Abstract
During the process of endochondral bone formation, chondrocytes and osteoblasts mineralize their extracellular matrix (ECM) by promoting the synthesis of hydroxyapatite (HA) seed crystals in the sheltered interior of membrane-limited matrix vesicles (MVs). Several lipid and proteins present in the membrane of the MVs mediate the interactions of MVs with the ECM and regulate the initial mineral deposition and posterior propagation. Among the proteins of MV membranes, ion transporters control the availability of phosphate and calcium needed for initial HA deposition. Phosphatases (orphan phosphatase 1, ectonucleotide pyrophosphatase/phosphodiesterase 1 and tissue-nonspecific alkaline phosphatase) play a crucial role in controlling the inorganic pyrophosphate/inorganic phosphate ratio that allows MV-mediated initiation of mineralization. The lipidic microenvironment can help in the nucleation process of first crystals and also plays a crucial physiological role in the function of MV-associated enzymes and transporters (type III sodium-dependent phosphate transporters, annexins and Na+/K+ ATPase). The whole process is mediated and regulated by the action of several molecules and steps, which make the process complex and highly regulated. Liposomes and proteoliposomes, as models of biological membranes, facilitate the understanding of lipid–protein interactions with emphasis on the properties of physicochemical and biochemical processes. In this review, we discuss the use of proteoliposomes as multiple protein carrier systems intended to mimic the various functions of MVs during the initiation and propagation of mineral growth in the course of biomineralization. We focus on studies applying biophysical tools to characterize the biomimetic models in order to gain an understanding of the importance of lipid–protein and lipid–lipid interfaces throughout the process.
Keywords: Lipid microenvironment, Biomineralization, Matrix vesicles, Liposome, Proteoliposome, Hydroxyapatite
Mineralization process and the biogenesis of matrix vesicles
Mineralization of cartilage and bone occurs by a series of physicochemical and biochemical processes that together facilitate the deposition of calcium phosphate. The deposited calcium phosphate is subsequently converted into hydroxyapatite (HA) [Ca10(PO4)6(OH)2] in specific areas of the extracellular matrix (ECM). Various experiments have revealed the presence of HA crystals both inside and outside collagen fibrils in the ECM (McNally et al. 2012) and also within the lumen of matrix vesicles (MVs) (Ali et al. 1970; Anderson 1969; Bonucci 1970). MVs are extracellular vesicles with a diameter ranging from approximately 100 to 300 nm (Anderson 2003; Anderson et al. 2010; Cui et al. 2016; Golub 2009; Wuthier and Lipscomb 2011) which harbor the biochemical machinery needed to establish the appropriate inorganic pyrophosphate (PPi) to inorganic phosphate (Pi) ratio (Ciancaglini et al. 2006, 2010; Harmey et al. 2004; Hessle et al. 2002; Johnson et al. 2000; Roberts et al. 2007; Yadav et al. 2011; Zhou et al. 2012) required to initiate the synthesis of apatitic mineral (Bechkoff et al. 2008; Thouverey et al. 2009; Wu et al. 1997), which will subsequently be propagated onto the collagenous ECM. These vesicles are released from the hypertrophic chondrocytes and mature osteoblasts, the cells responsible for endochondral and membranous ossification. Hypertrophic chondrocyte differentiation is related to high tissue nonspecific alkaline phosphatase (TNAP) activity and to the synthesis and secretion of type X and II collagens by proliferating and pre-hypertrophic chondrocytes (Golub 2009; McKee et al. 2013).
We hypothesize that mineralization progresses through the following steps: (1) crystal deposition starts inside MVs; (2) after budding, MVs bind to collagen fibers through molecular interactions mediated by specific proteins and lipids; (3) MVs break and expose HA crystals to the ECM; (4) osteopontin (OPN) binds to the exposed HA crystals and controls the propagation of HA onto adjacent collagen fibers.
The role of phosphatases in MV-mediated initiation of skeletal mineralization
Matrix vesicles harbor proteins and lipids that mediate the interactions of MVs with the ECM, regulate the extra-vesicular PPi/Pi ratio and control mineral deposition. Among the proteins of the MV membrane, phosphatases have a relevant effect on the regulation of mineralization.
Phosphatases play a crucial role in controlling the PPi/Pi ratio that allows MV-mediated initiation of mineralization: orphan phosphatase 1 (PHOSPHO1) present in the lumen of MVs produces Pi from phosphocholine (PC), which is derived from sphingomyelin (SM) by the action of sphingomyelin phosphodiesterase 3 (SMPD3) located in the inner surface of the MV membrane. Two ectophosphatases on the outer surface of the MV membrane act in concert to regulate the extracellular PPi/Pi ratio: ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1 or NPP1) produces PPi, as well as Pi, from ATP, and TNAP hydrolyzes both ATP and PPi to form Pi. Phosphate transporter 1 (PiT-1) helps to incorporate the Pi generated in the perivesicular space into the MVs. MVs isolated from growth plate cartilage have a higher content of cholesterol (Chol), SM and phosphatidylserine (PS) (Thouverey et al. 2009, 2011; Wuthier and Lipscomb 2011) than do the plasma membranes, as well as longer fatty acids (Abdallah et al. 2014). It is significant that MVs are enriched in SM as SM, via the enzymatic action of SMPD3, is converted to the PC to be used by PHOSPHO1 to produce Pi intravesicularly. PHOSPHO1 not only regulates MV-mediated initiation of mineralization, but also MV formation. SMPD3 has also been implicated in the biogenesis of vesicles by vascular smooth muscles cells (VSMCs) (Kapustin et al. 2015).
The biogenesis of MVs coincides with the sequence of events leading to apoptosis or programmed cell death (Anderson 1995) in hypertrophic chondrocytes (Anderson 1969; Garimella et al. 2004; Kirsch et al. 1997; Kirsch and Vondermark 1992) and osteoblasts (Dean et al. 1994), but MVs are distinct from apoptotic bodies (Kirsch et al. 2003). Bonucci (1970) reported that MVs are formed through the exfoliation of vesicles from the outer membrane of growth plate chondrocytes. These findings were subsequently confirmed by freeze-fracture analyses (Borg et al. 1978; Cecil and Anderson 1978; Hale and Wuthier 1987). Recent Cryo-electron microscopy analyses (Mahamid et al. 2010; Nollet et al. 2014) show that mineralization may be initiated within intracytoplasmic vesicles. MVs would appear to be a specialized form of microvesicle that is able to start mineralization inside as well as outside the cell. Understanding the pathways leading to MV biogenesis and function are fundamentally important to facilitate efforts to target either TNAP, PHOSPHO1 or both together for the prevention of soft-tissue calcification, particularly arterial calcification.
How HA crystals formed within the MV microenvironment can promote further propagation of mineral onto collagen fibrils matrix remains unclear. In vivo, collagen molecules assemble into three-dimensional (3D) structures in which each molecule is in a staggered arrangement with respect to its neighbors. Two regions have been identified in this model, namely, the hole (or gap, 47 nm in length) and the overlap zone (20 nm in length); these comprise the periodic staggered distance (Katz and Li 1973). Collagen has also been reported to pack through strict and contiguous alignment of its hole and overlap zones (Landis et al. 1996; Weiner and Traub 1986, 1989). It has been proposed that the pattern of holes and overlap sites among collagen molecules provide channels or gaps in their assemblage where HA crystals can nucleate (Landis et al. 1993). However, the majority of bone mineral is external to the collagen fibers (McNally et al. 2012; Pidaparti et al. 1996). In contrast with the well-documented events explaining collagen-mediated mineralization, it has been hard to visualize how apatite crystals from MVs could make their way to the collagen fibrils and help to promote further mineral propagation. Nascent HA crystals exposed to the extravesicular fluid have been found to bind OPN, a collagen-binding molecule (Chen et al. 1992; Liu et al. 2007; Martin et al. 2004). Given that MVs display collagen-binding molecules on their membranes (TNAP, Annexin A5 (AnxA5)) (Balcerzak et al. 2008; Wu et al. 1991; Xiao et al. 2007), it is conceivable that a specific binding of MVs to collagen mediates the physical transfer of HA crystals to the exterior of collagen fibrils. This model does not negate, nor is it incompatible with, data indicating that crystals are able to form in the gaps and holes of the collagen fibrils, as has been well demonstrated by the work of many investigators, but if proven correct it would help explain how crystals formed within MVs can contribute to ECM mineralization.
The role of transporters in MV-mediated initiation of skeletal mineralization
The MV membrane is enriched in transporter proteins associated with mineralization that are responsible for Ca2+ and Pi homeostasis by mediating the influx of these ions into the vesicles. Among these, the most abundant proteins detected by the proteomic assay are the annexins (Anx).
Anx belong to a family of structurally related proteins which bind to negatively charged phospholipids in a manner that is Ca2+ dependent and rapidly reversible by addition of Ca2+ chelators. Anx are amphipathic proteins, and as such they differ from soluble or integral membrane proteins; they are composed of four (or eight in the case of AnxA6) homologous repeat domains of about 70 amino acid residues each. These highly conserved domains hold sequence similarities of 40–70% and represent the protease-resistant phospholipid binding core of each protein. A distinct characteristic of each Anx is the amino-terminal region which varies in length and primary sequence and may influence the cellular functions. It has been described that AnxA2, AnxA5 and AnxA6 are involved in Ca2+ homeostasis by mediating Ca2+ influx into MVs (Kirsch et al. 2000; Wang and Kirsch 2002; Wang et al. 2005).
