Abstract
According to the World Health Organization (WHO), hundreds of millions of people of all ages and in all countries suffer from chronic respiratory diseases, with particular negative consequences such as poor health-related quality of life, impaired work productivity, and limitations in the activities of daily living. Chronic obstructive pulmonary disease, asthma, occupational lung diseases (such as silicosis), cystic fibrosis, and pulmonary arterial hypertension are the most common of these diseases, and none of them are curable with current therapies. The advent of nanotechnology holds great therapeutic promise for respiratory conditions, because non-viral vectors are able to overcome the mucus and lung remodeling barriers, increasing pharmacologic and therapeutic potency. It has been demonstrated that the extent of pulmonary nanoparticle uptake depends not only on the physical and chemical features of nanoparticles themselves, but also on the health status of the organism; thus, the huge diversity in nanotechnology could revolutionize medicine, but safety assessment is a challenging task. Within this context, the present review discusses some of the major new perspectives in nanotherapeutics for lung disease and highlights some of the most recent studies in the field.
Keywords: Nanotherapy, Lung diseases, Nanoparticles, Nanomedicine
Introduction
Nanoparticles (NPs) are one of the most widely studied classes of drug delivery systems, with more than 25,000 publications over the last 10 years (Anselmo and Mitragotri 2014). They have a wide range of unique properties and capabilities that can be used to improve on traditional drug administration; therefore, exhaustive efforts have focused on developing advanced nanotherapeutic delivery systems for improved efficacy, enhanced patient compliance, and optimal treatment safety (Peer et al. 2007).
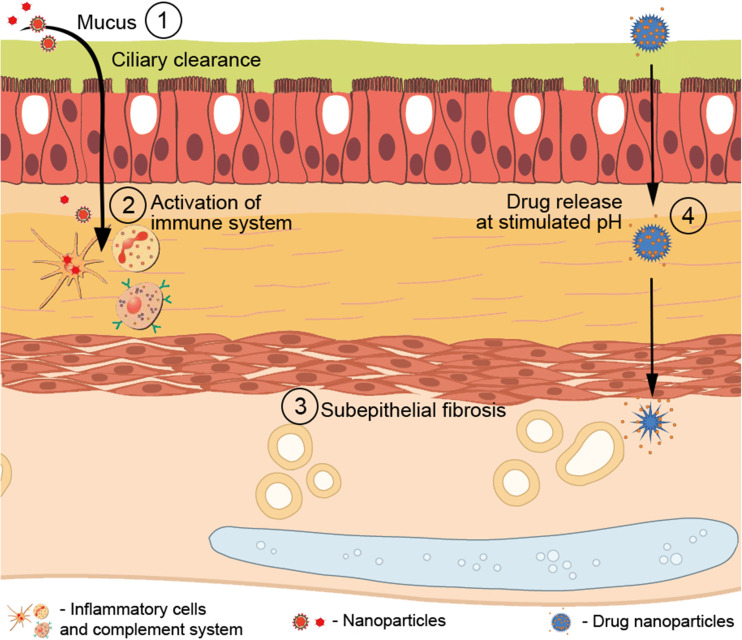
In this context, previous reports have observed that, to be efficient and safe for lung delivery, NPs must be resistant to a variety of changes in lung parenchyma with the development of lung diseases and external stimuli (Fig. 1), such as heat, the mucus hyperproduction barrier and its clearance, subepithelial fibrosis, activation of macrophages and neutrophils, the complement system, and changes in pH (Gupta et al. 2002; Boylan et al. 2012a; Lee and Thompson 2017).
Fig. 1.
The pulmonary barriers that nanoparticles should overcome in the injured tissue. After reaching the pulmonary epithelium, the nanoparticle must be stable and efficient enough to overcome the barriers and clearance processes to reach the underlying tissue, treating lung diseases: (1) mucus hyperproduction barrier and its mucociliary clearance could halt nanoparticles, preventing their passage; (2) after succeeding in reaching the lung parenchyma, nanoparticles could stimulate the activation of macrophages and neutrophils, as well as the complement system, leading to degradation of nanoparticles; (3) subepithelial fibrosis as a mechanical barrier that hinders the passage of nanoparticles; (4) changes in the pH of the organism may hinder drug release
Recently, nanomedicine has made great progress in targeting NPs to individual organs in one of the major advances achieved in this field of research (Nguyen et al. 2015; Uddin et al. 2016). Within an organ, diseases display a large degree of spatial heterogeneity, with the same organ containing both healthy and pathologic regions (González-García et al. 2002; Ju et al. 2014). Thus, studies have observed that some drug delivery formulations would be able to directly target the specific region affected by the disease (Brenner et al. 2017).
With the aim of reducing systemic toxicity induced by various medications, mainly anticancer drugs, a range of magnetic nanocarriers have been developed as a promising strategy (Xie et al. 2009; Wang et al. 2010). Magnetic NPs have been investigated for decades as potential drug delivery systems. Despite the described long clearance time for degradation and the accumulation in some tissues, mainly in the liver and kidney, consequently generating some inflammatory side effects (Wahajuddin and Arora 2012), some studies have been developing novel strategies to modify nanoparticle structure, improving their high magnetic responsiveness, biodegradability, biocompatibility, high delivery efficiency, and potential targeting function, reducing the frequency of injections (Emerich et al. 2002). Drug-loaded magnetic NPs are injected directly into the affected site; they are expected to be held in place by an external magnetic field and to release the drug in a controlled manner (Amirfazli 2007; Stocke et al. 2015).
Within this context, the present review summarizes some of the major new perspectives in the diagnostic and therapeutic use of nanocarriers for chronic lung diseases, including asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, occupational diseases (silicosis), and pulmonary artery hypertension, highlighting some of the most recent preclinical studies in gene therapy and drug delivery, discussing relevant clinical trials, and addressing the many pros and cons of this innovative technology.
Nanocarriers for chronic lung diseases
Asthma
Allergic asthma is a major global health problem. Approximately 334 million people worldwide are believed to be affected by asthma (Global Initiative for Asthma 2017, http://ginasthma.org), with negative consequences such as poor health-related quality of life, impaired work productivity, and limitations in the activities of daily living (Peters et al. 2006; Nelsen et al. 2017).
Classically, CD4+ Th2 cells have been considered the primary regulators of the allergic response through production of Th2 cytokines, which leads to airway inflammation and hyper-responsiveness (Holloway et al. 2010; Shinoda et al. 2017). Patients with asthma may also display airway hyper-responsiveness with no early- or late-phase inflammation present, which is likely caused by long-term changes in the structure of the airway, such as hypertrophy and/or hyperplasia of the smooth muscle layer (Murdoch and Lloyd 2010; Grenier et al. 2016). The most widely used therapies for asthma, including inhaled corticosteroids and bronchodilators (short- or long-acting β-adrenergic agonists or muscarinic antagonists), reduce disease symptoms (Kim et al. 2011; Raissy et al. 2013). However, corticosteroids cause long-lasting side effects and may not provide instant relief, thus decreasing treatment adherence by patients (Cooper et al. 2015; Lawani et al. 2017) and accelerating disease progression and lung remodeling, which leads to progressive loss of lung function (Pascual and Peters 2005). Moreover, long-term use of corticosteroids cannot revert lung remodeling and may result in immunosuppression, predisposing patients to lung infection with opportunistic microorganisms (Barnes 2012). Thus, alternative therapeutic approaches that can attenuate both inflammatory and remodeling processes in different asthma phenotypes and reduce airway resistance without leading to immunosuppression are needed.
