Abstract
BACKGROUND
Functional impairments often remain despite symptomatic improvement with antidepressant treatment, supporting the need for novel treatment approaches. The present study examined the extent to which exercise augmentation improved several domains of psychosocial functioning and quality of life among depressed participants. METHODS Data were collected from 122 partial responders to antidepressant medication. Participants were randomized to either high (16 kilocalories per kilogram of weight per week [KKW]) or low dose (4KKW) exercise. Participants completed a combination of supervised and home-based exercise for 12 weeks. The Short-Form Health Survey, Work and Social Adjustment Scale, Social Adjustment Scale, the Quality of Life Enjoyment and Satisfaction Questionnaire, and the Satisfaction with Life Scale were collected at 6 and 12 weeks. Participants with data for at least one of the two follow-up time points (n=106) were analyzed using a linear mixed model to assess change from baseline within groups and the difference between groups for each psychosocial outcome measure. All analyses controlled for covariates, including baseline depressive symptomatology.
RESULTS
Participants experienced significant improvements in functioning across tested domains, and generally fell within a healthy range of functioning on all measures at Weeks 6 and 12. While no differences were found between exercise groups, improvements were observed across a variety of psychosocial and quality-of-life domains, even in the low exercise dose group.
CONCLUSIONS
These findings support exercise augmentation of antidepressant treatment as a viable intervention for treatment-resistant depression to improve function in addition to symptoms.
Keywords: functional improvement, depression, physical activity, behavioral interventions, adjunct treatments
INTRODUCTION
Major Depressive Disorder (MDD) often adversely affects psychosocial functioning and quality of life. Patients with MDD report a wide range of functional impairments and reduced health-related quality of life (HRQoL)[1], including poor occupational functioning, role functioning, social functioning, and physical health [2–5], as well as high levels of disability [6; 7]. Specific functional consequences of depressive symptoms and diagnoses range from deficits in romantic competency [8] and interpersonal problem-solving [9] that impair social relationships, to high rates of presenteeism, or reduced productivity while at the workplace [10]. Despite the prevalence of psychosocial functioning deficits in patients with MDD, relatively few studies have examined the effects of MDD treatment on psychosocial functioning as a primary outcome measure [11–14]. The limited research available suggests that domains of psychosocial functioning do improve with treatment [11; 12; 15], although residual psychosocial and HRQoL dysfunction frequently remain despite improvements and even resolution of depressive symptoms [16–18]. Furthermore, subsequent deteriorations in psychosocial functioning have been found to predict future depression relapse and recurrence [19], suggesting adverse consequences for the course of the disorder if functional improvements are not achieved or maintained.
Given the pervasive and negative impact of functional deficits on individuals with depression, there is a need to focus on functional remission, in addition to symptomatic remission, as a target of antidepressant treatments [20; 21]. The prevalence of partial response to MDD treatment and persistent residual psychosocial dysfunction following treatment with traditional antidepressant medications highlights the need for novel augmentation strategies. One such promising augmentation treatment is exercise.
Exercise has been shown to be an effective treatment for mild to moderate depression, both as a monotherapy and as an adjunctive treatment [22–27]. Evidence also indicates improvements in psychosocial functioning following exercise. Increased physical activity has been positively associated with improvements in health-related quality of life in healthy individuals [28; 29], and has resulted in decreased fatigue and increased energy among patients with a variety of chronic illnesses [30]. A recent meta-analysis reported a moderate effect of exercise on improvements in quality of life in individuals with mental illness, with the authors noting that few studies report such outcomes [31]. An earlier review of studies investigating the effects of exercise on quality of life outcomes specifically in depression also noted moderate to large effects of exercise, again with the limitation that few studies examined such outcomes, and those that did had significant methodological limitations (e.g., restricted samples [small sample sizes; predominantly older adults], lack of allocation concealment and blinding of outcome measures) that weaken the conclusions [27]. Additional studies that have included patients with depression have noted improved global, social and family functioning among depressed adolescents [32] and increased motivation and self-esteem among depressed patients with traumatic brain injury [33]. In addition, in a small study [34; 35] comparing psychosocial outcomes between depressed patients randomized to maintain antidepressant treatment as usual (TAU) or to TAU plus exercise, exercise augmentation was associated with improvements in global functioning [35] as well as social and physical functioning [34], although some psychosocial domains did not change in either group. Similarly, Schuch et al. found significant improvements in physical and psychological QoL domains in severely depressed, hospitalized patients who added exercise to TAU, in comparison to patients who received TAU alone [36]. Taken together, these data support using exercise to target functional outcomes in depressed individuals, particularly as an augmentation treatment strategy, in trials that are designed to overcome some of the previously noted methodological concerns in this area.
This paper examines the effect of exercise augmentation on physical and social functioning as well as HRQoL and life satisfaction among participants in the Treatment with Exercise Augmentation for Depression (TREAD) study. The purpose of TREAD was to examine two doses of exercise as augmentation strategies for non-remitted MDD following SSRI treatment. The primary depressive symptom outcomes have been reported elsewhere [37]. The objectives of the current analyses were to examine the effect of the two exercise doses on several domains of psychosocial functioning and HRQoL among participants with partial response to SSRI treatment. We also clarify the extent to which participants achieved healthy functioning by comparing follow-up values of functional outcomes after exercise augmentation with established norms for healthy ranges of functioning. The TREAD study was designed [38] to address many methodological issues known to dilute the confidence of our understanding of the efficacy of exercise in depression and its impact on symptom and functional outcomes including: allocation concealment, clinical raters that are blind to treatment assignment, thorough diagnostic evaluation and requirement of a clinical diagnosis of MDD for inclusion, and the use of well-validated diagnostic and symptom severity measures [26; 27]. Additionally, TREAD utilized a comprehensive assessment to thoroughly capture treatment history, including adequacy of both dose and duration of previous antidepressant treatment. Furthermore, individualized aerobic exercise was prescribed to participants, conducted independently (i.e., in a non-group setting) in order to minimize social effects. Importantly, this report adds to a growing literature that focuses on functional outcomes, which are of critical importance to depressed patients, but have been understudied with respect to exercise specifically, as well as depression treatments in general.
METHODS
The study design and rationale have been published previously [38] and are described briefly below. The research was conducted in compliance with the Declaration of Helsinki and the standards of the UT Southwestern and the Cooper Institute’s Institutional Review Boards. Written informed consent was obtained from all participants after the study's protocol and procedures were fully explained.
Participants
Participants, aged 18–70, with nonpsychotic, non-remitted major depressive disorder (MDD) following adequate SSRI treatment were recruited. Participants reported some response to their pre-study antidepressant treatment, but were still symptomatic (i.e., non-remitted) at study entry, defined as a score of 14 or greater on the 17-item Hamilton Rating Scale for Depression (HRSD17) [39]. Participants had not engaged in regular physical activity over the past month and were medically cleared to exercise, based on physical examination, fasting blood draw, and a maximal exercise test. SSRI dose at study entry was maintained throughout the trial.
