Abstract
The success of a biomaterial relies on an appropriate interaction between the surface of that biomaterial and the surrounding environment; more specifically, the success of a biomaterial depends on how fluids, proteins, and cells interact with the foreign material. For this reason, the surface properties of biomaterial, such as composition, charge, wettability, and roughness, must be optimized for a desired application to be achieved. In this review we highlight different bioinspired approaches that are used to manipulate and fine-tune the interfacial properties of biomaterials. Inspired by noteworthy natural processes, researchers have developed materials with a functional anatomy that range from hierarchical hybrid structures to self-cleaning interfaces. In this review we focus on (1) the creation of particles and modified surfaces inspired by the structure and composition of biogenic mineralized tissues, (2) the development of biofunctional coatings, (3) materials inspired by biomembranes and proteins, and (4) the design of superwettable materials. Our intention is to point out different bioinspired methodologies that have been used to design materials for biomedical applications and to discuss how interfacial properties modified by manipulation of these materials determine their final biological response. Our objective is to present future research directions and to highlight the potential of bioinspired materials. We hope this review will provide an understanding of the interplay between interfacial properties and biological response so that successful biomaterials can be achieved.
Keywords: Biomaterials, Surface properties, Bioactivity, Biofunctional coatings
Introduction
Biomaterial is the name given to a synthetic or natural device that can be completely or partially put in contact with a biological system for a certain period of time to treat, improve, or replace a tissue, organ, or function of the body (Gemini-Piperni et al. 2014). During the course of biomaterial development three different generations of biomaterials have evolved. The first generation consisted of bioinert materials which only provided a suitable combination of physical properties that matched the properties of the replaced tissue with minimal toxic response from the host tissue (Hench and Wilson 1984). The second generation comprised bioactive materials that were able to interact with biological systems to promote a tissue/material bond. The third generation involved materials that stimulated a specific cellular response at the molecular level (Hench and Polak 2002).
Because the surface of the biomaterial is the first region that comes into contact with the physiological environment, the initial biological response depends on interfacial contact and on the improvement of the surface properties by modification of this surface. From this perspective, the field of bioinspired surfaces fits well into the general field of biomimetic materials. Additionally, extensive investigation of biological surfaces in recent years has revealed that these surfaces display unusual properties; for example, very water-repelling rough surfaces use the “lotus effect,” surfaces with high and adjustable adhesion use the “gecko effect,” and non-reflective surfaces use the “moth-eye effect,” among others (Drelich et al. 2011). Liu et al. (2014) pointed out that special surface wettability stems from a coordinated action between multiscaled surfaces and surface chemistry. According to Vogler (1998), hydrophilicity and hydrophobicity are the first parameters that affect the adsorption of proteins. Hydrophobic surfaces thermodynamically favor the adsorption of proteins from aqueous solutions, but they also induce irreversible adsorption, which can denature proteins. On the other hand, hydrophilic surfaces may prevent proteins from being adsorbed.
The emergence of new technologies and of devices with reduced size requires new materials and surfaces with specific properties, such as low adhesion and friction and non-wettability, among others. These specific properties will depend on increasing the surface-to-volume ratio so that surface effects will dominate over volume effects (Drelich et al. 2011).
Physical and chemical properties, such as size, crystalline structure, charge, composition, and hydrophilicity, determine whether a biomaterial is biocompatible. In other words, whether the best physical and chemical properties are suitable will depend on the application of the biomaterial. For example, cardiovascular articulation implants demand good cell adhesion, whereas catheters require that cell adhesion be kept to a minimum to avoid contamination. In general, a suitable biomaterial must interact well with body cells and show good cell proliferation; that is, the surface of the biomaterial must be hydrophilic (high wettability), rough (to promote adsorption of proteins), and highly reactive, as well as presenting high surface energy (Vogler 1998; Chang and Wang 2011). In this review we discuss these aspects of biomaterials, using polymeric, nanoparticulated, and titanium (Ti)-based biomaterials as examples.
Modification of the surface of polymers
Regarding composition, a biomaterial can be a synthetic polymer, a metal, a ceramic material, or a natural macromolecule. Polymers are among the most often used materials for biomedical applications, such as vascular grafts, heart valves, contact lenses, orthopedic implants, and tissue engineering (Kawachi et al. 2000). Polymers are preferred because they exhibit excellent mechanical and chemical properties, including high mechanical strength, elasticity, and resistance to corrosion, not to mention that they are inexpensive and non-toxic. Compared to metallic and ceramic materials, the surface of polymers is easier to modify (Yoshida et al. 2013).
Several types of polymers have been studied for biomedical use, such as polymethylmethacrylate (PMMA) (Lee et al. 2008) and polyethylene terephthalate (PET) (Liu et al. 2005). However, their application as biomaterial depends on characteristics like biodegradability (Maia et al. 2010) and bioactivity. Biodegradable synthetic polymers, such as polylactide (PLA) and poly(lactide-co-glycotide) (PLGA), are not sufficiently bioactive and cannot promote cell regeneration (Lee et al. 2008). Hence, researchers in the field of interfacial science have made numerous efforts to modify the surface of these materials to improve their biological response when they come into contact with the host tissue.
Several strategies have been used to modify the surface of polymers, including treatment with active gases (vapor or radiation) (Ma et al. 2007), plasma treatment (Khorasani and Mirzadeh 2004), layer-by-layer (LbL) deposition (Seo et al. 2008), and self-assembled monolayers (Chen and McCarthy 1997). All of these strategies have the aim to enhance the physical and chemical properties of the surface of the polymer, such as wettability and roughness, which play a key role in cell adhesion, spreading, and proliferation and in tissue formation (Liu and Ma 2004).
Just how modification of the surface of polymers affects the way biomaterials perform in biological systems has been widely discussed (Hallab et al. 2001). Lee and McCarthy (2007) verified the topographic effects of modifying the surface of two of the most common polymers, polyethylene (PE) and Teflon (FEP), observing that an altered topography induces enormous changes in wettability through, for example, alterations in the contact angle, thereby rendering a surface “super hydrophobic.”
Liu et al. (2005) modified PET films using hydrolysis and the LbL assembly technique with chitosan and chondroitin sulfate (CS, an important extra-cellular matrix component that plays an important role in maintaining cell functions). These authors verified that the surface of the modified PET is rougher and more hydrophilic, which allows it to adhere to endothelial cells more strongly than unmodified PET and to maintain the endothelial function.
The plasma treatment methodology can chemically insert reactive functional groups into polymeric substrates, using oxygen, ammonia, or air to generate carboxyl groups (Yoshida et al. 2013). This type of modification can create functional groups on the surface of the polymer, which will then be able to immobilize various types of extracellular matrix proteins, including collagen. The plasma modification technique also makes the polymer more hydrophilic, providing the modified surface with high adhesion power and cell proliferation.
Other studies involving modification of the surface by radiation have been conducted. Nechifor et al. (2009) used gamma radiation on porous polymeric membranes obtained through the alloying of poly(hydroxy-urethane) (PHU) and poly(vinyl alcohol) (PVA) at different concentrations. These authors found that porosity and hydrophilicity increase as the dose of gamma radiation augments, but that the samples are significantly less rough.
In an attempt to mimic the structure of lipids such as the phosphorylcholine group of phosphatidylcholine and sphingomyelin, two components of cell membranes, researchers have developed new polymer-based biomaterials based on the structure of biomembranes. Ishihara (2000) synthesized the polymer 2-methacrylooyloxyethyl phosphorylcholine (MPC) by adding a lipid polar group to the side chain, with the aim to improve the adsorption of proteins and resistance of cell adhesion to these surfaces. However, biomembrane-inspired methods are quite complex and require rigorous procedures under mild conditions, which prevent their general applicability.
The mussel adhesion process has inspired a simple method which can be used to modify the surface of polymers and has been widely used in polymer-based biomaterials. Mussels can strongly adhere to all surfaces regardless of roughness due to having catechol groups which mediate synergy between noncovalent and covalent chemical interactions (Lee et al. 2007). Catechol groups can be easily inserted into polymers by immersing the polymer in a solution of dopamine, a biological neurotransmitter. In this method, dopamine undergoes oxidative polymerization in the presence of oxygen under alkaline conditions. During polymerization, a tightly adherent layer of polydopamine emerges on the surface of the substrate that was immersed in the solution of the neurotransmitter for a certain period (Jiang et al. 2011). Mussel-inspired adhesiveness of polymeric biomaterials has been used to create hydrogels (Krogsgaard et al. 2013, Han et al. 2017), biofunctional coatings (Ryu et al. 2010), and scaffolds for tissue engineering (Ku and Park 2010).
Biomimetic modification of the surface of nanoparticles
The unique properties of nanoscaled materials that arise from their high surface area have been employed in many fields (Sanvicens and Marco 2008; Jayakumar et al. 2010; Moon et al. 2011). Growing interest in applying this kind of material in the biomedical field stems from their dimensions, which are comparable to those of cellular organelles and consequently enhance their interaction with biological media (Verma and Stellacci 2010). In this regard, the chemical and physical characteristics of nanoscaled materials must be investigated in an attempt to understand and improve their biological response (Chithrani et al. 2006).
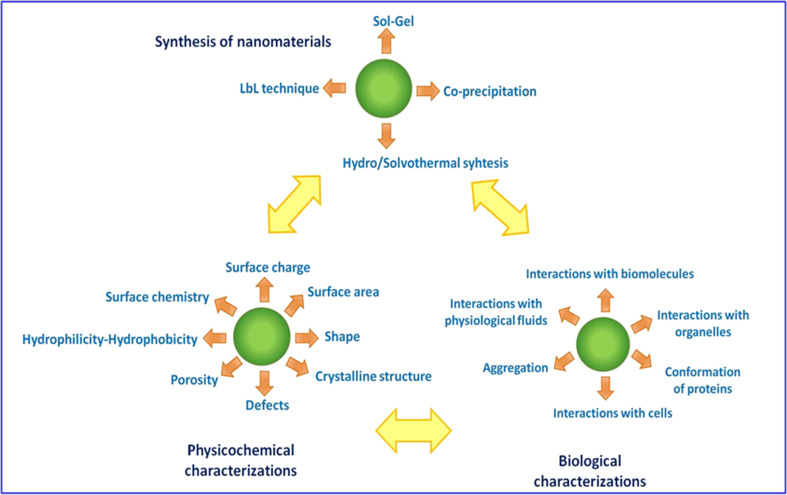
Recognition by biomolecules and cells is the key step to determine whether implantable nanomaterials are biocompatible (Nel et al. 2009). In this context, physical and chemical properties, such as size, crystalline structure, charge, composition, and wettability, must be tailored to mediate the interaction of the biomaterial with the physiological medium (Fig. 1) (Jiang et al. 2008). Biomimetic strategies have been applied to both fashion and modify the chemical and physical characteristics of nanoparticles, thereby enhancing their biological performance (Fan et al. 2006; Carmona-Ribeiro 2010).
Fig. 1.
