Abstract
Based on passive cable theory, an increase in membrane conductance produces a decrease in the membrane time constant and input resistance. Unlike the classical leak currents, voltage-dependent currents have a nonlinear behavior which can create regions of negative conductance, despite the increase in membrane conductance (permeability). This negative conductance opposes the effects of the passive membrane conductance on the membrane input resistance and time constant, increasing their values and thereby substantially affecting the amplitude and time course of postsynaptic potentials at the voltage range of the negative conductance. This paradoxical effect has been described for three types of voltage-dependent inward currents: persistent sodium currents, L- and T-type calcium currents and ligand-gated glutamatergic N-methyl-D-aspartate currents. In this review, we describe the impact of the creation of a negative conductance region by these currents on neuronal membrane properties and synaptic integration. We also discuss recent contributions of the quasi-active cable approximation, an extension of the passive cable theory that includes voltage-dependent currents, and its effects on neuronal subthreshold properties.
Keywords: Passive cable theory, Voltage-dependent inward currents, Neuronal membrane, Synaptic integration, Neuronal subthreshold properties
What is a negative slope conductance?
Ion channels are considered to be biological conductors in the neuronal membrane. By definition, the conductance of a single channel is positive following the linear Ohm’s law. According to the passive cable model of the biological membrane, the opening of the channels increases ionic permeability and decreases membrane resistance (R m) and the time constant (τ m). Each neuronal type has a distinct membrane resistance which affects its excitability, integrative and temporal properties. Potassium background leak channels are considered to be the classical determinants of R m (Enyedi and Czirják 2010). For example, in a heterogeneous neuronal population, membrane input resistance, action potential latency and threshold are inversely correlated with the expression of potassium leak currents (Dagostin et al. 2015). It is also well known that volatile anesthetics decrease membrane excitability by opening neuronal potassium leak channels (Ries and Puil 1999).
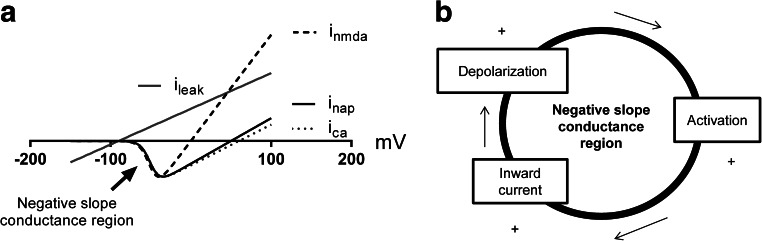
Ohmic leak currents have a linear current–voltage relationship (the I–V curve), producing a positive slope conductance (Fig. 1). However, voltage-gated channels present nonlinear I–V relationships and under certain circumstances can create regions of negative slope conductance by a positive feedback between a change of the membrane potential and the activation variables (Hutcheon and Yarom 2000; Izhikevich 2007; Remme and Rinzel 2011). In terms of current behavior, a negative slope conductance means that an inward current increases its magnitude with depolarization or that an outward current increases its magnitude with hyperpolarization. In the case of inward currents, a negative slope conductance region is created when the current is activated by depolarization which leads to a positive feedback between the activation and the amplitude of the current through a regenerative mechanism. The activation phase of the voltage-gated sodium and calcium currents are typical examples of regions of negative slope conductances (Fig. 1).
Fig. 1.
a Current–voltage characteristic (I–V curves) of the persistent sodium current (I nap), calcium current (I ca), N-methyl-D-aspartate current (I nmda) and leak current (I leak), and negative slope conductance region (arrow). b Positive feedback between depolarization and the activation variable that increases the inward currents regeneratively and creates the negative slope conductance region
However, what is exactly a negative slope conductance? The infinitesimal or differential definition of the membrane conductance in the steady state is G m = dI/dV ss, where V ss is the steady-state membrane potential. This definition is also commonly known as the slope conductance, since it corresponds to the slope of the steady-state I–V plot (Johnston and Wu 1994). The slope conductance at the steady state of a voltage-dependent current I v = g(V, t)(V − E rev) is obtained by differentiating its current equation, G = dI v/dV ss = g chord + G Der, where g chord is the so-called chord conductance, and G Der = (V − E rev)(dg/dV) is the so-called derivative conductance. Interestingly, g chord is always positive, whereas G Der can be positive or negative, and when G Der is negative and larger than g chord the slope conductance is negative (Koch 1998; Wessel et al. 1999). The membrane conductance G m of a membrane that contains linear ionic currents (IL) and voltage-dependent ionic currents (IV) is obtained by differentiating the sum of the currents G m = ∑ G V + ∑ g L where G V is the slope conductance of the voltage-dependent currents and g L is the conductance of the linear currents. The inverse of the membrane conductance is the slope resistance, or membrane resistance . In practical terms the R m is also referred as input resistance (R in) which is the sum of the membrane resistance R m and the series resistance R s of the electrode.
The basic concepts and mathematical analysis of the chord conductance and the slope conductance of voltage-dependent currents have been well described elsewhere (Finkelstein and Mauro 2011; Johnston and Wu 1994; Koch 1998). However, only recently have explicit equations been developed for the slope conductance of voltage-dependent currents. These equations use the Hodgkin–Huxley Formalism to describe currents and the Boltzmann equation to describe activation and inactivation kinetics (Ghigliazza and Holmes 2004; Remme and Rinzel 2011). This has allowed the possibility of an analytically tractable approach to study the effects of the negative slope conductance.