Exercise Intervention
Eligible participants (n=126) were randomized to receive augmentation with either a high dose (16 kilocalories per kilogram of body weight [KKW] (n=64) or a low dose (4 KKW) of exercise (n=62). The 16 KKW dose was selected to meet and exceed current public health physical activity recommendations (approximately 150 minutes per week of moderate intensity exercise) [40]. The exercise dose for the high dose group was increased gradually; the prescribed energy expenditure was 10 KKW during the first week, 13 KKW in the second week, and 16 KKW in weeks 3–12. Participants in both groups completed a combination of supervised and home-based exercise sessions. Supervised sessions were conducted by trained personnel at the Cooper Institute (CI), while home-based sessions were completed as needed to fulfill the weekly exercise prescription. Participants received three supervised sessions during Week 1 and two supervised sessions during Week 2. After Week 3, participants reported to CI weekly to complete one supervised exercise session and address any exercise-related concerns with staff. Participants used treadmills, cycle ergometers, or a combination of both in the exercise lab. Training intensity was self-selected, with a target of moderate intensity. Data from each home-based exercise session were recorded on a heart rate monitor (Polar SI610) and downloaded during visits to CI. Participants recorded frequency, duration, and intensity of all exercise sessions via the study website.
Assessment Visits and Outcome Measures
Trained personnel blinded to group assignment completed clinician-rated measures and administered self-report measures prior to the first exercise session of each week. All personnel received comprehensive training in human subjects research, HIPAA regulations, and assessment administration. Interrater reliability sessions for clinician-rated measures were conducted quarterly, and established safety protocols were maintained.
Psychosocial/HRQoL Measures
The following measures, administered at baseline, Week 6, and Week 12, were selected to assess changes in specific domains of perceived HRQoL and psychosocial functioning.
Physical and Social Functioning
The following assessments generally assess characteristics such as perceived health – both physical and emotional – and functioning in various areas of life (e.g., work, school).
Short-Form Health Survey (SF-36 - Version 2) [41; 42]
The SF-36 is a self-report inventory that contains eight scales measuring different domains of HRQoL: Physical Functioning, Physical Role Functioning, Bodily Pain, General Health, Vitality, Social Functioning, Emotional Role Functioning, and Mental Health. Each scale score ranges from 0 to 100, with higher scores indicating better perceived health and functioning. Reliability statistics for the eight scales exceed 0.70 in more than 25 studies [43], and factor-analytic studies have yielded good construct validity for the measurement of physical and mental components of health [44].
Social Adjustment Scale - Self-Report (SAS-SR) [45]
The SAS-SR is a 54-item self-report measure of instrumental and expressive role performance. Items are rated on a 5-point scale (1 to 5) and averaged to create a total scale score, with higher scores indicating impairment. The SAS-SR has adequate criterion validity (r = 0.72 with clinical interview) and interrater reliability (ICC = 0.62) for overall social adjustment in acute depression.
Work and Social Adjustment Scale (WSAS) [46]
The WSAS is a 5-item self-report measure designed to identify functional impairment that is attributed to an identified problem or condition and has been used in studies of depression and anxiety. Each question is rated on a 0 to 8 scale, with 0 indicating no impairment, and 8 indicating very severe impairment. WSAS scores above 20 suggest moderately severe or worse psychopathology. Cronbach’s α measure of internal consistency ranges from 0.80 to 0.94, with test-retest reliability of 0.73. The WSAS also has good convergent and discriminant validity (r = 0.76 with depression severity) and is sensitive to patient differences in severity, as well as treatment-related change.
Life Satisfaction
Assessments of life satisfaction assess general satisfaction, either globally or with respect to functional areas, rather than functioning itself. Such measures used in TREAD are as follows:
Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) [47]
The Q-LES-Q is a self-report measure designed to assess subjective satisfaction and enjoyment, as opposed to functioning, in various domains including physical health, feelings, work, household duties, school/course work, leisure time activities, social relations, and general activities. The raw score is converted into a percent of the maximum possible score and ranges from 0 to 100. Higher scores indicate greater enjoyment and satisfaction. The Q-LES-Q summary scales showed good test-retest reliability (ranging from 0.63 to 0.89) and high internal consistency (α ranging from 0.90 to 0.96) in a group of depressed outpatients. The Q-LES-Q also has high concurrent validity with measures of illness severity, depression, and change/improvement.
Satisfaction with Life Scale (SWLS) [48; 49]
The SWLS is a 5-item self-report inventory that measures global life satisfaction. Higher scores indicate greater satisfaction. The SWLS has high internal consistency (α = 0.87) and good construct validity with factorial invariance across male and female groups.
Depressive Symptom Severity Measures
Clinician-rated measures of depression included the Inventory of Depressive Symptomatology (IDS-C30) [50–52] and the HRSD17 [39]. Participants also completed the self-report version of the IDS (IDS-SR30) [51; 52] weekly.
Statistical Analyses
Only participants with a baseline visit and at least one post-baseline visit on the psychosocial measures (i.e., Week 6 or Week 12) were included in analyses. Groups were compared with regard to baseline characteristics using t-tests or chi square tests as appropriate. For each psychosocial outcome measure the change from baseline within groups and the difference between groups was assessed using a linear mixed model (also known as a random regression or mixed-effects model) as implemented in SAS V9.3 (Proc Mixed). The model contained terms for visit (weeks 6, 12), treatment group, and treatment group by visit interaction. The baseline value of the outcome measure was always used as a covariate. The following covariates were also included for each outcome measure: the 16-item Quick Inventory of Depressive Symptomatology–Clinician-Rated [51; 52] (QIDS-C16; derived from IDS-C30 scores), African-American race (yes/no), family history of mental illness (yes/no), recurrent MDD episode status, and gender. These covariates were determined based on an exhaustive model search conducted for the analysis of the primary outcome of IDS-C30 remission using a previously described method [37].
Conducting multiple tests on the same outcome measure will inflate the probability of erroneously concluding there is a significant difference within or between groups when there is no difference. The Bonferroni correction procedure (i.e., adjusting the level of significance by dividing the desired alpha level of 0.05 by the number of tests conducted) will guarantee the alpha level for each outcome measure remains at or below 0.05 [53]. The SAS-SR, WSAS, SWLS have no subscales, therefore the Bonferroni corrected p-value to assess significance for these outcomes is 0.05/2 = 0.025 because tests are conducted at Week 6 and Week 12. The SF- 36 has 8 subscales tested at two time points, therefore the p-value to assess significance is 0.05/16 = 0.003, and the adjusted p-value for the Q-LES-Q (with 7 subscales) is 0.05/14 = 0.004.
Effect sizes were measured by Cohen’s d. Applying benchmarks from Cohen [54], effect sizes of 0.2, 0.5, and 0.8 can be interpreted as small, medium, and large, respectively. In addition, the percentage of participants who were functioning in a healthy range at baseline, Week 6 and Week 12 was calculated. Data for normative comparisons to calculate the healthy range of functioning were obtained from the published reports of Q-LES-Q [55] and WSAS [56] with the largest numbers of healthy controls, the initial published reports of SAS-SR [45] and SWLS [48], and the SF-36 manual [42]. Healthy range of functioning was defined as 1.28 SD from the mean of the best available normative sample using the methods outlined by Vittengl and colleagues [57; 58]. As lower values on the SAS-SR and WSAS suggest greater functioning, participants with outcome measures lower than (Mean) + (1.28SD) were identified as normal/healthy. Conversely, as higher values on SF-36, Q-LES-Q, and SWLS suggest higher functioning, participants with outcome measures greater than (Mean) - (1.28SD) were identified as normal/healthy. The two treatment groups were also compared for the number of participants in the healthy range using the chi-square test. Changes from baseline to Week 6 and baseline to Week 12 were also tested using McNemar’s test.
RESULTS
Of 126 participants randomized to the intervention, 106 participants (58 in 4 KKW group and 48 in 16 KKW group) contributed data at Week 6 and 101 at Week 12. Participants who discontinued participation prior to those assessment timepoints discontinued for the following reasons: inconvenience/time (n=10), non-compliance (n=7), wanted a different treatment (n=5), AE (n=2), and lost to follow-up (n=1). Table 1 presents baseline participant characteristics for key variables. Participants were primarily Caucasian (86.7%) and female (84.9%) with moderate depressive symptom severity at study entry. No group differences were observed on any baseline variables.