In this context, nanotechnology has emerged as a new paradigm for the treatment of diseases refractory to conventional therapeutics, including asthma. The use of NPs facilitates the delivery of biological materials and drugs directly to target tissues, thus improving lung deposition of anti-asthma drugs and translating into improved clinical efficacy with sustained therapeutic effects, while mitigating adverse effects.
A variety of nanoparticulate systems (nanocomplexes) have been evaluated in the lung (da Silva et al. 2013; van Rijt et al. 2014; Kolte et al. 2017). However, several physiologic barriers, e.g., mucus in the airway lumen, make application of this therapeutic strategy to the lung challenging (Schneider et al. 2017). Therefore, nanometric size, the material used to manufacture NPs, and their sustained activity must be evaluated in order to avoid clearance by macrophages and allow transcytosis and treatment of the submucosa; crossing of the endothelium allows systemic treatment and avoids side effects. In the current review, we discussed which NPs have been developed and tested for asthma treatment, including: liposomes, solid lipid NPs, telodendrimer, poly(ethylene imine) (PEI), chitosan, dendrimers, poly(lactic-co-glycolic acid) (PLGA), and CK30PEG.
A recent study showed that stealth steroids compacted with NPs produce prolonged and greater benefits at the site of airway inflammation compared with free steroids (Matsuo et al. 2009). Furthermore, NPs compacted with salbutamol interact more with the lung membrane, leading to greater and more sustained relief of bronchospasm as a result of concentration of drug in the target area (Bhavna et al. 2009). Studies have also demonstrated that liposomes could increase the concentration and retention time of salbutamol sulfate in the lungs, thus prolonging its therapeutic effect for up to 10 h, more than that of the unencapsulated drug (Chen et al. 2012).
Solid lipid NPs have attracted increasing attention as a potential drug delivery carrier because of their unique structure and properties, such as good biocompatibility, protection of the incorporated compound against degradation, and controlled release (Wang et al. 2012). These authors reported enhanced bioavailability and efficiency of curcumin, a potent anti-inflammatory supplement that could be used as an add-on therapy in patients with bronchial asthma, as a formulation in solid lipid NPs.
Another compound widely used to prevent the wheezing, exercise-induced bronchospasm, chest tightness, and coughing caused by asthma is montelukast, one of a class of medications known as leukotriene receptor antagonists (LTRAs). Patil-Gadhe et al. (2014) and Patil-Gadhe and Pokharkar (2014) demonstrated that a montelukast-loaded nanostructured lipid carrier improved the systemic bioavailability and performance of the drug, bypassing hepatic metabolism and, thus, reducing hepatocellular toxicity.
Furthermore, a well-defined nontoxic telodendrimer has also been reported to be an efficient nanocarrier with greater loading capacity and superior stability (longer than 6 months) than micelles (Jackson et al. 2004). This nanocarrier allows delivery of slow-release formulations of hydrophobic drugs (such as dexamethasone) directly to the lung, decreasing allergic lung inflammation and reducing the number of eosinophils and inflammatory cytokines, thus improving airway hyper-responsiveness to a greater degree than equivalent doses of dexamethasone alone (Kenyon et al. 2013).
The expansion of novel NPs for gene therapy has emerged as an alternative for asthma treatment, with the aim of inhibiting Th2 transcription factors, cytokines, and the function or overexpression of Th2 antagonists. According to a recent study, synthetic NP-based gene delivery systems offer highly tunable platforms for delivery of therapeutic genes, but the inability to achieve sustained, high-level transgene expression in vivo presents a significant hurdle (Mastorakos et al. 2015). The respiratory system, although readily accessible, remains a challenging target, because effective gene therapy mandates colloidal stability in physiologic fluids and the ability to overcome biological barriers found in the lung; as nucleic acids are prone to degradation by nucleases, delivery vectors must mediate protection against degradation (Sanders et al. 2009).
A number of lipid- and polymer-based non-viral vectors have been developed to formulate nucleic acids into nanosized particles for pulmonary delivery (Di Gioia et al. 2015). One of the most prominent polymeric gene delivery vectors is poly(ethylene imine) (PEI) (Merdan et al. 2003). Use of the polysaccharide chitosan (Köping-Höggård et al. 2001), dendrimers (Rudolph et al. 2000), and poly(lactic-co-glycolic acid) (PLGA)-based polymers (Bivas-Benita et al. 2009) for pulmonary delivery of nucleic acids has also been described.
Kumar et al. (2003) showed that chitosan interferon (IFN)-γ-pDNA nanoparticle (CIN) treatment significantly lowered airway hyper-responsiveness to methacholine and reduced lung histopathology in a BALB/c mouse model of ovalbumin (OVA)-induced allergic asthma. In another study designed to elucidate the mechanism of these effects, the same group demonstrated that CIN treatment was able to reduce cytokine production by a population of OVA-specific proinflammatory CD8+ T lymphocytes in the lung, leading to decreased activation of dendritic cells. Because of the reciprocal regulation of T helper cells, it was anticipated that increasing IFN-γ levels would promote a Th1 response by blocking Th2 cytokine production (Kong et al. 2008).
More recently, researchers used highly compacted DNA NPs composed of pDNA compacted with block copolymers of poly-L-lysine and polyethylene glycol linked by a cysteine residue (CK30PEG), which have been shown to be nontoxic and nonimmunogenic in the lungs of mice and humans. Indeed, da Silva et al. (2014) used this NP system to deliver thymulin, a nonapeptide known for its anti-inflammatory and anti-fibrotic effects in the lung, to OVA-challenged allergic asthma in BALB/c mice. A single intratracheal instillation of DNA NPs carrying thymulin plasmids prevented lung inflammation, collagen deposition, and smooth muscle hypertrophy in the murine lungs up to 27 days after administration, leading to improved lung mechanics.
Because of its high cationic charge density, PEI is able to efficiently condense negatively charged DNA into nanosized complexes, thereby protecting it from degradation by nucleases. However, this positive charge causes toxicity and makes it a poor vehicle for asthma gene therapy. PEGylated PEI can also activate the complement system and induce expression of apoptosis-related genes (Merkel et al. 2011a, b). Therefore, the need for biodegradable vectors seems obvious. Mastorakos et al. (2015) presented a highly compacted, biodegradable DNA NP capable of overcoming the mucus barrier for inhaled lung gene therapy with a favorable safety profile and no signs of toxicity after intratracheal administration. In addition, a promising cytosine–phosphate–guanine (CpG) adjuvant-loaded, biodegradable NP-based vaccine for treatment of dust mite allergies has been developed recently and used as a potent adjuvant for shifting immune responses to the Th1 type, suppressing Th2-triggered asthma responses (Salem 2014).
In summary, it is well known that asthma is a very complex inflammatory disease, in which numerous cells and cellular factors are implicated. Thus, there are many potential molecular targets for therapy, including cytokines/chemokines, transcription factors, tyrosine kinases and their receptors, and co-stimulatory molecules for gene silencing or overexpression of specific targets, together with drug delivery by NPs.
