Abstract
Bile acids are synthesized in the liver and are the major component in bile. Impaired bile flow leads to cholestasis that is characterized by elevated levels of bile acid in the liver and serum, followed by hepatocyte and biliary injury. Although the causes of cholestasis have been extensively studied, the molecular mechanisms as to how bile acids initiate liver injury remain controversial. In this chapter, we summarize recent advances in the pathogenesis of bile acid induced liver injury. These include bile acid signaling pathways in hepatocytes as well as the response of cholangiocytes and innate immune cells in the liver in both patients with cholestasis and cholestatic animal models. We focus on how bile acids trigger the production of molecular mediators of neutrophil recruitment and the role of the inflammatory response in this pathological process. These advances point to a number of novel targets where drugs might be judged to be effective therapies for cholestatic liver injury.
Keywords: bile acids, cholestatic liver injury, inflammation, innate immunity, neutrophils
1. Introduction
Bile acids are amphipathic molecules synthesized from cholesterol in the liver and are the major component in bile. Secretion of bile acids and other choleretic compounds by hepatocytes generates bile flow and facilitates elimination of endogenous compounds and metabolites such as bilirubin and hormones, as well as xenobiotics including drugs (1). In humans, most primary bile acids are conjugated with glycine or taurine and form mixed micelles with phospholipids and cholesterol in the bile before they reach the small intestine, where they facilitate digestion and absorption of lipophilic nutrients such as cholesterol, fat and fat-soluble vitamins. At the terminal ileum, approximately 95% of bile acids are reclaimed and transported back to the liver via the portal circulation. The remaining fraction are transformed into secondary bile acids by gut microbiota where they either passively diffuse across the colon or are excreted in the feces.
Bile acid excretion is impaired in cholestatic liver injury either by direct inhibition or genetic deficiencies of canalicular bile acid transporters in hepatocytes or by mechanical or immune mediated obstruction of the biliary ducts. Whatever the cause, bile acid levels increase in the liver and serum, followed by hepatocyte injury and bile duct proliferation. If left untreated, cholestatic liver injury often progresses to liver fibrosis, cirrhosis and eventually liver failure. While the causes of cholestasis have been extensively studied, the molecular mechanisms as to how bile acids initiate liver injury are not well understood. Part of the reason may be due to the diverse physical properties of bile acids. Although all bile acids are metabolites of cholesterol, their structural variation determines their physical properties and physiological function (2). For example: 1) The hydrophilicity or water solubility of bile acids increases with the number of hydroxylation sites located either the nuclear ring or side chain, and is also affected by the carboxyl group conjugation; 2) Bile acids with lower water solubility are more cytotoxic; 3) While unconjugated bile acids freely diffuse across cell membranes, conjugated bile acids (which make up the majority in the bile acid pool) require specific protein transporters; 4) Different cell types may respond quite differently to the same bile acid.
Early work suggested that bile acids injured the liver directly through their detergent cytolytic effects, as submillimolar levels of toxic bile acids directly killed hepatocytes when added to these cells in vitro (3–5). However, the serum and tissue levels of toxic bile acids rarely reach these submillimolar levels in pathophysiological conditions, suggesting that their cytolytic properties may not be the cause of liver cell death Subsequently, it was proposed that bile acids induced apoptosis in hepatocytes. This hypothesis is supported by the observation that apoptosis was detected in rat hepatocyte cultures when they were treated with >50 μM glycochenodeoxycholic acid (GCDCA) (6, 7), as reviewed by Malhi et al (8)). However, 1) GCDCA is not a major bile acid in rats as the serum concentration of total chenodeoxycholic acid is only ~ 5 μM even in rats with complete bile duct obstruction (9); 2) In contrast, taurocholic acid (TCA), the major endogenous bile acid in rats does not induce apoptosis in rat hepatocytes (7).
Also, taurine conjugation is the major form of conjugates in rodents in contrast to glycine which is dominant in humans; 3) Normally, apoptotic cell death does not elicit an immune response; 4) Most importantly, apoptosis of hepatocytes has not been detected in vivo in the liver of bile duct ligated (BDL) mice or in vitro in bile acid treated human hepatocytes (10–13);Cai, 2017 1/id}; 5) Finally, depletion of macrophages in mice did not reduce liver injury after BDL (14, 15), indicating that inflammatory mediators from macrophages do not play a significant role, at least in the initiating stages. Rather, it is the infiltration of neutrophils that best correlates with liver injury in cholestasis (16–18). Altogether, these concerns suggest that under pathophysiological conditions, bile acids must injure the liver by alternative mechanisms rather than by their intrinsic toxicity. This review summarizes recent advances in the molecular mechanism of bile acid induced liver injury focusing on early events and the role of the inflammatory response in this pathological process (Table 1).
Table 1.
The effects of bile acids on inflammatory mediators in cholestatic liver injury depends upon cell type
| Cell type | Bile acid species | Response | Mediator/pathway | References |
|---|---|---|---|---|
| Mouse hepatocytes | TCA, DCA. CDCA, Bile | Induction of inflammatory cytokines | MAPKs/Egr1 | 10, 11 |
|
| ||||
| Mouse and human hepatocytes | Major endogenous bile acids | Induction of inflammatory cytokines, neutrophil chemotaxis | ER stress, Mitochondrial damage, Tlr9 activation. | 18 |
|
| ||||
| Mouse cholangiocytes | BDL model | Secretion and cleavage of osteopontin for immune cell recruitment Cell proliferation | Excessive pressure in the biliary system/MMPs TGR5/cSrc-EGFR-MEK-ERK1/2 | 48, 54, 61 |
| TLCA, TCA | Activation of COX-2 | S1PR2/ERK1/2/NF-kB | ||
|
| ||||
| Mouse and human neutrophils | BDL model | Activation, chemotaxis and cytotoxicity | DAMPs CXC/CCL chemokines Adhesion molecules Cytoskeletal proteins | 16–18, 72–74, 80, 82 |
|
| ||||
| Mouse and human monocytes/macrophages | BDL model TC, TCDC, GCDC, TLCA, CDCA | Production of pro-or/and anti-inflammatory cytokines | TGR5/NF-kB/JNK/Inflammasome | 50, 56, 87–92, 103 |
|
| ||||
| Mouse TH cells | BDL model | Production of pro- inflammatory and fibrotic cytokine IL-17 | 93, 97 | |
|
| ||||
| Mouse NK and invariant NK T cells | BDL model | Stimulation of anti- or suppression of pro- inflammatory cytokines produced in Kupffer cells | 98, 99 | |
2. Cholestatic hepatocytes initiate inflammatory response by releasing cytokines
More recently, Allen et al proposed that bile acid may induce liver injury via a hepatocyte initiated inflammatory response (10, 11). In these studies, exposure of cultured mouse hepatocytes to 200 μM of TCA, a major endogenous bile acid in this species significantly stimulated the expression (mRNA) of a series of cytokines and adhesion molecules, at pathophysiologically relevant concentrations, including MCP1 (Ccl2), MIP-2 (Cxcl2) and ICAM-1. Remarkably, bile acid treatment did not increase caspase 3 activity in these mouse hepatocytes or release alanine transaminase (ALT) activity in the culture medium, suggesting that neither apoptosis nor necrosis had occurred in these cells (10, 11). Interestingly, increased expression (mRNA) of the early growth response protein 1 (Egr1) was also detected in these bile acids treated cells. Egr1 is a transcription factor that plays an important role in regulating the expression of many genes, including inflammatory cytokines. Furthermore, the authors demonstrated that bile acid induced up-regulation of these inflammatory cytokines in mouse hepatocytes was partially Egr1-dependent but independent of farnesoid X receptor (Fxr/Nr1h4), the bile acid activated nuclear receptor, because these inflammatory genes were not reduced in bile duct ligated Fxr knockout mice. In contrast, Egr1-deficiency reduced bile acid induction of some of these cytokines and adhesion molecules in vitro in mouse hepatocyte cultures and in vivo in the liver of BDL mice, where reduced liver injury was also detected (10, 19). To explain how elevated levels of bile acid cause Egr1 activation, the authors proposed that MAP kinases may mediate this transactivation, speculating that there are cell surface receptors involved in this signaling pathway.