Table 1.
Baseline demographic and clinical characteristics* (N = 106)
| Baseline Characteristic | 4-KKW Exercise Group (n=58) | 16-KKW Exercise Group (n=48) | t or χ2 | df | p Value |
|---|---|---|---|---|---|
| Age, mean (SD), y | 48.9 (9.1) | 46.4 (10.2) | 1.3 | 95 | .18 |
| Sex , female, n (%) | 47 (81.0%) | 43 (89.6%) | 1.5 | 1 | .22 |
| Race, n (%) | 1.0 | 2 | .62 | ||
| White | 52 (89.7%) | 40 (83.3%) | |||
| Black | 5 (8.6%) | 7 (14.6%) | |||
| Other | 1 (1.7%) | 1 (2.1%) | |||
| Body mass index, mean (SD) | 31.4 (5.6) | 29.5 (6.3) | 1.6 | 93 | .12 |
| Family history of mental illness, n (%) | 42 (72.4%) | 31 (64.6%) | 0.8 | 1 | .39 |
| Depression, mean (SD) | |||||
| Age at onset, y | 27.3 (12.1) | 26.6 (10.6) | 0.3 | 104 | .75 |
| Length of current episode, mo | 91.2 (100) | 74.4 (90.9) | 0.9 | 103 | .37 |
| Weeks of adequate dose of SSRI | 6.7 (4.1) | 8.7 (8.6) | −1.4 | 57 | .17 |
| SSRI, n (%) | |||||
| Citalopram (%) | 3.4 | 4.2 | 0.0 | 1 | .85 |
| Escitalopram (%) | 70.7 | 77.1 | 0.6 | 1 | .46 |
| Fluoxetine (%) | 3.4 | 2.1 | 0.2 | 1 | .67 |
| Paroxetine CR (%) | 1.7 | 6.3 | 1.5 | 1 | .22 |
| Paroxetine (%) | 3.4 | 0.0 | 1.7 | 1 | .19 |
| Sertraline (%) | 15.5 | 10.4 | 0.6 | 1 | .44 |
| Depression symptom severity, mean (SD) | |||||
| HRSD17 | 18.2 (3.8) | 18.0 (4.0) | 0.2 | 99 | .83 |
| IDS-C30 | 35.1 (7.6) | 33.8 (7.5) | 0.9 | 101 | .39 |
| Physical and social functioning, mean (SD) | |||||
| SF-36 Physical Functioning | 79.4 (20.9) | 79.5 (19.5) | −0.0 | 102 | .98 |
| SF-36 Role-Physical | 74.8 (29.7) | 74.6 (28.0) | 0.0 | 93 | .97 |
| SF-36 Bodily Pain | 67.0 (24.2) | 72.2 (18.5) | −1.2 | 103 | .22 |
| SF-36 General Health | 60.8 (20.6) | 59.2 (20.1) | 0.4 | 101 | .69 |
| SF-36 Vitality | 28.2 (19.7) | 27.2 (17.3) | 0.3 | 104 | .78 |
| SF-36 Social Functioning | 49.4 (28.2) | 57.6 (23.6) | −1.6 | 104 | .11 |
| SF-36 Role-Emotional | 47.3 (26.1) | 50.3 (26.5) | −0.6 | 100 | .55 |
| SF-36 Mental Health | 49.1 (15.9) | 50.7 (13.9) | −0.5 | 104 | .58 |
| SAS-SR | 2.4 (0.4) | 2.5 (0.4) | −0.3 | 104 | .73 |
| WSAS | 20.8 (9.9) | 18.7 (7.1) | 1.3 | 102 | .20 |
| Life satisfaction, mean (SD) | |||||
| Q-LES-Q Physical Health | 52.4 (13.4) | 55.2 (10.3) | −1.2 | 99 | .24 |
| Q-LES-Q Subjective Feelings | 64.0 (11.2) | 62.9 (10.1) | 0.5 | 102 | .59 |
| Q-LES-Q Work | 68.2 (14.9) | 66.3 (13.4) | 0.6 | 86 | .54 |
| Q-LES-Q Household Duties | 57.3 (19.7) | 57.9 (14.9) | −0.2 | 92 | 88 |
| Q-LES-Q Leisure Time Activities | 62.2 (16.4) | 62.6 (15.8) | −0.1 | 102 | .90 |
| Q-LES-Q Social Relationships | 68.2 (13.0) | 67.8 (12.0) | 0.2 | 101 | .86 |
| Q-LES-Q General Activities | 60.7 (10.9) | 60.2 (10.2) | 0.2 | 101 | .82 |
| SWLS | 14.3 (6.7) | 13.4 (6.0) | 0.8 | 103 | .45 |
Abbreviations: KKW = kcal/kg/wk; SSRI = selective serotonin reuptake inhibitor; HRSD17 = 17-Item Hamilton Rating Scale for Depression; IDS-C30 = 30-Item Inventory of Depressive Symptomatology, Clinician-Rated; SF-36 = Medical Outcomes Study 36-Item Short-Form Health Survey; SAS-SR = Social Adjustment Scale, Self-Report; WSAS = Work and Social Adjustment Scale; Q-LES-Q = Quality of Life Enjoyment and Satisfaction Questionnaire; SWLS = Satisfaction with Life Scale
Tables 2 and 3 show the covariate adjusted means from baseline to Week 6 and Week 12 for each outcome measure within each exercise treatment group. As expected, by Week 12, the high dose group demonstrated benefit across nearly all measures of psychosocial functioning, with the exception of the Physical Functioning, Bodily Pain and Physical Role subscales from the SF-36 and the Work subscale of the Q-LES-Q. These effects were largely in the medium to large range, as defined by Cohen's d. Unexpectedly, the low dose group also demonstrated substantial psychosocial/HRQoL benefit across nearly all measures, also with predominantly medium to large effect sizes. Only one difference was found between the high and low dose exercise groups (SAS-SR at Week 6). However, this finding (p = .035) was no longer significant after applying the Bonferroni correction. Numerically greater improvements were observed for high dose exercise at Week 6 among certain domains of functioning. Additionally, the 16 KKW group showed significant improvements in SF-36 Social Functioning, Q-LES-Q Subjective Feelings, Q-LES-Q Social Relationships, and SWLS at the Week 6 timepoint that were not observed in the 4 KKW group. The percent of individuals in the healthy range are presented for each group at each timepoint.
Table 2.