Chronic obstructive pulmonary disease
COPD is the fourth leading cause of death by noncommunicable diseases, and is expected to be ranked third by 2030 (Vij 2011). COPD is a chronic disease, occurring primarily in the elderly, and characterized by an abnormal inflammatory response to inhaled noxious particles or gases, leading to airflow limitation. The pathophysiology of COPD involves chronic inflammation of the airways and destruction of lung parenchyma. Alveolar macrophages play a key role in this inflammatory response by releasing inflammatory mediators, including tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-8, monocyte chemotactic peptide (MCP)-1, leukotriene LTB4, and reactive oxygen species. Current treatment options for COPD include anticholinergics, β2-agonists, and inhaled corticosteroids, which can control the symptoms but do not cure the underlying disease (Global Initiative for Chronic Obstructive Lung Disease, GOLD 2017).
The major challenges in the delivery and efficacy of nanotherapeutic systems in chronic obstructive airway conditions are airway defense, severe inflammation, and mucus hypersecretion. Several groups have been seeking new therapeutic approaches, and despite the wide application of nano-based drug delivery systems, very few have been tested to date. In this scenario, Vij (2011) described a potential multifunctional polymeric vesicle formed by a mixture of poly(ethylene glycol) and PLGA (PLGAPEG) for combined delivery of COPD drugs (the corticosteroid prednisolone and/or the anti-inflammatory bronchodilator theophylline) and molecular probes that can be used for theranostic application in obstructive lung disease. Furthermore, Muralidharan et al. (2016) reported the successful design of advanced inhalable dry powders containing dimethyl fumarate, a first-in-class antioxidant Nrf2 activator known to treat pulmonary inflammation, using advanced particle engineering design technology for targeted delivery to the lungs as a single-component dry spray. Using in vitro predictive lung deposition modeling, the authors found that the aerosol deposition patterns of these particles were capable of reaching the lower airways to treat inflammation in this region in diseases such as COPD.
Metallic NPs, which have widespread application as contrast elements, are also used effectively for drug and gene delivery due to effective penetration in cells and tissues. However, these nanoparticles have toxic effects that are not yet completely understood (Sadeghi et al. 2015). Metallic NPs have also been assessed as potential nanocarriers for COPD treatment. Geiser et al. (2013) demonstrated that gold NP delivery is a successful strategy for therapeutic targeting of alveolar epithelial cells and macrophages in COPD. Titanium dioxide (TiO2) and carbon black (CB) NPs have biological effects that could aggravate pulmonary emphysema (Chen et al. 2006). On the other hand, TiO2 and CB NPs did not aggravate elastase-induced emphysema. However, CB NPs induced histologic inflammation and MMP-12 mRNA and protein expression in macrophages. Roulet et al. (2012) demonstrated that TiO2 and CB NPs did not aggravate elastase-induced emphysema and could be promising metallic nanocarriers for the treatment of COPD. NPs can also be used for molecular imaging of chronic lung diseases. Al Faraj et al. (2014) demonstrated that antibody-conjugated superparamagnetic iron oxide (SPIO) NPs could be used for noninvasive magnetic resonance imaging (MRI) of macrophage subpopulations. In lipopolysaccharide (LPS)-induced COPD mouse models, anti-CD86 and anti-CD206 antibody-conjugated SPIO NPs were instilled via the intrapulmonary route, and each type of NP showed specific affinity to the proinflammatory (M1) and resolutive (M2) macrophage subpopulations. Coupling of magnetic iron oxide NPs with a specific antibody targeted to a particular macrophage subpopulation could offer a promising strategy for early and better diagnosis of pulmonary inflammatory diseases using noninvasive MRI. Surface individualization with targeting molecules offers selective delivery of NPs to the target cells, and targeted delivery of NPs contributes to high-contrast molecular imaging as well as targeted therapy (Al Faraj et al. 2014).
Cystic fibrosis
Cystic fibrosis (CF) is the most common autosomal recessive inherited disorder, affecting nearly 70,000 patients worldwide, and its first mutation in the cystic fibrosis transmembrane regulator (CFTR) gene was described in 1989 (Riordan et al. 1989). The disease is characterized by an ionic imbalance that causes reduced volume of surface liquid in the airways, mucus dehydration, and reduced mucus clearance, thus leading to the secretion and accumulation of a viscous mucus in the airways that causes bronchial obstruction (Moreau-Marquis et al. 2008).
The amiloride-sensitive epithelial sodium channel (ENaC) plays a key role in salt and water resorption in the epithelium of patients with CF, in whom dehydration of respiratory secretions and impaired mucociliary clearance, caused by the hyperabsorption of Na+ through the ENaC in the absence of Cl− secretion, contributes heavily to lung pathology and respiratory insufficiency (Mall and Galietta 2015). Consequently, the tenacious mucus enables chronic bacterial infection by the opportunistic Gram-negative bacterium Pseudomonas aeruginosa, which is the most frequent pathogen identified in patients with CF (Ratjen et al. 2009).
Although these patients routinely take antibiotics, the thick mucus and bacterial biofilm contribute to poor lung penetration of antimicrobial agents because of partial immobilization and deactivation, leading to clinical exacerbations (Heijerman et al. 2009).
From this perspective, the development and optimization of pulmonary drug delivery systems engineered for improved interaction with local environment is needed. Thus, PLGA, which is already contained in several products approved by the US Food and Drug Administration, has low toxicity, and is biocompatible (Wacker 2013), was chosen as a nanocarrier material for the manufacture of ciprofloxacin complex-loaded PLGA NPs for pulmonary treatment of infections in CF (Günday Türeli et al. 2016).
Likewise, tobramycin-loaded nanostructured lipid carriers were found to exhibit high drug encapsulation, a sustained release profile, and activity against clinically isolated P. aeruginosa. Moreover, these lipid NPs did not decrease cell viability and were able to overcome an artificial mucus barrier in the presence of mucolytic agents, showing a wide nanosystem distribution in the lungs (Moreno-Sastre et al. 2016).
In the gene therapy context, 6 years after the cloning of CFTR, the first evidence was obtained that non-viral gene transfer mediated by liposomes complexed with CFTR cDNA could partially correct cAMP-mediated Cl− transport in the nasal epithelium of patients with CF (Caplen et al. 1995).
Nebulized cationic liposomes could also be used to effectively deliver siRNA against the α-subunit of ENaC to the airway epithelium, modulating sodium hyperabsorption and, thus, helping to restore the correct volume of surface liquid, mucus hydration, and mucociliary clearance in the airways. This approach might be promising as an adjuvant therapy for CF. However, an optimal dose is required to achieve therapeutic benefit without potential side effects (Manunta et al. 2017).
Monthly administration of pGM169 plasmid DNA delivered by liposomes (GL67A) led to benefit in FEV1 (forced expiratory volume in the first second of expiration) compared with placebo after 1 year, suggesting lung function stabilization in the treatment group (Alton et al. 2015). This was the first proof of concept that repeated administration of non-viral CFTR gene therapy can safely change clinically relevant parameters in CF. Nevertheless, the authors note that further improvements in efficacy and consistency of response are required before such therapy can be used in the clinical setting (Alton et al. 2015).