Methods for synthesizing nanoparticles, surface properties, and physiological interactions. LbL Layer-by-layer
Biomineralization has been one of the most inspiring processes to synthesize biocompatible inorganic nanoparticles as it enables a high control over physical and chemical properties. Biomineralization is the process through which living organisms form highly organized inorganic substances (Barabási and Oltvai 2004; Mann 2004). This process is mediated by an organic matrix consisting of biomolecules such as proteins and polysaccharides, which play a crucial role in the unique properties of biominerals (Didymus et al. 1993; Mann et al. 1993; Xu et al. 2007). The organic phase accounts for nucleation and growth of the inorganic phase. Also, by means of kinetic and thermodynamic effects, nature exerts a high control over the properties of biominerals, such as size, aggregation, texture, and crystalline structure, among others (Navrotsky 2004). Jiang et al. investigated nucleation of hydroxyapatite in the presence of CS to show that the latter organic molecule provides a lower nucleation barrier to precipitation of the inorganic phase due to molecular recognition between the organic matrix and the inorganic precursors (Jiang et al. 2005). In this case, formation of the mineral does not require supersaturation, which is the force that drives precipitation. In an extensive review, Meldrum and Colfen described how nature controls the morphology and structure of minerals (Meldrum and Colfen 2008).
Materials such as bone, nacre, and silica diatoms are just a few examples of complex hierarchically structured natural materials emerging at ambient conditions. Inspired by these structures, scientists have synthesized organic–inorganic hybrid particles with controlled hierarchical structures by mimicking the biomineralization process (Cai and Tang 2008; Xu et al. 2008; He et al. 2009). To this end, biological macromolecules have been used to assemble inorganic particles through biomimetic methodologies, including emulsions (Szcześ 2009), reverse emulsions (Tai 2008), confined spaces (Gautier et al. 2007; Stephens et al. 2011), and self-assembled polymeric systems (Cruz and Ramos 2016). In these studies, numerous kinds of organic matrixes can be used, including, for example, polyelectrolytes (Falini et al. 1994), block copolymers (Colfen et al. 2001), surfactants (Lin et al. 1994), proteins (Chiu et al. 2012), and polysaccharides (Nogueira et al. 2016).
Biomimetic strategies are more versatile than those which involve controlling the physical properties of inorganic materials only. A number of studies have shown that the organic matrix can also guide the nucleation and growth of the inorganic phase by changing surface properties. Wang et al. (2006) synthesized hydrophobic calcium carbonate (CaCO3) vaterite (the thermodynamically most unstable mineral phase of CaCO3) nanoparticles through a biomimetic approach in the presence of oleic acid, to obtain hydrophobic CaCO3 nanoparticles with a hybrid structure. Oleic acid molecules associate with Ca2+ by interfacial recognition, which leads the mineral phase to precipitate. The authors found that the organic matrix not only induces nucleation and growth of CaCO3 nanoparticles, but also changes surface wettability from hydrophilic to hydrophobic. The crystalline structure and morphology and the hydrophobic character of the surface of nanoparticles depend on the concentration of oleic acid. Chen et al. (2010) synthesized hydrophilic CaCO3 nanoparticles using PE glycol phosphate. Like Wang et al. (2006); Chen et al. (2010) verified that organic molecules guide nucleation and the growth of nanoparticles and coat the surface of these particles, to produce hydrophilic structures.
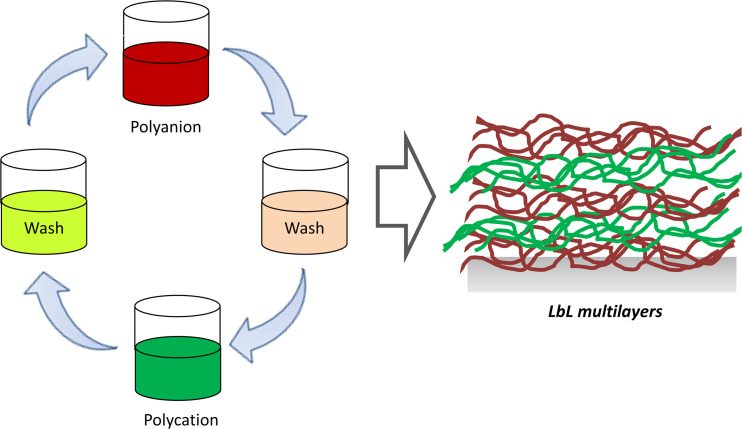
The versatile LbL technique (Fig. 2) has also been employed to precipitate biomimetic nanoparticles (Caruso 1998; (Wu and Zhang 2012). The LbL technique is based on the deposition of multilayers of opposite charge, or it can be mediated by covalent and hydrogen bonds. This technique allows the hydrophilic and hydrophobic character of nanoparticles to be controlled by modification of the surface. Other methods, such as the adsorption of polymeric dispersant, can be used to control the hydrophobic and hydrophilic character of a material in order to improve the interaction of the nanoparticles with the physiological medium, proteins, and cells (Cyster et al. 2005).
Fig. 2.
Layer-by-layer (LbL) multilayers are easily created by alternate and consecutive adsorption of oppositely charged polymers on a surface. This can be achieved by immersing the surface (a particle or a planar substrate) in a solution containing the desired polymer, followed by a washing step, to release weakly adsorbed molecules. The second layer is deposited by immersion in a solution of the oppositely charged polymer. To build the multilayers, immersion/washing cycles are repeated as many times as desired
The specific surface properties of a nanoparticle account for the adsorption of proteins, cellular interactions, and host responses (Walkey et al. 2012). Upon contact with the physiological environment, these materials interact first with biomolecules and ions and then with cells (Walkey et al. 2012). Due to their large surface area, and consequently high surface energy, nanoparticles tend to agglomerate and adsorb proteins, giving rise to a “corona” on the surface of the nanoparticles (Saptarshi et al. 2013). This “corona” will dictate the new surface properties of the material and hence its stability in physiological media (Saptarshi et al. 2013). The characteristics of the surface of the particles will strongly influence the spatial orientation of the amino acid residues in the adsorbed proteins and subsequent biological events,such as blood circulation, coagulation, and internalization (Saptarshi et al. 2013).
Although nanomaterials have been increasingly applied in living systems, investigations on the relationship between the physicochemical properties of these nanomaterials and their effect on biocompatibility are still lacking (Zhu et al. 2013). The chemical composition of the surface of nanoparticles affects hydrophilicity, which is one of the main factors related to the ability of nanoparticles to interact with biomolecules and cells (Verma and Stellacci 2010). A number of studies have shown that proteins adsorb onto hydrophilic surfaces mainly via electrostatic attraction, whereas their adsorption onto hydrophobic surfaces is driven by increased entropy assigned to the random orientation of proteins on this kind of surface (Kondo and Mihara 1996; Malmsten 1998). Zhu et al. (2013) investigated how the hydrophobicity of the surface of nanoparticles affects the spatial orientation of amino acids in adsorbed proteins. According to these authors, compared to proteins adsorbed on hydrophilic surfaces, the methyl groups of proteins adsorbed onto hydrophobic nanoparticles are more randomly oriented despite the higher amount of protein, which suggests denaturation. Saha and Das (2009) showed that the amount of basic and acidic functional groups on the surface of nanoparticles influences the adsorption of proteins more than the hydrophobic/hydrophilic character of the nanoparticles. These authors synthesized hydrophilic nanoparticles based on malachite, which has a basic nature that prompts an additional acid–base interaction between the nanoparticles and bovine serum albumin (BSA), to verify the higher adsorption of protein even at a pH higher than the isoelectric point of the nanoparticles. This result differs from those observed for hydrophilic inorganic oxide nanoparticles like magnetite and TiO2, where a lower amount of BSA adsorbs onto the surface at a pH higher than the isoelectric point. Gustafson (2015) reported that nanoparticles modified with hydrophobic molecules cause oxidative stress in cells. Liang et al. (2007) modified the surface of magnetite nanoparticles with chitosan to enhance hydrophilicity and colloidal stability. Adsorption of BSA onto the modified nanoparticles also increases as a consequence of higher hydrophilicity. The shape of nanoparticles is also an important factor. TiO2 nanorods and nanotubes adsorb proteins from human plasma in different ways (Deng et al. 2009). A faster proliferation of osteoblasts on TiO2 nanotubes has been attributed to the morphology of the tubes, which resembles the fibril arrangement of collagen in bone tissue (Suwandi et al. 2015).
Surface charge plays a key role in the biological performance of nanomaterials and drives their electrostatic interaction with proteins, cells, and biological fluids (Albanese et al. 2012). In this context, ionizable groups, such as acidic and amine groups, are often applied to modulate the charge of nanoparticles in order to generate negatively and positively charged surfaces, respectively (Mendes 2008). Experimentally, the surface charge of nanoparticles can be evaluated by measuring the zeta potential by means of electrophoretic mobility. Differently charged nanoparticles display appreciably distinct cellular behavior. For example, phosphate groups from lipids provide cell membranes with negative charge (Papahadjopoulos and Miller 1967). Positively charged nanoparticles generally exhibit a higher cellular binding and cellular uptake ability than negatively charged nanoparticles (Cho et al. 2009). Patil et al. (2007) showed that electrostatic interactions are the driving force behind the adsorption of proteins and cellular uptake in the case of Ce2O3 nanoparticles. The positive zeta potential of nanosized Ce2O3 samples favor the adsorption of albumin, whereas negative zeta potential favors the uptake of nanoparticles by cells.
The target application must be carefully evaluated before the physical and chemical properties of a nanomaterial are tailored. For example, when the goal is to obtain a nanomaterial to regenerate bone tissue, migration of proteins toward the surface of the nanomaterial is mandatory to obtain adequate cellular response (Wei and Ma 2004). In this case, biocompatibility will be dictated by the adsorption of proteins on the surface and subsequent cell migration, attachment, and differentiation. On the other hand, if the final goal is to obtain a drug-delivery system, the migration of proteins toward the surface of the nanoparticles can lead to aggregation, and the nanomaterial will be cleared by macrophages before it reaches the target tissue (Buzea et al. 2007). Therefore, in this specific example, it is necessary to modify the surface of the nanomaterial to increase the circulation of proteins, thereby avoiding their adsorption (Storm et al. 1995).
Hu et al. (2007) studied how the size of hydroxyapatite nanoparticles impacts the bioactivity of this mineral. These authors found that smaller nanocrystallites stimulate proliferation of the bone marrow mesenchymal stem cells. Nevertheless, predicting how nanoparticles will interact with physiological media is a difficult task, which leads to divergent results due to a lack of accurate physicochemical characterization (Sapsford and Russ Algar 2013). Although several studies have shown that compared to crystalline particles amorphous phosphate nanoparticles display enhanced bioactivity, Tang’s group demonstrated that most of these studies do not consider the size of the particles when different crystalline structures are compared (Hu et al. 2007). Tang’s group therefore synthesized amorphous and crystalline phosphate nanoparticles with the same size distribution to verify different results and found that crystalline phosphates affect the proliferation of osteoblasts more significantly than do amorphous calcium phosphates (Cai et al. 2007).
Despite countless studies on the physicochemical properties of nanomaterials and their biological response, to date we have been unable to come to a general conclusion on how size, shape, and surface chemistry influence the way nanomaterials and physiological media interact (Mu et al. 2016). Many studies have failed to accomplish complete characterization of the nanoparticles, providing the scientific community with unclear results (Lynch and Dawson 2008). A well-characterized system is mandatory for improved understanding and control of the biological responses of nanoparticles according to the desired applications (Barabási and Oltvai 2004).
Biomimetic modification of the surface of Ti
Pure Ti and its alloys are widely used as bone and dental implants due to their excellent mechanical properties and high chemical stability. Nevertheless, the inert surface of Ti and its alloys (considered to be due to the formation of a thin oxide layer) results in poor implant–tissue contact. The latter in turn leads to low osteointegration and to the formation of a fibrous tissue, a process which culminates in implant failure. Different methodologies have been proposed to modify the surface of Ti in order to improve osteointegration (Liang et al. 2015; Velasco-Ortega et al. 2016).