Means (standard deviations) for changes from baseline to follow-up for measures of physical and social functioning across both exercise groups
| Measure | 4-KKW Group | Effect Sizea | p | N (%) in Healthy Range | 16-KKW Group | Effect Sizea | p | N (%) in Healthy Range | p (btwn groups) |
|---|---|---|---|---|---|---|---|---|---|
| SF-36 Physical Functioning | |||||||||
| Baseline | 79.4 (20.9) | 51 (89.5) | 79.5 (19.5) | 41 (85.4) | |||||
| Week 6 | 82.5 (19.2) | 0.15 | 0.250 | 51 (92.7) | 87.8 (13.8) | 0.54 | <0.001* | 45 (100.0) | 0.052 |
| Week 12 | 85.0 (19.2) | 0.37 | 0.007 | 45 (91.8) | 85.3 (18.7) | 0.38 | 0.011 | 40 (88.9) | 0.993 |
| SF-36 Role- Physical | |||||||||
| Baseline | 74.8 (29.7) | 41 (78.9) | 74.6 (28.0) | 35 (79.6) | |||||
| Week 6 | 75.2 (26.4) | 0.03 | 0.829 | 47 (87.0) | 82.7 (17.8) | 0.33 | 0.029 | 41 (95.4) | 0.077 |
| Week 12 | 79.7 (24.8) | 0.17 | 0.230 | 41 (87.2) | 81.5 (20.0) | 0.23 | 0.120 | 41 (95.4) | 0.759 |
| SF-36 Bodily Pain | |||||||||
| Baseline | 67.0 (24.2) | 53 (91.4) | 72.2 (18.5) | 46 (95.8) | |||||
| Week 6 | 71.6 (23.5) | 0.07 | 0.579 | 51 (91.1) | 70.6 (20.5) | 0.01 | 0.957 | 42 (93.3) | 0.683 |
| Week 12 | 72.3 (24.5) | 0.16 | 0.259 | 43 (87.8) | 72.6 (21.1) | 0.08 | 0.606 | 42 (93.3) | 0.683 |
| SF-36 General Health | |||||||||
| Baseline | 60.8 (20.6) | 45 (77.6) | 59.2 (20.1) | 36 (75.0) | |||||
| Week 6 | 67.3 (18.5) | 0.45 | 0.001* | 49 (87.5) | 66.8 (18.9) | 0.51 | 0.001* | 40 (88.9) | 0.768 |
| Week 12 | 69.5 (18.5) | 0.57 | <0.001* | 43 (89.6) | 66.8 (19.7) | 0.54 | <0.001* | 36 (81.8) | 0.852 |
| SF-36 Vitality | |||||||||
| Baseline | 28.2 (19.7) | 22 (37.9) | 27.2 (17.3) | 15 (31.3) | |||||
| Week 6 | 39.8 (21.3) | 0.55 | <0.001* | 33 (60.0) | 39.2 (17.9) | 0.59 | <0.001* | 27 (60.0) | 0.830 |
| Week 12 | 44.1 (24.4) | 0.83 | <0.001* | 31 (63.3) | 43.5 (21.2) | 0.79 | <0.001* | 31 (68.9) | 0.823 |
| SF-36 Social Functioning | |||||||||
| Baseline | 49.4 (28.2) | 24 (41.4) | 57.6 (23.6) | 25 (52.1) | |||||
| Week 6 | 58.7 (28.8) | 0.33 | 0.014 | 34 (60.7) | 66.1 (21.9) | 0.52 | 0.001* | 30 (66.7) | 0.327 |
| Week 12 | 64.8 (26.0) | 0.64 | <0.001* | 32 (65.3) | 76.4 (21.8) | 0.95 | <0.001* | 36 (81.8) | 0.116 |
| SF-36 Role- Emotional | |||||||||
| Baseline | 47.3 (26.1) | 18 (31.0) | 50.3 (26.5) | 16 (33.3) | |||||
| Week 6 | 63.4 (31.6) | 0.61 | <0.001* | 31 (55.4) | 64.4 (26.0) | 0.61 | <0.001* | 21 (46.7) | 0.993 |
| Week 12 | 70.1 (26.4) | 0.91 | <0.001* | 33 (67.4) | 70.2 (25.3) | 0.83 | <0.001* | 30 (66.7) | 0.673 |
| SF-36 Mental Health | |||||||||
| Baseline | 49.1 (15.9) | 27 (46.6) | 50.7 (13.9) | 20 (41.7) | |||||
| Week 6 | 58.6 (18.0) | 0.52 | <0.001* | 35 (62.5) | 61.5 (17.8) | 0.62 | <0.001* | 30 (66.7) | 0.594 |
| Week 12 | 62.4 (21.0) | 0.80 | <0.001* | 34 (69.4) | 67.2 (18.5) | 0.95 | <0.001* | 33 (73.3) | 0.460 |
| SAS-SR | |||||||||
| Baseline | 2.4 (0.4) | 11 (19.0) | 2.5 (0.4) | 3 (6.3) | |||||
| Week 6 | 2.3 (0.4) | 0.38 | 0.004* | 16 (28.6) | 2.2 (0.4) | 0.80 | <0.001* | 18 (40.0) | 0.035 |
| Week 12 | 2.1 (0.5) | 0.99 | <0.001* | 24 (49.0) | 2.1 (0.4) | 0.93 | <0.001* | 20 (44.4) | 0.804 |
| WSAS | |||||||||
| Baseline | 20.8 (9.9) | 3 (5.2) | 18.7 (7.1) | 2 (4.2) | |||||
| Week 6 | 16.8 (9.7) | 0.43 | 0.001* | 5 (9.1) | 15.8 (9.2) | 0.42 | 0.006* | 5 (11.1) | 0.957 |
| Week 12 | 14.6 (10.8) | 0.75 | <0.001* | 9 (18.0) | 12.0 (9.8) | 0.81 | <0.001* | 12 (26.7) | 0.726 |
Note:
Effect sizes follow the convention for Cohen's d and measure the strength of the covariate-adjusted mean change between each time point and baseline;
significant change from baseline after applying Bonferroni correction. No significant between-group differences were found.
Table 3.
Means (standard deviations) for changes from baseline to follow-up for measures of life satisfaction across both exercise groups
| Measure | 4- KKW Group | Effect Sizea | p | N (%) in Healthy Range | 16- KKW Group | Effect Sizea | p | N (%)in Healthy Range | p (btwn groups) |
|---|---|---|---|---|---|---|---|---|---|
| Q-LES-Q Physical Health | |||||||||
| Baseline | 52.4 (13.4) | 19 (34.6) | 55.2 (10.3) | 16 (34.0) | |||||
| Week 6 | 59.3 (14.5) | 0.44 | 0.001* | 31 (57.4) | 61.7 (14/3) | 0.58 | <0.001* | 25 (56.8) | 0.494 |
| Week 12 | 62.7 (14.8) | 0.78 | <0.001* | 30 (62.5) | 66.2 (13.7) | 0.86 | <0.001* | 28 (63.6) | 0.686 |
| Q-LES-Q Subjective Feelings | |||||||||
| Baseline | 64.0 (11.2) | 21 (37.5) | 62.9 (10.1) | 12 (25.0) | |||||
| Week 6 | 66.2 (13.3) | 0.19 | 0.150 | 26 (46.4) | 68.6 (10.1) | 0.51 | 0.001* | 28 (62.2) | 0.112 |
