Abstract
Pharmacokinetic, pharmacodynamic and pharmacogenomic studies of midazolam are currently being performed in critically ill children to find suitable dose regimens. Sensitive assays using small volumes of plasma are necessary to determine the concentrations of midazolam and its respective metabolites in pediatric studies. Midazolam is metabolized to hydroxylated midazolam isomers, which are present as free as well as the corresponding glucuronide conjugates. A high-performance liquid chromatographic method with tandem mass spectrometry has been developed and validated for the quantification of midazolam, and free and total 1-hydroxymidazolam and 4-hydroxymidazolam metabolites in small volumes of plasma. Cleanup consisted of 96-well µ-elution solid phase extraction (SPE). The analytes were separated by gradient elution using a C18 analytical column with a total run time of 5 min. Multiple reaction monitoring was employed using precursor to product ion transitions of m/z 326.2 →291.3 for midazolam, m/z 342.1 →203.0 for 1-hydroxymidazolam, m/z 342.1 →325.1 for 4-hydroxymidazolam and m/z 330.2 →295.3 for 2H4-midazolam (internal standard). Since authentic hydroxymidazolamglucuronide standards are not available, samples were hydrolyzed with β-glucuronidase under optimized conditions. Assay conditions were modified and optimized to provide appropriate recovery and stability because 4-hydroxymidazolam was very acid sensitive. Standard curves were linear from 0.5 to 1,000 ng/mL for all three analytes. Intra- and inter day accuracy and precision for quality control samples (2, 20, 200 and 800 ng/mL) were within 85–115% and 15% (coefficient of variation), respectively. Stability in plasma and extracts were sufficient under assay conditions. Plasma samples were processed and analyzed for midazolam, and free 1-hydroxymidazolam and 4-hydroxymidazolam metabolites. Plasma samples that were hydrolyzed with β-glucuronidase were processed and analyzed for midazolam, and total 1-hydroxymidazolam and 4-hydroxymidazolam metabolites under the same assay conditions. The difference in concentration between the total and free hydroxymidazolam metabolites provided an estimate of conjugated hydroxymidazolam metabolites. The combination of 96-well µ-elution SPE and LC-MS/MS allows reliable quantification of midazolam and its metabolites in small volumes of plasma for pediatric patients. This assay is currently being successfully utilized for analysis of samples from ongoing clinical trials.
Keywords: midazolam, 1-hydroxymidazolam, 4-hydroxymidazolam, glucuronide metabolites, glucuronidase hydrolysis
1. Introduction
Midazolam is a short acting benzodiazepine that is routinely used in the treatment of critically ill children. There is a growing need to understand the pharmacokinetics, pharmacodynamics and pharmacogenomics of midazolam disposition in critically ill children [1–3]. For children who are mechanically ventilated, there is a need to quantitatively define the heritable and non-heritable factors that underlie the variability in midazolam and morphine exposure and response. The Pharmacologic Impact on Sedation Assessments (PISA, NCT01105663) study, which was recently conducted, examined heritable polymorphisms on drug exposure, metabolite formation and pharmacodynamic response in critically ill children. Hypothermia's Impact on Pharmacology (HIP, NCT01560338) study is being conducted to estimate the impact of hypothermia on the variability in midazolam pharmacokinetics in children after cardiac arrest and to estimate the impact of genetic factors on the variability in midazolam pharmacokinetics. The results will be valuable to optimize the prospective treatment of critically ill children. Hence, there is a need to develop a sensitive bioanalytical method for the quantification of midazolam, morphine and their metabolites in pediatric plasma samples.
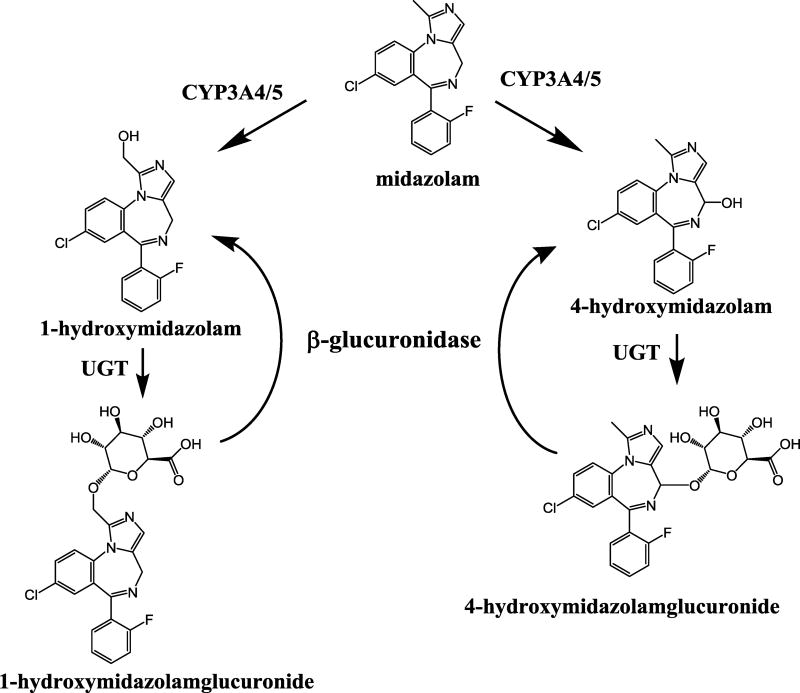
Midazolam is metabolized by cytochrome P450 enzymes 3A4 and 3A5 [4, 5] to 1-hydroxymidazolam and 4-hydroxymidazolam. Of these, 1-hydroxymidazolm is the major pharmacologically active metabolite and 4-hydroxymidazolam is the minor metabolite. Each hydroxylated metabolite is further metabolized by UGT to form the corresponding glucuronide conjugate. There are several LC-MS/MS methods reported for the analysis of midazolam and 1-hydroxymidazolam in human plasma and other biological samples [6–26]. However, there are few LC-MS/MS methods reported for the analysis of 4-hydroxymidazolam, which is an acid sensitive metabolite [8, 9, 16, 23]. Three of the reported methods [8, 16, 23] require a larger volume of plasma samples (0.5 to 1.0 mL), which is not feasible in pediatric studies where blood draws are limited to smaller volume to minimize risk. A recent study utilized 1-hydroxymidazolam glucuronide that was isolated from human urine as a standard for quantitation [6]. The goal of our study was to develop a sensitive assay for the measurements of midazolam, and free and glucuronide conjugated 1-hydroxymidazolam and 4-hydorxymidazolam in a small volume of pediatric plasma samples. However, authentic analytical standards of 1-hydroxymidazolam and 4-hydroxymidazolam glucuronides were not readily available. Hydrolysis with β-glucuronidase has previously been shown to provide quantitative hydrolysis of glucuronide metabolites [27]. Therefore, we employed β-glucuronidase hydrolysis to measure total 1-hydroxymidazolam and 4-hydroxymidazolam in plasma samples, obviating the need for the glucuronide standards. By analyzing plasma samples with and without hydrolysis utilizing our validated LC-MS/MS method, we were able to quantitate the levels of free and total 1-hydroxymidazolam and 4-hydroxymidazolam metabolites in small volumes for clinical samples. The respective difference between the total hydroxylated midazolam metabolites after hydrolysis and free hydroxylated midazolam metabolites concentration before hydrolysis provided a measure of 1-hydroxymidazolam and 4-hydroxymidazolam glucuronides in the plasma samples.