Silicosis
Silicosis is an occupational disease characterized by hyalinized and fibrotic nodules, thickening of the alveolar interstitium, and accumulation of inflammatory cells, such as alveolar macrophages and lymphocytes. The pathogenesis of silicosis has been related to the accumulation of inflammatory cells that produce fibrogenic and inflammatory cytokines and growth factors, including TNF-α, IL-1β, TGF-β, macrophage inflammatory proteins (MIP)-1 and MIP-2, and platelet-derived, insulin-like, and fibroblast growth factors (Lopes-Pacheco et al. 2016). Despite substantial research effort, no current treatment is able to reverse the pathologic changes or halt disease progression.
In this context, one promising medical application of nanomaterials is the use of inorganic NPs within mesenchymal stromal cell (MSC)-based therapies. Nanomaterials have facilitated not only the acquisition of knowledge of stem cell biology but also allowed the development of new approaches that have enhanced their homing, survival, and biological effects (Silva et al. 2016). Silva et al. (2016) magnetized MSCs with iron oxide NPs, administered these cells through the jugular vein of silicotic mice, and exposed the animals to a localized magnetic field around the thorax. The authors found that NPs were able to act as good agents for magnetic targeting of MSCs to the site of injury, resulting in increased cell retention in lung parenchyma and histologic improvement.
Pulmonary artery hypertension
Pulmonary artery hypertension (PAH) is a life-threatening disease characterized by progressively increased pulmonary vascular resistance (PVR) and pulmonary artery pressure. Increased PVR is derived from lung vasoconstriction, vascular remodeling (intimal and medial hypertrophy), and thrombosis. High levels of PVR cause severe right ventricular (RV) hypertrophy and failure, which are associated with poor prognosis. In the past 20 years, several drugs have become available for PAH treatment, including prostacyclin (prostaglandin I2), endothelin receptor antagonists (ERAs), phosphodiesterase type-5 inhibitors (PDE-5i), and a soluble guanylate cyclase stimulator. However, their full therapeutic effects are hampered by nonadherence and side effects, and PAH is still an aggressive and fatal disorder for many patients (Nakamura et al. 2017).
Several novel therapeutic strategies for PAH, including drug/gene delivery systems, have been proposed and tested with a view to optimizing efficacy and minimizing systemic adverse effects. In animal models of PAH, prostacyclin analogs (Ishihara et al. 2015; Akagi et al. 2016), imatinib (Akagi et al. 2015), pitavastatin (Chen et al. 2011), fasudil (Gupta et al. 2013), nitric oxide (NO)-based therapies and nitroglycerin (Ardekani et al. 2015; Mohamed et al. 2016), an NF-κB decoy (Kimura et al. 2009), and antimiRNA-145-incorporated NPs (McLendon et al. 2015) have been tested and proven to be effective.
A formulation of beraprost (a prostacyclin analog) incorporated with PLGA NPs was administered intratracheally in Sugen-hypoxia-normoxia and monocrotaline rat models of PAH and found to induce less vascular remodeling and cardiac hypertrophy and increased survival with no evident adverse effects (Akagi et al. 2016). In parallel, Ishihara et al. (2015) showed that intravenous administration of beraprost encapsulated in NPs derived from polylactide (PLA) and PEG-polylactide was associated with improvement in lung vascular remodeling and in cor pulmonale.
PLGA-based NPs incorporated with imatinib, a tyrosine kinase inhibitor, showed antiproliferative effects and inhibited disease development when administered intratracheally in a rat model of monocrotaline-induced PAH (Akagi et al. 2015). Chen et al. (2011) investigated the impact of intratracheal administration of pitavastatin-incorporated PLGA NPs in rats and found that this therapy was able to reduce inflammation, remodeling, and disease progression and improve survival rates in the same model.
Administration of intratracheal liposomal fasudil, a Rho kinase inhibitor, was effective in reducing lung arterial pressure by reducing vascular remodeling in the monocrotaline PAH model (Gupta et al. 2013).
Several groups have attempted to use inhaled NO gas in humans with both acute and chronic PAH. However, the effect of NO gas is limited by its short half-life and by the metabolism of cGMP by phosphodiesterases. Mohamed et al. (2016) developed and performed extensive preclinical testing of a new NO-nanomedicine formulation based on hydrogel-like polymeric composite NO-releasing NPs (NO-RP). The NO-RP produced concentration-dependent relaxation of pulmonary arteries in mice with hypoxia-induced PAH, enhancing the therapeutic potential of NO therapy for PAH. In this same context, Ardekani et al. (2015) developed a nanoliposomal formulation of nitroglycerin that revealed superior therapeutic effects compared with nitroglycerin alone, preventing the excessive mitochondrial superoxide production associated with high doses of nitroglycerin.
Currently, therapies that interfere with gene expression of pathogenic genes have been tested. Kimura et al. (2009) reported that intratracheal instillation of NF-κB decoy oligonucleotide-NPs (PEG-PLGA) attenuated the development of MCT-induced PAH in rats. Moreover, treatment with NF-κB decoy NPs 3 weeks after MCT injection improved the survival rate compared with vehicle administration. Interference RNA-based therapies have been tested as potential strategies in several diseases. McLendon et al. (2015) studied the impact of intravenous liposomal NP delivery of an antisense oligonucleotide against microRNA-145 and reported it that improved Sugen-hypoxia-induced PAH in rats.
Nanotechnology in medical diagnostic techniques
In the past few decades, imaging has become a critical tool in the diagnosis of disease. Advances in magnetic resonance techniques and computed tomography have been remarkable, but nanotechnology promises sensitive and extremely accurate tools for in vitro and in vivo diagnostics far beyond the reach of today’s state-of-the-art equipment (Rangger et al. 2013; Ali et al. 2016).
As with any advance in diagnostics, the ultimate goal is to enable physicians to identify a disease as early as possible. Nanotechnology is expected to make diagnosis possible at the cellular and even subcellular levels (Digesu et al. 2016). For instance, involvement of the immune system in tumor progression is at the forefront of cancer research, and analysis of the tumor immune microenvironment has been shown to yield a wealth of crucial information for prognostic indicators (Cuccarese et al. 2017).
To reduce the toxicity generated by traditional contrast agents, these substances have been formulated onto nanocarriers, seeking to facilitate intratumor uptake and minimize nephrotoxicity, as demonstrated with gadolinium NPs for MRI and liposomal iodinated contrast agents for computed tomography (Kong et al. 2007; Key and Leary 2014). These improvements have the potential to greatly improve lung cancer diagnosis and staging, particularly given the suboptimal diagnostic accuracy and sensitivity of conventional scans, as well as the risk for nephrotoxicity associated with each intravenous dose of traditional iodinated contrast.
Nanocrystal fluorophores, known as quantum dots, have finally made the transition from pure demonstration experiments to real applications in imaging. Quantum dots exhibit strong light absorbance, bright fluorescence, and marked photostability, facilitating their application as a powerful imaging tool (Azzazy et al. 2007). Despite significant interest, clinical translation of quantum dots has been slow because of concerns over heavy metal toxicity. Clinical applicability does remain a possibility; recent studies showed no observable toxicity in a long-term murine model of exposure to a novel formulation of non-cadmium-based quantum dots (Lin et al. 2015).
