Abstract
Actinoporins constitute a unique class of pore-forming toxins found in sea anemones that are able to bind and oligomerize in membranes, leading to cell swelling, impairment of ionic gradients and, eventually, to cell death. In this review we summarize the knowledge generated from the combination of biochemical and biophysical approaches to the study of sticholysins I and II (Sts, StI/II), two actinoporins largely characterized by the Center of Protein Studies at the University of Havana during the last 20 years. These approaches include strategies for understanding the toxin structure–function relationship, the protein–membrane association process leading to pore formation and the interaction of toxin with cells. The rational combination of experimental and theoretical tools have allowed unraveling, at least partially, of the complex mechanisms involved in toxin–membrane interaction and of the molecular pathways triggered upon this interaction. The study of actinoporins is important not only to gain an understanding of their biological roles in anemone venom but also to investigate basic molecular mechanisms of protein insertion into membranes, protein–lipid interactions and the modulation of protein conformation by lipid binding. A deeper knowledge of the basic molecular mechanisms involved in Sts–cell interaction, as described in this review, will support the current investigations conducted by our group which focus on the design of immunotoxins against tumor cells and antigen-releasing systems to cell cytosol as Sts-based vaccine platforms.
Keywords: Sticholysins, Actinoporins, Pore-forming toxins, Protein–membrane interaction, Hemolytic activity
Introducing the toxins
Pore-forming toxins (PFT) are one of the most ancient and spectacular weapons used by living beings to attack or self-defend. Secreted in soluble form, they undergo conformational changes upon different stimuli, allowing them to create a pore in the host membrane. These toxins exhibit a very broad taxonomic distribution from bacteria to mammals (Gonzalez et al. 2008; Bischofberger et al. 2012; Alves et al. 2014), with bacterial PFT the best characterized to date (Peraro and van der Goot 2016). PFT can be classified into two broad groups: α-pore-forming proteins (PFP) and β-PFP, depending on the secondary structural elements involved in pore architecture (Parker and Feil 2005; Iacovache et al. 2010). These toxins are characterized by displaying at least two conformational states, i.e. soluble monomers and membrane bound oligomers.
The two PFT from Stichodactyla helianthus
Sticholysins I and II (Sts, StI/II) are PFT produced by the Caribbean Sea anemone Stichodactyla helianthus (Lanio et al. 2001; Alvarez et al. 2009); they belong to the actinoporin protein family, a unique class of PFT found in sea anemones. Actinoporins are monomeric, soluble, α-helical barrel PFP characterized by a molecular mass of about 20 kDa, high pI (>9.0), lack of Cys residues and a high affinity for sphingomyelin (SM)-containing membranes (Anderluh and Macek 2002). StI and StII have molecular weights of 19,392 (± 2) Da and 19,283 (± 2) Da, respectively and exhibit high sequence similarity (99%) and identity (93%) (Huerta et al. 2001; Lanio et al. 2001). These toxins bind to and disturb lipidic membranes, leading to the formation of pores both in cells and model membranes. These transmembrane α-helical barrel pores disrupt cellular ionic gradients, cause osmotic swelling and eventually lead to cell death (Lanio et al. 2001; Tejuca et al. 2001).
Structure of StI and StII
The three-dimensional (3D) solution structures of four actinoporins have been solved: StI (García-Linares et al. 2013), StII (Mancheno et al. 2003), equinatoxin II (EqtII) from Actinia equina (Athanasiadis et al. 2001; Hinds et al. 2002) and fragaceatoxin C (FraC) from Actinia fragacea (Mechaly et al. 2011; Tanaka et al. 2015). The comparison of these structures in solution show a similar 3D fold formed by a central rigid and compact core consisting of two β sheets. On the opposite sides of this β-core, two α-helices are oriented perpendicularly to each other. The first α-helix located close to the N-terminus is amphipathic in nature, mobile and flexible (Mancheno et al. 2003; García-Linares et al. 2013). A distinctive feature of actinoporins is the existence of an aromatic amino acid cluster exposed to the aqueous medium. The determination of the crystallographic structure of the complex formed between StII and phosphorylcholine (POC), the common polar head group of the phospholipids SM and phosphatidylcholine (PC), enabled researchers to explain, from the structural point of view, the affinity of actinoporins for SM (Mancheno et al. 2003). Co-crystallization of StII with POC enabled the definition of the POC binding site, crucial for the specific recognition of SM. Residues involved in the POC binding site are strictly conserved in actinoporins, implying that the same mechanism of lipid headgroup recognition is followed by other members of this protein family (Bakrac et al. 2008). The POC binding site is a cavity formed by amino acids of the second α-helix, the compact β-sheet nucleus and the aromatic amino acid cluster (Fig. 1). This binding site together with the aromatic amino acid cluster forms an essential structural assembly for binding of these toxins to membranes and is known as the interfacial binding site (Bakrac et al. 2008).
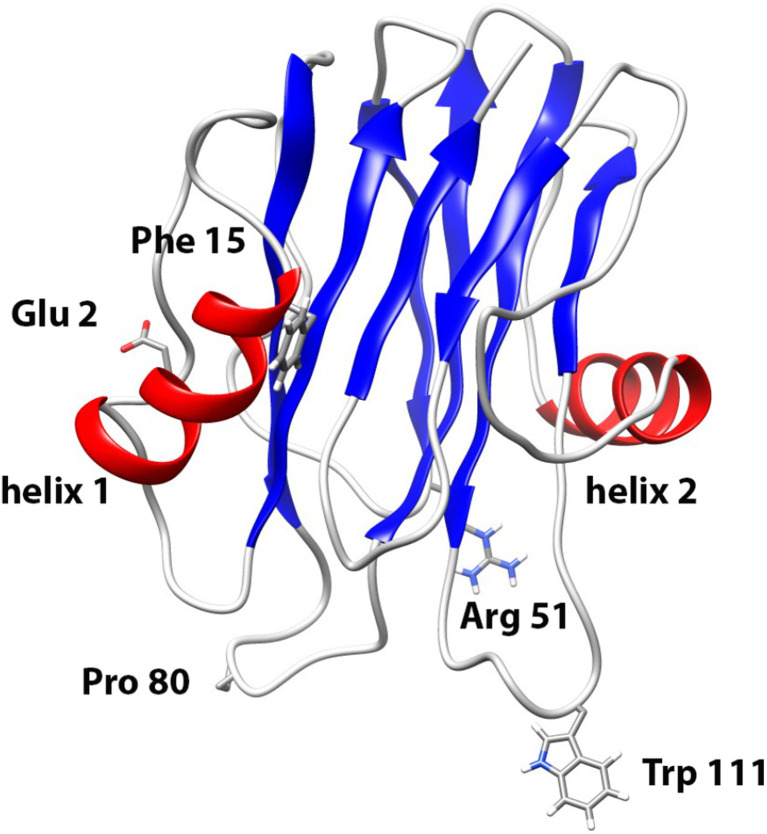
Fig. 1.
Structural elements of actinoporins. Ribbon representation of sticholysins I (StI) structure (Protein Data Base: 2KS4). α-Helices are represented by light gray ribbons, β-sheets are in dark gray and non-periodic structures are gray color. The amino acids Glu2, Phe15, Arg52, Pro80 and Trp111 replaced by Cys in the site-directed mutants are indicated in the figure. Images were produced with the UCSF Chimera program for Windows (Pettersen et al. 2004)
Recently, researchers have solved the crystallographic structure of FraC at four different stages of the lytic mechanism, namely the water-soluble state, the monomeric lipid-bound form, an intermediate assembly and the fully assembled transmembrane pore. These studies revealed the existence of multiple sites for lipid binding (Tanaka et al. 2015); in fact two of these sites (L2 and L3) were considered to be initial binding sites similar to the POC binding site described for StII (Mancheno et al. 2003), while L4 and L5 were hypothesized to be sites of low affinity for POC or perhaps high-affinity binding sites for lipids with headgroups other than POC (Tanaka et al. 2015).
The mechanism of pore formation by actinoporins in membranes
Pores are formed in membranes by a mechanism involving several stages, i.e. membrane attachment, oligomerization, detachment and insertion of the toxin N-terminal region into the hydrophobic core of the membrane and pore formation (Hong et al. 2002; Malovrh et al. 2003; Mancheno et al. 2003; García-Linares et al. 2013). The first step in this sequence of events is binding of the soluble monomers to the membrane. As this step does not involve significant protein conformational changes, the structure of the membrane-bound monomer is similar to that of the solution (Menestrina et al. 1999; Alvarez et al. 2003; Alegre-Cebollada et al. 2007). Once the toxin binds to the membrane, the association of several monomers and the transfer of the amino-terminal region from the body of the protein to the hydrophobic core of the bilayer must occur. However, the exact sequence of events taking place during the oligomerization process is probably the most controversial aspect of the actinoporin pore-forming mechanism (Cosentino et al. 2016). In this context, one proposal is that the partial detachment of the amino-terminal region from the body of the protein is triggered upon binding to the membrane (Alvarez et al. 2003, 2009; Alegre-Cebollada et al. 2007). According to this model, the transition of the N-terminus to the bilayer occurs in a non-concerted manner, prior to or concurrently with the oligomerization process, as has been proposed for StII (Antonini et al. 2014) or EqtII (Rojko et al. 2014). Thus, the pore is constructed from the successive addition of N-termini of various protomers and lipid molecules (Antonini et al. 2014). In contrast, there is debate about the possible existence of prepore structures in the mechanism of actinoporins (Mancheno et al. 2003, 2006; Mechaly et al. 2011; Tanaka et al. 2015). However, there is no evidence showing that such prepore structures can evolve into a conducting channel (Rojko et al. 2013, 2015).
The pore architecture of actinoporins
Three models have been proposed to describe the pore structure formed by actinoporins in membrane, the toroidal pore (Alvarez-Valcarcel et al. 2001), the conical pore (Mechaly et al. 2011) and the hybrid pore (Tanaka et al. 2015). The toroidal pore model describes a channel formed by three or four protein monomers lined by non-bilayer forming lipids (Alvarez-Valcarcel et al. 2001). In contrast, the conic pore hypothesis assumes that the pore is formed by nine toxin protomers, excluding the involvement of lipids in the structure, while the hybrid pore proposal assumes that the pore is composed of eight monomers with lipid participation (Tanaka et al. 2015).
The toroidal pore model was firstly suggested based on functional studies with Sts showing that the presence of non-lamellar forming lipids in the bilayer potentiates the toxin pore-forming activity. In the toroidal proteolipidic arrangement, the lipids that make up the pore assume a positive curvature in the plane perpendicular to the bilayer and a negative curvature in the membrane plane. The ability of Sts to promote lipid–transbilayer movement was an experimental finding that supported this hypothesis (Alvarez-Valcarcel et al. 2001). This hypothesis is consistent with results obtained from electronic paramagnetic resonance (EPR) studies, which showed that Sts do not cause the immobilization of lipidic spin probes located towards the center of the bilayer (Alvarez et al. 2003). The toroidal lipid model was subsequently proposed for EqtII (Anderluh et al. 2003).
