Abstract
Cellular oxidative stress is implicated not only in lung injury but also in contributing to the development of pulmonary fibrosis. We demonstrate that a cell-permeable superoxide dismutase (SOD) mimetic and peroxynitrite scavenger, manganese (III) tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP) significantly inhibited bleomycin-induced fibrogenic effects both in vitro and in vivo. Further investigation into the underlying mechanisms revealed that MnTBAP targets canonical Wnt and non-canonical Wnt/Ca2+ signaling pathways, both of which were upregulated by bleomycin treatment. The effect of MnTBAP on canonical Wnt signaling was significant in vivo but inconclusive in vitro and the non-canonical Wnt/Ca2+ signaling pathway was observed to be the predominant pathway regulated by MnTBAP in bleomycin-induced pulmonary fibrosis. Furthermore, we show that the inhibitory effects of MnTBAP involve regulation of VEGF which is upstream of the Wnt signaling pathway. Overall, the data show that the superoxide scavenger MnTBAP attenuates bleomycin-induced pulmonary fibrosis by targeting VEGF and Wnt signaling pathways.
Idiopathic pulmonary fibrosis (IPF) is a usually fatal disease of the lung characterized by abnormal tissue repair, scarring, and fibrosis. The latest statistics from the American Lung Association indicates that about 140,000 Americans have been diagnosed with pulmonary fibrosis. There is no cure for the disease and there are only two FDA-approved drugs to control and slow down the progression of pulmonary fibrosis in the United States. The average survival rate for newly diagnosed patients is approximately 5 years and the quality of life for IPF patients is very poor (Coultas et al., 1994).
VEGF is the central regulator of angiogenesis in various physiological and pathophysiological states (Carmeliet, 2000; Helmlinger et al., 2000) and inhibition of VEGF signaling is shown to attenuate fibrosis (Chaudhary et al., 2007; Kulkarni et al., 2016). The Wnt signaling pathways constitute an important group of signal transduction pathways that relay intracellular signals via signal receptors. Three signaling pathways, including the canonical Wnt pathway, the non-canonical cell polarity pathway, and the non-canonical Wnt/ Ca2+ pathway, have been characterized (Nusse and Varmus, 1992; Nusse, 2005). The canonical Wnt signaling is activated when the Wnt ligand binds to the receptor Frizzled (Fz) and its co-receptor low density lipoprotein receptor-related protein 6 (LRP6). This binding leads to recruitment of the dishevelled (Dvl) proteins that further leads to accumulation of β-catenin in the cytoplasm, and its subsequent translocation into the nucleus to activate T-cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors (Komiya and Habas, 2008; MacDonald et al., 2009). Recent studies have shown that canonical Wnt signaling plays a predominant role in fibrotic diseases including pulmonary fibrosis (Miao et al., 2013; Sun et al., 2014; Kulkarni et al., 2016). Wnt binding to Fz receptor can also lead to activation of a second type of non-canonical Wnt signaling, Wnt/Ca2+ pathway that functions by activating phospholipase C (PLC) which cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into 1,2-diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). This results in the activation of protein kinase C (PKC), the calcium/calmodulin-dependent protein kinase type II (CaMKII), and calcineurin that activate other transcription factors that determine cell fate and cell migration (Oishi et al., 2003; Liu et al., 2005; Grumolato et al., 2010). The non-canonical cell polarity pathway is activated when Wnt ligand binding also binds a co-receptor such as ROR1/2, Ryk, or PTK7 leading to activation of Rho and Rac GTPases, Rho-kinase (ROCK) and c-Jun N-terminal kinase (JNK) (Komiya and Habas, 2008). The LRP5/6 co-receptors, which are crucial receptors of the canonical Wnt signaling pathway, have also been observed to control the non-canonical Wnt signaling pathway (Grumolato et al., 2010). A recent study showed that targeting calcium signaling inhibited bleomycin-induced pulmonary fibrosis (Mukherjee et al., 2015). However, the role of non-canonical Wnt/Ca2+ signaling is not well characterized and is understudied in fibrotic conditions.
Reactive oxygen species (ROS) are normal byproducts of cellular metabolism that are implicated not only in lung injury but also in contributing to the development of pulmonary fibrosis (Hemnes et al., 2008; Bhattacharjee et al., 2015; Hua-Huy et al., 2015). The lungs have a well-developed antioxidant defense system involving enzymes such as superoxide dismutases (SODs), catalase, and glutathione peroxidases (GPxs) to maintain normal levels of ROS (Quinlan et al., 1994; Rahman, 2007; Azad et al., 2008). Among these, SODs are the class of enzymes that convert superoxide radicals to hydrogen peroxide, which is further neutralized to water by GPx so as to maintain normal oxidative status of the lungs. Three types of SODs, including extracellular SODs, copper-zinc SODs (Cu-ZnSOD), and manganese SODs (MnSOD), are known and they have specific distributions and functions (Kinnula and Crapo, 2003). Several reports have indicated the benefits of using antioxidants as a viable therapeutic option for pulmonary fibrosis (Kinnula et al., 2005; Liu et al., 2007; Verma et al., 2013; Tang et al., 2015; Bilgin et al., 2016; Divya et al., 2016). MnSOD, in particular, was reported to exhibit effects better than Cu-ZnSOD in inhibiting bleomycin-induced pulmonary fibrosis (Parizada et al., 1991). Recent studies have demonstrated protective effects of MnSOD in limiting inflammatory responses and fibrosis (Li and Zhou, 2011; Kwak et al., 2015).
In this study, we analyzed the effect of an MnSOD mimetic, manganese (III) tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP) on bleomycin-induced pulmonary fibrosis. We demonstrate that MnTBAP significantly inhibits bleomycin-induced fibrogenic changes both in vitro and in vivo. Furthermore, bleomycin induced upregulation of key protein components of the canonical Wnt and non-canonical Wnt/Ca2+ signaling pathways that were targeted by MnTBAP. MnTBAP significantly inhibited bleomycin-induced VEGF, which was found to be upstream of Wnt signaling. Overall, our data show that MnTBAP is capable of attenuating bleomycin-induced pulmonary fibrosis and reveal the underlying mechanisms that could have important implications in antifibrotic therapy and management of this fatal disease.
Materials and Methods
Chemicals and reagents
Antibodies against PLCγ1, PKCα, CaMKII, LRP6, Wnt5AB, Dvl2, and β-catenin were obtained from Cell Signaling Technology, Inc. (Beverly, MA). Antibody for β-actin was from Sigma Chemical, Inc. (St. Louis, MO). CBO-P11 and MnTBAP were from Calbiochem (San Diego, CA). Bleomycin sulfate, dihydroethidine (DHE), CID 11210285 hydrochloride and salinomycin were obtained from Sigma Chemical, Inc. RNeasy mini kit, RT2 first strand kit, RT2 SYBR® Green qPCR mastermix, and RT2 profiler PCR array (RT2 profiler™ PCR array human angiogenesis) were obtained from Qiagen (Valencia, CA). The remaining chemicals and solvents used were of standard analytical grade and HPLC grade, respectively.
Cell culture
Human lung CRL-1490 fibroblasts (ATCC; Manassas, VA) were maintained in Eagle’s Minimum Essential Medium (MEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 100 U/ml penicillin, 100 mg/ml streptomycin, and Amphotericin B (Invitrogen). Cells were cultured at 37°C in 5% CO2 incubator, and were passaged at preconfluent densities using a solution containing 0.25% trypsin and 0.5 mM EDTA (Invitrogen).