Regarding additional tools for the diagnosis of pulmonary diseases, there is a need for efficient techniques to assess abnormalities in the peripheral regions of the lungs, e.g., for early diagnosis of pulmonary emphysema (Fain et al. 2006). This disease is one of the most common causes of death globally, and clinical evaluation of peripheral airspaces is difficult, because this region is not readily accessible for analysis. The current gold-standard diagnostic test for COPD is spirometry (Vestbo et al. 2013), which can detect airflow obstruction but has limited sensitivity to changes in the small airways. Thus, airspace dimension assessment by NPs has great potential, mainly because it is likely to be less time consuming, considerably cheaper, simpler to use, and easier to interpret (Löndahl et al. 2017).
Advantages of the inhalation route and its possible limitations
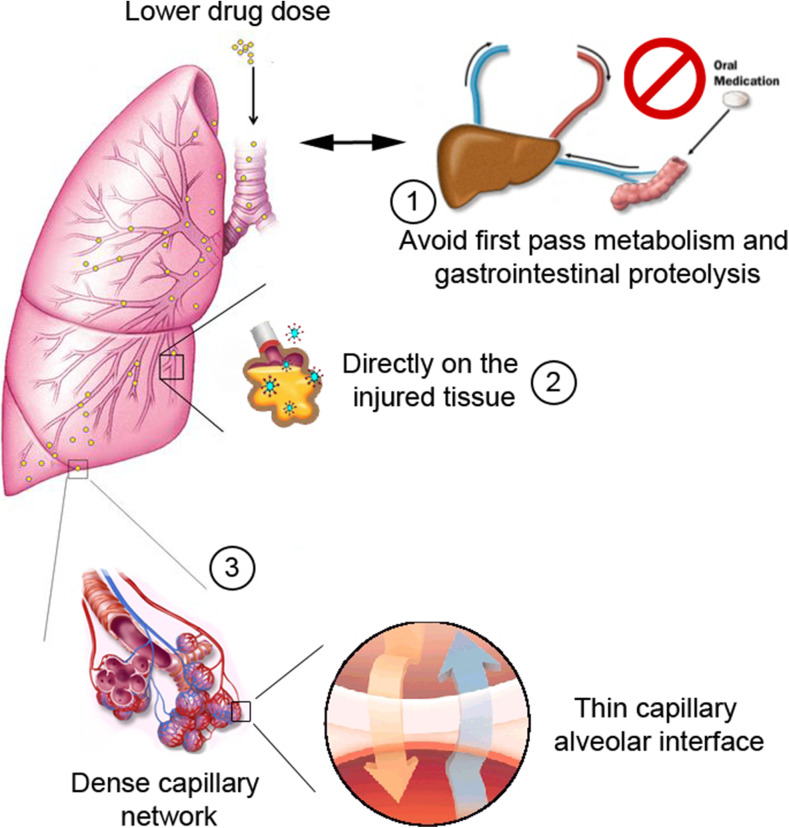
The lung, as an organ of gas exchange and dynamic portal of entry for airborne particles and pathogens, is an established route for delivery of therapeutic medications to treat pulmonary and systemic diseases (Paranjpe and Müller-Goymann 2014). In contrast to systemic administration, targeted drug delivery to the lung takes advantage of the extremely large alveolar surface area, dense capillary network, and very thin barrier for efficient drug absorption while avoiding gastrointestinal proteolysis and hepatic first-pass metabolism (Fig. 2) (Kuzmov and Minko 2015). Thus, to enhance the efficacy of treatment of various lung diseases and limit the exposure of healthy organs to potentially toxic drugs, it seems natural to deliver therapeutics directly to the lungs by inhalation.
Fig. 2.
Advantages of the inhalation route. The nanoparticle inhalation route has many advantages inherent in the physiology of the respiratory system: (1) avoids the first-pass metabolism effect as well as gastrointestinal proteolysis; (2) enables direct action on injured tissue; (3) makes it easier to reach the circulatory system because of the presence of the dense capillary network and its thin interface, contributing to lower drug doses
Despite these attractive advantages, the possible high lung toxicity of drugs, their degradation by lung macrophages, and the risk of drug-induced lung injury limit enthusiasm for inhalation as a route for systemic drug delivery (Iyer et al. 2015). Therefore, an ideal drug formulation and inhalation delivery method should be developed. To address this concern, recent advances in nanotechnology create an avenue to enhance the efficacy of inhalational treatments for different lung diseases. The application of nanotechnology to the design of drug delivery systems for effective delivery of therapeutics specifically to the tissues and cells affected by a disease allows for enhanced treatment outcomes and prevention of severe adverse side effects on tissues and cells, including those in the lungs, as well as entire organs.
Nanocarriers can support several aspects of pulmonary drug delivery, e.g., to achieve therapeutic effects in the lung at a lower drug dose (Pandey et al. 2003; Ahmad et al. 2005), to enhance delivery of hydrophobic molecules (Letsou et al. 1999; Wang et al. 1999; Naikwade et al. 2009), and to protect compounds against degradation (Sharma et al. 2013; Loira-Pastoriza et al. 2014) and from pulmonary clearance mechanisms (Suk et al. 2009; Salmaso and Caliceti 2013).
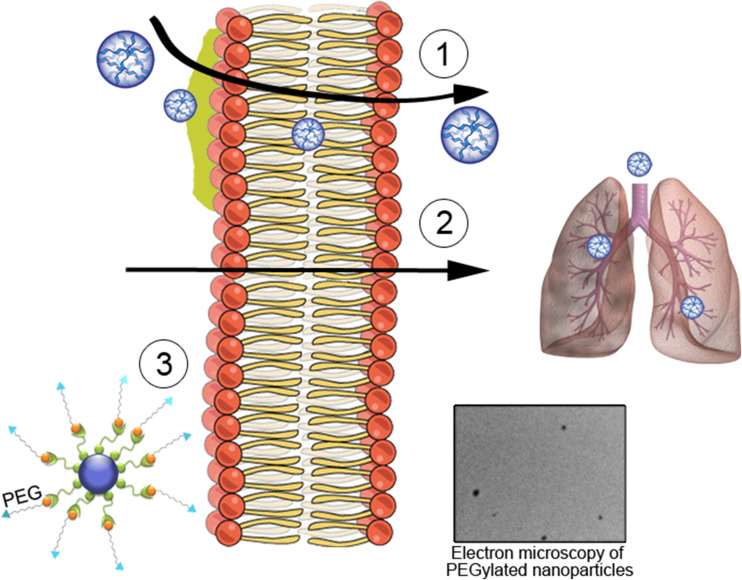
However, despite the potential advantages of inhaled nanocarriers, the unique microenvironment of the distal lung imposes some obstacles to their optimal delivery and therapeutic action. To prevent NP deposition in the lung, particle size, shape, surface charge, and surface properties should be optimized (Desai 2012; Dwivedi et al. 2014). This is particularly important for the delivery of proteins; a variety of approaches, including chemical modification and the addition of excipients such as buffers, sugars, and amino acids, may be used to improve protein stability and, thus, maintain the integrity and bioactivity of the compound (Depreter et al. 2013). In addition to the possibility of immunologic activation in vivo, including mucociliary clearance and activation of complement, alveolar macrophages, and mast cells, the recently escalating use of NPs has raised concerns regarding their potential toxicity. Many strategies have been used to mitigate these effects, such as incorporating metal oxide-based antioxidants and changing chemical structure through acylation, PEGylation, or addition of macromolecules (e.g., heparin) (Fig. 3) (Boylan et al. 2012b; Desai 2012).