2. Materials and methods
2.1 Chemicals and reagents
Midazolam, 1-hydroxymidazolam, and 2H4-midazolam (internal standard) were purchased from Cerilliant (Round Rock, TX, USA), and 4-hydroxymidazolam [Figure 1], reagent-grade formic acid (~98%), and β-glucuronidase from Helix promatia were purchased from Sigma-Aldrich (St. Louis, MO, USA). The different individual (either male or female) lots of drug-free (blank) human plasma prepared with citrate phosphate dextrose (CPD) as an anti-coagulant were obtained from the blood bank at The Children’s Hospital of Philadelphia. Six different lots of drug-free (blank) human plasma prepared with lithium heparin as an anti-coagulant were obtained from BioreclamationIVT (Baltimore MD). HPLC grade methanol and acetonitrile were purchased from Fisher-Scientific (Pittsburgh, PA, USA). Deionized water from Barnstead Nanopure™ water purifying system (Thermo Fisher Scientific, Marietta, OH, USA) was used for all experiments.
Figure 1.
Major metabolic pathways of midazolam
2.2. LC Conditions
The Shimadzu HPLC system consisted of two LC-20AD delivery pumps, a DGU-20A5 Shimadzu vacuum degasser, a SIL-20AC Shimadzu autosampler and a CBM-20A system controller (Shimadzu Scientific Instruments; Columbia, MD, USA). HPLC separations were performed on a Kinetex C18 analytical column (2.6 µm 100 A, 2 × 50 mm, Phenomenex Torrance, CA). For chromatographic separation, acetonitrile / water (10:90, v/v) with 0.1% acetic acid was used as mobile phase A and acetonitrile with 0.1% acetic acid was used as mobile phase B. The initial mobile phase condition was 95% mobile phase A and 5% mobile phase B. The linear gradient was as follows: 0.00–0.51 minutes mobile phase A 83%, mobile phase B 17% with divert valve off; 0.51–2.00 minutes mobile phase A 83%, mobile phase B 17%; 2.00–3.00 minutes mobile phase A 20%, mobile phase B 80%; 3.00–3.50 minutes mobile phase A 95%, mobile phase B 6%; and initial gradient maintained until 5 minutes. The flow rate was 0.30 mL/min and 2 µL of the sample was injected for each analysis. The column and autosampler were maintained at 40°C and 10°C, respectively. The LC flow was diverted to the waste for the first 1.5 minutes using an electronic valve actuator with a Rheodyne selector valve when the data acquisition was not taking place.
2.3. MS Conditions
An AB Sciex 4000 triple quadrupole mass spectrometer equipped with Turbo Ionspray was used for sample analysis. Software for controlling this equipment, acquiring and processing data was Analyst (Version 1.6.2; AB Sciex; Framingham, MA). The positive ionization mode for MS/MS analysis was utilized. Nitrogen was used as the nebulizer, auxillary, collision and curtain gases. Analytes were detected by tandem mass spectrometry using multiple reaction monitoring (MRM) with a dwell time of 100 msec. To determine the mass of the precursor and product ions, a solution of 5 µg/mL of midazolam, 1-hydroxymidazolam, 4-hydroxymidazolam, or internal standard 2H4-midazolam in mobile phase (acetonitrile: water (55/45, v/v) with 0.1% acetic acid) was infused directly into the ion sources with a Harvard Apparatus syringe pump at a flow rate of 10 µL/min. The following precursor-to-fragment transitions were monitored for quantitation: m/z 326.2 →291.3 for midazolam, m/z 342.1 →203.0 for 1-hydroxymidazolam, m/z 342.1 →325.1 for 4-hydroxymidazolam and m/z 330.2 →295.3 for 2H4-midazolam (internal standard). To further confirm the identity of 1-hydroxymidazolam and 4-hydroxymidazolam, a precursor-to-fragment transition of 342.1 →297.0 was utilized.
The conditions for ionization of midazolam, 1-hydroxymidazolam, 4-hydroxymidazolam, and 2H4-midazolam were optimized using individual standard solutions, each at 500 ng/mL in mobile phase (acetonitrile: water (55/45, v/v) with 0.1% acetic acid) at 10 µL/min. Midazolam, 1-hydroxymidazolam, 4-hydroxymidazolam, and 2H4-midazolam were infused by a syringe pump alone or through a Tee device at a flow rate of 10 µL/min into the stream of mobile phase (acetonitrile: water (55/45, v/v) with 0.1% acetic acid at 0.3 mL/min) eluting from the LC column through a mixing Tee and then into the turbo spray source, to mimic the LC-MS/MS conditions. The optimized gas parameters were: collision activate dissociation (CAD) gas, 5; curtain gas, 16; Gas 1 (nebulizer gas) 20; Gas 2 (heater gas) 48; source temperature 450 °C. The optimized compound parameters were: DP, EP, CE, and CXP were set at 30, 11, 37, and 10 for midazolam, 51, 11, 37, and 15 for 1-hydroxymidazolam, 66, 11, 43 and 8 for 4-hydroxymidazolam, and 51, 11, 37, and 8 for 2H4-midazolam, respectively.
2.4. Preparation of standards and quality controls
Pooled stock solutions (two independent sets) of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam at 25 µg/mL (of each analyte) in methanol/water (50/50, v/v) were prepared. Calibration standard solutions were prepared from one stock solution and quality control (QC) samples were prepared from the other stock solution. The stock solutions were stored at −20 °C in amber glass vials and were stable for at least 6 months. The primary stock solution was diluted in human plasma to prepare nine standards containing midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam concentrations of 0.5, 1, 5, 10, 25, 50, 100, 250, 500 and 1,000 ng/mL. These concentrations were prepared by adding the appropriate volume of corresponding stock or standard solution into 1.5 mL Eppendorf tubes containing plasma. Five QC concentrations were prepared in the same manner by adding appropriate volumes of corresponding QC stock solution or working solution to obtain concentrations of 0.5, 2, 20, 200 and 800 ng/mL. These concentrations represent the lowest limit of quantitation (LLOQ), low, medium 1, medium 2 and high QCs, respectively. The internal standard stock solution was prepared by diluting 1 mg/mL of 2H4-midazolam in methanol to a final concentration of 10 µg/mL. An internal standard working solution of 100 ng/mL of 2H4-midazolam in human plasma was used.
2.5. Sample preparation
Blood samples were collected in Microtainer tubes (BD, Franklin Lakes, NJ, USA) containing lithium heparin and placed on ice immediately after drawing. Tubes were centrifuged within 30 minutes at 3400 rpm for 15 min at 4 °C. The unknown plasma samples were collected and stored at −80 °C until analysis. As previously reported [16], 4-hydroxymidazolam is unstable under acidic conditions and the sample extraction procedure was optimized to provide acceptable stability and recovery. Sample preparation was performed with Waters Oasis HLB (2 mg sorbent per well) µ-Elution SPE 96-well plates with some modifications to the previously reported method [6]. SPE plates were conditioned with methanol (200 µL) and water (200 µL), then equilibrated with water containing 1% formic acid (200 µL). For the analysis of midazolam, free 1-hydroxymidazolam, and free 4-hydroxymidazolam, wells were loaded with 200 µL of water containing 1% formic acid, 50 µL of plasma sample (blank, standard, QC and unknown) and 50 µL of internal standard (100 ng/mL in human plasma) and ensured loading by applying vacuum to SPE manifold. Plate was washed with 10 mM ammonium carbonate (200 µL) and eluents from conditioning, equilibration, loading and washing steps were discarded. Analytes were eluted into a sample collection plate with methanol (2 × 25 µL) followed by 150 µL of water (dilution through wells).
The stability and recovery of 4-hydroxymidazolam [16] calibration standards under acidic conditions were optimized by exploring different loading and elution solvents in the SPE process. Sample loading was evaluated by comparing 1) water and 2) water with 1% formic acid, followed by elution with methanol. Recoveries of the analytes were low with water alone compared to water with 1% formic acid. Next, samples were loaded with water with 1% formic acid and SPE elution was performed with 1) methanol and 2) methanol with 1% formic acid. The calibration curves were analyzed three times (at 1, 10 and 24 h) to evaluate the autosampler stability of 4-hydroxymidazolam.