Remme and Rinzel (2011) studied the effects of a persistent sodium current (INaP) on synaptic inputs propagating through dendritic cables using the so-called quasi-active description of cables, an extension of the classical passive cable theory. This approach relies on linearizing voltage-dependent cable conductances around the membrane potential V R. The method was first employed by Sabah and Leibovic (1969) and further worked on by Koch (1984), and it elucidates certain general principles of quasi-active cables. This approach allowed classifying INaP as a regenerative current, which produces a positive feedback and amplifies membrane potential changes, boosting and broadening excitatory postsynaptic potentials (EPSPs). The effectiveness of INaP in modulating the shape of EPSPs is determined by the activation time constant: the faster the activation time constant, the stronger its effect on EPSP amplitude (Ceballos et al. 2017). These effects are controlled by a single dimensionless parameter μ.
where V R is the membrane potential around linearization, s ∞ is the activation variable in the steady state, γ is the ratio of the maximal conductance of the current to the leak conductance and the partial derivative conveys the slope of the activation curve at V R. The parameter μ denotes the strength of the positive feedback provided by INaP. The central parameters that qualitatively determine the effects of INaP on EPSP shape are the current-type μ, the activation time constant τ act and the relative current density γ. The parameter μ is negative for regenerative or amplifying currents, such as the persistent sodium current, which is activated by depolarization (i.e. the partial derivative is positive) and has a depolarized reversal potential (relative to V R) (Goldberg et al. 2007). These currents are called amplifying currents due to the amplified response in the low frequencies relative to the passive cable (Hutcheon and Yarom 2000). It is important to note that regenerative currents can be either inward or outward currents; for example, positive feedback results from both an inward current that activates or an outward current that deactivates upon depolarization.
The activation time constant of the amplifying current is central in determining the degree to which this current affects the shape of the EPSP. For example, a relatively slow current (τ act> > τ m) is not able to react to voltage transients and simply acts as a passive current on the time scale of an EPSP, attenuating and narrowing the EPSPs. The effect of a slowly gated active current on the EPSP shape is caused by its contribution to the chord conductance, since τ act is typically too slow for its dynamics (characterized by the parameter μ) to significantly affect the shape of a single EPSP. In contrast, a very fast current (τ act < <τ m) varies continuously in steady state with the voltage and will therefore effectively act via its negative slope conductance (Ceballos et al. 2017; Remme and Rinzel 2011). A disadvantage of the linear approximation is that it is only valid for small voltage changes; the larger the voltage changes, the more the linearized system will deviate from the nonlinear system. Therefore, in principle this approach is well suited for small EPSPs.
Electrical impedance of neurons characterizes their voltage response to sinusoidal current inputs with varying frequency. Impedance is the frequency-domain extension of the concept of resistance and links the amplitudes and phases of the output/input oscillatory signals (Hutcheon and Yarom 2000). Using the quasi-active cable approximation, Káli and Zemankovics (2012) recently derived an explicit mathematical expression for the somatic membrane impedance of a model neuron consisting of a somatic compartment coupled to an infinite dendritic cable with voltage-gated conductances. These authors conceptualized all voltage-gated conductances as parallel impedances in the cell membrane. Since the negative slope conductance of the regenerative inward currents represents negative admittance, the final result of the inclusion of this kind of current into the model neuron is a net increase in the total impedance. As expected, their study predicted a significant contribution from INaP in amplifying the impedance profile of CA1 pyramidal neurons. The effect of amplifying currents, mainly INaP, and their effects on the impedance profiles of simple neuron models have been studied in several other theoretical and computational works (Richardson et al. 2003; Rotstein 2015, 2016; Rotstein and Nadim 2014).
Interestingly, the parameter μ is also central in these works and determines the contribution of the voltage-gated conductances to the impedance profile. Furthermore, τ act is an explicit term of the impedance equation that divides the parameter μ: thus, the bigger the τ act, the smaller the effect of μ on the impedance. Making a parallel between the parameter μ obtained using quasi-active cable approximation and the mathematical description of the slope conductance obtained by differentiating the current equation as in Koch (1998), one can say that the parameter μ and the derivative conductance are equivalent (Moore et al. 1995). Recently, Ceballos et al. (2017) were able to measure experimentally the derivative conductance of the INaP, suggesting that it is possible to test these theories experimentally.
Negative slope conductances increase the membrane resistance, time constant and impedance
The concept of negative slope conductance creates an interesting paradox. If a neuron depolarizes and activates voltage-dependent inward conductances, which creates a region of negative slope conductance, would R m and τ m decrease, as expected by an increased ionic conductance (i.e. permeability), or they would increase by the negative slope conductance? Early observations (Hirsch and Oertel 1988; Stafstrom et al. 1982) related the presence of a negative slope conductance created by INaP with an increase in R m. Other studies found that activation of glutamatergic N-methyl-D-aspartate (NMDA) currents (INMDA) induced a region of negative slope conductance with an increased R m (Crunelli and Mayer 1984; MacDonald et al. 1982). Later studies showed that INaP can be activated during a single EPSP, slowing its decay phase (Hirsch and Oertel 1988; Stafstrom et al. 1985). Thomson et al. (1988) showed that the amplitude and duration of EPSPs are voltage dependent and that depolarization of the membrane potential to values close to the action potential threshold amplifies the EPSP amplitude and prolongs its decay phase. It was subsequently established that these effects are mediated mainly by INaP (Andreasen and Lambert 1999; Deisz et al. 1991; Lipowsky et al. 1996; Schwindt and Crill 1995; Stuart and Sakmann 1995; Urban et al. 1998). Voltage-dependent calcium currents (ICa) can similarly produce these effects (Gillessen and Alzheimer 1997). These three currents (INaP, ICa and INMDA) are generally accepted to be amplifying currents that increase R m and amplify EPSPs by their negative slope conductances at subthreshold voltages (MacDonald et al. 1982; Putzier et al. 2009; Stafstrom et al. 1982) (Fig. 1).