Animal model
For the animal studies, 6–8-week-old C57BL/6 mice were used (Jackson Laboratories, Bar Harbor, ME). Mice were housed in a barrier facility with specific pathogen-free conditions, and all experiments were performed using protocols approved by the Old Dominion University (ODU) animal facility. Briefly, mice were anesthetized with isoflurane. In the first set of experiments, either bleomycin sulfate or equal volumes of saline as control was administered intranasally. In a separate set, bleomycin-treated mice were co-treated every other day by intraperitoneal injection of MnTBAP (3 mg/kg) or CBO-P11 (0.3 mg/kg) starting at day 0 and continued until the mice were euthanized at day 28. Bronchoalveolar lavage (BAL) fluid was collected after the trachea was exposed and cannulated with a 20-gauge catheter. After instillation of 1 ml of cold sterile PBS three times through the trachea into the lung, BAL fluid was recovered at 90% of the original volume. The BAL fluid was centrifuged for 10 min at 1,500 rpm and the cell-free supernatant was stored at −80°C. The lung was perfused with 5 ml of cold saline through the left ventricle and surgically removed. The left lung was used to evaluate the fibrotic score by histological examination, and the right lung was homogenized to analyze protein expression levels. Additionally, liver samples were also obtained from the animals to screen for any adverse effects as a result of drug treatments.
Histopathology
Mice were euthanized and the left lung was fixed with 10% formalin overnight and embedded in paraffin. Paraffin sections (3-µm thick) were stained with hematoxylin-eosin (H&E). The pathological grade of fibrosis was evaluated under 10× magnification. Histological assessment of the extent and severity of pulmonary fibrosis was independently determined by six researchers and one pathologist in a blind study using the Ashcroft method (Ashcroft et al., 1988) on sections of the left lung stained with H&E.
ROS detection
Cellular ROS production was determined fluorometrically using dihydroethidine (DHE), a fluorescent probe for superoxide. After specific treatments, cells were incubated with the probe (10 µM) for 30 min at 37°C, after which they were washed, resuspended in PBS, and analyzed for fluorescence intensity using a multiwell plate reader (Synergy H1 Hybrid Reader, BioTek, Winooski, VT) at the excitation/emission wavelengths of 485/535 nm.
Thiobarbituric acid reactive assay (TBARS)
The assay was performed as described by Halliwell and Gutteridge (1999), in which the extent of lipid peroxidation was estimated from the concentration of malondialdehyde (MDA), a thiobarbituric acid reactive substance (TBARS). The lung and liver for the preparation of homogenate to be used in this assay were obtained from 6 to 8-week-old C57BL/6 mice. One milliliter of 0.15 M potassium chloride was added to the tubes followed by 0.5 ml of lung or liver homogenate (10% w/v in PBS; calcium, magnesium free). Peroxidation was initiated by the addition of 100 µl of 2 mM ferric chloride. After incubating the tubes for 30 min at 37°C, the peroxidation reaction was stopped by adding 2 ml of ice-cold HCl (0.25 N) containing 15% trichloroacetic acid and 0.38% thiobarbituric acid. The tubes were kept at 80°C for 1 h, cooled and centrifuged at 7,500 rpm. The absorbance of the supernatant, containing TBA-MDA complex was read at 532 nm.
Cell proliferation and collagen assays
Cells were seeded in six-well plates at a density of 50,000 cells/ well. After the treatments, 0.25% trypsin was added to the cells and counted using a hemocytometer. A minimum of three separate experiments were performed for each assay. Collagen content was determined by Sircol® assay (Biocolor Ltd., Belfast, UK), according to the manufacturer’s protocol. Briefly, sirius red reagent (1 ml) was added to cell culture supernatant or BAL fluids (100 µl) and mixed for 30 min. The collagen-dye complex was precipitated by centrifugation at 16,000g for 5 min, washed with ethanol, and dissolved in 0.5M NaOH. Absorbance was measured at 540 nm.
Scratch assay for cell migration
Cells were seeded in 12-well plates and a 1 ml pipette tip was used to scratch sub-confluent cultures for the scratch assay. Cells were washed with PBS and pretreated for 1 h with MnTBAP followed by bleomycin (10 mU/ml or 25 mU/ml) treatment for 48 h. Bright field pictures were taken at 0 and 48 h and relative cell migration was quantified using ImageJ software (Java image processing, NIH).
Western blot analysis
Cell lysates or mice lung homogenates were resolved on a 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto a nitrocellulose membrane. The protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL), and equal amount of protein was loaded per sample. The membrane was blocked with TBS-T (0.1% Tween-20 in TBS) containing 5% dry milk, and incubated with primary antibody overnight at 4°C. After three washes with TBS-T, the membrane was incubated with HRP-conjugated secondary antibody for 1 h and then washed with TBST. Immunoreactive proteins were detected by chemiluminescence (Supersignal® West Femto, Pierce Biotechnology) and quantified by imaging densitometry using myImageAnalysis Software (Thermo Scientific, Waltham, MA). Mean densitometry data from independent experiments were normalized to results obtained from untreated control cells.
Enzyme-linked immunosorbent assays (ELISA)
The expression levels of VEGF and IL-8 proteins were quantified using ELISA. Briefly, supernatant from treated cells or lavage fluid from mice treated with saline (control) or bleomycin (1 U/kg) with or without MnTBAP (3 mg/kg) co-treatment were collected and analyzed for VEGF levels using a Human VEGFA ELISA kit (Thermo Scientific, Waltham, MA) and for IL-8 levels using a Quantikine ELISA kit (R&D Systems, Minneapolis, MN) as per manufacturer’s protocol. Briefly, samples or reference standards (100 µl) were added to each well of a microplate pre-coated with monoclonal antibody specific to VEGF and IL-8. After washing out unbounded proteins, an HRP-conjugated polyclonal secondary antibody was added to the wells and incubated. After washing and adding 100 µl of substrate solution, optical density was determined at 450 nm (Synergy HI Hybrid Reader, BioTek).
Messenger RNA expression profiling and quantitative RT-PCR
Expression of angiogenesis-related mRNAs in CRL-1490 cells was determined by RT-PCR. Cells were treated, lysed, and mRNA isolated using the RNeasy Mini Kit (Qiagen). cDNA was prepared from extracted mRNA (1 µg) using the RT2 First Strand Kit (Qiagen). cDNA combined with RT2 SYBR Green Master Mix (Qiagen) was added to individual RT-PCR array wells (SABiosciences, Valencia, CA) for analysis. Data were collected using the iCycler RT-PCR system (BioRad, Hercules, CA). Gene arrays for angiogenesis were assayed for mRNA expression, and the data were normalized to blank housekeeping genes.
Statistical analysis
Representative data from three or more independent experiments are shown as mean value ± S.E.M. Statistical analysis was performed with two-way analysis of variance to identify differences between groups using GraphPad Prism Software (San Diego, CA) and P values <0.05 were considered significant.
Results
MnTBAP inhibits bleomycin-induced fibrogenic effects
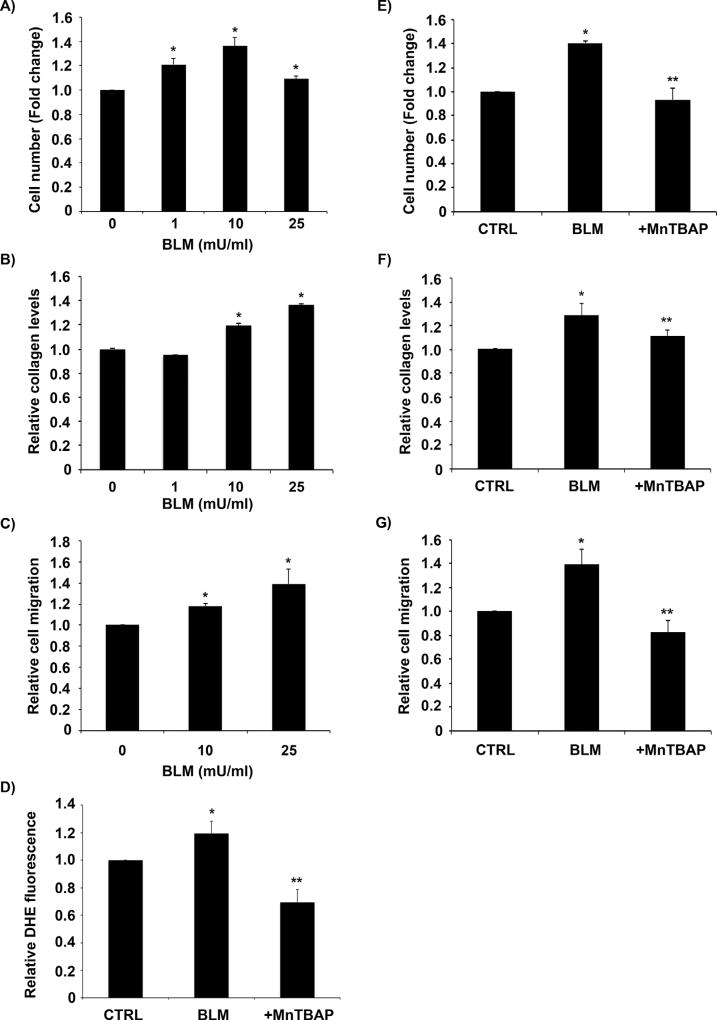
The effect of bleomycin on fibroblast proliferation and collagen levels was assessed to confirm bleomycin-induced fibrogenic response. Bleomycin induced a dose-dependent increase in fibroblast cell proliferation, total collagen content in cell supernatant and cell migration as compared to untreated control (Fig. 1A, B, and C). Furthermore, bleomycin treatment induced superoxide levels in fibroblast cells and pre-treating cells with a cell permeable SOD mimetic MnTBAP, significantly inhibited bleomycin-induced superoxide levels in fibroblast cells (Fig. 1D). Pre-treatment of cells with MnTBAP also significantly inhibited bleomycin-induced fibrogenic effects including fibroblast proliferation, collagen production, and cell migration (Fig. 1E, F, and G and Supplementary Fig. S1).