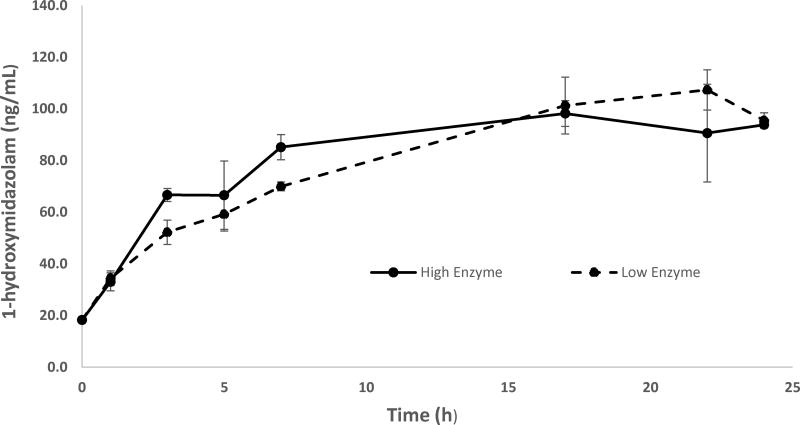
For the analysis of total 1-hydroxymidazolam, and total 4-hydroxymidazolam, samples were hydrolyzed with β-glucuronidase/arylsulfatase and extracted with Waters Oasis HLB µ-Elution SPE 96-well plates as described above. The conditions for enzyme hydrolysis were optimized for enzyme concentration and reaction time at 37 °C. Residual pooled authentic samples from a clinical study were exposed to different concentrations of β-glucuronidase (low enzyme: 208 units, high enzyme: 416 units) for different durations (0, 1, 3, 5, 7, 17, 22, and 24 h) at 37 °C. The samples were analyzed for 1-hydroxymidazolam and 4-hydroxymidazolam to determine optimal duration of enzymatic hydrolysis (18 h). β-Glucuronidase (1 mL) was diluted with 0.5 M KH2PO4 buffer (5 mL), then 25 µL of diluted enzyme (416 units) was added to 100 µL of plasma sample and incubated at 37 °C for 18 hours. Samples were centrifuged at 13,200 rpm for 5 minutes and sodium hydroxide solution (15 µL, 0.1 M) was added to each sample. A 70 µL aliquot of hydrolyzed sample was used for SPE.
2.6. Validation of the bioanalytical method
Method validation and documentation were performed according to guidelines set by the United States Food and Drug Administration (FDA) for bioanalytical method validation [28, 29]. This method was validated for linearity, specificity, intra- and inter-day accuracy and precision, recovery, and stability of the analytes [29, 30]. Analytical runs included a double blank (without internal standard), a blank (with internal standard), nine calibration standards (0.5 to 1,000 ng/mL), and six sets of QCs: LLOQ QC at 0.5 ng/mL, low QC at 2 ng/mL, medium QCs at 20 and 200 ng/mL, and high QC at 800 ng/mL.
2.6.1. Linearity
The linearity of the standard calibration curve was evaluated by the analyses of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam in plasma samples on three independent days using fresh preparations (n=2). The calibration curves (range: 0.5 to 1,000 ng/mL) were prepared at nine concentration levels of 0.5, 1, 5, 10, 25, 50, 100, 250, 500 and 1,000 ng/mL in human plasma. The calibration curve had a blank, a double blank, and nine calibrators, and another double blank, which were analyzed immediately following the highest concentration standard in each run to monitor for carryover of the analytes and the internal standard. Additional experiments were conducted to define the lowest plasma volume producing adequate sensitivity and reproducibility. Assay sensitivity with various sample volumes (5, 10, 25 and 50 µL) were tested by preparing and processing calibration curves (n=2) by SPE.
The calibration curves were analyzed following FDA guidance. The mean value should be within ±15% of the theoretical value, and it should not deviate by more than ±20% for LLOQ. The precision around the mean value should be within 15% CV, except for LLOQ, which should be within 20% CV. At least 75% of the non-zero standards should meet the above criteria and the linear correlation coefficient should be at least 0.99.
Calibration curves were established by plotting the analytes to internal standard peak area ratios (y) against the midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam concentrations (x) using a least-square linear regression fit (y=ax+b) with 1/x2 weighting using the Analyst ® Software. The parameters a and b were used to calculate the concentrations of the analytes.
2.6.2. Selectivity and specificity
The specificity was defined as non-interference at retention times for midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam from the endogenous plasma components and no cross-interference between these analytes and the internal standard using the proposed extraction procedure and LC-MS/MS conditions. Six different individual lots of blank human plasma and a pooled lot of the corresponding six individual lots (midazolam free plasma with CPD as anticoagulant) with a concentration of 0.5 ng/mL were evaluated with and without internal standard. The effect of co-administered drugs morphine (100 ng/mL) and fentanyl (20.0 ng/mL) at therapeutically relevant concentrations [31, 32] was evaluated on the quantitation of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam in human plasma at QC (n=6) levels of 20.0 and 800 ng/mL. The QC samples with co-administered drugs were processed and analyzed with freshly prepared calibration standards and QCs.
2.6.3. Accuracy and precision
The intra- and inter-assay precisions were determined using the CV (%), and the intra- and inter-assay accuracies were expressed as the percent difference between the measured concentration and the nominal concentration of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam. The % accuracy was expressed as the following: % accuracy= (measured concentration) / (nominal concentration) × 100 %. Intra-assay precision and accuracy were calculated using 6 replicate determinations at each concentration of the analytes in a single analytical run. For inter-assay precision and accuracy calculations, 6 replicate determinations of each QC prepared on three separate analytical runs on three different days (total of 18 measurements) were used.
2.6.4. Recovery and matrix effect
The recovery and matrix effect of midazolam and 1-hydroxymidazolam using Oasis HLB µ-Elution Plate were previously evaluated [6]. In the present study, the extraction efficiency of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam was determined by analyzing three replicates of plasma samples at four QC concentrations of 2, 20, 200 and 800 ng/mL. Recovery was calculated by comparing the peak areas of the analytes extracted using the SPE procedure with those obtained from analytes spiked directly into post-SPE eluent over four QC concentrations. The matrix effect was measured by comparing the peak area response of the post-extracted spiked sample with corresponding pure standards containing equivalent concentrations of the analytes in mobile phase solvents.
2.6.5. Stability
The stability of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam in human plasma was assessed by analyzing replicates of QC samples (n=6) at concentrations of 2, 20, 200 and 800 ng/mL during the sample handling and storage procedure (in 4 mL Amber vials). Stability studies were performed by comparing freshly prepared QCs and stability testing QCs using freshly prepared standard curves. The autosampler stability was assessed after exposure of the extracted plasma samples to room temperature for 24 hours (n=6). The long term stability was assessed after storage of the plasma samples −80 °C for 6 months (n=6). The freeze/thaw stability of analytes were determined after subjecting samples through three freeze/thaw cycles (−80 °C to room temperature, n=6). The concentrations measured from all stability QCs were compared to freshly prepared QC samples to calculate the percentage concentration deviation. The analyte was considered to be stable when the concentration difference was less than 15% between the freshly prepared QCs and the stability testing QCs.
2.7. Morphine and metabolites assay
An assay was validated for morphine and its glucuronide metabolites based on a previously published method [6] with some modifications. Samples were analyzed using 50 µL of human plasma samples by LC-MS/MS to quantify morphine, morphine 3-β-D-glucuronide (M3G) and morphine 6-β-D-glucuronide (M6G), while 2H3- morphine was used as an internal standard. Cleanup consisted of 96-well micro-elution SPE, followed by reversed-phase chromatographic separation (HPLC using Phenomenex Kinetex F5 column, 2.6 µm 100 A°, 4.6 × 50 mm) and selective detection with electro-spray ionization tandem mass spectrometry with a run time of 5 minutes per sample. The following precursor-to-fragment transitions were monitored for quantitation: m/z 286.2 →152.1 for morphine, m/z 462.2 →286.2 for M3G and M6G, m/z 289.2 →152.1 for 2H3- morphine. Chromatographic conditions were optimized to provide good separation for M3G and M6G. Linearity was observed for the calibration standard curve (5–2500 ng/mL) having sufficient limits of quantitation at 5.00 ng/mL for all three analytes.