The pore size of Sts (Tejuca et al. 2001) and EqtII (Belmonte et al. 1993; Macek et al. 1994) in erythrocytes and liposomes was determined using oligosaccharides and polyethylene glycols of different hydrodynamic radii as well as through conductance measurements in flat bilayers (Belmonte et al. 1993). The radius determined for the three proteins was ~ 1 nm, regardless of the type of osmoprotector or toxin concentration used (Tejuca et al. 2001). Accordingly, the toroidal pore model allowed researchers to explain how the dimensions of the pore determined experimentally (~ 2 nm) would be satisfied if lipid molecules would also contribute to the pore architecture, in addition to three or four α-helices of the protein.
The conical pore model was deduced from the structural determinations of the crystals of a FraC nonameric structure in the presence of detergent and the cryo-electron microscopy studies of FraC in the presence of large, unilamellar vesicles (LUV) composed of an equimolar mixture of dioleoyl-phosphatidylcholine (DOPC) and SM (Mechaly et al. 2011). From these studies, it was proposed that the pores of actinoporins are composed of the α-helices from the N-terminus of nine monomers, without the involvement of membrane lipids. The conical pore is depicted as being funnel-shaped and characterized by an upper diameter of 5 nm and a lower one of 1.5 nm, with the latter diameter similar to that previously calculated for EqtII and Sts (Belmonte et al. 1993; Tejuca et al. 2001). Initially, the nonameric structure of FraC in the presence of detergent was considered to be a prepore state because it did not exhibit the N-terminal helices detached from the protein body (Mechaly et al. 2011); however, this model was later reconsidered by its own authors, taking into account that it constitutes an oligomer with a low probability to evolve to a competent lytic structure due to steric problems (Tanaka et al. 2015).
The hybrid pore model was proposed from the determination of the crystal structure of the transmembrane pore of FraC (Tanaka et al. 2015). According to this model, FraC forms an octameric proteolipidic pore where each monomer is associated with three lipid molecules. In this model, the protein–protein interactions and the protein–lipid interactions are relevant for the stabilization of the oligomer, resulting in a hypothesis having features common to both the toroidal and the conical pore model. In the hybrid pore model, SM not only acts as a lipid receptor in the membrane, but also as an important structural element in pore assembly (Tanaka et al. 2015). The main limitation of the hybrid pore model is that it does not provide any information about the lipid membrane reorganization process (Cosentino et al. 2016). It is noteworthy that the octameric structure obtained for FraC (Tanaka et al. 2015) has motivated the reanalysis of the tetrameric structures previously observed for StII (Mancheno et al. 2003). A second inspection of the StII tetramers suggests that such structures may conceal an octameric structure similar to that of FraC. In fact, the fourfold symmetry of the StII pore, reflecting the P4 symmetry of its 2D crystals, masked the underlying octameric symmetry as found in the FraC structure. Indeed, there are eight density maxima around the perimeter of the StII pore; in the previous model these were treated as two lobes of density for four protein subunits laid on their side (Mancheno et al. 2003). The FraC structure suggests that a more accurate interpretation of the StII pore would be eight subunits rotated through 90° and viewed down their long axis, since that is the orientation of the subunits in the FraC pore (Tanaka et al. 2015). It is possible that the protein units identified for the toroidal pore model (Mancheno et al. 2003, 2006) correspond to dimeric rather than monomeric structures (Gilbert 2015).
Sts display permeabilizing activity in both the cell and model membranes. This activity is strongly dependent on the lipid composition of the membrane favored by the presence of SM, cholesterol and non-bilayer-forming lipids (Alvarez-Valcarcel et al. 2001; Alvarez et al. 2003; Martinez et al. 2007; Pedrera et al. 2015). In turn, this activity modifies the membrane properties by promoting the mixing of the lipid phases and an increase in the order of the system; therefore, the interaction of Sts with lipids entails a remodeling of membrane domains that probably facilitates the action of the toxin (Ros et al. 2013).
The protein structure–function connection
Two of the most extended experimental approaches to clarify the relationship between structure and function in actinoporins are the use of site-directed mutagenesis in positions relevant for binding and activity and the synthesis of peptides reproducing those sequences whose importance for protein function should be elucidated. Both strategies have been applied to unveil this relation in Sts and will be detailed below.
Cys mutants of Sts
Most actinoporins do not contain Cys residues (Anderluh and Macek 2002; Valle et al. 2015, 2016), therefore, site-specific mutagenesis introducing a Cys residue has been a useful strategy to analyze the relevance of several amino acid positions to toxin function. Inclusion of a Cys residue in a specific position has allowed (1) the assessment of the structural and functional importance of the replaced residue; (2) the stabilization of conformational states by the non-native S-S bond (Hong et al. 2002; Anderluh et al. 2003; Kristan et al. 2004; Penton et al. 2011; Valle et al. 2011; Hervis et al. 2014); (3) the assessment of thiol-specific fluorescent labels (Malovrh et al. 2003; Alegre-Cebollada et al. 2007) or spin probe labels. Thiol-linked conjugates have also been used for the construction of immunotoxins against tumor cells (Tejuca et al. 2009; Penton et al. 2011).
In order to obtain further information on the conformational relevance and the involvement of specific amino acid residues in the interaction and insertion of Sts into the membrane, we have designed and produced different Cys single mutants of recombinant StI (rStI). The positions for the Cys residue substitutions were selected taking into account their solvent exposure in models calculated by homology modeling, as well as their location in relevant functional regions of the protein: StIE2C and StIF15C (in the N-terminal region involved in channel formation) and StI R52C, StIW111C and StIP80C (in the membrane binding region) (Fig. 1). In all cases, circular dichroism (CD) spectroscopy and fluorescence data suggested that replacement of these specific amino acids by Cys residues did not noticeably change the conformation of the protein (Valle et al. 2011).
Functional characterization of StIR52C showed that the substitution of StI Arg52 by Cys did not change the ability of rStI to bind lipid vesicles; however, it did decrease its pore-forming activity. Accordingly, StIR52C showed lower hemolytic activity, suggesting an important role for the Arg52 residue in the functional ability of the toxin (Valle et al. 2011). Replacement of Trp111 by Cys caused an eightfold decrease in the hemolytic activity of rStI due to a loss in its ability to interact with membranes (Penton et al. 2011). These results are in agreement with those of García-Linares et al. (2016), who concluded that the Trp residues of actinoporins play a major role in membrane recognition and binding but they have only minor influence on the diffusion and oligomerization steps needed to assemble a functional pore. The Pro80 residue is located in one of the conformational loops of StI (Pardo-Cea et al. 2011) and is strictly conserved in actinoporins (Hervis et al. 2014). The substitution of Pro80 did not modify the affinity of the StI toxin for membranes, but it did decrease its pore-forming activity, suggesting that the participation of this residue occurs in a stage beyond the initial association to the membranes and prior to the formation of a functional pore (Hervis et al. 2014).
Interestingly, StIE2C and StIF15C exhibited equal or slightly higher hemolytic activity than their parental recombinant protein rStI. Studies with EqtII have revealed that the N-terminus of the toxin extends through the pore to the trans side of the membrane; moreover, the authors proposed that the first five amino acids help to anchor the amphipathic helix on the trans side of the membrane and consequently stabilize the final transmembrane pore (Kristan et al. 2007). In light of these observations, Valle et al. (2011) speculated that the elimination of a negative charge in StIE2C would favor the translocation of this sequence through the membrane. On the other hand, it was found that the substitution of Phe15 by Cys, a residue relatively more polar, seems to enhance the functional role of the N-terminus in pore formation. Valle et al. (2011) found that StIE2C, at 50 mM under non-reducing conditions, exhibited approximately 50% homodimeric structure stabilized by a disulfide bridge. In this condition, StIE2C showed a surprisingly high permeabilizing capacity, sixfold larger than that of rStI. This result suggested that the presence of oligomeric structures in solution, such as dimers, and the expected pre-aggregated protein at the bilayer surface could favor the complex oligomerization process (Valle et al. 2011).
Synthetic peptides as useful models of Sts
The main difference in the primary sequence between Sts lies in their N-terminus, where all the non-conservative substitutions and one conservative substitution are found (Huerta et al. 2001). Compared to StII, StI contains two additional anionic amino acid residues (Glu2 and Asp9), instead of the non-polar Ala, in positions 1 and 8 of StII. StI also has an extra polar residue (Ser) at position 1, rendering the N-terminus 1–10 sequence of StII more hydrophobic than its counterpart in StI. The lytic activity of StII is approximately three- to sixfold higher than that of StI in human red blood cells (Martinez et al. 2001). Since the N-terminal region of the toxins is involved in pore formation (Mancheno et al. 2003; Casallanovo et al. 2006), the different hemolytic activity of StI and StII could be due, at least partially, to differences in this region.
Thus, another strategy applied to study the structure–function relationship has been the use of synthetic N-terminal peptides as models of Sts function. This approach has been useful to gain an understanding of the influence of the N-terminus on folding and activity of Sts. To clarify the contribution of the first 30 (StII) or 31 (StI) N-terminal amino acid residues to the activity of the toxins, four peptides spanning residues 1–31 of StI (StI1–31, StI12–31) and residues 1–30 of StII (StII1–30, StII11–30) were synthesized and their structural and functional properties investigated (Casallanovo et al. 2006; Cilli et al. 2007; Ros et al. 2011, 2013). The peptides were characterized by a high structural plasticity; however, in solution, most of them were largely unordered, which was not unexpected as peptides that are not constrained by disulfide bonds are often very flexible and do not adopt regular secondary structures in aqueous solution. The exception was StII1–30, which was found to have a higher tendency to acquire secondary structure and/or aggregate in solution (Casallanovo et al. 2006; Ros et al. 2013). These differences clearly point to the modulating effect of the first ten hydrophobic residues on the aggregation and conformational properties of the peptides. However, in the presence of membrane mimetic systems they adopted an α-helical structure, similar to that adopted by these segments in the full-protein structure, which supports their suitability as models of the N-terminus structure of Sts. Biologically relevant peptides are active against human red blood cells (Casallanovo et al. 2006; Cilli et al. 2007) and liposomes (Ros et al. 2011, 2013, 2015) in a micromolar concentration range, despite their small size and the absence of the Sts binding site to the membrane (Casallanovo et al. 2006; Cilli et al. 2007; Ros et al. 2011, 2013). The fact they are active, albeit in a higher concentration range than the full-length proteins, reinforces the notion that these small molecules can mimic the functional behavior of actinoporins. Of relevance, the hemolytic and permeabilizing activity of the peptides reproduced qualitatively the behavior of their respective parental proteins (Cilli et al. 2007; Ros et al. 2011, 2013), an observation that we could even extend to the other members of the actinoporins family EqtII and FraC (Ros et al. 2015). This study of the four most studied actinoporins strongly suggest the importance of continuity of the 1–10 hydrophobic amino acid sequence in StII1–30 for displaying higher membrane binding and activity. Furthermore, we also demonstrated that StII1–30 forms pores of similar radius to that of the protein (approx. 1 nm) (Casallanovo et al. 2006), with its N-terminus oriented towards the hydrophobic core of the bilayer while the rest of the peptide is more exposed to the aqueous environment (Ros et al. 2013), as hypothesized for Sts. Altogether our studies demonstrate that synthetic peptides that reproduce the N-terminus of Sts are not only a good model of the structure and function of these toxins but, and due to its reduced molecular size, could be useful biotechnological tools, replacing their larger parental proteins.