Several studies have highlighted how the physical and chemical surface properties of Ti implants are important, including composition, topography, roughness, wettability, and surface free energy (Lampin et al. 1997; Costa and Maquis 1998; Elias et al. 2008; de Souza et al. 2014; Cruz and Ramos 2016). Roughness is a very important parameter for implants because it induces cell differentiation and enhances osteointegration (Buser et al. 2004; Rupp et al. 2006). Many methods to make the surface of Ti rougher have been described, including acid etching, plasma spraying, sandblasting, and electrochemical corrosion (Liang et al. 2015; Ferreira Ribeiro et al. 2016; Hotchkiss et al. 2016; Velasco-Ortega et al. 2016). Most studies have stated that higher roughness causes higher cell adhesion (Wang et al. 2013; Shibata and Tanimoto 2015; Velasco-Ortega et al. 2016). Wang et al. (2013) studied the roughness of the surface of Ti at three different levels, i.e., smooth, micro, and micro/nanostructured surfaces, respectively, and found higher adhesion and proliferation of osteoblasts with increasing roughness. Velasco-Ortega et al. reported that roughness and topography are the two factors which influence the success of Ti implants the most. These authors also reported that rougher surfaces stimulate cells to differentiate into osteoblasts (Velasco-Ortega et al. 2016.) Kikuchi et al. (2005) demonstrated that microtopography determines activation of human blood platelets as compared to calcium phosphate-modified Ti surface (2005). On the other hand, some studies have reported that cell adhesion does not depend on roughness (Ferreira Ribeiro et al. 2016; Kaliaraj et al. 2016). Ferreira Ribeiro et al. (2016) did not observe any differences in terms of bacterial adhesion and proliferation on acid-etched and laser-irradiated Ti samples characterized by different roughness. Goriainov et al. (2014) reported that a surface characterized by moderate roughness leads to good cell attachment and elicits relatively better interfacial response, which is beneficial to integration of the implant. These authors observed that a smooth/slightly rough surface hinders cell adhesion, whereas a highly rough surface gives rise to excessively distant peaks, which harms cellular nutrition (Goriainov et al. 2014).
The oxide layer on the surface of the implant can undergo hydrolysis in aqueous medium to form pH-dependent species. A negatively charged surface is important for positive cellular responses (Han et al. 2016). Liang et al. (2015) investigated cell adhesion to a novel electrochemically micro/nanotextured surface. These authors reported that samples with a TiO2 layer generated by anodization displayed higher wettability and surface free energy than samples that had not been treated by anodization. As expected, the authors verified that a more hydrophilic surface provides higher cell adhesion and proliferation (Liang et al. 2015).
The hydrophilicity of a surface not only accounts for the initial binding of proteins and macromolecules, it also influences the orientation and conformation of these molecules on the surface. This step drives cell attraction and adhesion because it activates receptors at the outer cell membrane (Gittens et al. 2014; Goriainov et al. 2014; Han et al. 2016). Using this knowledge, researchers have proposed biomimetic modifications that use naturally occurring species and methods that improve mainly the chemical properties, but also the physical properties, of the surface of Ti to favor implant–tissue contact. Among the methodologies employed for this purpose is modification of the surface of Ti with biomolecules, such as fibrin fibers, collagen, dopamine, LbL films, and Langmuir–Blodgett films (Tejero et al. 2014; Chou et al. 2015; Cai et al. 2016; Cruz et al. 2016), and with biominerals, such as like calcium phosphates and CaCO3 (de Souza et al. 2014; Cruz et al. 2016). Many methodologies involve the use of biomolecules and biominerals as the organic framework and inducers, respectively, to precipitate hydroxyapatite. The methodological procedure consists of dipping the modified titanium in a solution called simulated body fluid (SBF) that simulates the concentrations of the ions in human plasma(Kokubo et al. 1990).
Sánchez-Treviño et al. (2016) studied the self-assembled films of alkyl phosphonate as a coating on the surface of Ti to promote the growth of hydroxyapatite and to improve cell adhesion. These authors observed that this modification increases roughness and decreases the contact angle. They also reported that these changes, mainly higher hydrophilicity, underlie the better cell adhesion of these films as compared to that of pure Ti. Biopolymers like fibrin fibers and collagen can attach to the surface of Ti by simple physical adsorption or covalent binding, or they can be trapped on the surface of Ti by electrochemical methods, and so on (Tejero et al. 2014). Cai et al. (2016). described an antifouling Ti surface based on host–guest interactions. These authors covalently anchored dopamine derivatives to Ti as host molecules and used a zwitterionic and a hydrophilic polymer as guest molecules. They found that the contact angle of the modified Ti surface decreased relative to pure Ti, although adsorption of proteins and bacteria diminished due to the antifouling properties. Chou et al. (2015) investigated a modification technique that employs oxidized dopamine as an interlayer between a LbL of heparin/collagen. According to these authors, compared to pure Ti, the contact angle of the modified surface increases after modification, affording a less hydrophilic surface while promoting hemocompatibility. These authors showed that the combined effect of oxidized dopamine with several multilayers of biopolymer affects cell adhesion positively even on a hydrophobic surface (Chou et al. 2015). This result indicates that a more hydrophilic surface does not always lead to better adhesion of cells or proteins, which also depends on the chemical properties of the species attached to the surface (Chou et al. 2015, Cai et al. 2016).
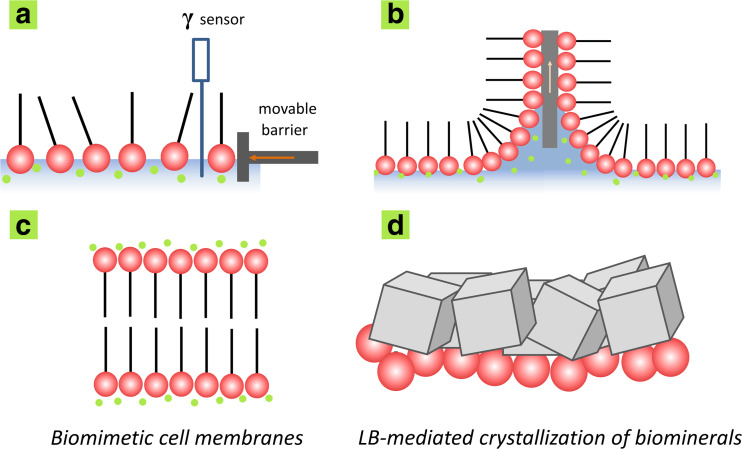
Langmuir–Blodgett (LB) films can also be used to create a biomimetic lipid membrane on metallic surfaces (Fig. 3). LB films are molecularly organized matrixes composed of amphiphilic molecules arranged in a bidimensional membrane that resembles the structure and composition of biological plasma membranes (Caseli et al. 2015). Spreading a solution of an insoluble amphiphilic molecule on an aqueous subphase causes the molecule to adsorb at the air–liquid interface. This process can be studied with the aid of a Langmuir trough (Fig 3a), which measures changes in liquid surface tension (γ) resulting from the adsorption of molecules at the air–liquid interface. A movable barrier can reduce the interfacial area by causing the amphiphilic molecules to condense at the interface. After reaching a specific state of condensation, these molecules can be transferred to solid supports by passing the support vertically through the air–liquid interface to produce LB films where the number of layers depends on immersion and withdrawal cycles (Fig. 3b). The proper choice of the amphiphilic molecule and ionic composition leads the resulting organic matrix to act as a framework for the crystallization of biominerals (Fig. 3d).
Fig. 3.
a, b Representation of a Langmuir trough used to study the behavior of insoluble amphiphilic molecules at the air–liquid interface (a) and to build Langmuir–Blodgett (LB) films (b). c, d The resulting LB film (c) exhibits a structure and composition that resembles the structure and composition of biological plasma membranes (d) and can be used as a biomimetic system to mediate crystallization of biominerals
Besides containing lipids, LB films can carry ions and other molecules during formation of the matrix (de Souza et al. 2014; Cruz et al. 2016; Ruiz et al. 2017). Cruz et al. (2016) recently developed a method that consists of transferring dihexadecyl phosphate (DHP) with Ca2+ ions by the LB technique and exposing the film to a CO2-rich atmosphere, with the aim to generate CaCO3 on the surface of the matrix and to stimulate further growth of the hydroxyapatite therein. According to these authors, these modifications result in a rougher and more hydrophilic surface as compared to pure Ti, and viable osteoblasts reveal improved recovery of surface cells (Cruz et al. 2016). Ruiz et al. (2017) used LB films to deposit collagen fibers on the surface of Ti and to induce precipitation of hydroxyapatite. These authors showed that this modification enhances cell viability due to increased wettability, surface free energy, and roughness (Ruiz et al. 2017). De Souza et al. (2014) provided evidence that surfaces with comparable roughness but different composition induce different responses from osteoblasts. In their study, sand-blasted Ti presented lower cell viability than to hydroxyapatite-modified Ti (de Souza et al. 2014). This result indicates that initial contact of the cell with biomimetic coatings is important.
In addition, roughness and wettability are both extremely important for biocompatibility and osteointegration of Ti implants. Tejero et al. (2014) explained that it is difficult to separate the contribution of these two parameters to the final implant material . Wenzel’s equation relates roughness with wettability,
where r is the average roughness, θ W is the measured contact angle, and θ Y is the Young contact angle for an ideal smooth surface (Wenzel 1936; Wolansky and Marmur 1999). This equation shows that increased roughness reduces the contact angle, which means that a more hydrophilic surface arises. However, Wenzel’s equation cannot be applied to general surfaces originating from several modifications along the years (Rodríguez-Valverde 2008; Yamaguchi et al. 2014; Jardim et al. 2016).
Development of super-wettable bioinspired materials
The focus of this section is the recent advances in bioinspired, extremely wettable surfaces for biomedical applications. Surface wettability is an important property for plants and other living organisms because it affects photosynthesis, absorption of water, infection with pathogens, and other physiological processes (Liu et al. 2014). For this reason, naturally occurring materials with unique properties of surface wettability (such as lotus leaves, rice leaves, peanut leaves, red rose petals, cicada wings, butterfly wings, mosquito compound eyes, fly eyes, gecko feet, nepenthes pitcher plants, Salvinia molesta floating leaves, desert beetles, spider silk, cactus, and fish scales, among others) have long served as sources of inspiration to scientists and engineers (Liu et al. 2014; Shin et al. 2016). Extreme wetting properties, i.e., superhydrophobicity and superhydrophilicity (two terms used to describe incongruous behavior of water on a solid surface), can be found in living species (Drelich et al. 2011).
Superhydrophilic and superhydrophobic behaviors have attracted considerable attention because they may help to further out understanding of how nature solves engineering problems. A better understanding could, in turn be the basis for obtaining materials and surfaces with potential applications in industry, agriculture, and daily life, such as in self-cleaning fabrics, anti-fog windows, anti-corrosive coatings, and drag-reduction systems, among others (Nosonovsky and Bhushan 2008; Liu et al. 2014; Shin et al. 2016).