AnxA5 (~ 33 kDa) is characterized by a predominant cluster of α-helices that define a hydrophilic pore through the center of the protein. This pore was thought to serve as a Ca2+ channel in the MV membrane in the form of a trimer arrangement that binds to the surface of acidic phospholipid-containing bilayers. Experimental evidence shows that these trimers may be involved in the formation of the nucleational core (NC) within MVs and that they contribute to the ability of MVs to induce mineral formation. More than any other protein so far studied, AnxA5 greatly accelerates the nucleational activity of the acidic phospholipid–Ca2+–Pi complexes present in the NC that triggers de novo calcium phosphate mineral formation in MVs (Genge et al. 2007; Wuthier and Lipscomb 2011).
AnxA6, the largest member of the Anx family found in MVs, is present on the surface of the external leaflet of the MV membrane, on the surface of the internal leaflet, inserted into the hydrophobic bilayer and co-localized with regions enriched in Chol. Different localizations and ways of interaction with MV membrane indicate that AnxA6 has distinct functions during the mineralization process (Theobald et al. 1994; Wu et al. 1997). Expression of AnxA6 in cells reduces cell proliferation (Theobald et al. 1994; Wu et al. 1997), suggesting that AnxA6 may play a role in growth plate cartilage in the zones of maturation and hypertrophy where cell division stops. Many studies have reported that VSMC-derived MVs are enriched in AnxA6 and AnxA2 and that these annexins can stimulate mineralization when associated with type I, but not type II, collagen (Chen et al. 2008). Curiously, VSMC-derived MVs appear to be devoid of AnxA5, in contrast to cartilage MVs. These studies suggest that AnxA6, and possibly AnxA2, may be important for MV binding to type I collagen in smooth muscle calcification (Wuthier and Lipscomb 2011) and that AnxA5 may be important for MV binding to type II collagen in cartilage calcification (Bolean et al. 2017).
Cellular Pi levels are controlled by type III Na+-dependent Pi co-transporters (Virkki et al. 2007). This SLC20 family of transporters includes PiT-1 (SLC20A1) and PiT-2 (SLC20A2) (Collins et al. 2004). Some authors have suggested that PiT-1 plays important roles in vascular and bone physiology (Lau et al. 2010; Villa-Bellosta et al. 2009b), whereas PiT-2 has a role in renal Pi reabsorption (Breusegem et al. 2009; Villa-Bellosta et al. 2009a; Villa-Bellosta and Sorribas 2008, 2010). In previous studies PiT-1 was found to be expressed in hypertrophic chondrocytes during endochondral ossification in mice (Palmer et al. 1999) and also in chicken chondrocytes (Mansfield et al. 2001). PiT-1 plays important roles in the regulation of Pi homeostasis in bone and cartilage in vitro (Cecil et al. 2005; Solomon et al. 2007; Sugita et al. 2011; Wang et al. 2005), and extracellular Pi, epinephrine, insulin-like growth factor 1 and bone morphogenic protein 2 are known to regulate PiT-1 levels in osteoblast-like cells (Suzuki et al. 2006; Villa-Bellosta et al. 2009b). Moreover, it has been demonstrated that Pi modulates chondrocyte differentiation (Cecil et al. 2005; Fujita et al. 2001; Guicheux et al. 2000; Montessuit et al. 1991; Wang et al. 2001; Wu et al. 2002) and apoptosis (Magne et al. 2003; Mansfield et al. 2001). Sugita et al. (2011) suggested that ATP synthesis mediated by Pi influx via PiT-1 is critical for the regulation of late chondrogenesis, including apoptosis and mineralization, as the differentiation of cartilage is an ATP-dependent event. Lau et al. (2010) demonstrated that Na+/Pi co-transport activity through PiT-1 is associated with osteoblast differentiation and that extracellular Pi affects the differentiation of chondrogenic cells induced by PiT-1 functions. Suzuki et al. (2010) also investigated the effects of transgenic PiT-1 overexpression on calcium/phosphate and bone metabolism. PiT-1-transgenic rats showed abnormal mineral metabolism and also reduced alkaline phosphatase activity in their osteoblasts, but the mineralization of the bone matrix and skeletal development were normal (Suzuki et al. 2010). In adult PiT-1-transgenic rats, hyperphosphatemia affects bone formation and is associated with a reduced bone mass (Suzuki et al. 2010). In this in vitro study, PiT-1 overexpression in osteoblasts led to a marked increase in Pi transport and the downregulation of alkaline phosphatase expression. However, no effect on matrix mineralization was reported. These observations suggest that compensatory mechanisms may support the calcification of the collagenous matrix (Suzuki et al. 2010).
Another relevant transporter protein abundantly present in MV membrane is Na+/K+ ATPase (NKA) (Hsu and Anderson 1996), which can promote mineralization by increasing the local concentration of Pi and by decreasing the concentration of PPi (Anderson et al. 2004). NKA is an active cation transport protein ubiquitous in the membrane of all mammalian cells that moves three Na+ outside and two K+ inside per molecule of ATP. The enzyme functional structure is a heterodimer composed of two main alpha (α) and beta (β) subunits. The α subunit (110 kDa) has ten transmembrane segments and three cytoplasmic domains (A, actuator; N, nucleotide-binding; P, phosphorylation), whereas the β subunit (55 kDa) has only one transmembrane segment and a highly glycosylated extracellular portion (Kaplan 2002; Morth et al. 2011). Almost 40 years after the existence of the NKA pump was proposed, Hsu and Anderson (1996) described that an ATPase inhibited by vanadate, but not by alkaline phosphatase, could be also responsible for initiating Ca2+ and Pi deposition into intact MVs (Hsu and Anderson 1996). In a study carried out much later on the proteome of the chicken embryo, Balcerzak et al. (2008) revealed MVs to be a very complex biological environment where they identified 23 transporter proteins. Among them, five α and β subunits from NKA self-assemble in specific dimers that can subsequently associate into multimeric species (Tokhtaeva et al. 2012; Yoneda et al. 2016). Taken together, these studies suggest a model of NKA actively participating in MV-driven mineralization processes. However, it has also been suggested that the NKA pump is in a right-side-out orientation, with the ATP binding site protruding from the internal surface of the MV membrane (Wuthier and Lipscomb 2011). Since the concentrations of ATP and K+ are low in the MV lumen and that of Na+ is high, with respect to the ECM, this NKA pump configuration would be the opposite of what is expected when the pump is active (Wuthier and Lipscomb 2011). Thus, the contribution of NKA to mineralization is still a matter of debate.
What are the biomimetic systems of MVs?
Since biological membranes are very complex systems, it is not easy to understand the physicochemical behavior of the lipid bilayer or to explain many of its properties. In this context, “models” of biological membranes that consist of simplified lipid monolayers or bilayers have been developed. These mimetic systems are constituted of a pure lipid, mixtures of more than one type of lipid and a single or multiple proteins (Rigaud et al. 1995; Silvius 1992; Singer 2004). The most studied membrane model systems are the liposomes, which can be obtained in solution. In an aqueous suspension, most lipids (generally phospholipids) organize spontaneously to form multilamellar systems, i.e. a stacking of bilayers. This event is driven by the entropy of the water molecules and by the interaction between the lipid hydrophobic chains constituted by fatty acids. To form unilamellar vesicles with a single concentric bilayer similar to natural membranes, multilamellar vesicles can be sonicated or extruded to nanometric dimensions. Sonication as well as extrusion processes supply the necessary energy to break the different lamellas and re-organize the bilayers into uniform and control-sized vesicles (Camolezi et al. 2002; Daghastanli et al. 2004; de Lima Santos et al. 2005; Ierardi et al. 2002; Rigos et al. 2008; Simao et al. 2015).
Proteoliposomes are mimic systems of natural vesicles to which proteins have been incorporated or inserted. The biochemical characteristics of inserted proteins will dictate the methodology for their incorporation into the vesicular systems. When the protein to be reconstructed in a membrane system has a large hydrophobic domain (e.g. an integral or transmembrane protein), it is difficult to force it to dislocate from the micellar system into the liposomal space, even at low concentrations of detergent. In this case, the co-solubilization technique is the most recommended. In this methodology, the solubilized protein in detergent is incubated with a lipid suspension (or a mixture of more than one lipid). After this ternary system has been incubated for a sufficiently long time, the detergent is removed using an appropriate technique (for example, dialysis, gel filtration or the use of hydrophobic resins). Following detergent removal, the lipid molecules tend to organize themselves so as to form a bilayer structure in which their hydrophobic tails are isolated from the aqueous medium, guided by the entropy of the water molecules. In addition, proteins tend to accommodate among the lipid groups, creating lipid–protein domains and forming the proteoliposomes. By changing the type and proportion of the lipids, incubation time, method used for detergent removal or even the velocity by which the detergent is removed, different vesicular systems can be obtained, varying in the type and quantity of proteins that can be reconstituted (Bolean et al. 2010, 2011; Camolezi et al. 2002; Daghastanli et al. 2004; de Lima Santos et al. 2005; Ierardi et al. 2002; Rigos et al. 2008, 2010; Simao et al. 2010a), 2015. Anchored membrane proteins are a class of proteins with a covalently bound lipid or alkyl chain; this chain provides a hydrophobic moiety that allows attachment to the membrane and plays specific functions in cells (Yeagle 1993). For this class of proteins, the method of incorporation via direct insertion guarantees that the protein is associated with the external leaflet of the liposome’s membrane (Bolean et al. 2010, 2011; Camolezi et al. 2002; Daghastanli et al. 2004; de Lima Santos et al. 2005; Ierardi et al. 2002; Rigos et al. 2008, 2010; Simao et al. 2010a, 2015). The incorporation of the protein/enzyme is time-dependent and the incorporation yield depends on the lipid composition used to form the vesicles (Simao et al. 2015). Alternatively, the soluble proteins can also be sequestered into the aqueous cavity of the liposome.