| Week 12 | 71.6 (13.5) | 0.77 | <0.001* | 32 (65.3) | 72.8 (12.2) | 0.84 | <0.001* | 32 (71.1) | 0.750 |
| Q-LES-Q Work | |||||||||
| Baseline | 68.2 (14.9) | 33 (70.2) | 66.3 (13.4) | 25 (61.0) | |||||
| Week 6 | 70.4 (13.9) | 0.17 | 0.187 | 39 (79.6) | 70.3 (12.8) | 0.18 | 0.219 | 32 (82.1) | 0.977 |
| Week 12 | 73.3 (17.4) | 0.39 | 0.006 | 34 (77.3) | 74.2 (14.6) | 0.43 | 0.004 | 31 (79.5) | 0.838 |
| Q-LES-Q Household Duties | |||||||||
| Baseline | 57.3 (19.7) | 32 (61.5) | 57.9 (14.9) | 26 (60.5) | |||||
| Week 6 | 62.5 (18.8) | 0.37 | 0.006 | 33 (63.5) | 60.4 (13.9) | 0.24 | 0.105 | 31 (72.1) | 0.514 |
| Week 12 | 69.8 (16.4) | 0.79 | <0.001* | 37 (80.4) | 64.2 (15.9) | 0.47 | 0.002* | 32 (71.1)b | 0.087 |
| Q-LES-Q Leisure Time Activities | |||||||||
| Baseline | 62.2 (16.4) | 29 (50.0) | 62.6 (15.8) | 30 (62.5) | |||||
| Week 6 | 67.8 (16.5) | 0.38 | 0.005 | 36 (66.7) | 67.4 (13.1) | 0.41 | 0.006 | 33 (73.3) | 0.902 |
| Week 12 | 69.7 (18.9) | 0.53 | <0.001* | 35 (70.0) | 71.3 (14.9) | 0.66 | <0.001* | 34 (77.3) | 0.509 |
| Q-LES-Q Social Relationships | |||||||||
| Baseline | 68.2 (13.0) | 46 (79.3) | 67.8 (12.0) | 36 (76.6) | |||||
| Week 6 | 72.3 (13.5) | 0.37 | 0.005 | 47 (83.9) | 73.7 (12.7) | 0.55 | <0.001* | 43 (95.6) | 0.361 |
| Week 12 | 76.3 (14.3) | 0.77 | <0.001* | 44 (88.0) | 78.0 (10.9) | 0.85 | <0.001* | 44 (97.8) | 0.646 |
| Q-LES-Q General Activities | |||||||||
| Baseline | 60.7 (10.9) | 30 (51.7) | 60.2 (10.2) | 24 (51.1) | |||||
| Week 6 | 66.3 (11.9) | 0.53 | <0.001* | 42 (76.4) | 67.0 (11.5) | 0.57 | <0.001* | 31 (68.9) | 0.839 |
| Week 12 | 70.2 (13.5) | 0.93 | <0.001* | 38 (77.6) | 70.3 (11.9) | 0.85 | <0.001* | 36 (80.0) | 0.737 |
| SWLS | |||||||||
| Baseline | 14.3 (6.7) | 22 (37.9) | 13.4 (6.0) | 17 (35.4) | |||||
| Week 6 | 15.7 (6.9) | 0.28 | 0.035 | 26 (47.3) | 16.6 (7.1) | 0.49 | 0.001* | 24 (53.3) | 0.294 |
| Week 12 | 17.7 (8.4) | 0.61 | <0.001* | 28 (56.0) | 19.1 (8.0) | 0.93 | <0.001* | 28 (62.2) | 0.114 |
Note:
Effect sizes follow the convention for Cohen's d and measure the strength of the covariate-adjusted mean change between each time point and baseline;
More people were in the healthy range in Week 12 than in Week 6, but the percentage is lower - this was due to availability of data for 43 subjects at Week 6 and 45 in Week 12.
Significant change from baseline after applying Bonferroni correction. No significant between-group differences were found.
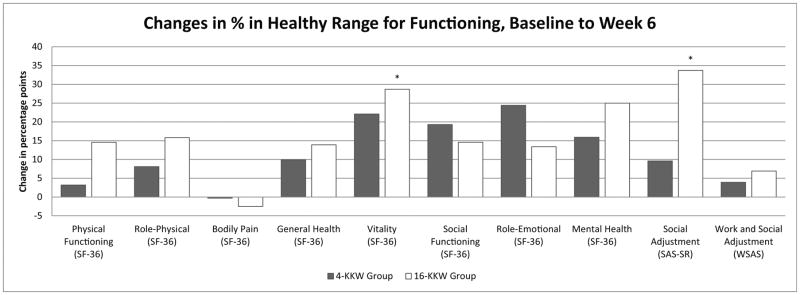
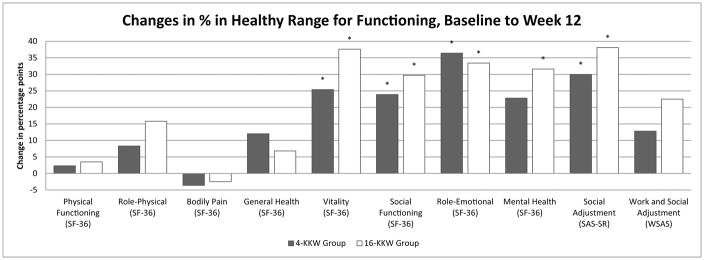
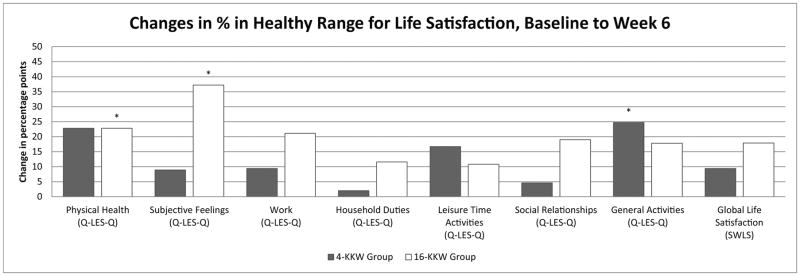
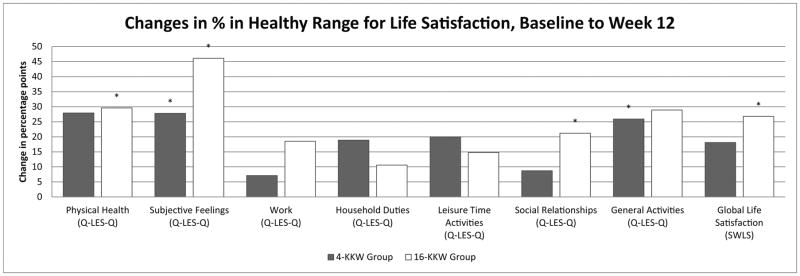
Changes in percentage points of individuals in the healthy range from baseline to Weeks 6 and baseline to Week 12 across psychosocial/HRQoL and life satisfaction measures are depicted in Figures 1 and 2, respectively. There was no significant difference between the two treatment groups in terms of the percentage of participants with functioning in the healthy range at Week 6 or Week 12. However, the high dose group showed significantly higher increases in percent of individuals in the healthy range on the SF-36 Vitality subscale, SAS-SR, and Q-LES-Q Physical Health and Subjective Feelings subscales at the Week 6 timepoint that were not observed at that timepoint in the low dose group. In the Q-LES_Q Subjective Feelings and General Activities subscales, the SAS-SR, and the SF-36 Vitality, Role Emotional, and Social Functioning subscales, the low dose group achieved significant increases in percent in the healthy range by the Week 12 timepoint. Additionally, the Q-LES-Q Physical Functioning and Social Relationships subscales, SF-36 Mental Health subscale, and SWLS measures showed a significant increase in percent of individuals in the high dose group in the healthy range from baseline to Week 12; percent of individuals in the healthy range did not change significantly in the low dose group. Interestingly, percent of healthy individuals in the low dose group for the Q-LES-Q General Activities subscale increased from baseline to Week 6 and Week 12 in the low dose group, but not the high dose group.
Figure 1.
(a&b). Percentage point changes of individuals in the healthy range on physical and social functioning measures from baseline to Week 6 (1a) and baseline to Week 12 (1b) by exercise group.
Figure 2.
(a&b). Percentage point changes of individuals in the healthy range on life satisfaction measures from baseline to Week 6 (2a) and baseline to Week 12 (2b) by exercise group.