Among these applications, self-cleaning surfaces have attracted much interest for both reasons of fundamental research and practical applications (Liu and Jiang 2012). Lotus leaves are one of the most promising bioinspired self-cleaning surfaces. Lotus roots are embedded in muck, but the leaves of this plant are seemingly never dirty. Water droplets fall onto and roll off of the leaves, and rainwater washes dirt from the lotus leaves. Therefore, these leaves can accomplish self-cleaning, which is known as the lotus effect. Liu et al. ( 2010) reviewed several studies that explain the self-cleaning mechanism of lotus leaves on the basis of micropapillae that have diameters ranging from 5 to 9 μm and are randomly distributed on the surface of lotus leaves. Each papilla contains fine branch-like nanostructures with a diameter of approximately 120 nm. Multiscaled structures form air pockets, resulting in a smaller contact area between the surface of lotus leaves and water droplets. Hydrophobic three-dimensional epicuticular waxes with a tubular structure also exist on the surface of lotus leaves. In addition, Liu et al. (2012) reported that cooperation of surface micro- and nanoscaled hierarchical structures and hydrophobic epicuticular waxes confers a high water contact angle and small sliding angle of approximately 160° and 2°, respectively, giving rise to superhydrophobic and low-adhesion characteristics (Nosonovsky and Bhushan 2008; Liu and Jiang 2012). Superhydrophilicity is another wetting behavior of solid surfaces defined by their static contact angle (Koch and Barthlott 2009). Surfaces are termed superhydrophilic when the contact angle is <10°; surfaces with a contact angle of >10° and <90° are termed hydrophilic (Liu et al. 2010).
Inspired by superwettable biomaterials, researchers have proposed a great number of innovative strategies to fabricate novel and advanced materials by tailoring the geometric structure and chemical composition of surfaces (Drelich et al. 2011; Liu and Jiang 2012; Liu et al. 2014). Shin et al. (2016) stated that the non-wetting behavior of water droplets on superhydrophobic surfaces is governed not only by topography, but also by its combination with surface chemistry. In their study Nosonovsky and Bhushan (2008) discussed the role(s) of roughness and hierarchical structure in superhydrophobicity. Their experiments showed that both hierarchical and non-hierarchical surfaces can be superhydrophobic, suggesting that roughness itself has an important part in superwettable properties but that hierarchical structure, while beneficial, is not mandatory. Moreover, in their study, Nosonovsky and Bhushan (2008) noted that roughness is more important than low surface energy because an extremely low surface energy is not necessary to achieve superhydrophobicity. In certain cases, even initially hydrophilic surfaces can exhibit superhydrophobicity after roughening. Therefore, roughness is the central property for bioinspired superhydrophobic surfaces.
Among the different areas of application of superhydrophobic surfaces, Shin et al. (2016) excelled in their work on the engineering of surface wettability by manipulating chemical properties and structure to elucidate biomedical applications ranging from high-throughput cell culture platforms to biomedical devices. For implanted medical devices to be applied in biomedicine, undesired biological matter must be prevented from adhering to the surface of the implant. It is crucial that the surface possess antibacterial property to prevent inflammation of the implanted device or contamination during cell/tissue culture. To eliminate or substantially reduce the extent of bacterial attachment, intensive efforts have focused on the fabrication of antibacterial surfaces based on bioinspired superhydrophobic or superhydrophilic surfaces (Hasan et al. 2013; Shin et al. 2016). However, despite all the efforts to date to apply bioinspired surfaces in the biomedical field, these surfaces are still in the early stages as compared to their conventional use in other industries. A number of critical issues need to be addressed for the wide use of bioinspired surfaces as advanced biomedical platforms. For example, the durability and long-term stability of surfaces with extreme wetting properties must be improved.
Surface modifications that lead to superhydrophobicity
Ma and Hill (2006) emphasized that it is important that materials for biomedical applications display a non-wettable character. In this section, we highlight a number of studies that contextualize both chemical and physical methods for the modification of the wetting properties of biomaterials. Antibacterial surfaces have been developed by different strategies that are used to modify surface chemistry (Hasan et al. 2013), including modification of the surface by immobilization of an antimicrobial agent, functionalization of the surface with an antimicrobial agent, and derivatization (these approaches involve the introduction of positively charged coatings with a long, hydrophobic chain, such as alkylated PE amines or of a functional group via polymerization, covalent linkages, and/or plasma treatment).
There are many examples of superhydrophilic and superhydrophobic antibacterial surfaces. Wang et al. (2017) developed a novel {2-(dimethylamino)-ethyl methacrylate-co-2-methacryloyloxyethyl phosphorylcholine [p(DMAEMA-co-MPC]} brush that had been chemically modified with 1-bromo-heptane and exhibited low roughness, high hydrophilicity, and bactericidal function. Hasan et al. (2015) fabricated a nanostructured “super-surface” by using a simple recipe based on deep reactive ion etching of a silicon wafer. The resulting topography consists of nanopillars with height of 4 μm and diameter of 220 nm as well as random inter-pillar spacing and resembles the surface topographical features of the wings of a dragonfly. This surface is superhydrophobic and has a static water contact angle of 154.0° and contact angle hysteresis of 8.3°. It kills bacterial cells because the sharp surface nanopillars rupture the bacterial cell membrane.
In terms of chemical and physical modifications, the study of Whitehead et al. (2005) is noteworthy. These authors used ion beam sputtering technology to coat silicon wafers and wafers attached to nucleopore® filters (Whatman plc, GE Healthcare, Little Chalfont, UK) and quantifoils with Ti. This technique produces irregularly spaced, but regularly featured surface pits measuring 0.2 and 0.5 μm as well as regularly spaced pits with regular diameters of 1 and 2 μm. The authors found that only the contact angle of the surfaces measuring 1 and 2 μm are significantly different from the smooth surfaces of Ti (Whitehead et al. 2005). They also showed that the dimension of surface defect is important to the size of the cell and its subsequent retention. Ma and Hill (2006) highlighted that techniques to develop superhydrophobic surfaces, such as the ones mentioned above, can be divided into two categories, namely, making a rough surface from a material with low surface energy and modifying a rough surface with a material with low surface energy. In the first case, compounds such as fluorinated polymers and poly(dimethylsiloxane) (PMDS) (silicones) have been widely used to develop superhydrophobic surfaces because they have extremely low surface energy. For example, Singh et al. (2005) reported a simple and effective way to achieve superhydrophobic nanofibers of poly[bis(2,2,2-trifluoroethoxy)phosphazene] by electrospinning solutions in tetrahydrofuran, methylethyl ketone, and acetone. According to these authors, hydrophobicity varies as a function of the diameter of the fiber and of surface morphology, with contact angles relative to water ranging from 135° to 159°. Extremely high hydrophobicity results from a combination of fluorinated surface with inherent surface roughness. These same authors (Singh et al. 2005) also obtained superhydrophobic surfaces by developing two PMDS templates using a nanocasting method. These authors showed that, despite the distinct composition and consequent different surface energy of the lotus leaf and the PDMS replica, the positive PDMS template surprisingly has the same surface structures and almost the same superhydrophobicity as the lotus leaf. Ma et al. (2005) proposed another way to exploit the low surface energy of PDMS, namely, by developing a superhydrophobic membrane composed of fibers of the block copolymer poly(styrene-b-dimethylsiloxane) (PS-PDMS) with diameters ranging from 150 to 400 nm. The authors attributed the resulting superhydrophobicity to combined effects of surface enrichment in siloxane and roughness (Ma et al. 2005; Ma and Hill 2006). They also highlighted that flexibility, breathability, and the free-standing feature of the membrane are of particular interest in areas such as textile and biomedical applications.
Other compounds can also be employed to obtain superhydrophobic surfaces (Ma and Hill 2006; Nosonovsky and Bhushan 2008). Lu et al. (2004) produced a highly porous superhydrophobic PE surface from an organic material, which enabled them to control crystallization. Inspired by the combination of two amazing natural abilities, the superhydrophobic property of lotus leaves and the adhesiveness of mussel protein, Zhang et al. (2012) synthesized silver nanoparticles. These authors highlighted that a clever choice of core materials paves the way for new applications in the medical and biological fields. By modifying a rough surface with a material of low surface energy, Ma and Hill (2006) noted that methods leading to superhydrophobic surfaces are mostly one-step, simple processes although they are always limited to a small set of materials. Etching and lithography, sol–gel processing, LbL, and electrochemical deposition are examples of these processes (Ma and Hill 2006; Nosonovsky and Bhushan 2008).
In terms of etching, which is a straightforward and effective way to make rough surfaces, Teshima et al. (2005) produced ultra-water-repellent polymer sheets from a nanotexture on the surface of a substrate consisting of PET to achieve a transparent material. Using lithography, a well-established technique to create periodic micro/nanopatterns with a large area, Abdelsalam et al. (2005) studied the wettability of structured gold surfaces formed by the electrodeposition of monolayer templates of closely packed uniform submicrometer spheres. These authors explained that the thickness of the gold layer deposited through the template controls the depth of the pores and the topography of the surface and influences the apparent contact angle of the surface with water, consequently increasing the thickness of the porous film relative to the radius of the pores. In this study, the contact angle increased from 70° to >130°, even though gold itself is hydrophilic. Consequently, the study of Abdelsalam et al. (2005) is an example of how a hydrophilic surface can be changed to a hydrophobic surface purely by controlling the topography of the surface.
Both the energy and the roughness of a surface can be controlled by using colloidal silica particles and fluoroalkylsilane in the sol–gel technique (Hikita et al. 2005). In this technique, monomers are transformed into a colloidal solution (sol) that will act as a precursor for the formation of a complex network of particles (gel) (Hench and West 1990). The LbL technique can also be used to develop surfaces with super-wettability. Lopez-Torres et al. (2015) reported a nanocoating process at ambient temperature that provides the required level of roughness for a material to display superhydrophilic and superhydrophobic behavior without the need to use nanoparticles. The authors fabricated a superhydrophilic nanocoating by using poly(allylamine hydrochloride) (PAH) and poly(sodiumphosphate) (PSP) with different numbers of bilayers. Nanofilms with >20 bilayers exhibited a contact angle close to 0°. After functionalization with 1H,1H,2H,2H–perfluorodecyltriethoxysilane, the films were transformed into hydrophobic coatings with contact angle of 165° for the 40-bilayer film (Lopez-Torres et al. (2015).
Electrochemical deposition has been extensively used to prepare super-wettable nanocoatings through low-cost, reproducible, and fast methods (Si and Guo 2015). One of the main advantages of this methodology is the possibility to produce surfaces with different morphologies and a precise control of the structure at the micro- or nanoscale. Han et al. (2005) proposed manufacturing lotus leaf-like superhydrophobic metal surfaces from a simple electrochemical reaction of Cu or Cu–Sn.
In light of the last two methodologies mentioned herein, LbL can also be combined with electrochemical deposition to prepare superhydrophobic surfaces. Shi et al. (2005) proposed combining these two techniques to develop a superhydrophobic coating on gold threads to mimic the legs of water striders.
Application of superhydrophobicity in the biomedical field
Application of superhydrophobic surfaces in the biomedical field entails more than just obtaining antibacterial materials. In the field of bone regeneration, Lima et al. (2013, 2015) obtained hybrid materials by immobilizing fibronectin and cells onto biomimetic superhydrophobic surfaces. Huang et al. (2015) fabricated coatings consisting of superhydrophobic anatase TiO2 nanotubes on 316 L stainless steel, with the aim to reduce adhesion/activation of platelets and to improve resistance to corrosion.
However, superhydrophobicity can impair the regeneration of bone tissue. Alves et al. (2009) verified that surfaces which repel water may prevent adhesion and proliferation of bone marrow-derived cells. With respect to other applications in the biomedical field, Desrousseaux et al. (2013) noted that biofouling of medical devices generally causes adverse complications, such as thrombosis, infection, and pathogenic calcification. These authors assumed that modification of the surface of silicone, a material that is widely employed in medical applications, encounters undesirable “hydrophobic recovery,” which results in a deterioration of surface engineering. They consequently proposed developing a material with opposite behavior toward superwettability in order to obtain a substrate with excellent bioinertness upon exposure to solutions containing bacteria, proteins, and lipids (Desrousseaux et al. 2013). Based on these studies, it is clear that there are cases requiring materials with a wettable character, or superhydrophilicity.