Biophysical and biochemical characterization of biomimetic models
Biophysical tools applied to biomineralization studies
A large number of studies have been carried out using proteoliposomes as chondrocyte- and osteoblast-derived MVs biomimetics (Andrade et al. 2016; Bolean et al. 2010, 2011, 2015, 2017; Ciancaglini et al. 2010; Favarin et al. 2017; Simao et al. 2010a, b, 2013).
Several experiments have confirmed the interaction between detergent-solubilized TNAP, free of detergent excess, and the lipid bilayer of liposomes via the glycophosphatidylinositol (GPI)-anchor of the enzyme (Camolezi et al. 2002). A relevant point to be observed is that the enzyme bound to liposomes retains the ability to hydrolyze different substrates, such as ATP, ADP, AMP, PPi, and p-nitrophenylphosphate (Bolean et al. 2015; Ciancaglini et al. 2006, 2010; Simao et al. 2010a, b).
The size of a dipalmitoylphosphatidylcholine (DPPC) proteoliposome harboring TNAP is around 300 nm, as determined by dynamic light scattering (Bolean et al. 2010; Simao et al. 2010a). It is thus comparable to the median size of a natural MV (Bolean et al. 2017; Ciancaglini et al. 2006) and can adequately serve as a vesicular mimetic system to examine TNAP function in the context of a lipid membrane environment that mimics the MV environment. Electron microscopy of empty DPPC liposomes and TNAP-proteoliposomes showed that enzyme reconstitution did not affect the morphology of the liposomes (Simao et al. 2010a).
Garcia et al. (2015) used electron spin resonance measurements along with spin labeled phospholipids to probe the possible dynamic changes prompted by the interaction of GPI-anchored TNAP with model membranes. In this study, these authors showed that TNAP is probably close to the membrane surface, suggesting that this proximity can be related to the modulation of TNAP activity by the lipid composition of the vesicles, as previously reported (Bolean et al. 2010, 2011, 2015; Ciancaglini et al. 2012; Garcia et al. 2015; Simao et al. 2010a).
The fact that MVs isolated from growth plate cartilage have a higher content of Chol, SM and PS (Balcerzak et al. 2007; Peress et al. 1974; Wuthier 1975) and longer fatty acids (Abdallah et al. 2014) than extracellular vesicles isolated from homogeneous membranes indicate that MVs from both chondrocytes and osteoblasts can originate from lipid raft domains. Among the usual protein markers of lipid rafts domains (Foster et al. 2003), AnxA6 and TNAP have been found in MVs isolated from epiphyseal growth plate cartilage (Balcerzak et al. 2008), suggesting that the lipid composition is as important as the protein composition for the role of MVs in the mineralization process. Bolean et al. (2010, 2011) therefore carried out experiments to better understand the role of the lipids present in lipid rafts and their interactions with TNAP by means of differential scanning calorimetry using the substrates DPPC, Chol, SM and the ganglioside GM1. Calorimetry analysis of liposomes and proteoliposomes indicated that lateral phase segregation occurred only in the presence of Chol, with the formation of Chol-rich microdomains. The gradual increase in the complexity of the vesicles decreased the activity of the incorporated TNAP, and the presence of the enzyme also fluidified the vesicles (Bolean et al. 2010, 2011). Therefore, the study of different microdomains and their biophysical characterization may contribute to knowledge of the interactions between the lipids present in MVs and their interactions with TNAP.
The production of stable DPPC and DPPC:DPPS (1,2-dipalmitoyl-sn-glycero-3-phosphoserine) proteoliposomes harboring AnxA5 and TNAP has also been described, and these proteoliposomes have been investigated for whether the presence of AnxA5 impacts the kinetic parameters for hydrolysis of TNAP substrates at physiological pH (Bolean et al. 2015). The best catalytic efficiency was achieved with 9:1 DPPC:DPPS proteoliposomes (molar ratio), conditions that also increased the specificity of TNAP hydrolysis of PPi. AnxA5 was able to mediate Ca2+ influx into both lipid compositions (DPPC and 9:1 DPPC:DPPS vesicles) at physiological Ca2+ concentrations (~ 2 mM). This process was not affected by the presence of TNAP in the proteoliposomes. However, AnxA5 significantly affected the hydrolysis of these substrates by TNAP. These proteoliposomes may be used as MVs biomimetics, since they successfully transport Ca2+ and possess the ability to hydrolyze phosphosubstrates in the lipid/water interface.
The TNAP interaction with a lipid interface can also be investigated in monolayer systems by monitoring simultaneously the surface tension of pendant drop and the phosphohydrolytic activities, using the UV-Vis spectra of the axisymmetric drop shape analysis technique coupled to diffuse reflectance spectrophotometry (Andrade et al. 2016). Studies combining saturated (DPPC) and unsaturated (DOPC) phospholipids with different sterols, e.g. Chol, ergosterol (Ergo) and cholestenone (Achol), showed a higher affinity of TNAP for saturated lipid monolayer, reflected on its higher incorporation into DPPC liposomes. The presence of Chol in the monolayers resulted in higher exclusion pressure as compared to pure DPPC or pure DOPC liposomes, indicating that GPI–TNAP penetrates into the saturated lipid monolayer to a greater extent. The organization of the lipids and the structure of the sterols influenced the surface tension, the phosphohydrolytic activity of TNAP in the monolayer and the catalytic efficiency of TNAP in the bilayers. Membranes in the swollen lamellar (Lα) phase (Achol) provided better kinetic parameters than membranes in the liquid ordered (Lo) phase (Chol and Ergo), showing that thermodynamic factors, such as phase state, decreased transition enthalpy, with a loss of pre-transition. In addition, the presence of surface charges from the polar heads of the phospholipids can also lead to modifications of TNAP conformation, culminating in its different positioning or different access to its catalytic site (Bolean et al. 2015).
Proteoliposomes as a tool to study the propagation of the biomineralization process
The MV lumen contains a NC that consists mostly of AnxA5, amorphous calcium phosphate complexes and PS and is capable of inducing mineral formation (Wu et al. 1997, 1993). These findings contributed to the design of liposome models containing PS, Ca2+ and Pi complexes. The addition of other lipids, such as phosphatidylethanolamine or SM, with PS strongly inhibited the nucleational activity while the addition of AnxA5 promoted it (Genge et al. 2007).
The main goal of propagation studies using MV biomimetic models (Fig. 1) is to replicate in vitro the key events leading to the initiation of HA crystal synthesis in chondrocyte- and osteoblast-derived MVs. Once these proteoliposomes have been built and characterized, they can be added to fixed amounts of MVs, either wild-type (WT) MVs or those deficient in specific enzymes, as a way of modulating the in vitro calcification properties of the MVs. Such MV biomimetic proteoliposomes would also be useful for many important translational applications. Enzymatic mutations associated with diseases, such as those found in hypophosphatasia (Di Mauro et al. 2002) could be further elucidated in a membrane vesicle that better mimics the enzyme’s in vivo biological environment. Since these artificial vesicles adequately mimic the kinetic behavior of enzymes in the natural vesicular MV environment (Bolean et al. 2015; Ciancaglini et al. 2010; Simao et al. 2010a, b), these proteoliposomes could also be used for the screening of small molecule compounds able to modulate (inhibit or activate) the activity of MV enzymes for potential therapeutic usage (Lanier et al. 2010; Sergienko et al. 2009).
Fig. 1.
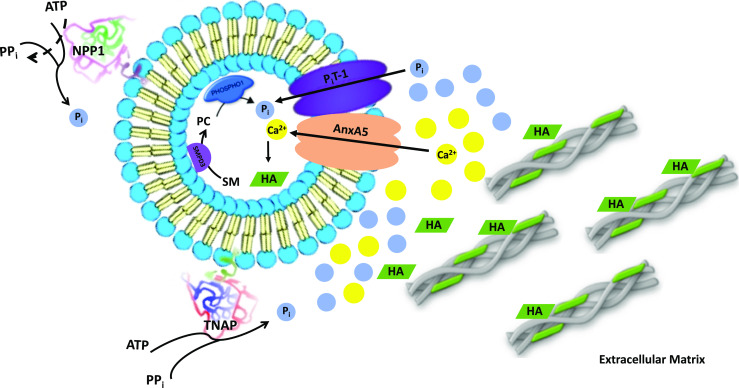
Proteoliposomes as matrix vesicles (MVs) biomimetic systems. The construction of MV models has the aim to provide an environment that would allow the initial nucleation of apatite inside of liposomes as well as to provide the surface nucleation that can also help in the propagation of apatite onto the extracellular matrix. The proteoliposomes internal nucleation can be initiated by orphan phosphatase 1 (PHOSPHO1) action producing inorganic phosphate (Pi) from the hydrolysis of phosphocoline (PC), which itself is derived from sphingomyelin (SM) by the action of sphingomyelin phosphodiesterase 3 (SMPD3) located in the inner surface of the MV membrane. In addition, protein transporters, such as annexin A5 (AnxA5) and phosphate transporter 1 (PiT-1), provide ions for nucleation inside the proteoliposomes. The events outside the vesicles are studied by the incorporation of the regulated proteins, tissue nonspecific alkaline phosphatase (TNAP) and ectonucleotide pyrophosphatase/phosphodiesterase (NPP1), which play a crucial role controlling appropriate inorganic pyrophosphate (PPi)/Pi ratio. Furthermore, calcium channels accelerate the nucleational activity of calcium–phosphate–lipid complexes (CPLX) inside the MVs. AnxA5 also shows binding affinity to type II collagen and when present in the proteoliposome membrane can drive the vesicles’ attachment onto to collagenous extracellular matrix stimulating mineral propagation. Hydroxyapatite (HA)
Recently, an in vitro biomineralization assay showed that proteoliposomes containing either TNAP, NPP1 or both together can induce mineral formation when incubated in synthetic cartilage lymph containing 1 mM ATP as substrate. The induction of mineralization was equivalent at pH 7.5 and 8, but considerably less at pH 9 (Simao et al. 2013). However, proteoliposomes harboring both TNAP and NPP1 triggered significantly more extensive Pi-dependent mineralization than proteoliposomes harboring TNAP alone, despite the lower TNAP content in the former proteoliposomes (Simao et al. 2010a), suggestive of additive hydrolytic activities for NPP1 and TNAP.
