Abstract
Background
Cholangiocytes are injured in many biliary tract diseases that result in cirrhosis, cholangiocarcinoma and need for liver transplantation. Recent studies demonstrate that the hormone Fibroblast Growth Factor 19 (FGF19) is produced in the ileum and regulates hepatic gene expression via the enterohepatic circulation. However, the role of FGF19 on cholangiocytes remains largely unknown. The purpose of this study was to elucidate the effect of FGF19 on cholangiocyte gene and protein expression.
Methods
Cultured human cholangiocyte-derived H69 cells were treated with FGF19 (0-50ng/ml) and expression of genes and proteins involved in the Unfolded Protein Response (UPR) and mitogen-activated protein kinase (MAPK) pathways were studied using RT-PCR and Western blot analysis.
Results
FGF19-induced gene and protein expression of the UPR genes BiP and CHOP increased in a dose-responsive pattern. The UPR protein P-eIF2a displayed a bimodal pattern of protein expression, with 10ng/ml of FGF19 maximally reducing and 50ng/ml maximally increasing expression. MAPK pathway protein expression (P-JNK, P-ERK, P-38) displayed a similar bimodal pattern of expression with 2.5ng/ml of FGF19 decreasing expression and 25ng/ml of FGF19 increasing expression.
Conclusions
FGF19 treatment of H69 cells selectively activates BiP and CHOP in a dose-dependent manner. FGF19 also regulates P-eIF2a and MAPK protein expression with a bimodal response. We speculate that FGF19 has an important role in the pathogenesis of many human cholangiopathies and cholestatic liver disorders.
Keywords: Fibroblast Growth Factor 19, Unfolded Protein Response, Mitogen-Activated Protein Kinases
Background
Cholangiocytes, the epithelial cells that line the bile ducts, are affected in a wide variety of cholangiopathies, such as primary sclerosing cholangitis and primary biliary cirrhosis [1, 2]. The etiologies of these diseases are unknown and their pathogeneses are poorly understood. These cholestatic diseases often result in inflammation, cholangiocarcinoma, and the need for liver transplantation [1-4]. No effective treatments have been shown to slow the progression of PSC, and a greater understanding of the various processes that govern cholangiocyte activity may be integral to the development of therapies for these chronic liver diseases.
Fibroblast growth factor 19 (FGF19, and its murine ortholog FGF15) is secreted in the ileum in response to stimulation by the nuclear bile acid receptor FXR (farsenoid-X receptor) in order to regulate bile acid synthesis [5-8]. FGF19 expression has been observed in the small intestine and the gallbladder, and has an important role in gallbladder filling and the enterohepatic circulation of bile salts [9, 10]. FGF19 binds to a complex of FGF receptor 4 (FGFR4)-βKlotho in the liver, which results in the activation of mitogen-activated protein kinase (MAPK) pathways and reduced transcription of cholesterol 7 α-hydroxylase (CYP7A1), which is a key rate-limiting step in the bile acid synthesis pathway [6, 7, 11-15]. FGF19 has also been shown to metabolically regulate hepatic protein and glycogen synthesis in the liver [16, 17]. In addition, FGF19 increases metabolic rate and improves glucose homeostasis in diabetic mice [18-21]. Therefore, studies indicate that FGF19 has broad metabolic and protective roles in the liver; however, its role in the biliary tract remains unknown.
In contrast to its putative protective roles in the liver, studies have also shown that FGF19 may play an important role in the pathogenesis of hepatocellular carcinoma (HCC). Hepatocyte proliferation is increased in the presence of FGF19 through its activation of FGFR4, a process which can eventually lead to HCC formation [22]. Transgenic mice overexpressing FGF19 in skeletal muscle were shown to have increased HCC formation [23]. Furthermore, FGF19 and FGFR4 are expressed in primary human liver, colon, and lung tumors, and administration of an antibody against their interaction inhibited tumor growth in two human colon cancer models and FGF19 transgenic hepatocellular carcinomas [24].
Cholangiocytes are routinely exposed to high concentrations of bile salts. The apical sodium-dependent bile salt transporter (ASBT) is primarily responsible for regulating the transport of bile acids across cholangiocytes and its up-regulation in chronic cholestasis plays a role in causing bile acid-induced liver injury [25]. FGF19 in the presence of βKlotho has been shown to inhibit ASBT in human cholangiocytes [26]. FGF19 is also highly expressed in livers of patients with extrahepatic cholestasis, which is part of a number of recognized adaptations to protect the liver from the toxicity of bile salts in those environments [27].