The authors of a number of other studies have compared superhydrophobic and superhydrophilic surfaces. Ishizaki et al. (2010) investigated the physicochemical properties of superhydrophobic, superhydrophilic, and superhydrophobic/superhydrophilic surfaces, with the aim to verify that cells adhered and proliferated on both superhydrophobic and superhydrophilic surfaces. Nevertheless, these authors found that constant contact was necessary to facilitate cell division and proliferation on the superhydrophobic surface. In the same study, they also examined the adsorption behavior of proteins on flat hydrophobic and hydrophilic surfaces to observe whether a higher amount of protein adsorbed onto the flat hydrophilic surface (Ishizaki et al. 2010). Therefore, if we consider biomedical applications, exploring surface modification strategies that result in superhydrophilic surfaces is essential.
Surface modifications that lead to superhydrophilicity
Research into superhydrophilicity has emerged in the last few years. The number of publications has increased significantly since 2000, just after the boost in the number of published studies on superhydrophobic surfaces. These studies are now moving toward several possible applications and toward the commercialization of different products and devices (Drelich et al. 2011).
Roughening of the surface of hydrophilic materials has been conducted to improve adhesion in composites, biocompatibility in implant devices, and spreading of liquids. Drelich et al. ( 2011) highlighted that, at least theoretically, any natural or synthetic material can be converted to a superhydrophilic material by chemical treatment and mechanical roughening on the basis of the same principles and techniques mentioned above for the development of superhydrophobic surfaces.
Among inorganic materials, the use of TiO2 (Nakata et al. 2010) and ZnO (Wu et al. 2011) is noteworthy. These two oxides have been widely studied due to their photoinduced self-cleaning ability (Drelich et al. 2011; Nakata et al. 2011; Wu et al. 2011; He 2015). Ganesh et al. (2012) used electrospinning to develop a photocatalytic, superhydrophilic, transparent, porous TiO2 film consisting of rice-shaped nano/mesostructures deposited on glass substrates. In turn, Wang et al. (2014) employed the sol–gel spin-coating technique to produce a superhydrophilic Cu-doped TiO2 thin film with excellent anti-fogging behavior. The wide availability and low cost of SiO2 motivated Kou and Gao (2011) to design a strategy to synthesize nanohybrids consisting of graphene oxide coated with silica nanoparticles. Their procedure provided a material with an excellent hydrophilic nature and potential direct application as a general kind of building block to construct large-area superhydrophilic surfaces on arbitrary substrates through the simple drop-coating method (Kou and Gao 2011).
Besides the use of inorganic materials, we can highlight the use of polymers, which are attractive materials for superhydrophilic coatings. Improving the hydrophilicity of polymeric surfaces typically requires oxidation, which must also affect the roughness of the surface or must be performed in conjunction with surface roughening (Drelich et al. 2011). Agrawal et al. (2014) used argon ion to modify the surface of nanocomposite polymer membranes, with the aim to diminish bacterial cell adhesion and to increase wettability. Zogbi et al. (2014) applied an electron beam to obtain a superhydrophilic composite consisting of vertically aligned multi-walled carbon nanotubes and hydroxyapatite on a pure Ti alloy substrate. Chen and Su (2011) introduced –COOH groups on the surface of poly(lactic acid) nanofibers with cationized gelatin in order to improve compatibility of the polymer with chondrocytes.
Application of superhydrophilicity in the biomedical field
According to Drelich et al. (2011), superhydrophilic coatings have also attracted the interest of researchers in the field of biomedical engineering. These authors emphasize that both polymers and inorganic compounds can be used to make more biocompatible and hydrophilic implantable materials. Superhydrophilic coatings are also becoming applicable in antifouling, antimicrobial, and/or biologically active surfaces that perform tasks other than imparting lubricity and reducing friction. For example, biomedical applications of polymers include the development of lenses and catheters (Kim et al. 2008; Babcock et al. 2013). To avoid adverse clinical events due to the adhesion of bacteria to these materials, researchers have prepared biologically non-fouling surfaces to which proteins, lipids, and cells do not adhere. For this reason, applied research on surface modification should be substantially tied to the purpose of rendering the surface of coatings superhydrophilic. In addition, superhydrophilic coatings, which provide better lubricity than superhydrophobic coatings, are interesting in terms of fabricating catheters, which require low friction to facilitate their handling within the patient’s vasculature. In the case of lenses, wearer comfort and better biocompatibility are achieved through wetting of the coating with tear fluid, which allows lenses to move relatively freely on the eye. Cui et al. (2014) developed low-fouling surfaces for biomedical applications. These authors verified that substrate-independent, low-fouling surfaces produced by immobilization of mussel-inspired polydopamine coatings and zwitterionic glutamic acid- and lysine-based peptides on substrates like noble metals, metal oxides, polymers, and semiconductors result in promising materials for various applications, including biosensing and drug delivery (Cui et al. 2014).
The importance of superhydophilicity to the design of three dimensional (3D) scaffolds for application as orthopedic implants and in tissue engineering is worth highlighting (Lai et al. 2010). Specific studies in this area have concentrated on improving the bioactivity and biocompatibility of core materials used in orthopedic applications, such as Ti-based alloys (Oh et al. 2005; Mohan et al. 2012; Madhan Kumar and Rajendran 2013) and polymers (Gentile et al. 2012; Kunjukunju et al. 2013; Ma and Tang 2014). These studies have focused on coatings containing biomimetic calcium phosphate bioactive layers or on chemical modifications that enhance formation of hydroxyapatite on the surface of biomaterials upon contact with the bone tissue (Baker et al. 2006). In the study of Oh et al. (2005), the vertically aligned TiO2 nanotube array fabricated on the surface of a Ti substrate became bioactive after treatment with a solution of NaOH, thereby inducing the growth of hydroxyapatite in SBF. Nanostructures accelerate the kinetics of hydroxyapatite formation significantly because they induce the nucleation and growth of a nanoscaled hydroxyapatite phase. However, the biological properties of coated implants and scaffolds depend not only on the chemical composition of the coating but also on its structure (Drelich et al. 2011). An ideal coating should resemble the structure of natural bone, which favors cell anchoring and cell culture and should be a run-through 3D structure (Drelich et al. 2011). Another point is that superhydrophilicity favors deposition of Ca-based bioactive coatings on biomaterials (Lai et al. 2010). This is an important property to explore during the development of 3D structures of materials for application as orthopedic implants and tissue engineering scaffolds (Wu et al. 2008). Kizuki et al. (2015) used the sol–gel process to modify the substrate polyetheretherketone, a polymer that is widely employed in spinal fusion devices in orthopedic implants, with a bioactive and superhydrophilic TiO2 coating. This modification added bone-bonding properties to the substrate and demonstrated that positively charged TiO2 facilitates the formation of apatite in SBF within a short period.
Acknowledgements
The authors thank Cynthia M.C.P. Manso for the revision of the English language.
Compliance with ethical standards
Funding information
The authors thank the National Council for Scientific and Technological Development (CNPq) for the financial support (442834/2014-4). T.T. Paterlini thanks the Coordination for the Improvement of Higher Education Personnel (CAPES) for the master studentship. L.F.B Nogueira, M.A.E. Cruz, Rafael Derradi, and C.B. Tovani thanks São Paulo Research Foundation for their master and doctoral studentships (2016/25955-1, 2015/08774-0, 2015/03594-4, and 2014/24249-0, respectively).
Conflicts of interest
Thais T. Paterlini declares that he has no conflicts of interest. Lucas F.B. Nogueira declares that he has no conflicts of interest. Camila B. Tovani declares that she has no conflicts of interest. Marcos A.E. Cruz declares that he has no conflicts of interest. Rafael Derradi declares that he has no conflicts of interest. Ana P. Ramos declares that she has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a special issue on ‘Latin America’ edited by Pietro Ciancaglini and Rosangela Itri.
References
- Abdelsalam ME, Bartlett PN, Kelf T, Baumberg J. Wetting of regularly structured gold surfaces. Langmuir. 2005;21:1753–1757. doi: 10.1021/la047468q. [DOI] [PubMed] [Google Scholar]
- Agrawal NK, Agarwal R, Awasthi K, et al. Surface modification of nanocomposite polymer membranes by ion plasma irradiation for improving biocompatibility of polymer. Adv Mater Lett. 2014;5:645–651. doi: 10.5185/amlett.2014.nib502. [DOI] [Google Scholar]
- Albanese A, Tang PS, Chan WCW. The effect of Nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- Alves NM, Shi J, Oramas E, et al. Bioinspired superhydrophobic poly(L-lactic acid) surfaces control bone marrow derived cells adhesion and proliferation. J Biomed Mater Res A. 2009;91:480–488. doi: 10.1002/jbm.a.32210. [DOI] [PubMed] [Google Scholar]
- Babcock DE, Hergenrother RW, Craig DA, et al. In vivo distribution of particulate matter from coated angioplasty balloon catheters. Biomaterials. 2013;34:3196–3205. doi: 10.1016/j.biomaterials.2013.01.040. [DOI] [PubMed] [Google Scholar]
- Baker KC, Anderson MA, Oehlke SA, et al. Growth, characterization and biocompatibility of bone-like calcium phosphate layers biomimetically deposited on metallic substrata. Mater Sci Eng C. 2006;26:1351–1360. doi: 10.1016/j.msec.2005.08.015. [DOI] [Google Scholar]
- Barabási A-L, Oltvai ZN. Network biology: understanding the cell’s functional organization. Nat Rev Genet. 2004;5:101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- Buser D, Broggini N, Wieland M, et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529–533. doi: 10.1177/154405910408300704. [DOI] [PubMed] [Google Scholar]