Fig. 3.
Advances in nanotechnology. Some developments have been made to improve the performance of nanoparticles in the treatment of respiratory diseases, such as: (1) the development of polymers with faster mucus penetration and do not remain stuck, overcoming this barrier; (2) the creation of biodegradable nanoparticles with the stability to overcome the cell membrane and act in the lung with minimal levels of toxicity, causing no lesions during treatment; (3) modification of the chemical structure of nanoparticles by adding, e.g., polyethylene glycol (PEG), in order to increase solubility as a result of PEG hydrophilicity and decrease accessibility for proteolytic enzymes and antibodies. The electron micrograph shows an example of PEGylated nanoparticles, demonstrating that there is no significant aggregation between nanoparticles
Clinical trials of nanotherapy
Despite several preclinical studies showing promising effects, few clinical trials have been conducted to test the potential of nanotherapy in lung diseases.
A phase I, investigator-initiated clinical trial to test the efficacy of PLGA NP-mediated delivery of pitavastatin (UMIN000014940) has recently been completed (Nakamura et al. 2017). Future clinical trials may prove the safety and efficacy of a nano-drug delivery system for PAH.
Currently, two liposomal products in the last stage of clinical development are the dry-powder liposomes amikacin (Arikace, Insmed, Monmouth Junction, NJ, USA) and ciprofloxacin (Pulmaquin, Aradigm Corp., Hayward, CA, USA) for the treatment of lung infections. Amikacin, a liposomal preparation consisting of dipalmitoyl-phosphatidylcholine and cholesterol, is undergoing a phase II trial for the treatment of CF-associated lung infection with P. aeruginosa. In a recent double-blind randomized clinical trial of 105 patients with CF with P. aeruginosa infection, the authors found satisfactory safety and tolerability, improved lung functions, and decreased density of P. aeruginosa in the sputum of patients treated with the amikacin preparation (Paranjpe and Müller-Goymann 2014).
Despite the survival advantage seen with adjuvant chemotherapy in patients with advanced lung cancer, the overall benefits of systemic treatment in early-stage lung cancer are often outweighed by systemic side effects of chemotherapeutic agents. Currently, there are 27 clinical trials registered in ClinicalTrials.gov (https://clinicaltrials.gov) testing the effect of nanotherapy in lung diseases. Several NP-based gene/drug delivery platforms have been described to improve tissue-specific delivery, decrease toxic side effects, increase bioavailability, and prevent drug resistance secondary to enhanced cellular efflux in the treatment of non-small cell lung cancer (Lee et al. 2016).
Conclusions
This review summarizes the potential beneficial effects of nanoparticle (NP) drug and gene therapy in lung diseases, including asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), silicosis, and pulmonary artery hypertension (PAH). Nanotechnology has generated much enthusiasm as a potentially beneficial approach for the treatment and diagnosis of lung diseases. Preclinical studies using NPs demonstrated promising advancements, and current trials show that many NPs are safe for administration, with few adverse effects. Nevertheless, substantial challenges still have to be overcome before nanotherapeutic approaches can be used in clinical practice. Further studies focusing on understanding the mechanisms of action of NPs and improving their chemical structure are warranted to continue the development of rational approaches for clinical trials.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests. This study was supported by the Brazilian Council for Scientific and Technological Development (CNPq), the Rio de Janeiro State Research Foundation (FAPERJ), the Coordination for the Improvement of Higher Education Personnel (CAPES), and the Department of Science and Technology, Brazilian Ministry of Health (DECIT/MS).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
The original version of this article was revised: The original version of this article unfortunately contains an error. The third author’s name “Patricia Rieken Macedo Rocco” was incorrectly spelled with “Roccco”. The correct author name is now presented in the authorgroup.
This article is part of a Special Issue on ‘Latin America’ edited by Pietro Ciancaglini and Rosangela Itri.
Change history
10/6/2017
The original version of this article unfortunately contains an error. The third author’s name “Patricia Rieken Macedo Rocco” was incorrectly spelled with “Roccco”. The correct author name is now presented in the authorgroup.
References
- Ahmad Z, Sharma S, Khuller GK. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int J Antimicrob Agents. 2005;26(4):298–303. doi: 10.1016/j.ijantimicag.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Akagi S, Nakamura K, Matsubara H, et al. Intratracheal administration of prostacyclin analogue-incorporated nanoparticles ameliorates the development of monocrotaline and sugen-hypoxia-induced pulmonary arterial hypertension. J Cardiovasc Pharmacol. 2016;67(4):290–298. doi: 10.1097/FJC.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi S, Nakamura K, Miura D, et al. Delivery of imatinib-incorporated nanoparticles into lungs suppresses the development of monocrotaline-induced pulmonary arterial hypertension. Int Heart J. 2015;56(3):354–359. doi: 10.1536/ihj.14-338. [DOI] [PubMed] [Google Scholar]
- Al Faraj A, Shaik AS, Afzal S, et al. MR imaging and targeting of a specific alveolar macrophage subpopulation in LPS-induced COPD animal model using antibody-conjugated magnetic nanoparticles. Int J Nanomedicine. 2014;9:1491–1503. doi: 10.2147/IJN.S59394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali W, Moghaddam FJ, Raza MU, et al. Electromechanical transducer for rapid detection, discrimination and quantification of lung cancer cells. Nanotechnology. 2016;27(19):195101. doi: 10.1088/0957-4484/27/19/195101. [DOI] [PubMed] [Google Scholar]
- Alton EW, Armstrong DK, Ashby D, et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015;3(9):684–691. doi: 10.1016/S2213-2600(15)00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirfazli A. Nanomedicine: magnetic nanoparticles hit the target. Nat Nanotechnol. 2007;2(8):467–468. doi: 10.1038/nnano.2007.234. [DOI] [PubMed] [Google Scholar]