Understanding the interaction of actinoporins with membranes
Model membranes used to understand the functioning of Sts
Given that Sts exert their first action in membranes, over the years we have applied several model membrane systems to gain an understanding of binding and pore formation by Sts (for review, see Alvarez et al. 2009). Among the most commonly used artificial membrane systems are flat membranes (i.e. lipid monolayers and supported membranes, either monolayers or bilayers), micelles and liposomes. When amphiphilic lipid molecules are dissolved in an organic solvent and deposited at an air–water interface they become oriented to minimize contact of their non-polar regions with water and maximize contact of their polar regions with water. The result is a lipid or Langmuir’s monolayer that constitutes one of the most elemental lipid systems (Brown and Brockman 2007). The changes in surface pressure (π) originated from binding of diverse molecules to the lipid monolayer constitute the physical principle that allows evaluating the degree of interaction of the proteins with lipids. The interaction of proteins with monolayers can be measured by two methods. In the first of them, the initial pressure (π0) of the monolayer is kept constant and the interaction is followed from the increase of the surface area that is necessary to maintain π constant. In the second method, which is the more employed of the two, the area is kept constant and the increase in π that is produced by injecting the protein into the subphase is measured, as a function of time, until equilibrium is reached (Brockman 1999; Maget-Dana 1999). Monolayers have been used to study the membrane binding of diverse families of proteins, such as lipolytic toxins (Maget-Dana 1999), some cell transcription factors, myelin protein components (Maggio et al. 2008), sphingomyelinases (Fanani et al. 2010) and several members of the actinoporin family (Barlic et al. 2004; Bellomio et al. 2009) including Sts (Martinez et al. 2007; Pedrera et al. 2014, 2015). On the other hand, liposomal vesicles are the most accepted systems as membrane models due to their bilayer organization and vesicular geometry. Among the different liposomal lipidic systems, the small unilamellar vesicles (SUV), LUV and the so-called giant unilamellar vesicles (GUV) are the most widely used for the study of PFT. In the following sections we describe several of the versatile applications of these systems.
Spectroscopic methods to study the binding of Sts to model membranes
The relatively high concentration of aromatic amino acid residues, in particular Trp and Tyr, in the Sts binding region to the membrane allows the use of fluorescence spectroscopy in the evaluation of the conformational changes leading to protein insertion into the membrane. These changes involve an increase in the intensity of the fluorescence and a shift of the fluorescence emission maximum to lower wavelengths, both phenomena resulting from the modification of the microenvironment of aromatic residues to a more apolar environment. Most of these binding studies have been carried out with SUV (diameter 30–50 nm) in spite of the limitations that their great curvature might introduce into the system, which should be taken into consideration. However, in compensation, they do not introduce significant turbidity into the test, a prerequisite for achieving a reliable result in spectroscopy studies (Martinez et al. 2001). The use of freshly obtained SUV over short periods of experimental time is recommended to circumvent the metastability of these vesicles. On the other hand, LUV, with a lower stressed curvature, also constitute a good model for these binding experiments, provided a control of light dispersion and other corrections are carefully implemented as recommended (Ladokhin et al. 2000). Selective Trp fluorescence quenching by different agents, such hydrophilic neutral quencher (acrylamide) or lipid-confined phosphatidyltype quenchers [bis(9,10-dibromostearoyl)-sn-glycero-3-phosphocholine and 1-palmitoyl-2-(1-pyrenedecanoyl)-sn-glycero-3-phosphocholine] have been used to assess the exposure of the emitting centers to the solvent and/or to the lipid environment. Quenching studies together with binding isotherms offer complementary information on toxin binding to membranes (Macek et al. 1995; Martinez et al. 2001; Alvarez et al. 2003).
Circular dichroism and Fourier transform infrared spectroscopies (FTIR) have been used by our group to monitor the Sts conformational transitions from a water-soluble state to a membrane-bound one.
CD spectroscopy is one of the most used procedures to characterize the conformational changes during PFP binding to membranes. CD spectroscopy in the far ultraviolet (UV) region is a well-established tool for the determination of the secondary structure content of proteins and peptides. Usually, the protein or peptide CD spectra are acquired in solution and titrated with increasing amounts of vesicles. As in fluorescence studies, CD spectroscopy in the presence of vesicles should be carried out in translucent solutions to minimize light scattering; therefore, it is advisable to use homogeneous small vesicles (SUV; Alvarez et al. 2003). Another approach to assess the conformational changes resulting from toxin binding to membranes is to use other less legitimized systems than liposomal vesicles, such as (1) the solvent trifluoroethanol (Casallanovo et al. 2006), which is a medium that induces peptide conformational changes similar to those occurring in membranes, and (2) detergent micelles (Lanio et al. 2002, 2003, 2007). Another method to follow protein conformational changes is to evaluate transitions in the near-UV-CD range (250–350 nm). Analysis of the spectra in this area provides a sort of protein fingerprint; therefore, it is possible to monitor changes by measuring the intensity and position of the absorption bands in the presence and absence of a membrane mimetic system (Alvarez et al. 2003).
FTIR has been extensively used in the structural characterization of proteins and peptides. Although the method does not provide accurate structural information at the atomic level, it is extremely sensitive to conformational changes, including those originating from protein interaction with membranes. FTIR in attenuated total reflection mode (ATR-FTIR) is a sensitive, surface version of the method that has been useful to study proteins and lipids of the membrane, protein–membrane interactions, the molecular architecture of membrane pores and protein/peptide-forming channels, as well as the structure and orientation of membrane-bound hydrophobic proteins and peptides (Shai 2013). The assessment of changes in the secondary structure content of Sts upon their interaction with lipids is an example of the application of the ATR-FTIR to the study of the PFP interaction with membranes. Frequency component analysis of the amide I′ band indicated that Sts are composed predominantly of beta structure, comprising 44–50% beta-sheet, 18–20% beta-turn, 12–15% alpha-helix and 19–22% random coil. Upon interaction with lipid membranes, a slight increase in the alpha-helical and beta-sheet structures was observed with a concomitant decrease of the unordered structure. Polarization experiments indicated that both toxins had some disordering effect on the lipid layers (Menestrina et al. 1999). Subsequent studies have supported these results, for example, the proposal of a toroidal lipid pore which favors lipid exchange between the external and internal lipidic monolayers (Alvarez-Valcarcel et al. 2001) and the lipid-mixing effect of membranes microdomains by Sts (Ros et al. 2013).
Electronic paramagnetic resonance spectroscopy is an absorption technique largely employed to study the association of proteins/peptides with membranes. This technique requires the presence of the so-called spin labels, which are stable radicals used as probes in the system. Structural and dynamic information on the system can be obtained by studying the EPR spectrum of these probes. Nitroxide radicals, due to their high stability, have been extensively used as spin probes, covalently attached to specific functional groups or intercalated in regions of the system to be analyzed. EPR studies make it possible to evaluate not only the association of proteins or peptides with the membrane but also the fluidity of these structures. In a study of the interaction of Sts with liposomal membranes by EPR, we incorporated free fatty acid carrying a nitroxide moiety on different carbon atoms (5, 7, 12 and 16) of the hydrocarbon chain to the lipidic mixture. The addition of toxins resulted in a spectrum with two components for the probe labeled in C-12. The broadest component corresponded to a strongly immobilized spin probe population, which was attributed to the boundary lipids, suggesting a direct interaction of the toxin with membrane lipids up to that position. However, these spectral modifications were absent when the label was at C-16, indicating a loss of lipid–toxin interaction at this depth. This result was considered to be further evidence of the toroidal pore hypothesis that would justify the preferential interaction of Sts with the carbon atoms near the polar head groups of the fatty acids (Alvarez et al. 2003).
Functional studies: pore-forming activity in membrane mimetic systems
Liposomes are the most commonly used membrane mimetic systems to evaluate the pore-forming activity of proteins/peptides, given the ability of these vesicles to encapsulate molecules in their water compartment, such as fluorescent dyes. Carboxyfluorescein and calcein are among the most employed fluorescent probes. Their fluorescence is self-quenched inside the liposomes due to the high concentrations of these probes in the intravesicular medium. Another variant is the combination of a fluorescent probe with its quencher in the aqueous compartment of the vesicles. In both cases, the experimental approach is based on the increase of the fluorescent signal as a consequence of the quenching relief when vesicles are perforated by the PFT and the fluorophore/fluorophore + quencher are released into the extravesicular medium. Usually, extruded LUV with different pore sizes are used (Tejuca et al. 1996), although SUV can also be employed (Pedrera et al. 2015) to understand pore-forming activity. Because the size of GUV is of the same order of cells, and single vesicles can be directly observed under the microscope, GUV also allow evaluation of the pore-forming activity of PFP by means of optical microscopy with phase contrast (Mally et al. 2002; Ros et al. 2011; Pedrera et al. 2015) and confocal microscopy (Schon et al. 2008). In the first case, the permeabilizing activity can be evaluated based on the loss of phase contrast resulting from the mixture of intra- and extravesicular medium with different refractive indexes. In a second approach, the liposomes are resuspended in a solution containing a fluorophore, and the filling of the vesicle is followed after pore formation. For example, pore activity of actinoporin EqtII was measured by adding both a buffered solution containing the fluorophore Alexa Fluor 488 as a marker and EqtII to GUV, followed by gentle mixing of the sample to achieve a largely homogeneous distribution of vesicles, fluorescent label and toxin (Schon et al. 2008). After 45 min the number of GUV into which the marker had penetrated was determined in relation to the total number of vesicles in several regions of the sample. The degree of filling was calculated by taking a sample image every 30 s and comparing the intensity of the fluorescence marker within a vesicle with the intensity just outside the GUV (Schon et al. 2008).
Another system used to evaluate the pore-forming ability of proteins are the planar (black) lipid membranes (PLM). PLM are stable lipid bilayers adhered to a teflon septum with a hole in contact with two compartments filled with different aqueous solutions. PLM are particularly useful for the study of the electrophysiological properties of channels. One of the advantages of PLM is that they allow easy control of different physicochemical parameters, such as transmembrane voltage and the current passing through a single channel, which allows researchers to follow the events of pore opening and closing. The system can provide useful information on pore properties at the molecular level; for example, PLM can be used to determine the size of the pore and its selectivity to anions or cations, as well as to characterize the charge distribution along the toxin channel (Tejuca et al. 1996). On the other hand, this type of PLM experimentation has also contributed to corroboration of the participation of lipids in the structure of the channel in a process started by EqtII, which is in agreement with the toroidal pore hypothesis (Anderluh et al. 2003).
Membrane physico-chemical requirements for the binding and activity of Sts
Sticholysins are effective pore formers on model lipid membranes (Tejuca et al. 1996; Alvarez-Valcarcel et al. 2001); thus it is assumed that a protein receptor is not strictly required for their action. There are different interpretations of the role of lipid composition in the interaction of actinoporins with membranes. Some authors suggest that each consecutive step in the complex pore-forming process could be influenced by a single physico-chemical characteristic of individual lipid molecules, such as SM, or by physical parameters arising from the collective nature of lipids in membranes (Alvarez et al. 2009). It was a long-standing assumption that actinoporins possess a unique lipid binding site (Mancheno et al. 2003), but recent studies have found the presence of multiple lipid binding sites in the structure of the actinoporin FraC. Therefore, lipid multivalency has emerged as a new concept for describing the interaction of actinoporins with membranes. This property seems to be crucial to increasing their membrane affinity. Two of these sites are proposed to represent the primary sites for protein attachment to the membrane and possess a high affinity with POC, which is part of the headgroup of both SM and PC (Tanaka et al. 2015). In addition, two other binding sites have been described as having lower affinity for POC, which could explain why these proteins are able to recognize other lipids to a lesser extent (Tanaka et al. 2015).