To further elucidate the role of inflammatory cytokines in cholestatic liver injury and also to gain insights into the mechanism of bile acid induction of cytokines expression, we recently examined this hypothesis in vitro using isolated mouse liver cells and human hepatocytes, and in vivo in cholestatic murine models. First, we confirmed that a 24-hour exposure to TCA stimulated inflammatory cytokines expression in a collagen sandwich culture of mouse hepatocyte, a system closely resembling cholestatic liver conditions. In addition, we found that at pathophysiological concentrations (25 – 200 μM) only hepatocytes, but not isolated liver non-parenchymal cells or cholangiocytes, responded to bile acid stimulation with increased cytokine expression. Furthermore, the cytokines released into the medium of the bile acid treated hepatocyte cultures significantly enhanced neutrophil chemotaxis in a transwell experiment, emphasizing the functional importance of these hepatocyte specific cytokines in initiating the inflammatory response. Knockout of chemokine Ccl2 significantly reduced hepatic neutrophils infiltration in two cholestatic mouse models, i.e. 1% cholic acid feeding and 7-day BDL, where less liver injury was also detected.
To understand why hepatocytes are uniquely susceptible to bile acids, we demonstrated that the hepatocyte-specific basolateral bile acid transporter NTCP/SLC10A1 is required for this event because knocking down NTCP or inhibiting the bile acid uptake transporters reduced bile acid induction of chemokines. This is consistent with recent reports in an NTCP-deficient patient and in Ntcp knockout mice. Both the patient and the mice were completely protected from cholestatic liver injury, despite extremely high levels of bile acid in the blood (20, 21). These findings also indicate that bile acids must first enter and accumulate in hepatocytes in order to stimulate cytokine expression, rather than the effect being mediated by a specific cell membrane receptor as proposed by others (10, 22). Once accumulated in the cell, bile acid caused ER stress and mitochondrial damage, as has been previously reported (23–27). However, there was no evidence of caspase 3 cleavage in these cells, in agreement with the findings of others that apoptosis is not playing a role (10–12, 18).
Because mitochondria damage was detected in bile acid treated hepatocytes, we hypothesized that the injured mitochondria may release “damage-associated molecular patterns” (DAMPs) that could activate toll-like receptors (Tlr). Tlr9 is one of them and is an intracellular DNA sensor. Previous studies have demonstrated that mitochondrial DNA can activate Tlr9 and stimulate inflammatory cytokines expression (28). To examine whether Tlr9 plays a role in bile acid induced liver injury, we treated Tlr9 −/− mouse hepatocytes with bile acid and found reduced induction of Cxcl2 in these cells. The involvement of Tlr9 is further supported by the observations that Cxcl2 induction was also significantly reduced in MyD88/Trif double knock out mouse hepatocytes. Of note, MyD88 and Trif are downstream molecules in Tlr9 signaling pathway. Reduced liver injury was also found in Tlr9−/− mouse liver after BDL (18, 29). However, Tlr9 normally resides on the endoplasmic reticulum and endosomes so that the mechanism of activation remains unclear. One possibility is that injured mitochondria undergo autophagy/mitophagy and present mitochondrial DNA to Tlr9 (30) Bile acid induction of cytokines are reduced in mouse hepatocytes when mitochondria are protected by cyclosporine A or norursodeoxycholic acid, (18). Norursodeoxycholic acid also improves liver function in several cholestatic rodent models and in patients with sclerosing cholanagitis in a recent phase II trial (31–33). Together, these findings support a role for mitochondrial damage in the pathogenesis of the cholestatic liver and suggests that bile acids activate the innate immune system.
Similarly, the major human bile acid, GCDCA, also stimulated cytokine expression when applied to human hepatocyte cultures at pathophysiological levels (≥50 μM), including CCL2, CCL15, CCL20, CXCL1 and IL-8, where mitochondrial damage was also detected(18). Periportal neutrophil infiltration in cholestatic patient livers also correlated with levels of serum ALT in these patients, consistent with a role for neutrophils in the pathogenesis of liver injury in human cholestatic disorders(18). Together, these findings in both humans and mice support the hypothesis that when bile acids accumulate in hepatocytes, they initiate liver injury by triggering an inflammatory response.
Is this hypothesis also correct in patients with obstructive cholestasis where high concentrations of bile acids regurgitate from bile into the hepatic parenchyma? In a recent report, Woolbright et al (13) found that human hepatocytes were much more resistant to bile acid treatment than rodent hepatocytes as only a minimal increase of IL-8 mRNA expression was detected when they were exposed to 5 mM TCA. However, in this study, the cells were cultured in Williams’ Medium E (Life Technologies, Grand Island, NY) shortly after cell preparation, rather than maintained in Hepatocyte Maintenance Medium (Lonza, Walkersville, MD) with matrigel sandwich culture configuration, a condition that better maintains the expression of NTCP and cell polarity, which is needed for bile acids to enter hepatocytes. Further studies will be needed to resolve this discrepancy.
Once inflammation is initiated in hepatocytes, they will either undergo irreversible injury and cell death or hepatoprotective mechanisms will attempt to restore tissue homeostasis. Details of these signaling pathways are described in recent reviews (34–36). For example, in response to cholestasis, hepatocytes minimize bile acid accumulation in hepatocytes by down regulating NTCP and OATPs as well as altering the metabolism of bile acids in order to increase their hydroxylation sites which decreases their hydrophobicity. At the same time, Up-regulation of the export transporters MRP3 and MRP4 and OSTα/β also occurs. See (37).
3. The response of cholangiocytes to cholestasis
Cholangiocytes are epithelial cells that line the lumen of the bile ducts and are responsible for secretion of an aqueous fluid rich in bicarbonate in response to meal induced excretion of the hormones secretin, vasoactive intestinal peptide and bombesin (37). Cholangiocytes also express bile acid transporters (i.e. ASBT/SLC10A2 and OSTα/SLC51A-OSTβ/SLC51B on the apical and basolateral membranes, respectively) that facilitate the cholehepatic circulation of bile acids. In contrast to hepatocytes, cholangiocytes are continuously exposed to much higher concentrations of bile acids in the millimolar range, yet these cells normally do not show signs of injury. It is possible that the concentrations of bile acids do not reach levels in these cells that would activate an inflammatory cascade as seen in hepatocytes, since Ostα-Ostβ, which transport bile acids by facilitated diffusion, should prevent an intracellular concentration gradient of bile acids from accumulating. In addition, other protective mechanisms include the secretion of a layer of mucous that is rich in bicarbonate. This “biliary HCO3− umbrella” maintains an alkaline environment on the surface of the luminal apical membrane of cholangiocytes, which should prevent the protonation of glycine-conjugated bile salts like GCDCA, a major conjugated bile acid in human bile. The pKa of glycine bile acid conjugates are ~ 4, so that an alkaline bile will maintain these bile acids in a charged non-protonated state, thus preventing their diffusion across the apical lipid membranes into the cholangiocytes (38). This hypothesis has been supported by the identification of a 20- to 40-nm-thick extracellular, juxta membranous layer of glycocalyx on the apical membrane of human and mouse biliary epithelium, as well as by studies showing that bile acid uptake and toxicity were dependent on pH and the key HCO3− exporter, anion exchanger 2 (AE2) in immortalized human cholangiocytes (39, 40). The potential role of the biliary HCO3− umbrella has also been implicated in the pathogenesis of primary biliary cholangitis (PBC), a cholestatic, chronic inflammatory autoimmune liver disease, as reduced expression of AE2 has been found in liver biopsies from patients with PBC (41–44). Interestingly, Ae2a, b-deficient mice exhibit features resembling PBC, including portal inflammation with CD8(+) and CD4(+) T lymphocytes surrounding damaged bile ducts and altered gene expression profiles in isolated cholangiocytes (45).