- Anselmo AC, Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release. 2014;190:15–28. doi: 10.1016/j.jconrel.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani S, Scott HA, Gupta S, et al. Nanoliposomal nitroglycerin exerts potent anti-inflammatory effects. Sci Rep. 2015;5:16258. doi: 10.1038/srep16258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzazy HM, Mansour MM, Kazmierczak SC. From diagnostics to therapy: prospects of quantum dots. Clin Biochem. 2007;40(13–14):917–927. doi: 10.1016/j.clinbiochem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Severe asthma: advances in current management and future therapy. J Allergy Clin Immunol. 2012;129(1):48–59. doi: 10.1016/j.jaci.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Bhavna, Ahmad FJ, Mittal G, et al. Nano-salbutamol dry powder inhalation: a new approach for treating broncho-constrictive conditions. Eur J Pharm Biopharm. 2009;71(2):282–291. doi: 10.1016/j.ejpb.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Bivas-Benita M, Lin MY, Bal SM, et al. Pulmonary delivery of DNA encoding mycobacterium tuberculosis latency antigen Rv1733c associated to PLGA-PEI nanoparticles enhances T cell responses in a DNA prime/protein boost vaccination regimen in mice. Vaccine. 2009;27(30):4010–4017. doi: 10.1016/j.vaccine.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Boylan NJ, Kim AJ, Suk JS, et al. Enhancement of airway gene transfer by DNA nanoparticles using a pH-responsive block copolymer of polyethylene glycol and poly-L-lysine. Biomaterials. 2012;33(7):2361–2371. doi: 10.1016/j.biomaterials.2011.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan NJ, Suk JS, Lai SK, et al. Highly compacted DNA nanoparticles with low MW PEG coatings: in vitro, ex vivo and in vivo evaluation. J Control Release. 2012;157(1):72–79. doi: 10.1016/j.jconrel.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner JS, Bhamidipati K, Glassman PM, et al. Mechanisms that determine nanocarrier targeting to healthy versus inflamed lung regions. Nanomedicine. 2017;13(4):1495–1506. doi: 10.1016/j.nano.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplen NJ, Alton EW, Middleton PG, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1(1):39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- Chen HW, Su SF, Chien CT, et al. Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. FASEB J. 2006;20(13):2393–2395. doi: 10.1096/fj.06-6485fje. [DOI] [PubMed] [Google Scholar]
- Chen L, Nakano K, Kimura S, et al. Nanoparticle-mediated delivery of pitavastatin into lungs ameliorates the development and induces regression of monocrotaline-induced pulmonary artery hypertension. Hypertension. 2011;57(2):343–350. doi: 10.1161/HYPERTENSIONAHA.110.157032. [DOI] [PubMed] [Google Scholar]
- Chen X, Huang W, Wong BC, et al. Liposomes prolong the therapeutic effect of anti-asthmatic medication via pulmonary delivery. Int J Nanomedicine. 2012;7:1139–1148. doi: 10.2147/IJN.S28011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper V, Metcalf L, Versnel J, et al. Patient-reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side-effect prevalence: a UK-wide, cross-sectional study. NPJ Prim Care Respir Med. 2015;25:15026. doi: 10.1038/npjpcrm.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuccarese MF, Dubach JM, Pfirschke C, et al. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat Commun. 2017;8:14293. doi: 10.1038/ncomms14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva AL, Martini SV, Abreu SC, et al. DNA nanoparticle-mediated thymulin gene therapy prevents airway remodeling in experimental allergic asthma. J Control Release. 2014;180:125–133. doi: 10.1016/j.jconrel.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva AL, Santos RS, Xisto DG, et al. Nanoparticle-based therapy for respiratory diseases. An Acad Bras Cienc. 2013;85(1):137–146. doi: 10.1590/s0001-37652013005000018. [DOI] [PubMed] [Google Scholar]
- Depreter F, Pilcer G, Amighi K. Inhaled proteins: challenges and perspectives. Int J Pharm. 2013;447(1–2):251–280. doi: 10.1016/j.ijpharm.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14(2):282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gioia S, Trapani A, Castellani S, et al. Nanocomplexes for gene therapy of respiratory diseases: targeting and overcoming the mucus barrier. Pulm Pharmacol Ther. 2015;34:8–24. doi: 10.1016/j.pupt.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Digesu CS, Hofferberth SC, Grinstaff MW, Colson YL. From diagnosis to treatment: clinical applications of nanotechnology in thoracic surgery. Thorac Surg Clin. 2016;26(2):215–228. doi: 10.1016/j.thorsurg.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi MV, Harishchandra RK, Koshkina O, et al. Size influences the effect of hydrophobic nanoparticles on lung surfactant model systems. Biophys J. 2014;106(1):289–298. doi: 10.1016/j.bpj.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich DF, Snodgrass P, Lafreniere D, et al. Sustained release chemotherapeutic microspheres provide superior efficacy over systemic therapy and local bolus infusions. Pharm Res. 2002;19(7):1052–1060. doi: 10.1023/a:1016434926649. [DOI] [PubMed] [Google Scholar]
- Fain SB, Panth SR, Evans MD, et al. Early emphysematous changes in asymptomatic smokers: detection with 3He MR imaging. Radiology. 2006;239(3):875–883. doi: 10.1148/radiol.2393050111. [DOI] [PubMed] [Google Scholar]
- Geiser M, Quaile O, Wenk A, et al. Cellular uptake and localization of inhaled gold nanoparticles in lungs of mice with chronic obstructive pulmonary disease. Part Fibre Toxicol. 2013;10:19. doi: 10.1186/1743-8977-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) (2017) Global strategy for the diagnosis, management, and prevention of COPD
- González-García I, Solé RV, Costa J. Metapopulation dynamics and spatial heterogeneity in cancer. Proc Natl Acad Sci U S A. 2002;99(20):13085–13089. doi: 10.1073/pnas.202139299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier PA, Fetita CI, Brillet PY. Quantitative computed tomography imaging of airway remodeling in severe asthma. Quant Imaging Med Surg. 2016;6(1):76–83. doi: 10.3978/j.issn.2223-4292.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günday Türeli N, Türeli AE, Schneider M. Optimization of ciprofloxacin complex loaded PLGA nanoparticles for pulmonary treatment of cystic fibrosis infections: design of experiments approach. Int J Pharm. 2016;515(1–2):343–351. doi: 10.1016/j.ijpharm.2016.10.025. [DOI] [PubMed] [Google Scholar]
- Gupta P, Vermani K, Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today. 2002;7(10):569–579. doi: 10.1016/s1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- Gupta V, Gupta N, Shaik IH, et al. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J Control Release. 2013;167(2):189–199. doi: 10.1016/j.jconrel.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijerman H, Westerman E, Conway S, et al. Inhaled medication and inhalation devices for lung disease in patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2009;8(5):295–315. doi: 10.1016/j.jcf.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Holloway JW, Yang IA, Holgate ST. Genetics of allergic disease. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S81–S94. doi: 10.1016/j.jaci.2009.10.071. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Hayashi E, Yamamoto S, et al. Encapsulation of beraprost sodium in nanoparticles: analysis of sustained release properties, targeting abilities and pharmacological activities in animal models of pulmonary arterial hypertension. J Control Release. 2015;197:97–104. doi: 10.1016/j.jconrel.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Iyer R, Hsia CC, Nguyen KT. Nano-therapeutics for the lung: state-of-the-art and future perspectives. Curr Pharm Des. 2015;21(36):5233–5244. doi: 10.2174/1381612821666150923095742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JK, Zhang X, Llewellen S, Hunter WL, Burt HM. The characterization of novel polymeric paste formulations for intratumoral delivery. Int J Pharm. 2004;270(1–2):185–198. doi: 10.1016/j.ijpharm.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Ju J, Li R, Gu S, et al. Impact of emphysema heterogeneity on pulmonary function. PLoS One. 2014;9(11):e113320. doi: 10.1371/journal.pone.0113320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon NJ, Bratt JM, Lee J, et al. Self-assembling nanoparticles containing dexamethasone as a novel therapy in allergic airways inflammation. PLoS One. 2013;8(10):e77730. doi: 10.1371/journal.pone.0077730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J, Leary JF. Nanoparticles for multimodal in vivo imaging in nanomedicine. Int J Nanomedicine. 2014;9:711–726. doi: 10.2147/IJN.S53717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ye YM, Lee HY, Sin HJ, Park HS. Combined pharmacogenetic effect of ADCY9 and ADRB2 gene polymorphisms on the bronchodilator response to inhaled combination therapy. J Clin Pharm Ther. 2011;36(3):399–405. doi: 10.1111/j.1365-2710.2010.01196.x. [DOI] [PubMed] [Google Scholar]
