual LNvs neurons with Epac1-camps (Shafer et al., 2008; Shang et al., 2008; Shang et al., 2011; Klose et al., 2016). Thus, we expressed UAS-Epac1-camps using pdf-GAL4 and examined cAMP responses in lLNvs in response to dopamine. To begin we asked whether THIP could increase sleep in pdf-GAL4/+>UAS-Epac; UAS-tau/+ flies. As seen in Fig. 7A–C, THIP increased both total sleep time and sleep consolidation during the day compared to age-matched, vehicle-fed siblings. Consistent with previous reports, dopamine modulates cAMP levels in single LNv neurons (Fig. 7D,E). Importantly, the cAMP responses of LNv neurons are not modulated in flies maintained on 0.1 mg/mL of THIP for two days (Fig. 7E). Expressing tau in pdf-GAL4>UAS-Epac-camps expressing neurons substantially reduced the increase in cAMP usually seen following the administration of Dopamine (DA; Fig. 7F, red) (Shang et al., 2011). Interestingly, five days of THIP- induced sleep can restore the response properties of clock neurons (Fig. 7F green).
Fig. 7.
Sleep reverses the abnormal response properties of ventrolateral clock neurons (LNvs) expressing tau. A) pdf-GAL4/+>UAS-Epac; UAS-tau/+ flies increased sleep when placed onto 0.1 T; 2 (Veh, 0.1 T)×24 (time) repeated measures ANOVA F[23,506]=6.94; p=9.99E-16; n=12 flies/condition. B) Total Sleep Time was significantly increased in 0.1 T fed pdf-GAL4/+>UAS-Epac; flies compared to Vehicle-fed siblings; n=12 flies/condition; p=9.25E-06, t-test. C) Daytime sleep bout duration was significantly increased in 0.1 T fed pdf-GAL4/+>UAS-Epac flies compared to Vehicle-fed siblings; n=12 flies/condition; p=5.6E-04, t-test. D) Confocal image of pdf-GAL4>GFP flies showing the location of the LNvs E) THIP-induced sleep does not alter cAMP levels in wild-type pdf>Epac-camps flies in response to 3e-5M Dopamine(DA). F) Expressing UAS-tau reduces the change in cAMP in response to DA (red) compared to controls (blue). Interestingly, 5 days of THIP-induced sleep fully restored cAMP levels to normal in flies expressing tau (green). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In the present study, we tested the hypothesis that sleep could be used as a therapeutic to reverse memory impairments and the underlying pathology in Drosophila models of Alzheimer's disease. We used two established models of Alzheimer's disease previously shown to produce memory impairments and age-dependent brain degeneration (Wittmann et al., 2001, Chakraborty et al., 2011, Mhatre et al., 2013, Mhatre et al., 2014). Each of the Alzheimer's model evaluated, human APP:BACE and tau mutants, disrupted sleep and produced memory impairments. Although recent studies suggest that the onset of Alzheimer's pathology can be delayed by preventing sleep deficits (Ju et al., 2013, Yaffe et al., 2014), we wished to know whether sleep could also reverse these deficits after the onset of pathology had been established. The ability to reverse aspects of Alzheimer's pathology could prove valuable since it is frequently difficult to diagnose and treat the disorder prior to the point where pathology is clearly evident (Ryman et al., 2014). Our data indicate that enhancing sleep can reverse both behavioral and physiological deficits in Alzheimer's flies.
Recent studies have focused on a prophylactic role of preventing sleep deficits in treating Alzheimer's disease (Musiek et al., 2015). That is, current-hypotheses propose that sleep disturbances early in life might aggravate Alzheimer's pathology in susceptible individuals (Kang et al., 2009, Yaffe et al., 2014, Colby-Milley et al., 2015, Tabuchi et al., 2015, Branger et al., 2016). This view is supported by a growing body of data in humans, rodents and flies. Indeed, acute sleep deprivation increased levels of Aβ in the interstitial fluid of in Tg2576 mice, while chronic sleep restriction significantly and enhanced Aβ plaque deposition (Kang et al., 2009). Moreover, when sleep was chronically disrupted in 3xTg mice, which express PS1(M146V), APP(Swe), and tau(P301L) transgenes, they exhibited significant memory impairments, altered tau metabolism, and lower levels of the post-synaptic density protein 95 (PSD95) (Di Meco et al., 2014). An increase in Aβ and Tau levels were also found in the cortex of 3xTg mice undergoing chronic sleep restriction for 6 weeks (Rothman et al., 2013). Interestingly, in humans, poor sleep quality in pre-clinical patients is associated with increased amyloid deposition (Ju et al., 2013, Branger et al., 2016). In addition, women with sleep disrupted breathing show an increased risk of developing cognitive impairment or dementia (Yaffe et al., 2011). Importantly, sleep deprivation also increases Aβ burden in fly models of Alzheimer's disease (Tabuchi et al., 2015). The observation that insufficient sleep aggravates Alzheimer's pathology across such diverse species highlights the importance of sleep deficits in disease progression.