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–MR71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- Cai XY, Li NN, Chen JC, et al. Biomimetic anchors applied to the host-guest antifouling functionalization of titanium substrates. J Colloid Interface Sci. 2016;475:8–16. doi: 10.1016/j.jcis.2016.04.034. [DOI] [PubMed] [Google Scholar]
- Cai Y, Liu Y, Yan W, et al. Role of hydroxyapatite nanoparticle size in bone cell proliferation. J Mater Chem. 2007;17:3780. doi: 10.1039/b705129h. [DOI] [Google Scholar]
- Cai Y, Tang R. Calcium phosphate nanoparticles in biomineralization and biomaterials. J Mater Chem. 2008;18:3775. doi: 10.1039/b805407j. [DOI] [Google Scholar]
- Carmona-Ribeiro AM. Biomimetic nanoparticles: preparation, characterization and biomedical applications. Int J Nanomedicine. 2010;5:249–259. doi: 10.2147/IJN.S9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso F (1998) Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 80(282):1111–1114. doi: 10.1126/science.282.5391.1111 [DOI] [PubMed]
- Caseli L, Nobre TM, Ramos AP, et al. The role of Langmuir monolayers to understand biological events. ACS Symp Ser. 2015;1215:65–88. doi: 10.1021/bk-2015-1215.ch004. [DOI] [Google Scholar]
- Chang H, Wang Y (2011) Cell responses to surface and architecture of tissue engineering scaffolds. In: Eberli D (ed) Regenerative medicine and tissue engineering-cells and biomaterials, 1st edn. InTech, Croatia, pp 569-588
- Chen JP, Su CH. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011;7:234–243. doi: 10.1016/j.actbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Chen W, McCarthy TJ. Layer-by-layer deposition: a tool for polymer surface modification. Macromolecules. 1997;30:78–86. doi: 10.1021/ma961096d. [DOI] [Google Scholar]
- Chen X, Zhu Y, Zhou B, et al. Hydrophilic CaCO3 nanoparticles designed for poly (ethylene terephthalate) Powder Technol. 2010;204:21–26. doi: 10.1016/j.powtec.2010.07.002. [DOI] [Google Scholar]
- Chithrani BD, Ghazani AA, Chan WCW. Determing the size and shape dependence of goldnanoparticles uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- Chiu D, Zhou W, Kitayaporn S, et al. Biomineralization and size control of stable calcium phosphate core–protein shell noparticles: potential for vaccine applications. Bioconjug Chem. 2012;23:610–617. doi: 10.1021/bc200654v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EC, Xie J, Wurm PA, Xia Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I 2/KI etchant. Nano Lett. 2009;9:1080–1084. doi: 10.1021/nl803487r. [DOI] [PubMed] [Google Scholar]
- Chou CC, Hsin SW, Lin HC, et al. Oxidized dopamine as the interlayer between heparin/collagen polyelectrolyte multilayers and titanium substrate: an investigation of the coating’s adhesion and hemocompatibility. Surf Coatings Technol. 2015;303:277–282. doi: 10.1016/j.surfcoat.2016.03.098. [DOI] [Google Scholar]
- Colfen H, Cölfen H, Colfen H, Cölfen H. Double-hydrophilic block copolymers: synthesis and application as novel surfactants and crystal growth modifiers. Macromol Rapid Commun. 2001;22:219–252. doi: 10.1002/1521-3927(20010201)22:4<219::AID-MARC219>3.0.CO;2-G. [DOI] [Google Scholar]
- Costa N, Maquis PM. Biomimetic processing of calcium phosphate coating. Med Eng Phys. 1998;20:602–606. doi: 10.1016/S1350-4533(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Cruz MAE, Ramos AP. Bioactive CaCO3/poly(acrylic acid)/chitosan hybrid coatings deposited on titanium. Surf Coatings Technol. 2016;294:145–152. doi: 10.1016/j.surfcoat.2016.03.084. [DOI] [Google Scholar]
- Cruz MAE, Ruiz GCM, Faria AN, et al. Calcium carbonate hybrid coating promotes the formation of biomimetic hydroxyapatite on titanium surfaces. Appl Surf Sci. 2016;370:459–468. doi: 10.1016/j.apsusc.2015.12.250. [DOI] [Google Scholar]
- Cui J, Ju Y, Liang K, et al. Nanoscale engineering of low-fouling surfaces through polydopamine immobilisation of zwitterionic peptides. Soft Matter. 2014;10:2656–2663. doi: 10.1039/C3SM53056F. [DOI] [PubMed] [Google Scholar]
- Cyster LA, Grant DM, Howdle SM, et al. The influence of dispersant concentration on the pore morphology of hydroxyapatite ceramics for bone tissue engineering. Biomaterials. 2005;26:697–702. doi: 10.1016/j.biomaterials.2004.03.017. [DOI] [PubMed] [Google Scholar]
- de Souza ID, Cruz MAE, de Faria AN, et al. Formation of carbonated hydroxyapatite films on metallic surfaces using dihexadecyl phosphate-LB film as template. Colloids Surf B Biointerfaces. 2014;118:31–40. doi: 10.1016/j.colsurfb.2014.03.029. [DOI] [PubMed] [Google Scholar]
- Deng Z, Mortimer G, Schiller T. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology. 2009;20:455101. doi: 10.1088/0957-4484/20/45/455101. [DOI] [PubMed] [Google Scholar]
- Desrousseaux C, Sautou V, Descamps S, Traoré O. Modification of the surfaces of medical devices to prevent microbial adhesion and biofilm formation. J Hosp Infect. 2013;85:87–93. doi: 10.1016/j.jhin.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Didymus JM, Oliver P, Mann S, et al. Influence of low-molecular-weight and macromolecular organic additives on the morphology of calcium carbonate. J Chem Soc Faraday Trans. 1993;89:2891. doi: 10.1039/ft9938902891. [DOI] [Google Scholar]
- Drelich J, Chibowski E, Meng DD, Terpilowski K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter. 2011;7:9804. doi: 10.1039/c1sm05849e. [DOI] [Google Scholar]
- Elias CN, Oshida Y, Lima JHC, Muller CA. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J Mech Behav Biomed Mater. 2008;1:234–242. doi: 10.1016/j.jmbbm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Falini G, Gazzano M, Ripamonti a (1994) Crystallization of calcium-carbonate in presence of magnesium and polyelectrolytes. J Cryst Growth 137:577–584. doi: 10.1016/0022-0248(94)91001-4
- Fan X, Lin L, Messersmith PB. Surface-initiated polymerization from TiO2 nanoparticle surfaces through a biomimetic initiator: a new route toward polymer-matrix nanocomposites. Compos Sci Technol. 2006;66:1195–1201. doi: 10.1016/j.compscitech.2005.10.001. [DOI] [Google Scholar]
- Ferreira Ribeiro C, Cogo-Müller K, Franco GC, et al. Initial oral biofilm formation on titanium implants with different surface treatments: an in vivo study. Arch Oral Biol. 2016;69:33–39. doi: 10.1016/j.archoralbio.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Ganesh VA, Nair AS, Raut HK, et al. Photocatalytic superhydrophilic TiO2 coating on glass by electrospinning. RSC Adv. 2012;2:2067. doi: 10.1039/c2ra00921h. [DOI] [Google Scholar]
- Gautier C, Lopez PJ, Livage J, Coradin T. Influence of poly-l-lysine on the biomimetic growth of silica tubes in confined media. J Colloid Interface Sci. 2007;309:44–48. doi: 10.1016/j.jcis.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Gemini-Piperni S, Takamori ER, Sartoretto SC, et al. Cellular behavior as a dynamic field for exploring bone bioengineering: a closer look at cell-biomaterial interface. Arch Biochem Biophys. 2014;561:88–98. doi: 10.1016/j.abb.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Gentile P, Mattioli-Belmonte M, Chiono V, et al. Bioactive glass/polymer composite scaffolds mimicking bone tissue. J Biomed Mater Res A. 2012;100A:2654–2667. doi: 10.1002/jbm.a.34205. [DOI] [PubMed] [Google Scholar]
- Gittens RA, Scheideler L, Rupp F, et al. A review on the wettability of dental implant surfaces II: biological and clinical aspects. Acta Biomater. 2014;10:2907–2918. doi: 10.1016/j.actbio.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriainov V, Cook R, Latham JM, et al. Bone and metal: an orthopaedic perspective on osseointegration of metals. Acta Biomater. 2014;10:4043–4057. doi: 10.1016/j.actbio.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Gustafson HH, Holt-Casper D, Grainger DW, et al. Nanoparticle uptake: the phagocyte problem HHS public access. Nano Today. 2015;10:487–510. doi: 10.1016/j.nantod.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallab NJ, Bundy KJ, O’Connor K, et al. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng. 2001;7:55–71. doi: 10.1089/107632700300003297. [DOI] [PubMed] [Google Scholar]
- Han A, Tsoi JKH, Pires F, et al. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int J Adhes Adhes. 2016;69:58–71. doi: 10.1016/j.ijadhadh.2016.03.022. [DOI] [Google Scholar]
- Han JT, Jang Y, Lee DY, et al. Fabrication of a bionic superhydrophobic metal surface by sulfur-induced morphological development. J Mater Chem. 2005;15:3089. doi: 10.1039/b504850h. [DOI] [Google Scholar]
- Han L, Lu X, Liu K, et al. Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano. 2017;11:2561–2574. doi: 10.1021/acsnano.6b05318. [DOI] [PubMed] [Google Scholar]
- Hasan J, Crawford RJ, Ivanova EP. Antibacterial surfaces: the quest for a new generation of biomaterials. Trends Biotechnol. 2013;31:295–304. doi: 10.1016/j.tibtech.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Hasan J, Raj S, Yadav L, Chatterjee K. Engineering a nanostructured “super surface” with superhydrophobic and superkilling properties. RSC Adv. 2015;5:44953–44959. doi: 10.1039/C5RA05206H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY. Photoinduced superhydrophilicity and high photocatalytic activity of ZnO-reduced graphene oxide nanocomposite films for self-cleaning applications. Mater Sci Semicond Process. 2015;31:200–208. doi: 10.1016/j.mssp.2014.11.029. [DOI] [Google Scholar]
- He W, Zhou W, Wang Y, et al. Biomineralization of iron phosphate nanoparticles in yeast cells. Mater Sci Eng C. 2009;29:1348–1350. doi: 10.1016/j.msec.2008.10.030. [DOI] [Google Scholar]
- Hench LL, Polak JM (2002) Third-generation biomedical materials. Science 295:1014–1017. doi: 10.1126/science.1067404 [DOI] [PubMed]
- Hench L, Wilson J (1984) Surface-active biomaterials. Science 226:630–636. doi: 10.1126/science.6093253 [DOI] [PubMed]
- Hench LL, West JK. The sol-gel process. Chem Rev. 1990;90:33–72. doi: 10.1021/cr00099a003. [DOI] [Google Scholar]
- Hikita M, Tanaka K, Nakamura T, et al. Super-liquid-repellent surfaces prepared by colloidal silica nanoparticles covered with fluoroalkyl groups. Langmuir. 2005;21:7299–7302. doi: 10.1021/la050901r. [DOI] [PubMed] [Google Scholar]
- Hotchkiss KM, Reddy GB, Hyzy SL, et al. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016;31:425–434. doi: 10.1016/j.actbio.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Tan Z, Liu Y, et al. Effect of crystallinity of calcium phosphate nanoparticles on adhesion, proliferation, and differentiation of bone marrow mesenchymal stem cells. J Mater Chem. 2007;17:4690. doi: 10.1039/b710936a. [DOI] [Google Scholar]
- Huang Q, Yang Y, Hu R, et al. Reduced platelet adhesion and improved corrosion resistance of superhydrophobic TiO2-nanotube-coated 316L stainless steel. Colloids Surfaces B Biointerfaces. 2015;125:134–141. doi: 10.1016/j.colsurfb.2014.11.028. [DOI] [PubMed] [Google Scholar]
