Abstract
Glioblastoma, also known as glioblastoma multiforme (GBM), is the most recurrent and malignant astrocytic glioma found in adults. Biologically, GBMs are highly aggressive tumors that often show diffuse infiltration of the brain parenchyma, making complete surgical resection difficult. GBM is not curable with surgery alone because tumor cells typically invade the surrounding brain, rendering complete resection unsafe. Consequently, present-day therapy for malignant glioma remains a great challenge. The location of the invasive tumor cells presents several barriers to therapeutic delivery. The blood–brain barrier regulates the trafficking of molecules to and from the brain. While high-grade brain tumors contain some “leakiness” in their neovasculature, the mechanisms of GBM onset and progression remain largely unknown. Recent advances in the understanding of the signaling pathways that underlie GBM pathogenesis have led to the development of new therapeutic approaches targeting multiple oncogenic signaling aberrations associated with the GBM. Among these, drug delivery nanosystems have been produced to target therapeutic agents and improve their biodistribution and therapeutic index in the tumor. These systems mainly include polymer or lipid-based carriers such as liposomes, metal nanoparticles, polymeric nanospheres and nanocapsules, micelles, dendrimers, nanocrystals, and nanogold. Photodynamic therapy (PDT) is a promising treatment for a variety of oncological diseases. PDT is an efficient, simple, and versatile method that is based on a combination of a photosensitive drug and light (generally laser-diode or laser); these factors are separately relatively harmless but when used together in the presence of oxygen molecules, free radicals are produced that initiate a sequence of biological events, including phototoxicity, vascular damage, and immune responses. Photodynamic pathways activate a cascade of activities, including apoptotic and necrotic cell death in both the tumor and the neovasculature, leading to a permanent lesion and destruction of GBM cells that remain in the healthy tissue. Glioblastoma tumors differ at the molecular level. For example, gene amplification epidermal growth factor receptor and its receptor are more highly expressed in primary GBM than in secondary GBM. Despite these distinguishing features, both types of tumors (primary and secondary) arise as a result dysregulation of numerous intracellular signaling pathways and have standard features, such as increased cell proliferation, survival and resistance to apoptosis, and loss of adhesion and migration, and may show a high degree of invasiveness. PDT may promote significant tumor regression and extend the lifetime of patients who experience glioma progression.
Keywords: Glioblastoma multiforme, Drug delivery nanosystems, Signaling pathways
Introduction
Glioma
Glioma is a common type of primary tumor of the central nervous system (CNS) in adults. According to the World Health Organization (WHO), glioma is classified into four grades: pilocytic astrocytoma (grade I), low-grade astrocytoma (grade II), anaplastic astrocytoma (grade III), and glioblastoma multiforme (GBM) (grade IV), where increasing grade number is associated with increased malignancy and aggressiveness (Kleihues and Cavenee 2000).
Because of the complexity of the brain and its central role in maintaining homeostasis in an organism, secondary symptoms of gliomas, both neurological and non-neurological, affect patients over the course of the disease, such as by causing epilepsy, confusion, personality change, vision problems, hearing, locomotor, cardiorespiratory change, and other effects. With increased severity, pulmonary embolism and thrombosis may occur, leading to patient death (Pytel and Lukas 2009). Moreover, gliomas rarely metastasize (Van Meir et al. 2010).
In recent years, improvements in diagnostic techniques (such as magnetic resonance imaging and computed tomography) have led to an increase in the number of new cases diagnosed with glioma and other neoplastic diseases observed in CNS tumors (Van Meir et al. 2010). This has also led to the development of new treatment strategies. Another important factor affecting the emergence of new cases is exposure to environmental factors (risk factors) that may cause the development of not only brain tumors, but also other types of tumors (Preston-Martin 1996).
The WHO’s glioma classification is based on molecular and epidemiological data of the tumor (Brat et al. 2007; Fuller and Scheithauer 2007; Louis et al. 2007; Nakazato 2008). Thus, the classification of gliomas is described using three parameters: cellular type, tumor location, and malignancy degree.
Glioma: classification
Cellular type
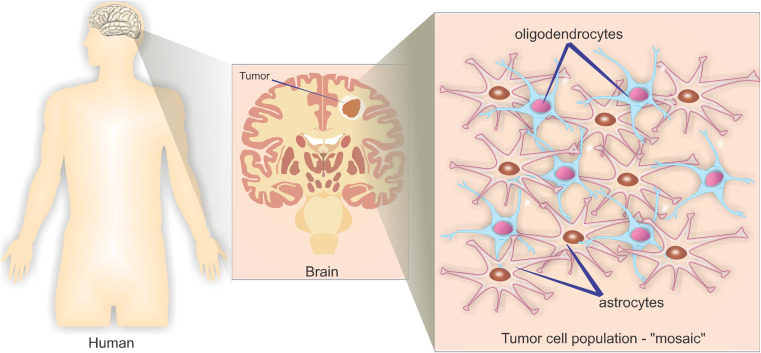
Cell types are classified based on histological characteristics according to the phenotypic similarity of cancer cells and different types of glial cells (support cells and functional support CNS). According to their morphology, gliomas are classified as astrocytomas, oligodendrogliomas, and ependymomas. It is also possible to detect the development of “mosaic” tumors that present characteristics of both astrocytomas and oligoastrocytomas—these are known as oligoastrocytomas (Fig. 1) and may be present at low prevalence in glioblastoma.
Fig. 1.
Mosaic population of different tumor cell types
According to the WHO, gliomas, astrocytoma, and oligodendroglioma account for approximately 90% of cases of GBM. In addition to histological differences, types of cancer cells also show different therapeutic sensitivities and are associated to different patient prognoses. Each type is divided into subtypes that are histologically defined from the classification based on malignancy and tumor development.
Tumor location
Clinically, gliomas are rated based on their location, which relies on a membranous structure that defines the brain cerebellum, the cerebellum tentorium (or “roof” cerebellum). Thus, gliomas can be classified as supratentorial because they develop above the tentorium (in the brain), which accounts for 70% of gliomas in adults, and as infratentorial because they develop below the tentorium (cerebellum) (Brat et al. 2007; Fuller and Scheithauer 2007; Louis et al. 2007; Nakazato 2008).
Tumor malignancy
The malignancy scale ranges from grade I (low-grade, slow growing, and likely to require eventual surgical removal) to grade IV (high-grade, highly aggressive growth, and a recurrence rate rarely amenable to surgical resection). The WHO rating of glioma tumor malignancy is as follows (Brat et al. 2007; Fuller and Scheithauer 2007; Louis et al. 2007; Nakazato 2008):
Low-grade gliomas: grade I and II tumors, differentiated (not anaplastic, state of dedifferentiation and cell pleomorphism, with high-capacity multiplication of cells, common for highly malignant tumors) and slow growth. Grade I tumors are typically found in children.
Low-grade gliomas have five subtypes:
-
i.
Diffuse astrocytoma: most common; mostly affects adults aged 30–40 years; slow but diffuse growth, enabling surgical removal
-
ii.
Pilocytic astrocytoma: found nearly exclusively in individuals under the age of 25 years; the main characteristic is slow progression; typically encapsulated and fully removable by surgery
-
iii.
Oligodendroglioma: second-most common type of low-grade glioma; grows slowly
-
iv.
Gangliogliomas: glioneuronal tumor (mixed gliomas, time pilocytic astrocytoma, and neuronal cells); affects individuals aged 20–30 years and develops mainly in the temporal lobe; surgically removable with excellent prognosis for patients
-
v.
Mixed glioma: typically shows characteristics of diffuse astrocytoma and oligodendroglioma; behaves similarly to diffuse astrocytomas.
Low-grade gliomas are not benign, but patients with these gliomas do have a better prognosis than those with high-grade tumors. Patients with grade II tumors, for example, have a mean survival of 5–8 years compared to 12–14 months survival for patients with grade IV tumors. The symptoms presented by patients depend on the location of the tumor and the rate of tumor development. However, early symptoms include convulsive seizures followed by psychomotor disorders, such as headaches, nausea, and vomiting. Secondary symptoms are treated pharmacologically. The primary therapy against tumors is based on surgical resection followed by radiation therapy and chemotherapy according to each individual case and tumor type. On rare occasions low-grade gliomas grow to become encapsulated, making complete surgical resection difficult. Additionally, depending on the region of tumor growth, surgical approaches may not be feasible. In such cases, chemotherapy and radiotherapy are used as primary therapies (Brat et al. 2007; Fuller and Scheithauer 2007; Louis et al. 2007; Nakazato 2008).
-
2)
High-grade gliomas: tumors typically are undifferentiated or anaplastic, show high proliferation, are resistant to therapy, and have a poor prognosis. The two most common and aggressive subtypes are anaplastic astrocytoma (grade III) and glioblastoma multiforme (grade IV).
-
i.
Anaplastic astrocytoma: these tumor types are diffusely infiltrative, focal, anaplastic sound, and dispersed. They have a high proliferative index and are defined histologically by nuclear atypia and pronounced mitotic activity. Patients survive for a median of 3 years (Brat et al. 2007; Fuller and Scheithauer 2007; Louis et al. 2007; Nakazato 2008).
-
ii.
Glioblastoma multiforme: type of astrocytoma accounting for over 70% of high-grade gliomas diagnosed. It is also the most aggressive type of primary brain tumor (grade IV) (Brat et al. 2007; Fuller and Scheithauer 2007; Louis et al. 2007; Nakazato 2008).