Sleep disturbances are common features of Alzheimer's disease. Thus, even for individuals that may not have experienced sleep-disturbances early in life, sleep will most likely be disrupted when Alzheimer's related neural degeneration begins to impact sleep and circadian circuits (Colby-Milley et al., 2015, Branger et al., 2016). Unfortunately, ameliorating sleep deficits in this population is difficult (see Musiek et al. (2015) for discussion). Given the increased risk of adverse events when older adults are administered sedative-hypnotics, few studies have systematically examined the impact of long-term use of sleep agents during Alzheimer's disease. Interestingly, two weeks of Trazadone administration improved both sleep and circadian parameters in Alzheimer's patients (Camargos et al., 2014, Grippe et al., 2015). Unfortunately, two weeks of Trazadone did not improve cognition in these individuals (Camargos et al., 2014). In rodents, the administration of Almorexant significantly reduced Aβ plaque formation in Tg2576 mice suggesting that hypocretin/orexin antagonists might be useful for ameliorating some Alzheimer's pathology (Kang et al., 2009). Interestingly, increasing sleep by administering Growth Hormone-releasing hormone in transgenic mice that overexpress mutant forms of APP and PSEN1 also resulted in a decrease in Aβ levels suggesting that hypnotics, in general, may eventually prove useful in offsetting some Alzheimer's pathology (Liao et al., 2015). However, hypnotics can also have a negative impact on plasticity and identifying an appropriate compound that can increase sleep but does not also produce negative side-effects is non-trivial (Aton et al., 2009, Vienne et al., 2012). If too many side-effects are found with currently available hypnotics, it will be of interest to determine whether newer genetic methods for enhancing sleep in rodents can reverse/attenuate or slow-down Alzheimer's pathology in rodent models (Jego et al., 2013, Konadhode et al., 2013, Anaclet et al., 2014).
Although we have identified genetic tools that can be used to enhance sleep in Drosophila (Donlea et al., 2011), a goal of the present study is to determine whether pharmacological agents might be useful for increasing sleep in the context of Alzheimer's related memory impairments. Clearly, a pharmacological agent will be required if sleep is to be useful as a therapeutic for treating Alzheimer's disease in humans (Musiek et al., 2015). Thus, we chose to enhance sleep pharmacologically by administering the GABA-A agonist THIP. THIP has recently been identified as a powerful sleep promoting agent in flies (Berry et al., 2015, Dissel et al., 2015a). THIP increases sleep-time as well as sleep-consolidation via the Ligand-gated chloride channel homolog 3 and the Glycine receptor GABA-A receptors which have homology with human GABA-A beta3 and alpha6 subunits, respectively (Dissel et al., 2015a) (http://www.flyrnai.org/diopt). Importantly, the sleep seen following THIP administration meets all of the criteria needed for identifying sleep including increased arousal thresholds, rapid reversibility and homeostatic regulation (Hendricks et al., 2000, Shaw et al., 2000). Moreover, the sleep induced by THIP produces similar effects on molecular and physiological parameters that are modulated by spontaneous sleep including synaptic markers, immune-related transcripts and changes in local field potentials (van Alphen et al., 2013, Dissel et al., 2015a). Indeed, while THIP substantially increases sleep, the total amount of sleep and sleep architecture observed closely resemble other conditions of high sleep drive such as during recovery sleep following sleep deprivation, sleep during the early stages of adult life, sleep following social enrichment, and sleep following training protocols that induce LTM using courtship conditioning (Donlea et al., 2009, Seugnet et al., 2011, Thimgan et al., 2015). Importantly, the effects of THIP-induced sleep have been shown to be identical to that observed using two molecularly-distinct methods of genetically-increasing sleep (Berry et al., 2015, Dissel et al., 2015a). Together with the observation that the beneficial effects of THIP are not observed in the absence of sleep (Fig. 3F and Dissel et al. (2015a)), the data strongly indicates that the benefits of THIP are due to sleep-itself and not due to non-specific effects of the drug. In fact, our data using THIP to reverse Alzheimer's pathology are consistent with a recent report showing that genetically-enhancing sleep reduces Aβ deposition in flies (Tabuchi et al., 2015). Thus, these results provide proof-of-principle data that sleep can be enhanced pharmacologically to offset pathology associated with Alzheimer's disease.