- Ishihara K. Bioinspired phospholipid polymer biomaterials for making high performance artificial organs. Sci Technol Adv Mater. 2000;1:131–138. doi: 10.1016/S1468-6996(00)00012-7. [DOI] [Google Scholar]
- Ishizaki T, Saito N, Takai O. Correlation of cell adhesive behaviors on superhydrophobic, superhydrophilic, and micropatterned superhydrophobic/superhydrophilic surfaces to their surface chemistry. Langmuir. 2010;26:8147–8154. doi: 10.1021/la904447c. [DOI] [PubMed] [Google Scholar]
- Jardim PLG, Horowitz F, Felde N, et al. Determination of the Wenzel roughness parameter by the power spectral density of functional alumina surfaces. Thin Solid Films. 2016;606:57–62. doi: 10.1016/j.tsf.2016.03.027. [DOI] [Google Scholar]
- Jayakumar R, Menon D, Manzoor K, et al. Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydr Polym. 2010;82:227–232. doi: 10.1016/j.carbpol.2010.04.074. [DOI] [Google Scholar]
- Jiang H, Liu XY, Zhang G, Li Y. Kinetics and template nucleation of self-assembled hydroxyapatite nanocrystallites by chondroitin sulfate. J Biol Chem. 2005;280:42061–42066. doi: 10.1074/jbc.M412280200. [DOI] [PubMed] [Google Scholar]
- Jiang J, Zhu L, Zhu L, et al. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir. 2011;27:14180–14187. doi: 10.1021/la202877k. [DOI] [PubMed] [Google Scholar]
- Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- Kaliaraj GS, Bavanilathamuthiah M, Kirubaharan K, et al. Bio-inspired YSZ coated titanium by EB-PVD for biomedical applications. Surf Coatings Technol. 2016;307:227–235. doi: 10.1016/j.surfcoat.2016.08.039. [DOI] [Google Scholar]
- Kawachi E, Bertran C, Reis RR, Alves OD. Bioceramicas: tencdencias e perspectivas de uma área interdiciplinar. Quim Nova. 2000;23:518–522. doi: 10.1590/S0100-40422000000400015. [DOI] [Google Scholar]
- Khorasani MT, Mirzadeh H. Laser surface modification of silicone rubber to reduce platelet adhesion in vitro. J Biomater Sci Polym Ed. 2004;15:59–72. doi: 10.1163/156856204322752237. [DOI] [PubMed] [Google Scholar]
- Kikuchi L, Park JY, Victor C, Davies JE. Platelet interactions with calcium-phosphate-coated surfaces. Biomaterials. 2005;26:5285–5295. doi: 10.1016/j.biomaterials.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Kim J, Conway A, Chauhan A. Extended delivery of ophthalmic drugs by silicone hydrogel contact lenses. Biomaterials. 2008;29:2259–2269. doi: 10.1016/j.biomaterials.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Kizuki T, Matsushita T, Kokubo T. Apatite-forming PEEK with TiO2 surface layer coating. J Mater Sci Mater Med. 2015;26:1–9. doi: 10.1007/s10856-014-5359-1. [DOI] [PubMed] [Google Scholar]
- Koch K, Barthlott W. Superhydrophobic and superhydrophilic plant surfaces: an inspiration for biomimetic materials. Philos Trans R Soc A Math Phys Eng Sci. 2009;367:1487–1509. doi: 10.1098/rsta.2009.0022. [DOI] [PubMed] [Google Scholar]
- Kokubo T, Kushitani H, Sakka S, et al. Solutions able to reproducein vivo surface-structure changes in bioactive glass-ceramic A-W3. J Biomed Mater Res. 1990;24:721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- Kondo A, Mihara J. Comparison of adsorption and conformation of hemoglobin and myoglobin on various inorganic ultrafine particles. J Colloid Interface Sci. 1996;177:214–221. doi: 10.1006/jcis.1996.0023. [DOI] [PubMed] [Google Scholar]
- Kou L, Gao C. Making silicananoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nano. 2011;3:519–528. doi: 10.1039/C0NR00609B. [DOI] [PubMed] [Google Scholar]
- Krogsgaard M, Behrens MA, Pedersen JS, Birkedal H. Self-healing mussel-inspired multi-pH-responsive hydrogels. Biomacromolecules. 2013;14:297–301. doi: 10.1021/bm301844u. [DOI] [PubMed] [Google Scholar]
- Ku SH, Park CB. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials. 2010;31:9431–9437. doi: 10.1016/j.biomaterials.2010.08.071. [DOI] [PubMed] [Google Scholar]
- Kunjukunju S, Roy A, Ramanathan M, et al. A layer-by-layer approach to natural polymer-derived bioactive coatings on magnesium alloys. Acta Biomater. 2013;9:8690–8703. doi: 10.1016/j.actbio.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Lai Y, Huang Y, Wang H, et al. Selective formation of ordered arrays of octacalcium phosphate ribbons on TiO2 nanotube surface by template-assisted electrodeposition. Colloids Surfaces B Biointerfaces. 2010;76:117–122. doi: 10.1016/j.colsurfb.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Lampin M, Warocquier-Clérout R, Legris C, et al. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J Biomed Mater Res. 1997;36:99–108. doi: 10.1002/(SICI)1097-4636(199707)36:1<99::AID-JBM12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318:426–430. doi: 10.1126/science.1147241 [DOI] [PMC free article] [PubMed]
- Lee H, Lee Y, Statz AR, et al. Substrate-independent layer-by-layer assembly by using mussel-adhesive-inspired polymers. Adv Mater. 2008;20:1619–1623. doi: 10.1002/adma.200702378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, McCarthy TJ. Polymer surface modification: topography effects leading to extreme wettability behavior. Macromolecules. 2007;40:3965–3969. doi: 10.1021/ma070061i. [DOI] [Google Scholar]
- Liang YY, Zhang, LM, Li, W, Chen, RF (2007) Polysaccharide-modified iron oxide nanoparticles as an effective magnetic affinity adsorbent for bovine serum albumin. Colloid Polym Sci 285:1193-1199. doi:10.1007/s00396-007-1672-2
- Liang J, Song R, Huang Q, et al. Electrochemical construction of a bio-inspired micro/nano-textured structure with cell-sized microhole arrays on biomedical titanium to enhance bioactivity. Electrochim Acta. 2015;174:1149–1159. doi: 10.1016/j.electacta.2015.06.100. [DOI] [Google Scholar]
- Lima AC, Batista P, Valente TAM, et al. Novel methodology based on biomimetic superhydrophobic substrates to immobilize cells and proteins in hydrogel spheres for applications in bone regeneration. Tissue Eng Part A. 2013;19:1175–1187. doi: 10.1089/ten.tea.2012.0249. [DOI] [PubMed] [Google Scholar]
- Lima AC, Mano JF, Concheiro A, Alvarez-Lorenzo C. Fast and mild strategy, using superhydrophobic surfaces, to produce collagen/platelet Lysate gel beads for skin regeneration. Stem Cell Rev Rep. 2015;11:161–179. doi: 10.1007/s12015-014-9548-6. [DOI] [PubMed] [Google Scholar]
- Lin J, Cates E, Bianconi PA. A synthetic analog of the biomineralization process: controlled crystallization of an inorganic phase by a polymer matrix. J Am Chem Soc. 1994;116:4738–4745. doi: 10.1021/ja00090a021. [DOI] [Google Scholar]
- Liu K, Jiang L. Bio-inspired self-cleaning surfaces. Annu Rev Mater Res. 2012;42:231–263. doi: 10.1146/annurev-matsci-070511-155046. [DOI] [Google Scholar]
- Liu K, Yao X, Jiang L. Recent developments in bio-inspired special wettability. Chem Soc Rev. 2010;39:3240. doi: 10.1039/b917112f. [DOI] [PubMed] [Google Scholar]
- Liu KS, Fujishima A, Jiang L, Cao M. Bio-inspired titanium dioxide materials with special Wettability and their applications. Chem Rev. 2014;114:10044–10094. doi: 10.1021/cr4006796. [DOI] [PubMed] [Google Scholar]
- Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32:477–486. doi: 10.1023/B:ABME.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- Liu Y, He T, Gao C. Surface modification of poly(ethylene terephthalate) via hydrolysis and layer-by-layer assembly of chitosan and chondroitin sulfate to construct cytocompatible layer for human endothelial cells. Colloids Surf B Biointerfaces. 2005;46:117–126. doi: 10.1016/j.colsurfb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lopez-Torres D, Elosua C, Hernaez M, et al. From superhydrophilic to superhydrophobic surfaces by means of polymeric layer-by-layer films. Appl Surf Sci. 2015;351:1081–1086. doi: 10.1016/j.apsusc.2015.06.004. [DOI] [Google Scholar]
- Lu X, Zhang C, Han Y. Low-density polyethylene superhydrophobic surface by control of its crystallization behavior. Macromol Rapid Commun. 2004;25:1606–1610. doi: 10.1002/marc.200400256. [DOI] [Google Scholar]
- Lynch I, Dawson KA. Protein-nanoparticle interactions. Nano Today. 2008;3:40–47. doi: 10.1016/S1748-0132(08)70014-8. [DOI] [Google Scholar]
- Ma M, Hill RM. Superhydrophobic surfaces. Curr Opin Colloid Interface Sci. 2006;11:193–202. doi: 10.1016/j.cocis.2006.06.002. [DOI] [Google Scholar]
- Ma M, Hill RM, Lowery JL, et al. Electrospun poly(styrene-block-dimethylsiloxane) block copolymer fibers exhibiting superhydrophobicity. Langmuir. 2005;21:5549–5554. doi: 10.1021/la047064y. [DOI] [PubMed] [Google Scholar]
- Ma R, Tang T. Current strategies to improve the bioactivity of PEEK. Int J Mol Sci. 2014;15:5426–5445. doi: 10.3390/ijms15045426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Mao Z, Gao C. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids Surfaces B Biointerfaces. 2007;60:137–157. doi: 10.1016/j.colsurfb.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Madhan Kumar A, Rajendran N. Electrochemical aspects and in vitro biocompatibility of polypyrrole/TiO2 ceramic nanocomposite coatings on 316L SS for orthopedic implants. Ceram Int. 2013;39:5639–5650. doi: 10.1016/j.ceramint.2012.12.080. [DOI] [Google Scholar]
- Maia M, Klein ES, Monje TV, Pagliosa C. Reconstrução da estrutura facial por biomateriais:revisão de literatura. 2010;25:566–572. [Google Scholar]
- Malmsten M. Formation of adsorbed protein layers. J Colloid Interface Sci. 1998;207:186–199. doi: 10.1006/jcis.1998.5763. [DOI] [PubMed] [Google Scholar]
- Mann S (2004) Materials that naturally assemble themselves. Chem Commun (Camb) 2004:1–4. doi:10.1039/b310010n [DOI] [PubMed]
- Mann S, Archibald DD, Didymus JM, et al (1993) Organic interfaces: biominerals. Science 261:1286–1292. doi: 10.1126/science.261.5126.1286 [DOI] [PubMed]
- Meldrum FC, Colfen H. Controlling mineral morphologies and structures in biological and synthetic systems. Chem Rev. 2008;108:4332–4432. doi: 10.1021/cr8002856. [DOI] [PubMed] [Google Scholar]
- Mendes PM. Stimuli-responsive surfaces for bio-applications. Chem Soc Rev. 2008;37:2512. doi: 10.1039/b714635n. [DOI] [PubMed] [Google Scholar]
- Mohan L, Durgalakshmi D, Geetha M, et al. Electrophoretic deposition of nanocomposite (HAp + TiO2) on titanium alloy for biomedical applications. Ceram Int. 2012;38:3435–3443. doi: 10.1016/j.ceramint.2011.12.056. [DOI] [Google Scholar]
- Moon RJ, Martini A, Nairn J, et al. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. 2011;40:3941. doi: 10.1039/c0cs00108b. [DOI] [PubMed] [Google Scholar]
- Mu Q, Jiang G, Chen L, et al. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem Rev. 2016;i:7740–7781. doi: 10.1021/cr400295a.Chemical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Nishimoto S, Yuda Y, et al. Rewritable superhydrophilic-superhydrophobic patterns on a sintered titanium dioxide substrate. Langmuir. 2010;26:11628–11630. doi: 10.1021/la101947y. [DOI] [PubMed] [Google Scholar]