Fig. 1. MnTBAP inhibits bleomycin-induced fibrogenic effects.
(A) CRL-1490 cells were treated with the indicated concentrations of bleomycin for 24 h after which they were trypsinized and counted using a hemocytometer. (B) Supernatant from CRL-1490 cells treated with varying concentrations of bleomycin (0–25 mU/ml) for 24 h were collected and analyzed for soluble collagen content by Sircol® assay. (C) Subconfluent CRL-1490 cells were treated with bleomycin (10 mU/ml or 25 mU/ml) for 48 h and relative cell migration was analyzed by in vitro scratch assay. (D) CRL-1490 cells were pre-treated for 1 h with MnTBAP (100 µM) followed by bleomycin (10 mU/ml) treatment and analyzed for ROS production by measuring DHE fluorescence intensity. Plots show relative fluorescence intensities over non-treated control at a peak response time of 3 h after treatment. (E) CRL-1490 cells were pre-treated for 1 h with MnTBAP (100 µM), and then treated with bleomycin (10 mU/ml) for 24 h. The cells were trypsinized and counted using a hemocytometer. (F) CRL-1490 cells were pre-treated for 1 h with MnTBAP (100 µM), and then treated with bleomycin (10 mU/ml) for 24 h. Cell supernatants were collected and analyzed for soluble collagen content by Sircol® assay. (G) Subconfluent CRL-1490 cells were pre-treated for 1 h with MnTBAP (100 µM), treated with bleomycin (25 mU/ml) for 48 h and relative cell migration was analyzed by in vitro scratch assay. Plots are mean ± S.E.M (n = 4). *P < 0.05 versus non-treated control. **P < 0.05 versus bleomycin treatment.
Inhibitory effects of MnTBAP on bleomycin-induced fibrosis in vivo
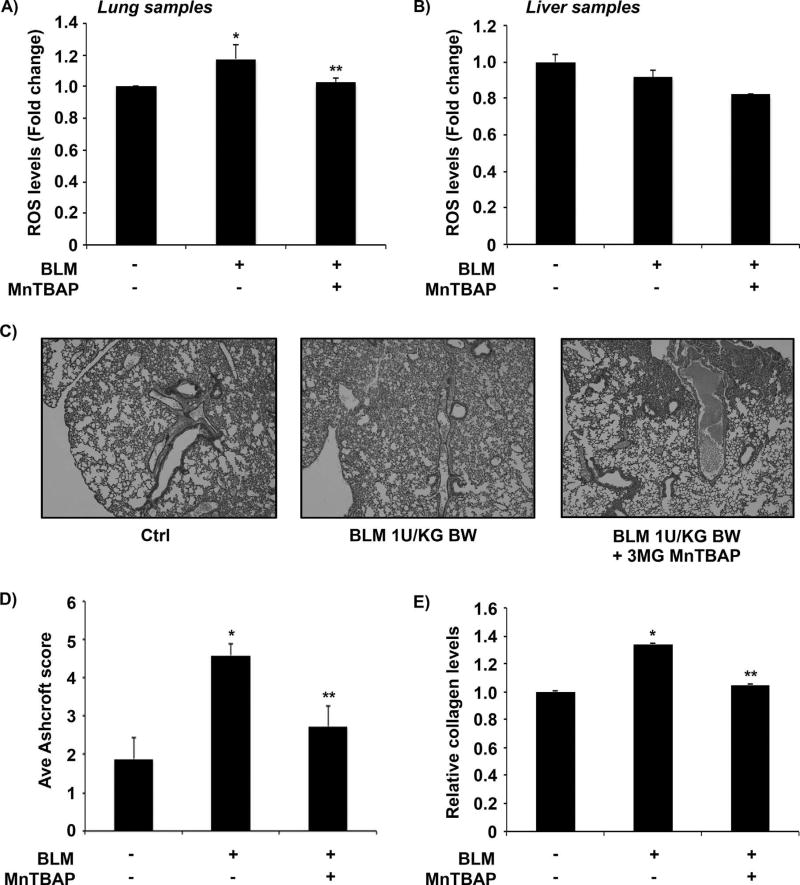
Bleomycin-treated mice lung and liver homogenates were analyzed for total ROS levels using the TBARS assay. Figure 2A shows that bleomycin-instillation led to significant ROS generation in lung samples obtained from bleomycin-treated mice and co-treatment with MnTBAP decreased ROS levels. No significant changes in ROS levels were observed in liver tissue samples (Fig. 2B). No adverse effects or fatalities were observed in animals throughout the experimental period. Lung histology data showed characteristic inflammatory cells with changes in alveolar wall of the lungs of mice treated with bleomycin as compared to the control group (Fig. 2C). Quantitative histology showed a significant reduction in fibrosis in animals co-treated with MnTBAP, which brought the fibrotic response down to control levels (Fig. 2D). Bleomycin-induced collagen production was significantly inhibited in MnTBAP-pretreated mice (Fig. 2E).
Fig. 2. Inhibitory effects of MnTBAP on bleomycin-induced fibrosis in vivo.
Mice treated with bleomycin (1 U/kg) with or without MnTBAP (3 mg/kg) or equal volume of saline as control were euthanized at day 28. (A) Lung tissue homogenates and (B) liver tissue homogenates from control and treated mice were analyzed for lipid peroxidation and total ROS levels by the TBARS assay. (C) Representative immunohistochemical micrographs of mice lung tissue stained with H&E. (D) Calculated fibrosis score based on the histopathological assessment of H&E-stained slides. (E) BAL fluid from control and treated mice were analyzed for soluble collagen levels by Sircol® assay. Plots are mean ± S.E.M of five animals per sample group. *P <0.05 versus non-treated control. **P <0.05 versus bleomycin treatment.