Discovery experiments intended to better elucidate the pathophysiology of Alzheimer's disease have been hampered both by the complexity of the disorder and also by the fact that Alzheimer's pathology does not impact all types of neurons equally. An advantage of using Drosophila is that tools are available to monitor the structure and physiology of specific, genetically-accessible neurons. A set of 16 clock neurons, known as the small and large ventral lateral neurons (sLNVs and lLNvs, respectively) are well suited for elucidating the relationship between sleep and Alzheimer's disease. First, these clock neurons influence a surprising number of important behaviors (e.g., memory, sleep, social enrichment, age-dependent cognitive decline, ethanol sensitivity, cocaine sensitization) (Andretic et al., 1999; Shang et al., 2008; Donlea et al., 2009; Eddison et al., 2011; Donlea et al., 2014). Second, clock neurons display stereotyped, plastic changes in their anatomical projection patterns that can be easily quantified during disease and subsequent therapeutic interventions (Fernandez et al., 2008). Third, the physiological properties of individual clock neurons can be quantified using a variety of imaging techniques to better understand their underlying physiology (Shafer et al., 2008; Yao et al., 2012; Cao et al., 2013). Fourth, clock neurons have been the subject of several intensive transcriptomic studies and thus a great deal is known about genes that are present in these neurons (Kula-Eversole et al., 2010; Hadzic et al., 2015; Petsakou et al., 2015). Finally, recent studies have found that expressing transgenic Alzheimer's related genes in clock neurons can alter their morphology, physiology and behavior, sometimes in surprising ways (Pirooznia et al., 2012; Chen et al., 2014; Blake et al., 2015; Bouleau and Tricoire 2015; Tabuchi et al., 2015). Since previous studies had focused on the behavioral, physiological and molecular effects of APP processing and the expression of Aβ-like peptides in clock neurons (Chen et al., 2014; Blake et al., 2015; Tabuchi et al., 2015), we examined the physiological consequences of expressing human tau. The overexpression of human tau had profound negative effects on the physiology of LNv neurons as indicated by reduced cAMP signaling in response to dopamine. Clock neurons are believed to support behavioral flexibility by regulating the integration of diverse environmental cues across the circadian day (Shang et al., 2011; Choi et al., 2012; Duvall and Taghert, 2012; Zhang and Emery, 2013). Within clock neurons, both the dopamine receptor and pigment dispersing factor receptor (Pdfr) exert their effects, in part, by modulating cAMP (Shafer et al., 2008; Shang et al., 2011; Li et al., 2014). Indeed, the ability of the clock to gate when external signals can drive cAMP oscillations may be crucial for synchronizing circadian circuits. (Collins et al., 2014). In addition to its role in circadian behavior, cAMP also plays important role in sleep and memory consolidation (Hendricks et al., 2001; Havekes et al., 2016). Thus, by restoring cAMP signaling in w;pdf>Epac; tau, flies sleep has the potential to positively influence a number of important behaviors regulated by the clock. Nonetheless, these data emphasize that sleep can restore normal functioning to unhealthy neurons expressing a toxic protein. How sleep restores the response of clock neurons to cAMP and whether sleep improves behavior by modulating other signaling pathways will undoubtedly be addressed in future studies.