Cholangiocytes express and secrete osteopontin (OPN), a multifunctional glycophosphoprotein that can bind to integrin receptors expressed on inflammatory cells and function as a chemoattractant to neutrophils, macrophages and natural killer T cells in the liver (46, 47). Both the expression of osteopontin in biliary epithelial cells and its cleaved form in bile are significantly increased after BDL in mice, presumably caused by the stress on bile epithelial cells that results from the pressure in the biliary systems after obstruction of the bile duct. OPN−/− mice showed a delayed inflammatory response after BDL, as neutrophil infiltration was dramatically reduced and bile infarcts were nearly absent at one but not three days after BDL, suggesting that osteopontin plays a role in attracting neutrophils only at the very early stages of liver injury in this model (48).
TGR5 is a newly discovered G-protein coupled receptor that is activated in response to unconjugated > conjugated bile acids. TGR5 activation leads to an increase in cAMP and PKA activation and has been shown to modulate bile acid homeostasis and inflammation (49–51). TGR5 has been detected in cholangiocytes, Kupffer cells, and sinusoidal endothelial cells but not hepatocytes (52). Recent studies suggest that TGR5 is required for bile acid induced cholangiocyte proliferation and activation of TGR5 protects cholangiocytes from death-receptor mediated apoptosis (53, 54). In addition, TGR5 has also been shown to enhance bile flow and HCO3− secretion in mice after partial hepatectomy and in Mdr2−/− (Abcb4−/−) mice with sclerosing cholangitis like bile duct injury (55–57).
Although cholangiocytes respond to “pathogen associated molecular patterns” (PAMPs) and activators such as LPS with secretion of a number of cytokines including IL-1, IL-6, IL-8, and IFN-γ (58), there is little evidence to support the notion that bile acids directly elicit a cytokine response in normal cells. This is demonstrated by our recent studies, where exposure of isolated mouse cholangiocytes to pathophysiological concentrations (25 – 200 μM) of bile acids did not stimulate cytokine production (18). Lamireau et al used an immortalized BEC line in culture for 6 days and then exposed them to a range of bile acids from 20–500 μM for 72 hours. Only taurocholate (TC) stimulated release of cytokines MCP-1(Ccl2) and IL-6 in this somewhat artificial system at all concentrations and without a dose effect (59). Recently, Hisamoto et al pre-treated primary human biliary epithelial cells with 200 μM GCDCA for 3 days followed by LPS or poly (I:C) stimulation for 24 hours and found increased production of IL-6, IL-8 and CXCL10 in these cells. However, the authors did not elucidate any direct effect of GCDCA treatment on the cytokine production without LPS or poly (I:C) stimulation in these isolated cholangiocytes (60). Wang et al recently present evidence that conjugated bile acids can activate the sphiinogsine-1-Receptor 2 in mouse cholangiocyte plasma membranes which initiates an AKT and ERK1/2 signaling pathway that elicits an inflammatory and fibrotic response. Total serum bile acid levels, ALP and liver fibrosis were greatly diminished in bile duct ligated mice where this receptor was deleted, although little evidence was provided for an attenuated inflammatory response in these mice other than an absence of COX-2 induction in isolated cholangiocytes (61).
Patients with primary sclerosing cholangitis (PSC) have high levels of IL-8 in their bile compared to other non-PSC patients. Immunostaining revealed increasing expression of IL-8 protein as well as IL-8 receptors (CXCR1 and CXCR2) and CXCL5 as the disease advanced. IL-8 also caused cell proliferation when added to primary human cholangiocytes cultures and stimulated production of profibrotic genes suggesting that IL-8 may be involved in the pathogenesis of PSC (62). It remains unclear what role if any bile acids play in this process.
4. The role of neutrophils in cholestatic liver injury
The liver is constantly exposed to bacterial and viral components from the blood system as well as toxins and food-derived antigens. As such, it plays a key role in the innate immune defense response. The innate immune system in the liver is composed of a multiple population of immune cells, such as neutrophils, natural killer cells, natural killer T cells and dendritic cells (63, 64). Normally, neutrophils that circulate in the blood act as the first-responders of inflammatory cells and migrate towards the site of inflammation following chemical signals in a process known as chemotaxis. Due to their high abundance and mobility as well as potent cytotoxicity, neutrophils play a critical role in immediate response to pathogens as well as in sterile inflammation, where they respond to DAMPs released from stressed or damaged cells in the absence of pathogens. These molecules, such as high-mobility group box-1 (HMGB1) protein, heat shock proteins, ATP, nuclear and mitochondrial DNA, are recognized by toll-like receptors expressed in liver cells, including hepatocytes. This in turn activates the transcription of pro-inflammatory genes and initiates the recruitment of cytotoxic cells to the site of inflammation (65). In the liver, as in other organs, an excessive activation of neutrophils induces additional tissue damage as demonstrated during hepatic ischemia-reperfusion, as well as in viral hepatitis, non-alcoholic fatty liver disease, alcoholic liver disease, liver fibrosis/cirrhosis and other causes of liver failure (66–68).
Neutrophils execute their cytotoxicity through production of reactive oxygen species (ROS) and hypochlorous acid, a potent oxidant generated via myeloperoxidase. Increased myeloperoxidase activity, which reflects the number of neutrophils, has been found in the liver of bile duct ligated rats (69). The adhesion and locomotion of neutrophils during their extravasation across the endothelial barrier and recruitment to the site of inflammation is mediated through the interaction of intercellular adhesion molecule-1 (ICAM-1) on the endothelium and β2 integrins on the neutrophil cell surface(70, 71). A growing body of evidence suggests that the expression levels of these adhesion molecules as well as the hepatic accumulation of neutrophils directly correlate with the degree of cholestatic injury in both humans and in animal models. Normally in humans ICAM-1 is only detected at low levels in the endothelium of some portal vessels and sinusoidal lining cells, and is not found in hepatocytes. However, in patients with cholestasis due to extrahepatic obstruction, ICAM- 1 expression increases on sinusoidal endothelial and Kupffer cells. In addition, de novo ICAM-l expression is now observed on the sinusoidal membrane of hepatocytes in areas of parenchymal cell injury (72). An increase in ICAM-1 expression was also found on the endothelium of microvessels in chronic cholangitis patients with complete bile duct obstruction (73). In mice deficient of ICAM-1 or β2 integrin CD18, liver necrosis is dramatically reduced after BDL, accompanied by decreased hepatic neutrophil accumulation, compared with the wild-type control (16, 17).
The cytoskeletal proteins ezrin-radixin-moesin (ERM) and Na+/H+ exchanger regulatory factor-1 (NHERF-1, also known as ERM-binding phosphoprotein 50 (EBP50)), also play a role in neutrophil mediated liver injury in BDL mice (74). ERM proteins and NHERF-1 are located beneath plasma membranes of hepatocytes and biliary epithelial cells (75–77). They function as scaffolding proteins that tether membrane proteins to underlying F-actin network in microvilli-like membrane projections. These “docking” structures anchor and partially embrace leukocytes, including neutrophils, to promote firm adhesion and initiate leukocyte transmigration across endothelial and epithelial cells such as hepatocytes (78, 79). Our results show that NHERF-1 assembles ERM proteins, ICAM-1 and F-actin into a macromolecule complex that is increased in mouse liver at the plasma membranes after BDL and participates in transendothelial and -hepatocyte migratory uptake of neutrophils induced by BDL. In contrast, mice deficient in NHERF-1 exhibit lower levels of activated ERM and ICAM-1 protein in the liver and hepatocytes. Compared with wild-type controls, Nherf-1−/− mice have significantly reduced hepatic neutrophil infiltration as well as attenuated liver injury after BDL. These findings suggest that NHERF-1 plays a key role in the formation of ICAM-1/ERM/NHERF-1 macromolecule complexes that are important in the neutrophil mediated inflammatory response in cholestatic liver injury.