Role of SM as a lipid receptor or modulator of bilayer properties suitable for Sts–membrane interaction
Sphingomyelin has been proposed as the lipid receptor of actinoporins in the membrane. The essential role of SM in the interaction of Sts with membranes has been discussed by several authors. However, some studies have found that this phospholipid is not essential for the permeabilizing activity of Sts in liposomes (de los Rios et al. 1998). It appears that actinoporins recognize SM both at the level of the headgroup and at the ceramide (Cer) moiety (Alvarez et al. 2009; Soto et al. 2017). SM strongly promotes irreversible binding and pore formation in model membranes (Alvarez-Valcarcel et al. 2001; Martinez et al. 2007). In general, SM not only acts as a lipid receptor of actinoporins on the membrane surface, but also as a structural element of the pore, where it plays the role of an assembly co-factor (Tanaka et al. 2015). It is also likely that the lipid environment modulates SM function as lipid receptor of Sts. For example, lipid partners like Chol or Cer have an effect on the tilt, orientation and dynamics of the SM headgroup, and consequently on the activity of actinoporins (Alm et al. 2015). The role of SM in the mechanism of action of Sts has also been investigated in the context of SM function as a modulator of membrane properties. In recent years, it has been postulated that the affinity of actinoporins for membranes is greatly enhanced by the coexistence of lipid phases, leading to a focus of the role of SM on its ability to form raft-like structures (Barlic et al. 2004; Schon et al. 2008). Our recent investigations have challenged this long-standing assumption. It would appear that Sts binding results from an interplay between the presence of SM and membrane fluidity, with negligible influence of the presence of domain boundaries. We generalized that once the membrane has a high availability of SM, its phase state and rheological properties acquire a major role in the recognition of Sts. Consequently, we hypothesize that more fluid phases characterized by weaker lipid cohesion and high toxin–SM H-bonding potentiality provide a suitable environment for toxin binding and penetration of the toxin into the membrane (Pedrera et al. 2014).
Cholesterol and other sterols
The presence of cholesterol (Chol) in PC membranes leads to pore formation, even in the absence of SM; however, binding of the toxin to this lipid ensemble is low (de los Rios et al. 1998). Although the joint presence of SM and Chol in membranes significantly increases the binding and permeabilizing activity of actinoporins, this enhancement has been related to the coexistence of lipid phases (Barlic et al. 2004; Martinez et al. 2007; Schon et al. 2008; Rojko et al. 2014; Garcia-Linares et al. 2015). In model membrane systems, the liquid ordered (Lo) domains occur usually when Chol associates with saturated glycerophospholipids or sphingophospholipids to render phospholipid–Chol complexes. In particular, SM has emerged as the major partner of Chol for Lo phase formation, and ternary mixtures of dioleoylphosphatidylcholine (DOPC), SM and Chol are the most widely studied systems used to characterize the Lo/liquid-disordered (Ld) phase coexistence (de Almeida et al. 2003). In this regard, the influence of Lo/Ld phase coexistence in bilayers and their equivalent Lo and liquid-expanded phases in monolayers have been the most studied systems in the actinoporin family (Barlic et al. 2004; Martinez et al. 2007; Schon et al. 2008; Rojko et al. 2014; Garcia-Linares et al. 2015). For example, lateral defects related to the presence of Lo domains have been considered the preferential binding sites for EqtII (de Almeida et al. 2003; Barlic et al. 2004). However, more recently it has been proposed for EqtII that the lateral interphases between the Lo and Ld phases act as primary binding sites before the toxin accumulates in the Ld phase, where the pore formation takes place predominantly (Rojko et al. 2014).
We recently studied the functional activity of StI in monolayers and liposomes composed of DOPC:SM:sterols (1:1:1), including sterols promoting [ergosterol (Erg) and Chol] or not [cholestenone (Cln)] Lo/Ld phase coexistence (Fig. 2) (Pedrera et al. 2015). We found that the presence of any of the analyzed sterols into PC:SM mixtures favored toxin–membrane association and pore-forming ability in the order Chol > Erg ≥ Cln > PC:SM and that this order was related to the higher molecular heterogeneity of the membrane resulting from the inclusion of sterols (Table 1). Membrane heterogeneity reaches its maximum expression in the mixtures that contain Chol or Erg as a result of the coexistence of the lipid phases, but it is also probably related to the different solvation degree of the polar headgroups, lipid mismatch, lipid packing and the propensity to adopt a nonlamellar structure. In all model membranes, Chol emerged as the strongest promoter of binding and pore-forming ability of StI due to its unique capacity to combine a concentrating effect of the toxin in its smaller Ld area with the relatively higher fluidity of this phase, compared to Erg, and its capacity to promote nonlamellar structures.
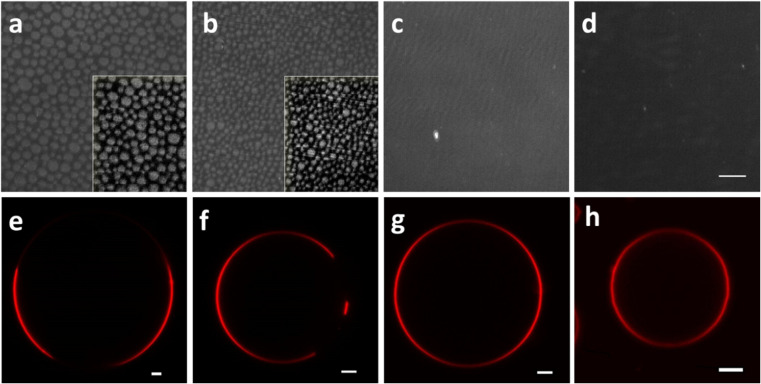
Fig. 2.
Characterization of lipid monolayer topography and giant unilamellar vesicles (GUV) composed of dioleoylphosphatidylcholine (DOPC):sphingomyelin (SM):sterols (1:1:1), with the sterols being of different chemical nature. a, e DOPC:egg yolk (e) SM:cholesterol (Chol), b, f DOPC:eSM:ergosterol (Erg), c, g DOPC:eSM:Cln, d, h DOPC:eSM. a–d Visualization of lipid monolayers by Brewster angle microscopy. Images were taken at 20 mN m−1. For better visualization, the lower 0–90 Gy level range (from the 0 to 255 original scale) was selected to maintain the gray level:film thickness ratio. Scale bar (d) 100 μm, also for images a–c. Insets to a and b show a piece of both images enhanced by a bandpass filter. e–h Visualization of GUV by confocal microscopy. All vesicles contain the fluorescence probe DiD (0.1 mol%). Scale bars (e–h) 5 μm. T = 23 ± 2 °C
Table 1.
Effect of sterols in binding and pore-forming activity of sticholysin I
| Lipid composition | StI-monolayer binding | SUV permeabilization | GUV permeabilization |
|---|---|---|---|
| πc (mN m−1)a | C50% (mol:mol)b | permeabilized GUV (%)c | |
| DOPC:eSM:Chol | 55.8 ± 0.6 | 2715.8 ± 193.4 a | 64.37 ± 9.53 x |
| DOPC:eSM:Erg | 48.5 ± 0.8 | 1243.6 ± 114.9 b | 57.19 ± 6.81 y |
| DOPC:eSM:Cln | 44.8 ± 0.8 | 1170.9 ± 61.7 b | 53.94 ± 9.41 y |
| DOPC:eSM | 40.7 ± 0.6 | 238.4 ± 8.4 c | 52.82 ± 5.44 y |
StI, Sticholysin I, Suv, small unilamellar vesicles; Guv, giant unilamellar vesicles; for definition of lipids, see Fig. 2 caption
aπc: Pressure that must be applied to avoid incorporation of StI into the monolayer. This parameter indicates the affinity of StI for the lipid monolayers and is calculated by extrapolating regression lines from plots of π (surface pressure) versus π0 (initial surface pressure)
bLipid/toxin molar ratio necessary to promote the release carboxyfluorescein (50%) entrapped in SUV. The parameter was calculated by fitting dose-dependence curves of permeabilization induced by StI to a Hill sigmoid (R2 > 0.96) using Origin 8.0, Microcal Inc. (Studio City, CA). Mean ± standard deviation from two independent experiments are shown. Statistical analysis was performed with one-way analysis of variance with Tukey as a post hoc test. Values in row followed by different lowercase letters are independent groups with significant differences among them (p < 0.05)
cPercentage of permeabilized vesicles 30 min after the addition of StI. Values in row followed by different lowercase letters are independent groups with significant differences among them (p < 0.05). Between 1000 and 1800 vesicles were analyzed for each composition
Role of other membrane lipids: phospholipids and ceramides
In addition to the main cell-membrane lipid components, the influence of other phospholipids in the functional activity of Sts has been examined. The inclusion of even small proportions (5 mol%) of negatively charged lipids [e.g. phosphatidic acid (PA), phosphatidylserine, phosphatidylglycerol, phosphatidylinositol, or cardiolipin] into PC:SM vesicles increases pore formation by these toxins. Strikingly, some boosting effects were also obtained by including the zwitterionic lipid phosphatidylethanolamine (PE) or even, albeit to a lesser extent, the positively charged lipid stearylamine. These results indicate that the effect is not mediated by electrostatic interactions between the cytolysin and the negative surface of the vesicles, leading to the hypothesis that the presence of minor amounts of lipids favoring this non-lamellar organization could also augment the efficiency of pore formation. In fact, these results represent a first clue that Sts form pore structures with toroidal shape (Alvarez-Valcarcel et al. 2001). In this model, both polypeptide chains and lipid headgroups form the walls of the protein channel. To avoid the high energetic cost of exposing their hydrophobic acyl chains to the aqueous environment, lipids bend and form a highly curved, non-bilayer structure at the pore edge that connects the two monolayers of the membrane with a continuous surface. Lipids can then easily exchange monolayers by simple diffusion at the pore (Ros and Garcia-Saez 2015). Of relevance, Sts strongly promote the rate of transbilayer movement of lipid molecules, indicating local disruption of the lamellar structure (Alvarez-Valcarcel et al. 2001). Other indirect experimental evidence supporting the hypothesis of the toroidal pore has been derived from EPR spectra of intercalated fatty acid spin probes carrying the nitroxide moiety at different carbons. Upon addition of Sts, a component ascribed to the boundary lipid was clearly detectable for the C-12-labeled probe, but it was absent when the label was at C-16, indicating a lack of lipid–protein interaction close to the lipid terminal methyl group probably associated to a toroidal pore (Alvarez et al. 2003).