We have shown that pharmacologically enhancing sleep can reverse cognitive deficits in mutants with opposing neurophysiological deficits without repairing the precise underlying genetic lesion (Dissel et al., 2015a). The observation that sleep can independently restore memory when brains are disrupted in different ways, suggests that sleep may play a more fundamental role in modulating plasticity than has been previously recognized. This observation may have several potential ramifications for treating Alzheimer's disease. First, sleep is well suited for treating a complex disease that involves more than one toxic protein and that can impact multiple physiological systems. Second, sleep may be useful as a co-therapy in conjunction with promising compounds that may produce negative side effects on their own (Vassar, 2014, Blake et al., 2015). That is, sleep may not only reduce the pathology associated with Alzheimer's disease (thereby allowing for lower doses of the therapeutic) it may also work independently to offset any negative side-effects of the therapeutic compound. Third, sleep may be beneficial, even if indirectly, by restoring neuronal-functioning sufficiently to re-open the therapeutic window for patients whose disease has progressed beyond the point where compounds can be effective. Fourth, identifying the molecular mechanisms underlying the positive effects of sleep may create additional treatment opportunities for slowing or offsetting disease progression. Together with the recent work by other labs (Kang et al., 2009, Chen et al., 2014, Mhatre et al., 2014, Blake et al., 2015, Tabuchi et al., 2015), these data provide new avenues for harnessing the power of Drosophila to better understand the therapeutic potential of sleep for reversing the pathophysiology of Alzheimer's disease.
Conflicts of interest
None of the authors have any conflicts of interest to declare.
Acknowledgments
We thank Drs. Holtzman and Kirszenblat for discussions and comments on this project. This study was funded by NIH Grants R01 NS051305-11, R01 NS076980-05, to PJS and the NIH Neuroscience Blueprint Core Grant, #NS057105.
References
- Anaclet C., Ferrari L., Arrigoni E., Bass C.E., Saper C.B. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat. Neurosci. 2014;17:1217–1224. doi: 10.1038/nn.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R., Chaney S., Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- Aton S.J., Seibt J., Dumoulin M.C., Coleman T., Shiraishi M. The sedating antidepressant trazodone impairs sleep-dependent cortical plasticity. PLoS One. 2009;4:e6078. doi: 10.1371/journal.pone.0006078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Gedalya T., Moll L., Bejerano-Sagie M., Frere S., Cabral W.A. Alzheimer’s disease-causing proline substitutions lead to presenilin 1 aggregation and malfunction. EMBO J. 2015;34:2820–2839. doi: 10.15252/embj.201592042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J.A., Cervantes-Sandoval I., Chakraborty M., Davis R.L. Sleep facilitates memory by blocking dopamine neuron-mediated forgetting. Cell. 2015;161:1656–1667. doi: 10.1016/j.cell.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M.R., Holbrook S.D., Kotwica-Rolinska J., Chow E.S., Kretzschmar D. Manipulations of amyloid precursor protein cleavage disrupt the circadian clock in aging Drosophila. Neurobiol. Dis. 2015;77:117–126. doi: 10.1016/j.nbd.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouleau S., Tricoire H. Drosophila models of Alzheimer’s disease: advances, limits, and perspectives. J. Alzheimers Dis. 2015;45:1015–1038. doi: 10.3233/JAD-142802. [DOI] [PubMed] [Google Scholar]
- Bourdet I., Lampin-Saint-Amaux A., Preat T., Goguel V. Amyloid-beta peptide exacerbates the memory deficit caused by amyloid precursor protein loss-of-function in drosophila. PLoS One. 2015;10:e0135741. doi: 10.1371/journal.pone.0135741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Branger P., Arenaza-Urquijo E.M., Tomadesso C., Mezenge F., Andre C. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol. Aging. 2016;41:107–114. doi: 10.1016/j.neurobiolaging.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Camargos E.F., Louzada L.L., Quintas J.L., Naves J.O., Louzada F.M. Trazodone improves sleep parameters in Alzheimer disease patients: a randomized, double-blind, and placebo-controlled study. Am. J Geriatr. Psychiatry. 2014;22:1565–1574. doi: 10.1016/j.jagp.2013.12.174. [DOI] [PubMed] [Google Scholar]