DISCUSSION
Our analyses revealed significant improvements in psychosocial functioning and HRQoL following exercise augmentation in participants with nonremitted MDD. Specifically, we found that across both levels of exercise group and follow-up timepoints, the majority of our indices of psychosocial functioning and HRQoL were significantly improved after exercise augmentation, compared to baseline level of functioning. There were no significant differences between doses after applying the Bonferroni correction, suggesting that both exercise groups yielded psychosocial improvements. The breadth of improvements was substantial, spanning several domains of functioning including physical and mental health, social/interpersonal relationships, work and other role functioning, and general life satisfaction. Psychosocial improvements continued to be statistically significant even after controlling for baseline depression severity. Given that psychosocial impairments often remain after pharmacological treatment and resolution of depressive symptoms [16–18], these findings are particularly important and highlight the significance of using exercise as augmentation in MDD to improve residual deficits in psychosocial functioning.
Using normative values across all domains, we also found that after receiving either high or low dose exercise augmentation, the percentage of individuals in a healthy range of functioning was substantially increased. Several measures showed an increased percentage of individuals in the healthy range for the high dose group that occurred earlier than for those in the low dose group, suggesting that high dose exercise confers a quicker positive response in those functional domains. Additionally, some measures showed increases in percent of individuals in the healthy range only for participants in the high dose group, again showing some advantage of the higher dose of exercise.
Our findings are in agreement with other studies suggesting that as little as 60–70 minutes of moderate intensity physical activity weekly (as compared to the recommended guidelines of 150 minutes per week) has significant functional benefit. For example, both stretching (< 4 KKW) and vigorous exercise (> 12 KKW) significantly reduced depressive symptomatology and improved adolescent’s functioning at school and in interpersonal relationships with friends and parents [32]. Furthermore, a study comparing the benefits of three doses of supervised exercise to a no-exercise control in sedentary, overweight, postmenopausal women with high blood pressure also found functional improvements across exercise doses [59]. In addition to improvements in fitness, exercise training resulted in improvements on all measures of the SF-36 except Bodily Pain and Role Physical, with the low dose (4 KKW) group demonstrating significant improvements in the General Health, Vitality and Mental Health subscales. These findings suggest dose-dependent effects, with numerically greater benefits in individuals receiving higher levels of exercise [60]. Nonetheless, significant benefits were observed even when individuals receiving a low amount of exercise were compared to control groups who received no exercise.
Our previously reported results suggested reductions in depressive symptomatology with both exercise groups in the TREAD study [37]. However, dose-dependent effects were seen with respect to rates of remission at Week 12, with a trend in favor of the high-exercise dose group (28.3%, p <0.06). The current report also describes significant improvements over time following both low and high dose exercise, with potential additional benefits of high dose exercise, such as improvements earlier in the course of treatment. Four functional measures significantly improved from baseline in the high dose exercise group at Week 6, but significant improvements on these same measures were not achieved until Week 12 in the lower dose exercise group.
It is conceivable that different mechanisms are at work with respect to the high and low exercise doses; the higher exercise dose could be essential for physiologically-based antidepressant effects, such as the reduction of inflammation and oxidative stress via neuroimmune mechanisms [61; 62] or the production of endorphins and increases in levels of BDNF, endocannabinoids, serotonin, dopamine, and norepinephrine [63–65], while the low exercise dose could elicit effects through behavioral activation [66] and increased self-efficacy [67]. Given that psychosocial impairment following treatment is associated with an increased risk of recurrence of depression [68], our finding that psychosocial improvements can be realized even with low dose exercise are promising. Added to our previous findings in this sample suggesting that exercise decreases depressive symptoms [37] and improves self-reported sleep quality [69] and cognitive function [70], this research would suggest that exercise augmentation is likely to improve long-term treatment outcomes by reducing several risk factors for depression recurrence.
The major limitation of the current study is the lack of a true control group – because both low and high exercise doses improved psychosocial functioning to a similar degree, it is unclear whether these effects were due to the exercise intervention or due to nonspecific factors such as increased interactions and support from research staff. However, it is important to note that the study was designed to minimize social interactions, with supervised exercise conducted individually (i.e., non-group settings), and the majority of sessions conducted at home. Future work should be designed with a non-exercise active control group in order to tease apart potential nonspecific effects. Other limitations include the relative homogeneity of the sample (i.e., predominantly middle-class Caucasian females, selection of partial responders to antidepressant treatment), which may limit external validity. Future work should aim to replicate these findings with a more diverse population. Furthermore, participants completed exercise under the supervision of CI staff, thereby limiting the possibility of social interaction through workout "buddies" or groups and introducing an accountability procedure that may be lacking from the majority of exercise programs. While this is a design strength, it may reduce generalizability. Allowing participants to exercise at home is a noteworthy strength that improved our generalizability and likely reduced participant burden. The comprehensive evaluation of psychosocial symptoms is an additional strength of the study.
The results of the current study also suggest potential areas for future work. First, given the apparent effect of a low dose of exercise, research is needed to determine the optimal dose required for alleviation of depressive symptoms and co-occurring psychosocial dysfunction. Additional work will inform whether high dose exercise offers improvements above and beyond those obtained at lower doses. Additionally, this study manipulated volume of exercise in terms of energy expenditure, but future research may inform whether effects vary by additional factors such as intensity, exercise type (aerobic vs. weight training), exercise setting, etc. In addition, other factors that may mediate or moderate the effect of exercise on QoL in depression should be examined. The current analysis included covariates known to be relevant to the interpretation of exercise efficacy on depressive outcomes, but further exploration of covariates specifically related to functional outcomes and/or response to exercise (e.g., medical comorbidities, fat/muscle mass, duration of illness) is an important area of future investigation.
CONCLUSIONS
Our findings support the use of exercise augmentation as a potentially efficacious way to improve psychosocial functioning and quality-of-life. This work further supports the use of exercise as an augmentation strategy for non-remitted MDD, as well as the need to assess functional outcomes both as part of clinical care and as targeted research outcomes.
Acknowledgments
The authors would like to thank Mariam Andersen, M.A., Tyson Bain, M.S., Elizabeth Darr, B.A., Erica Dickson, M.S., Andrea Dunn, Ph.D., Natalie Dufresne, R.N., Carrie Finley, M.S., Ariell Flood, M.A., Daniel I. Galper, Ph.D., Shailesh Jain, M.D., Shaily Jain, M.D., Alex Jordan, M.S., Dino Jurca, Ph.D., Heather Kitzman-Ulrich, Ph.D., Beverly Kleiber, Ph.D., Benji Kurian, M.D., Jennifer Kupper, M.S., Lucille Marcoux, R.N., Britney McGill, B.A., David W. Morris, Ph.D., Anne Marie Preston, M.A., A. John Rush, M.D., Jennifer Schroeder, B.A., Robin Selman, B.A., Erin Sinclair, B.A., Julie Smith, B.A., Prabha Sunderajan, M.D., Mei Sui, M.D., MPH, Kim Warren, B.A., Paul Watson, Bradley Witte, B.S., and Beth Wright, M.S. for assisting with this project. We would additionally like to recognize Dr. Galper, now deceased, for his commitment and leadership on the TREAD project. We also recognize, with great appreciation, all of the study participants who contributed to this project. We thank Carol A. Tamminga, M.D., Lou and Ellen McGinley Distinguished Chair and the McKenzie Chair in Psychiatry and Chair, Department of Psychiatry, University of Texas Southwestern Medical Center, and Savitha Kalidas, Ph.D. for administrative support.