MnTBAP inhibits bleomycin-induced upregulation of non-canonical Wnt/Ca2+ signaling
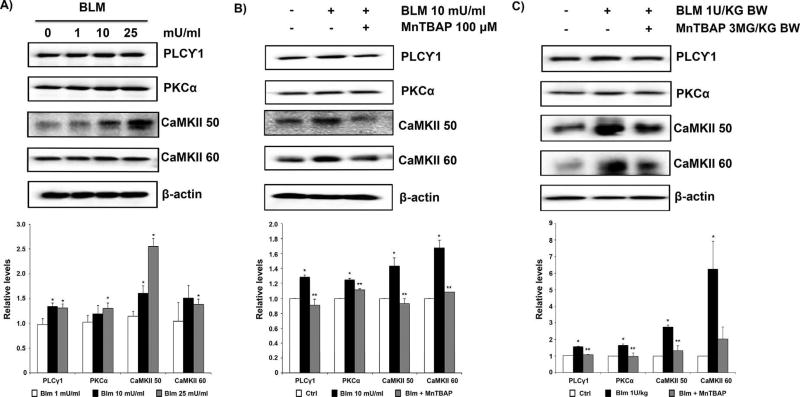
The effect of bleomycin on non-canonical Wnt/Ca2+ signaling was studied by investigating the key protein components of the pathway including PLCγ1, PKCα, and CaMKII. Bleomycin induced a dose-dependent increase in the expression levels of these proteins in CRL-1490 cells (Fig. 3A). MnTBAP pre-treatment significantly inhibited bleomycin-induced upregulation of PLCγ1, PKCα, and CaMKII (Fig. 3B). Similarly, lung tissue homogenates from mice pretreated with MnTBAP showed significant downregulation of bleomycin-induced PLCγ1, PKCα, and CaMKII protein levels (Fig. 3C). The observed effect of MnTBAP on PKCα was marginal in comparison to the effects observed on CaMKII proteins in both in vitro and in vivo samples.
Fig. 3. MnTBAP inhibits bleomycin-induced upregulation of non-canonical Wnt/Ca2+ signaling.
(A) CRL-1490 cells were treated with the indicated concentrations of bleomycin for 24 h and probed for non-canonical Wnt/Ca2+ proteins PLCγ1, PKCα, and CaMKII. (B) CRL-1490 cells were pretreated for 1 h with MnTBAP (100 µM), and then treated with bleomycin (10 mU/ml) for 24 h and probed for PLCγ1, PKCα, and CaMKII proteins. (C) Mice treated with bleomycin (1 U/kg) with or without MnTBAP (3 mg/kg) or equal volume of saline as control were euthanized at day 28. Lung tissue homogenates obtained from control and treated mice were probed for PLCγ1, PKCα, and CaMKII proteins. Blots were reprobed with β-actin antibody to confirm equal loading of the samples. Representative blots from three independent experiments (in vitro) or three animals per sample group (lung tissue homogenates) are shown. The immunoblot signals were quantified by densitometry. Values are mean ± S.E.M. (n = 3). *P <0.05 versus non-treated control. **P <0.05 versus bleomycin treatment.
Regulation of canonical Wnt signaling by MnTBAP
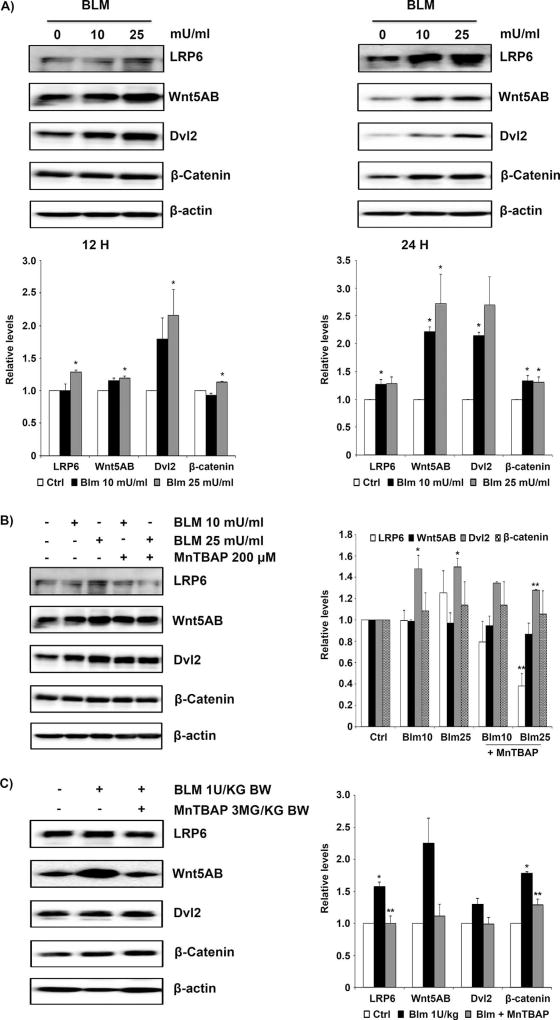
We next investigated the effects of bleomycin on canonical Wnt signaling. Protein components of the canonical Wnt signaling, including LRP6, Wnt5AB, Dvl2, and β-catenin, were all upregulated by bleomycin in a dose-dependent manner in lung fibroblast cells (Fig. 4A). MnTBAP, which at a concentration of 100 µM exhibited significant inhibition of bleomycin-induced non-canonical Wnt/Ca2+ upregulation (Fig. 3B), required a higher concentration of 200 µM to exhibit effects on canonical Wnt upregulation (Fig. 4B). The observed effects of MnTBAP to inhibit bleomycin-induced upregulation of the canonical Wnt proteins were only marginal at 12 h and the effects were less pronounced at 24 h (data not shown). Although the in vitro data were inconclusive, lung tissue homogenates from mice pretreated with MnTBAP showed significant inhibition of LRP6 and Wnt5AB proteins but the effects were less pronounced for Dvl2 and β-catenin proteins (Fig. 4C).
Fig. 4. Regulation of canonical Wnt signaling by MnTBAP.
(A) CRL-1490 cells were treated with the indicated concentrations of bleomycin for either 12 or 24 h, and then probed for canonical Wnt proteins LRP6, Wnt5AB, Dvl2, and β-catenin. (B) CRL-1490 cells were pretreated for 1 h with MnTBAP (200 µM), and then treated with bleomycin (10 mU/ml or 25 mU/ml) for 12 h and probed for LRP6, Wnt5AB, Dvl2, and β-catenin proteins. (C) Mice treated with bleomycin (1 U/kg) with or without MnTBAP (3 mg/kg) or equal volume of saline as control were euthanized at day 28. Lung tissue homogenates obtained from control and treated mice were probed for LRP6, Wnt5AB, Dvl2, and β-catenin proteins. Blots were reprobed with β-actin antibody to confirm equal loading of the samples. Representative blots from three independent experiments (in vitro) or three animals per sample group (lung tissue homogenates) are shown. The immunoblot signals were quantified by densitometry. Values are mean ± S.E.M. (n = 3). *P <0.05 versus non-treated control. **P <0.05 versus bleomycin treatment.