Comparisons of proteoliposomes and osteoblast-derived MVs or MVs deficient in TNAP, NPP1 or PHOSPHO1, using natural substrates such as ATP, ADP, PPi, have confirmed the validity of the proteoliposome models (Ciancaglini et al. 2010). TNAP- and PHOSPHO1-deficient MVs showed reduced calcification ability, while NPP1-deficient MVs hypercalcified. These results reveal that the cooperativity as well as the competition of TNAP, NPP1 and PHOSPHO1 for the biomineralization of substrates provide an additional level of regulation of metabolite flow for the control of the calcification process.
Favarin and collaborators (2017) showed that the presence of Chol and Achol in DPPC proteoliposomes increased the mineralization in vitro. DPPC proteoliposomes elicited a 1.3-fold increase in the mineralization, whereas DPPC:Chol proteoliposomes and DPPC:Achol proteoliposomes increased mineralization by four- and fivefold, respectively. However, the presence of Ergo did not significantly affect mineralization as compared to pure DPPC proteoliposomes. Proteoliposomes composed of unsaturated lipid (DOPC, a naturally occurring phospholipid) favored mineralization (approximately threefold higher mineralization) relative to proteoliposomes composed of a saturated lipid (DPPC). The exception was DOPC:Ergo proteoliposomes, which increased mineralization by 1.6-fold as compared to DPPC:Ergo proteoliposomes. DPPC:Achol and DOPC:Ergo proteoliposomes had similar abilities to propagate mineralization (Favarin et al. 2017). On the basis of these results, the addition of Chol and Ergo to a membrane in the Lα phase (DOPC) reduced the free volume between the phospholipids, thereby increasing the order of the membrane (Almeida et al. 2005) and affecting the ability of proteoliposomes to conduct biomineralization. However, it is important to bear in mind that the mineralization process is highly complex and depends on many factors, such as the physical properties, and the lateral organization of lipids in proteoliposomes has been revealed to be very important to control mineral propagation mediated by TNAP activity during mineralization. Proteoliposomes like MV biomimetics harboring other lipids and proteins shall help to elucidate even more specific interactions in further studies.
Advanced microscopy techniques to study lipid–protein interactions during the biomineralization process
General principles of atomic force microscopy-based studies
Scanning probe microscopy (SPM) is a large “family” of related technologies used to investigate the physical and chemical properties immediately above (typically a few tens of nanometers high) or immediately beneath (typically a few tens of nanometers deep) the surface of a sample. Scanning probe microscopes use a probe to raster-scan the sample in the XY plane. For each X, Y coordinate pair, the probe–sample interaction leads to a change in a property of the probe that is recorded by the detector as one data point. The pool of data points collected by the detector for a scan field is then elaborated into an SPM image. SPM images are mostly topographic images, where the relative height of the sample surface is reported as the third dimension (Z) for each X, Y coordinate pair. In non-topographic images, Z is a measure of the variation in a detectable probe–sample interaction caused by the change in a physical and/or chemical property of sample surface or of the layer immediately beneath sample surface. Topographic and non-topographic SPM images are usually recorded simultaneously and displayed side-by-side in order to facilitate the revelation of correlations between variations in surface height and changes in surface chemical and/or physical properties.
Due to the ability of SPM to identify surface chemical and/or physical properties by simply raster-scanning a sample with a probe, hundreds of new SPM techniques have been developed in the last decades. These techniques can be broadly divided into two main groups, i.e. imaging techniques and non-imaging techniques. Imaging techniques can in turn be subdivided into “primary” and “derivative” imaging modes. In terms of the function of the type of probe–sample interaction, four types of primary imaging modes can be identified: contact mode atomic force microscopy (AFM), dynamic (vertical or lateral) mode AFM, scanning tunneling microscopy and scanning near-field optical microscopy. Each primary imaging mode can be operated by using different methods (“operative” modes). For example, dynamic vertical mode AFM can be operated in either non-contact, or frequency modulation or tapping mode. Irrespective of the operative method, each primary imaging mode enables a host of derivative imaging modes, which have proven to be very useful to understand several sample properties, including viscoelasticity, adhesiveness and stiffness, at the nanometer scale. For instance, contact mode AFM enables lateral force microscopy, which provides information about the friction force between the probe and the sample, whereas dynamic mode AFM enables phase imaging, which has been proven to be very useful in polymer research to assess changes in viscoelasticity due to variations in the chemical composition of the surface of the sample or of the layer immediately beneath sample surface (see below) (Magonov et al. 1997).
In contact mode, the AFM probe (a cantilevered tip) is in perpetual contact with the sample surface. Variations in surface height lead to changes in the cantilever’s deflection, which are recorded by a detector by using a reflected laser beam. As a result of the constant contact between the probe–sample combination, lateral shear forces are generated, which may damage the sample during raster scanning and introduce topographic artifacts. In dynamic mode AFM, a rapidly oscillating cantilevered tip is employed to raster-scan the sample, and this technique minimizes, or nearly eliminates, lateral shear forces that may alter the tip or damage the sample surface by reducing the duration of probe–sample contact. As a result, soft materials are amenable for imaging at nanoscale resolution. Nevertheless, dynamic mode AFM does not completely eliminate probe–sample interactions. When the tip interacts with the sample, it experiences both repulsive and attractive forces, leading to energy dissipation and a shift (Δφ) in the phase angle of the oscillations of the interacting probe relative to the free probe. These changes can be measured by phase imaging, thus providing (qualitative) information on the viscoelastic properties and, in turn, on differences in material composition of the sample surface as well of the layer immediately beneath sample surface (Aytun et al. 2008; Dong and Yu 2003; Ruozi et al. 2009; Scott and Bhushan 2003).
SPM non-imaging techniques, which are generally referred to as “nano-manipulation”, also rely on probe–sample interactions as do imaging techniques; however, they extend the utility of SPM beyond simple imaging. Nano-manipulation techniques can be subdivided into “in-plane” and “out-of-plane” modes. Examples of in-plane nano-manipulation techniques are nano-scratching and nano-indenting, which use the SPM probe to alter the location of the sample’s surface atoms (Moon et al. 2001). An example of out-of-plane nano-manipulation technique is AFM single molecule force spectroscopy, where the AFM probe is used as a nanoscopic tweezer to grab and pull one end of a biomacromolecule bound to a substrate through the other end and unfold the biomacromolecule’s secondary and tertiary structure to assess the intra-molecular forces. This technique has enabled researchers to unravel key aspects of folding–unfolding of proteins designed to behave as mechanotransducers, i.e., polypeptides that perform biological functions under mechanical stress (Fowler et al. 2002; Ng et al. 2007).
AFM applications using topographic and phase imaging investigations of mineralization-competent matrix vesicles
Yadav and collaborators (2016) have recently used a combination of AFM topography and phase imaging to show that MV biogenesis and function are regulated by PHOSPHO1. For this study, MVs produced by chondrocytes isolated from WT and Phospho1 −/− mice and grown in non-mineralizing conditions were used. MVs were imaged through an AFM operated in dynamic vertical tapping mode. Topographic images showed that WT MVs were in greater number and had a greater size than Phospho1 −/− MVs, thus suggesting that PHOSPHO1 regulates MV biogenesis and development. Topographic images also revealed that the surface of WT MVs had irregularities, whose height increased with vesicle size, whereas most of Phospho1 −/− MVs showed a smooth surface. This result was interpreted taking into account that while WT MVs were filled with mineral aggregates (the NC), Phospho1 −/− MVs’ lumen was mostly devoid of the NC— i.e. PHOSPHO1 also regulates the formation of the NC within MVs. In order to validate this hypothesis, AFM phase analysis was used. AFM phase images showed that the surface of WT MVs had an irregular distribution of Δφ, with spots with high values of Δφ surrounded by regions with lower values of Δφ. The spots with high values of Δφ corresponded to morphological irregularities on the MV surface and were interpreted as being due to the presence of highly viscoelastic mineral aggregates (the NC) beneath the surface of the MVs. These aggregates were surrounded by less crowded regions that led to lower probe–sample energy dissipation when the membrane covering these regions was raster scanned by the AFM probe. On the contrary, Phospho1 −/− MVs did not show any appreciable changes in surface Δφ, which validated the hypothesis that the lumens of Phospho1 −/− MVs’ were mostly devoid of the NC. Thus, these studies, using an innovative combination of AFM topography and phase imaging, showed that the genetic ablation of PHOSPHO1 impairs MV biogenesis and NC formation. This result, in turn, suggests that the intra-vesicular production of Pi is necessary for the correct Ca2+/Pi stoichiometry for the formation of the NC (Yadav et al. 2016).