In order to look deeper into the lipid structural determinants governing Sts–membrane affinity, we examined the interaction between StII and different Cer-derived lipids that, even in minor concentrations at the cell surface, could contribute to binding and pore formation by StII. Of note, StII recognized lipids other than SM, such as PC, Cer and gangliosides, but not PA, and, as expected, SM recognition by the toxin was higher than that observed with other lipids. Our results reinforce the notion that both POC and Cer groups are responsible for the higher affinity of StII for SM. The absence of recognition of glucosylceramide and lactosylceramide by StII could be due, firstly, to the absence of a POC group and, secondly, as a consequence of a minor access of the toxin to the Cer base of both lipids. This limited access could be a direct consequence of hydrogen bonding between the toxin and hydroxyl groups of the sugar residues. Thus, StII would be trapped by this purported H bond network and, consequently, its access to Cer-binding sites would be lower. In contrast, StII binding to gangliosides was significantly larger than that to the neutral glucosphyngolipids, probably due to the presence of the anionic sialic acid unit in gangliosides (GM3, GM1 and GD1) that would facilitate electrostatic interactions with the protein basic amino acid residues. Nevertheless, the structural complexity of gangliosides reduces StII association with these lipids in relation with Cer and SM. In short, the lower recognition of gangliosides by StII in comparison with SM could be related with the absence of the POC group and the presence of sugar residues that would immobilize StII, thereby restricting its access to the Cer moiety. GM3 contains two sugar residues in contrast to GM1 and GD1 that contain four. It would appear that the higher the number of sugar residues, the larger the difficulty for StII to reach the relevant binding sites. Apparently, the number of sialic acids units compensate for the effect of neutral sugars, since GD1 (two sialic acid units) shows higher binding than its GM1 counterpart containing just one anionic sialic acid moiety. Moreover, due to the structural complexity of these gangliosides, they would not probably be accommodated easily in the ligand binding site of StII. Taken together our results reinforce the notion that both POC and CER groups are responsible for the higher affinity of StII for SM. Furthermore, the presence of sugar residues in glycosphingolipids seems to modulate binding and pore formation by actinoporins, probably by hindering StII from reaching relevant structural motifs in the membrane for binding or inducing a noncompetent adsorption to membrane (Soto et al. 2017).
Effect of Sts on membrane organization
Sticholysin do not just passively interact with the membrane but they can also influence its physical properties and organization. Our investigations support the notion that Sts decrease the line tension between phases, a key parameter determining the lateral membrane organization and domain sizes. Although phase separation could promote pore-forming activity by acting as concentrating platforms, the tight molecular packing and acyl chain within ordered domains create a locally ordered environment that does not readily favor protein insertion and the consequent pore formation. In fact, proteins and peptides preferentially bind to interfaces and function as detergents, reducing line tension and leading to domain dispersion (Ros and Garcia-Saez 2015). By decreasing line tension between lipid domains, Sts might promote the formation of more disordered regions (i.e. more disordered than the so-called raft Lo domains), but still SM enriched. This lipid environment should be more suitable for N-terminus insertion and pore formation (Ros et al. 2013).
Cellular mechanisms triggered upon Sts-membrane interaction: experimental approaches
Pore-forming toxins form holes in membranes, thereby provoking devastating damage to a target cell. However, target organisms have evolved a regulated response against this damage, including cell membrane repair. PFT from bacteria constitute the best characterized group (Peraro and van der Goot 2016), with aerolysin produced by Aeromonas hydrophila (Iacovache et al. 2010) and listeriolysin O (LLO) from Listeria monocytogenes representative examples of this wide assembly of proteins (Hotze and Tweten 2012). By contrast, eukaryotic PFT have been less studied than their bacterial counterparts, perhaps due to their lower impact on human health.
Despite the great diversity of PFT in terms of source, structure or physiological role, they all follow a similar mode of action (Bischofberger et al. 2012; Ros and Garcia-Saez 2015). PFT are produced as soluble molecules and then become membrane-associated proteins forming water-filled pores in the membrane of the target cells that disrupt cell homeostasis through the increase of the non-selective passage of molecules. However, pores from different PFT have diverse properties that could induce dissimilar phenotypes of cell death (Ros and Garcia-Saez 2015; Cosentino et al. 2016; Peraro and van der Goot 2016). Different families of PFT form pores by distinctive mechanisms, irrespective of the producing organism. Therefore, large differences between bacteria and eukaryotic PFT are not expected. In fact, structural aspects are more important in defining pore-forming function and mechanism of action. The lesions of the plasma membrane are highly dissimilar in size and nature, and these characteristics determine how cells handle membrane repair mechanisms. The events following pore formation in the plasmatic membrane can differ according to cell type. From the most simple cellular model (non-nucleated cells, such as erythrocytes) to the more complex one (nucleated cells), there is a need for simple and accurate bioassays to study the impact of the increased cell membrane permeability which results from pore formation by PFT. In the following sections we attempt to summarize the procedures and results reported by our group regarding the characterization of the lesions due to Sts in non-nucleated and nucleated cells and their impact on these cells.
Interaction with erythrocytes
The lytic activity of actinoporins can be straightforwardly evaluated by a hemolytic activity assay, namely by their pore-forming ability in the erythrocyte membrane. Cell lysis occurs because of the colloid–osmotic shock induced by the formation of such pores in membranes; in fact, hemolysis can be prevented by osmotic protectants of adequate size. Colloid–osmotic hemolysis results from an osmotic outward gradient of non-permeating molecules [e.g. hemoglobin (Hb)] and the consequent increase in cell volume because of water influx to compensate osmotic imbalance, leading to the loss of membrane integrity (MacGregor and Tobias 1972). The lesion resulting from StI or StII action on membranes in both erythrocytes and liposomes exhibits a radius of approximately 1.0 nm and permeability to small molecules and solutes (Tejuca et al. 2001).
There are at least two ways to assess the hemolytic activity: (1) the estimation of Hb release following aggression by the toxin (Alvarez et al. 1998); (2) the turbidity loss of an erythrocyte suspension occurring as a result of cell lysis (Martinez et al. 2001). In both cases, relatively simple mathematical processing allows estimation of a parameter such as HC50, which refers to the toxin concentration required to lyse 50% of the red cells in the assay. The decrease in turbidity of a cellular suspension can be more easily recorded using a microplate reader (Alvarez-Valcarcel et al. 2001; Tejuca et al. 2001).
Interaction with nucleated cells
Few studies have reported the interaction of actinoporins with nucleated cells. In the same way that occurs in membrane mimetic systems and erythrocytes, these proteins are able to open pores in membranes of nucleated eukaryotic cells. The presence of SM in these membranes suggests that actinoporins may open pores in the membrane of almost any eukaryotic cells (Tejuca et al. 1999, 2004). Studies on Sts–erythrocyte interaction have shown that trans-membrane pore formation interrupts ionic gradients by inducing the influx of chloride and calcium ions as well as the efflux of potassium ions, leading to an increase in cell volume and ultimately to cell death (Celedon et al. 2005, 2009). Beyond this information, little is known how “target” cells respond at a molecular level to this insult by actinoporins and whether these cells can recover or not after membrane damage. In this sense, the interaction between nucleated cells and bacterial PFT is yet not fully understood.
Studies with aerolysin and LLO have shown that sub-lytic doses of these proteins produce a primary effect on the target cells characterized by a decrease in intracellular potassium and a concomitant increase in calcium (Gonzalez et al. 2011). These changes in cytoplasmic ionic composition trigger what are known as side effects that result in the activation of various signaling pathways of mitogen-activated protein (MAP) kinases (Huffman et al. 2004; Gurcel et al. 2006; Bischof et al. 2008), which are considered to be essential for survival following toxin-mediated membrane disruption. Such activation results in the transcriptional regulation of a broad range of physiological activities involved in the recovery of plasma membrane integrity, cell survival and adaptation (Kao et al. 2011; Wald et al. 2014).
The intracellular K+ content is considered to be the main indicator of damage to the plasma membrane. In the case of non-nucleated erythrocyte cells, K+ output from the cellular interior precedes hemolysis and can be used as an early indicator of loss of cellular integrity (Martinez et al. 2001). K+ release can be measured by an ion selective electrode or by emission flame photometry (Martinez et al. 2001; Cabezas et al. 2017). In the case of nucleated cells, adherent cells are frequently used to avoid the centrifugation steps of culture between treatments. Cells (~ 106cells/ml) can be incubated with different toxin concentrations for periods ranging from 10 min to 24 h. For StI and StII in particular, protein amounts fluctuating in the range of nanomolar and micromolar concentrations have been evaluated. Following the challenge, the supernatant containing the toxin is eliminated and the cellular monolayer washed out with a potassium-free buffer (Gonzalez et al. 2011). The rupture of the cellular membrane to quantify intracellular potassium concentration is achieved using a washing buffer containing Triton X-100.
It is advisable to evaluate at least three conditions in one culture plate: cells without treatment (negative control), cells which are incubated with the problem toxin and cells that are incubated with a toxin having a well-known membranolytic effect (positive control). Aerolysin and LLO are the most frequently used PFT as positive control due to their potent lytic activities (Abrami et al. 2000; Heuck et al. 2010). The level of intracellular K+ can be directly correlated with the pore-forming activity of the PFP studied. PFT at sub-lytic concentrations and during the first hours of incubation with cells provoke a significant loss of intracellular K+ due to membrane damage. However, in the following hours, intracellular K+ level can increase as an indication of cell recovery (Bischofberger et al. 2012). Strikingly, it has been found that membrane recovery upon StII damage takes place in a time scale similar to that upon LLO damage even though these two PFT form pores that differ greatly in size. In addition, for StII, membrane recovery occurs in a completely different time span to that observed for other bacterial PFT also producing small pores (Husmann et al. 2009; Gonzalez et al. 2011). At sub-lytic concentrations (100 ng/ml) in BHK cells, StII is able to cause a drop of intracellular potassium (<40%) after 20 min of incubation. Nevertheless, the intracellular K+ concentration recovers rapidly, close to 80%, by 1 h post-incubation, which would indicate that the fall in the intracellular potassium triggers mechanisms of cell recovery (Cabezas et al. 2017). It has been hypothesized that there is an inverse correlation between the size of the pores and the time required to repair the membrane; this has been a non-intuitive concept for a long time and remains far from completely understood. Furthermore, the results obtained when comparing the effect of StII with LLO and PA contradict this suggestion and reinforce the notion that pore heterogeneity and stability are more important than pore size for membrane repair (Cabezas et al. 2017).
The phospho-kinases array procedure allows, in a simple and direct manner, the identification of different kinases that are phosphorylated upon cell–PFT interaction. Using this methodology, the intracellular kinase proteins activated as a consequence of pore formation by StII have been elucidated (Cabezas et al. 2017). Employing 46 antibodies against 29 different kinases, it was observed that the phosphorylation of MAP kinases p38 and ERK was similar to what has been described for other PFT, such as streptolysin O, pneumolysin O, anthrolysin O, Cry5B, aerolysin and LLO (Ratner et al. 2006; Aguilar et al. 2009; Porta et al. 2011; Gonzalez et al. 2011).
It remains to be elucidated why toxins which are so different are able to specifically activate the intracellular phosphorylation of two kinases (p38 and ERK). On possible explanations is that it may be a common mechanism of cell repair induced by PFT. Furthermore, a common role of K+, as well as MAP kinases in the mechanism that cells use to cope with the PFT injury is also supported by the findings obtained by StII. The identification of other cellular mechanisms that contribute to the recovery of the plasma membrane after K+ loss due to the cell–StII interaction is also an area which has not yet been investigated.