- Cao G., Platisa J., Pieribone V.A., Raccuglia D., Kunst M. Genetically targeted optical electrophysiology in intact neural circuits. Cell. 2013;154:904–913. doi: 10.1016/j.cell.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmine-Simmen K., Proctor T., Tschape J., Poeck B., Triphan T. Neurotoxic effects induced by the Drosophila amyloid-beta peptide suggest a conserved toxic function. Neurobiol. Dis. 2009;33:274–281. doi: 10.1016/j.nbd.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R., Vepuri V., Mhatre S.D., Paddock B.E., Miller S. Characterization of a Drosophila Alzheimer’s disease model: pharmacological rescue of cognitive defects. PLoS One. 2011;6:e20799. doi: 10.1371/journal.pone.0020799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.F., Possidente B., Lomas D.A., Crowther D.C. The central molecular clock is robust in the face of behavioural arrhythmia in a Drosophila model of Alzheimer’s disease. Dis. Model Mech. 2014;7:445–458. doi: 10.1242/dmm.014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C., Cao G., Tanenhaus A.K., McCarthy E.V., Jung M. Autoreceptor control of peptide/neurotransmitter corelease from PDF neurons determines allocation of circadian activity in drosophila. Cell Rep. 2012;2:332–344. doi: 10.1016/j.celrep.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B.Y., Kilman V.L., Keath J.R., Pitman J.L., Allada R. The GABA(A) receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr. Biol. 2009;19:386–390. doi: 10.1016/j.cub.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby-Milley J., Cavanagh C., Jego S., Breitner J.C., Quirion R. Sleep-wake cycle dysfunction in the TgCRND8 mouse model of Alzheimer’s disease: from early to advanced pathological stages. PLoS One. 2015;10:e0130177. doi: 10.1371/journal.pone.0130177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B., Kaplan H.S., Cavey M., Lelito K.R., Bahle A.H. Differentially timed extracellular signals synchronize pacemaker neuron clocks. PLoS Biol. 2014;12:e1001959. doi: 10.1371/journal.pbio.1001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.L. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meco A., Joshi Y.B., Pratico D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol. Aging. 2014;35:1813–1820. doi: 10.1016/j.neurobiolaging.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Dissel S., Melnattur K., Shaw P.J. Sleep, performance, and memory in flies. Curr. Sleep Med. Rep. 2015;1:47–54. doi: 10.1007/s40675-014-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissel S., Angadi V., Kirszenblat L., Suzuki Y., Donlea J. Sleep restores behavioral plasticity to Drosophila mutants. Curr. Biol. 2015;25:1270–1281. doi: 10.1016/j.cub.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissel S., Seugnet L., Thimgan M.S., Silverman N., Angadi V. Differential activation of immune factors in neurons and glia contribute to individual differences in resilience/vulnerability to sleep disruption. Brain Behav. Immun. 2015;47:75–85. doi: 10.1016/j.bbi.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea J., Leahy A., Thimgan M.S., Suzuki Y., Hughson B.N. Foraging alters resilience/vulnerability to sleep disruption and starvation in Drosophila. Proc. Natl. Acad. Sci. USA. 2012;109:2613–2618. doi: 10.1073/pnas.1112623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea J.M., Ramanan N., Shaw P.J. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea J.M., Ramanan N., Silverman N., Shaw P.J. Genetic rescue of functional senescence in synaptic and behavioral plasticity. Sleep. 2014;37:1427–1437. doi: 10.5665/sleep.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea J.M., Thimgan M.S., Suzuki Y., Gottschalk L., Shaw P.J. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–1576. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J., Chiang A.S. Systems memory consolidation in Drosophila. Curr. Opin. Neurobiol. 2013;23:84–91. doi: 10.1016/j.conb.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Duvall L.B., Taghert P.H. The circadian neuropeptide PDF signals preferentially through a specific adenylate cyclase isoform AC3 in M pacemakers of Drosophila. PLoS Biol. 2012;10:e1001337. doi: 10.1371/journal.pbio.1001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddison M., Guarnieri D.J., Cheng L., Liu C.H., Moffat K.G. Arouser reveals a role for synapse number in the regulation of ethanol sensitivity. Neuron. 2011;70:979–990. doi: 10.1016/j.neuron.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Fernandez M.P., Berni J., Ceriani M.F. Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol. 2008;6:e69. doi: 10.1371/journal.pbio.0060069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Funez P., de Mena L., Rincon-Limas D.E. Modeling the complex pathology of Alzheimer’s disease in Drosophila. Exp. Neurol. 2015;274:58–71. doi: 10.1016/j.expneurol.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly-Fitzgerald I., Donlea J., Shaw P.J. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–1781. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- Gerstner J.R., Lenz O., Vanderheyden W.M., Chan M.T., Pfeiffenberger C. Amyloid-beta induces sleep fragmentation that is rescued by fatty acid binding proteins in Drosophila. J. Neurosci. Res. 2016 doi: 10.1002/jnr.23778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeve I., Kretzschmar D., Tschape J.A., Beyn A., Brellinger C. Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. J. Neurosci. 2004;24:3899–3906. doi: 10.1523/JNEUROSCI.0283-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippe T.C., Goncalves B.S., Louzada L.L., Quintas J.L., Naves J.O. Circadian rhythm in Alzheimer disease after trazodone use. Chronobiol. Int. 2015;32:1311–1314. doi: 10.3109/07420528.2015.1077855. [DOI] [PubMed] [Google Scholar]
- Hadzic T., Park D., Abruzzi K.C., Yang L., Trigg J.S. Genome-wide features of neuroendocrine regulation in Drosophila by the basic helix-loop-helix transcription factor DIMMED. Nucleic Acids Res. 2015;43:2199–2215. doi: 10.1093/nar/gku1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes R., Park A.J., Tudor J.C., Luczak V.G., Hansen R.T. Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. Elife. 2016:5. doi: 10.7554/eLife.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks J.C., Finn S.M., Panckeri K.A., Chavkin J., Williams J.A. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Hendricks J.C., Williams J.A., Panckeri K., Kirk D., Tello M. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat. Neurosci. 2001;4:1108–1115. doi: 10.1038/nn743. [DOI] [PubMed] [Google Scholar]
- Iijima K., Liu H.P., Chiang A.S., Hearn S.A., Konsolaki M. Dissecting the pathological effects of human Abeta40 and Abeta42 in Drosophila: a potential model for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2004;101:6623–6628. doi: 10.1073/pnas.0400895101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeri L., Opp M.R. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H., Wang Z., Rao Y., Wu C.F., Kitamoto T. A novel role for ecdysone in Drosophila conditioned behavior: linking GPCR-mediated non-canonical steroid action to cAMP signaling in the adult brain. PLoS Genet. 2013;9:e1003843. doi: 10.1371/journal.pgen.1003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego S., Glasgow S.D., Herrera C.G., Ekstrand M., Reed S.J. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat. Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D., Berg D.K. Long-lasting changes in neural networks to compensate for altered nicotinic input. Biochem Pharmacol. 2015;97:418–424. doi: 10.1016/j.bcp.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner W.J., Crocker A., White B.H., Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- Ju Y.E., McLeland J.S., Toedebusch C.D., Xiong C., Fagan A.M. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsai L., Zars T. Learning and memory in Drosophila: behavior, genetics, and neural systems. Int. Rev. Neurobiol. 2011;99:139–167. doi: 10.1016/B978-0-12-387003-2.00006-9. [DOI] [PubMed] [Google Scholar]
- Kang J.E., Lim M.M., Bateman R.J., Lee J.J., Smyth L.P. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis a researcher’s handbook. Prentice-Hall Englewood Cliffs; NJ: 1982. [Google Scholar]
- Klose M., Duvall L.B., Li W., Liang X., Ren C. Functional PDF signaling in the drosophila circadian neural circuit is gated by Ral A-dependent modulation. Neuron. 2016;90:781–794. doi: 10.1016/j.neuron.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konadhode R.R., Pelluru D., Blanco-Centurion C., Zayachkivsky A., Liu M. Optogenetic stimulation of MCH neurons increases sleep. J. Neurosci. 2013;33:10257–10263. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kula-Eversole E., Nagoshi E., Shang Y., Rodriguez J., Allada R. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc. Natl. Acad. Sci. USA. 2010;107:13497–13502. doi: 10.1073/pnas.1002081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourg E. Effects of aging on learned suppression of photopositive tendencies in Drosophila melanogaster. Neurobiol. Aging. 2004;25:1241–1252. doi: 10.1016/j.neurobiolaging.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Le Bourg E., Buecher C. Learned suppression of photopositive tendencies in Drosophila melanogaster. Anim. Learn Behav. 2002;30:330–341. doi: 10.3758/bf03195958. [DOI] [PubMed] [Google Scholar]