2.8. Clinical application
This assay was successfully implemented for analysis of pediatric plasma samples from two clinical trials: Pharmacologic Impact on Sedation Assessments (PISA, NCT01105663) study and Hypothermia's Impact on Pharmacology (HIP, NCT01560338) study. The effect of anti-coagulant (CPD versus lithium heparin) on the quantitation of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam was evaluated by comparing the performance of QC samples prepared in CPD versus lithium heparin human plasma at QC concentrations (n=6) of 20 and 800 ng/mL. A representative pharmacokinetic profile of midazolam, and free and total 1-hydroxymidazolam and 4-hydroxymidazolam for a subject who received an intravenous infusion of midazolam for sedation is presented here. The subject (29.3 kg) initially received continuous infusion of midazolam at 0.1 mg/kg/h with adjustments in infusion rate between 0.05 to 0.2 mg/kg/h during the course of a 110 h pharmacokinetic sampling period. Blood samples (1 mL) were collected at 18 time points (with nominal time of every 6 hours) in lithium heparin tubes and placed on ice immediately. These samples were centrifuged at 3400 rpm for 15 min at 4 °C and plasma samples were collected and stored at −78 °C until analysis.
3. Results
3.1. Method development
Initial method development was performed with a 3 micron Luna C18 (150 × 2 mm) column with midazolam, 1-hydroxymidazolam and 4-hydroxymidazolam and resulted in a good chromatographic separation with a run time of 12 minutes. We have evaluated sample extraction with protein precipitation using methanol and acetonitrile as well as liquid-liquid extraction with ethyl acetate. Samples prepared with protein precipitation had higher background noise at 0.5 ng/mL and the samples prepared by liquid-liquid extraction had an improved signal to noise ratio at 0.5 ng/mL. However, liquid-liquid extraction was not a suitable method for working with a large number of clinical samples containing small volumes of plasma. We have employed Waters Oasis HLB µ-elution plate, which was previously utilized for analysis of midazolam and 1-hydroxymidazolam [6]. A previously reported method did not work for 4-hydroxymidazolam since it was an acid sensitive compound and had very limited stability in the autosampler. We have modified and optimized elution conditions to provide acceptable stability for all three analytes. By using the Kinetex C18 column, chromatography conditions were optimized resulting in an improved run time of 5 minutes. This was achievable while maintaining good separation and sensitivity.
Acid instability of 4-hydroxymidazolam [16] was addressed by modifying SPE elution solvent. Elution with methanol containing 1% formic acid showed significant loss of response (>80%) for 4-hydroxymidazolam after 10 h and complete degradation of 4-hydroxymidazolam after 24 h. Sample loading with water and elution with methanol showed poor recovery. Sample loading with water containing 1% formic acid and elution with methanol showed consistently robust response for 4-hydroxymidazolam after 1, 10 and 24 h. Optimized sample loading and elution conditions were used to address acid instability of 4-hydroxymidazolam and implemented for this assay.
Enzymatic hydrolysis of glucuronide conjugated hydroxymidazolam isomers with β-glucuronidase was optimized using residual pooled authentic samples by exposing different concentrations of β-glucuronidase for different durations at 37 °C and measuring the concentration of 1-hydroxymidazolam and 4-hydroxymidazolam. The concentration versus incubation time data showed the hydrolysis is gradually increasing from 0 to 7 h and reaching its maximum level within 17 h for 1-hydroxymidazolam at low and high enzyme concentration levels (Figure 2). The concentration of 4-hydroxymidazolam in the hydrolyzed sample was low (1 – 3 ng/mL). The results confirmed that high enzyme concentration (416 units) with overnight incubation at 37 °C was optimal for enzyme hydrolysis.
Figure 2.
Concentration - time plot for the optimization of β-glucuronide hydrolysis: Pooled residual clinical samples (n=3) were treated with low (208 units) and high (416 units) concentrations of β-glucuronide for different time points at 37 °C. Samples were extracted and analyzed for 1-hydroxymidazolam.
3.2. Linearity, sensitivity, specificity and selectivity
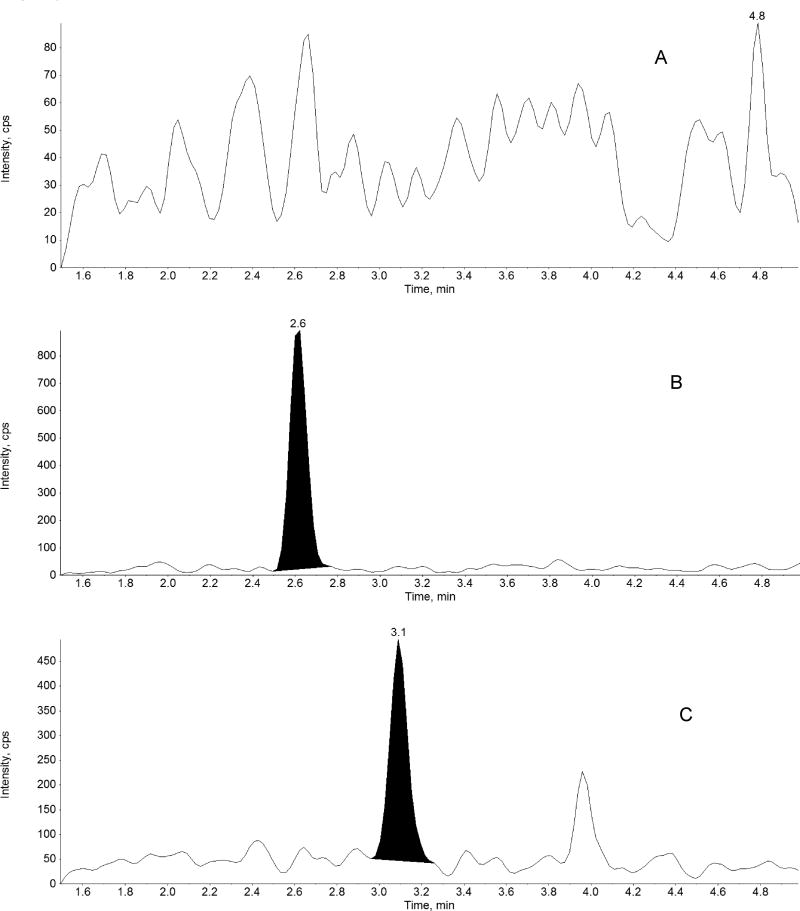
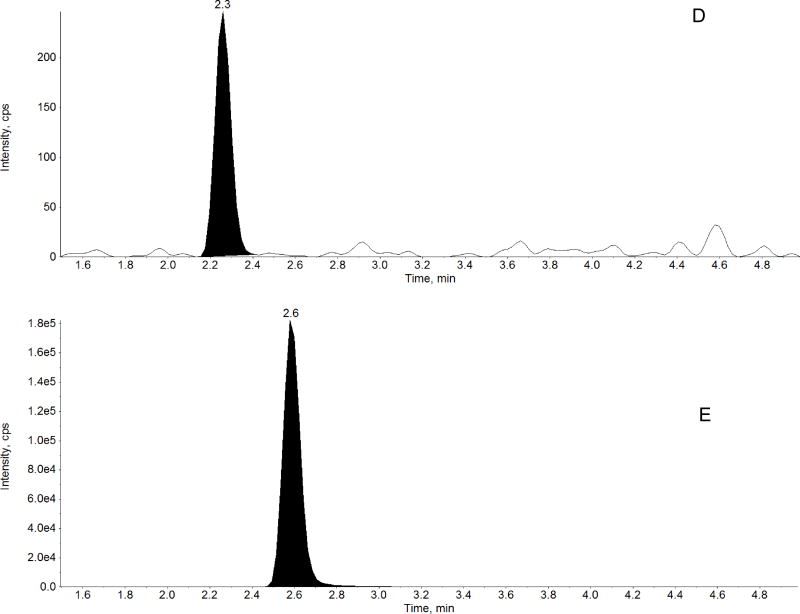
The method was linear over the range of 0.5 – 1000 ng/mL for midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam. A representative chromatogram of double blank, midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam at LLOQ, internal standard are shown in Figure 3. The linear correlation coefficient (r) was greater than 0.98 for all three analytes in all analytical runs. The LLOQ was 0.5 ng/mL for all three analytes with a %CV less than 20%, accuracy greater than 91%, and a signal-to-noise (S/N) ratio of at least 10 (Figure 3, Tables 1 to 3). LLOQ was determined as the concentration at which the measured value was within ±20% of the nominal value and all acceptable criteria were met. Results from the evaluation of various sample volumes (5, 10, 25 and 50 µL) to define the lowest plasma volume producing adequate sensitivity and reproducibility showed that a 50 µL of sample volume is required to achieve LLOQ of 0.500 ng/mL for midazolam and the 1’- and 4’-hydroxy metabolites. Analysis of different lots of blank plasma samples (n =6) and LLOQ (0.5 ng/mL) prepared in the corresponding plasma lots and a pooled lot of the corresponding six individual lots showed no interference from biological matrix components. The effect of co-administered drugs, morphine and fentanyl, on the quantitation of midazolam and metabolites in human plasma was tested at therapeutically relevant concentration [31, 32]. The accuracy ± precision for replicates of QC samples (n=6) at 20 and 800 ng/mL (with 100 ng/mL of morphine and 20.0 ng/mL of fentanyl) were: 103 ± 3.69 and 104 ± 1.65 for midazolam, 97.9 ± 4.05 and 106 ± 3.91 for 1-hydroxymidazolam and 89.6 ± 6.59 and 94.5 ± 3.26 for 4-hydroxymidazolam. The results showed that the presence of morphine and fentanyl did not impact the quantitation of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam. Carryover peaks were not present at the retention times of the analytes and the internal standard.