In the present study, we examined the function of FGF19 on human-derived cholangiocyte cell cultures in an effort to elucidate its effect on cholangiocyte function and its role in the regulation of cholangiocyte gene expression.
Materials and Methods
Cell Culture
The H-69 biliary cell line [28], was kindly provided by Dr. Cara Mack (Denver, CO). The cell line was cultured in DMEM and DMEM/Ham's F12 (ATCC, Manassas, VA) supplemented with 10% fetal bovine serum, penicillin/streptomycin, adenine (Sigma-Aldrich, St. Louis, MO), insulin (Sigma-Aldrich, St. Louis, MO), epinephrine (Sigma-Aldrich, St. Louis, MO), T3-T (Sigma-Aldrich, St. Louis, MO), EGF (Sigma-Aldrich, St. Louis, MO), and hydrocortisone (Sigma-Aldrich, St. Louis, MO). Cells were maintained at 37°C in 10% CO2. HepG2 cells (ATCC, Manassas, VA) were also cultured in DMEM with 10% fetal bovine serum and maintained at 37°C in 5% CO2. Treatments were carried out for 24 h in serum-free supplemented DMEM for all experiments, after which cells were harvested for RNA isolation or protein extraction.
Analysis of Gene Expression by Real-time Quantitative PCR
Total RNA was obtained using TRIzol reagent (Invitrogen, Carlsbad, CA). With samples from three wells that had been pooled together, two micrograms of total RNA were prepared using a SuperScript First Strand kit (Invitrogen, Carlsbad, CA) for reverse transcription-PCR. Real-time quantitative PCR was performed using 2μl of cDNA from each sample in a 25μl reaction mixture containing Quantitect SYBR Green PCR Mastermix (Qiagen, Valencia, CA) and the primers specific for the gene of interest. Human Ubiquitin C was utilized as a housekeeping gene. The primer sequences are shown in Supplementary Table 1.
Analysis of Protein Expression by Western blotting
Protein was extracted from H-69 and HepG2 cells using a mixture of T-Per (Thermo Scientific, Hanover Park, IL), protease mixture inhibitor (Thermo Scientific, Hanover Park, IL), and Halt phosphatase inhibitor (Thermo Scientific, Hanover Park, IL). The Bradford assay was utilized to determine protein concentrations of the homogenates in Coomassie Blue reagent (Pierce, Rockford, IL). 3.75μg of sample protein were separated through electrophoresis using 10% or 12% SDS-polyacrylamide gels. Protein samples represent either pooled samples of four separate samples or single samples. Protein detection was performed using polyclonal rabbit antibodies to total SAPK/JNK (1:1000, Cell Signaling Technology, Danvers, MA), phospho-SAPK/JNK (1:1000, Cell Signaling Technology, Danvers, MA), total p44/p42 MAPK (ERK1/2) (1:1000, Cell Signaling Technology, Danvers, MA), phospho-p44/p42 MAPK (ERK1/2) (1:1000, Cell Signaling Technology, Danvers, MA), p38 MAPK (1:1000, Cell Signaling Technology, Danvers, MA), phospho-p38 MAPK (1:1000, Cell Signaling Technology, Danvers, MA), BiP (1:1000, Cell Signaling Technology, Danvers, MA), C/EBP homologous transcription factor (CHOP) (1:500, Cell Signaling Technology, Danvers, MA), and a monoclonal mouse antibody to β-actin (1:5000, Sigma-Aldrich, St. Louis, MO). HepG2 phospho-ERK1/2 expression was used as a positive control. The bound antibody was detected using goat-anti rabbit or goat anti-mouse polyclonal HRP antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and an ECL Western blotting substrate (Pierce, Rockford, IL) was used.
FGF19 Dose Response
In order to examine the effects of FGF19 on cholangiocyte gene and protein expression, the H-69 and HepG2 cells were treated for 24 h with increasing concentrations of recombinant FGF19 (0-50ng/ml). Protein or RNA extraction was then performed as previously described.
Statistical Analysis
Data analysis was performed using Student's t-test or ANOVA to compare data between groups. Results are stated as mean ± standard deviation. Data was deemed statistically significant if p ≤ 0.05.