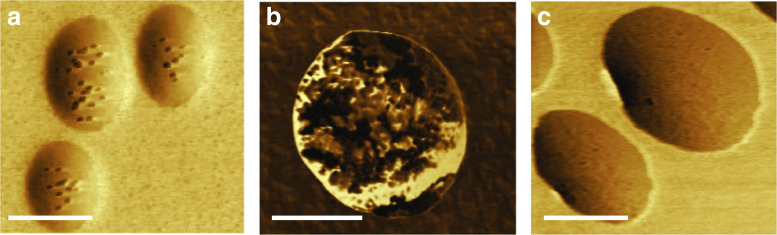
AFM phase analysis can be also used to characterize the structure of liposomes and proteoliposomes in order to assess how the addition of proteins to liposomes affects the membrane properties of the vesicles, induce the formation of surface protrusions and affect the function of proteoliposomes in biomineralization, thus mimicking MV activity (Bolean et al. 2017). AFM images of proteoliposomes harboring TNAP revealed the presence of protrusions with distinct viscoelasticity, thus suggesting that the presence of the protein induced local changes in membrane fluidity, compatible with the generation of protrusions (Fig. 2a). The protrusions were barely detectable in AnxA5-proteoliposomes (Fig. 2c). A more complex surface structure was observed for the mixed proteoliposomes harboring both TNAP and AnxA5, resulting in a lower affinity for type II collagen fibrils compared to proteoliposomes harboring AnxA5 exclusively (Bolean et al. 2017). Since these AFM studies may provide basic yet crucial information on the structure of lipid–protein clusters in more complex protein-containing lipid vesicles, they can be exploited to shed light on processes involving lateral heterogeneity on cellular membranes, including domain-induced budding and possibly MV formation, both of which are considered to be critical for the biomineralization process (Kirsch 2012; Wuthier and Lipscomb 2011).
Fig. 2.
Use of atomic force microscopy to image and characterize the structure of liposomes and proteoliposomes harboring relevant proteins present in the membrane of MVs: a 9:1 DPPC:DPPS (molar ratio) proteoliposomes harboring TNAP (scale bar 250 nm), b DPPC proteoliposomes harboring Na+/K+ ATPase (scale bar 500 nm), c 9:1 DPPC:DPPS (molar ratio) proteoliposomes harboring AnxA5 (scale bar 250 nm).
Proteoliposomes harboring NKA, a transmembrane protein present in MVs, were also structurally characterized by AFM (Fig. 2b). Topographic and phase images exhibited the presence on the vesicle surface of dark protrusions ranging from 38 to 115 nm in diameter and associated to the (αβ)2 heterodimer and its oligomers (Fig. 2b). Dark protrusions in phase images were associated to reconstituted NKA molecules. Further research is warranted to unveil the effect of NKA on the structure of MVs.
Optical microscopy applications on biomineralization studies
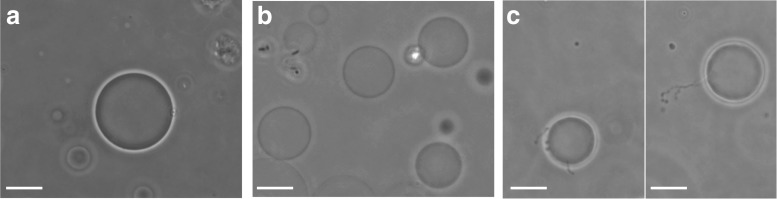
Several types of optical microscopy techniques can be applied to study specific lipid–protein interactions which play important roles in the growth of HA crystals. These techniques have provided us with additional information through the direct observation of the model vesicles. Using giant unilamellar vesicles (GUVs) coupled to phase contrast microscopy, it was possible to observe GUVs composed of DOPC (Fig. 3a) and, following protein incorporation, the effects of AnxA5 (Fig. 3b) and TNAP (Fig. 3c) on the membrane of the GUVs. As shown, AnxA5–membrane interactions are impaired on GUV membranes composed of DOPC, promoting changes in membrane permeability. As a consequence, optical contrast fading is observed without membrane rupture (Fig. 3b). Additionally, GUVs under an electric field initially change their morphology into prolate ellipsoids, but the spherical shape is recovered over time (Bolean et al. 2015). This finding must be due to the release of salt from the GUV lumen into the solution, thus equilibrating the internal and external conductivities. The same behavior is observed in vesicles made up of 9:1 DOPC:DPPS (molar ratio). Taken together, these results confirm the functional insertion of AnxA5 in both systems constituted by DOPC and 9:1 DOPC:DPPS, showing its Ca2+-transport role. Future experiments will address how AnxA5 forms Ca2+ channels and how the ions are transported through the membrane.
Fig. 3.
Different effects of osteogenic proteins on the morphology of GUV membrane composed of DOPC (2 mg/mL). a GUVs exhibiting spherical shape, b the AnxA5–membrane interaction with DOPC allowing changes between the internal and external media and subsequent loss of the optical contrast without membrane disruption, c the effect on GUV membrane morphology after TNAP interaction, showing excess area and filament formation. Magnification ×60, scale bars 20 μm
TNAP–membrane interactions promote an area excess with the formation of filaments in a localized area, which extends to the whole membrane perimeter with increasing incubation time (Fig. 3c). TNAP promotes an increase in membrane area, as observed by an enhancement of membrane fluctuations, followed by the protrusion of lipid filaments to re-establish membrane tension.
Additionally, the use of fluorescent probes bound to lipids, inserted in the membranes of interest, fluorescent proteins and the intrinsic tryptophan enable the making of fluorescence images of the protein–membrane interactions (Simeonov et al. 2013). Confocal microscopes are very useful due to their higher spatial resolution when compared to conventional wide field fluorescence microscopes, allowing analysis even with proteoliposomes as small as MVs (Mathiasen et al. 2014). An additional property that can be followed using fluorescence is the fluorescence lifetime. The coupling between pulsed laser excitation, confocal set-up, sensitive detection and scanning through the sample permits the detection of fluorescence lifetime in each point of the sample. This technique is called fluorescence lifetime imaging microscopy (FLIM) and provides us with a 2D map of fluorescence lifetimes (Fujiwara and Cieslik 2006). This property is very sensitive to microenvironmental changes due to micro-viscosity properties, ion concentrations, membrane fluidity, among others. As membrane fluidity changes with protein incorporation, it is possible to monitor protein–membrane interactions and the effects of different lipid compositions and lipid phases (de Almeida et al. 2009; Stockl and Herrmann 2010). Another possible use of fluorescence microscopy is Förster Resonance Energy Transfer (FRET) (Raicu and Singh 2013), which allows the distance on the order of few nanometers to be determined between a pair of donor and acceptor fluorescent molecules in the system.
Conclusion
This review provides basic and meaningful information on lipid–lipid and lipid–protein interactions on the surface of MVs. A fundamental understanding of the interplay between lipid composition and membrane behavior is crucial for the validation of these systems as mimetic of the process of biomineralization. Taken together, biophysical and biochemical approaches will be of great help to unveil the complex mechanisms involved in the interaction of specific proteins with the lipid bilayer components within the framework of the biomineralization process.
Acknowledgements
The authors thank FAPESP (2014/11941-3, 2014/00371-1 and 2016/21236-0), CAPES (7124/12-0) and CNPq (306166/2013-5, 400146/2014-2 and 443758/2014-0) for the financial support given to our laboratory.
Compliance with ethical standards
Conflicts of interest
All authors state that they have no conflicts of interest to declare.
Financial information
MB and AMSS received a FAPESP and CAPES scholarship, respectively. PC and RI also acknowledges CNPq for research fellowships. This work was also supported in part by grants DE12889 and AR53102 from the National Institutes of Health (USA) and by grants from the Arthritis National Research Foundation, respectively.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Latin America’ edited by Pietro Ciancaglini and Rosangela Itri.
Contributor Information
Maytê Bolean, Email: maytebolean@usp.br.
Pietro Ciancaglini, Email: pietro@ffclrp.usp.br.