After bile duct ligation in mice, neutrophils are the predominant infiltrating immune cells during the acute phase of liver injury. They can be detected within 8 hours after BDL and reach maximum levels around 2 to 3 days, mainly in areas of injured hepatocytes and in surrounding sinusoids (16, 80). In a model of intrahepatic cholestasis caused by α-naphthylisothiovyanate (ANIT), neutrophil depleted-mice exhibited much less hepatocyte injury (81). In Mdr2−/− mice, neutrophils accumulate in response to elevated cytokines prior to histologic and biochemical evidence of liver cell injury (82). Together these data strongly support the hypothesis that neutrophils are the principal cause of hepatocyte toxicity in the early stages of cholestatic liver injury. In summary, neutrophils are activated and recruited to the liver by the pro-inflammatory mediators induced by high levels of bile acids, as discussed above, where they target and kill stressed or injured hepatocytes.
5. The role of other immune cells in cholestatic liver injury
In addition to neutrophils, other immune cells in the liver also participate in cholestatic liver injury. Hepatic macrophages consist of Kupffer cells, the tissue-resident macrophages in the liver, and infiltrated bone marrow-derived monocytes/macrophages that are recruited to the liver during injury. Traditionally, macrophages have been classified as “M1” or “M2” subsets. One functional subset, M1 macrophages, can be classically activated by interferon gamma (IFNγ) or toxins such as bacterial lipopolysaccharide (LPS). Activated M1 macrophages produce pro-inflammatory mediators including cytokines such as tumor necrosis factor (TNF)-α, IL-1β, and reactive oxygen species (ROS), which contribute to liver inflammation and injury as the disease progresses. The other subset, M2 macrophages, which are alternatively activated by IL-4 and IL-13, release IL-4, IL-10 and IL-13, have an anti-inflammatory phenotype. However, increasing evidence shows that during liver injury, macrophages are highly plastic as “mixed” macrophage phenotypes are also observed and they can rapidly change from a pro- inflammatory to an anti-inflammatory phenotype in response to changes in the hepatic microenvironment(83, 84). The various populations of hepatic macrophages display different forms of activation and exert diverse functional properties in liver inflammation, including phagocytosis of apoptotic cells and cell debris, initiation of an immune response in other liver cells such as hepatocytes, antigen presentation and immune cell recruiting (85, 86).
During cholestasis, various functional alterations of macrophages have been reported, such as delayed clearance of bacteria in BDL mice and higher levels of IL-10 and reciprocally lower levels of IL-12 production in response to LPS stimulation in Kupffer cells isolated from these mice (87). Monocytes and macrophages express TGR5, the G-protein coupled bile acid receptor, that can be activated by both conjugated and unconjugated bile acids, with lithocholic acid(LCA), deoxycholic acid (DCA), chenodeoxycholic acid (CDCA), and cholic acid (CA) being the most potent activators in descending order (50). Activation of TGR5 in macrophages reduces pro-inflammatory cytokines while maintaining anti-inflammatory cytokine expression thus promoting the development of an anti-inflammatory macrophage phenotype (88). In primary human macrophages, bile acids inhibit the LPS-induced expression of proinflammatory cytokines without affecting the expression of the anti-inflammatory cytokine IL-10, resulting in a macrophage phenotype with an increased IL-10/IL-12 ratio as well as a suppressed basal phagocytic activity (89). In the liver, TGR5 has been identified in Kupffer cells and is upregulated in rats after BDL (90). Compared with wild-type mice, TGR5-deficient mice have higher AST levels after cholic acid feeding, as well as increased necrotic areas on liver sections 2 or 3 days after BDL, accompanied with significantly higher levels of serum CCL2 (MCP-1), further demonstrating a role for TGR5 in the protection of cholestatic liver injury (54, 56). The anti-inflammatory effect of TGR5 in macrophages is mediated by inhibiting of NF-kB and JNK signaling pathways (50, 91, 92), as well as through inhibition of NLRP3 inflammasome activation as discussed in detail below in Section 6.
Recent studies show that Kupffer cells can be regulated by IL-17 during cholestasis. In BDL mice, IL-17 is released from T helper cells and neutrophils, and its upregulation starts on day 5, peaks on day 7, and remains elevated at 14 days after surgery (93, 94). Induction of IL-17 and its receptors, as well as IL-1β, TGF-β1, IL-6, and TNF-α were observed in isolated mouse Kupffer cells in response to IL-17 stimulation. Decreased expression of TGF-β1, IL-6, IL-1β, and TNF-α mRNA was also detected in IL-17A receptor deficient Kupffer cells (95). Neutralization of IL-17 with anti-IL17-A antibody significantly reduced BDL-induced hepatocellular necrosis, pro-inflammatory cytokine production and neutrophil infiltration in mice after 9 or 14 days of bile duct obstruction, although there are conflicting results as to whether of the accumulation of macrophages in the liver was reduced in these IL-17 blocked mice (93, 94). These findings indicate that Kupffer cells may be orchestrated with other liver cells and contribute to cholestatic liver injury.
Nevertheless, the role of Kupffer cells in cholestatic liver injury remains controversial. One study in BDL rats showed that administration of gadolinium chloride, a Kupffer cell inhibitor, attenuated liver injury and fibrosis, indicating that Kupffer cells promote BDL-induced liver injury (96). However, other studies using liposome-encapsulated dichloromethylene diphosphonate or alendronate for Kupffer cell depletion demonstrated that Kupffer cell-depleted mice have increased liver injury, as well as decreased hepatocyte regeneration and liver fibrosis than control mice, 7 or 10 days after BDL, suggesting that Kupffer cells have a protective role for hepatocyte injury and promote cell survival, regeneration and fibrosis in cholestasis (14, 15). In addition, these studies showed that Kupffer cells from BDL mice at 6 hours but not at 24 hours after surgery produced more IL-6 that suppressed liver injury, whereas no significant differences in liver histology and ALT levels were found 24 hours after common BDL. These studies indicate that Kupffer cells may play different roles at different stages of liver injury induced by BDL. Further investigation is needed to clarify whether and how Kupffer cells promote or protect from liver injury in cholestasis.
Other innate immune cells in the liver also participate in cholestatic liver injury. TH 17 cells are the major source of the pro-inflammatory and fibrogenic cytokine IL-17. Increased numbers of TH 17 cells as well as upregulation of IL-17 were observed in the livers of BDL mice (93, 97). In contrast, hepatic natural killer cells and invariant natural killer T cells have been shown to suppress cholestatic liver injury by stimulating anti-inflammatory or suppressing pro-inflammatory cytokines produced in Kupffer cells (98, 99).
6. The role of the inflammasome in cholestatic liver injury
Inflammsomes are multiprotein complexes that detect signals from injured cells and pathogens known as DAMPs and PAMPs respectively. These complexes assemble to activate caspase-1 which then proteolytically activates cytokine IL-1β and IL-18. IL-1β which amplifies the inflammatory response by further stimulating production of inflammatory cytokines. Activation of inflammasomes has been seen primarily in alcoholic hepatitis, NASH, chronic HCV, ischemia-reperfusion injury and acetaminophen toxicity (100). The role of inflammasomes in cholestatic liver injury is less clear. Inflammasomes consist of multiprotein complexes that sense DAMPs and PAMPs via nucleotide-binding oligomerization domain receptors known as NOD-like receptors (NLRs). In liver, these proteins consist primarily of NLRP3, NLRP1 and AIM2 (absent in melanoma protein, also known as interferon-inducible protein). These assemblies recruit apoptosis associate speck-like protein (ASC) and caspase–1 which subsequently leads to activation of caspase-1 and cleavage of pro-IL-1β. These proteins are prominently expressed in Kupffer cells and in liver sinusoidal endothelial cells and normally are essentially absent from hepatocytes. LPS is a major activator of the inflammasome in Kupffer cells. Recently pathophysiologic levels of bile acids have been shown to inhibit NLRP3 inflammasome activation in isolated macrophages via the TGR5-cAMP-PKA axis and phosphorylation of NLRP3 on Ser 291. TLCA or INT-777 treatment of mice subjected to LPS-induced sepsis or alum induced peritoneal inflammation significantly reduced IL-1β and IL-18 in Nlrp3 wild type mice but had no effect in Nlrp3 −/− mice. These findings suggest that bile acids place significant constraints on NLRP3 inflammasome -related inflammation (101), a finding supported by several earlier studies (90, 102). Opposite effects have been reported with chenodeoxycholic acid, which has been shown to activate the NLRP3 inflammasome in isolated macrophages as well as Kupffer cells initially sensitized to LPS(103). However non-pathophysiologic concentrations of this unconjugated bile acid, which are never seen in cholestatic serum or liver were used in these in vitro assays in this study. Interestingly, taurocholic acid did not produce these effects in macrophages as we have recently confirmed in the non-parenchymal cell fraction of mouse livers (18). This study also showed that a caspase-1 inhibitor improved inflammation, AST and ALT levels and fibrosis after bile duct ligation in mice for 21 days (103), effects most likely related to inflammasome activation in non-parenchymal cells as liver injury progressed in time.