It has been observed in several types of neurons that R m and τ m increase in a voltage-dependent manner and that this effect is abolished by tetrodotoxin (TTX) application (Agrawal et al. 2001; Curti et al. 2008; Economo et al. 2014; Fernandez et al. 2015; Hirsch and Oertel 1988; Klink and Alonso 1993; Stafstrom et al. 1982; Yamada-Hanff and Bean 2015). The authors of these studies suggested that the negative slope conductance region generated by INaP caused this paradoxical increase in R m. Recently, Yamada-Hanff and Bean (2015) have shown, in hippocampal pyramidal cells, that INaP activation increases R m and τm simultaneously, whereas Ih, which does not produce a negative slope conductance region, decreases both R m and τ m. The increase or decrease of these parameters matches with regions of negative and positive slope conductance of the I–V curves, respectively. Similar results were obtained by Hirsch and Oertel (1988) in fusiform cells of the dorsal cochlear nucleus of mice where a TTX-sensitive increase in R m with depolarization was observed. These authors also observed a concomitant decrease in R m and τ m with hyperpolarization, in parallel with the deactivation of INaP which is present in these neurons (Leao et al. 2012).
Activation of NMDA receptors can also produce an increase in R m. A pioneering study by MacDonald et al. (1982) showed that L-aspartic acid induced a region of negative slope conductance in cultured spinal cord neurons concomitantly with an increased in R m. In accordance with the negative conductance hypothesis, Crunelli and Mayer (1984) observed that in the absence of Mg2+, which abolishes NMDA-receptor current outward rectification, the depolarizing response to NMDA was accompanied by a decrease in R m, but that in the presence of Mg2+ the response to NMDA was accompanied by an increase in R m. Later, Moore et al. (1994) observed that the increase in R m after the application of the glutamate agonist NMDA was voltage dependent and that the Mg2+ block of NMDA channels at the hyperpolarized membrane potential produced this voltage dependency. All of this evidence suggests that the anomalous R m increase observed during the activation of NMDA receptors can be explained as a consequence of a negative slope conductance region.
Compelling evidence that the increase in R m is the result of the negative slope conductance comes from the work of Fernandez et al. (2015). These authors injected artificial linear negative and positive conductance using a dynamic clamp in entorhinal stellate cells and observed that negative conductance increased R m while a positive conductance decreased R m, suggesting that is the negative slope conductance—and not the positive chord conductance—that determines R in and τ m (Ceballos et al. 2017). In a case like this, slope conductances (positive and negative) from each current (linear and voltage-dependent) are simply added up algebraically, and it is this sum that determines R m and τ m following a simple mathematical rule: R m = 1/ΣG and τ m = R mC m, (where C m is membrane capacitance) (Buchanan et al. 1992; Moore et al. 1994; Yoshii et al. 1988). Thus, in the subthreshold region, the negative slope conductances of INaP and INMDA oppose the positive slope conductance of the leak current, which leads to a decrease in the total positive slope conductance and increases in both R m and τ m.
In order to increase R m and τ m, a critical feature for a current with a negative slope conductance is a fast activation (Ceballos et al. 2017; Manuel et al. 2007; Remme and Rinzel 2011). But how fast? In principle, the speed should be faster than the cell membrane time constant because the negative slope conductance is created proportionally to the rate of change of the conductance. Thus, an almost instantaneously activated current (current time constant <<τ m) is able to reach its steady state so fast that it might be able to control R m and τ m by its steady state slope conductance. Since INaP has the fastest activation of all the voltage-activated inward currents (τact ≈ 250 μs; Carter et al. 2012), we speculate that the effect of INaP on R m and τ m is more powerful than that of the other currents.
It has been observed that INaP increases the impedance of neurons (Boehlen et al. 2012; Curti et al. 2008; Economo et al. 2014; Gutfreund and Segev 1995; Hu et al. 2002; Hutcheon et al. 1996a; Hutcheon et al. 1996b; Jacobson et al. 2005; Klink and Alonso 1997; Manuel et al. 2007; Pape and Driesang 1998; Saint Mleux and Moore 2000; Sun et al. 2014; Wu et al. 2001; Wu et al. 2005; Yang et al. 2009; Yaron-Jakoubovitch et al. 2008). This increase in the membrane impedance is thus a function of membrane voltage, increasing as the neuron is depolarized towards the spike threshold. Buchanan et al. (1992) showed that the activation of INaP causes an impedance increase that leads directly to an amplification of the EPSPs. Other studies have also reported that Ca++ currents amplify the neuronal membrane impedance (Manuel et al. 2007; Matsumoto-Makidono et al. 2016; Saint Mleux and Moore 2000). Theoretical modeling has also shown that ICa has an amplifying effect on neuronal impedance (Ghaffari et al. 2015). Finally, there are some reports showing that NMDA currents increase the impedance in neurons (Moore et al. 1994, 1995, 1999) due to negative slope conductance.
Summarizing, the main effect of the negative slope conductance on neuronal impedance is to increase it. One the most important consequences of increases in R m, τ m and impedance is the amplification of synaptic inputs, i.e. their amplitude is increased and the decay phase is prolonged. This will be discussed in depth in the following section.