References
- Abdallah D, Hamade E, Merhi RA, Bassam B, Buchet R, Mebarek S. Fatty acid composition in matrix vesicles and in microvilli from femurs of chicken embryos revealed selective recruitment of fatty acids. Biochem Biophys Res Commun. 2014;446:1161–1164. doi: 10.1016/j.bbrc.2014.03.069. [DOI] [PubMed] [Google Scholar]
- Ali SY, Sajdera SW, Anderson HC. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci USA. 1970;67:1513–1520. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida PF, Pokorny A, Hinderliter A. Thermodynamics of membrane domains. Biochim Biophys Acta. 2005;1720:1–13. doi: 10.1016/j.bbamem.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HC (1995) Molecular-biology of matrix vesicles. Clin Orthop Relat Res 314:266–280 [PubMed]
- Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- Anderson HC, Sipe JB, Hessle L et al (2004) Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol 164:841–847 [DOI] [PMC free article] [PubMed]
- Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Investig. 2010;90:1549–1557. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- Andrade MA, Favarin B, Derradi R et al (2016) Pendant-drop method coupled to ultraviolet-visible spectroscopy: a useful tool to investigate interfacial phenomena. Colloids Surf A Physicochem Eng Asp 504:305–311. 10.1016/j.colsurfa.2016.05.085 [DOI] [PMC free article] [PubMed]
- Aytun T, Mutaf OF, El-Atwani OJ, Ow-Yang CW. Nanoscale composition mapping of segregation in micelles with tapping-mode atomic force microscopy. Langmuir. 2008;24:14183–14187. doi: 10.1021/la802384x. [DOI] [PubMed] [Google Scholar]
- Balcerzak M, Radisson J, Azzar G et al (2007) A comparative analysis of strategies for isolation of matrix vesicles. Anal Biochem 361:176–182. 10.1016/j.ab.2006.10.001 [DOI] [PubMed]
- Balcerzak M, Malinowska A, Thouverey C et al (2008) Proteome analysis of matrix vesicles isolated from femurs of chicken embryo. Proteomics 8:192–205. 10.1002/pmic.200700612 [DOI] [PubMed]
- Bechkoff G, Radisson J, Bessueille L, Bouchekioua-Bouzaghou K, Buchet R. Distinct actions of strontium on mineral formation in matrix vesicles. Biochem Biophys Res Commun. 2008;373:378–381. doi: 10.1016/j.bbrc.2008.06.044. [DOI] [PubMed] [Google Scholar]
- Bolean M, Simao AM, Favarin BZ, Millan JL, Ciancaglini P. The effect of cholesterol on the reconstitution of alkaline phosphatase into liposomes. Biophys Chem. 2010;152:74–79. doi: 10.1016/j.bpc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Bolean M, Simao AM, Favarin BZ, Millan JL, Ciancaglini P. Thermodynamic properties and characterization of proteoliposomes rich in microdomains carrying alkaline phosphatase. Biophys Chem. 2011;158:111–118. doi: 10.1016/j.bpc.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolean M, Simao AM, Kiffer-Moreira T et al (2015) Proteoliposomes with the ability to transport Ca(2+) into the vesicles and hydrolyze phosphosubstrates on their surface. Arch Biochem Biophys 584:79–89. 10.1016/j.abb.2015.08.018 [DOI] [PMC free article] [PubMed]
- Bolean M, Borin IA, Simão AMS et al (2017) Topographic analysis by atomic force microscopy of proteoliposomes matrix vesicle mimetics harboring TNAP and AnxA5. Biochim Biophys Acta 1859:1911–1920. 10.1016/j.bbamem.2017.05.010 [DOI] [PMC free article] [PubMed]
- Bonucci E. Fine structure and histochemistry of “calcifying globules” in epiphyseal cartilage. Z Zellforsch Mikrosk Anat. 1970;103:192–217. doi: 10.1007/BF00337312. [DOI] [PubMed] [Google Scholar]
- Borg TK, Runyan RB, Wuthier RE. Correlation of freeze-fracture and scanning electron-microscopy of epiphyseal chondrocytes. Calcif Tissue Res. 1978;26:237–241. doi: 10.1007/Bf02013264. [DOI] [PubMed] [Google Scholar]
- Breusegem SY, Takahashi H, Giral-Arnal H et al (2009) Differential regulation of the renal sodium-phosphate cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 in dietary potassium deficiency. Am J Physiol Ren Physiol 297:F350–F361. 10.1152/ajprenal.90765.2008 [DOI] [PMC free article] [PubMed]
- Camolezi FL, Daghastanli KR, Magalhaes PP, Pizauro JM, Ciancaglini P (2002) Construction of an alkaline phosphatase–liposome system: a tool for biomineralization study. Int J Biochem Cell Biol 34:1091–1101 [DOI] [PubMed]
- Cecil RNA, Anderson HC. Freeze-fracture studies of matrix vesicle calcification in epiphyseal growth plate. Metab Bone Dis Relat. 1978;1:89–95. doi: 10.1016/0221-8747(78)90043-7. [DOI] [Google Scholar]
- Cecil DL, Rose DM, Terkeltaub R, Liu-Bryan R. Role of interleukin-8 in PiT-1 expression and CXCR1-mediated inorganic phosphate uptake in chondrocytes. Arthritis Rheum. 2005;52:144–154. doi: 10.1002/art.20748. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bal BS, Gorski JP. Calcium and collagen binding properties of osteopontin, bone sialoprotein, and bone acidic glycoprotein-75 from bone. J Biol Chem. 1992;267:24871–24878. [PubMed] [Google Scholar]
- Chen NX, O'Neill KD, Chen X, Moe SM. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J Bone Miner Res. 2008;23:1798–1805. doi: 10.1359/jbmr.080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciancaglini P, Simao AM, Camolezi FL, Millan JL, Pizauro JM. Contribution of matrix vesicles and alkaline phosphatase to ectopic bone formation. Braz J Med Biol Res. 2006;39:603–610. doi: 10.1590/S0100-879X2006000500006. [DOI] [PubMed] [Google Scholar]
- Ciancaglini P, Yadav MC, Simão AM et al (2010) Kinetic analysis of substrate utilization by native and TNAP-, NPP1-, or PHOSPHO1-deficient matrix vesicles. J Bone Miner Res 25:716–723. 10.1359/jbmr.091023 [DOI] [PMC free article] [PubMed]
- Ciancaglini P, Simão AMS, Bolean M et al (2012) Proteoliposomes in nanobiotechnology. Biophys Rev 4:67–81. 10.1007/s12551-011-0065-4 [DOI] [PMC free article] [PubMed]
- Collins JF, Bai L, Ghishan FK. The SLC20 family of proteins: dual functions as sodium-phosphate cotransporters and viral receptors. Pflugers Arch. 2004;447:647–652. doi: 10.1007/s00424-003-1088-x. [DOI] [PubMed] [Google Scholar]
- Cui L, Houston DA, Farquharson C, MacRae VE. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone. 2016;87:147–158. doi: 10.1016/j.bone.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Daghastanli KR, Ferreira RB, Thedei G, Jr, Maggio B, Ciancaglini P. Lipid composition-dependent incorporation of multiple membrane proteins into liposomes. Colloids Surf B Biointerfaces. 2004;36:127–137. doi: 10.1016/j.colsurfb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- de Almeida RFM, Loura LMS, Prieto M. Membrane lipid domains and rafts: current applications of fluorescence lifetime spectroscopy and imaging. Chem Phys Lipids. 2009;157:61–77. doi: 10.1016/j.chemphyslip.2008.07.011. [DOI] [PubMed] [Google Scholar]
- de Lima Santos H, Lopes ML, Maggio B, Ciancaglini P. Na,K-ATPase reconstituted in liposomes: effects of lipid composition on hydrolytic activity and enzyme orientation. Colloids Surf B Biointerfaces. 2005;41:239–248. doi: 10.1016/j.colsurfb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Dean DD, Schwartz Z, Bonewald L et al (1994) Matrix vesicles produced by osteoblast-like cells in culture become significantly enriched in proteoglycan-degrading metalloproteinases after addition of beta-glycerophosphate and ascorbic-acid. Calcified Tissue Int 54:399–408. 10.1007/Bf00305527 [DOI] [PubMed]
- Di Mauro S, Manes T, Hessle L et al (2002) Kinetic characterization of hypophosphatasia mutations with physiological substrates. J Bone Miner Res 17:1383–1391. 10.1359/jbmr.2002.17.8.1383 [DOI] [PubMed]
- Dong R, Yu LYE. Investigation of surface changes of nanoparticles using TM-AFM phase imaging. Environ Sci Technol. 2003;37:2813–2819. doi: 10.1021/es034071k. [DOI] [PubMed] [Google Scholar]
- Favarin BZ, Andrade MAR, Bolean M et al (2017) Effect of the presence of cholesterol in the interfacial microenvironment on the modulation of the alkaline phosphatase activity during in vitro mineralization. Colloids Surf B Biointerfaces 155:466–476. 10.1016/j.colsurfb.2017.04.051 [DOI] [PubMed]
- Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SB, Best RB, Toca Herrera JL et al (2002) Mechanical unfolding of a titin Ig domain: structure of unfolding intermediate revealed by combining AFM, molecular dynamics simulations, NMR and protein engineering. J Mol Biol 322:841–849. 10.1016/S0022-2836(02)00805-7 [DOI] [PubMed]
- Fujita T, Meguro T, Izumo N et al (2001) Phosphate stimulates differentiation and mineralization of the chondroprogenitor clone ATDC5. Jpn J Pharmacol 85:278–281 [DOI] [PubMed]
- Fujiwara M, Cieslik W. Fluorescence lifetime imaging microscopy: two-dimensional distribution measurement of fluorescence lifetime. Method Enzymol. 2006;414:633–642. doi: 10.1016/S0076-6879(06)14033-1. [DOI] [PubMed] [Google Scholar]
- Garcia AF, Simao AM, Bolean M et al (2015) Effects of GPI-anchored TNAP on the dynamic structure of model membranes. Phys Chem Chem Phys: PCCP 17:26295–26301. 10.1039/c5cp02377g [DOI] [PMC free article] [PubMed]