Glioblastoma multiforme (GBM)
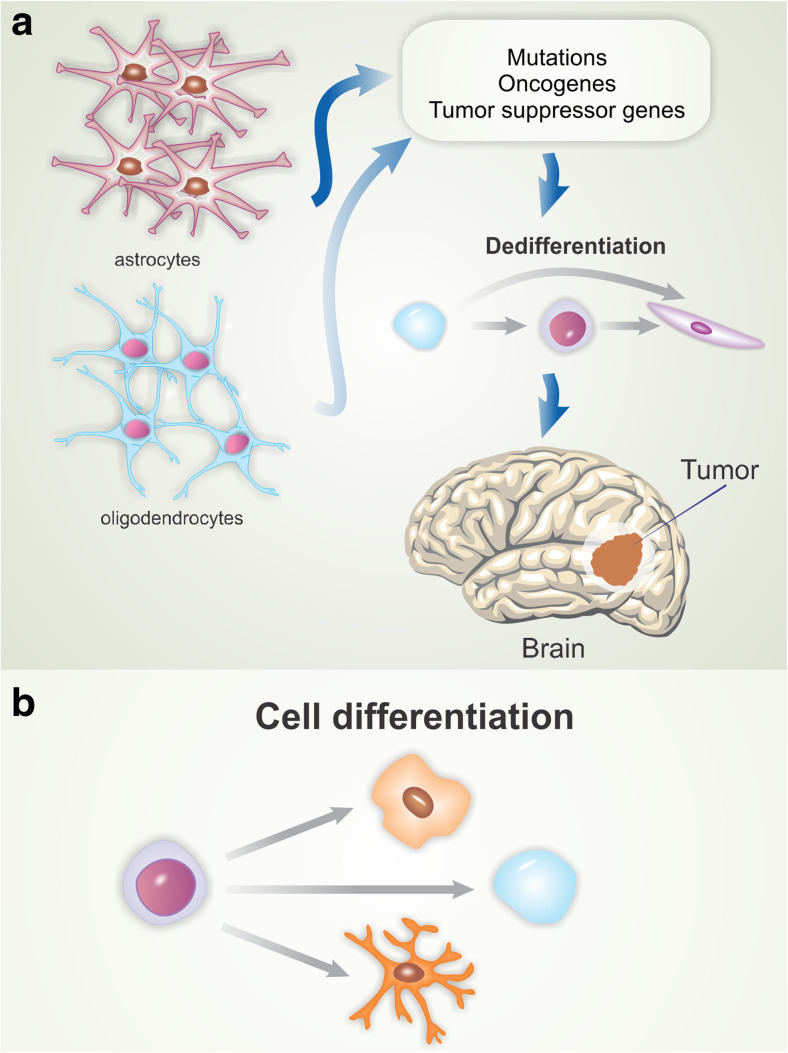
There are two main theories regarding cells that give rise to glioblastoma. The first proposes that glioblastomas are derived from mature glial cells (astrocytes and oligodendrocytes) that undergo mutations in oncogenes and tumor suppressor genes, leading to cell dedifferentiation and tumor development (Fig. 2a) (Maher et al. 2001). The second and most accepted theory suggests that these tumors originate from progenitor cells that undergo transformation events during development (Fig. 2b) (Van Meir et al. 2010). Such cells, upon oncogenic transformation, become tumor-initiating cells (Singh et al. 2004).
Fig. 2.
Theories on the origin of glioblastomas. The first theory (a) suggests that glioblastomas are derived from mature glial cells that undergo mutations in oncogenes and tumor suppressor genes, leading to cell dedifferentiation and tumor development. The second theory (b) suggests that these tumors originate from progenitor cells that undergo transformation events during development
GBM can develop quickly without clinical, radiological or morphological evidence of a less malignant precursor tumor or it may grow from healthy glial cells or their precursors, known as primary GBM. Secondary GBM arises progressively from low-grade astrocytomas, which are less common than primary GBMs (Collins et al. 2005). Glioblastoma tumors differ at the molecular level. The “multiforme” term refers to the different conditions under which the same phenotype may result from mutations in different subsets of genes.
Histological and tumor characteristics of GBMs
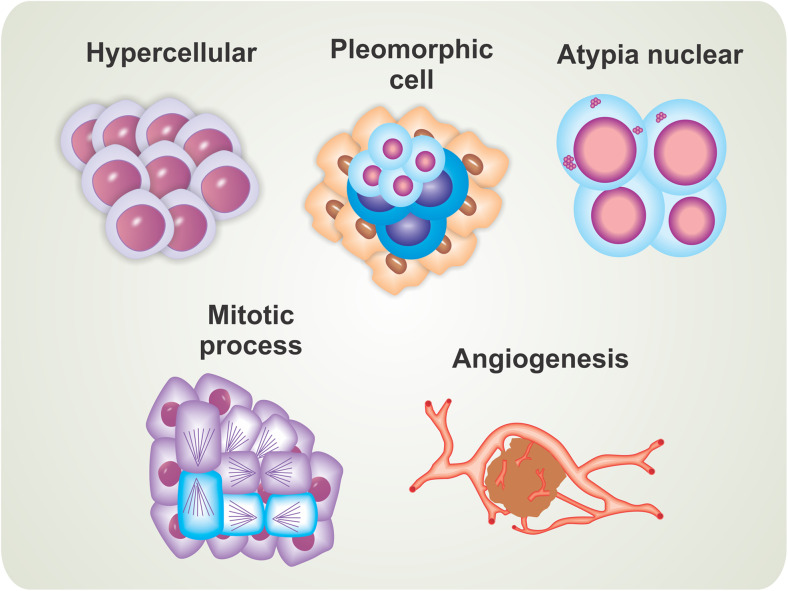
Glioblastoma are extremely aggressive tumors that are resistant to conventional therapies used to treat other tumor types (Krakstad and Chekenya 2010). The main cellular/histological characteristics that cause high malignancy are the presence of an excess of cells (hypercellularity), wide cytomorphologic and architectural tumor diversity (cellular pleomorphic), unusual appearance of cell nuclei (nuclear atypia), uncontrolled cell division (large numbers of mitotic cells), and formation and differentiation of blood vessels (angiogenesis) (Fig. 3). GBM tumors are highly proliferative and show a pattern of multifocal growth (do not form a single solid mass, but rather form multiple tumors scattered throughout the tissue), abruptly generating several symptoms in patients.
Fig. 3.
Cellular and histological characteristics associated with high-level tumor malignancy
GBM cells also show intrinsic apoptosis and radiation resistance, and thus proapoptotic anticancer therapies often fail to induce tumoral regression (Furnari et al. 2007; Van Meir et al. 2010). Accordingly, these cells have been shown to be more sensitive to therapies that induce other mechanisms of cell death, such as autophagic processes, senescence, and mitotic catastrophe (Van Meir et al. 2010).
Genetic alterations in GBMs
Amplification of the epidermal growth factor receptor (EGFR) was the first genetic modification described in most GBM tumors (Libermann et al. 1985). However, amplification of the EGFR gene with consequent elevation of EGFR expression is more prominent in primary glioblastoma than in secondary glioblastoma (Nicholas et al. 2006).
In 1989, karyotypic studies and loss of heterozygosity identified tumor suppressor loci on chromosomes 9, 10, and 17 (James et al. 1989). These changes were later found to target the tumor suppressor gene TP53, located on chromosome 17, which monitors DNA damage, modulates apoptosis, controls cell cycle dynamics, and allows for repair of cells from injury or triggers cell death mechanisms (Rao and James 2004). Inactivation of the TP53 tumor suppressor gene occurs more frequently in secondary glioblastoma than in primary glioblastoma. However, primary glioblastoma shows a higher amplification of the murine double minute 2 gene MDM2. The protein MDM2 binds to TP53 and controls cell growth mediated by the p53 protein. Two other targets modified in GBMs and showing high significance were reported in 1993 and 1997, including the progression inhibitor of the cell cycle and tumor suppressor PTEN, which encodes the homologous proteins phosphatase and tensin. These genes were identified as tumor suppressor genes that are lost on chromosomes 9 and 10, respectively (Van Meir et al. 2010). The complete deletion or heterozygous loss on chromosome 10 or mutations in the tumor suppressor gene PTEN are more common in primary glioblastoma than in secondary glioblastoma.
The last common genetic alteration in GBM was described in 2008. Genes encoding isocitrate dehydrogenase 1 (IDH1) are mutated in low-grade gliomas and a group of GBMs (Dang et al. 2009). Mutations in IDH1 have an indirect oncogenic effect by activating a transcription factor induced by hypoxia (HIF1), allowing for metabolic adaptation of tumor cells under hypoxic and nutrient deprivation conditions (Jansen et al. 2010).