Little is known about the role of inflammsomes in human cholestatic liver disease. NLRP3 and the adaptor protein ASC and the downstream activation of caspasae-1 and IL-1β were up-regulated in liver tissue from patients with primary biliary cholangitis. In addition, Galectin-3 (Gal3), a macrophage produced lectin that was thought to be mediating activation of the inflammasome, was also increased on both the mRNA and the protein levels in these patients. Immunostaining of Gal-3 and NLFP3 were increased in liver sections but whether they were in macrophages or stellate cells was not clear (104). Thus, the role of inflammasomes in cholestatic liver injury remains elusive both in cholestataic animal models and in man and needs much further study.
7. Bile acids as therapeutics for liver diseases
In addition to TGR5, bile acids activate ligand-activated nuclear receptors, such as Fxr/Nr1h4, to regulate their synthesis, transport, metabolism and immunity. Therefore, bile acids have emerged as attractive therapeutic agents in treating metabolic and inflammatory diseases. Ursodeoxycholic acid (UDCA), a major component of the bile of black bears, has been used as a remedy for cholestasis in Chinese traditional medicine for more than a thousand years. Currently UDCA is used as the primary treatment of PBC. UDCA has been shown to improve serum liver tests and slow the progression to cirrhosis and prolong the time needed for liver transplantation. UDCA exerts multiple benefits including increase of bile flow, induction of the “biliary HCO3− umbrella” and immunosuppressive effects (105, 106). A derivate of UDCA, norUDCA, cannot be conjugated and is reabsorbed by cholangiocytes from bile and returned to the sinusoids via the periductular capillary plexus of the liver, which results in increased bicarbonate secretion and hyperchloresis, as well as “ductular targeting” anti-inflammatory and anti-proliferative effects to injured bile ducts (107). In Mdr2−/− (Abcb4−/−) mice, norUDCA reverse injury in this model of sclerosing cholangitis. Recently another BA analogue, obeticholic acid (also known as INT-747), was approved by the FDA for the treatment of PBC in combination with UDCA in patients with an inadequate response to UDCA. Treatment with obeticholic acid results in decreases in alkaline phosphatase, γ-glutamyl transpeptidase and alanine aminotransferase in PBC patients (108, 109). The anti-inflammtory effects of obeticholic acid in PBC patients are likely related to FXR mediated inhibition of cholesterol 7α-hydroxylase (CYP7A1) and a reduction in the bile acid pool (110).
8. Future perspectives
As the molecular basis for bile acid homeostasis in the enterohepatic circulation has progressed, a number of therapeutic targets have emerged that are leading to pharmacologic trials (Table 2) (111). A key target is CYP7A1 since it regulates the synthesis of bile acids from cholesterol and its inhibition results in a diminution of the bile acid pool size. This is a major mechanism that Fxr agonists like obeticholic acid exploit. Reductions in the bile acid pool size limit the intracellular concentration of bile acids and lessen their ability to aggravate an inflammatory response. The modified form of FGF19 as well as all-trans retinoic acid appear to also act by inhibiting CYP7A1. Other potential targets at the level of the hepatocyte include the PPARs. Bezafibrate and Fenofibrate that act as pan PPAR and PPARα isoform agonists respectively, improve liver function in primary biliary cholangitis and in a few patients with PSC by inhibiting bile acid synthesis and also by stimulating phospholipid excretion (112). The terminal ileum is another site that regulates bile acid metabolism and several inhibitors of the apical sodium dependent bile salt transporter (ABST) are under clinical trial and increase fecal excretion of bile acids. Inhibition of ABST in the proximal tubule of the kidney also increases bile acid excretion in the urine.
Table 2.
Targets for Therapeutic Interventions in cholestatic liver injury (Current and Projected)
| Current Targets | Mechanisms of Action | (Drugs) |
|---|---|---|
| FXR/NR1H4 | Alter bile acid homeostasis by repressing CYP7A1 | Obetacholic acid, Fibrates, All-trans retinoic acid*, FGF19*) |
| MDR3/ABCB4 | Inducers enhances phosphatidyl choline synthesis and excretion | Fenofibrate, Benzafibrate |
| BSEP and MRP2 | Increase bile acid excretion and bile flow | UDCA and FXR agonists |
| ASBT | Inhibitors of bile acid uptake in ileum increases bile acid fecal and urinary exception | A4250*; LLuM001* |
| AE2 | Stimulates Cholangiocyte HC03− excretion | norURSO* |
| Potential Targets: (Suggested by basic mechanistic studies of the pathogenesis of cholestasis) | ||
| NTCP/SLC10A1 | block hepatic bile acid uptake | Myrcludex B & others in development* |
| OSTα/β (SLC51A/B) | block enterohepatic bile acid circulation and decreases bile acid pool size | ?? |
| Endoplasmic reticulum | Reducers of ER stress and reactive oxygen species | UDCA |
| Mitochondria | Stabilize ATPases & MMP | Cyclosporins, others? |
| Cytokine Receptors | Repress inflammatory response | CVC |
Undergoing clinical trials. See (111) for more details.
Over the past two decades, mounting studies suggest that in addition to their role in lipid digestion and absorption, bile acids have emerged as signaling molecules that participate in the inflammatory response associated with cholestatic liver injury. These studies that are examining the molecular mechanism by which bile acids injure the liver, as summarized in this review, point to a number of novel sites that might be exploited to minimize liver injury. These include inhibiting the uptake of bile acids into hepatocytes by blocking NTCP, protecting the hepatocyte from bile acid induced ER stress and or mitochondrial injury and developing cytokine receptor blockers that prevent neutrophil chemotaxis as recently described (18). Figure 1 illustrates the various sites where drugs might be judged to be effective therapies for cholestatic liver injury.
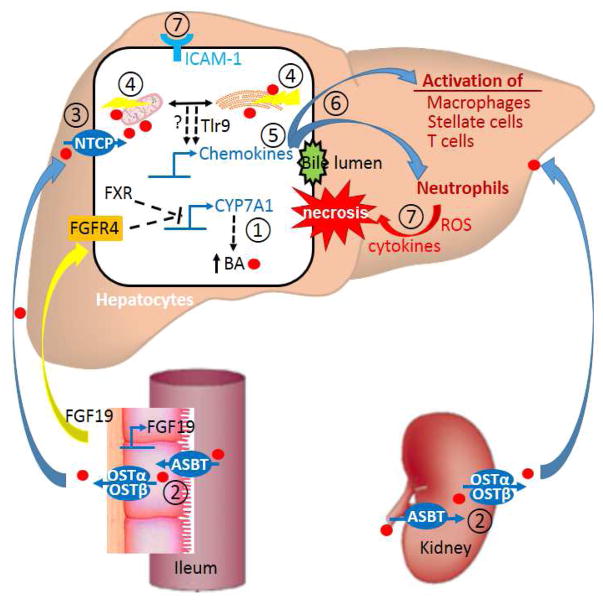
Figure 1.