Negative slope conductance amplifies postsynaptic potentials
It is known that the amplitude and duration of EPSPs are voltage dependent and that depolarization of the membrane potential to values close to action potential threshold amplifies the EPSP amplitude and prolongs its decay phase (Deisz et al. 1991; Thomson et al. 1988; Zsiros and Hestrin 2005). Experiments using sodium and calcium blockers in the perfusion bath could be performed in order to determine whether these postsynaptic conductances are responsible for this effect, but since these blockers also impair glutamate release by the presynaptic fiber it is complicated to assess their effect on the EPSPs. Several approaches have been considered to circumvent this limitation, such as the injection of artificial EPSCs directly into the postsynaptic neuron (Stuart and Sakmann 1995; Ceballos et al. 2017), local application of sodium or calcium blockers to the postsynaptic neuron (González-Burgos and Barrionuevo 2001; Stuart and Sakmann 1995) and the use of intracellular sodium channel blockers, such as QX 314 (Deisz et al. 1991; González-Burgos and Barrionuevo 2001). Recently, more advanced techniques have been used, such as glutamate uncaging (Carter et al. 2012; Liu and Shipley 2008) or knockout animals of Nav channels (Branco et al. 2016). Additional strategies, such as canceling the endogenous INaP or applying an artificial INaP with dynamic clamp (Ceballos et al. 2017; Vervaeke et al. 2006), have also been employed to analyze the effects of this conductance on the EPSPs.
The amplification of EPSPs by INaP has been extensively observed in neurons from the neocortex (Andreasen and Lambert 1999; Carter et al. 2012; Deisz et al. 1991; Fricker and Miles 2000; González-Burgos and Barrionuevo 2001; Hirsch and Gilbert 1991; Lipowsky et al. 1996; Rotaru et al. 2007; Schwindt and Crill 1995; Stafstrom et al. 1985; Stuart and Sakmann 1995; Thomson et al. 1988; Zsiros and Hestrin 2005), hippocampus (Ceballos et al. 2017; Urban et al. 1998; Vervaeke et al. 2006), entorhinal cortex (Economo et al. 2014; Rosenkranz and Johnston 2007), dorsal cochlear nucleus (Hirsch and Oertel 1988), subthalamic nucleus (Farries et al. 2010), hypothalamus (Branco et al. 2016), olfactory bulb (Liu and Shipley 2008), dorsal horn of the spinal cord (Prescott and De Koninck 2005), medial superior olive (Scott et al. 2010) and substantia nigra pars compacta (Yamashita and Isa 2004). In most of these experiments, blocking INaP with TTX abolished the voltage dependence of the EPSP amplitude and area.
The mechanism for the amplification of the EPSP amplitude is attributed to the almost instantaneous activation of INaP, which boosts depolarization and increases R m by its negative slope conductance (Ceballos et al. 2017). EPSP amplification was observed upon the injection of both an artificial INaP or a linear negative conductance after TTX application, using the dynamic clamp method (Ceballos et al. 2017; Economo et al. 2014). Further evidence supporting the hypothesis that the negative slope conductance of INaP prolongs EPSP decay is provided by the recently proposed quasi-active cable theory approximation (Remme and Rinzel 2011). Moreover, Zsiros and Hestrin (2005) showed that injecting a positive conductance shortens the prolongation of the EPSP decay enhanced by INaP.
Pharmacological blockage of calcium currents also decreases the voltage dependence of the amplitude and area of EPSPs (Connelly et al. 2016; Gillessen and Alzheimer 1997; Liu and Shipley 2008; Prescott and De Koninck 2005; Urban et al. 1998). Amplification of EPSP by calcium currents seems to be mediated mostly by T-type Ca2+ currents rather than by L-type Ca2+ currents (Liu and Shipley 2008). In addition, calcium currents tend to prolong EPSP decay rather than amplify EPSP amplitude (Prescott and De Koninck 2005).
On the other hand, the negative slope conductance of NMDA currents has very small effect on EPSP amplitude and decay in neocortical neurons. Blocking NMDA currents with AP5 did not affect substantially the EPSP (Deisz et al. 1991; González-Burgos and Barrionuevo 2001; Hirsch and Gilbert 1991; Stuart and Sakmann 1995; Thomson et al. 1988). However, the effect of NMDA currents in some other areas appears to be more significant. For example, Fricker and Miles (2000) showed a decrease in EPSP area when blocked by AP5 in hippocampal pyramidal cells, and Connelly et al. (2016) showed strong EPSP amplification by NMDA currents in thalamocortical neurons. In addition, Porres et al. (2011) observed an EPSP decay phase prolongation in neurons of the dorsal nucleus of the lateral lemniscus caused by NMDA currents.
Interestingly, regenerative inward currents also amplify inhibitory postsynaptic potentials (IPSPs). For example, INaP amplifies IPSPs in neocortical pyramidal neurons (Stuart 1999; Williams and Stuart 2003), hippocampal pyramidal neurons (Hardie and Pearce 2006) and stellate cells of the medial entorhinal cortex (Economo et al. 2014). Ca++ currents also amplify IPSPs in cat motoneurons (Bui et al. 2008). These observations suggest that a negative slope conductance amplifies the voltage changes independently of the direction of the membrane potential change, i.e. amplifies both depolarization and hyperpolarization of the membrane.
Reinforcing that negative slope conductance creates amplification of EPSPs, the activation of Ih, which is an inward depolarizing current activated by hyperpolarization, decreases the amplitude of EPSPs and reduces their decay time (Magee 1998), similar to the effect of some outward potassium currents (Hoffman et al. 1997; Mathews et al. 2010). In addition, the inwardly rectifying potassium current, an outward potassium current that has a negative slope conductance region in depolarized potentials, amplifies hyperpolarizations in endothelial cells (Jackson 2016), striatal cholinergic interneurons (Wilson 2005) and vascular smooth muscles (Smith et al. 2008), and also amplifies EPSPs in the AP cell of the leech (Wessel et al. 1999). All of these observations together suggest that the amplification of membrane voltage changes is caused by negative slope conductance rather than by current amplitude.