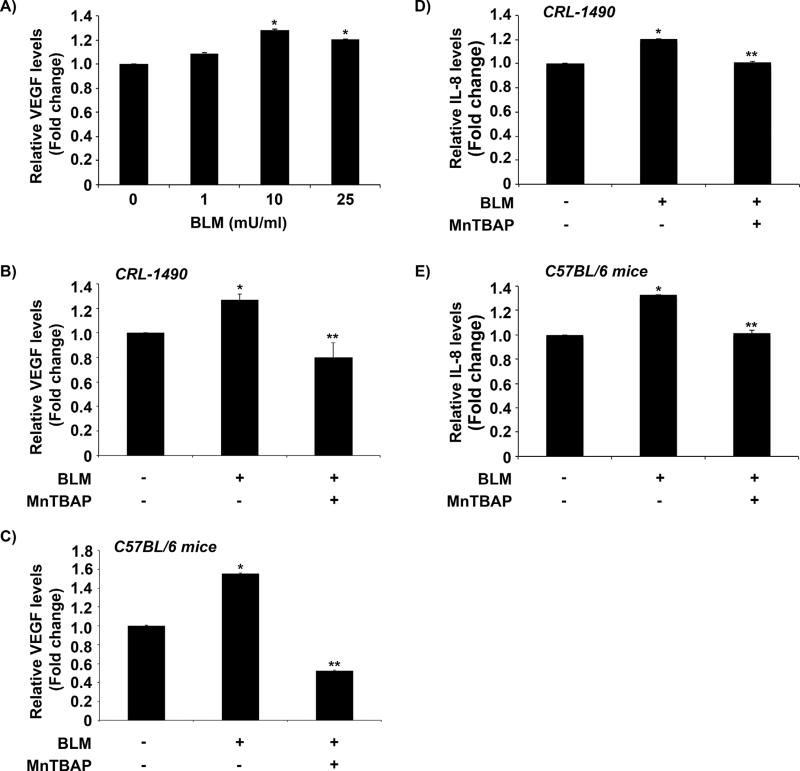
Effect of MnTBAP on bleomycin-induced VEGF and other angiogenic markers
Bleomycin treatment resulted in a dose-dependent upregulation of VEGF levels in human lung fibroblasts (Fig. 5A) and MnTBAP pre-treatment significantly inhibited this effect in treated CRL-1490 cells (Fig. 5B). Similarly, VEGF levels analyzed in BAL fluid obtained from mice co-treated with bleomycin and MnTBAP indicated a significant inhibition in VEGF upregulation as compared to bleomycin-treated mice (Fig. 5C). mRNA profiling using a human angiogenesis array revealed that MnTBAP pre-treatment also downregulated angiogenesis markers involving Chemokine (C-C motif) ligands (CCL2, CXCL1, CXCL5, CXCL6, CXCL10), fibroblast growth factors (FGF1, FGF2, FGFR3), and interleukins (IL1B, IL6, CXCL8 (IL8)) upregulated by bleomycin treatment (Table 1). To further validate the observed effects of MnTBAP, levels of IL-8, an important angiogenesis marker identified by mRNA profiling and reported for its role in pulmonary fibrosis was investigated. MnTBAP pre-treatment significantly inhibited IL-8 levels in bleomycin-treated lung fibroblasts (Fig. 5D) and in BAL fluid obtained from mice co-treated with bleomycin and MnTBAP as compared to bleomycin-treated mice (Fig. 5E). Overall, the inhibitory effects of MnTBAP in bleomycin-induced fibrosis may in part be due to its regulatory control of VEGF signaling and other angiogenic markers identified.
Fig. 5. MnTBAP regulates bleomycin-induced VEGF levels.
(A) Supernatant from CRL-1490 cells treated with varying concentrations of bleomycin (0–25 mU/ml) for 24 h were collected and analyzed for VEGF by ELISA. (B) Cells were left untreated or pretreated with MnTBAP (100 µM) for 1 h followed by bleomycin (10 mU/ml) treatment for 24 h. Cell supernatant was collected and analyzed for VEGF protein by ELISA. Data represent mean values (± standard error [S.E.M]) of duplicate determinations from three independent experiments. (C) Mice treated with bleomycin (1 U/kg) with or without MnTBAP (3 mg/kg) or equal volume of saline as control were euthanized at day 28. BAL fluid from control and treated mice was analyzed for VEGF protein by ELISA. Plots are mean ± S.E.M of five animals per sample group. (D) Cells were left untreated or pretreated with MnTBAP (100 µM) for 1 h followed by bleomycin (10 mU/ml) treatment for 24 h. Cell supernatant were collected and analyzed for IL-8 protein by ELISA. Data represent mean values (± standard error [S.E.M]) of duplicate determinations from three independent experiments. (E) Mice treated with bleomycin (1 U/kg) with or without MnTBAP (3 mg/kg) or equal volume of saline as control were euthanized at day 28. BAL fluid from control and treated mice was analyzed for IL-8 protein by ELISA. Plots are mean ± S.E.M of five animals per sample group. *P <0.05 versus non-treated control. **P <0.05 versus bleomycin treatment.
TABLE 1.
MnTBAP regulates bleomycin induced angiogenesis markers
| Relative fold regulation (with respect to untreated control cells) in cells treated with |
|||
|---|---|---|---|
| Angiogenesis markers |
Description | Bleomycin | Bleomycin + MnTBAP |
| CCL2 | Chemokine (C-C motif) ligand 2 | 1.815 | −1.0718 |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity, alpha) | 1.3755 | −1 |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 | 1.5801 | −2.2974 |
| CXCL5 | Chemokine (C-X-C motif) ligand 5 | 1.4743 | −1.3195 |
| CXCL6 | Chemokine (C-X-C motif) ligand 6 (granulocyte chemotactic protein 2) | 1.815 | 1.3195 |
| EFNB2 | Ephrin-B2 | 1.1975 | −1.3195 |
| F3 | Coagulation factor III (thromboplastin, tissue factor) | 1.4743 | −1.0718 |
| FGF1 | Fibroblast growth factor 1 (acidic) | 1.1173 | −1.0718 |
| FGF2 | Fibroblast growth factor 2 (basic) | 3.1602 | 2.639 |
| FGFR3 | Fibroblast growth factor receptor 3 | 1.1173 | −1 |
| FLT1 | Fms-related tyrosine kinase 1 (vascular endothelial growth factor/vascular permeability factor receptor) | 1.3755 | −1.3195 |
| HGF | Hepatocyte growth factor (hepapoietin A; scatter factor) | 1.0425 | −1.1487 |
| HIF1A | Hypoxia inducible factor 1, alpha subunit (basic helix-loop-helix transcription factor) | 1.1173 | −1 |
| IL1B | Interleukin 1, beta | 1.815 | 1.0718 |
| IL6 | Interleukin 6 (interferon, beta 2) | 3.387 | 2.2974 |
| CXCL8 | Interleukin 8 | 2.0849 | 1.7411 |
| ITGAV | Integrin, alpha V (vitronectin receptor, alpha polypeptide, antigen CD51) | 1.0425 | −1 |
| MDK | Midkine (neurite growth-promoting factor 2) | 1.0425 | −2.639 |
| NRP1 | Neuropilin 1 | 1.2834 | −1.2311 |
| SERPINE1 | Serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1 | 1.1975 | −1.4142 |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | 1.0425 | −1.1487 |
| TIMP3 | TIMP metallopeptidase inhibitor 3 | 1.6935 | 1.4142 |
| VEGFB | Vascular endothelial growth factor B | 1.0425 | −1.0718 |
| VEGFC | Vascular endothelial growth factor C | 1.6935 | 1.5157 |
| B2M | Beta-2-microglobulin | 1.3755 | 1.2311 |
mRNA was extracted from untreated CRL-1490 cells or cells treated with bleomycin (10 mU/ml) or cells pre-treated for 1 h with MnTBAP (100 µM) followed by bleomycin (10 mU/ml) treatment for 24 h and transcribed into cDNA. Abundance of angiogenesis specific genes was analyzed by qRT-PCR. Fold regulation between control and treated cells is shown.
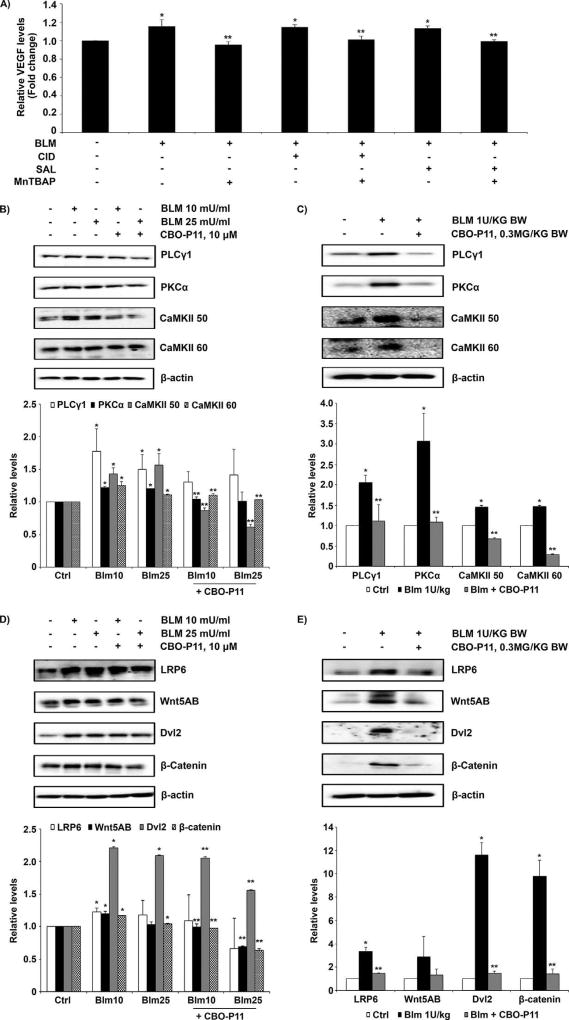
Inhibitory effects of MnTBAP involves regulation of VEGF and Wnt signaling
We investigated the potential crosstalk between VEGF and Wnt signaling induced by bleomycin. We modulated Wnt and VEGF using the Wnt activator CID 11210285 hydrochloride and small molecule Wnt inhibitor salinomycin, as well as the VEGF inhibitor CBO-P11. The dose of CID 11210285 hydrochloride and salinomycin used did not show significant cellular toxicity as tested by the MTT assay (Supplementary Fig. S2). Figure 6A shows that CID 11210285 hydrochloride (500 nM) and salinomycin (50 nM) did not significantly affect bleomycin-induced VEGF levels, and the effect of MnTBAP on bleomycin-induced VEGF levels was independent of Wnt. VEGF inhibitor CBO-P11 significantly inhibited bleomycin-induced upregulation of proteins of the non-canonical Wnt/Ca2+ and canonical Wnt pathways both in vitro and in vivo (Fig. 6B, C, D, and E). These data indicate that MnTBAP exhibits its effect by downregulating VEGF which further regulates Wnt signaling pathways.