- Li L., Zhang L., Yang C.C. Multi-Target Strategy and Experimental Studies of Traditional Chinese Medicine for Alzheimer’s Disease Therapy. Curr. Top. Med. Chem. 2016;16:537–548. doi: 10.2174/1568026615666150813144003. [DOI] [PubMed] [Google Scholar]
- Li Y., Guo F., Shen J., Rosbash M. PDF and cAMP enhance PER stability in Drosophila clock neurons. Proc. Natl. Acad. Sci. USA. 2014;111:E1284–E1290. doi: 10.1073/pnas.1402562111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F., Zhang T.J., Mahan T.E., Jiang H., Holtzman D.M. Effects of growth hormone-releasing hormone on sleep and brain interstitial fluid amyloid-beta in an APP transgenic mouse model. Brain Behav. Immun. 2015;47:163–171. doi: 10.1016/j.bbi.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey B.P., Holtzman D.M. How amyloid, sleep and memory connect. Nat. Neurosci. 2015;18:933–934. doi: 10.1038/nn.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander B.A., Winer J.R., Jagust W.J., Walker M.P. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s Disease? Trends Neurosci. 2016 doi: 10.1016/j.tins.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z., Roman G., Zong L., Davis R.L. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. Proc. Natl. Acad. Sci. USA. 2004;101:198–203. doi: 10.1073/pnas.0306128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride S.M., Choi C.H., Wang Y., Liebelt D., Braunstein E. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- McBride S.M., Choi C.H., Schoenfeld B.P., Bell A.J., Liebelt D.A. Pharmacological and genetic reversal of age-dependent cognitive deficits attributable to decreased presenilin function. J. Neurosci. 2010;30:9510–9522. doi: 10.1523/JNEUROSCI.1017-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mershin A., Pavlopoulos E., Fitch O., Braden B.C., Nanopoulos D.V. Learning and memory deficits upon TAU accumulation in Drosophila mushroom body neurons. Learn Mem. 2004;11:277–287. doi: 10.1101/lm.70804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre S.D., Paddock B.E., Saunders A.J., Marenda D.R. Invertebrate models of Alzheimer’s disease. J. Alzheimers Dis. 2013;33:3–16. doi: 10.3233/JAD-2012-121204. [DOI] [PubMed] [Google Scholar]
- Mhatre S.D., Satyasi V., Killen M., Paddock B.E., Moir R.D. Synaptic abnormalities in a Drosophila model of Alzheimer’s disease. Dis. Model Mech. 2014;7:373–385. doi: 10.1242/dmm.012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek E.S., Xiong D.D., Holtzman D.M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med. 2015;47:e148. doi: 10.1038/emm.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet R.M., Polanco J.C., Ittner L.M., Gotz J. Tau aggregation and its interplay with amyloid-beta. Acta Neuropathol. 2015;129:207–220. doi: 10.1007/s00401-014-1371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder T., Yoon K.S., White B.H., Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. USA. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisky K.M., Agosto J., Pulver S.R., Shang Y., Kuklin E. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsakou A., Sapsis T.P., Blau J. Circadian Rhythms in Rho1 Activity regulate neuronal plasticity and network hierarchy. Cell. 2015;162:823–835. doi: 10.1016/j.cell.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirooznia S.K., Chiu K., Chan M.T., Zimmerman J.E., Elefant F. Epigenetic regulation of axonal growth of Drosophila pacemaker cells by histone acetyltransferase tip60 controls sleep. Genetics. 2012;192:1327–1345. doi: 10.1534/genetics.112.144667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J.H., Jiang H., Finn M.B., Stewart F.R., Mahan T.E. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J. Exp. Med. 2014;211:2487–2496. doi: 10.1084/jem.20141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman S.M., Herdener N., Frankola K.A., Mughal M.R., Mattson M.P. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Abeta and pTau in a mouse model of Alzheimer’s disease. Brain Res. 2013;1529:200–208. doi: 10.1016/j.brainres.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman D.C., Acosta-Baena N., Aisen P.S., Bird T., Danek A. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology. 2014;83:253–260. doi: 10.1212/WNL.