Computational insights into the mechanism of actinoporin pore formation
Understanding the assembly mechanism of actinoporins has dramatically increased after determination of the crystal structure of FraC, which complemented a body of biochemical and biophysical data previously obtained by different groups (Tejuca et al. 1996; Alvarez-Valcarcel et al. 2001; Hong et al. 2002; Alvarez et al. 2003; Anderluh et al. 2003; Rojko et al. 2013; Antonini et al. 2014; Subburaj et al. 2015). However, the exact sequence of events that takes place during the assembly of actinoporins and the identification of the functionally relevant intermediates in the membrane remain under debate. It is now generally accepted that actinoporins bind to membranes mainly as monomers (Tejuca et al. 1996; Barlic et al. 2004; Bakrac et al. 2008; Pedrera et al. 2014; Rojko et al. 2014) and then undergo a conformational change that involves only the N-terminal segment (Rojko et al. 2013, 2015; Tanaka et al. 2015). The monomeric units then oligomerize to form pores in which the N-terminal α-helix lines the channel walls in conjunction with lipids (Tanaka et al. 2015). Some studies suggest the relevance of protein–protein interactions (Mechaly et al. 2011; Tanaka et al. 2015; Morante et al. 2016), while others assume that there is no need for such protein interfaces to stabilize oligomeric intermediates for the final pore assembly (Mancheno et al. 2003, 2006).
Here we review two studies carried out by our group that focused on understanding (1) the role of lipid components in actinoporin binding (Soto et al. 2017) and (2) the relevance of protein–protein interactions in the actinoporin oligomerization step (Mesa-Galloso et al. 2017). We combined comparative modeling, molecular dynamics simulations and free energy calculations with the Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) method (Jayaram et al. 1998) to figure out the structural and energetic basis of the higher affinity of StII for SM, PC and Cer. We found that the major energetic contributors to the better affinity of StII for SM compared to PC are the electrostatic interactions, while the van der Waals energies are the major driving forces of the better affinity of StII for SM with respect to Cer. For the first time, we reported that the difference in the pairwise interactions of SM and PC with StII residues A81, F106 and D107 explains the stronger binding of StII to SM than to PC. The lower binding of StII to neutral glycosphingolipids was found to be related to the absence of a POC group and a minor access of the toxin to the Cer moiety due to the presence of sugar residues. These simple theoretical models can be useful to understand the initial steps of the StII binding process with lipid bilayers (Soto et al. 2017).
To test the hypothesis that the dimeric interface of actinoporins is conserved and plays a key role in pore formation, we successfully applied a combination of molecular dynamics simulations, free energy calculations with the MM-GBSA method and site-directed mutagenesis. This approach also revealed the driving forces for the spontaneous formation of FraC dimeric structure using free energy calculations with the MM-GBSA approach. In particular, this model predicts that V60–F163 is the most relevant pair of interacting residues in the dimeric structure. Van der Waals interactions and non-polar desolvation are the most relevant energetic components of this interacting pair, suggesting that their geometric complementarity plays a key role in the stability of the dimer. FraC oligomers have a small protein–protein oligomerization surface and are held together by shape complementarity and hydrophobic interactions (Tanaka et al. 2015), two features that are easily compromised and result in stability reduction (Morante et al. 2015). We predicted that the introduction of anionic amino acid residues (D60 and D163) in the dimeric interface of FraC would result in its partial disruption as a consequence of electrostatic repulsion. The reorganization observed would generate an incompatible orientation of one protomer to detach its N-terminal segment once the dimer binds to the membrane (Mesa-Galloso et al. 2017). Since V60 and F163 are FraC residues conserved among actinoporins (Tanaka et al. 2015), we evaluated the effect of introducing homologous mutations in EqtII and StII in order to verify our hypothesis in other members of this family. Introducing two anionic charged amino acid residues at positions 60 and 163 dramatically abolished the activity of these three actinoporins. The complete activity loss of the double mutants is not due to the impairment of protein folding in solution and seems not to be related with differences in the affinity of the mutants for the lipid bilayer. Of mechanistic significance, we extend the importance of the FraC oligomerization interface to other members of the actinoporins family and reveal that they follow a common pathway of assembly and membrane disruption (Mesa-Galloso et al. 2017).
Concluding remarks
The combination of biochemical and biophysical approaches to study StI and StII, the two PFT produced by the Caribbean Sea anemone S. helianthus, has contributed to our increased understanding of the toxin structure–function relationship, the protein–membrane association process as well as the toxin interaction with cells and its molecular implications. The rational combination of multiple experimental techniques and, more recently, bioinformatics tools have unveiled several of the complex mechanisms involved in toxin–membrane interaction based on the conformational changes undergone by Sts as well as the molecular pathways triggered upon this interaction. Through the application of these strategies, it became possible to describe for the first time the involvement of lipids in the pore structure, i.e. the formulation of the toroidal pore hypothesis, the impact of a continuous hydrophobic sequence in the N-terminus of actinoporins to exert more efficient permeabilizing action on Sts and the membrane lipidic factors that modulate protein binding and pore formation. Thus, it has been demonstrated that the presence of SM, Chol and other sterols promotes the binding of Sts and pore formation, irrespective of their ability to form laterally segregated domains, with Chol being the strongest promoter. Moreover, the Sts–membrane association is a bidirectional process where membrane fluidity modulates toxin functioning and, in turn, the membrane itself is modified in a complex interplay where both the toxin and the membrane perform their roles in a concerted manner. Furthermore, we have also demonstrated the validity of using synthetic peptides as a model of relevant toxin sequences for protein function in conjunction with site-directed mutagenesis. As such, our results reinforce the relevance of dimer formation as a necessary intermediate structure in the mechanism of actinoporins. We have proposed that actinoporins follow a common pathway of assembly where protein–protein interactions stabilize the oligomeric structure in the membrane. A deeper knowledge of the basic molecular mechanisms involved in Sts–cell interaction as described in this contribution will support the current research developed in our group, which focuses on the design of immunotoxins against tumor cells and antigen-releasing systems to cell cytosol as vaccine platforms based on Sts.
Funding
UR (F/4616-1/2), AV (F/4574-1/2), LP (F/5194-1/2), CS (F4617-1) and SC (5193-1) were recipients of International Foundation for Science Grants (Sweden). This work has been partially supported by a CAPES-MES (Brazil–Cuba) Binational Collaboration Project (111/11).
Compliance with ethical standards
Conflict of interest
Carlos Alvarez declares that he has no conflict of interest. Uris Ros declares that she has no conflict of interest. Aisel Valle declares that he has no conflict of interest. Lohans Pedrera declares that she has no conflict of interest. Carmen Soto declares that she has no conflict of interest. Yadira P. Hervis declares that she has no conflict of interest. Sheila Cabezas declares that she has no conflict of interest. Pedro A. Valiente declares that he has no conflict of interest. Fabiola Pazos declares that she has no conflict of interest. Maria E. Lanio declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Latin America’ edited by Pietro Ciancaglini and Rosangela Itri.
References
- Abrami L, Fivaz M, van Der Goot FG. Adventures of a pore-forming toxin at the target cell surface. Trends Microbiol. 2000;8:168–172. doi: 10.1016/S0966-842X(00)01722-4. [DOI] [PubMed] [Google Scholar]
- Aguilar JL, Kulkarni R, Randis TM, et al. Phosphatase dependent regulation of epithelial mitogenactivated protein kinase responses to toxin-induced membrane pores. PLoS One. 2009;4:e8076. doi: 10.1371/journal.pone.0008076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegre-Cebollada J, Oñaderra M, Gavilanes JG, del Pozo AM. Sea anemone actinoporins: the transition from a folded soluble state to a functionally active membrane-boundoligomeric pore. Curr Protein Pept Sci. 2007;8:558–572. doi: 10.2174/138920307783018686. [DOI] [PubMed] [Google Scholar]
- Alm I, Garcia-Linares S, Gavilanes JG, Martinez-Del-Pozo A, Slotte JP. Cholesterol stimulates and ceramide inhibits sticholysin II-induced pore formation in complex bilayer membranes. Biochim Biophys Acta. 2015;1848:925–931. doi: 10.1016/j.bbamem.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Alvarez C, Lanio ME, Tejuca M, et al. The role of ionic strength on the enhancement of the hemolytic activity of sticholysin I, acytolysin from Stichodactyla helianthus. Toxicon. 1998;36:165–178. doi: 10.1016/S0041-0101(97)00069-X. [DOI] [PubMed] [Google Scholar]
- Alvarez C, Casallanovo F, Shida CS, et al. Binding of sea anemone pore-forming toxins sticholysins I and II to interfaces-Modulation of conformation and activity, and lipid-protein interaction. Chem Phys Lipids. 2003;122:97–105. doi: 10.1016/S0009-3084(02)00181-0. [DOI] [PubMed] [Google Scholar]
- Alvarez C, Mancheno JM, Martinez D, Tejuca M, Pazos F, Lanio ME. Sticholysins, two pore-forming toxins produced by the Caribbean Sea anemone Stichodactyla helianthus: their interaction with membranes. Toxicon. 2009;54:1135–1147. doi: 10.1016/j.toxicon.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Alvarez-Valcarcel CA, Dalla Serra M, Potrich C, et al. Effects of lipid composition on membrane permeabilization by sticholysin I and II, two cytolysins of the sea anemone Stichodactyla helianthus. Biophys J. 2001;80:2761–2774. doi: 10.1016/s0006-3495(01)76244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves GG, Machado de Avila RA, Chavez-Olortegui CD, Lobato FC. Clostridium perfringens epsilon toxin: the third most potent bacterial toxin known. Anaerobe. 2014;30:102–107. doi: 10.1016/j.anaerobe.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Anderluh G, Macek P. Cytolytic peptide and proteintoxins from sea anemones (Anthozoa: Actiniaria) Toxicon. 2002;40:111–124. doi: 10.1016/S0041-0101(01)00191-X. [DOI] [PubMed] [Google Scholar]