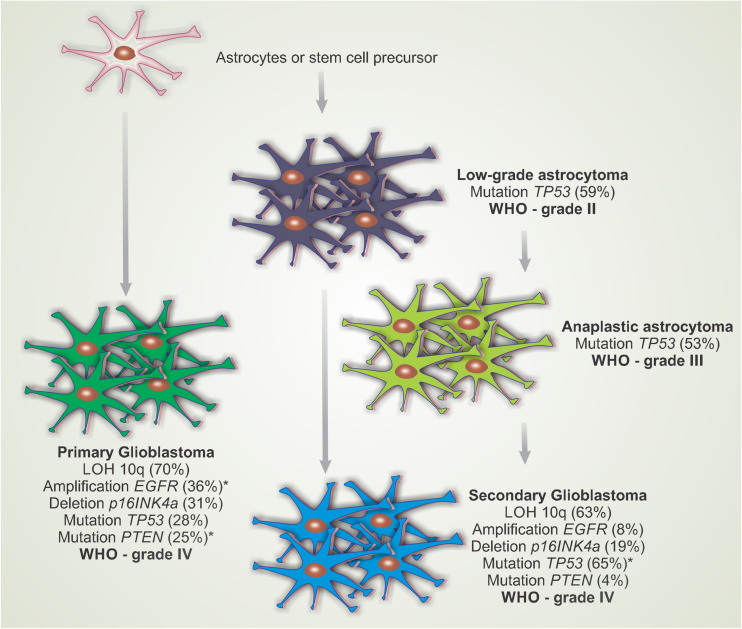
GBMs are classified as primary (originating from astrocytic cells or progenitor cells) or secondary (originating from tumor grade astrocytomas II and III) based on the process of gliomagenesis and signaling pathways that are altered in the tumor cells (Fig. 4). Several genetic changes occur in the progression of diffuse astrocytomas and events, such as EGFR gene amplification, and overexpression occurs in approximately 40–60% of primary glioblastomas, which are rare events in the progression of secondary glioblastomas (Ohgaki and Kleihues 2007).
Fig. 4.
Most common pathways of genetic alterations involved in the progression of diffuse astrocytomas [according to the World Health Organization (WHO) classification]. The diagram shows that primary glioblastoma manifests with no clinical or pathological evidence of malignant lesions and that a previous secondary glioblastoma develops from a low-grade astrocytoma or anaplastic astrocytoma. LOH 10q Loss of heterozygosity on chromosome 10q, EGFR epidermal growth factor receptor, TP53, PTEN, p16INKa tumor suppressor genes. Adapted from Ohgaki and Kleihues (2007)
In 2005, the pilot project Atlas of Cancers Genome [The Cancer Genome Atlas (TCGA); http://tcga.cancer.gov] was initiated, the largest such project developed to date. Because of the available high-efficiency and low-malignancy therapies, GBM was among the first tumor types analyzed by the TCGA. In 2008, the first set of data obtained by TCGA was published on GBMs (McLendon et al. 2008). Based on these data, cell death and three proliferation control pathways were found to be modified in GBMs, explaining more than 90% of the sources of these tumors. Specifically, the receptor tyrosine kinase/RAS/PI3K pathway was altered in 88% of GBMs, the p53 protein pathway was altered in 87% of GBMs, and the retinoblastoma protein (Rb) pathway was altered in 77% of GBMs.
Based on these results, primary and secondary gliomas share genetic alterations, but with some differences in the frequency at which they occur. Primary glioblastomas exhibit amplification of EGFR and a 36% mutation rate in the tumor suppressor gene PTEN in 25% of cases, while only 25% of primary tumors contain mutations in the tumor suppressor protein p53. This tendency changes in secondary GBMs: only 8% of cases show EGFR amplification, 4% have mutations in PTEN, and 65% show changes in TP53 (Van Meir et al., 2010). Distinctive features of both tumor types (primary and secondary) that arise from the deregulation of many intracellular signaling pathways and their common features, particularly increased cell proliferation, survival, and resistance to apoptosis and loss adhesion and migration, which may reveal a high grade of invasiveness (Merlo 2003; Wachsberger et al. 2012; Zahonero and Sánchez-Gómez 2014).
Prognosis and therapy
Compounds for the treatment of gliomas have been studied since the 1980s. Among these compounds are efficient chemotherapeutics for other types of solid tumors, such as cisplatin, carmustine, and lomustine ( Kaup et al. 2001; Kim et al. 2004; Natsume et al. 2008; Jiguet Jiglaire et al. 2014; Pastwa et al. 2014), as well as modulators of the immune system, cell metabolism and proliferation (EGFR), and angiogenesis [vascular EGFR (VEGFR)] (Atkins et al. 2015). However, none of these compounds effectively increased survival or improved patient prognosis, and they are no longer used as primary therapies for GBMs. Additionally, unlike other tumor types, the prognosis for patients with GBM has changed little in the last 10 years.
Grade IV tumors exhibit marked characteristics that include excessive proliferation, necrosis, genetic instability, and chemoresistance (Furnari et al. 2007). Because of these characteristics, GBMs are difficult to treat and have a poor prognosis with an average survival of less than 1 year (Collins et al. 2005).
The first breakthrough for the treatment of GBMs occurred after approval of the chemotherapy agent temozolomide in 2003, an alkylating agent which increased the survival of patients by 12–15 months (Stupp et al. 2005). The difficulties of processing GBMs are multifactorial and related to our current lack of understanding of disease resistance to drugs and ionizing radiation in addition to the blood–brain barrier (Kanu et al. 2009). Current treatment is based on a protocol developed by Stupp et al. (2009), which consists of surgical resection of the tumor, if possible, followed by radiotherapy (60 Gy typically in 2-Gy fractions) and concomitant chemotherapy with temozolomide (75 mg/m2 for 42 days). Thus, GBMs are often considered by oncologists as difficult to treat (Almeida et al. 2015; Missios et al. 2015; Tran and Rosenthal 2010).
Photodynamic therapy
Photodynamic therapy (PDT) is a treatment modality that employs a combination of light, a photosensitive drug, and molecular oxygen present in cells; when used independently these components do not exhibit toxicity to the body (Torchilin 2005; Somani et al. 2010; Lin et al. 2015). The treatment of skin tumors by PDT consists of first administering the drug, followed by irradiation with a monochromatic laser at the maximum absorption wavelength of the photosensitive drug, which is typically in the red region of the electromagnetic spectrum (Lin et al. 2015).
The primary objective of PDT is to induce the death of tumor tissue through a photosensitization process that will reduce tumor mass, while minimizing damage to surrounding tissue and possible side effects. These are the main advantages of PDT compared to the other classical treatments against cancer, such as chemotherapy, radiotherapy, and surgery. In the last decade, an increasing number of studies has examined PDT because of its recognition by the U.S. Food and Drug Administration as an effective therapy for the treatment of various diseases, including cancer (Retèl et al. 2009; Chen et al. 2013; Fan et al. 2014;).
Photosensitization occurs in a cell when a drug associated with the plasma membrane enters the cytosol passively by diffusion or osmosis or actively by active transport or endocytosis (Castano et al. 2004). PDT is based on photooxidative reactions in which a photosensitive drug, following excitation by light at an appropriate wavelength, causes the drug to change from its ground state (S0) to the first excited singlet state (S1), followed by intersystem crossing that leads to the triplet state (T1) according to the Jablonski diagram (Lakowicz and Masters 2008).
Photoactivation can promote the irreversible destruction of tumor tissue in three main ways: (1) production of reactive oxygen species (ROS) which directly cause the death of tumor cells by apoptosis and/or necrosis; (2) antivascular effects that can cause bleeding and thrombosis of tumor vessels, leading to the death of cancer cells by deprivation of oxygen and nutrients; (3) activation of an immune response against tumor cells by acute inflammation processes and the release of cytokines into the tumor, resulting in an influx of macrophages and leukocytes that may contribute to tumor destruction and stimulate the immune system to recognize and eliminate cancer cells (Ficheux 2009; Karioti and Bilia 2010; Ormond and Freeman 2013).
The mechanisms of PDT action are classified into Type I and Type II (Castano et al. 2004) and result from a combination of physical phenomena (light interaction with the molecule), chemical events (production of ROS), and biological aspects (tumor destruction) (Plaetzer et al. 2003). The Type I mechanism involves hydrogen atom abstraction or electron transfer reactions between the excited states of the photosensitive drug and a biological substrate, resulting in the production of free radicals and radical ions. These radicals are highly reactive to molecular oxygen-generating superoxide anions or hydroxyl radicals that cause irreparable damage to the tumor tissue (Plaetzer et al. 2003). The Type II mechanism involves energy transfer between the excited triplet state of the photosensitive drug and molecular oxygen, generating the first excited state of oxygen, singlet oxygen (1O2). This reactive molecule damages various cellular structures, such as by breaking genomic DNA and disrupting the plasma membrane, lysosomes, and mitochondria (Kübler et al. 2005; Wong et al. 2007; Ficheux 2009).
In vitro studies (de Paula et al. 2013, 2012, 2015) using nanoemulsion as a drug delivery system combined with a photosensitizer and PDT in a biological model of human glioblastoma cells were conducted by our group. Anaplastic astrocytoma (grade III) and glioblastoma (grade IV) cells (U343 and U87MG) were incubated with a chloroaluminum–phthalocyanine nanoemulsion (NE/ClAlPc: 0.5 μM) for 3 h. Activation of the photosensitizer delivered intracellularly by the NE was achieved by laser excitation of ClAlPc at 670 nm; subsequent cell death was measured by cell viability assays using the Giemsa method (Fig. 5).
Fig. 5.