One interesting consequence of the EPSP prolongation by INaP is the near-perfect synaptic integration (i.e. the opposite of ideal coincidence detection) that has been observed in different brain regions and which is physiologically relevant since it enhances temporal summation (Prescott and De Koninck 2005). Farries et al. (2010) showed in neurons of the subthalamic nucleus that negative slope conductance of INaP can oppose the positive slope conductance produced by other subthreshold currents, creating a wide region with zero slope conductance which establishes an infinite time constant and strongly reduces the decay phase of EPSPs. Moreover, Branco et al. (2016) showed that in hypothalamic neurons, the prolonged EPSPs produced by INaP are critical for the regulation of body weight.
Conclusion
We have reviewed the nature and physiological impact of the negative conductance created by inward voltage-dependent currents such as INaP, ICa and INMDA. Normally the effects of these currents on the membrane potential and neuronal excitability are attributed only to their depolarizing effects on the membrane potential, but it is clear that the dynamical effects they produce in membrane resistance and the time constant and in their activation potentials have a profound impact on neuronal synaptic integration in the subthreshold range.
Acknowledgments
This work was supported by FAPESP (2016/01607-4) and CNPq (470745/2012-6) grants to RML, and FAPESP (2013/07699-0) and CNPq (306251/2014-0) grants to ACR. CCC is a PhD scholarship recipient from CAPES.
Compliance with ethical standards
Conflicts of interest
All of the authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Latin America’ edited by Pietro Ciancaglini and Rosangela Itri
Contributor Information
Antonio C. Roque, Email: antonior@ffclrp.usp.br
Ricardo M. Leão, Email: leaor@fmrp.usp.br
References
- Agrawal N, Hamam BN, Magistretti J, Alonso A, Ragsdale DS. Persistent sodium channel activity mediates subthreshold membrane potential oscillations and low-threshold spikes in rat entorhinal cortex layer V neurons. Neuroscience. 2001;102:53–64. doi: 10.1016/s0306-4522(00)00455-3. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Lambert JD. Somatic amplification of distally generated subthreshold EPSPs in rat hippocampal pyramidal neurones. J Physiol. 1999;519:85–100. doi: 10.1111/j.1469-7793.1999.0085o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehlen A, Henneberger C, Heinemann U, Erchova I. Contribution of near-threshold currents to intrinsic oscillatory activity in rat medial entorhinal cortex layer II stellate cells. J Neurophysiol. 2012;109:445–463. doi: 10.1152/jn.00743.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T, Tozer A, Magnus CJ, Sugino K, Tanaka S, Lee AK, Wood JN, Sternson SM. Near-perfect synaptic integration by Na v 1.7 in hypothalamic neurons regulates body weight. Cell. 2016;165:1749–1761. doi: 10.1016/j.cell.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JT, Moore LE, Hill R, Wallén P, Grillner S. Synaptic potentials and transfer functions of lamprey spinal neurons. Biol Cybern. 1992;67:123–131. doi: 10.1007/BF00201019. [DOI] [PubMed] [Google Scholar]
- Bui TV, Grande G, Rose PK. Multiple modes of amplification of synaptic inhibition to motoneurons by persistent inward currents. J Neurophysiol. 2008;99:571–582. doi: 10.1152/jn.00717.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BC, Giessel AJ, Sabatini BL, Bean BP. Transient sodium current at subthreshold voltages: activation by EPSP waveforms. Neuron. 2012;75:1081–1093. doi: 10.1016/j.neuron.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos C, Roque A, Leao R (2017) A negative slope conductance of the persistent sodium current prolongs subthreshold depolarizations. Biophys J. doi:10.1016/j.bpj.2017.06.047 [DOI] [PMC free article] [PubMed]
- Connelly WM, Crunelli V, Errington AC. Passive synaptic normalization and input synchrony-dependent amplification of cortical feedback in thalamocortical neuron dendrites. J Neurosci. 2016;36:3735–3754. doi: 10.1523/JNEUROSCI.3836-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Mayer ML. Mg 2+ dependence of membrane resistance increases evoked by NMDA in hippocampal neurones. Brain Res. 1984;311:392–396. doi: 10.1016/0006-8993(84)90107-0. [DOI] [PubMed] [Google Scholar]
- Curti S, Gomez L, Budelli R, Pereda AE. Subthreshold sodium current underlies essential functional specializations at primary auditory afferents. J Neurophysiol. 2008;99:1683–1699. doi: 10.1152/jn.01173.2007. [DOI] [PubMed] [Google Scholar]
- Dagostin AA, Lovell PV, Hilscher MM, Mello CV, Leão RM. Control of Phasic firing by a background leak current in avian forebrain auditory neurons. Front Cell Neurosci. 2015;9:471. doi: 10.3389/fncel.2015.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz RA, Fortin G, Zieglgänsberger W. Voltage dependence of excitatory postsynaptic potentials of rat neocortical neurons. J Neurophysiol. 1991;65:371–382. doi: 10.1152/jn.1991.65.2.371. [DOI] [PubMed] [Google Scholar]