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L., Suzuki Y., Stidd R., Shaw P.J. Aversive phototaxic suppression: evaluation of a short-term memory assay in Drosophila melanogaster. Genes Brain Behav. 2009;8:377–389. doi: 10.1111/j.1601-183X.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L., Suzuki Y., Vine L., Gottschalk L., Shaw P.J. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr. Biol. 2008;18:1110–1117. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L., Suzuki Y., Donlea J.M., Gottschalk L., Shaw P.J. Sleep deprivation during early-adult development results in long-lasting learning deficits in adult Drosophila. Sleep. 2011;34:137–146. doi: 10.1093/sleep/34.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer O.T., Kim D.J., Dunbar-Yaffe R., Nikolaev V.O., Lohse M.J. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Griffith L.C., Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc. Natl. Acad. Sci. USA. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Haynes P., Pirez N., Harrington K.I., Guo F. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat. Neurosci. 2011;14:889–895. doi: 10.1038/nn.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P.J., Cirelli C., Greenspan R.J., Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Shaw P.J., Tononi G., Greenspan R.J., Robinson D.F. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- Sheeba V., Fogle K.J., Kaneko M., Rashid S., Chou Y.T. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr. Biol. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R., Johnson K. To sleep, perchance to delay dementia. Arch. Neurol. 2012;69:118–120. doi: 10.1001/archneurol.2011.1901. [DOI] [PubMed] [Google Scholar]
- Stewart B.A., Atwood H.L., Renger J.J., Wang J., Wu C.F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol [A] 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Stickgold R., Walker M.P. Sleep-dependent memory triage: evolving generalization through selective processing. Nat. Neurosci. 2013;16:139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi M., Lone S.R., Liu S., Liu Q., Zhang J. Sleep interacts with abeta to modulate intrinsic neuronal excitability. Curr. Biol. 2015;25:702–712. doi: 10.1016/j.cub.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimgan M.S., Seugnet L., Turk J., Shaw P.J. Identification of genes associated with resilience/vulnerability to sleep deprivation and starvation in Drosophila. Sleep. 2015;38:801–814. doi: 10.5665/sleep.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G., Cirelli C. Sleep function and synaptic homeostasis. Sleep. Med. Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- van Alphen B., Yap M.H., Kirszenblat L., Kottler B., van Swinderen B. A dynamic deep sleep stage in Drosophila. J. Neurosci. 2013;33:6917–6927. doi: 10.1523/JNEUROSCI.0061-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swinderen B. The aversive phototaxic suppression assay for individual adult Drosophila. Cold Spring Harb. Protoc. 2011;2011:1203–1205. doi: 10.1101/pdb.prot065896. [DOI] [PubMed] [Google Scholar]
- Vassar R. BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimers Res. Ther. 2014;6:89. doi: 10.1186/s13195-014-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vienne J., Lecciso G., Constantinescu I., Schwartz S., Franken P. Differential effects of sodium oxybate and baclofen on EEG, sleep, neurobehavioral performance, and memory. Sleep. 2012;35:1071–1083. doi: 10.5665/sleep.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinters H.V. Emerging concepts in Alzheimer’s disease. Annu Rev. Pathol. 2015;10:291–319. doi: 10.1146/annurev-pathol-020712-163927. [DOI] [PubMed] [Google Scholar]
- Wittmann C.W., Wszolek M.F., Shulman J.M., Salvaterra P.M., Lewis J. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- Xie L., Kang H., Xu Q., Chen M.J., Liao Y. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K., Falvey C.M., Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13:1017–1028. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- Yaffe K., Laffan A.M., Harrison S.L., Redline S., Spira A.P. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z., Macara A.M., Lelito K.R., Minosyan T.Y., Shafer O.T. Analysis of functional neuronal connectivity in the Drosophila brain. J. Neurophysiol. 2012;108:684–696. doi: 10.1152/jn.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Emery P. GW182 controls Drosophila circadian behavior and PDF-receptor signaling. Neuron. 2013;78:152–165. doi: 10.1016/j.neuron.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]