A hypothetic model of bile acid induced liver injury and potential therapeutic targets for treating cholestatic disorders. The intervention sites are listed as ① repress bile acid synthesis in hepatocytes; ② block bile acid reabsorption in intestine and kidney; ③ block hepatic uptake of bile acid; ④ reduce bile acid caused mitochondrial damage and ER stress in hepatocytes; ⑤ inhibit inflammatory chemokine production; ⑥ prevent inflammatory response by antagonize cytokine receptors in immune cells; ⑦ prevent neutrophils and immune cells from attacking injured hepatocytes. An agent targeting a specific site or the combination of agents targeting multiple sites are predicted to be beneficial for patients with cholestasis.
Acknowledgments
This work was supported by National Institutes of Health Grants DK34989 (to Yale Liver Center), DK25636 (to J.L.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003 Apr;83(2):633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008 Aug;65(16):2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholmerich J, Becher MS, Schmidt K, Schubert R, Kremer B, Feldhaus S, et al. Influence of hydroxylation and conjugation of bile salts on their membrane-damaging properties--studies on isolated hepatocytes and lipid membrane vesicles. Hepatology. 1984 Jul;4(4):661–666. doi: 10.1002/hep.1840040416. [DOI] [PubMed] [Google Scholar]
- 4.Attili AF, Angelico M, Cantafora A, Alvaro D, Capocaccia L. Bile acid-induced liver toxicity: relation to the hydrophobic-hydrophilic balance of bile acids. Med Hypotheses. 1986 Jan;19(1):57–69. doi: 10.1016/0306-9877(86)90137-4. [DOI] [PubMed] [Google Scholar]
- 5.Galle PR, Theilmann L, Raedsch R, Otto G, Stiehl A. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology. 1990 Sep;12(3 Pt 1):486–491. doi: 10.1002/hep.1840120307. [DOI] [PubMed] [Google Scholar]
- 6.Patel T, Bronk SF, Gores GJ. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest. 1994 Dec;94(6):2183–2192. doi: 10.1172/JCI117579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster CR, Anwer MS. Cyclic adenosine monophosphate-mediated protection against bile acid-induced apoptosis in cultured rat hepatocytes. Hepatology. 1998 May;27(5):1324–1331. doi: 10.1002/hep.510270519. [DOI] [PubMed] [Google Scholar]
- 8.Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010 Jul;90(3):1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinugasa T, Uchida K, Kadowaki M, Takase H, Nomura Y, Saito Y. Effect of bile duct ligation on bile acid metabolism in rats. J Lipid Res. 1981 Feb;22(2):201–207. [PubMed] [Google Scholar]
- 10.Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011 Jan;178(1):175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012 Jan;32(1):58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolbright BL, Antoine DJ, Jenkins RE, Bajt ML, Park BK, Jaeschke H. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol Appl Pharmacol. 2013 Dec 15;273(3):524–531. doi: 10.1016/j.taap.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolbright BL, Dorko K, Antoine DJ, Clarke JI, Gholami P, Li F, et al. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol. 2015 Mar 15;283(3):168–177. doi: 10.1016/j.taap.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehring S, Dickson EM, San Martin ME, van RN, Papa EF, Harty MW, et al. Kupffer cells abrogate cholestatic liver injury in mice. Gastroenterology. 2006 Mar;130(3):810–822. doi: 10.1053/j.gastro.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Osawa Y, Seki E, Adachi M, Suetsugu A, Ito H, Moriwaki H, et al. Role of acid sphingomyelinase of Kupffer cells in cholestatic liver injury in mice. Hepatology. 2010 Jan;51(1):237–245. doi: 10.1002/hep.23262. [DOI] [PubMed] [Google Scholar]
- 16.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003 Aug;38(2):355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 17.Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004 Mar;286(3):G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- 18.Cai SY, Ouyang X, Chen Y, Soroka CJ, Wang J, Mennone A, et al. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight. 2017 Mar 9;2(5):e90780. doi: 10.1172/jci.insight.90780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim ND, Moon JO, Slitt AL, Copple BL. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006 Apr;90(2):586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- 20.Vaz FM, Paulusma CC, Huidekoper H, de RM, Lim C, Koster J, et al. Sodium taurocholate cotransporting polypeptide (SLC10A1) deficiency: conjugated hypercholanemia without a clear clinical phenotype. Hepatology. 2015 Jan;61(1):260–267. doi: 10.1002/hep.27240. [DOI] [PubMed] [Google Scholar]
- 21.Slijepcevic D, Kaufman C, Wichers CG, Gilglioni EH, Lempp FA, Duijst S, et al. Impaired uptake of conjugated bile acids and hepatitis b virus pres1-binding in na(+) -taurocholate cotransporting polypeptide knockout mice. Hepatology. 2015 Jul;62(1):207–219. doi: 10.1002/hep.27694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen K, Kim ND, Moon JO, Copple BL. Upregulation of early growth response factor-1 by bile acids requires mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol. 2010 Feb 15;243(1):63–67. doi: 10.1016/j.taap.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botla R, Spivey JR, Aguilar H, Bronk SF, Gores GJ. Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: a mechanism of UDCA cytoprotection. J Pharmacol Exp Ther. 1995 Feb;272(2):930–938. [PubMed] [Google Scholar]
- 24.Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 2001 Mar;33(3):616–626. doi: 10.1053/jhep.2001.22702. [DOI] [PubMed] [Google Scholar]
- 25.Iizaka T, Tsuji M, Oyamada H, Morio Y, Oguchi K. Interaction between caspase-8 activation and endoplasmic reticulum stress in glycochenodeoxycholic acid-induced apoptotic HepG2 cells. Toxicology. 2007 Nov 30;241(3):146–156. doi: 10.1016/j.tox.2007.08.095. [DOI] [PubMed] [Google Scholar]
- 26.Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med. 2008 Aug;14(8):828–836. doi: 10.1038/nm.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, et al. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol. 2008 Feb;294(2):G498–G505. doi: 10.1152/ajpgi.00482.2007. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010 Mar 4;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabele E, Muhlbauer M, Dorn C, Weiss TS, Froh M, Schnabl B, et al. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008 Nov 14;376(2):271–276. doi: 10.1016/j.bbrc.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 30.Carchman EH, Whelan S, Loughran P, Mollen K, Stratamirovic S, Shiva S, et al. Experimental sepsis-induced mitochondrial biogenesis is dependent on autophagy, TLR4, and TLR9 signaling in liver. FASEB J. 2013 Dec;27(12):4703–4711. doi: 10.1096/fj.13-229476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006 Feb;130(2):465–481. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Moustafa T, Fickert P, Magnes C, Guelly C, Thueringer A, Frank S, et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology. 2012 Jan;142(1):140–151. doi: 10.1053/j.gastro.2011.09.051. [DOI] [PubMed] [Google Scholar]
- 33.Halilbasic E, Steinacher D, Trauner M. Nor-Ursodeoxycholic Acid as a Novel Therapeutic Approach for Cholestatic and Metabolic Liver Diseases. Dig Dis. 2017;35(3):288–292. doi: 10.1159/000454904. [DOI] [PubMed] [Google Scholar]
- 34.Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013 Sep;59(3):583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 35.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014 Sep;147(3):577–594. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 36.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014 Oct;147(4):765–783. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyer JL. Adaptive Regulation of Hepatocyte Transporters in cholestasis. In: Arias, Alter, Boyer, Cohen, Fausto, Shaftritz, Wolkoff, editors. The Liver: Biology and Pathobiology (Fifth Edition) John Wiley & Sons, Lad; 2009. [Google Scholar]