Figure 3.
Representative chromatograms of: A) blank human plasma, B) midazolam in human plasma at LLOQ (0.5 ng/mL), C) 1-hydroxymidazolam in human plasma at LLOQ (0.5 ng/mL), D) 4-hydroxymidazolam in human plasma at LLOQ (0.5 ng/mL) and E) D4-Midazolam (Internal standard, 10 ng/mL) in human plasma
Table 1.
Summary of validation outcomes for midazolam in human plasma
| Nominal Conc. (ng/mL) |
Day 1 (n=6) | Day 2 (n=6) | ||||
|---|---|---|---|---|---|---|
| Midazolam | (Mean±SD) (ng/mL) |
CV (%) | Accuracy (%) | (Mean±SD) (ng/mL) |
CV(%) | Accuracy (%) |
| 0.500 | 0.430±0.034 | 7.95 | 86.0 | 0.504±0.033 | 16.5 | 101 |
| 2.00 | 1.97±0.211 | 10.7 | 98.6 | 2.06±0.143 | 6.96 | 103 |
| 20.0 | 21.8±0.455 | 2.09 | 109 | 20.8±0.686 | 3.29 | 104 |
| 200 | 211±4.82 | 2.28 | 106 | 207±4.69 | 2.27 | 104 |
| 800 | 768±24.1 | 3.13 | 96.0 | 745±14.1 | 1.89 | 93.2 |
| Nominal Conc. (ng/mL) | Day 3 (n=6) | Inter-day (n=18, 3 days) | ||||
| Midazolam | (Mean±SD) (ng/mL) | CV (%) | Accuracy (%) | (Mean±SD) (ng/mL) | CV(%) | Accuracy (%) |
| 0.500 | 0.437±0.033 | 7.49 | 87.4 | 0.457±0.062 | 13.6 | 91.4 |
| 2.00 | 2.05±0.200 | 9.74 | 103 | 2.03±0.180 | 8.89 | 101 |
| 20.0 | 20.9±0.848 | 4.06 | 104 | 21.1±0.761 | 3.60 | 106 |
| 200 | 204±3.97 | 1.94 | 102 | 207±5.12 | 2.47 | 104 |
| 800 | 755±14.5 | 1.92 | 94.3 | 756±19.6 | 2.59 | 94.5 |
Table 3.
Summary of validation outcomes for 4-hydroxymidazolam in human plasma
| Nominal Conc. (ng/mL) |
Day 1 (n=6) | Day 2 (n=18, 3 days) | ||||
|---|---|---|---|---|---|---|
| 4- hydroxymidazolam |
(Mean±SD) (ng/mL) |
CV (%) |
Accuracy (%) |
(Mean±SD) (ng/mL) |
CV(%) | Accuracy (%) |
| 0.500 | 0.437±0.036 | 8.20 | 87.5 | 0.430±0.083 | 19.2 | 86.0 |
| 2.00 | 2.08±0.309 | 14.8 | 104 | 1.71±0.144 | 8.43 | 85.3 |
| 20.0 | 20.0±1.49 | 7.46 | 100 | 20.6±1.47 | 7.14 | 103 |
| 200 | 215±9.81 | 4.57 | 107 | 215±13.0 | 6.05 | 107 |
| 800 | 835±67.0 | 8.02 | 104 | 823±58.9 | 7.16 | 103 |
| Nominal Conc. (ng/mL) | Day 3 (n=6) | Inter-day (n=18, 3 days) | ||||
| 4-hydroxymidazolam | (Mean±SD) (ng/mL) | CV (%) | Accuracy (%) | (Mean±SD) (ng/mL) | CV(%) | Accuracy (%) |
| 0.500 | 0.495±0.094 | 19.0 | 99.0 | 0.461±0.076 | 16.6 | 92.1 |
| 2.00 | 1.95±0.255 | 13.1 | 97.5 | 1.92±0.286 | 14.9 | 96.1 |
| 20.0 | 20.8±1.45 | 6.99 | 104 | 20.5±1.42 | 6.93 | 102 |
| 200 | 208±7.86 | 3.78 | 104 | 212±10.3 | 4.86 | 106 |
| 800 | 841±89.8 | 10.7 | 105 | 833±68.1 | 8.17 | 104 |
Dilution integrity is essential to this assay since clinical samples often require dilution and reanalysis. This was evaluated by diluting 1000 ng/mL (n=6) by 50-fold and analyzing for midazolam, 1-hydroxymidazolam and 4-hydroxymidazolam (expected concentration of 20.0 ng/mL). Accuracy and precision (% CV) were determined for the QC concentration of 20.0 ng/mL illustrating values of 110 ± 5.19 for midazolam, 103 ± 5.56 for 1-hydroxymidazolam and 90.9 ± 3.59 for 4-hydroxymidazolam. Results showed that dilution integrity is reliable and acceptable for this assay.
3.3. Accuracy and precision
Validation results for the replicates of QCs (at 0.5, 2, 20, 200 and 800 ng/mL) for midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam are depicted in Tables 1 to 3. The intra-day precision (n=6) ranged from 2.09–14.8% with the accuracy ranging from 86.0–115%. The inter-day (n=18, 3 days) precision ranged from 2.47–16.7% with the accuracy ranging from 91.4– 106%. The results confirm that the present assay is robust and has acceptable accuracy and precision for the quantification of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam in human plasma with the desired concentration range.