Fig. 6. Inhibitory effects of MnTBAP involve regulations between bleomycin-induced VEGF and Wnt signaling.
(A) CRL-1490 cells were left untreated or pretreated with MnTBAP (100 µM), CID 11210285 hydrochloride (500 nM), salinomycin (50 nM), combination of MnTBAP (100 µM) and CID 11210285 hydrochloride (500 nM), or a combination of MnTBAP (100 µM) and salinomycin (50 nM) for 1 h followed by bleomycin (10 mU/ml) treatment for 24 h. Cell supernatant were collected and analyzed for VEGF protein by ELISA. Plots are mean ± S.E.M (n = 4). *P <0.05 versus non-treated control. **P <0.05 versus bleomycin treatment (B) CRL-1490 cells were pretreated for 1 h with CBO-P11 (10 µM), and then treated with bleomycin (10 mU/ml or 25 mU/ml) for 24 h and probed for PLCγ1, PKCα, and CaMKII proteins. (C) Mice treated with bleomycin (1 U/kg) with or without CBO-P11 (0.3 mg/kg) or equal volume of saline as control were euthanized at day 28. Lung tissue homogenates obtained from control and treated mice were probed for non-canonical Wnt/Ca2+ signaling proteins PLCγ1, PKCα, and CaMKII. (D) CRL-1490 cells were pretreated for 1 h with CBO-P11 (10 µM), and then treated with bleomycin (10 mU/ml or 25 mU/ml) for 24 h and probed for LRP6, Wnt5AB, Dvl2, and β-catenin. (E) Mice treated with bleomycin (1 U/kg) with or without CBO-P11 (0.3 mg/kg) or equal volume of saline as control were euthanized at day 28. Lung tissue homogenates obtained from control and treated mice were probed for canonical Wnt signaling proteins LRP6, Wnt5AB, Dvl2, and β-catenin. Blots were reprobed with β-actin antibody to confirm equal loading of the samples. Representative blots from three independent experiments (in vitro) or three animals per sample group (lung tissue homogenates) are shown. The immunoblot signals were quantified by densitometry. Values are mean ± S.E.M. (n = 3). *P <0.05 versus non-treated control. **P <0.05 versus bleomycin treatment.
Discussion
ROS has been implicated in the progression of pulmonary fibrosis and several studies have focused on scavenging these free radicals to prevent lung scarring and fibrosis progression (Lu et al., 2010; Andersson-Sjöland et al., 2015). ROS has been observed to elevate angiogenesis markers, mitochondrial DNA damage, apoptosis, and signal modulations all of which contribute to the process of fibrosis in various organs including lung (Cheresh et al., 2013). We observe an increase in cellular superoxide levels in response to bleomycin treatment in lung fibroblasts (Fig. 1D) and an overall increase in ROS levels in lung tissue samples obtained from bleomycin-treated animals (Fig. 2A). The relative ROS levels were significantly reduced in animals co-treated with the SOD mimetic, MnTBAP (Fig. 2A). Furthermore, MnTBAP significantly inhibited bleomycin-induced fibrogenic responses both in vitro and in vivo (Figs. 1 and 2). The bleomycin-induced pulmonary fibrosis mouse model has been successfully used to study the molecular basis of fibrotic changes (Cai and Kimura, 2015; Uji et al., 2015; Fernandez et al., 2016; Zhou et al., 2016). Immunohistochemical analysis further confirmed that MnTBAP has a protective effect in bleomycin-induced pulmonary fibrosis (Fig. 2C and D). The use of antioxidants for therapeutic benefits in pulmonary fibrosis has been widely reported (Tang et al., 2015; Bilgin et al., 2016; Divya et al., 2016). In a recent study, Muramatsu et al. (2016) observed that N-acetylcysteine (NAC), an antioxidant, improved the free radical imbalance in IPF patients. In another study, administration of the antioxidant echinochrome A was found to inhibit bleomycin-induced pulmonary fibrosis (Lebed’ko et al., 2015). Similarly, MnTBAP was reported for its inhibitory effects on bleomycin-induced pulmonary fibrosis (Oury et al., 2001). While existing reports present promising antioxidants, knowledge of the underlying signaling mechanisms is still unclear.
Canonical Wnt signaling pathway is widely reported for its involvement in fibrotic disease (Königshoff et al., 2008; Lam and Gottardi, 2011; Aumiller et al., 2013; Sun et al., 2014). On the other hand, the non-canonical Wnt signaling pathways, including Wnt/Ca2+ signaling and Wnt/planar cell polarity, have been understudied in fibrosis, but is reported for its involvement in tumor progression and metastasis (Jessen, 2009; Luna-Ulloa et al., 2011). We show that bleomycin induced a dose-dependent upregulation of components of the non-canonical Wnt/Ca2+ signaling and co-treatment with MnTBAP significantly inhibited these changes (Fig. 3A and B). The inhibitory effects of MnTBAP were through signal modulation of PLCγ1-CaMKII regulation (Fig. 3C). Bleomycin also induced a dose-dependent upregulation of components of the canonical Wnt signaling pathway and it was interesting to observe that the inhibitory effects of MnTBAP on bleomycin-induced canonical Wnt proteins were very significant in in vivo samples but were minimal in vitro (Fig. 4). While we present novel findings for involvement of both canonical Wnt and non-canonical Wnt/Ca2+ signaling pathways in response to MnTBAP and bleomycin treatment, the data indicate that the non-canonical Wnt/Ca2+ signaling pathway, through PLCγ1-CaMKII signal transduction, was the predominant pathway regulated by MnTBAP.