- Economo MN, Martínez JJ, White JA. Membrane potential-dependent integration of synaptic inputs in entorhinal stellate neurons. Hippocampus. 2014;24:1493–1505. doi: 10.1002/hipo.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- Farries MA, Kita H, Wilson CJ. Dynamic spike threshold and zero membrane slope conductance shape the response of subthalamic neurons to cortical input. J Neurosci. 2010;30:13180–13191. doi: 10.1523/JNEUROSCI.1909-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez FR, Malerba P, White JA. Non-linear membrane properties in entorhinal cortical stellate cells reduce modulation of input-output responses by voltage fluctuations. PLoS Comput Biol. 2015;11:e1004188. doi: 10.1371/journal.pcbi.1004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A, Mauro A. Physical principles and formalisms of electrical excitability. In: Kandel ER, editor. Comprehensive physiology. Bethesda: American Physiological Society; 2011. pp. 161–213. [Google Scholar]
- Fricker D, Miles R. EPSP amplification and the precision of spike timing in hippocampal neurons. Neuron. 2000;28:559–569. doi: 10.1016/s0896-6273(00)00133-1. [DOI] [PubMed] [Google Scholar]
- Ghaffari BV, Kouhnavard M, Aihara T, Kitajima T. Mathematical modeling of subthreshold resonant properties in pyloric dilator neurons. Biomed Res Int. 2015;2015:21. doi: 10.1155/2015/135787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigliazza RM, Holmes P. Minimal models of bursting neurons: how multiple currents, conductances, and timescales affect bifurcation diagrams. SIAM J App Dyn Syst. 2004;3:636–670. [Google Scholar]
- Gillessen T, Alzheimer C. Amplification of EPSPs by low Ni2+- and amiloride-sensitive Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J Neurophysiol. 1997;77:1639–1643. doi: 10.1152/jn.1997.77.3.1639. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Deister CA, Wilson CJ. Response properties and synchronization of rhythmically firing dendritic neurons. J Neurophysiol. 2007;97:208–219. doi: 10.1152/jn.00810.2006. [DOI] [PubMed] [Google Scholar]
- González-Burgos G, Barrionuevo G. Voltage-gated sodium channels shape subthreshold EPSPs in layer 5 pyramidal neurons from rat prefrontal cortex. J Neurophysiol. 2001;86:1671–1684. doi: 10.1152/jn.2001.86.4.1671. [DOI] [PubMed] [Google Scholar]
- Gutfreund Y, Segev I. Subthreshold oscillations and resonant frequency in guinea-pig cortical neurons: physiology and modelling. J Physiol. 1995;483:621–640. doi: 10.1113/jphysiol.1995.sp020611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie JB, Pearce RA. Active and passive membrane properties and intrinsic kinetics shape synaptic inhibition in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:8559–8569. doi: 10.1523/JNEUROSCI.0547-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Gilbert CD. Synaptic physiology of horizontal connections in the cat's visual cortex. J Neurosci. 1991;11:1800–1809. doi: 10.1523/JNEUROSCI.11-06-01800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Oertel D. Intrinsic properties of neurones in the dorsal cochlear nucleus of mice, in vitro. J Physiol. 1988;396:535–548. doi: 10.1113/jphysiol.1988.sp016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol. 2002;545:783–805. doi: 10.1113/jphysiol.2002.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon B, Yarom Y. Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 2000;23:216–222. doi: 10.1016/s0166-2236(00)01547-2. [DOI] [PubMed] [Google Scholar]
- Hutcheon B, Miura RM, Puil E. Models of subthreshold membrane resonance in neocortical neurons. J Neurophysiol. 1996;76:698–714. doi: 10.1152/jn.1996.76.2.698. [DOI] [PubMed] [Google Scholar]
- Hutcheon B, Miura RM, Puil E. Subthreshold membrane resonance in neocortical neurons. J Neurophysiol. 1996;76:683–697. doi: 10.1152/jn.1996.76.2.683. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM. Dynamical systems in neuroscience. Cambridge: MIT Press; 2007. [Google Scholar]
- Jackson WF (2016) Boosting the signal: endothelial inward rectifier K+ channels. Microcirculation 24(3):e12319. doi:10.1111/micc.12319 [DOI] [PMC free article] [PubMed]
- Jacobson GA, Diba K, Yaron-Jakoubovitch A, Oz Y, Koch C, Segev I, Yarom Y. Subthreshold voltage noise of rat neocortical pyramidal neurones. J Physiol. 2005;564:145–160. doi: 10.1113/jphysiol.2004.080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Wu SMS. Foundations of cellular neurophysiology. Cambridge: MIT press; 1994. pp. 39–53. [Google Scholar]
- Káli S, Zemankovics R. The effect of dendritic voltage-gated conductances on the neuronal impedance: a quantitative model. J Comput Neurosci. 2012;33:257–284. doi: 10.1007/s10827-012-0385-9. [DOI] [PubMed] [Google Scholar]
- Klink R, Alonso A. Ionic mechanisms for the subthreshold oscillations and differential electroresponsiveness of medial entorhinal cortex layer II neurons. J Neurophysiol. 1993;70:144–157. doi: 10.1152/jn.1993.70.1.144. [DOI] [PubMed] [Google Scholar]
- Klink R, Alonso A. Ionic mechanisms of muscarinic depolarization in entorhinal cortex layer II neurons. J Neurophysiol. 1997;77:1829–1843. doi: 10.1152/jn.1997.77.4.1829. [DOI] [PubMed] [Google Scholar]
- Koch C. Cable theory in neurons with active, linearized membranes. Biol Cybern. 1984;50:15–33. doi: 10.1007/BF00317936. [DOI] [PubMed] [Google Scholar]
- Koch C. Biophysics of computation: information processing in single neurons. New York: Oxford University Press; 1998. pp. 381–400. [Google Scholar]