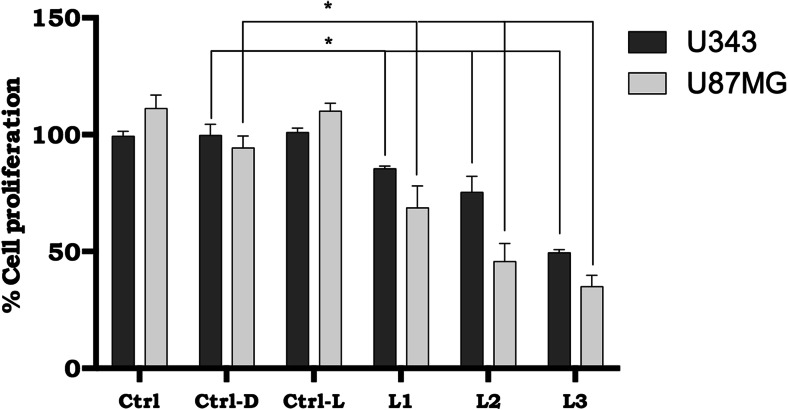
Presence of chloroaluminum–phthalocyanine nanoemulsion (NE/ClAlPc: 0.5 μM) under different irradiation conditions after 48 h of treatment of anaplastic astrocytoma (grade III) and glioblastoma (grade IV) (U343 and U87MG cell lines). Ctrl: Control cells cultured with 3% fetal bovine serum, Ctrl-D: control samples with NE/ClAlPc in the absence of sunlight light (darkness), Ctrl-L: control samples subjected to sunlight at a dose of 700 mJ/cm2 in the absence of NE/ClAlPc, L1: samples with NE/ClAlPc subjected to sunlight at a dose of 100 mJ/cm2, L2: samples with NE/ClAlPc subjected to sunlight at a dose of 200 mJ/cm2, L3: samples with NE/ClAlPc subjected to sunlight at a dose of 700 mJ/cm2. Statistical analysis was performed by two-way analysis of variance followed by Tukey’s test. Statistical significance was set at *p < 0.05. All data are expressed as the mean ± standard error of the mean of three independent experiments
U343 and U87MG cell lines were subjected to three treatments: (1) NE/ClAlPc in the absence of sunlight (dark assay), (2) sunlight at a dose of 700 mJ/cm2 in the absence of NE/ClAlPc, or (3) sunlight at doses of 100, 200, and 700 mJ/cm2, respectively, in the presence of NE/CIAIPc (NE/CIAIPc–PDT). After 3 h of incubation and after 48 h of treatment, the accumulation of NE/ClAlPc with PDT in U343 cells showed cell death rates of 85, 70, and 50% in the presence of sunlight at doses of 100, 200, and 700 mJ/cm2, while U87MG cells showed cell death rates of 75, 45, and 30 compared to the dark assay (Fig. 5).
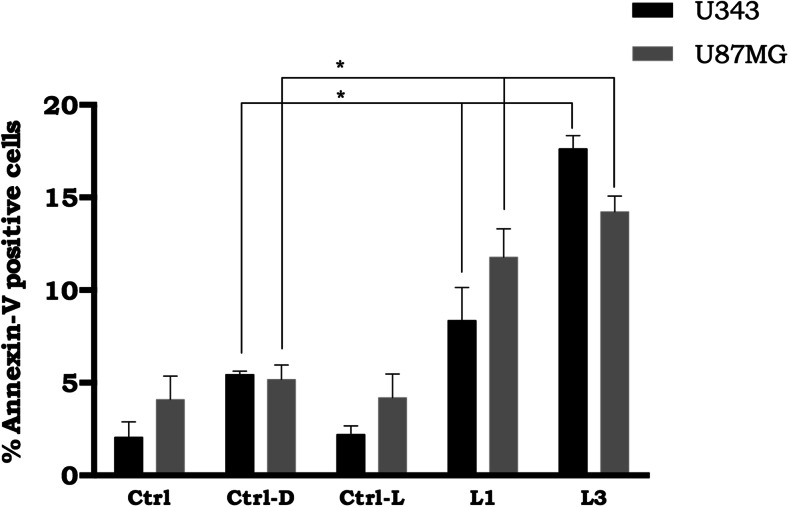
These data indicated that while NE/ClAlPc–PDT efficiently induced cell death in U343 and U87MG cells, NE/ClAlPc in the absence of sunlight light (dark assay) as well as the presence of sunlight at a dose of 700 mJ/cm2 in the absence of NE/ClAlPc alone did not cause such cytotoxicity. The cytotoxic effects observed after PDT with different doses of treatments represents not only decreased cell proliferation, but also cell death based on the percentage of apoptotic cells after labeling with annexin-V. The assay was performed within 48 h after treatment with two doses of sunlight, 100 (L1) and 700 (L2) mJ/cm2 (Fig. 6). For both doses, cells showed significant apoptosis rates (p < 0.05) following treatment (PDT) compared to cells that were only incubated with NE/ClAlPc (Ctrl-D). After 48 h of treatment, annexin-V staining increased by two-to threefold for the L1 and L3 treatments (U343 cells: 9.6 and 18%; U87MG cells: 12.8 and 15%) relative to the Ctrl-D treatment (darkness).
Fig. 6.
Cell death assay of glioblastoma lines by flow cytometry in the presence of NE/ClAlPc (0.5 μM) under different irradiation conditions after 48 h of treatment. Ctrl: Control samples cultured with 3% fetal bovine serum, Ctrl-D: control samples with NE/ClAlPc in the absence of sunlight light (darkness), Ctrl-L: control samples subjected to sunlight at a dose of 700 mJ/cm2 in the absence of NE/ClAlPc, L1: samples with NE/ClAlPc subjected to sunlight light at a dose of 100 mJ/cm2, L3: samples with NE/ClAlPc subjected to sunlight light at a dose of 700 mJ/cm2. Statistical significance was set at *p < 0.05
These results support the premise that combined drug delivery systems (DDS) and PDT are feasible for treating all grades of glioblastoma but that photosensitizers (ClAlPc) under proper DDS may address all tumor cells.
Drug delivery system
The effectiveness of a therapeutic device against cancer is measured by its ability to reduce and eliminate tumors without harming healthy surrounding tissue. The ultimate goal of cancer treatment is to increase survival and the quality of life of the patient. Thus, increasing local specificity and internalization may improve the effectiveness of treatment and patient adherence to treatment as well as reduce the possibility of common side effects experienced during traditional therapies. For a drug to be effective against a tumor, some physiological and biochemical barriers must be overcome, such as tumor drug resistance, distribution, biotransformation, and drug inactivation in the body (Lim et al. 2013; Almeida et al. 2014).
The cellular mechanisms of drug resistance include changes in the activities of specific enzyme systems involved in the regulation of apoptosis and transport mechanisms. One of these changes is the development of multiple drug resistance (MDR) (Tada 2007; Bae et al. 2011; del Burgo et al. 2014). MDR is a generic term used to define strategies adopted by cancer cells to inhibit the effects of conventional anticancer drugs; such strategies can be intrinsic or acquired during treatment (Jabr-Milane et al. 2008; del Burgo et al. 2014). MDR is multifactorial and may result in decreased intracellular accumulation of an antitumor substance, thereby preventing it from entering cancer cells at effective concentrations. This resistance mechanism of drug efflux occurs outside of cells, mainly because of overexpression of a transport protein known as P-glycoprotein, which nonspecifically carries several molecules outside of the cell or to other cell compartments (Persidis 1999; Wager et al. 2011; Rubenstein and Rakic 2013; Abdallah et al. 2015).
Nanoparticulate systems offer the mean to improve cancer therapy because they provide local specificity, the ability to avoid MDR, and an efficient means to release of anticancer drugs. A series of nanoparticulate systems are currently being explored for the treatment of cancer. These materials were designed to promote specific release at the tumor site. For example, hydrophobic surfaces can be used to enhance circulation time and to positively charge surfaces to enhance endocytosis (Oh et al. 2009; Bolfarini et al. 2014).
Nanoparticles used in studies against cancer include dendrimers (Primo et al. 2008; Nishiyama et al. 2009), liposomes (Siqueira-Moura et al. 2013; Bolfarini et al. 2014), nanoparticulate polymer, micelles, protein nanoparticles, ceramic nanoparticles, viral nanoparticles, metal nanoparticles (de Paula et al. 2012, 2015), and carbon nanotubes (Nishiyama et al. 2009; Spencer et al. 2015). Functionalization of these systems can be used to create a stealth surface opsonization, bind plasma proteins, and increase circulation time, thereby avoiding the particle removal from the blood circulation by the reticuloendothelial system.
Additionally, tumor tissues exhibit limited diffusion because of their rapid and uncontrolled cell proliferation. This limited spreading mainly affects the nutrition, excretion, and oxygenation of the tumor tissue. Incomplete vascularization of the tumor interstitial space results in the formation of tumors ranging in size from 100 nm to 1 μm, which are easily permeated by nanoparticles. A combination of poor vascularity and poor lymphatic drainage results in increased retention and permeation, which provides selective retention release by tumor nanoparticulate systems (Krasnici et al. 2003).
The development of DDS has been proposed as an excellent strategy for overcoming cellular resistance mechanisms and improving the selectivity of the drug for cancer cells while also reducing side effects (Chatterjee et al. 2008; Primo et al. 2008, 2012; de Paula et al. 2013, 2015; Bolfarini et al. 2014). These systems are also responsible for the stability of unstable cytotoxic compounds in a biological medium (Niziolek et al. 2003; Tada 2007). Lipid DDS, such as liposomes (Macaroff et al. 2005; Molnar et al. 2007; Sadzuka et al. 2008; Tapajós et al. 2008) and nanoparticle lipids significantly reduce drug scattering throughout the body and increase its concentration in tumor tissues (Chatterjee et al. 2008; Jabr-Milane et al. 2008; Bolfarini et al. 2012, 2014; de Paula et al. 2012).