Results
FGF19 globally activates MAPK proteins in a bimodal pattern in H-69 cells
Mitogen-activated protein kinases have previously been shown to be activated by FGF19 in the HepG2 cell line [13, 15, 16]. Therefore, we investigated whether the MAPK pathways were activated in human cholangiocyte cells. H-69 cells treated with FGF19 in varying concentrations display a bimodal pattern of phosphorylation of MAPK proteins, especially ERK1/2 (Fig. 1A and 1B). FGF19 activates ERK1/2 in a bimodal manner, with an initial decrease in phosphorylation from 2.5-10ng/ml FGF19 and then a return to baseline at 25ng/ml FGF19. Western blotting of HepG2 ERK1/2 activation was used as a positive control.
Figure 1. ERK activation in H-69 human cholangiocyte cells (A and B) by FGF19.
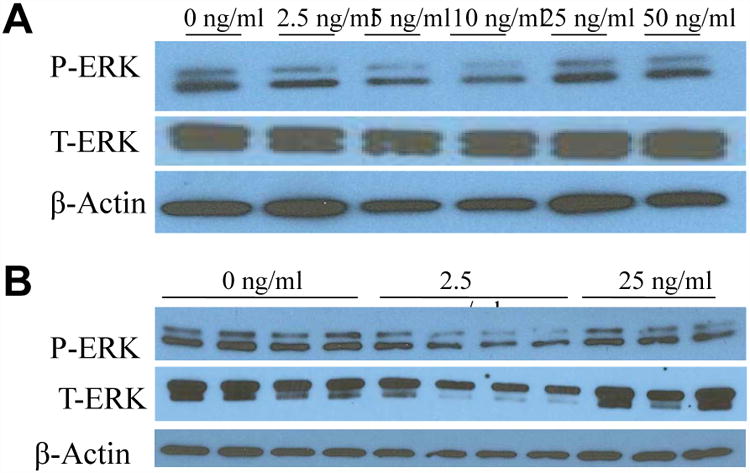
Western blotting of cholangiocyte phosphorylated (P-) and total (T-) ERK after a 24h incubation period with various concentrations of FGF19. Data represent pooled samples with n = 4 (A) and unpooled single samples (B).
We further observed the activation of JNK1/2 and p38 proteins in the H69 human cholangiocyte cell line. Treatment with FGF19 induces phosphorylation of these two proteins in a similar bimodal manner with reduced phosphorylation from 2.5-10ng/ml and then a return to baseline at 25ng/ml (Fig. 2A and 2B).
Figure 2. Activation of other MAPK proteins in a bimodal manner in cholangiocytes with FGF19 treatment.
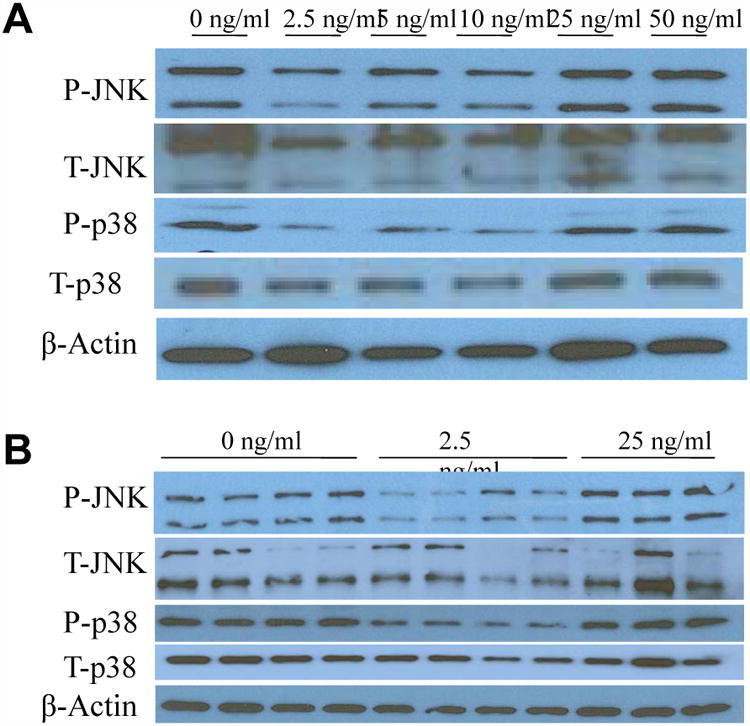
Western blotting of human cholangiocyte phosphorylated (P-) and total (T-) JNK and phosphorylated and total p38 after a 24h incubation period with various concentrations of FGF19. Data represent pooled samples with n = 4 (A) and unpooled single samples (B).