- Garimella R, Bi XH, Camacho N, Sipe JB, Anderson HC. Primary culture of rat growth plate chondrocytes: an in vitro model of growth plate histotype, matrix vesicle biogenesis and mineralization. Bone. 2004;34:961–970. doi: 10.1016/j.bone.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Genge BR, Wu LN, Wuthier RE. Kinetic analysis of mineral formation during in vitro modeling of matrix vesicle mineralization: effect of annexin A5, phosphatidylserine, and type II collagen. Anal Biochem. 2007;367:159–166. doi: 10.1016/j.ab.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Golub EE. Role of matrix vesicles in biomineralization. Biochim Biophys Acta. 2009;1790:1592–1598. doi: 10.1016/j.bbagen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicheux J, Palmer G, Shukunami C, Hiraki Y, Bonjour JP, Caverzasio J. A novel in vitro culture system for analysis of functional role of phosphate transport in endochondral ossification. Bone. 2000;27:69–74. doi: 10.1016/S8756-3282(00)00302-1. [DOI] [PubMed] [Google Scholar]
- Hale JE, Wuthier RE. The mechanism of matrix vesicle formation. Studies on the composition of chondrocyte microvilli and on the effects of microfilament-perturbing agents on cellular vesiculation. J Biol Chem. 1987;262:1916–1925. [PubMed] [Google Scholar]
- Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millan JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessle L, Johnson KA, Anderson HC et al (2002) Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA 99:9445–9449. 10.1073/pnas.142063399 [DOI] [PMC free article] [PubMed]
- Hsu HH, Anderson HC. Evidence of the presence of a specific ATPase responsible for ATP-initiated calcification by matrix vesicles isolated from cartilage and bone. J Biol Chem. 1996;271:26383–26388. doi: 10.1074/jbc.271.42.26383. [DOI] [PubMed] [Google Scholar]
- Ierardi DF, Pizauro JM, Ciancaglini P. Erythrocyte ghost cell-alkaline phosphatase: construction and characterization of a vesicular system for use in biomineralization studies. Biochim Biophys Acta. 2002;1567:183–192. doi: 10.1016/S0005-2736(02)00615-6. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Hessle L, Vaingankar S et al (2000) Osteoblast tissue-nonspecific alkaline phosphatase antagonizes and regulates PC-1. Am J Physiol Regul Integr Comp Physiol 279:R1365–R1377 [DOI] [PubMed]
- Kaplan JH (2002) Biochemistry of Na,K-ATPase. Annu Rev Biochem 71:511–535 10.1146/annurev.biochem.71.102201.141218 [DOI] [PubMed]
- Kapustin AN, Chatrou ML, Drozdov I et al (2015) Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res 116:1312–1323. 10.1161/Circresaha.116.305012 [DOI] [PubMed]
- Katz EP, Li ST. The intermolecular space of reconstituted collagen fibrils. J Mol Biol. 1973;73:351–369. doi: 10.1016/0022-2836(73)90347-1. [DOI] [PubMed] [Google Scholar]
- Kirsch T. Biomineralization--an active or passive process? Connect Tissue Res. 2012;53:438–445. doi: 10.3109/03008207.2012.730081. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Vondermark K. Remodeling of collagen type-I, type-ii and type-X and calcification of human fetal cartilage. Bone Miner. 1992;18:107–117. doi: 10.1016/0169-6009(92)90851-4. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Nah HD, Shapiro IM, Pacifici M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J Cell Biol. 1997;137:1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T, Harrison G, Golub EE, Nah HD. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J Biol Chem. 2000;275:35577–35583. doi: 10.1074/jbc.M005648200. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Wang W, Pfander D. Functional differences between growth plate apoptotic bodies and matrix vesicles. J Bone Miner Res. 2003;18:1872–1881. doi: 10.1359/jbmr.2003.18.10.1872. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Song MJ, Leith A, McEwen L, McEwen BF. Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J Struct Biol. 1993;110:39–54. doi: 10.1006/jsbi.1993.1003. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Hodgens KJ, Song MJ et al (1996) Mineralization of collagen may occur on fibril surfaces: evidence from conventional and high-voltage electron microscopy and three-dimensional imaging. J Struct Biol 117:24–35. 10.1006/jsbi.1996.0066 [DOI] [PubMed]
- Lanier M, Sergienko E, Simao AM et al (2010) Design and synthesis of selective inhibitors of placental alkaline phosphatase. Bioorg Med Chem 18:573–579. 10.1016/j.bmc.2009.12.012 [DOI] [PMC free article] [PubMed]
- Lau WL, Festing MH, Giachelli CM. Phosphate and vascular calcification: emerging role of the sodium-dependent phosphate co-transporter PiT-1. Thromb Haemost. 2010;104:464–470. doi: 10.1160/TH09-12-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Qin C, Butler WT, Ratner BD, Jiang S. Controlling the orientation of bone osteopontin via its specific binding with collagen I to modulate osteoblast adhesion. J Biomed Mater Res A. 2007;80:102–110. doi: 10.1002/jbm.a.30858. [DOI] [PubMed] [Google Scholar]
- Magne D, Bluteau G, Faucheux C et al (2003) Phosphate is a specific signal for ATDC5 chondrocyte maturation and apoptosis-associated mineralization: possible implication of apoptosis in the regulation of endochondral ossification. J Bone Miner Res 18:1430–1442. 10.1359/jbmr.2003.18.8.1430 [DOI] [PMC free article] [PubMed]
- Magonov SN, Elings V, Whangbo MH. Phase imaging and stiffness in tapping-mode atomic force microscopy. Surf Sci. 1997;375:L385–L391. doi: 10.1016/S0039-6028(96)01591-9. [DOI] [Google Scholar]
- Mahamid J, Aichmayer B, Shimoni E et al (2010) Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc Natl Acad Sci USA 107:6316–6321. 10.1073/pnas.0914218107 [DOI] [PMC free article] [PubMed]
- Mansfield K, Teixeira CC, Adams CS, Shapiro IM. Phosphate ions mediate chondrocyte apoptosis through a plasma membrane transporter mechanism. Bone. 2001;28:1–8. doi: 10.1016/S8756-3282(00)00409-9. [DOI] [PubMed] [Google Scholar]
- Martin SM, Schwartz JL, Giachelli CM, Ratner BD. Enhancing the biological activity of immobilized osteopontin using a type-1 collagen affinity coating. J Biomed Mater Res A. 2004;70:10–19. doi: 10.1002/jbm.a.30052. [DOI] [PubMed] [Google Scholar]
- Mathiasen S, Christensen SM, Fung JJ et al (2014) Nanoscale high-content analysis using compositional heterogeneities of single proteoliposomes. Nat Methods 11:931–934. 10.1038/Nmeth.3062 [DOI] [PMC free article] [PubMed]
- McKee MD, Hoac B, Addison WN, Barros NM, Millan JL, Chaussain C. Extracellular matrix mineralization in periodontal tissues: noncollagenous matrix proteins, enzymes, and relationship to hypophosphatasia and X-linked hypophosphatemia. Periodontol. 2013;63:102–122. doi: 10.1111/prd.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally EA, Schwarcz HP, Botton GA, Arsenault AL. A model for the ultrastructure of bone based on electron microscopy of ion-milled sections. PLoS One. 2012;7:e29258. doi: 10.1371/journal.pone.0029258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montessuit C, Caverzasio J, Bonjour JP. Characterization of a pi transport system in cartilage matrix vesicles. Potential role in the calcification process. J Biol Chem. 1991;266:17791–17797. [PubMed] [Google Scholar]
- Moon WC, Yoshinobu T, Iwasaki H. Nanotribology of Si oxide layers on Si by atomic force microscopy. Ultramicroscopy. 2001;86:49–53. doi: 10.1016/S0304-3991(00)00089-9. [DOI] [PubMed] [Google Scholar]
- Morth JP, Pedersen BP, Buch-Pedersen MJ et al (2011) A structural overview of the plasma membrane Na+,K+−ATPase and H+−ATPase ion pumps. Nat Rev Mol Cell Biol 12:60–70. 10.1038/nrm3031 [DOI] [PubMed]
- Ng SP, Randles LG, Clarke J. Single molecule studies of protein folding using atomic force microscopy. Methods Mol Biol. 2007;350:139–167. doi: 10.1385/1-59745-189-4:139. [DOI] [PubMed] [Google Scholar]
- Nollet M, Santucci-Darmanin S, Breuil V et al (2014) Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy 10:1965–1977. 10.4161/auto.36182 [DOI] [PMC free article] [PubMed]
- Palmer G, Zhao J, Bonjour J, Hofstetter W, Caverzasio J. In vivo expression of transcripts encoding the Glvr-1 phosphate transporter/retrovirus receptor during bone development. Bone. 1999;24:1–7. doi: 10.1016/S8756-3282(98)00151-3. [DOI] [PubMed] [Google Scholar]
- Peress NS, Anderson HC, Sajdera SW. The lipids of matrix vesicles from bovine fetal epiphyseal cartilage. Calcif Tissue Res. 1974;14:275–281. doi: 10.1007/BF02060301. [DOI] [PubMed] [Google Scholar]
- Pidaparti RM, Chandran A, Takano Y, Turner CH. Bone mineral lies mainly outside collagen fibrils: predictions of a composite model for osteonal bone. J Biomech. 1996;29:909–916. doi: 10.1016/0021-9290(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Raicu V, Singh DR. FRET spectrometry: a new tool for the determination of protein quaternary structure in living cells. Biophys J. 2013;105:1937–1945. doi: 10.1016/j.bpj.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud JL, Pitard B, Levy D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim Biophys Acta. 1995;1231:223–246. doi: 10.1016/0005-2728(95)00091-V. [DOI] [PubMed] [Google Scholar]
- Rigos CF, Nobre TM, Zaniquelli ME, Ward RJ, Ciancaglini P. The association of Na,K-ATPase subunits studied by circular dichroism, surface tension and dilatational elasticity. J Colloid Interface Sci. 2008;325:478–484. doi: 10.1016/j.jcis.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Rigos CF, de Lima SH, Yoneda JS, Montich G, Maggio B, Ciancaglini P. Cytoplasmatic domain of Na,K-ATPase alpha-subunit is responsible for the aggregation of the enzyme in proteoliposomes. Biophys Chem. 2010;146:36–41. doi: 10.1016/j.bpc.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Roberts S, Narisawa S, Harmey D, Millan JL, Farquharson C. Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization. J Bone Miner Res. 2007;22:617–627. doi: 10.1359/jbmr.070108. [DOI] [PubMed] [Google Scholar]