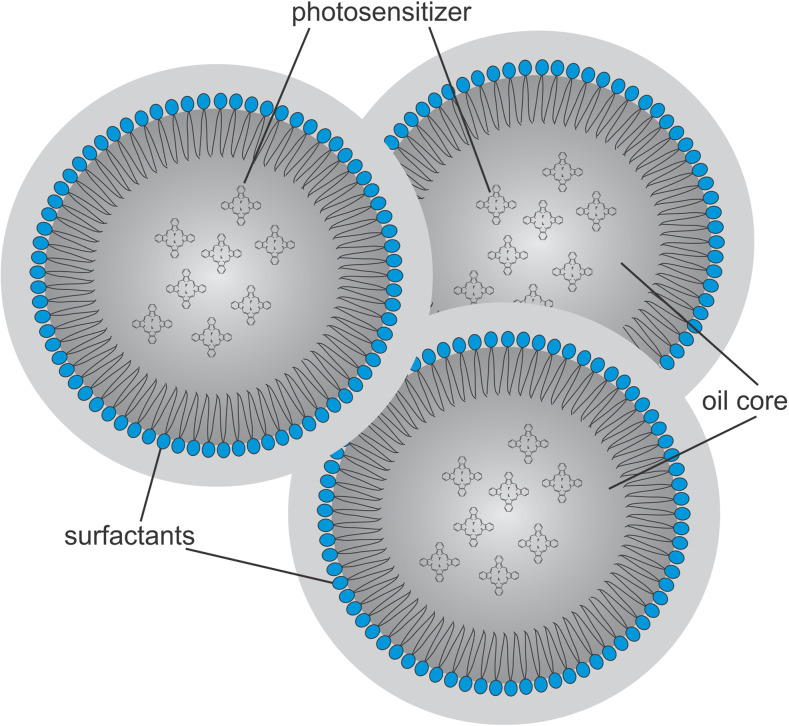
Nanoemulsions (NEs) are colloidal systems (Fig. 7) in which the internal phase is a dimensionally restricted microenvironment with specific properties that can connect to or associate with molecules of different polarities, acting as spherical aggregates with diameters of <1000 Å (de Oliveira et al. 2004; Primo et al. 2008). According to the pharmaceutical industry, NEs can be defined as emulsions that are typically transparent in which an oil or a lipophilic drug is dispersed in an aqueous medium containing a surfactant, with or without a cosurfactant, generating a thermodynamically stable system (Fig. 8).
Fig. 7.
Model representative of the structure of a colloidal particle of a nanoemulsion of oil and water
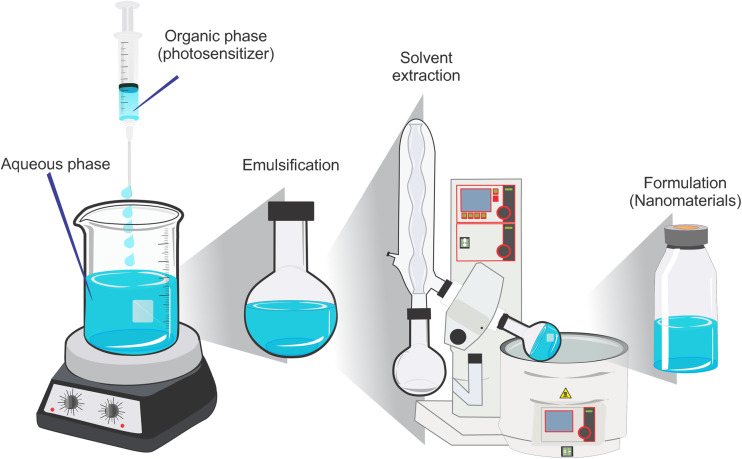
Fig. 8.
Steps used to prepare a nanoemulsion formulation
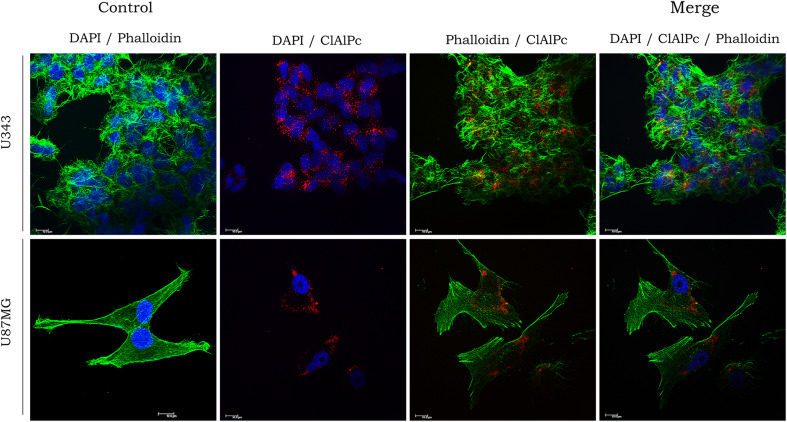
Confocal studies conducted by our laboratory using two cell cultures of anaplastic astrocytoma and glioblastoma (U343 and U87MG) facilitated the precise subcellular localization of ClAlPc in the designed DDS. U343 and U87MG cells internalized NE/ClAlPc after incubation for 3 h at 37 °C (Fig. 9). Subsequent analysis revealed that NE/ClAlPc–PDT is predominantly localized at the cytoplasmic level with a homogenous distribution. Cellular uptake of NE as a nanocarrier was also observed in mesenchymal stem cells (de Paula et al. 2015) and in the tissue of human glioblastomas (Trylcova et al. 2015). Muehlmann et al. (2014) also found that ClAlPc internalization was concentrated in the cytosol of cancerous and non-cancerous cells. These results also reinforce the use of PDT for treatment of late-stage GBM.
Fig. 9.
Subcellular localization of nanoemulsion loaded with photosensitive drug (NE/ClAlPc) in in vitro U343 and U87MG cells. DAPI (blue) nuclear staining; phalloidin (green) labeling of actin filaments, ClAlPc (red) labeling by nanoemulsion loaded with ClAlPc
These findings indicate the potential of a multifunctional DDS associated with PDT for targeting malignant tumor cells, mainly glioblastomas, and reducing photosensitizer cytotoxicity within the surrounding milieu.
Photosensitive drugs: phthalocyanines
In recent years, research in the field for PDT has focused on development studies and the administration and biodistribution of possible candidates for photosensitive drugs employable in the photochemical–therapeutic treatment of tumor tissue (Saavedra et al. 2014; Lucky et al. 2015). Tumoral photosensitive drugs typically show high chemical purity, a high coefficient of molar extinction in the red region (620–650 nm) of the electromagnetic spectrum, a low tendency to aggregate in aqueous medium, high quantum yield of singlet oxygen and other ROS, good selectivity, fast absorption into the body with low or complete absence of toxicity in the dark, minimum accumulation in the skin, and the absence of mutagenic potential (Rosenthal and Ben-Hur 1995).
First-generation photosensitive drugs included derivatives of hematoporphyrin compounds (Yang et al. 2013; Ren et al. 2014). However, the low selectivity towards neoplastic tissue and weak absorption in the red region have led to the development of new molecules. Second- and third-generation photosensitizers include phthalocyanine, chlorins, and bacteriochlorins. Among second-generation compounds, phthalocyanines have received particular attention because of their strong absorption band in the range of 600–800 nm or in the absorption range known as the “therapeutic window”, as this spectral range the light penetrates tissue in the absence of interference from endogenous chromophores in a biological system (Sharman et al. 1999).
The absorption spectrum of phthalocyanines is formed by a surface set of bands known as the B-bands (or Soret band) and Q-bands, which are in the ultraviolet region or ultraviolet radiation (300–350 nm) and sunlight light region (UV-VIS) (600–700 nm) (Siqueira-Moura et al. 2010, 2013). The chemical structure of phthalocyanine mimics that of porphyrins, with a central macrocycle consisting of a cyclic tetrapirrólica unit (Siqueira-Moura et al. 2010). However, in pyrrolic phthalocyanines, the subunits are joined by hydrogen atoms, whereas porphyrin subunits are linked via a methylene bridge (Nonell et al. 1995; Lo et al. 2008). The phthalocyanine macrocycle is extended by a combination of pyrrolic units composed of four benzene rings, resulting in a strong absorption band in the red region of the sunlight spectrum (650–680 nm). Complexation of phthalocyanines with diamagnetic metal ions, such as Zn2+ and Al3+, produces a complex with a long lifetime in the excited singlet and triplet states, good quantum yield, and excellent photochemical and photodynamic activities (Idowu and Nyokong 2007).
Among the metal phthalocyanines, ClAlPc presents the best photophysical characteristics, with a long lifetime in the excited state and high quantum yield of singlet oxygen; it also shows good selectivity for tumor tissue and has a low toxicity (Rosenthal 1991). However, the primary factor limiting the use of ClAlPc in PDT is its significant hydrophobicity, which renders it impossible to administer in the physiological environment. To overcome this problem, substantial efforts have been directed towards the development of DDS using nanotechnology (Konan et al. 2002).