Recent reports indicate that VEGF, a central regulator of angiogenesis, is dysregulated in patients with fibrotic diseases (Smadja et al., 2014; Bien et al., 2015; Roels et al., 2015). Furthermore, VEGF inhibitors have been shown to attenuate bleomycin-induced pulmonary fibrosis in mice (Ou et al., 2009; Iyer et al., 2015). Recently, our group reported key protein targets involved in VEGF inhibitor-mediated attenuation of bleomycin-induced pulmonary fibrosis using a proteomics approach (Kulkarni et al., 2016). In this study, pre-treatment with MnTBAP significantly inhibited bleomycin-induced upregulation of VEGF and IL-8 both in vitro and in vivo (Fig. 5). mRNA profiling revealed that MnTBAP pre-treatment also downregulated angiogenesis markers involving chemokine (C-C motif) ligands, fibroblast growth factors, and interleukins that are upregulated by bleomycin treatment (Table 1). Involvement of these angiogenesis markers in pulmonary fibrosis is also reported by other investigators (Ziegenhagen et al., 1998; Besnard et al., 2013; Deng et al., 2013; Le et al., 2014; MacKenzie et al., 2015). Recent evidences suggest an important role for canonical Wnt signaling in fibrosis and VEGF is reported to be a downstream target of Wnt (Park et al., 2011; Villar et al., 2011). We observed no significant changes in VEGF levels in cells pretreated with either Wnt activator or inhibitor (Fig. 6A). The inhibitory effect of MnTBAP on VEGF was also not affected in the presence or absence of Wnt modulators. These data indicated that bleomycin-mediated induction of VEGF was mediated through superoxide and was independent of its effect on Wnt signaling. On the other hand, pre-treatment of cells with CBO-P11 or co-treatment of bleomycin-treated mice with CBO-P11 significantly inhibited all the key proteins of the canonical Wnt and non-canonical Wnt/Ca2+ pathways (Fig. 6). This indicated that bleomycin-mediated Wnt activation is downstream of VEGF. The first study to provide insights into Wnt signaling being directly controlled by VEGF was the study on colorectal cancer (CRC) by Naik et al. (2009). The investigators using RNA interference to VEGFR1 showed a direct control of the Wnt/β-catenin pathway by VEGF receptor not known before. A similar novel control of VEGF over Wnt signaling in bleomycin-induced pulmonary fibrosis was observed in the current study.
In summary, this study reveals a significant role of superoxide and superoxide scavenger MnTBAP in bleomycin-induced pulmonary fibrosis through regulation of Wnt and VEGF signaling. We for the first time show the critical signaling pathways regulated by MnTBAP to exert its effect. MnTBAP inhibited bleomycin-induced activation of canonical Wnt and non-canonical Wnt/Ca2+signaling pathways and the effect was more prominent on non-canonical Wnt/Ca2+ pathway. We also report that MnTBAP mediates its effect through regulation of VEGF signaling which in turn regulates the Wnt signaling pathways in bleomycin-induced pulmonary fibrosis. Oxidative stress is known to play a key role in lung injury and pulmonary fibrosis. Using antioxidants, such as MnTBAP, and targeting the key signaling mechanisms involved may aid in the development of more efficient preventive and therapeutic strategies against pulmonary fibrosis.
Supplementary Material
Acknowledgments
Contract grant sponsor: NHLBI.
Contract grant sponsor: National Institutes of Health;
Contract grant numbers: HL112630, CA173069.
This work was supported by grants from National Institutes of Health (HL112630 and CA173069). We thank the Animal facility staff at ODU for maintaining the animals.
Footnotes
Conflict of interest: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Andersson-Sjöland A, Karlsson JC, Rydell-Törmänen K. ROS-induced endothelial stress contributes to pulmonary fibrosis through pericytes and Wnt signaling. Lab Invest. 2015;96:206–217. doi: 10.1038/labinvest.2015.100. [DOI] [PubMed] [Google Scholar]
- Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller V, Balsara N, Wilhelm J, Günther A, Königshoff M. WNT/β-catenin signaling induces IL-1β expression by alveolar epithelial cells in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2013;49:96–104. doi: 10.1165/rcmb.2012-0524OC. [DOI] [PubMed] [Google Scholar]
- Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: Roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- Besnard AG, Struyf S, Guabiraba R, Fauconnier L, Rouxel N, Proost P, Uyttenhove C, Van Snick J, Couillin I, Ryffel B. CXCL6 antibody neutralization prevents lung inflammation and fibrosis in mice in the bleomycin model. J Leukoc Biol. 2013;94:1317–1323. doi: 10.1189/jlb.0313140. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Basu A, Biswas J, Bhattacharya S. Nano-Se attenuates cyclophosphamide-induced pulmonary injury through modulation of oxidative stress and DNA damage in Swiss albino mice. Mol Cell Biochem. 2015;405:243–256. doi: 10.1007/s11010-015-2415-1. [DOI] [PubMed] [Google Scholar]
- Bien MY, Wu MP, Chen WL, Chung CL. VEGF correlates with inflammation and fibrosis in tuberculous pleural effusion. Scientific World Journal. 2015;2015:417124. doi: 10.1155/2015/417124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgin G, Kismet K, Kuru S, Kaya F, Senes M, Bayrakceken Y, Yumusak N, Celikkan FT, Erdemli E, Celemli OG, Sorkun K, Koca G. Ultrastructural investigation of the protective effects of propolis on bleomycin induced pulmonary fibrosis. Biotech Histochem. 2016;91:195–203. doi: 10.3109/10520295.2015.1123294. [DOI] [PubMed] [Google Scholar]
- Cai Y, Kimura S. Secretoglobin 3A2 exhibits anti-fibrotic activity in bleomycin-induced pulmonary fibrosis model mice. PLoS ONE. 2015;10:e0142497. doi: 10.1371/journal.pone.0142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Chaudhary NI, Roth GJ, Hilberg F, Müller-Quernheim J, Prasse A, Zissel G, Schnapp A, Park JE. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007;29:976–985. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]
- Cheresh P, Kim SJ, Tulasiram S, Kamp DW. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta. 2013;1832:1028–1040. doi: 10.1016/j.bbadis.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150:967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- Deng X, Xu M, Yuan C, Yin L, Chen X, Zhou X, Li G, Fu Y, Feghali-Bostwick CA, Pang L. Transcriptional regulation of increased CCL2 expression in pulmonary fibrosis involves nuclear factor-κB and activator protein-1. Int J Biochem Cell Biol. 2013;45:1366–1376. doi: 10.1016/j.biocel.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Divya T, Dineshbabu V, Soumyakrishnan S, Sureshkumar A, Sudhandiran G. Celastrol enhances Nrf2 mediated antioxidant enzymes and exhibits anti-fibrotic effect through regulation of collagen production against bleomycin-induced pulmonary fibrosis. Chem Biol Interact. 2016;246:52–62. doi: 10.1016/j.cbi.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Fernandez IE, Amarie OV, Mutze K, Königshoff M, Yildirim AÖ, Eickelberg O. Systematic phenotyping and correlation of biomarkers with lung function and histology in lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2016;310:L919–L927. doi: 10.1152/ajplung.00183.2015. [DOI] [PubMed] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- Helmlinger G, Endo M, Ferrara N, Hlatky L, Jain RK. Formation of endothelial cell networks. Nature. 2000;405:139–141. doi: 10.1038/35012132. [DOI] [PubMed] [Google Scholar]
- Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L24–L33. doi: 10.1152/ajplung.00245.2007. [DOI] [PubMed] [Google Scholar]
- Hua-Huy T, Le-Dong NN, Duong-Quy S, Bei Y, Rivière S, Tiev KP, Nicco C, Chèreau C, Batteux F, Dinh-Xuan AT. Increased exhaled nitric oxide precedes lung fibrosis in two murine models of systemic sclerosis. J Breath Res. 2015;9:036007. doi: 10.1088/1752-7155/9/3/036007. [DOI] [PubMed] [Google Scholar]