- Leao RM, Li S, Doiron B, Tzounopoulos T. Diverse levels of an inwardly rectifying potassium conductance generate heterogeneous neuronal behavior in a population of dorsal cochlear nucleus pyramidal neurons. J Neurophysiol. 2012;107:3008–3019. doi: 10.1152/jn.00660.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky R, Gillessen T, Alzheimer C. Dendritic Na+ channels amplify EPSPs in hippocampal CA1 pyramidal cells. J Neurophysiol. 1996;76:2181–2191. doi: 10.1152/jn.1996.76.4.2181. [DOI] [PubMed] [Google Scholar]
- Liu S, Shipley MT. Intrinsic conductances actively shape excitatory and inhibitory postsynaptic responses in olfactory bulb external tufted cells. J Neurosci. 2008;28:10311–10322. doi: 10.1523/JNEUROSCI.2608-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JF, Porietis AV, Wojtowicz JM. L-aspartic acid induces a region of negative slope conductance in the current-voltage relationship of cultured spinal cord neurons. Brain Res. 1982;237:248–253. doi: 10.1016/0006-8993(82)90575-3. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D. Resonant or not, two amplification modes of proprioceptive inputs by persistent inward currents in spinal motoneurons. J Neurosci. 2007;27:12977–12988. doi: 10.1523/JNEUROSCI.3299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews PJ, Jercog PE, Rinzel J, Scott LL, Golding NL. Control of submillisecond synaptic timing in binaural coincidence detectors by Kv1 channels. Nat Neurosci. 2010;13:601–609. doi: 10.1038/nn.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Makidono Y, Nakayama H, Yamasaki M, Miyazaki T, Kobayashi K, Watanabe M, Kano M, Sakimura K, Hashimoto K. Ionic basis for membrane potential resonance in neurons of the inferior olive. Cell Rep. 2016;16:994–1004. doi: 10.1016/j.celrep.2016.06.053. [DOI] [PubMed] [Google Scholar]
- Moore LE, Buchanan JT, Murphey CR (1995) Localization and interaction of N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors of lamprey spinal neurons. Biophys J 68:96–103 [DOI] [PMC free article] [PubMed]
- Moore LE, Buchanan JT, Murphey CR. Anomalous increase in membrane impedance of neurons during NMDA activation. In: Eeckman FH, editor. Computation in neurons and neural systems. Boston: Kluwer Academic; 1994. pp. 9–14. [Google Scholar]
- Moore LE, Chub N, Tabak J, O’Donovan M. NMDA-induced dendritic oscillations during a soma voltage clamp of chick spinal neurons. J Neurosci. 1999;19:8271–8280. doi: 10.1523/JNEUROSCI.19-19-08271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Driesang RB. Ionic mechanisms of intrinsic oscillations in neurons of the basolateral amygdaloid complex. J Neurophysiol. 1998;79:217–226. doi: 10.1152/jn.1998.79.1.217. [DOI] [PubMed] [Google Scholar]
- Porres CP, Meyer EM, Grothe B, Felmy F. NMDA currents modulate the synaptic input–output functions of neurons in the dorsal nucleus of the lateral lemniscus in mongolian gerbils. J Neurosci. 2011;31:4511–4523. doi: 10.1523/JNEUROSCI.6054-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Integration time in a subset of spinal lamina I neurons is lengthened by sodium and calcium currents acting synergistically to prolong subthreshold depolarization. J Neurosci. 2005;25:4743–4754. doi: 10.1523/JNEUROSCI.0356-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzier I, Kullmann PH, Horn JP, Levitan ES. Cav1. 3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J Neurosci. 2009;29:15414–15419. doi: 10.1523/JNEUROSCI.4742-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remme MW, Rinzel J. Role of active dendritic conductances in subthreshold input integration. J Comput Neurosci. 2011;31:13–30. doi: 10.1007/s10827-010-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MJ, Brunel N, Hakim V. From subthreshold to firing-rate resonance. J Neurophysiol. 2003;89:2538–2554. doi: 10.1152/jn.00955.2002. [DOI] [PubMed] [Google Scholar]
- Ries CR, Puil E. Ionic mechanism of isoflurane’s actions on thalamocortical neurons. J Neurophysiol. 1999;81:1802–1809. doi: 10.1152/jn.1999.81.4.1802. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. State-dependent modulation of amygdala inputs by dopamine-induced enhancement of sodium currents in layer V entorhinal cortex. J Neurosci. 2007;27:7054–7069. doi: 10.1523/JNEUROSCI.1744-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru DC, Lewis DA, Gonzalez-Burgos G. Dopamine D1 receptor activation regulates sodium channel-dependent EPSP amplification in rat prefrontal cortex pyramidal neurons. J Physiol. 2007;581:981–1000. doi: 10.1113/jphysiol.2007.130864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein HG. Subthreshold amplitude and phase resonance in models of quadratic type: nonlinear effects generated by the interplay of resonant and amplifying currents. J Comput Neurosci. 2015;38:325–354. doi: 10.1007/s10827-014-0544-2. [DOI] [PubMed] [Google Scholar]
- Rotstein HG. The shaping of intrinsic membrane potential oscillations: positive/negative feedback, ionic resonance/amplification, nonlinearities and time scales. J Comput Neurosci. 2016;42:133–166. doi: 10.1007/s10827-016-0632-6. [DOI] [PubMed] [Google Scholar]