An increasing number of studies have examined the association between DDS and PDT, mainly to better understand the mechanism and biology of cancer through the gene expression of distinct signaling pathways that are critical to tumor development and progression. Many of these signaling pathways involve EGF. EGFR signaling has been found to be important in the pathogenesis of many cancers, particularly glioblastoma, and many tumor types show overexpression or abnormal activity of EGFR (Libermann et al. 1985; Mellinghoff et al. 2005; Zahonero and Sánchez-Gómez 2014), leading to the unregulated growth of cells and their malignant transformation.
EGFR is overexpressed or mutated in approximately 60% of primary glioblastomas, but in only 8% of secondary glioblastomas (Nicholas et al. 2006), suggesting that activation of EGFR is linked to tumorigenesis in primary GBMs. The increased expression of this receptor may result from gene amplification, increased translation, or both (Huang et al. 2009). EGFR overexpression in primary GBMs is occasionally accompanied by increased secretion of its ligands. For example, many glioma cells produce EGF and transforming growth factor alpha, which activates EGFRs in an autocrine manner, resulting in deregulated signaling via EGFR (Ekstrand et al. 1991). The binding of EGF-like molecules to the extracellular domain of the receptor induces activation and subsequent formation of homo- or heterodimers. Tyrosine residues in the intracellular domain of a receptor are phosphorylated cross-members on the other dimer, recruiting cellular signaling proteins that bind to them and propagating the signal (Yarden 2001; Yarden and Sliwkowski 2001). The main pathways activated by these receptors, PI3K/Akt and Ras/MAPK, are involved in oncogenesis (Nicholas et al. 2006) (Fig. 10).
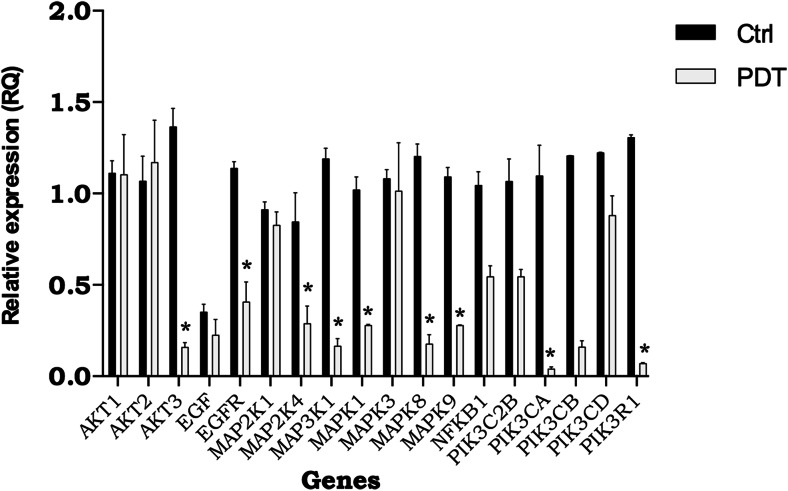
Fig. 10.
Gene expression analysis of genes involved in the epidermal growth factor (EGF) signaling pathway in glioblastoma cells (U87MG cell line) after 24 h of treatment. Ctrl: Control samples cultured cells with 3% fetal bovine serum, PDT: (photodynamic therapy) samples NE/ClAlPc subjected to sunlight at a dose of 100 mJ/cm2. Genes: AKT1, AKT2, AKT3: RAC–alpha serine/threonine-protein kinase, EFGR: epidermal growth factor receptor, MAP2K1: mitogen-activated protein kinase kinase 1, MAP2K4: mitogen-activated protein kinase kinase 4, MAP3K1: mitogen-activated protein kinase kinase kinase 1, MAPK1: mitogen-activated protein kinase 1, MAPK3: mitogen-activated protein kinase 3, MAPK8: mitogen-activated protein kinase 8, MAPK9: mitogen-activated protein kinase 9, NFKB1: nuclear factor NF-kappa-B p105 subunit, PIK3C2B: phosphatidylinositol-4-phosphate 3-kinase C2 domain-containing beta polypeptide, PIK3CA: phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha, PIK3CB: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta isoform, PIK3CD: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta isoform, PIK3R1: phosphatidylinositol 3-kinase regulatory subunit alpha. Statistical significance was set at *p < 0.05
Expression analysis of genes involved in the EGF pathway is expected to reveal several possible associations between the expression profiles of glioblastoma lines in tumor progression. This will increase our understanding of cancer biology and lead to developments that can be used to treat patients more efficiently and with reduced side effects. Further studies of drug delivery associated with PDT systems should be performed, particularly in vivo, to consolidate new alternatives for therapeutic procedures in cancer treatment.
Future prospects
Careful monitoring of GBM patients, particularly by neuroimaging, is essential for providing optimal care and conducting high-quality clinical trials. The introduction of new therapies targeting angiogenesis and affecting vascular permeability, as well as radiotherapy and immunotherapies that produce “pseudoprogression,” have complicated the assessment of response and progression.
Nanomedicines associated with nanoparticles have emerged as potential tools for the delivery of molecules into the brain and can overcome problems associated with current strategies. Nanoparticles can serve as targeted delivery devices for novel therapies, including PDT, gene therapy, radiotherapy, anti-angiogenic therapy, and thermotherapy. The results of our previous studies clearly demonstrate the potential of nanoparticles to play key roles in the diagnosis and imaging of brain tumors by revolutionizing both pre-operative and intraoperative brain tumor detection, allowing for early detection of pre-cancerous cells and providing real-time, longitudinal, non-invasive monitoring/imaging of treatment effects. A combination of nanotechnology, tissue engineering, and photoprocessing will be essential for supporting Regenerative Medicine of the twenty-first century. Additional efforts should focus on developing biomaterial systems that are uniquely capable of delivering tumor-associated antigens and immunotherapeutic agents or programming immune cells in situ to facilitate immune-mediated tumor cell killing.
The volume of residual tumor after surgical resection also appears to be a significant adverse prognostic factor for the recurrence of high-grade glioma, and complete tumor resection may be the next logical step in managing these locally malignant cancers. It is essential that the bulk of the tumor be removed and that the post-resection cavity is treated by PDT. PDT-associated surgical resection may be particularly suitable because improved local control can result in increased survival. The goals of developing synergic nanoparticles combining chemotherapeutic agents and photoactive compounds are the same as those of DDS. Phthalocyanine and its derivative molecules, as well as third-generation dyes, are the most commonly used photosensitizers in brain tumors. Several photosensitizers have been tested, but these are not currently licensed for treating brain tumors in most countries, and their use in brain tumors is off-label on an individual patient basis. PDT is effective because photosensitizers are preferentially taken up and retained by brain tumor cells. Many new methods of light delivery are also being actively studied.
Studies aim to develop tools for early diagnosis which play an essential role in every stage of brain tumors PDT, from disease detection to treatment planning, dosimetry, therapy monitoring, and outcome assessment. As demonstrated in in vivo models, imaging (anatomic, functional, and metabolic) combined with PDT offers the potential to revolutionize therapeutic strategies for brain tumors. Clinical imaging before the use of PDT appears to be an essential step in therapeutic planning. The functional information gathered through such pre-treatment imaging is important for treatment planning, light delivery, and treatment dose assessment.
Acknowledgments
The study was supported by grants from the Brazilian agencies, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) Thematic Project (# 2013/50181-1 A.C.T.), and Post Doc project (# 2015/18684-9 L.B.P). The authors also thank Financiadora de Estudos e Projeto (FINEP) 01.10.0758.01 and CNPq-National Institute of Science and Technology-INCT of Nanobiotechnology (Project # 573880/2008-5 A.C.T.).
Compliance with ethical standards
Conflict of interest statement
The authors declare that thhe have no conflicts of interest regarding this article.
Ethical approval statement
This article does not contain any studies with human participants or animals performed by the authors.
Footnotes
This article is part of a Special Issue on ‘Latin America’ edited by Pietro Ciancaglini and Rosangela Itri.