3.4. Recovery
Recovery for midazolam and 1-hydroxymidazolam was evaluated with Oasis HLB µ-elution plate previously [6]. In the present study, the extraction recoveries (mean ± % SD, n=3) for QC concentrations (2, 20, 200 and 800 ng/mL) were 76.1 ± 17.7, 88.5 ± 6.85, 82.9 ± 2.12 and 90.7 ± 3.76 for midazolam, 85.05 ± 18.2, 89.0 ± 5.45, 80.0 ± 3.07 and 85.5 ± 4.49 for 1-hydroxymidazolam and 67.7± 1.44, 91.4 ± 8.94 and 87.2 ± 11.4 and 85.5 ± 6.55 for 4-hydroxymidazolam respectively. These results confirmed that the extraction efficiency for the three analytes using Oasis HLB µ-elution plate was satisfactory and not concentration dependent. These results are consistent with previously reported results for midazolam and 1-hydroxymidazolam [6].
3.5. Assay specificity and matrix effect
Matrix effect (ion suppression or enhancement) is caused by co-eluting components of biological samples which can affect the quantitation of analytes in the assay. The matrix effect of midazolam, 1-hydroxymidazolam, 4-hydroxymidazolam and the internal standard were evaluated by comparing the peak areas of the analytes in spiked plasma post-precipitation with the peak area responses of the pure standards prepared in mobile phases. A response greater than 100% implies ionization enhancement, and a response less than 100% implies ionization suppression. The matrix effect for four QC concentrations (n=3) and pooled lots (n=6) of blank plasma were evaluated. The accuracies of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam were within 87 to 112% at the QC concentrations of 2, 20, 200 and 800 ng/mL. Matrix effect was further investigated by analyzing extracts from 6 different lots of blank human plasma with a post-column infusion methodology [33]. No significant interference or suppression was seen at the retention times of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam in the infusion chromatograms, confirming that there was minimal or no matrix effect observed for three analytes after SPE and the HPLC.
3.6. Stability
The stability of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam was assessed under the conditions of sample processing and storage, which included freeze/thaw (3 cycles), and short and long-term stability studies. The precision and accuracy for freeze/thaw QCs (n=6) at 2 and 800 ng/mL ranged from 1.80 –12.4% and 96.4 – 102% for midazolam, 2.59 – 11.2% and 100 – 102% for 1-hydroxymidazolam, and 2.15 – 8.14% and 100 – 110% for 4-hydroxymidazolam, respectively. For autosampler stability (24 h at room temperature) of the extracted plasma samples at 2, 20, 200 and 800 ng/mL (n=6), the precision and accuracy ranged from 2.27 –15.0% and 94.7–104% for midazolam, 2.84 –15.0% and 99.3–107% for 1-hydroxymidazolam, and 3.84 –11.5% and 94.0–110% for 4-hydroxymidazolam, respectively. After 6 months of storage at −80 °C, the precision and accuracy of the replicates (n=6) of QCs at 2 and 800 ng/mL ranged from 4.0 – 8.73% and 96.2 – 97.2 % for midazolam, 4.51 – 14.1% and 94.3 –100% for 1-hydroxymidazolam and 12.0 –15.8% and 99.6 102% for 4-hydroxymidazolam, respectively. This data showed acceptable stability for all three compounds during the analytical runs and under the storage conditions. These results are also consistent with previously reported results for midazolam and 1-hydroxymidazolam [6, 8] and 4-hydroxymidazolam [16].
3.7. Clinical application
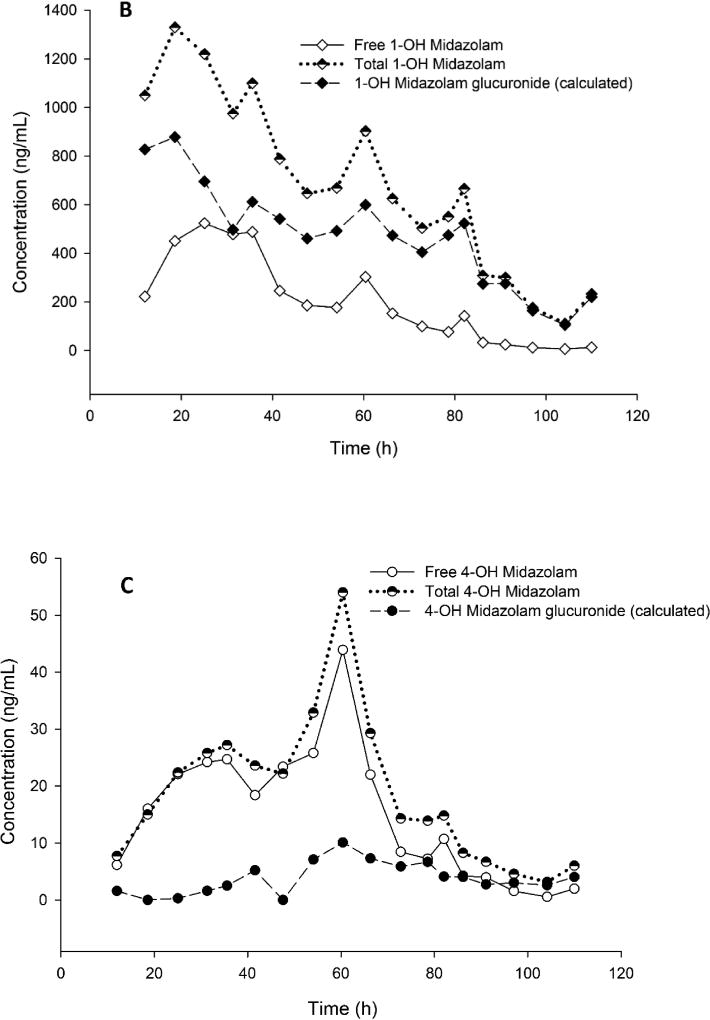
The representative PK profile of midazolam, free and total 1-hydroxymidazolam, and 4-hydroxymidazolam in a subject is shown in Figure 4. The measured levels of total 1-hydroxymidazolam were 2 to 18-fold higher than the free 1-hydroxymidazolam. However, the measured levels of total 4-hydroxymidazolam were up to 5-fold higher than the free 4-hydroxymidazolam. The concentrations of midazolam measured before and after hydrolysis remained consistent within 15% of analytical variability. The results show that the assay range was appropriate for analysis of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam in pediatric plasma samples from the clinical trials. However, some hydrolyzed samples for 1-hydroxymidazolam required dilution and reanalysis because the concentrations exceeded 1,000 ng/mL. The levels of glucuronide conjugated hydroxymidazolam metabolites were estimated as follows:
(Total 1-hydroxymidazolam) – (free 1-hydroxymidazolam) = (conjugated 1-hydroxymidazolam)
(Total 4-hydroxymidazolam) – (free 4-hydroxymidazolam) = (conjugated 4-hydroxymidazolam)
Figure 4.
Representative concentration-time profile of A) midazolam, B) free and total 1-hydroxymidazolam, and calculated concentrations of 1-hydroxymidazolam glucuronide, and C) free and total 4-hyrdoxymidazolam, and calculated concentrations of 4-hydroxymidazolam glucuronide in the plasma of a subject (29.3 kg) receiving intravenous infusion of midazolam
Since the method validation was performed with human plasma samples prepared with CPD as an anti-coagulant and clinical samples were collected with lithium heparin as an anti-coagulant, the impact of this difference on the quantitation of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam was evaluated. The accuracy and precision (%CV) for replicates of lithium heparin human plasma (n=6) at QC concentrations of 20 and 800 ng/mL were: 104 ± 1.50 and 106 ± 3.02 for midazolam, 111 ± 3.25 and 114 ± 1.94 for 1-hydroxymidazolam, and 99.6 ± 10.5 and 101 ± 3.50 for 4-hydroxymidazolam, respectively. The accuracy and precision (%CV) for replicates of human plasma with CPD (n=6) at QC concentrations of 20 and 800 ng/mL were: 106 ± 3.12 and 101 ± 2.34 for midazolam, 107 ± 3.93 and 103 ± 3.93 for 1-hydroxymidazolam, and 96.3 ± 5.05 and 90.0 ± 5.71 for 4-hydroxymidazolam, respectively. This data showed that the difference in anticoagulant used between study samples and calibration standards had no significant effect on the quantitation of midazolam, 1-hydroxymidazolam, and 4-hydroxymidazolam in human plasma.