- Nakata K, Sakai M, Ochiai T, et al. Antireflection and self-cleaning properties of a moth-eye-like surface coated with TiO2 particles. Langmuir. 2011;27:3275–3278. doi: 10.1021/la200438p. [DOI] [PubMed] [Google Scholar]
- Navrotsky A. Energetic clues to pathways to biomineralization: precursors, clusters, and nanoparticles. Proc Natl Acad Sci USA. 2004;101:12096–12101. doi: 10.1073/pnas.0404778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechifor C-D, Dorohoi DO. The influence of gamma radiations on physico- chemical properties of some polymer membranes. Rom J Phys. 2009;54:349–359. [Google Scholar]
- Nel AE, Mädler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Nogueira LFB, Maniglia BC, Pereira LS, et al. Formation of carrageenan-CaCO3 bioactive membranes. Mater Sci Eng C. 2016;58:1–6. doi: 10.1016/j.msec.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Nosonovsky M, Bhushan B. Biologically inspired surfaces: broadening the scope of roughness. Adv Funct Mater. 2008;18:843–855. doi: 10.1002/adfm.200701195. [DOI] [Google Scholar]
- Oh SH, Finõnes RR, Daraio C, et al. Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials. 2005;26:4938–4943. doi: 10.1016/j.biomaterials.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D, Miller N. Phospholipid model membranes. I Structural characteristics of hydrated liquid crystals. Biochim Biophys Acta. 1967;35:624–638. doi: 10.1016/0005-2736(67)90094-6. [DOI] [PubMed] [Google Scholar]
- Patil S, Sandberg A, Heckert E, et al. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials. 2007;28:4600–4607. doi: 10.1016/j.biomaterials.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-valverde MA. Mechanical derivation of the Wenzel and Cassie equations using a statistical interpretation of drop dispensation. J Colloid Interface Sci. 2008;327:477–479. doi: 10.1016/j.jcis.2008.08.048. [DOI] [PubMed] [Google Scholar]
- Rupp F, Scheideler L, Olshanska N, et al. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006;76:323–334. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- Ryu J, Ku SH, Lee H, Park CB. Mussel-inspired Pplydopamine coating as a universal route to hydroxyapatite crystallization. Adv Funct Mater. 2010;20:2132–2139. doi: 10.1002/adfm.200902347. [DOI] [Google Scholar]
- Ruiz GCM, Cruz MAE, Faria AN, Zancanela DC, Pietro Ciancaglini APR (2017) Biomimetic collagen/phospholipid coatings improve formation of hydroxyapatite nanoparticles on titanium. Mater Sci Eng C. doi:10.1016/j.msec.2017.03.204 [DOI] [PubMed]
- Saha B, Das G. Malachite nanoparticle: a new basic hydrophilic surface for pH-controlled adsorption of bovine serum albumin with a high loading capacity. J Phys Chem C. 2009;113:15667–15675. doi: 10.1021/jp903082e. [DOI] [Google Scholar]
- Sánchez-Treviño AY, García-Martínez O, Blasco-Avellaneda D, et al. Effect of the terminal group of phosphonate self-assembled films formed on Ti surfaces on the biomimetic layer formation and cell adhesion. Appl Surf Sci. 2016;362:304–314. doi: 10.1016/j.apsusc.2015.11.174. [DOI] [Google Scholar]
- Sanvicens N, Marco MP. Multifunctional nanoparticles—properties and prospects for their use in human medicine. Trends Biotechnol. 2008;26:425–433. doi: 10.1016/j.tibtech.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Sapsford KE, Russ Algar W, Lorenzo B. Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev. 2013;113:1904–2074. doi: 10.1021/cr300143v. [DOI] [PubMed] [Google Scholar]
- Saptarshi SR, Duschl A, Lopata AL. Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J Nanobiotechnol. 2013;11:26. doi: 10.1186/1477-3155-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Lutkenhaus JL, Kim J, et al. Effect of the layer-by-layer (LbL) deposition method on the surface morphology and wetting behavior of hydrophobically modified PEO and PAA LbL films. Langmuir. 2008;24:7995–8000. doi: 10.1021/la800906x. [DOI] [PubMed] [Google Scholar]
- Shi F, Wang Z, Zhang X. Combining a layer-by-layer assembling technique with electrochemical deposition of gold aggregates to mimic the legs of water striders. Adv Mater. 2005;17:1005–1009. doi: 10.1002/adma.200402090. [DOI] [Google Scholar]
- Shibata Y, Tanimoto Y. A review of improved fixation methods for dental implants. Part I: Surface optimization for rapid osseointegration. J Prosthodont Res. 2015;59:20–33. doi: 10.1016/j.jpor.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Shin S, Seo J, Han H et al (2016) Bio-inspired extreme wetting surfaces for biomedical applications. Materials 9(2):116. doi:10.3390/ma9020116 [DOI] [PMC free article] [PubMed]
- Si Y, Guo Z. Superhydrophobic nanocoatings: from materials to fabrications and to applications. Nano. 2015;7:5922–5946. doi: 10.1039/C4NR07554D. [DOI] [PubMed] [Google Scholar]
- Singh A, Steely L, Allcock HR. Poly[bis(2,2,2-trifluoroethoxy)phosphazene] superhydrophobic nanofibers. Langmuir. 2005;21:11604–11607. doi: 10.1021/la052110v. [DOI] [PubMed] [Google Scholar]
- Stephens CJ, Kim Y-Y, Evans SD, et al. Early stages of crystallization of calcium carbonate revealed in picoliter droplets. J Am Chem Soc. 2011;133:5210–5213. doi: 10.1021/ja200309m. [DOI] [PubMed] [Google Scholar]
- Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev. 1995;17:31–48. doi: 10.1016/0169-409X(95)00039-A. [DOI] [Google Scholar]
- Suwandi JS, Toes REM, Nikolic T, Roep BO. Inducing tissue specific tolerance in autoimmune disease with tolerogenic dendritic cells. Clin Exp Rheumatol. 2015;33:97–103. doi: 10.1002/jbm.a. [DOI] [PubMed] [Google Scholar]
- Szcześ A. Influence of the surfactant nature on the calcium carbonate synthesis in water-in-oil emulsion. J Cryst Growth. 2009;311:1129–1135. doi: 10.1016/j.jcrysgro.2008.12.044. [DOI] [Google Scholar]
- Tai CY, Kuang CC. Particle morphology, habit, and size control of CaCO3 using reverse microemulsion technique. Chem Eng Sci. 2008;63:3632–3642. doi: 10.1016/j.ces.2008.04.022. [DOI] [Google Scholar]
- Tejero R, Anitua E, Orive G. Toward the biomimetic implant surface: biopolymers on titanium-based implants for bone regeneration. Prog Polym Sci. 2014;39:1406–1447. doi: 10.1016/j.progpolymsci.2014.01.001. [DOI] [Google Scholar]
- Teshima K, Sugimura H, Inoue Y, et al. Transparent ultra water-repellent poly(ethylene terephthalate) substrates fabricated by oxygen plasma treatment and subsequent hydrophobic coating. Appl Surf Sci. 2005;244:619–622. doi: 10.1016/j.apsusc.2004.10.143. [DOI] [Google Scholar]
- Velasco-Ortega E, Alfonso-Rodríguez CA, Monsalve-Guil L, et al. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater Sci Eng C. 2016;64:1–10. doi: 10.1016/j.msec.2016.03.049. [DOI] [PubMed] [Google Scholar]
- Verma A, Stellacci F. Effect of surface properties on nanoparticle–cell interactions. Small. 2010;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- Vogler EA. Structure and reactivity of water at biomaterial surfaces. Adv Colloid Interf Sci. 1998;74:69–117. doi: 10.1016/S0001-8686(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Walkey CD, Olsen JB, Guo HB, Emili ACW. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134:2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- Wang B, Ye Z, Tang Y, et al. Fabrication of nonfouling, bactericidal, and bacteria corpse release multifunctional surface through surface-initiated RAFT polymerization. Int J Nanomedicine. 2017;12:111–125. doi: 10.2147/IJN.S107472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Xiao P, Zhao J, et al. Biomimetic synthesis of hydrophobic calcium carbonate nanoparticles via a carbonation route. Powder Technol. 2006;170:31–35. doi: 10.1016/j.powtec.2006.08.016. [DOI] [Google Scholar]
- Wang F, Shi L, He W, et al. Applied surface science bioinspired micro/nano fabrication on dental implant–bone interface. Appl Surf Sci. 2013;265:480–488. doi: 10.1016/j.apsusc.2012.11.032. [DOI] [Google Scholar]
- Wang S, Meng KK, Zhao L, et al. Superhydrophilic cu-doped TiO2 thin film for solar-driven photocatalysis. Ceram Int. 2014;40:5107–5110. doi: 10.1016/j.ceramint.2013.09.028. [DOI] [Google Scholar]
- Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25:4749–4757. doi: 10.1016/j.biomaterials.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Wenzel RN. Resistance of solid surfaces to wetting by water. Ind Eng Chem. 1936;28:988–994. doi: 10.1021/ie50320a024. [DOI] [Google Scholar]
- Whitehead KA, Colligon J, Verran J. Retention of microbial cells in substratum surface features of micrometer and sub-micrometer dimensions. Colloids Surfaces B Biointerfaces. 2005;41:129–138. doi: 10.1016/j.colsurfb.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Wolansky G, Marmur A. Apparent contact angles on rough surfaces: the Wenzel equation revisited. Colloids Surfaces A Physicochem Eng Asp. 1999;156:381–388. doi: 10.1016/S0927-7757(99)00098-9. [DOI] [Google Scholar]
- Wu G, Zhang X. Layer-by-layer assembly: from conventional to unconventional methods. Multilayer Thin Film Seq Assem Nanocomposite Mater Second Ed. 2012;1:43–67. doi: 10.1002/9783527646746.ch3. [DOI] [Google Scholar]
- Wu J, Xia J, Lei W, Wang BP. A one-step method to fabricate lotus leaves-like ZnO film. Mater Lett. 2011;65:477–479. doi: 10.1016/j.matlet.2010.10.029. [DOI] [Google Scholar]
- Wu S, Liu X, Hu T, et al. A biomimetic hierarchical scaffold: natural growth of nanotitanates on three-dimensional microporous Ti-based metals. Nano Lett. 2008;8:3803–3808. doi: 10.1021/nl802145n. [DOI] [PubMed] [Google Scholar]
- Xu A-W, Ma Y, Cölfen H. Biomimetic mineralization. J Mater Chem. 2007;17:415. doi: 10.1039/b611918m. [DOI] [Google Scholar]
- Xu AW, Antonietti M, Yu SH, Cölfen H. Polymer-mediated mineralization and self-similar mesoscale-organized calcium carbonate with unusual superstructures. Adv Mater. 2008;20:1333–1338. doi: 10.1002/adma.200701723. [DOI] [Google Scholar]
- Yamaguchi M, Suzuki S, Sasaki S, et al. Fabrication of nano-periodic structures and modification of the Wenzel model to estimate contact angle. Sensors Actuators A Phys. 2014;212:87–92. doi: 10.1016/j.sna.2014.03.006. [DOI] [Google Scholar]
- Yoshida S, Hagiwara K, Hasebe T, Hotta A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf Coatings Technol. 2013;233:99–107. doi: 10.1016/j.surfcoat.2013.02.042. [DOI] [Google Scholar]
- Zhang L, Wu J, Wang Y, et al. Combination of bioinspiration: a general route to superhydrophobic particles. J Am Chem Soc. 2012;134:9879–9881. doi: 10.1021/ja303037j. [DOI] [PubMed] [Google Scholar]
- Zhu M, Nie G, Meng H, et al. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc Chem Res. 2013;46:622–631. doi: 10.1021/ar300031y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogbi MM, Saito E, Zanin H, et al. Hydrothermal-electrochemical synthesis of nano-hydroxyapatite crystals on superhydrophilic vertically aligned carbon nanotubes. Mater Lett. 2014;132:70–74. doi: 10.1016/j.matlet.2014.06.033. [DOI] [Google Scholar]