FGF19 treatments lead to the upregulation of BiP in a dose-dependent manner
We next examined the effects of FGF19 on various components of the unfolded protein response (UPR) pathway. BiP protein expression increases in a dose-dependent manner (Fig. 3A). This is similarly noted in its mRNA expression, which displayed the same pattern of expression (Fig. 3B). BiP mRNA was significantly upregulated as a result of FGF19 treatments, with over a two-fold increase in doses greater than 25ng/ml FGF19 (1.02 ± 0.24 vs. 2.16 ± 0.62, p ≤ 0.01) (Supplementary Table 2A).
Figure 3. FGF19 supplementation leads to upregulation of BiP in a dose-dependent manner.
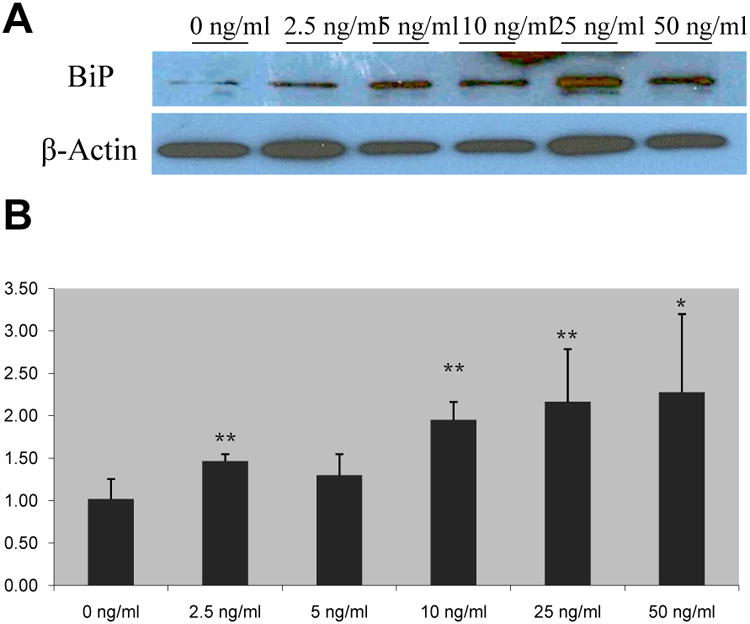
Human cholangiocyte protein expression of BiP was analyzed via Western blotting (A) and mRNA expression was analyzed through Real-time qPCR (B). Representative Western blots of pooled samples (n = 4). Relative mRNA expression, mean ± SD, * p ≤ 0.05 and ** p ≤ 0.01 versus control.
CHOP is upregulated in a dose-dependent manner as a result of FGF19 treatments
CHOP is also upregulated by FGF19 treatment. Protein expression of CHOP is increased in dose-dependent pattern (Fig. 4A). Furthermore, mRNA expression of CHOP is also increased in response to FGF19 supplementation (Fig. 4B). CHOP mRNA is significantly upregulated only at 50ng/ml FGF19 with nearly a 2.5-fold increase over control levels of expression; however, lower concentrations of FGF19 also displayed a trend towards a dose-dependent increase in expression of CHOP (1.05 ± 0.36 vs. 2.42 ± 0.56, p ≤ 0.01) (Supplementary Table 2A).
Figure 4. FGF19 supplementation leads to upregulation of CHOP in a dose-dependent manner.
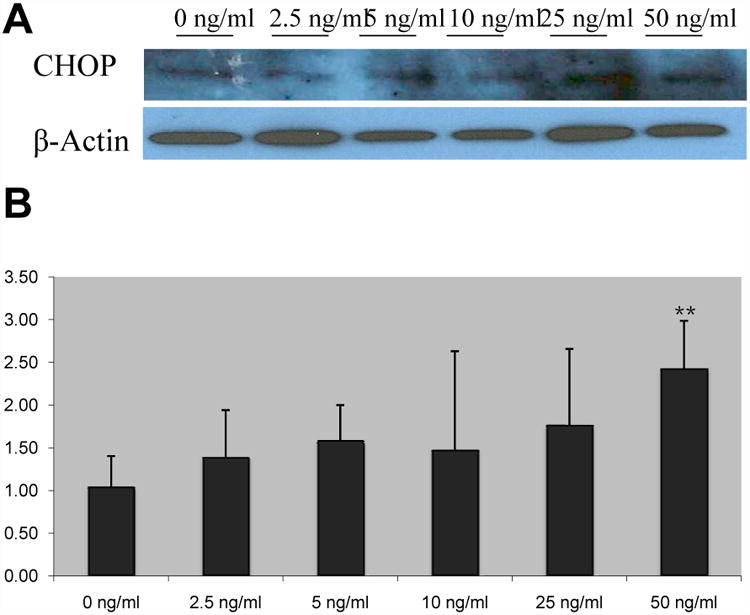
Cholangiocyte protein expression of CHOP was analyzed through Western blotting (A) and mRNA expression was analyzed via Real-time qPCR (B). Representative Western blots of pooled samples (n = 4). Relative mRNA expression, mean ± SD, ** p ≤ 0.01 versus control.