- 38.Beuers U, Maroni L, Elferink RO. The biliary HCO(3)(−) umbrella: experimental evidence revisited. Curr Opin Gastroenterol. 2012 May;28(3):253–257. doi: 10.1097/MOG.0b013e328352aab2. [DOI] [PubMed] [Google Scholar]
- 39.Hohenester S, Wenniger LM, Paulusma CC, van Vliet SJ, Jefferson DM, Elferink RP, et al. A biliary. Hepatology. 2012 Jan;55(1):173–183. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 40.Maillette de Buy Wenniger LJ, Hohenester S, Maroni L, van Vliet SJ, Oude Elferink RP, Beuers U. The Cholangiocyte Glycocalyx Stabilizes the ‘Biliary HCO3 Umbrella’: An Integrated Line of Defense against Toxic Bile Acids. Dig Dis. 2015;33(3):397–407. doi: 10.1159/000371864. [DOI] [PubMed] [Google Scholar]
- 41.Concepcion AR, Lopez M, Ardura-Fabregat A, Medina JF. Role of AE2 for pHi regulation in biliary epithelial cells. Front Physiol. 2013;4:413. doi: 10.3389/fphys.2013.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poupon R. Primary biliary cirrhosis: a 2010 update. J Hepatol. 2010 May;52(5):745–758. doi: 10.1016/j.jhep.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 43.Prieto J, Qian C, Garcia N, Diez J, Medina JF. Abnormal expression of anion exchanger genes in primary biliary cirrhosis. Gastroenterology. 1993 Aug;105(2):572–578. doi: 10.1016/0016-5085(93)90735-u. [DOI] [PubMed] [Google Scholar]
- 44.Medina JF, Martinez A, Vazquez JJ, Prieto J. Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology. 1997 Jan;25(1):12–17. doi: 10.1002/hep.510250104. [DOI] [PubMed] [Google Scholar]
- 45.Salas JT, Banales JM, Sarvide S, Recalde S, Ferrer A, Uriarte I, et al. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008 May;134(5):1482–1493. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Wen Y, Jeong S, Xia Q, Kong X. Role of Osteopontin in Liver Diseases. Int J Biol Sci. 2016;12(9):1121–1128. doi: 10.7150/ijbs.16445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramaiah SK, Rittling S. Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicol Sci. 2008 May;103(1):4–13. doi: 10.1093/toxsci/kfm246. [DOI] [PubMed] [Google Scholar]
- 48.Yang M, Ramachandran A, Yan HM, Woolbright BL, Copple BL, Fickert P, et al. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol Lett. 2014 Jan 13;224(2):186–195. doi: 10.1016/j.toxlet.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008 Aug;7(8):678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 50.Perino A, Schoonjans K. TGR5 and Immunometabolism: Insights from Physiology and Pharmacology. Trends Pharmacol Sci. 2015 Dec;36(12):847–857. doi: 10.1016/j.tips.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Guo C, Chen WD, Wang YD. TGR5, Not Only a Metabolic Regulator. Front Physiol. 2016;7:646. doi: 10.3389/fphys.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keitel V, Haussinger D. Perspective: TGR5 (Gpbar-1) in liver physiology and disease. Clin Res Hepatol Gastroenterol. 2012 Oct;36(5):412–419. doi: 10.1016/j.clinre.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Keitel V, Haussinger D. TGR5 in cholangiocytes. Curr Opin Gastroenterol. 2013 May;29(3):299–304. doi: 10.1097/MOG.0b013e32835f3f14. [DOI] [PubMed] [Google Scholar]
- 54.Reich M, Deutschmann K, Sommerfeld A, Klindt C, Kluge S, Kubitz R, et al. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut. 2016 Mar;65(3):487–501. doi: 10.1136/gutjnl-2015-309458. [DOI] [PubMed] [Google Scholar]
- 55.Jourdainne V, Pean N, Doignon I, Humbert L, Rainteau D, Tordjmann T. The Bile Acid Receptor TGR5 and Liver Regeneration. Dig Dis. 2015;33(3):319–326. doi: 10.1159/000371668. [DOI] [PubMed] [Google Scholar]
- 56.Pean N, Doignon I, Garcin I, Besnard A, Julien B, Liu B, et al. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology. 2013 Oct;58(4):1451–1460. doi: 10.1002/hep.26463. [DOI] [PubMed] [Google Scholar]
- 57.Baghdasaryan A, Claudel T, Gumhold J, Silbert D, Adorini L, Roda A, et al. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO(−)(3) output. Hepatology. 2011 Oct;54(4):1303–1312. doi: 10.1002/hep.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Savard CE, Blinman TA, Choi HS, Lee SK, Pandol SJ, Lee SP. Expression of cytokine and chemokine mRNA and secretion of tumor necrosis factor-alpha by gallbladder epithelial cells: response to bacterial lipopolysaccharides. BMC Gastroenterol. 2002 Oct 11;2:23. doi: 10.1186/1471-230X-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamireau T, Zoltowska M, Levy E, Yousef I, Rosenbaum J, Tuchweber B, et al. Effects of bile acids on biliary epithelial cells: proliferation, cytotoxicity, and cytokine secretion. Life Sci. 2003 Feb 7;72(12):1401–1411. doi: 10.1016/s0024-3205(02)02408-6. [DOI] [PubMed] [Google Scholar]
- 60.Hisamoto S, Shimoda S, Harada K, Iwasaka S, Onohara S, Chong Y, et al. Hydrophobic bile acids suppress expression of AE2 in biliary epithelial cells and induce bile duct inflammation in primary biliary cholangitis. J Autoimmun. 2016 Dec;75:150–160. doi: 10.1016/j.jaut.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Aoki H, Yang J, Peng K, Liu R, Li X, et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017 Jan 24; doi: 10.1002/hep.29076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zweers SJ, Shiryaev A, Komuta M, Vesterhus M, Hov JR, Perugorria MJ, et al. Elevated interleukin-8 in bile of patients with primary sclerosing cholangitis. Liver Int. 2016 Sep;36(9):1370–1377. doi: 10.1111/liv.13092. [DOI] [PubMed] [Google Scholar]
- 63.Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016 Feb;13(2):88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 64.Doherty DG. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J Autoimmun. 2016 Jan;66:60–75. doi: 10.1016/j.jaut.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 65.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012 Nov;143(5):1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007 Oct;35(6):757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- 67.Xu R, Huang H, Zhang Z, Wang FS. The role of neutrophils in the development of liver diseases. Cell Mol Immunol. 2014 May;11(3):224–231. doi: 10.1038/cmi.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH, et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology. 2012 Nov;56(5):1971–1982. doi: 10.1002/hep.25801. [DOI] [PubMed] [Google Scholar]
- 69.Demirbilek S, Akin M, Gurunluoglu K, Aydin NE, Emre MH, Tas E, et al. The NF-kappaB inhibitors attenuate hepatic injury in bile duct ligated rats. Pediatr Surg Int. 2006 Aug;22(8):655–663. doi: 10.1007/s00383-006-1721-9. [DOI] [PubMed] [Google Scholar]
- 70.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009 Jan;61(1):22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 71.Futosi K, Fodor S, Mocsai A. Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013 Dec;17(4):1185–1197. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 72.Gulubova MV. Intercellular adhesion molecule-1 (ICAM-1) expression in the liver of patients with extrahepatic cholestasis. Acta Histochem. 1998 Feb;100(1):59–74. doi: 10.1016/s0065-1281(98)80006-8. [DOI] [PubMed] [Google Scholar]
- 73.Gulubova M, Vlaykova T, Manolova I, Hadjipetkov P, Popharitov A. Implication of adhesion molecules in inflammation of the common bile duct in patients with secondary cholangitis due to biliary obstruction. Hepatogastroenterology. 2008 May;55(84):836–841. [PubMed] [Google Scholar]