- Ruozi B, Tosi G, Tonelli M et al (2009) AFM phase imaging of soft-hydrated samples: a versatile tool to complete the chemical-physical study of liposomes. J Liposome Res 19:59–67. 10.1080/08982100802584071 [DOI] [PubMed]
- Scott WW, Bhushan B. Use of phase imaging in atomic force microscopy for measurement of viscoelastic contrast in polymer nanocomposites and molecularly thick lubricant films. Ultramicroscopy. 2003;97:151–169. doi: 10.1016/S0304-3991(03)00040-8. [DOI] [PubMed] [Google Scholar]
- Sergienko E, Su Y, Chan X et al (2009) Identification and characterization of novel tissue-nonspecific alkaline phosphatase inhibitors with diverse modes of action. J Biomol Screen 14:824–837. 10.1177/1087057109338517 [DOI] [PMC free article] [PubMed]
- Silvius JR. Solubilization and functional reconstitution of biomembrane components. Annu Rev Biophys Biomol Struct. 1992;21:323–348. doi: 10.1146/annurev.bb.21.060192.001543. [DOI] [PubMed] [Google Scholar]
- Simao AMS, Yadav MC, Ciancaglini P, Millan JL. Proteoliposomes as matrix vesicles’ biomimetics to study the initiation of skeletal mineralization. Braz J Med Biol Res. 2010;43:234–241. doi: 10.1590/S0100-879x2010007500008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simao AMS, Yadav MC, Narisawa S et al (2010b) Proteoliposomes harboring alkaline phosphatase and nucleotide pyrophosphatase as matrix vesicle biomimetics. J Biol Chem 285:7598–7609. 10.1074/jbc.M109.079830 [DOI] [PMC free article] [PubMed]
- Simao AM, Bolean M, Hoylaerts MF, Millan JL, Ciancaglini P. Effects of pH on the production of phosphate and pyrophosphate by matrix vesicles’ biomimetics. Calcif Tissue Int. 2013;93:222–232. doi: 10.1007/s00223-013-9745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simao AMS, Bolean M, Cury TAC, Stabeli RG, Itri R, Ciancaglini P. Liposomal systems as carriers for bioactive compounds. Biophys Rev. 2015;7:391–397. doi: 10.1007/s12551-015-0180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonov P, Werner S, Haupt C, Tanabe M, Bacia K. Membrane protein reconstitution into liposomes guided by dual-color fluorescence cross-correlation spectroscopy. Biophys Chem. 2013;184:37–43. doi: 10.1016/j.bpc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Singer SJ. Some early history of membrane molecular biology. Annu Rev Physiol. 2004;66:1–27. doi: 10.1146/annurev.physiol.66.032902.131835. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Wilkins RJ, Meredith D, Browning JA. Characterisation of inorganic phosphate transport in bovine articular chondrocytes. Cell Physiol Biochem. 2007;20:99–108. doi: 10.1159/000104158. [DOI] [PubMed] [Google Scholar]
- Stockl MT, Herrmann A. Detection of lipid domains in model and cell membranes by fluorescence lifetime imaging microscopy. Biochim Biophys Acta. 2010;1798:1444–1456. doi: 10.1016/j.bbamem.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Sugita A, Kawai S, Hayashibara T et al (2011) Cellular ATP synthesis mediated by type III sodium-dependent phosphate transporter pit-1 is critical to chondrogenesis. J Biol Chem 286:3094–3103. 10.1074/jbc.M110.148403 [DOI] [PMC free article] [PubMed]
- Suzuki A, Ghayor C, Guicheux J et al (2006) Enhanced expression of the inorganic phosphate transporter pit-1 is involved in BMP-2-induced matrix mineralization in osteoblast-like cells. J Bone Miner Res 21:674–683. 10.1359/jbmr.020603 [DOI] [PubMed]
- Suzuki A, Ammann P, Nishiwaki-Yasuda K et al (2010) Effects of transgenic pit-1 overexpression on calcium phosphate and bone metabolism. J Bone Miner Metab 28:139–148. 10.1007/s00774-009-0121-3 [DOI] [PubMed]
- Theobald J, Smith PD, Jacob SM, Moss SE. Expression of annexin VI in A431 carcinoma cells suppresses proliferation: a possible role for annexin VI in cell growth regulation. Biochim Biophys Acta. 1994;1223:383–390. doi: 10.1016/0167-4889(94)90099-X. [DOI] [PubMed] [Google Scholar]
- Thouverey C, Bechkoff G, Pikula S, Buchet R. Inorganic pyrophosphate as a regulator of hydroxyapatite or calcium pyrophosphate dihydrate mineral deposition by matrix vesicles. Osteoarthr Cartil. 2009;17:64–72. doi: 10.1016/j.joca.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Thouverey C, Malinowska A, Balcerzak M et al (2011) Proteomic characterization of biogenesis and functions of matrix vesicles released from mineralizing human osteoblast-like cells. J Proteome 74:1123–1134. 10.1016/j.jprot.2011.04.005 [DOI] [PubMed]
- Tokhtaeva E, Clifford RJ, Kaplan JH, Sachs G, Vagin O. Subunit isoform selectivity in assembly of Na,K-ATPase alpha-beta heterodimers. J Biol Chem. 2012;287:26115–26125. doi: 10.1074/jbc.M112.370734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Bellosta R, Sorribas V. Role of rat sodium/phosphate cotransporters in the cell membrane transport of arsenate. Toxicol Appl Pharmacol. 2008;232:125–134. doi: 10.1016/j.taap.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Villa-Bellosta R, Sorribas V. Compensatory regulation of the sodium/phosphate cotransporters NaPi-IIc (SCL34A3) and pit-2 (SLC20A2) during pi deprivation and acidosis. Pflugers Arch. 2010;459:499–508. doi: 10.1007/s00424-009-0746-z. [DOI] [PubMed] [Google Scholar]
- Villa-Bellosta R, Levi M, Sorribas V (2009a) Vascular smooth muscle cell calcification and SLC20 inorganic phosphate transporters: effects of PDGF, TNF-alpha, and pi. Pflugers Arch 458:1151–1161. 10.1007/s00424-009-0688-5 [DOI] [PubMed]
- Villa-Bellosta R, Ravera S, Sorribas V et al (2009b) The Na+−Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary pi. Am J Physiol Ren Physiol 296:F691–F699. 10.1152/ajprenal.90623.2008 [DOI] [PMC free article] [PubMed]
- Virkki LV, Biber J, Murer H, Forster IC. Phosphate transporters: a tale of two solute carrier families. Am J Physiol Renal Physiol. 2007;293:F643–F654. doi: 10.1152/ajprenal.00228.2007. [DOI] [PubMed] [Google Scholar]
- Wang W, Kirsch T. Retinoic acid stimulates annexin-mediated growth plate chondrocyte mineralization. J Cell Biol. 2002;157:1061–1069. doi: 10.1083/jcb.200203014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Canaff L, Davidson D et al (2001) Alterations in the sensing and transport of phosphate and calcium by differentiating chondrocytes. J Biol Chem 276:33995–34005. 10.1074/jbc.M007757200 [DOI] [PubMed]
- Wang W, Xu J, Du B, Kirsch T. Role of the progressive ankylosis gene (ank) in cartilage mineralization. Mol Cell Biol. 2005;25:312–323. doi: 10.1128/MCB.25.1.312-323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner S, Traub W. Organization of hydroxyapatite crystals within collagen fibrils. FEBS Lett. 1986;206:262–266. doi: 10.1016/0014-5793(86)80993-0. [DOI] [PubMed] [Google Scholar]
- Weiner S, Traub W. Crystal size and organization in bone. Connect Tissue Res. 1989;21:259–265. doi: 10.3109/03008208909050015. [DOI] [PubMed] [Google Scholar]
- Wu LN, Genge BR, Lloyd GC, Wuthier RE. Collagen-binding proteins in collagenase-released matrix vesicles from cartilage. Interaction between matrix vesicle proteins and different types of collagen. J Biol Chem. 1991;266:1195–1203. [PubMed] [Google Scholar]
- Wu LN, Yoshimori T, Genge BR et al (1993) Characterization of the nucleational core complex responsible for mineral induction by growth plate cartilage matrix vesicles. J Biol Chem 268:25084–25094 [PubMed]
- Wu LN, Genge BR, Dunkelberger DG, LeGeros RZ, Concannon B, Wuthier RE. Physicochemical characterization of the nucleational core of matrix vesicles. J Biol Chem. 1997;272:4404–4411. doi: 10.1074/jbc.272.7.4404. [DOI] [PubMed] [Google Scholar]
- Wu LN, Guo Y, Genge BR, Ishikawa Y, Wuthier RE. Transport of inorganic phosphate in primary cultures of chondrocytes isolated from the tibial growth plate of normal adolescent chickens. J Cell Biochem. 2002;86:475–489. doi: 10.1002/jcb.10240. [DOI] [PubMed] [Google Scholar]
- Wuthier RE. Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochim Biophys Acta. 1975;409:128–143. doi: 10.1016/0005-2760(75)90087-9. [DOI] [PubMed] [Google Scholar]
- Wuthier RE, Lipscomb GF. Matrix vesicles: structure, composition, formation and function in calcification. Front Biosci. 2011;16:2812–2902. doi: 10.2741/3887. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Camalier CE, Nagashima K et al (2007) Analysis of the extracellular matrix vesicle proteome in mineralizing osteoblasts. J Cell Physiol 210:325–335. 10.1002/jcp.20826 [DOI] [PubMed]
- Yadav MC, Simao AM, Narisawa S et al (2011) Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification. J Bone Miner Res 26:286–297. 10.1002/jbmr.195 [DOI] [PMC free article] [PubMed]
- Yadav MC, Bottini M, Cory E et al (2016) Skeletal mineralization deficits and impaired biogenesis and function of chondrocyte-derived matrix vesicles in Phospho1(−/−) and Phospho1/Pi t1 double-knockout mice. J Bone Miner Res 31:1275–1286. 10.1002/jbmr.2790 [DOI] [PMC free article] [PubMed]
- Yeagle PL. Phosphorus-31 nuclear magnetic resonance in membrane fusion studies. Methods Enzymol. 1993;220:68–79. doi: 10.1016/0076-6879(93)20074-D. [DOI] [PubMed] [Google Scholar]
- Yoneda JS, Scanavachi G, Sebinelli HG et al (2016) Multimeric species in equilibrium in detergent-solubilized Na,K-ATPase. Int J Biol Macromol 89:238–245. 10.1016/j.ijbiomac.2016.04.058 [DOI] [PubMed]
- Zhou X, Cui Y, Zhou X, Han J. Phosphate/pyrophosphate and MV-related proteins in mineralisation: discoveries from mouse models. Int J Biol Sci. 2012;8:778–790. doi: 10.7150/ijbs.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]