For the clinical studies, PISA (NCT01105663) and HIP (NCT01560338), the clinical care team determined midazolam intravenous dose and dose titrations. Sensitivity and applicability of the current assay to quantify the levels of midazolam and hydroxymidazolam isomers in plasma samples depend on several factors such as dose, age, weight, drug tolerance, body temperature and polymorphism in metabolizing enzymes.
The plasma assay was further extended and validated for analysis of midazolam, free and total 1-hydroxymidazolam and 4-hydroxymidazolam in human urine samples. Sample preparation and analysis of urine samples was identical to that described for plasma samples. Validation results for urine sample analysis are shown in the supplementary materials (Tables S1–S3). One of the unique challenges seen with few urine samples was an increase of midazolam after hydrolysis. This is likely due to hydrolysis of midazolam N-glucuronide that is present only in urine samples as 1 – 2 % of the administered dose [34]. Further studies are required to quantify the levels of midazolam N-glucuronide in urine samples. For morphine and metabolites (M3G and M6G) plasma assay, intra- and inter-day results were within 85–115% for accuracy and 15% (coefficient of variation) for precision (supplementary materials, Tables S4 – S6). This assay was successfully utilized for the analysis of samples from clinical trials (NCT01105663 and NCT01560338).
4. Conclusion
The reported LC-MS/MS method with β-glucuronidase hydrolysis and µ-elution solid phase extraction provides an efficient determination of midazolam, free and total 1-hydroxymidazolam and 4-hydroxymidazolam in human plasma. Midazolam, 1-hydroxymidazolam and 4-hydroxymidazolam were measured using 50 µL of plasma. Total 1-hydroxymidazolam and 4-hydroxymidazolam were measured using 100 µL of plasma following β-glucuronidase hydrolysis. The method demonstrated acceptable sensitivity, accuracy and precision and is currently being utilized for analysis on clinical samples.
Supplementary Material
Table 2.
Summary of validation outcomes for 1-hydroxymidazolam in human plasma
| Nominal Conc. (ng/mL) |
Day 1 (n=6) | Day 2 (n=6) | ||||
|---|---|---|---|---|---|---|
| 1- hydroxymidazolam |
(Mean±SD) (ng/mL) |
CV (%) |
Accuracy (%) |
(Mean±SD) (ng/mL) |
CV(%) | Accuracy (%) |
| 0.500 | 0.430±0.057 | 13.2 | 85.9 | 0.485±0.052 | 10.7 | 97.0 |
| 2.00 | 2.18±0.156 | 7.17 | 109 | 2.02±0.174 | 8.65 | 101 |
| 20.0 | 20.5±0.978 | 4.77 | 103 | 21.4±2.06 | 9.65 | 107 |
| 200 | 216±11.7 | 5.44 | 108 | 210±7.28 | 3.46 | 106 |
| 800 | 807±32.7 | 4.06 | 101 | 790±15.9 | 2.01 | 98.7 |
| Nominal Conc. (ng/mL) | Day 3 (n=6) | Inter-day (n=18, 3 days) | ||||
| 1-hydroxymidazolam | (Mean±SD) (ng/mL) | CV (%) | Accuracy (%) | (Mean±SD) (ng/mL) | CV(%) | Accuracy (%) |
| 0.500 | 0.575±0.036 | 6.34 | 115 | 0.495±0.083 | 16.7 | 99.1 |
| 2.00 | 2.11±0.122 | 5.77 | 105 | 2.10±0.158 | 7.53 | 105 |
| 20.0 | 20.0±1.06 | 5.28 | 100 | 20.7±1.47 | 7.10 | 103 |
| 200 | 207±13.9 | 6.73 | 103 | 211±11.3 | 5.34 | 105 |
| 800 | 789±20.3 | 2.57 | 98.6 | 795±24.2 | 3.04 | 99.4 |
Highlights.
Midazolam is a short acting benzodiazepine that is routinely used in the treatment of critically ill children.
The sensitive LC-MS/MS method was developed and validated for the quantification of midazolam and its metabolites in small volumes of plasma.
Hydrolysis with β-glucuronidase was employed to measure total1-hydroxymidazolam and 4-hydroxymidazolam metabolites in plasma samples.
Solid phase extraction with Waters Oasis HLB µ-elution 96 well plates provided a robust and reliable sample cleanup for large number of clinical samples.
A representative pharmacokinetic profile of midazolam in a critically ill children demonstrates the disposition of midazolam and its metabolites.
Acknowledgments
This work was supported by NHLBI R01 HL098087 (Zuppa) Impact of Pharmacology on Duration of Ventilation in Patients with Respiratory Failure and NHLBI R01HL112745 (Zuppa) NHLBI Impact of Hypothermia on Midazolam/ Morphine Pharmacokinetics
Authors thank Benjamin Duy Tran, Mei-Ling Chen, Praveen Srivastava, Vu Nguyen, Janice Prodell and Ahila Moorthy for technical assistance and helpful discussions. This work was supported by NHLBI grants 5R01HL112745 (Zuppa) Impact of Hypothermia on Midazolam/Morphine Pharmacokinetics and 5R01HL098087 (Zuppa) Impact of Pharmacology on Duration of Ventilation in Patients with Respiratory Failure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ince I, de Wildt SN, Peeters MY, Murry DJ, Tibboel D, Danhof M, Knibbe CA. Critical illness is a major determinant of midazolam clearance in children aged 1 month to 17 years. Ther Drug Monit. 2012;34:381–389. doi: 10.1097/FTD.0b013e31825a4c3a. [DOI] [PubMed] [Google Scholar]
- 2.Ince I, de Wildt SN, Wang C, Peeters MY, Burggraaf J, Jacqz-Aigrain E, van den Anker JN, Tibboel D, Danhof M, Knibbe CA. A novel maturation function for clearance of the cytochrome P450 3A substrate midazolam from preterm neonates to adults. Clin Pharmacokinet. 2013;52:555–565. doi: 10.1007/s40262-013-0050-0. [DOI] [PubMed] [Google Scholar]
- 3.Peeters MY, Prins SA, Knibbe CA, Dejongh J, Mathot RA, Warris C, van Schaik RH, Tibboel D, Danhof M. Pharmacokinetics and pharmacodynamics of midazolam and metabolites in nonventilated infants after craniofacial surgery. Anesthesiology. 2006;105:1135–1146. doi: 10.1097/00000542-200612000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Guengerich FP, Hosea NA, Parikh A, Bell-Parikh LC, Johnson WW, Gillam EM, Shimada T. Twenty years of biochemistry of human P450s: purification, expression, mechanism, and relevance to drugs. Drug Metab Dispos. 1998;26:1175–1178. [PubMed] [Google Scholar]
- 5.Thummel KE, Wilkinson GR. In vitro and in vivo drug interactions involving human CYP3A. Annu Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- 6.Ahsman MJ, van der Nagel BC, Mathot RA. Quantification of midazolam, morphine and metabolites in plasma using 96-well solid-phase extraction and ultra-performance liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2010;24:969–976. doi: 10.1002/bmc.1394. [DOI] [PubMed] [Google Scholar]
- 7.Burhenne J, Halama B, Maurer M, Riedel KD, Hohmann N, Mikus G, Haefeli WE. Quantification of femtomolar concentrations of the CYP3A substrate midazolam and its main metabolite 1'-hydroxymidazolam in human plasma using ultra performance liquid chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem. 2012;402:2439–2450. doi: 10.1007/s00216-011-5675-y. [DOI] [PubMed] [Google Scholar]
- 8.de Loor H, de Jonge H, Verbeke K, Vanrenterghem Y, Kuypers DR. A highly sensitive liquid chromatography tandem mass spectrometry method for simultaneous quantification of midazolam, 1'-hydroxymidazolam and 4-hydroxymidazolam in human plasma. Biomed Chromatogr. 2011;25:1091–1098. doi: 10.1002/bmc.1576. [DOI] [PubMed] [Google Scholar]