- Anderluh G, Dalla Serra M, Viero G, Guella G, Macek P, Menestrina G. Pore formation by equinatoxin II, a eukaryotic protein toxin, occurs by induction of nonlamellar lipid structures. J Biol Chem. 2003;278:45216–45223. doi: 10.1074/jbc.M305916200. [DOI] [PubMed] [Google Scholar]
- Antonini V, Perez-Barzaga V, Bampi S, et al. Functional characterization of sticholysin I and W111C mutant reveals the sequence of the actinoporin's pore assembly. PLoS One. 2014;9:e110824. doi: 10.1371/journal.pone.0110824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiadis A, Anderluh G, Macek P, Turk D. Crystal structure of the soluble form of equinatoxin II, a pore-forming toxin from the sea anemone Actinia equina. Structure. 2001;9:341–346. doi: 10.1016/S0969-2126(01)00592-5. [DOI] [PubMed] [Google Scholar]
- Bakrac B, Gutierrez-Aguirre I, Podlesek Z, et al. Molecular determinants of sphingomyelin specificity of a eukaryotic pore-forming toxin. J Biol Chem. 2008;283:18665–18677. doi: 10.1074/jbc.M708747200. [DOI] [PubMed] [Google Scholar]
- Barlic A, Gutierrez-Aguirre I, Caaveiro J, et al. Lipid phase coexistence favors membrane insertion of equinatoxin-II, a pore-forming toxin from Actinia equina. J Biol Chem. 2004;279:34209–34216. doi: 10.1074/jbc.M313817200. [DOI] [PubMed] [Google Scholar]
- Bellomio A, Morante K, Barlič A, Gutiérrez-Aguirre I, Viguera AR, González-Mañas JM. Purification, cloning and characterization of fragaceatoxin C, a novel actinoporin from the sea anemone Actinia fragacea. Toxicon. 2009;54:869–880. doi: 10.1016/j.toxicon.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Belmonte G, Pederzolli C, Maček P, Menestrina G. Pore formation by the sea anemone cytolysin equinatoxin II in red blood cells and model lipid membranes. J Membr Biol. 1993;131:11–22. doi: 10.1007/BF02258530. [DOI] [PubMed] [Google Scholar]
- Bischof LJ, Kao CY, Los FC, et al. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog. 2008;4:e1000176. doi: 10.1371/journal.ppat.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger M, Iacovache I, van der Goot FG. Pathogenic pore-forming proteins: function and host response. Cell Host Microbe. 2012;12:266–275. doi: 10.1016/j.chom.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Brockman H. Lipid monolayers: why use half a membrane to characterize protein-membrane interactions? Curr Opin Struct Biol. 1999;9:438–443. doi: 10.1016/S0959-440X(99)80061-X. [DOI] [PubMed] [Google Scholar]
- Brown RE, Brockman HL. Using monomolecular films to characterize lipid lateral interactions. Methods Mol Biol. 2007;398:41–58. doi: 10.1007/978-1-59745-513-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas S, Ho S, Ros U, Lanio ME, Alvarez C, van der Goot FG. Damage of eukaryotic cells by the pore-formingtoxin sticholysin II: Consequences of the potassium efflux. Biochim Biophys Acta. 2017;1859:982–992. doi: 10.1016/j.bbamem.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Casallanovo F, de Oliveira FJ, de Souza FC, et al. Model peptides mimic thestructure and function of the N-terminus of the pore-formingtoxin sticholysin II. Biopolymers. 2006;84:169–180. doi: 10.1002/bip.20374. [DOI] [PubMed] [Google Scholar]
- Celedon G, Venegas F, Campos AM, et al. Role of endogenous channels in redblood cells response to their exposure to the pore formingtoxin Sticholysin II. Toxicon. 2005;46:297–307. doi: 10.1016/j.toxicon.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Celedon G, Gonzalez G, Lissi E, et al. Effect of calcium on the hemolytic activity of Stichodactyla helianthus toxin sticholysin II on human erythrocytes. Toxicon. 2009;54:845–850. doi: 10.1016/j.toxicon.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Cilli EM, Pigossi FT, Crusca E, Jr, et al. Correlations between differences in amino-terminal sequences and different hemolytic activity of sticholysins. Toxicon. 2007;50:1201–1204. doi: 10.1016/j.toxicon.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Cosentino K, Ros U, Garcia-Saez AJ. Assembling the puzzle: oligomerization of alpha-pore forming proteins in membranes. Biochim Biophys Acta. 2016;1858:457–466. doi: 10.1016/j.bbamem.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida RF, Fedorov A, Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys J. 2003;85:2406–2416. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Rios V, Mancheno JM, Lanio ME, Onaderra M, Gavilanes JG. Mechanism of the leakage induced on lipid model membranes by the hemolytic protein sticholysin II from the sea anemone Stichodactyla helianthus. Eur J Biochem. 1998;252:284–289. doi: 10.1046/j.1432-1327.1998.2520284.x. [DOI] [PubMed] [Google Scholar]
- Fanani ML, Hartel S, Maggio B, et al. The action of sphingomyelinase in lipidmonolayers as revealed by microscopic image analysis. Biochim Biophys Acta. 2010;1798:1309–1323. doi: 10.1016/j.bbamem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- García-Linares S, Castrillo I, Bruix M, et al. Three-dimensional structure of the actinoporin sticholysin I Influence of long-distance effects on protein function. ArchBiochem Biophys. 2013;532:39–45. doi: 10.1016/j.abb.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Garcia-Linares S, Alm I, Maula T, et al. The effect of cholesterol on the long-range network of interactions established among sea anemone sticholysin II residues at the water-membrane interface. Mar Drugs. 2015;13:1647–1665. doi: 10.3390/md13041647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Linares S, Maula T, Rivera-de-Torre E, Gavilanes JG, Slotte JP, Martínez-Del-Pozo Á. Role of the tryptophan residues in the specific interaction of the sea anemone Stichodactyla helianthus’s actinoporin sticholysin II with biological membranes. Biochemistry. 2016;55:6406–6420. doi: 10.1021/acs.biochem.6b00935. [DOI] [PubMed] [Google Scholar]
- Gilbert RJ. Protein-lipid interactions and non-lamellar lipidic structures in membrane pore formation and membrane fusion. Biochim Biophys Acta. 2015;1858:487–499. doi: 10.1016/j.bbamem.2015.11.026. [DOI] [PubMed] [Google Scholar]
- Gonzalez MR, Bischofberger M, Pernot L, van der Goot FG, Freche B. Bacterial pore-forming toxins: the (w)hole story? Cell Mol Life Sci. 2008;65:493–507. doi: 10.1007/s00018-007-7434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MR, Bischofberger M, Freche B, Ho S, Parton RG, van der Goot FG. Pore-forming toxins induce multiplecellular responses promoting survival. Cell Microbiol. 2011;13:1026–1043. doi: 10.1111/j.1462-5822.2011.01600.x. [DOI] [PubMed] [Google Scholar]
- Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways inresponse to bacterial pore-forming toxins promotes cellsurvival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Hervis Y, Valle A, Canet L, Alvarez C, Lanio M, Pazos F. Relevance of Pro80 for membrane interaction and poreformation by sticholysin I, a toxin from Stichodactyla helianthus (Anthozoa: Stichodactylidae) Rev Cubana Cienc Biol. 2014;3:27–40. [Google Scholar]
- Heuck AP, Moe PC, Johnson BB. The cholesterol-dependent cytolysin family of gram-positive bacterial toxins. Subcell Biochem. 2010;51:551–577. doi: 10.1007/978-90-481-8622-8_20. [DOI] [PubMed] [Google Scholar]
- Hinds MG, Zhang W, Anderluh G, Hansen PE, Norton RS. Solution structure of the eukaryotic poreformingcytolysin equinatoxin II: implications for pore formation. J Mol Biol. 2002;315(5):1219–1229. doi: 10.1006/jmbi.2001.5321. [DOI] [PubMed] [Google Scholar]
- Hong Q, Gutierrez-Aguirre I, Barlic A, et al. Two-step membrane binding byequinatoxin II, a poreforming toxin from the sea anemone, involves an exposed aromatic cluster and a flexible helix. J Biol Chem. 2002;277:41916–41924. doi: 10.1074/jbc.M204625200. [DOI] [PubMed] [Google Scholar]
- Hotze EM, Tweten RK. Membrane assembly of the cholesterol-dependent cytolysin pore complex. Biochim Biophys Acta. 2012;1818:1028–1038. doi: 10.1016/j.bbamem.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta V, Morera V, Guanche Y, et al. Primary structure of two cytolysin isoforms from Stichodactyla helianthus differing in their hemolytic activity. Toxicon. 2001;39:1253–1256. doi: 10.1016/S0041-0101(00)00247-6. [DOI] [PubMed] [Google Scholar]
- Huffman DL, Abrami L, Sasik R, Corbeil J, van der Goot FG, Aroian RV. Mitogen-activated protein kinase pathwaysdefend against bacterial pore-forming toxins. Proc Natl Acad Sci U S A. 2004;101:10995–11000. doi: 10.1073/pnas.0404073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husmann M, Beckmann E, Boller K, et al. Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett. 2009;583:337–344. doi: 10.1016/j.febslet.2008.12.028. [DOI] [PubMed] [Google Scholar]
- Iacovache I, Bischofberger M, van der Goot FG (2010) Structure and assembly of pore-forming proteins. Curr Opin Struct Biol 20:241–246. 10.1016/j.sbi.2010.01.013 [DOI] [PubMed]
- Jayaram B, Sprous D, Beveridge D. Solvation free energy of biomacromolecules: parameters for a modified generalized born model consistent with the AMBER forcefield. J Phys Chem B. 1998;102:9571–9576. doi: 10.1021/jp982007x. [DOI] [Google Scholar]
- Kao CY, Los FCO, Huffman DL et al (2011) Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog 7:E1001314. 10.1371/journal.ppat.1001314%209 [DOI] [PMC free article] [PubMed]
- Kristan K, Podlesek Z, Hojnik V et al (2004) Pore formation by equinatoxin, a eukaryotic pore-forming toxin, requires a flexible N-terminal region and a stable β-sandwich. J Biol Chem 279:46509–46517. 10.1074/jbc.M406193200 [DOI] [PubMed]
- Kristan K, Viero G, Macek P, Dalla Serra M, Anderluh G. The equinatoxin N-terminus is transferred acrossplanar lipid membranes and helps to stabilize thetransmembrane pore. FEBS J. 2007;274:539–550. doi: 10.1111/j.1742-4658.2006.05608.x. [DOI] [PubMed] [Google Scholar]
- Ladokhin AS, Jayasinghe S, White SH. How tomeasure and analyze tryptophan fluorescence in membranesproperly, and why bother? Anal Biochem. 2000;285:235–245. doi: 10.1006/abio.2000.4773. [DOI] [PubMed] [Google Scholar]
- Lanio ME, Morera V, Alvarez C, et al. Purification and characterization of two hemolysins from Stichodactyla helianthus. Toxicon. 2001;39:187–194. doi: 10.1016/S0041-0101(00)00106-9. [DOI] [PubMed] [Google Scholar]
- Lanio ME, Alvarez C, Martinez D, et al. Effect of azwitterionic surfactant (HPS) on the conformation and hemolytic activity of St I and St II, two Isotoxins purified from Stichodactyla helianthus. J Protein Chem. 2002;21:401–405. doi: 10.1023/A:1021130516229. [DOI] [PubMed] [Google Scholar]
- Lanio ME, Alvarez C, Pazos F, et al. Effects of sodium dodecyl sulfate on the conformation and hemolytic activity of St I and St II, two isotoxins purified from Stichodactyla helianthus. Toxicon. 2003;41:65–70. doi: 10.1016/S0041-0101(02)00210-6. [DOI] [PubMed] [Google Scholar]
- Lanio ME, Alvarez C, Ochoa C, et al. Sticholysins I and II interaction with cationic micelles promotes toxins’ conformational changes and enhanced hemolytic activity. Toxicon. 2007;50:731–739. doi: 10.1016/j.toxicon.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Macek P, Belmonte G, Pederzolli C, Menestrina G. Mechanism of action of equinatoxin II, a cytolysin from thesea anemone Actinia equina L. belonging to the family ofactinoporins. Toxicology. 1994;87:205–227. doi: 10.1016/0300-483X(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Macek P, Zecchini M, Pederzolli C, Dalla Serra M, Menestrina G. Intrinsic tryptophan fluorescence ofequinatoxin II, a pore-forming polypeptide from the sea anemone Actinia equina L, monitors its interaction with lipidmembranes. Eur J Biochem. 1995;234:329–335. doi: 10.1111/j.1432-1033.1995.329_c.x. [DOI] [PubMed] [Google Scholar]