Footnotes
DISCLOSURES
This work was supported by the National Institute for Mental Health (1-R01-MH067692-01; PI: MH Trivedi) Washington, D.C., and in part by a National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award (MHT) and Young Investigator Award (TLG) and a fellowship award (JMT) under 5 T32 MH067543-10 (PI: MH Trivedi). Chad D. Rethorst is supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K01MH097847. The study sponsors have had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of this report; nor in the decision to submit the paper for publication.
Dr. Greer (for the past 36 months) has received honoraria, speakers or advisory boards and/or consultant fees from H. Lundbeck A/S. Dr. Trombello currently owns stock in Merck and Gilead Sciences and within the past 36 months previously owned stock in Johnson & Johnson. Dr. Rethorst, Dr. Carmody, Dr. Jha, Dr. Chambliss, Dr. Church, Dr. Liao and Mr. Grannemann have no disclosures to report. Dr. Trivedi is or has been an advisor/consultant to, or on the Speakers’ Bureaus for (for the past 36 months) Alkermes, AstraZeneca, Cerecor, Eli Lilly & Company, Lundbeck, Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Pfizer Inc., SHIRE Development and Takeda. In addition, he has received grants/research support from: National Institute of Mental Health and National Institute on Drug Abuse.
References
- 1.IsHak WW, Greenberg JM, Balayan K, et al. Quality of life: the ultimate outcome measure of interventions in major depressive disorder. Harv Rev Psychiatry. 2011;19(5):229–39. doi: 10.3109/10673229.2011.614099. [DOI] [PubMed] [Google Scholar]
- 2.Hays RD, Wells KB, Sherbourne CD, et al. Functioning and well-being outcomes of patients with depression compared with chronic general medical illnesses. Arch Gen Psychiatry. 1995;52(1):11–9. doi: 10.1001/archpsyc.1995.03950130011002. [DOI] [PubMed] [Google Scholar]
- 3.Wells KB, Sherbourne CD. Functioning and utility for current health of patients with depression or chronic medical conditions in managed, primary care practices. Arch Gen Psychiatry. 1999;56(10):897–904. doi: 10.1001/archpsyc.56.10.897. [DOI] [PubMed] [Google Scholar]
- 4.Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. Jama. 1989;262(7):914–9. [PubMed] [Google Scholar]
- 5.Gaynes BN, Burns BJ, Tweed DL, Erickson P. Depression and health-related quality of life. J Nerv Ment Dis. 2002;190(12):799–806. doi: 10.1097/00005053-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ormel J, Vonkorff M, Oldehinkel AJ, et al. Onset of disability in depressed and non-depressed primary care patients. Psychol Med. 1999;29(4):847–53. doi: 10.1017/s0033291799008600. [DOI] [PubMed] [Google Scholar]
- 7.Judd LL, Schettler PJ, Solomon DA, et al. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Affect Disord. 2008;108(1–2):49–58. doi: 10.1016/j.jad.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Herzberg DS, Hammen C, Burge D, et al. Social competence as a predictor of chronic interpersonal stress. Personal Relationships. 1998;5(2):207–218. [Google Scholar]
- 9.Davila J, Hammen C, Burge D, et al. Poor interpersonal problem solving as a mechanism of stress generation in depression among adolescent women. J Abnorm Psychol. 1995;104(4):592–600. doi: 10.1037//0021-843x.104.4.592. [DOI] [PubMed] [Google Scholar]
- 10.Stewart WF, Ricci JA, Chee E, et al. Cost of lost productive work time among US workers with depression. Jama. 2003;289(23):3135–44. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- 11.Hirschfeld RM, Montgomery SA, Aguglia E, et al. Partial response and nonresponse to antidepressant therapy: current approaches and treatment options. J Clin Psychiatry. 2002;63(9):826–37. doi: 10.4088/jcp.v63n0913. [DOI] [PubMed] [Google Scholar]
- 12.Miller IW, Keitner GI, Schatzberg AF, et al. The treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramine. J Clin Psychiatry. 1998;59(11):608–19. doi: 10.4088/jcp.v59n1108. [DOI] [PubMed] [Google Scholar]
- 13.Papakostas GI, Petersen T, Denninger JW, et al. Psychosocial functioning during the treatment of major depressive disorder with fluoxetine. J Clin Psychopharmacol. 2004;24(5):507–11. doi: 10.1097/01.jcp.0000138761.85363.d5. [DOI] [PubMed] [Google Scholar]
- 14.Lam RW, Filteau MJ, Milev R. Clinical effectiveness: the importance of psychosocial functioning outcomes. J Affect Disord. 2011;132(Suppl 1):S9–s13. doi: 10.1016/j.jad.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 15.Matsunaga M, Okamoto Y, Suzuki S, et al. Psychosocial functioning in patients with Treatment-Resistant Depression after group cognitive behavioral therapy. BMC Psychiatry. 2010;10:22. doi: 10.1186/1471-244X-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy N, Foy K, Sherazi R, et al. Long-term social functioning after depression treated by psychiatrists: a review. Bipolar Disord. 2007;9(1–2):25–37. doi: 10.1111/j.1399-5618.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 17.Coryell W, Scheftner W, Keller M, et al. The enduring psychosocial consequences of mania and depression. Am J Psychiatry. 1993;150(5):720–7. doi: 10.1176/ajp.150.5.720. [DOI] [PubMed] [Google Scholar]
- 18.Greco T, Eckert G, Kroenke K. The outcome of physical symptoms with treatment of depression. J Gen Intern Med. 2004;19(8):813–8. doi: 10.1111/j.1525-1497.2004.30531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vittengl JR, Clark LA, Jarrett RB. Deterioration in psychosocial functioning predicts relapse/recurrence after cognitive therapy for depression. J Affect Disord. 2009;112(1–3):135–43. doi: 10.1016/j.jad.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greer TL, Kurian BT, Trivedi MH. Defining and measuring functional recovery from depression. CNS Drugs. 2010;24(4):267–84. doi: 10.2165/11530230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman M, McGlinchey JB, Posternak MA, et al. Remission in depressed outpatients: more than just symptom resolution? J Psychiatr Res. 2008;42(10):797–801. doi: 10.1016/j.jpsychires.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Blumenthal JA, Smith PJ, Hoffman BM. Is Exercise a Viable Treatment for Depression? ACSMs Health Fit J. 2012;16(4):14–21. doi: 10.1249/01.FIT.0000416000.09526.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bridle C, Spanjers K, Patel S, et al. Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. 2012;201(3):180–5. doi: 10.1192/bjp.bp.111.095174. [DOI] [PubMed] [Google Scholar]
- 24.Rethorst CD, Trivedi MH. Evidence-based recommendations for the prescription of exercise for major depressive disorder. J Psychiatr Pract. 2013;19(3):204–12. doi: 10.1097/01.pra.0000430504.16952.3e. [DOI] [PubMed] [Google Scholar]
- 25.Silveira H, Moraes H, Oliveira N, et al. Physical exercise and clinically depressed patients: a systematic review and meta-analysis. Neuropsychobiology. 2013;67(2):61–8. doi: 10.1159/000345160. [DOI] [PubMed] [Google Scholar]
- 26.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;9:Cd004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuch FB, Vasconcelos-Moreno MP, Fleck MP. The impact of exercise on Quality of Life within exercise and depression trials: A systematic review. Ment Health Phys Act. 2011;4(2):43–48. [Google Scholar]
- 28.Kruger J, Bowles HR, Jones DA, et al. Health-related quality of life, BMI and physical activity among US adults (>/=18 years): National Physical Activity and Weight Loss Survey, 2002. Int J Obes (Lond) 2007;31(2):321–7. doi: 10.1038/sj.ijo.0803386. [DOI] [PubMed] [Google Scholar]