References
- Abdallah HM, Al-Abd AM, El-Dine RS, El-Halawany AM. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J Adv Res. 2015;6:45–62. doi: 10.1016/j.jare.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JP, Chaichana KL, Rincon-Torroella J, Quinones-Hinojosa A. The value of extent of resection of glioblastomas: clinical evidence and current approach. Curr Neurol Neurosci Rep. 2015;15:1–13. doi: 10.1007/s11910-014-0517-x. [DOI] [PubMed] [Google Scholar]
- Almeida JPM, Figueroa ER, Drezek RA. Gold nanoparticle mediated cancer immunotherapy. Nanomedicine. 2014;10:503–514. doi: 10.1016/j.nano.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins RJ, Ng W, Stylli SS, Hovens CM, Kaye AH. Repair mechanisms help glioblastoma resist treatment. J Clin Neurosci. 2015;22:14–20. doi: 10.1016/j.jocn.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Bae KH, Chung HJ, Park TG (2011) Nanomaterials for cancer therapy and imaging. Mol Cells 31:295–302 [DOI] [PMC free article] [PubMed]
- Bolfarini GC, Siqueira-Moura MP, Demets GJ, Morais PC, Tedesco AC. In vitro evaluation of combined hyperthermia and photodynamic effects using magnetoliposomes loaded with cucurbituril zinc phthalocyanine complex on melanoma. J Photochem Photobiol B Biol. 2012;115:1–4. doi: 10.1016/j.jphotobiol.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Bolfarini GC, Siqueira-Moura MP, Demets GJ, Tedesco AC. Preparation, characterization, and in vitro phototoxic effect of zinc phthalocyanine cucurbit [7] uril complex encapsulated into liposomes. Dyes Pigments. 2014;100:162–167. [Google Scholar]
- Brat DJ, Scheithauer BW, Fuller GN, Tihan T. Newly codified glial neoplasms of the 2007 WHO classification of tumours of the central nervous system: angiocentric glioma, pilomyxoid astrocytoma and pituicytoma. Brain Pathol. 2007;17:319–324. doi: 10.1111/j.1750-3639.2007.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008;60:1627–1637. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Chen J, Shao R, Zhang XD, Chen C. Applications of nanotechnology for melanoma treatment, diagnosis, and theranostics. Int J Nanomedicine. 2013;8:2677. doi: 10.2147/IJN.S45429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739–744 [DOI] [PMC free article] [PubMed]
- de Oliveira AG, Scarpa MV, Correa MA, Cera LFR, Formariz TP. Microemulsões: estrutura e aplicações como sistema de liberação de fármacos. Química Nova. 2004;27:131–138. [Google Scholar]
- de Paula LB, Primo FL, Jardim DR, Morais PC, Tedesco AC. Development, characterization, and in vitro trials of chloroaluminum phthalocyanine-magnetic nanoemulsion to hyperthermia and photodynamic therapies on glioblastoma as a biological model. J Appl Phys. 2012;111:07B307. [Google Scholar]
- de Paula CS, Tedesco AC, Primo FL, Vilela JMC, Andrade MS, Mosqueira VCF. Chloroaluminium phthalocyanine polymeric nanoparticles as photosensitisers: photophysical and physicochemical characterisation, release and phototoxicity in vitro. Eur J Pharm Sci. 2013;49:371–381. doi: 10.1016/j.ejps.2013.03.011. [DOI] [PubMed] [Google Scholar]
- de Paula LB, Primo FL, Pinto MR, Morais PC, Tedesco AC. Combination of hyperthermia and photodynamic therapy on mesenchymal stem cell line treated with chloroaluminum phthalocyanine magnetic-nanoemulsion. J Magn Magn Mater. 2015;380:372–376. [Google Scholar]
- del Burgo LS, Pedraz J, Orive G. Advanced nanovehicles for cancer management. Drug Discov Today. 2014;19:1659–1670. doi: 10.1016/j.drudis.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor α, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- Fan Z, Fu PP, Yu H, Ray PC. Theranostic nanomedicine for cancer detection and treatment. J Food Drug Anal. 2014;22:3–17. doi: 10.1016/j.jfda.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficheux H (2009) Photodynamic therapy: principles and therapeutic indications. Ann Pharmaceut Fr 1:32–40 [DOI] [PubMed]
- Fuller GN, Scheithauer BW. The 2007 revised World Health Organization (WHO) classification of tumours of the central nervous system: newly codified entities. Brain Pathol. 2007;17:304–307. doi: 10.1111/j.1750-3639.2007.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM et al (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21:2683–2710 [DOI] [PubMed]
- Huang PH, Xu AM, White FM (2009) Oncogenic EGFR signaling networks in glioma. Sci Signal 2:re6. doi: 10.1126/scisignal.287re6 [DOI] [PubMed]
- Idowu M, Nyokong T. Photophysical and photochemical properties of zinc and aluminum phthalocyanines in the presence of magnetic fluid. J Photochem Photobiol A Chem. 2007;188:200–206. [Google Scholar]
- Jabr-Milane L, van Vlerken L, Devalapally H, Shenoy D, Komareddy S, Bhavsar M, Amiji M. Multi-functional nanocarriers for targeted delivery of drugs and genes. J Control Release. 2008;130:121–128. doi: 10.1016/j.jconrel.2008.04.016. [DOI] [PubMed] [Google Scholar]
- James CD, Carlbom E, Nordenskjold M, Collins VP, Cavenee WK. Mitotic recombination of chromosome 17 in astrocytomas. Proc Natl Acad Sci USA. 1989;86:2858–2862. doi: 10.1073/pnas.86.8.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M, Yip S, Louis DN. Molecular pathology in adult gliomas: diagnostic, prognostic, and predictive markers. Lancet Neurol. 2010;9:717–726. doi: 10.1016/S1474-4422(10)70105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiguet Jiglaire C, Baeza-Kallee N, Denicolaï E et al (2014) Ex vivo cultures of glioblastoma in three-dimensional hydrogel maintain the original tumor growth behavior and are suitable for preclinical drug and radiation sensitivity screening. Exp Cell Res 321:99–108 [DOI] [PubMed]
- Kanu OO, Hughes B, Di C, Lin N, Fu J, Bigner DD, Yan H et al (2009) Glioblastoma multiforme oncogenomics and signaling pathways. Clin Med Oncol 3:39–52 [DOI] [PMC free article] [PubMed]
- Karioti A, Bilia AR. Hypericins as potential leads for new therapeutics. Int J Mol Sci. 2010;11:562–594. doi: 10.3390/ijms11020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup B, Schindler I, Knüpfer H, Schlenzka A, Preiβ R, Knüpfer M. Time-dependent inhibition of glioblastoma cell proliferation by dexamethasone. J Neuro-Oncol. 2001;51:105–110. doi: 10.1023/a:1010684921099. [DOI] [PubMed] [Google Scholar]
- Kim JW, Kim SY, Park SY, Kim YM, Kim JM, Lee MH, Ryu HM. Mesenchymal progenitor cells in the human umbilical cord. Ann Hematol. 2004;83:733–738. doi: 10.1007/s00277-004-0918-z. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Webster K, Cavenee WK (2000) Pathology and genetics of tumours of the nervous system. International Agency for Research on Cancer Press, Lyon
- Konan YN, Gurny R, Allémann E. State of the art in the delivery of photosensitizers for photodynamic therapy. J Photochem Photobiol B Biol. 2002;66:89–106. doi: 10.1016/s1011-1344(01)00267-6. [DOI] [PubMed] [Google Scholar]
- Krakstad C, Chekenya M. Survival signalling and apoptosis resistance in glioblastomas: opportunities for targeted therapeutics. Mol Cancer. 2010;9:135. doi: 10.1186/1476-4598-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnici S, Werner A, Eichhorn ME et al (2003) Effect of the surface charge of liposomes on their uptake by angiogenic tumor vessels. Int J Cancer 105:561–567 [DOI] [PubMed]
- Kübler A, Niziol C, Sidhu M, Dünne A, Werner J. Analysis of cost effectiveness of photodynamic therapy with Foscan (Foscan-PDT) in comparison with palliative chemotherapy in patients with advanced head-neck tumors in Germany. Laryngo-Rhino-Otologie. 2005;84:725–732. doi: 10.1055/s-2005-861048. [DOI] [PubMed] [Google Scholar]
- Lakowicz JR, Masters BR. Principles of fluorescence spectroscopy. J Biomed Opt. 2008;13:9901. [Google Scholar]
- Libermann TA, Nusbaum HR, Razon N et al (1985) Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature 313:144–147 [DOI] [PubMed]
- Lim CK, Heo J, Shin S et al (2013) Nanophotosensitizers toward advanced photodynamic therapy of cancer. Cancer Lett 334:176–187 [DOI] [PubMed]
- Lin L, Xiong L, Wen Y et al (2015) Active targeting of nano-photosensitizer delivery systems for photodynamic therapy of cancer stem cells. J Biomed Nanotechnol 11:531–554 [DOI] [PubMed]
- Lo PC, Fong WP, Ng DK. Effects of peripheral chloro substitution on the photophysical properties and in vitro photodynamic activities of galactose-conjugated silicon (IV) phthalocyanines. ChemMedChem. 2008;3:1110–1117. doi: 10.1002/cmdc.200800042. [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109 [DOI] [PMC free article] [PubMed]
- Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- Macaroff PP, Oliveira DM, Ribeiro KF, Lacava ZG, Lima EC, Morais PC, Tedesco AC. Studies of cell toxicity of complexes of magnetic fluids and biological macromolecules. J Magn Magn Mater. 2005;293:293–297. [Google Scholar]
- Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- McLendon R, Friedman A, Bigner D, et al (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455(7216):1061–1068 [DOI] [PMC free article] [PubMed]
- Mellinghoff IK, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- Merlo A. Genes and pathways driving glioblastomas in humans and murine disease models. Neurosurg Rev. 2003;26:145–158. doi: 10.1007/s10143-003-0267-8. [DOI] [PubMed] [Google Scholar]