- MacGregor RD, II, Tobias CA. Molecular sieving of redcell membranes during gradual osmotic hemolysis. J MembrBiol. 1972;10:345–356. doi: 10.1007/BF01867865. [DOI] [PubMed] [Google Scholar]
- Maget-Dana R. The monolayer technique: a potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochim Biophys Acta. 1999;1462:109–140. doi: 10.1016/S0005-2736(99)00203-5. [DOI] [PubMed] [Google Scholar]
- Maggio B, Borioli GA, Del Boca M, et al. Composition-driven surface domain structuring mediated by sphingolipids and membrane-active proteins. Above the nano- but under the micro-scale: mesoscopic biochemical/structural cross-talk in biomembranes. Cell Biochem Biophys. 2008;50:79–109. doi: 10.1007/s12013-007-9004-1. [DOI] [PubMed] [Google Scholar]
- Mally M, Majhenc J, Svetina S, Zeks B. Mechanismsof equinatoxin II-induced transport through the membrane ofa giant phospholipid vesicle. Biophys J. 2002;83:944–953. doi: 10.1016/s0006-3495(02)75220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malovrh P, Viero G, Serra MD, et al. Novel mechanism ofpore formation: membrane penetration by the N-terminalamphipathic region of equinatoxin. J Biol Chem. 2003;278:22678–22685. doi: 10.1074/jbc.M300622200. [DOI] [PubMed] [Google Scholar]
- Mancheno JM, Martin-Benito J, Martinez-Ripoll M, Gavilanes JG, Hermoso JA (2003) Crystal and electron microscopy structures of sticholysin II actinoporin reveal insights into the mechanism of membrane pore formation. Structure (London, England : 1993) 11:1319–1328 [DOI] [PubMed]
- Mancheno JM, Martin-Benito J, Gavilanes JG, Vazquez L. A complementary microscopy analysis of Sticholysin II crystals on lipid films: atomic force and transmission electron characterizations. Biophys Chem. 2006;119:219–223. doi: 10.1016/j.bpc.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Martinez D, Campos AM, Pazos F, et al. Properties of St I and St II, two isotoxins isolated from Stichodactyla helianthus: a comparison. Toxicon. 2001;39:1547–1560. doi: 10.1016/S0041-0101(01)00127-1. [DOI] [PubMed] [Google Scholar]
- Martinez D, Otero A, Alvarez C, et al. Effect of sphingomyelin and cholesterol on the interaction of St II with lipidic interfaces. Toxicon. 2007;49:68–81. doi: 10.1016/j.toxicon.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Mechaly AE, Bellomio A, Gil-Carton D, et al. Structural insights into the oligomerization and architecture of eukaryotic membrane pore-forming toxins. Structure. 2011;19:181–191. doi: 10.1016/j.str.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Menestrina G, Cabiaux V, Tejuca M. Secondary structure of sea anemone cytolysins in soluble and membrane bound form by infrared spectroscopy. Biochem Biophys Res Commun. 1999;254:174–180. doi: 10.1006/bbrc.1998.9898. [DOI] [PubMed] [Google Scholar]
- Mesa-Galloso H, Delgado-Magnero KH, Cabezas S, et al. Disrupting a key hydrophobic pair in the oligomerization interface of the actinoporins impairs their pore-forming activity. Protein Sci. 2017;26:550–565. doi: 10.1002/pro.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morante K, Caaveiro JM, Viguera AR, Tsumoto K, Gonzalez-Manas JM (2015) Functional characterization of Val60, a key residue involved in the membrane-oligomerization of fragaceatoxin C, an actinoporin from Actinia fragacea. FEBS Lett 589:1840–1846. 10.1016/j.febslet.2015.06.012%2015 [DOI] [PubMed]
- Morante K, Bellomio A, Gil-Carton D et al (2016) Identification of a membrane-bound prepore species clarifies the lytic mechanism of actinoporins. J Biol Chem 291:19210–19219. 10.1074/jbc.M116.734053 [DOI] [PMC free article] [PubMed]
- Pardo-Cea MA, Castrillo I, Alegre-Cebollada J, Martínez-del- Pozo A, Gavilanes JG, Bruix M. Intrinsic local disorder and a network of charge-charge interactions are key to actinoporin membrane disruption and cytotoxicity. Biomol NMR Assign. 2011;4:69–72. doi: 10.1007/s12104-010-9214-0. [DOI] [PubMed] [Google Scholar]
- Parker MW, Feil SC. Pore-forming protein toxins: from structure to function. Prog Biophys Mol Biol. 2005;88:91–142. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Pedrera L, Fanani ML, Ros U, Lanio ME, Maggio B, Alvarez C. Sticholysin I-membrane interaction: an interplay between the presence of sphingomyelin and membrane fluidity. Biochim Biophys Acta. 2014;1838:1752–1759. doi: 10.1016/j.bbamem.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Pedrera L, Gomide AB, Sanchez RE, et al. The presence of sterols favors sticholysin I-membrane association and pore formation regardless of their ability to form laterally segregated domains. Langmuir. 2015;31:9911–9923. doi: 10.1021/acs.langmuir.5b01687. [DOI] [PubMed] [Google Scholar]
- Penton D, Pérez-Barzaga V, Díaz I, et al. Validation of a mutant of the pore-forming toxin sticholysin-I for the construction of proteinase-activated immunotoxins. Protein Eng Des Sel. 2011;24:485–493. doi: 10.1093/protein/gzr002. [DOI] [PubMed] [Google Scholar]
- Peraro MD, van der Goot FG. Pore-forming toxins: ancient, but never really out of fashion. Nat Rev Microbiol. 2016;14:77–92. doi: 10.1038/nrmicro.2015.3. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, et al. UCSF chimera a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Porta H, Cancino-Rodezno A, Soberon M, Bravo A. Role of MAPK p38 in the cellular responses to pore-forming toxins. Peptides. 2011;32:601–606. doi: 10.1016/j.peptides.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner AJ, Hippe KR, Aguilar JL, Bender MH, Nelson AL, Weiser JN. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J Biol Chem. 2006;281:12994–12998. doi: 10.1074/jbc.M511431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojko N, Kristan KC, Viero G, et al. Membrane damage by an alpha helical pore-forming protein, equinatoxin II, proceeds through a succession of ordered steps. J Biol Chem. 2013;288:23704–23715. doi: 10.1074/jbc.M113.481572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojko N, Cronin B, Danial JS, Baker MA, Anderluh G, Wallace MI. Imaging the lipid-phase-dependent pore formation of equinatoxin II in droplet interface bilayers. Biophys J. 2014;106:1630–1637. doi: 10.1016/j.bpj.2013.11.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojko N, Dalla Serra M, Macek P, Anderluh G. Pore formation by actinoporins, cytolysins from sea anemones. Biochim Biophys Acta. 2016;1858(3):446–456. doi: 10.1016/j.bbamem.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Ros U, Garcia-Saez AJ. More than a pore: the interplay of pore-forming proteins and lipid membranes. J Membr Biol. 2015;248:545–561. doi: 10.1007/s00232-015-9820-y. [DOI] [PubMed] [Google Scholar]
- Ros U, Pedrera L, Diaz D, et al. The membranotropic activity of N-terminal peptides from the poreforming proteins sticholysin I and II is modulated by hydrophobic and electrostatic interactions as well as lipid composition. J Biosci. 2011;36:781–791. doi: 10.1007/s12038-011-9156-4. [DOI] [PubMed] [Google Scholar]
- Ros U, Edwards MA, Epand R, et al. The sticholysin family of pore-forming toxins induces the mixing of lipids in membrane domains. Biochim Biophys Acta. 2013;1828:2757–2762. doi: 10.1016/j.bbamem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Ros U, Rodríguez-Vera W, Pedrera L, et al. Differences in activity of actinoporins are related with the hydrophobicity of their N-terminus. Biochimie. 2015;116:70–78. doi: 10.1016/j.biochi.2015.06.024. [DOI] [PubMed] [Google Scholar]
- Schon P, Garcia-Saez AJ, Malovrh P, Bacia K, Anderluh G, Schwille P. Equinatoxin II permeabilizing activity depends on the presence of sphingomyelin and lipid phase coexistence. Biophys J. 2008;95:691–698. doi: 10.1529/biophysj.108.129981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai Y. ATR-FTIR studies in pore forming and membrane induced fusion peptides. Biochim Biophys Acta. 2013;1828:2306–2313. doi: 10.1016/j.bbamem.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Soto C, Del Valle A, Valiente PA, et al. Differential binding and activity of the pore-forming toxin sticholysin II in model membranes containing diverse ceramide-derived lipids. Biochimie. 2017;138:20–31. doi: 10.1016/j.biochi.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Subburaj Y, Ros U, Hermann E, Tong R, Garcia-Saez AJ. Toxicity of an alpha-pore-forming toxin depends on the assembly mechanism on the target membrane as revealed by single molecule imaging. J Biol Chem. 2015;290:4856–4865. doi: 10.1074/jbc.M114.600676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Caaveiro JM, Morante K, Gonzalez-Manas JM, Tsumoto K. Structural basis for selfassembly of a cytolytic pore lined by protein and lipid. Nat Commun. 2015;6:6337. doi: 10.1038/ncomms7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejuca M, Serra MD, Ferreras M, Lanio ME, Menestrina G. Mechanism of membrane permeabilization by sticholysin I, a cytolysin isolated from the venom of the sea anemone Stichodactyla helianthus. Biochemistry. 1996;35:14947–14957. doi: 10.1021/bi960787z. [DOI] [PubMed] [Google Scholar]
- Tejuca M, Anderluh G, Macek P, et al. Antiparasite activity of sea-anemone cytolysins on Giardia duodenalis and specific targeting with anti-Giardia antibodies. Int J Parasitol. 1999;29:489–498. doi: 10.1016/S0020-7519(98)00220-3. [DOI] [PubMed] [Google Scholar]
- Tejuca M, Dalla Serra M, Potrich C, Alvarez C, Menestrina G. Sizing the radius of the pore formed in erythrocytes and lipid vesicles by the toxin sticholysin I from the sea anemone Stichodactyla helianthus. J Membr Biol. 2001;183:125–135. doi: 10.1007/s00232-001-0060-y. [DOI] [PubMed] [Google Scholar]
- Tejuca M, Diaz I, Figueredo R, et al. Construction of an immunotoxin with the pore forming protein StI and ior C5, a monoclonal antibody against a colon cancer cell line. Int J Immunopharmacol. 2004;4:731–744. doi: 10.1016/j.intimp.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Tejuca M, Anderluh G, Dalla Serra M. Sea anemone cytolysins as toxic components of immunotoxins. Toxicon. 2009;54:1206–1214. doi: 10.1016/j.toxicon.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Valle A, López-Castilla A, Pedrera L, et al. Cys mutants in functional regions of sticholysin I clarify the participation of these residues in pore formation. Toxicon. 2011;58:8–17. doi: 10.1016/j.toxicon.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Valle A, Alvarado-Mesen J, Lanio ME, Alvarez C, Barbosa JA, Pazos IF. The multigene families of actinoporins (part I): isoforms and genetic structure. Toxicon. 2015;103:176–187. doi: 10.1016/j.toxicon.2015.06.028. [DOI] [PubMed] [Google Scholar]
- Valle A, Hervis YP, Socas LB, et al. The multigene families of actinoporins (part II): strategies for heterologous production in Escherichia coli. Toxicon. 2016;118:64–81. doi: 10.1016/j.toxicon.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Wald T, Petry-Podgorska I, Fiser R, et al. Quantification of potassium levels in cells treated with Bordetella adenylate cyclase toxin. Anal Biochem. 2014;450:57–62. doi: 10.1016/j.ab.2013.10.039. [DOI] [PubMed] [Google Scholar]