eIF2α is activated in a bimodal manner due to FGF19 supplementation
In addition, FGF19 also increases the phosphorylation of the transcription factor eIF2α, which is also involved in the UPR pathway. Interestingly, eIF2α phosphorylation also displayed a bimodal pattern of activation, similar to what was observed in the MAPK proteins, with 10ng/ml FGF19 maximally reducing and 50ng/ml maximally increasing expression (Fig. 5A and 5B).
Fig. 5. eIF2α activation in a bimodal manner as a result of FGF19 treatment.
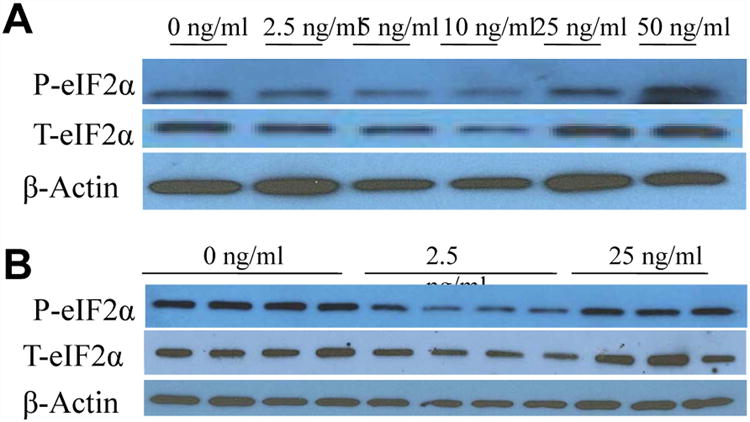
Western blotting of cholangiocyte phosphorylated (P-) and total (T-) eIF2α after a 24h incubation period with various concentrations of FGF19. Data represent pooled samples with n = 4 (A) and unpooled single samples (B).
Supplementary Table 2 displays the raw data for the UPR genes discussed above in addition to several other genes that were tested to identify FGF19's effects on the H-69 cell line.
Discussion
FGF19 is an important regulator of bile salt synthesis through its regulation of hepatic CYP7A1 expression. It is produced in the ileum in response to intestinal bile salt stimulation and is then secreted into the portal circulation, where it can be taken up into the liver and regulate hepatic genes by binding to FGFR4. FGF19 is also produced in the human liver in response to extrahepatic cholestasis, but is not expressed in healthy livers [27]. FGF19 also has important metabolic roles involving glucose homeostasis and hepatic lipid metabolism. Little is known, however, about the role of FGF19 signaling in cholangiocytes. Therefore, our study sought to elucidate the role of FGF19 signaling in H69 human cholangiocyte cells.
Our findings indicate that FGF19 activated the UPR pathway through its stimulation of BiP and CHOP in H69 cholangiocyte cells. We observed that BiP and CHOP were activated in a dose-dependent manner with increased activation at higher doses of FGF19. In addition, eIF2α was also activated in response to FGF19, but it displayed a bimodal pattern of activation. BiP is the major upstream activator of all three arms of the UPR pathway, and our data demonstrates that CHOP is the major downstream target of FGF19 signaling in H69 cholangiocyte cells. Previous studies on FGF19 in the liver have shown that it stimulates the MAPK/ERK1/2 pathway [15]. However, no prior study reports activation of the UPR pathway or the effect of FGF19 in cholangiocyte cells. The UPR pathway is activated in response to ER stress, which leads to an accumulation of unfolded or misfolded proteins in the lumen of the endoplasmic reticulum. It has been increasingly recognized to be important in several liver diseases, including viral hepatitis, alpha 1-antitrypsin deficiency, alcohol-induced liver injury, and fatty liver disease [29]. The activation of this pathway through FGF19 signaling in H69 cholangiocyte cells may also serve an important role in the pathogenesis of cholestatic liver disease and cholangiocarcinoma. It has previously been shown that circulating levels of FGF19 are elevated in response to extrahepatic cholestasis [27]. The variety of concentrations we used in our study ranges from 10- to 250-fold higher than serum FGF19 concentrations in humans [30]; however, our study more closely models tissue levels, which have not been reported in the literature. The higher concentrations of FGF19 in our study may represent a situation resembling cholestatic liver disease, where FGF19 levels are elevated, and the activation of the UPR pathway through BiP, CHOP, and eIF2α may serve as a protective measure under this kind of environmental stress to cholangiocyte cells.