- 74.Li M, Mennone A, Soroka CJ, Hagey LR, Ouyang X, Weinman EJ, et al. Na(+)/H(+) exchanger regulatory factor 1 knockout mice have an attenuated hepatic inflammatory response and are protected from cholestatic liver injury. Hepatology. 2015 Oct;62(4):1227–1236. doi: 10.1002/hep.27956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fouassier L, Duan CY, Feranchak AP, Yun CH, Sutherland E, Simon F, et al. Ezrin-radixin-moesin-binding phosphoprotein 50 is expressed at the apical membrane of rat liver epithelia. Hepatology. 2001 Jan;33(1):166–176. doi: 10.1053/jhep.2001.21143. [DOI] [PubMed] [Google Scholar]
- 76.Li M, Wang W, Soroka CJ, Mennone A, Harry K, Weinman EJ, et al. NHERF-1 binds to Mrp2 and regulates hepatic Mrp2 expression and function. J Biol Chem. 2010 Jun 18;285(25):19299–19307. doi: 10.1074/jbc.M109.096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, et al. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006 Sep;131(3):878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002 Jun 24;157(7):1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol. 2003 Dec 1;171(11):6135–6144. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 80.Georgiev P, Jochum W, Heinrich S, Jang JH, Nocito A, Dahm F, et al. Characterization of time-related changes after experimental bile duct ligation. Br J Surg. 2008 May;95(5):646–656. doi: 10.1002/bjs.6050. [DOI] [PubMed] [Google Scholar]
- 81.Kodali P, Wu P, Lahiji PA, Brown EJ, Maher JJ. ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am J Physiol Gastrointest Liver Physiol. 2006 Aug;291(2):G355–G363. doi: 10.1152/ajpgi.00458.2005. [DOI] [PubMed] [Google Scholar]
- 82.Cai SY, Boyer JL. The Role of Inflammation in the Mechanisms of Bile Acid-Induced Liver Damage. Dig Dis. 2017;35(3):232–234. doi: 10.1159/000450916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calmus Y, Poupon R. Shaping macrophages function and innate immunity by bile acids: mechanisms and implication in cholestatic liver diseases. Clin Res Hepatol Gastroenterol. 2014 Oct;38(5):550–556. doi: 10.1016/j.clinre.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017 Jun;66(6):1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 85.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016 May;13(3):316–327. doi: 10.1038/cmi.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sato K, Hall C, Glaser S, Francis H, Meng F, Alpini G. Pathogenesis of Kupffer Cells in Cholestatic Liver Injury. Am J Pathol. 2016 Sep;186(9):2238–2247. doi: 10.1016/j.ajpath.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abe T, Arai T, Ogawa A, Hiromatsu T, Masuda A, Matsuguchi T, et al. Kupffer cell-derived interleukin 10 is responsible for impaired bacterial clearance in bile duct-ligated mice. Hepatology. 2004 Aug;40(2):414–423. doi: 10.1002/hep.20301. [DOI] [PubMed] [Google Scholar]
- 88.Reich M, Klindt C, Deutschmann K, Spomer L, Haussinger D, Keitel V. Role of the G Protein-Coupled Bile Acid Receptor TGR5 in Liver Damage. Dig Dis. 2017;35(3):235–240. doi: 10.1159/000450917. [DOI] [PubMed] [Google Scholar]
- 89.Haselow K, Bode JG, Wammers M, Ehlting C, Keitel V, Kleinebrecht L, et al. Bile acids PKA-dependently induce a switch of the IL-10/IL-12 ratio and reduce proinflammatory capability of human macrophages. J Leukoc Biol. 2013 Dec;94(6):1253–1264. doi: 10.1189/jlb.0812396. [DOI] [PubMed] [Google Scholar]
- 90.Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008 Jul 18;372(1):78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 91.Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology. 2011 Oct;54(4):1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lou G, Ma X, Fu X, Meng Z, Zhang W, Wang YD, et al. GPBAR1/TGR5 mediates bile acid-induced cytokine expression in murine Kupffer cells. PLoS One. 2014;9(4):e93567. doi: 10.1371/journal.pone.0093567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Brien KM, Allen KM, Rockwell CE, Towery K, Luyendyk JP, Copple BL. IL-17A synergistically enhances bile acid-induced inflammation during obstructive cholestasis. Am J Pathol. 2013 Nov;183(5):1498–1507. doi: 10.1016/j.ajpath.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang S, Huang D, Weng J, Huang Y, Liu S, Zhang Q, et al. Neutralization of Interleukin-17 Attenuates Cholestatic Liver Fibrosis in Mice. Scand J Immunol. 2016 Feb;83(2):102–108. doi: 10.1111/sji.12395. [DOI] [PubMed] [Google Scholar]
- 95.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012 Sep;143(3):765–776. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zandieh A, Payabvash S, Pasalar P, Morteza A, Zandieh B, Tavangar SM, et al. Gadolinium chloride, a Kupffer cell inhibitor, attenuates hepatic injury in a rat model of chronic cholestasis. Hum Exp Toxicol. 2011 Nov;30(11):1804–1810. doi: 10.1177/0960327111400106. [DOI] [PubMed] [Google Scholar]
- 97.Licata LA, Nguyen CT, Burga RA, Falanga V, Espat NJ, Ayala A, et al. Biliary obstruction results in PD-1-dependent liver T cell dysfunction and acute inflammation mediated by Th17 cells and neutrophils. J Leukoc Biol. 2013 Oct;94(4):813–823. doi: 10.1189/jlb.0313137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng CW, Duwaerts CC, Rooijen N, Wintermeyer P, Mott S, Gregory SH. NK cells suppress experimental cholestatic liver injury by an interleukin-6-mediated, Kupffer cell-dependent mechanism. J Hepatol. 2011 Apr;54(4):746–752. doi: 10.1016/j.jhep.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duwaerts CC, Sun EP, Cheng CW, van RN, Gregory SH. Cross-activating invariant NKT cells and kupffer cells suppress cholestatic liver injury in a mouse model of biliary obstruction. PLoS One. 2013;8(11):e79702. doi: 10.1371/journal.pone.0079702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015 Jul;12(7):387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 101.Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016 Oct 18;45(4):802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 102.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003 Mar 14;278(11):9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 103.Gong Z, Zhou J, Zhao S, Tian C, Wang P, Xu C, et al. Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis. Oncotarget. 2016 Dec 20;7(51):83951–83963. doi: 10.18632/oncotarget.13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tian J, Yang G, Chen HY, Hsu DK, Tomilov A, Olson KA, et al. Galectin-3 regulates inflammasome activation in cholestatic liver injury. FASEB J. 2016 Dec;30(12):4202–4213. doi: 10.1096/fj.201600392RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beuers U, Trauner M, Jansen P, Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol. 2015 Apr;62(1 Suppl):S25–S37. doi: 10.1016/j.jhep.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 106.Yoshikawa M, Tsujii T, Matsumura K, Yamao J, Matsumura Y, Kubo R, et al. Immunomodulatory effects of ursodeoxycholic acid on immune responses. Hepatology. 1992 Aug;16(2):358–364. doi: 10.1002/hep.1840160213. [DOI] [PubMed] [Google Scholar]
- 107.Trauner M, Halilbasic E, Claudel T, Steinacher D, Fuchs C, Moustafa T, et al. Potential of nor-Ursodeoxycholic Acid in Cholestatic and Metabolic Disorders. Dig Dis. 2015;33(3):433–439. doi: 10.1159/000371904. [DOI] [PubMed] [Google Scholar]
- 108.Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015 Apr;148(4):751–761. doi: 10.1053/j.gastro.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 109.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016 Aug 18;375(7):631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 110.Xu Y, Li F, Zalzala M, Xu J, Gonzalez FJ, Adorini L, et al. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology. 2016 Oct;64(4):1072–1085. doi: 10.1002/hep.28712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trauner M, Fuchs CD, Halilbasic E, Paumgartner G. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology. 2017 Apr;65(4):1393–1404. doi: 10.1002/hep.28991. [DOI] [PubMed] [Google Scholar]
- 112.Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology. 2015 Aug;62(2):635–643. doi: 10.1002/hep.27744. [DOI] [PMC free article] [PubMed] [Google Scholar]