- Iyer AK, Ramesh V, Castro CA, Kaushik V, Kulkarni YM, Wright CA, Venkatadri R, Rojanasakul Y, Azad N. Nitric oxide mediates bleomycin-induced angiogenesis and pulmonary fibrosis via regulation of VEGF. J Cel Biochem. 2015;116:2484–2493. doi: 10.1002/jcb.25192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen JR. Noncanonical Wnt signaling in tumor progression and metastasis. Zebrafish. 2009;6:21–28. doi: 10.1089/zeb.2008.0571. [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: A possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Königshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE. 2008;3:e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni YM, Dutta S, Iyer AK, Venkatadri R, Kaushik V, Ramesh V, Wright CA, Semmes OJ, Yakisich JS, Azad N. A proteomics approach to identifying key protein targets involved in VEGF inhibitor mediated attenuation of bleomycin-induced pulmonary fibrosis. Proteomics. 2016;16:33–46. doi: 10.1002/pmic.201500171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HB, Lee Y, Kim JH, Van Remmen H, Richardson AG, Lawler JM. MnSOD overexpression reduces fibrosis and pro-apoptotic signaling in the aging mouse heart. J Gerontol A Biol Sci Med Sci. 2015;70:533–544. doi: 10.1093/gerona/glu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AP, Gottardi CJ. β-catenin signaling: A novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol. 2011;23:562–567. doi: 10.1097/BOR.0b013e32834b3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TT, Karmouty-Quintana H, Melicoff E, Le TT, Weng T, Chen NY, Pedroza M, Zhou Y, Davies J, Philip K, Molina J, Luo F, George AT, Garcia-Morales LJ, Bunge RR, Bruckner BA, Loebe M, Seethamraju H, Agarwal SK, Blackburn MR. Blockade of IL-6 trans signaling attenuates pulmonary fibrosis. J Immunol. 2014;193:3755–3768. doi: 10.4049/jimmunol.1302470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebed’ko OA, Ryzhavskii BY, Demidova OV. Effect of antioxidant echinochrome A on bleomycin-induced pulmonary fibrosis. Bull Exp Biol Med. 2015;159:351–354. doi: 10.1007/s10517-015-2960-3. [DOI] [PubMed] [Google Scholar]
- Li C, Zhou HM. The role of manganese superoxide dismutase in inflammation defense. Enzyme Res. 2011;2011:387176. doi: 10.4061/2011/387176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Ahmed KM, Nantajit D, Rosenthal FS, Hai CX, Li JJ. Therapeutic effects of alpha-lipoic acid on bleomycin-induced pulmonary fibrosis in rats. Int J Mol Med. 2007;19:865–873. [PubMed] [Google Scholar]
- Liu G, Bafico A, Aaronson SA. The mechanism of endogenous receptor activation functionally distinguishes prototype canonical and noncanonical Wnts. Mol Cell Biol. 2005;25:3475–3482. doi: 10.1128/MCB.25.9.3475-3482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Azad N, Wang L, Iyer AKV, Castranova V, Jiang BH, Rojanasakul Y. Phosphatidylinositol-3-Kinase/Akt regulates bleomycin-induced fibroblast proliferation and collagen production. Am J Respir Cell Mol Biol. 2010;42:432–441. doi: 10.1165/rcmb.2009-0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Ulloa LB, Hernández-Maqueda JG, Castañeda-Patlán MC, Robles-Flores M. Protein kinase C in Wnt signaling: Implications in cancer initiation and progression. IUBMB Life. 2011;63:915–921. doi: 10.1002/iub.559. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie B, Korfei M, Henneke I, Sibinska Z, Tian X, Hezel S, Dilai S, Wasnick R, Schneider B, Wilhelm J, El Agha E, Klepetko W, Seeger W, Schermuly R, Günther A, Bellusci S. Increased FGF1-FGFRc expression in idiopathic pulmonary fibrosis. Respir Res. 2015;16:83. doi: 10.1186/s12931-015-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao CG, Yang YY, He X, Huang C, Huang Y, Zhang L, Lv XW, Jin Y, Li J. Wnt signaling in liver fibrosis: Progress, challenges and potential directions. Biochimie. 2013;95:2326–2335. doi: 10.1016/j.biochi.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ayaub EA, Murphy J, Lu C, Kolb M, Ask K, Janssen LJ. Disruption of calcium signaling in fibroblasts and attenuation of bleomycin-induced fibrosis by nifedipine. Am J Respir Cell Mol Biol. 2015;53:450–458. doi: 10.1165/rcmb.2015-0009OC. [DOI] [PubMed] [Google Scholar]
- Muramatsu Y, Sugino K, Ishida F, Tatebe J, Morita T, Homma S. Effect of inhaled N-acetylcysteine monotherapy on lung function and redox balance in idiopathic pulmonary fibrosis. Respir Investig. 2016;54:170–178. doi: 10.1016/j.resinv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Naik S, Dothager RS, Marasa J, Lewis CL, Piwnica-Worms D. Vascular Endothelial Growth Factor Receptor-1 is synthetic lethal to aberrant β-catenin activation in colon cancer. Clin Cancer Res. 2009;15:7529–7537. doi: 10.1158/1078-0432.CCR-09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Ou XM, Li WC, Liu DS, Li YP, Wen FQ, Feng YL, Zhang SF, Huang XY, Wang T, Wang K, Wang X, Chen L. VEGFR-2 antagonist SU5416 attenuates bleomycin-induced pulmonary fibrosis in mice. Int Immunopharmacol. 2009;9:70–79. doi: 10.1016/j.intimp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Oury TD, Thakker K, Menache M, Chang LY, Crapo JD, Day BJ. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25:164–169. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- Parizada B, Werber MM, Nimrod A. Protective effects of human recombinant MnSOD in adjuvant arthritis and bleomycin-induced lung fibrosis. Free Radic Res Commun. 1991;15:297–301. doi: 10.3109/10715769109105225. [DOI] [PubMed] [Google Scholar]
- Park K, Lee K, Zhang B, Zhou T, He X, Gao G, Murray AR, Ma JX. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol. 2011;31:3038–3051. doi: 10.1128/MCB.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan T, Spivack S, Mossman BT. Regulation of antioxidant enzymes in lung after oxidant injury. Environ Health Perspect. 1994;102:79–87. doi: 10.1289/ehp.9410279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- Roels E, Krafft E, Antoine N, Farnir F, Laurila HP, Holopainen S, Rajamäki MM, Clercx C. Evaluation of chemokines CXCL8 and CCL2, serotonin, and vascular endothelial growth factor serum concentrations in healthy dogs from seven breeds with variable predisposition for canine idiopathic pulmonary fibrosis. Res Vet Sci. 2015;101:57–62. doi: 10.1016/j.rvsc.2015.05.020. [DOI] [PubMed] [Google Scholar]
- Smadja DM, Nunes H, Juvin K, Bertil S, Valeyre D, Gaussem P, Israel-Biet D. Increase in both angiogenic and angiostatic mediators in patients with idiopathic pulmonary fibrosis. Pathol Biol. 2014;62:391–394. doi: 10.1016/j.patbio.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Sun Z, Wang C, Shi C, Sun F, Xu X, Qian W, Nie S, Han X. Activated Wnt signaling induces myofibroblast differentiation of mesenchymal stem cells, contributing to pulmonary fibrosis. Int J Mol Med. 2014;33:1097–1109. doi: 10.3892/ijmm.2014.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Gao L, Mao J, He H, Liu J, Cai X, Lin H, Wu T. Salidroside protects against bleomycin-induced pulmonary fibrosis: Activation of Nrf2-antioxidant signaling, and inhibition of NF-κB and TGF-β1/Smad-2/-3 pathways. Cell Stress Chaperones. 2015;21:239–249. doi: 10.1007/s12192-015-0654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uji M, Nakada A, Nakamura T, Hirata K. Effect of intratracheal administration of adipose-derived stromal cells on bleomycin-induced lung injury in a rat model. Osaka City Med J. 2015;61:81–91. [PubMed] [Google Scholar]
- Verma R, Kushwah L, Gohel D, Patel M, Marvania T, Balakrishnan S. Evaluating the ameliorative potential of quercetin against the bleomycin-induced pulmonary fibrosis in Wistar rats. Pulm Med. 2013;2013:921724. doi: 10.1155/2013/921724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J, Cabrera NE, Valladares F, Casula M, Flores C, Blanch L, Quilez ME, Santana-Rodríguez N, Kacmarek RM, Slutsky AS. Activation of the Wnt/β-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS ONE. 2011;6:e23914. doi: 10.1371/journal.pone.0023914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XM, Cao ZD, Xiao N, Shen Q, Li JX. Inhibitory effects of amines from Citrus reticulata on bleomycin-induced pulmonary fibrosis in rats. Int J Mol Med. 2016;37:339–346. doi: 10.3892/ijmm.2015.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenhagen MW, Zabel P, Zissel G, Schlaak M, Müller-Quernheim J. Serum level of interleukin 8 is elevated in idiopathic pulmonary fibrosis and indicates disease activity. Am J Respir Crit Care Med. 1998;157:762–768. doi: 10.1164/ajrccm.157.3.9705014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.