We also report that the three main branches of the MAPK pathway are upregulated in a bimodal manner in response to FGF19 in H69 cholangiocyte cells. The activation of MAPK proteins in response to FGF19 has previously been reported in the literature, and our study confirms that this pathway is also activated in H69 cholangiocyte cells; however, this bimodal pattern of activation is another novel finding. We hypothesize that this pattern is because a small amount of FGF19 may be necessary for the normal functioning of the tissue; therefore, an absence of any FGF19 in these cells may lead to the activation of this pathway and induce inflammation of the tissue. Furthermore, elevated levels of FGF19 may be a marker for cholestasis, and the upregulation of the MAPK in this case could also serve as a protective measure. The dyregulation of Raf/MEK/ERK1/2 pathway has been shown to be responsible for cancer in several tissues [31]; therefore, we hypothesize that chronically elevated levels of FGF19 could induce cholangiocarcinoma formation.
The upregulation of both MAPK and UPR pathways may be to protect the liver during cholestatic conditions. The combined activation of these pathways could favor inflammation and a pro-apoptotic drive. In addition, their activation in response to chronically elevated levels of FGF19 may display the effect of aberrant signaling.
Conclusions
These data support a potentially protective role of FGF19 in cholangiocytes through its activation of the MAPK and UPR pathways. FGF19 activates an array of downstream signaling molecules in H69 cholangiocyte cells, which if chronically stimulated could possibly lead to dysregulated inflammation and proliferation. Further research on the impact of FGF19 signaling in vivo would be beneficial in elucidating its protective effects and its therapeutic potential.
Supplementary Material
Supplementary Table 1. Quantitative RT-PCR Primer Sequences.
Supplementary Table 2A. Relative mRNA expression levels for all tested UPR genes.
Supplementary Table 2B. Relative mRNA expression levels for all other testedgenes.
Acknowledgments
This research was funded by the PSC Partners Seeking a Cure Foundation.
Abbreviations
- FGF
Fibroblast growth factor
- UPR
Unfolded protein response
- MAPK
Mitogen-activated protein kinase
- FXR
farsenoid-X receptor
- FGFR4
FGF receptor 4
- CYP7A1
cholesterol 7 alpha-hydroxylase
- HCC
Hepatocellular carcinoma
- ASBT
Apical sodium-dependent bile salt transporter
- ERK
Extracellular-signal-relagulated kinase
- JNK
c-Jun N-terminal kinase
- BiP
Binding immunoglobulin protein
- CHOP
CCAAT/enhancer binding protein (C/EBP) homologous protein
- eIF2α
eukaryotic initiation factor 2 alpha
- ER
endoplasmic reticulum
- PSC
primary sclerosing cholangitis
- PBC
primary biliary cirrhosis
Footnotes
Competing Interests: The authors declare that they have no competing interests.
Author Contributions: HAQ performed cell culture, carried out molecular genetic studies, participated in performing western immunoblotting, completed the data analysis, and drafted the manuscript. JAP and RMG conceived of the study, participated in its design and coordination, and helped to draft the manuscript. KAA aided in performing western immunoblotting. All authors read and approved the final manuscript.