- 9.Dostalek M, Macwan JS, Chitnis SD, Ionita IA, Akhlaghi F. Development and validation of a rapid and sensitive assay for simultaneous quantification of midazolam, 1'-hydroxymidazolam, and 4-hydroxymidazolam by liquid chromatography coupled to tandem mass-spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1629–1633. doi: 10.1016/j.jchromb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Frerichs VA, Zaranek C, Haas CE. Analysis of omeprazole, midazolam and hydroxy-metabolites in plasma using liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;824:71–80. doi: 10.1016/j.jchromb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Jabor VA, Coelho EB, Dos Santos NA, Bonato PS, Lanchote VL. A highly sensitive LC-MS-MS assay for analysis of midazolam and its major metabolite in human plasma: applications to drug metabolism. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;822:27–32. doi: 10.1016/j.jchromb.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Kim T, London A, Kharasch ED. Simultaneous determination of alfentanil and midazolam in human plasma using liquid chromatography and tandem mass spectrometry. J Pharm Biomed Anal. 2011;55:487–493. doi: 10.1016/j.jpba.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lepper ER, Baker SD, Permenter M, Ries N, van Schaik RH, Schenk PW, Price DK, Ahn D, Smith NF, Cusatis G, Ingersoll RG, Bates SE, Mathijssen RH, Verweij J, Figg WD, Sparreboom A. Effect of common CYP3A4 and CYP3A5 variants on the pharmacokinetics of the cytochrome P450 3A phenotyping probe midazolam in cancer patients. Clin Cancer Res. 2005;11:7398–7404. doi: 10.1158/1078-0432.CCR-05-0520. [DOI] [PubMed] [Google Scholar]
- 14.Lepper ER, Hicks JK, Verweij J, Zhai S, Figg WD, Sparreboom A. Determination of midazolam in human plasma by liquid chromatography with mass-spectrometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;806:305–310. doi: 10.1016/j.jchromb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Luo S, Smith HT, Tse FL. Simultaneous determination of midazolam and 1'-hydroxymidazolam in human plasma by liquid chromatography with tandem mass spectrometry. Biomed Chromatogr. 2007;21:841–851. doi: 10.1002/bmc.829. [DOI] [PubMed] [Google Scholar]
- 16.Link B, Haschke M, Wenk M, Krahenbuhl S. Determination of midazolam and its hydroxy metabolites in human plasma and oral fluid by liquid chromatography/electrospray ionization ion trap tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:1531–1540. doi: 10.1002/rcm.2987. [DOI] [PubMed] [Google Scholar]
- 17.Marquet P, Baudin O, Gaulier JM, Lacassie E, Dupuy JL, Francois B, Lachatre G. Sensitive and specific determination of midazolam and 1-hydroxymidazolam in human serum by liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl. 1999;734:137–144. doi: 10.1016/s0378-4347(99)00340-0. [DOI] [PubMed] [Google Scholar]
- 18.Muchohi SN, Ward SA, Preston L, Newton CR, Edwards G, Kokwaro GO. Determination of midazolam and its major metabolite 1'-hydroxymidazolam by high-performance liquid chromatography-electrospray mass spectrometry in plasma from children. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;821:1–7. doi: 10.1016/j.jchromb.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Quintela O, Cruz A, Concheiro M, de Castro A, Lopez-Rivadulla M. A sensitive, rapid and specific determination of midazolam in human plasma and saliva by liquid chromatography/electrospray mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:2976–2982. doi: 10.1002/rcm.1719. [DOI] [PubMed] [Google Scholar]
- 20.Rogers JF, Morrison AL, Nafziger AN, Jones CL, Rocci ML, Jr, Bertino JS., Jr Flumazenil reduces midazolam-induced cognitive impairment without altering pharmacokinetics. Clin Pharmacol Ther. 2002;72:711–717. doi: 10.1067/mcp.2002.128866. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu M, Uno T, Tamura HO, Kanazawa H, Murakami I, Sugawara K, Tateishi T. A developed determination of midazolam and 1'-hydroxymidazolam in plasma by liquid chromatography-mass spectrometry: application of human pharmacokinetic study for measurement of CYP3A activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;847:275–281. doi: 10.1016/j.jchromb.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Shiran MR, Gregory A, Rostami-Hodjegan A, Tucker GT, Lennard MS. Determination of midazolam and 1'-hydroxymidazolam by liquid chromatography-mass spectrometry in plasma of patients undergoing methadone maintenance treatment. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;783:303–307. doi: 10.1016/s1570-0232(02)00673-6. [DOI] [PubMed] [Google Scholar]
- 23.Streetman DS, Kashuba AD, Bertino JS, Jr, Kulawy R, Rocci ML, Jr, Nafziger AN. Use of midazolam urinary metabolic ratios for cytochrome P450 3A (CYP3A) phenotyping. Pharmacogenetics. 2001;11:349–355. doi: 10.1097/00008571-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Wermeling DP, Record KA, Archer SM, Rudy AC. A pharmacokinetic and pharmacodynamic study, in healthy volunteers, of a rapidly absorbed intranasal midazolam formulation. Epilepsy Res. 2009;83:124–132. doi: 10.1016/j.eplepsyres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Wermeling DP, Record KA, Kelly TH, Archer SM, Clinch T, Rudy AC. Pharmacokinetics and pharmacodynamics of a new intranasal midazolam formulation in healthy volunteers. Anesth Analg. 2006;103:344–349. doi: 10.1213/01.ane.0000226150.90317.16. table of contents. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Han F, Guo P, Zhao H, Lin ZJ, Huang MQ, Bertelsen K, Weng N. Simultaneous determination of tolbutamide, omeprazole, midazolam and dextromethorphan in human plasma by LC-MS/MS--a high throughput approach to evaluate drug-drug interactions. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1169–1177. doi: 10.1016/j.jchromb.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Bertol E, Vaiano F, Borsotti M, Quercioli M, Mari F. Comparison of immunoassay screening tests and LC-MS-MS for urine detection of benzodiazepines and their metabolites: results of a national proficiency test. J Anal Toxicol. 2013;37:659–664. doi: 10.1093/jat/bkt063. [DOI] [PubMed] [Google Scholar]
- 28.FDA. Guidance for Industry: Bioanalytical Methods Validation. 2001 [Google Scholar]
- 29.Kadian N, Raju KS, Rashid M, Malik MY, Taneja I, Wahajuddin M. Comparative assessment of bioanalytical method validation guidelines for pharmaceutical industry. J Pharm Biomed Anal. 2016;126:83–97. doi: 10.1016/j.jpba.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 30.Bansal S, DeStefano A. Key elements of bioanalytical method validation for small molecules. AAPS J. 2007;9:E109–114. doi: 10.1208/aapsj0901011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clavijo CF, Thomas JJ, Cromie M, Schniedewind B, Hoffman KL, Christians U, Galinkin JL. A low blood volume LC-MS/MS assay for the quantification of fentanyl and its major metabolites norfentanyl and despropionyl fentanyl in children. J Sep Sci. 2011;34:3568–3577. doi: 10.1002/jssc.201100422. [DOI] [PubMed] [Google Scholar]
- 32.Pacifici GM. Metabolism and pharmacokinetics of morphine in neonates: A review. Clinics. 2016;71:474–480. doi: 10.6061/clinics/2016(08)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonfiglio R, King RC, Olah TV, Merkle K. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun Mass Spectrom. 1999;13:1175–1185. doi: 10.1002/(SICI)1097-0231(19990630)13:12<1175::AID-RCM639>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Hyland R, Osborne T, Payne A, Kempshall S, Logan YR, Ezzeddine K, Jones B. In vitro and in vivo glucuronidation of midazolam in humans. Br J Clin Pharmacol. 2009;67:445–454. doi: 10.1111/j.1365-2125.2009.03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.