- Missios S, Abbassy M, Vogelbaum MA, Recinos PF. Use of image fluorescence in the resection of gliomas. Current Surgery Reports. 2015;3:1–6. [Google Scholar]
- Molnar A, Dědic R, Svoboda A, Hala J. Singlet oxygen production by lipophilic photosensitizers in liposomes studied by time and spectral resolved phosphorescence. J Mol Struct. 2007;834:488–491. [Google Scholar]
- Muehlmann LA, Ma BC, Longo JPF, Santos MFMA, Azevedo RB. Aluminum–phthalocyanine chloride associated to poly (methyl vinyl ether-co-maleic anhydride) nanoparticles as a new third-generation photosensitizer for anticancer photodynamic therapy. Int J Nanomed. 2014;9:1199. doi: 10.2147/IJN.S57420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato Y. The 4th edition of WHO classification of tumours of the central nervous system published in 2007 no shinkei geka. Neurol Surg. 2008;36:473. [PubMed] [Google Scholar]
- Natsume A, Wakabayashi T, Ishii D et al (2008) A combination of IFN-β and temozolomide in human glioma xenograft models: implication of p53-mediated MGMT downregulation. Cancer Chemother Pharmacol 61:653–659 [DOI] [PubMed]
- Nicholas MK, Lukas RV, Jafri NF, Faoro L, Salgia R. Epidermal growth factor receptor–mediated signal transduction in the development and therapy of gliomas. Clin Cancer Res. 2006;12:7261–7270. doi: 10.1158/1078-0432.CCR-06-0874. [DOI] [PubMed] [Google Scholar]
- Nishiyama N, Morimoto Y, Jang W-D, Kataoka K. Design and development of dendrimer photosensitizer-incorporated polymeric micelles for enhanced photodynamic therapy. Adv Drug Deliv Rev. 2009;61:327–338. doi: 10.1016/j.addr.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Niziolek M, Korytowski W, Girotti AW. Nitric oxide inhibition of free radical-mediated lipid peroxidation in photodynamically treated membranes and cells. Free Radic Biol Med. 2003;34:997–1005. doi: 10.1016/s0891-5849(03)00026-1. [DOI] [PubMed] [Google Scholar]
- Nonell S, Bou N, Borrell J, Teixidó J, Villanueva A, Juarranz A, Cañete M. Synthesis of 2, 7, 12, 17-tetraphenylporphycene (TPPo). First aryl-substituted porphycene for the photodynamic therapy of tumors. Tetrahedron Lett. 1995;36:3405–3408. [Google Scholar]
- Oh JM, Choi SJ, Lee GE, Han SH, Choy JH. Inorganic drug delivery nanovehicle conjugated with cancer cell-specific ligand. Adv Funct Mater. 2009;19:1617–1624. [Google Scholar]
- Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormond AB, Freeman HS. Dye sensitizers for photodynamic therapy. Materials. 2013;6:817–840. doi: 10.3390/ma6030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastwa E, Poplawski T, Lewandowska U, Somiari SB, Blasiak J, Somiari RI. Wortmannin potentiates the combined effect of etoposide and cisplatin in human glioma cells. Int J Biochem Cell Biol. 2014;53:423–431. doi: 10.1016/j.biocel.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Persidis A. Cancer multidrug resistance. Nat Biotechnol. 1999;17:94–95. doi: 10.1038/5289. [DOI] [PubMed] [Google Scholar]
- Plaetzer K, Kiesslich T, Verwanger T, Krammer B. The modes of cell death induced by PDT: an overview. Medical Laser Application. 2003;18:7–19. [Google Scholar]
- Preston-Martin S. Epidemiology of primary CNS neoplasms. Neurol Clin. 1996;14:273–290. doi: 10.1016/s0733-8619(05)70256-5. [DOI] [PubMed] [Google Scholar]
- Primo FL, Bentley MV, Tedesco AC. Photophysical studies and in vitro skin permeation/retention of Foscan®/nanoemulsion (NE) applicable to photodynamic therapy skin cancer treatment. J Nanosci Nanotechnol. 2008;8:340–347. [PubMed] [Google Scholar]
- Primo FL, De Paula LB, de Siqueira-Moura MP, Tedesco AC. Photobiostimulation on wound healing treatment by ClAlPc-nanoemulsion from a multiple-wavelength portable light source on a 3D-human stem cell dermal equivalent. Curr Med Chem. 2012;19:5157–5163. doi: 10.2174/092986712803530502. [DOI] [PubMed] [Google Scholar]
- Pytel P, Lukas RV. Update on diagnostic practice: tumors of the nervous system. Archives of Pathology & Laboratory Medicine. 2009;133:1062–1077. doi: 10.5858/133.7.1062. [DOI] [PubMed] [Google Scholar]
- Rao RD, James CD (2004) Altered molecular pathways in gliomas: an overview of clinically relevant issues. In: Seminars in Oncology, vol 5. Elsevier, New York Amsterdam, pp 595–604 [DOI] [PubMed]
- Ren Y, Wang R, Liu Y et al (2014) A hematoporphyrin-based delivery system for drug resistance reversal and tumor ablation. Biomaterials 35:2462–2470 [DOI] [PubMed]
- Retèl VP, Hummel MJ, van Harten WH. Review on early technology assessments of nanotechnologies in oncology. Mol Oncol. 2009;3:394–401. doi: 10.1016/j.molonc.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal I. Phthalocyanines as photodynamic sensitizers. Photochem Photobiol. 1991;53:859–870. doi: 10.1111/j.1751-1097.1991.tb09900.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal I, Ben-Hur E. Role of oxygen in the phototoxicity of phthalocyanines. Int J Radiat Biol. 1995;67:85–91. doi: 10.1080/09553009514550111. [DOI] [PubMed] [Google Scholar]
- Rubenstein J, Rakic P (eds) (2013) Cellular migration and formation of neuronal connections. Comprehensive developmental neuroscience, vol 2. Academic Press, New York vol 2
- Saavedra R, Rocha LB, Dąbrowski JM, Arnaut LG. Modulation of biodistribution, pharmacokinetics, and photosensitivity with the delivery vehicle of a bacteriochlorin photosensitizer for photodynamic therapy. ChemMedChem. 2014;9:390–398. doi: 10.1002/cmdc.201300449. [DOI] [PubMed] [Google Scholar]
- Sadzuka Y, Iwasaki F, Sugiyama I et al (2008) Phototoxicity of coproporphyrin as a novel photodynamic therapy was enhanced by liposomalization. Toxicol Lett 182:110–114 [DOI] [PubMed]
- Sharman WM, Allen CM, Van Lier JE. Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today. 1999;4:507–517. doi: 10.1016/s1359-6446(99)01412-9. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID et al (2004) Identification of human brain tumour initiating cells. Nature 432:396–401 [DOI] [PubMed]
- Siqueira-Moura MP, Primo FL, Espreafico EM, Tedesco AC. Development, characterization, and photocytotoxicity assessment on human melanoma of chloroaluminum phthalocyanine nanocapsules. Mater Sci Eng C. 2013;33:1744–1752. doi: 10.1016/j.msec.2012.12.088. [DOI] [PubMed] [Google Scholar]
- Siqueira-Moura MP, Primo FL, Peti APF, Tedesco AC. Validated spectrophotometric and spectrofluorimetric methods for determination of chloroaluminum phthalocyanine in nanocarriers. Pharmazie. 2010;65:9–14. [PubMed] [Google Scholar]
- Somani B, Moseley H, Eljamel M, Nabi G, Kata S. Photodynamic diagnosis (PDD) for upper urinary tract transitional cell carcinoma (UT-TCC): evolution of a new technique. Photodiagn Photodyn Ther. 2010;7:39–43. doi: 10.1016/j.pdpdt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Spencer DS, Puranik AS, Peppas NA. Intelligent nanoparticles for advanced drug delivery in cancer treatment. Curr Opin Chem Eng. 2015;7:84–92. doi: 10.1016/j.coche.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996 [DOI] [PubMed]
- Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466 [DOI] [PubMed]
- Tada DB (2007) Desenvolvimento de nanopartículas fotossensibilizadoras. Universidade de São Paulo, São Paulo
- Tapajós EC, Longo JP, Simioni AR et al (2008) In vitro photodynamic therapy on human oral keratinocytes using chloroaluminum-phthalocyanine. Oral Oncol 44:1073–1079 [DOI] [PubMed]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- Tran B, Rosenthal M. Survival comparison between glioblastoma multiforme and other incurable cancers. J Clin Neurosci. 2010;17:417–421. doi: 10.1016/j.jocn.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Trylcova J, Busek P, Smetana Jr K, Balaziova E, Dvorankova B, Mifkova A, Sedo A (2015) Effect of cancer-associated fibroblasts on the migration of glioma cells in vitro. Tumor Biol 36(8):5873–5879 [DOI] [PubMed]
- Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsberger PR, Lawrence YR, Liu Y, Daroczi B, Xu X, Dicker AP. Epidermal growth factor receptor expression modulates antitumor efficacy of vandetanib or cediranib combined with radiotherapy in human glioblastoma xenografts. Int J Radiat Oncol Biol Phys. 2012;82:483–491. doi: 10.1016/j.ijrobp.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Wager TT, Villalobos A, Verhoest PR, Hou X, Shaffer CL. Strategies to optimize the brain availability of central nervous system drug candidates. Expert Opin Drug Discovery. 2011;6:371–381. doi: 10.1517/17460441.2011.564158. [DOI] [PubMed] [Google Scholar]
- Wong KK, deLeeuw RJ, Dosanjh NS et al (2007) A comprehensive analysis of common copy-number variations in the human genome. Am J Hum Genet 80:91–104 [DOI] [PMC free article] [PubMed]
- Yang Y-T, Chen C-T, Tsai T. Absorption and fluorescence spectral properties of hematoporphyrin in liposomes, micelles, and nanoparticles. Dyes Pigments. 2013;96:763–769. [Google Scholar]
- Yarden Y. The EGFR family and its ligands in human cancer: signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37:3–8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Zahonero C, Sánchez-Gómez P (2014) EGFR-dependent mechanisms in glioblastoma: towards a better therapeutic strategy. Cell Mol Life Sci:1–24 [DOI] [PMC free article] [PubMed]