References
- 1.Park SM. The crucial role of cholangiocytes in cholangiopathies. Gut Liver. 2012;6(3) doi: 10.5009/gnl.2012.6.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendes FD, Kim WR, Pedersen R, Therneau T, Lindor KD. Mortality attributable to cholestatic liver disease in the United States. Hepatology. 2008;47(4):1241–1247. doi: 10.1002/hep.22178. [DOI] [PubMed] [Google Scholar]
- 3.Lindor KD. Characteristics of primary sclerosing cholangitis in the USA. Hepatol Res. 2007;37(Suppl 3):S474–S477. doi: 10.1111/j.1872-034X.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924–933. doi: 10.1056/NEJM199504063321406. [DOI] [PubMed] [Google Scholar]
- 5.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3):235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17(13):1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48(12):2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Zweers SJ, Booij KA, Komuta M, Roskams T, Gouma DJ, Jansen PL, Schaap FG. The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology. 2012;55(2):575–583. doi: 10.1002/hep.24702. [DOI] [PubMed] [Google Scholar]
- 10.Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, Suino-Powell K, Xu HE, Richardson JA, Gerard RD, Mangelsdorf DJ, Kliewer SA. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12(11):1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 11.Xie MH, Holcomb I, Deuel B, Dowd P, Huang A, Vagts A, Foster J, Liang J, Brush J, Gu Q, Hillan K, Goddard A, Gurney AL. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine. 1999;11(10):729–735. doi: 10.1006/cyto.1999.0485. [DOI] [PubMed] [Google Scholar]
- 12.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282(37):26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin BC, Wang M, Blackmore C, Desnoyers LR. Liver-specific activities of FGF19 require Klotho beta. J Biol Chem. 2007;282(37):27277–27284. doi: 10.1074/jbc.M704244200. [DOI] [PubMed] [Google Scholar]
- 14.Tomiyama K, Maeda R, Urakawa I, Yamazaki Y, Tanaka T, Ito S, Nabeshima Y, Tomita T, Odori S, Hosoda K, Nakao K, Imura A, Nabeshima Y. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc Natl Acad Sci U S A. 2010;107(4):1666–1671. doi: 10.1073/pnas.0913986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49(1):297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;31(6024):1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin DJ, Osborne TF. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. J Biol Chem. 2009;284(17):11110–11120. doi: 10.1074/jbc.M808747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu L, John LM, Adams SH, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145(6):2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 19.Wu AL, Coulter S, Liddle C, Wong A, Eastham-Anderson J, French DM, Peterson AS, Sonoda J. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS One. 2011;6(3):e17868. doi: 10.1371/journal.pone.0017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, Stewart TA. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143(5):1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 21.Bhatnagar S, Damron HA, Hillgartner FB. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem. 2009;284(15):10023–10033. doi: 10.1074/jbc.M808818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Ge H, Lemon B, Vonderfecht S, Weiszmann J, Hecht R, Gupte J, Hager T, Wang Z, Lindberg R, Li Y. FGF19-induced hepatocyte proliferation is mediated through FGFR4 activation. J Biol Chem. 2010;285(8):5165–5170. doi: 10.1074/jbc.M109.068783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholes K, Guillet S, Tomlinson E, Hillan K, Wright B, Frantz GD, Pham TA, Dillard-Telm L, Tsai SP, Stephan JP, Stinson J, Stewart T, French DM. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am J Pathol. 2002;160(6):2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desnoyers LR, Pai R, Ferrando RE, Hötzel K, Le T, Ross J, Carano R, D'Souza A, Qing J, Mohtashemi I, Ashkenazi A, French DM. Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene. 2008;27(1):85–97. doi: 10.1038/sj.onc.1210623. [DOI] [PubMed] [Google Scholar]
- 25.Xia X, Francis H, Glaser S, Alpini G, LeSage G. Bile acid interactions with cholangiocytes. World J Gastroenterol. 2006;12(22):3553–3563. doi: 10.3748/wjg.v12.i22.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha J, Chen F, Miloh T, Burns RC, Yu Z, Shneider BL. beta-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G996–G1003. doi: 10.1152/ajpgi.90343.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salthomeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49(4):1228–1235. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- 28.Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, Mulberg AE, Cherington V, Jefferson DM. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol Gastrointest Liver Physiol. 1994;226(6 Pt 1):G1060–1070. doi: 10.1152/ajpgi.1994.266.6.G1060. [DOI] [PubMed] [Google Scholar]
- 29.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54(4):795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundåsen T, Gälman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260(6):530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 31.De Luca A, Maiello MR, D'Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets. 2012;16(Suppl 2):S17–27. doi: 10.1517/14728222.2011.639361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Quantitative RT-PCR Primer Sequences.
Supplementary Table 2A. Relative mRNA expression levels for all tested UPR genes.
Supplementary Table 2B. Relative mRNA expression levels for all other testedgenes.