- Rotstein HG, Nadim F. Frequency preference in two-dimensional neural models: a linear analysis of the interaction between resonant and amplifying currents. J Comput Neurosci. 2014;37:9–28. doi: 10.1007/s10827-013-0483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabah NH, Leibovic KN (1969) Subthreshold oscillatory responses of the Hodgkin–Huxley cable model for the squid giant axon. Biophys J 9:1206–1222 [DOI] [PMC free article] [PubMed]
- Saint Mleux B, Moore LE (2000) Active Dendritic membrane properties of Xenopus larval spinal neurons analyzed with a whole cell soma voltage clamp. J Neurophysiol 83:1381–1393 [DOI] [PubMed]
- Schwindt PC, Crill WE. Amplification of synaptic current by persistent sodium conductance in apical dendrite of neocortical neurons. J Neurophysiol. 1995;74:2220–2224. doi: 10.1152/jn.1995.74.5.2220. [DOI] [PubMed] [Google Scholar]
- Scott LL, Mathews PJ, Golding NL. Perisomatic voltage-gated sodium channels actively maintain linear synaptic integration in principal neurons of the medial superior olive. J Neurosci. 2010;30:2039–2050. doi: 10.1523/JNEUROSCI.2385-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, Welsh DG. KIR channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol. 2008;586:1147–1160. doi: 10.1113/jphysiol.2007.145474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Schwindt PC, Crill WE. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res. 1982;236:221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Schwindt PC, Chubb MC, Crill WE. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985;53:153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- Stuart G. Voltage–activated sodium channels amplify inhibition in neocortical pyramidal neurons. Nat Neurosci. 1999;2:144–150. doi: 10.1038/5698. [DOI] [PubMed] [Google Scholar]
- Stuart G, Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- Sun H, An S, Luhmann HJ, Kilb W. Resonance properties of GABAergic interneurons in immature GAD67-GFP mouse neocortex. Brain Res. 2014;1548:1–11. doi: 10.1016/j.brainres.2013.12.032. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Girdlestone D, West DC. Voltage-dependent currents prolong single-axon postsynaptic potentials in layer III pyramidal neurons in rat neocortical slices. J Neurophysiol. 1988;60:1896–1907. doi: 10.1152/jn.1988.60.6.1896. [DOI] [PubMed] [Google Scholar]
- Urban NN, Henze DA, Barrionuevo G. Amplification of perforant-path EPSPs in CA3 pyramidal cells by LVA calcium and sodium channels. J Neurophysiol. 1998;80:1558–1561. doi: 10.1152/jn.1998.80.3.1558. [DOI] [PubMed] [Google Scholar]
- Vervaeke K, Hu H, Graham LJ, Storm JF. Contrasting effects of the persistent Na+ current on neuronal excitability and spike timing. Neuron. 2006;49:257–270. doi: 10.1016/j.neuron.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Wessel R, Kristan WB, Kleinfeld D. Supralinear summation of synaptic inputs by an invertebrate neuron: dendritic gain is mediated by an “inward rectifier” K+ current. J Neurosci. 1999;19:5875–5888. doi: 10.1523/JNEUROSCI.19-14-05875.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Voltage-and site-dependent control of the somatic impact of dendritic IPSPs. J Neurosci. 2003;23:7358–7367. doi: 10.1523/JNEUROSCI.23-19-07358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron. 2005;45:575–585. doi: 10.1016/j.neuron.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Wu N, Enomoto A, Tanaka S, Hsiao CF, Nykamp DQ, Izhikevich E, Chandler SH. Persistent sodium currents in mesencephalic v neurons participate in burst generation and control of membrane excitability. J Neurophysiol. 2005;93:2710–2722. doi: 10.1152/jn.00636.2004. [DOI] [PubMed] [Google Scholar]
- Wu N, Hsiao CF, Chandler SH. Membrane resonance and subthreshold membrane oscillations in mesencephalic V neurons: participants in burst generation. J Neurosci. 2001;21:3729–3739. doi: 10.1523/JNEUROSCI.21-11-03729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada-Hanff J, Bean BP. Activation of Ih and TTX-sensitive sodium current at subthreshold voltages during CA1 pyramidal neuron firing. J Neurophysiol. 2015;114:2376–2389. doi: 10.1152/jn.00489.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Isa T. Enhancement of excitatory postsynaptic potentials by preceding application of acetylcholine in mesencephalic dopamine neurons. Neurosci Res. 2004;49:91–100. doi: 10.1016/j.neures.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Yang RH, Wang WT, Chen JY, Xie RG, Hu SJ. Gabapentin selectively reduces persistent sodium current in injured type-a dorsal root ganglion neurons. Pain. 2009;143:48–55. doi: 10.1016/j.pain.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Yaron-Jakoubovitch A, Jacobson GA, Koch C, Segev I, Yarom Y (2008) A paradoxical isopotentiality: a spatially uniform noise spectrum in neocortical pyramidal cells. Front Cell Neurosci 2:1–9. doi:10.3389/neuro.03.003.2008 [DOI] [PMC free article] [PubMed]
- Yoshii K, Moore LE, Christensen BN. Effect of subthreshold voltage-dependent conductances on the transfer function of branched excitable cells and the conduction of synaptic potentials. J Neurophysiol. 1988;59:706–716. doi: 10.1152/jn.1988.59.3.706. [DOI] [PubMed] [Google Scholar]
- Zsiros V, Hestrin S. Background synaptic conductance and precision of EPSP-spike coupling at pyramidal cells. J Neurophysiol. 2005;93:3248–3256. doi: 10.1152/jn.01027.2004. [DOI] [PubMed] [Google Scholar]