- 29.Vuillemin A, Boini S, Bertrais S, et al. Leisure time physical activity and health-related quality of life. Prev Med. 2005;41(2):562–9. doi: 10.1016/j.ypmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Puetz TW, O'Connor PJ, Dishman RK. Effects of chronic exercise on feelings of energy and fatigue: a quantitative synthesis. Psychol Bull. 2006;132(6):866–76. doi: 10.1037/0033-2909.132.6.866. [DOI] [PubMed] [Google Scholar]
- 31.Rosenbaum S, Tiedemann A, Sherrington C, et al. Physical activity interventions for people with mental illness: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(9):964–74. doi: 10.4088/JCP.13r08765. [DOI] [PubMed] [Google Scholar]
- 32.Hughes CW, Barnes S, Barnes C, et al. Depressed Adolescents Treated with Exercise (DATE): A pilot randomized controlled trial to test feasibility and establish preliminary effect sizes. Ment Health Phys Act. 2013;6(2) doi: 10.1016/j.mhpa.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwandt M, Harris JE, Thomas S, et al. Feasibility and effect of aerobic exercise for lowering depressive symptoms among individuals with traumatic brain injury: a pilot study. J Head Trauma Rehabil. 2012;27(2):99–103. doi: 10.1097/HTR.0b013e31820e6858. [DOI] [PubMed] [Google Scholar]
- 34.Mota-Pereira J, Carvalho S, Silverio J, et al. Moderate physical exercise and quality of life in patients with treatment-resistant major depressive disorder. J Psychiatr Res. 2011;45(12):1657–9. doi: 10.1016/j.jpsychires.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Mota-Pereira J, Silverio J, Carvalho S, et al. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005–11. doi: 10.1016/j.jpsychires.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Schuch FB, Vasconcelos-Moreno MP, Borowsky C, et al. Exercise and severe major depression: effect on symptom severity and quality of life at discharge in an inpatient cohort. J Psychiatr Res. 2015;61:25–32. doi: 10.1016/j.jpsychires.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Trivedi MH, Greer TL, Church TS, et al. Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. J Clin Psychiatry. 2011;72(5):677–84. doi: 10.4088/JCP.10m06743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trivedi MH, Greer TL, Grannemann BD, et al. TREAD: TReatment with Exercise Augmentation for Depression: study rationale and design. Clin Trials. 2006;3(3):291–305. doi: 10.1191/1740774506cn151oa. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 41.Ware JE., Jr Conceptualization and measurement of health-related quality of life: comments on an evolving field. Arch Phys Med Rehabil. 2003;84(4 Suppl 2):S43–51. doi: 10.1053/apmr.2003.50246. [DOI] [PubMed] [Google Scholar]
- 42.Ware JE., Jr . SF-36 Physical and mental health summary scales: A user’s guide. Quality Metrics, Inc; 1997. Version 2. [Google Scholar]
- 43.Tsai C, Bayliss MS, Ware JE. SF-36 Health Survey Annotated Bibliography: Second Edition (1988–1996) Boston, MA: Health Assessment Lab, New England Medical Center; 1997. [Google Scholar]
- 44.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Weissman MM, Prusoff BA, Thompson WD, et al. Social adjustment by self-report in a community sample and in psychiatric outpatients. J Nerv Ment Dis. 1978;166(5):317–26. doi: 10.1097/00005053-197805000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry. 2002;180:461–4. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- 47.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–6. [PubMed] [Google Scholar]
- 48.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71–5. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 49.Shevlin M, Brunsden V, Miles JNV. Satisfaction With Life Scale: Analysis of factorial invariance, mean structures and reliability. Personality and Individual Differences. 1998;25(5):911–916. [Google Scholar]
- 50.Bernstein IH, Rush AJ, Thomas CJ, et al. Item response analysis of the Inventory of Depressive Symptomatology. Neuropsychiatr Dis Treat. 2006;2(4):557–564. doi: 10.2147/nedt.2006.2.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 52.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 53.Dunn OJ. Multiple comparisons among means. Journal of the American Statistical Association. 1961;56:52–64. [Google Scholar]
- 54.Cohen J. Statistical power analyses for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 55.Eisen JL, Mancebo MA, Pinto A, et al. Impact of obsessive-compulsive disorder on quality of life. Compr Psychiatry. 2006;47(4):270–5. doi: 10.1016/j.comppsych.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tchanturia K, Hambrook D, Curtis H, et al. Work and social adjustment in patients with anorexia nervosa. Compr Psychiatry. 2013;54(1):41–5. doi: 10.1016/j.comppsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 57.Vittengl JR, Clark LA, Jarrett RB. Improvement in social-interpersonal functioning after cognitive therapy for recurrent depression. Psychol Med. 2004;34(4):643–58. doi: 10.1017/S0033291703001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jarrett RB, Vittengl JR, Doyle K, Clark LA. Changes in cognitive content during and following cognitive therapy for recurrent depression: substantial and enduring, but not predictive of change in depressive symptoms. J Consult Clin Psychol. 2007;75(3):432–46. doi: 10.1037/0022-006X.75.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. Jama. 2007;297(19):2081–91. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 60.Martin CK, Church TS, Thompson AM, et al. Exercise dose and quality of life: a randomized controlled trial. Arch Intern Med. 2009;169(3):269–78. doi: 10.1001/archinternmed.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eyre H, Baune BT. Neuroimmunological effects of physical exercise in depression. Brain Behav Immun. 2012;26(2):251–66. doi: 10.1016/j.bbi.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Eyre HA, Papps E, Baune BT. Treating depression and depression-like behavior with physical activity: an immune perspective. Front Psychiatry. 2013;4:3. doi: 10.3389/fpsyt.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dinas PC, Koutedakis Y, Flouris AD. Effects of exercise and physical activity on depression. Ir J Med Sci. 2011;180(2):319–25. doi: 10.1007/s11845-010-0633-9. [DOI] [PubMed] [Google Scholar]
- 64.Heyman E, Gamelin FX, Goekint M, et al. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology. 2012;37(6):844–51. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 65.Dishman RK, Berthoud HR, Booth FW, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14(3):345–56. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- 66.Dimidjian S, Barrera M, Jr, Martell C, et al. The origins and current status of behavioral activation treatments for depression. Annual Review of Clinical Psychology. 2011;7:1–38. doi: 10.1146/annurev-clinpsy-032210-104535. [DOI] [PubMed] [Google Scholar]
- 67.Chang FH, Latham NK, Ni P, Jette AM. Does self-efficacy mediate functional change in older adults participating in an exercise program after hip fracture? A randomized controlled trial. Arch Phys Med Rehabil. 2015 doi: 10.1016/j.apmr.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solomon DA, Leon AC, Endicott J, et al. Psychosocial impairment and recurrence of major depression. Compr Psychiatry. 2004;45(6):423–30. doi: 10.1016/j.comppsych.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Rethorst CD, Sunderajan P, Greer TL, et al. Does exercise improve self-reported sleep quality in non-remitted major depressive disorder? Psychol Med. 2013;43(4):699–709. doi: 10.1017/S0033291712001675. [DOI] [PubMed] [Google Scholar]
- 70.Greer TL, Grannemann BD, Chansard M, et al. Dose-dependent changes in cognitive function with exercise augmentation for major depression: Results from the TREAD study. Eur Neuropsychopharmacol. 2015;25(2):248–56. doi: 10.1016/j.euroneuro.2014.10.001. [DOI] [PubMed] [Google Scholar]