- Kimura S, Egashira K, Chen L, et al. Nanoparticle-mediated delivery of nuclear factor kappaB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension. 2009;53(5):877–883. doi: 10.1161/HYPERTENSIONAHA.108.121418. [DOI] [PubMed] [Google Scholar]
- Kolte A, Patil S, Lesimple P, Hanrahan JW, Misra A. PEGylated composite nanoparticles of PLGA and polyethylenimine for safe and efficient delivery of pDNA to lungs. Int J Pharm. 2017;524(1–2):382–396. doi: 10.1016/j.ijpharm.2017.03.094. [DOI] [PubMed] [Google Scholar]
- Kong WH, Lee WJ, Cui ZY, et al. Nanoparticulate carrier containing water-insoluble iodinated oil as a multifunctional contrast agent for computed tomography imaging. Biomaterials. 2007;28(36):5555–5561. doi: 10.1016/j.biomaterials.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Kong X, Hellermann GR, Zhang W, et al. Chitosan interferon-gamma nanogene therapy for lung disease: modulation of T-cell and dendritic cell immune responses. Allergy Asthma Clin Immunol. 2008;4(3):95–105. doi: 10.1186/1710-1492-4-3-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köping-Höggård M, Tubulekas I, Guan H, et al. Chitosan as a nonviral gene delivery system. Structure–property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Ther. 2001;8(14):1108–1121. doi: 10.1038/sj.gt.3301492. [DOI] [PubMed] [Google Scholar]
- Kumar M, Kong X, Behera AK, et al. Chitosan IFN-gamma-pDNA nanoparticle (CIN) therapy for allergic asthma. Genet Vaccines Ther. 2003;1(1):3. doi: 10.1186/1479-0556-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmov A, Minko T. Nanotechnology approaches for inhalation treatment of lung diseases. J Control Release. 2015;219:500–518. doi: 10.1016/j.jconrel.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Lawani MA, Zongo F, Breton MC et al (2017) Factors associated with adherence to asthma treatment with inhaled corticosteroids: a cross-sectional exploratory study. J Asthma (in press) [DOI] [PubMed]
- Lee HY, Mohammed KA, Nasreen N. Nanoparticle-based targeted gene therapy for lung cancer. Am J Cancer Res. 2016;6(5):1118–1134. [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Thompson DH (2017) Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol 9(5) [DOI] [PMC free article] [PubMed]
- Letsou GV, Safi HJ, Reardon MJ, et al. Pharmacokinetics of liposomal aerosolized cyclosporine a for pulmonary immunosuppression. Ann Thorac Surg. 1999;68(6):2044–2048. doi: 10.1016/s0003-4975(99)01183-2. [DOI] [PubMed] [Google Scholar]
- Lin G, Ouyang Q, Hu R, et al. In vivo toxicity assessment of non-cadmium quantum dots in BALB/c mice. Nanomedicine. 2015;11(2):341–350. doi: 10.1016/j.nano.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Loira-Pastoriza C, Todoroff J, Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014;75:81–91. doi: 10.1016/j.addr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Löndahl J, Jakobsson JK, Broday DM, Aaltonen HL, Wollmer P. Do nanoparticles provide a new opportunity for diagnosis of distal airspace disease? Int J Nanomedicine. 2017;12:41–51. doi: 10.2147/IJN.S121369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Pacheco M, Bandeira E, Morales MM. Cell-based therapy for silicosis. Stem Cells Int. 2016;2016:5091838. doi: 10.1155/2016/5091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall MA, Galietta LJ. Targeting ion channels in cystic fibrosis. J Cyst Fibros. 2015;14(5):561–570. doi: 10.1016/j.jcf.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Manunta MD, Tagalakis AD, Attwood M, et al. Delivery of ENaC siRNA to epithelial cells mediated by a targeted nanocomplex: a therapeutic strategy for cystic fibrosis. Sci Rep. 2017;7(1):700. doi: 10.1038/s41598-017-00662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos P, da Silva AL, Chisholm J, et al. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc Natl Acad Sci U S A. 2015;112(28):8720–8725. doi: 10.1073/pnas.1502281112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo Y, Ishihara T, Ishizaki J, et al. Effect of betamethasone phosphate loaded polymeric nanoparticles on a murine asthma model. Cell Immunol. 2009;260(1):33–38. doi: 10.1016/j.cellimm.2009.07.004. [DOI] [PubMed] [Google Scholar]
- McLendon JM, Joshi SR, Sparks J, et al. Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension. J Control Release. 2015;210:67–75. doi: 10.1016/j.jconrel.2015.05.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdan T, Callahan J, Petersen H, et al. Pegylated polyethylenimine-Fab′ antibody fragment conjugates for targeted gene delivery to human ovarian carcinoma cells. Bioconjug Chem. 2003;14(5):989–996. doi: 10.1021/bc0340767. [DOI] [PubMed] [Google Scholar]
- Merkel OM, Beyerle A, Beckmann BM, et al. Polymer-related off-target effects in non-viral siRNA delivery. Biomaterials. 2011;32(9):2388–2398. doi: 10.1016/j.biomaterials.2010.11.081. [DOI] [PubMed] [Google Scholar]
- Merkel OM, Urbanics R, Bedocs P, et al. In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine-graft-poly(ethylene glycol) block copolymers. Biomaterials. 2011;32(21):4936–4942. doi: 10.1016/j.biomaterials.2011.03.035. [DOI] [PubMed] [Google Scholar]
- Mohamed NA, Ahmetaj-Shala B, Duluc L, et al. A new NO-releasing nanoformulation for the treatment of pulmonary arterial hypertension. J Cardiovasc Transl Res. 2016;9(2):162–164. doi: 10.1007/s12265-016-9684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Marquis S, Stanton BA, O’Toole GA. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm Pharmacol Ther. 2008;21(4):595–599. doi: 10.1016/j.pupt.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Sastre M, Pastor M, Esquisabel A, et al. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int J Pharm. 2016;498(1–2):263–273. doi: 10.1016/j.ijpharm.2015.12.028. [DOI] [PubMed] [Google Scholar]
- Muralidharan P, Hayes D, Jr, Black SM, Mansour HM. Microparticulate/nanoparticulate powders of a novel Nrf2 activator and an aerosol performance enhancer for pulmonary delivery targeting the lung Nrf2/Keap-1 pathway. Mol Syst Des Eng. 2016;1(1):48–65. doi: 10.1039/C5ME00004A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutat Res. 2010;690(1–2):24–39. doi: 10.1016/j.mrfmmm.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naikwade SR, Bajaj AN, Gurav P, Gatne MM, Singh Soni P. Development of budesonide microparticles using spray-drying technology for pulmonary administration: design, characterization, in vitro evaluation, and in vivo efficacy study. AAPS PharmSciTech. 2009;10(3):993–1012. doi: 10.1208/s12249-009-9290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsubara H, Akagi S et al (2017) Nanoparticle-mediated drug delivery system for pulmonary arterial hypertension. J Clin Med 6(5) [DOI] [PMC free article] [PubMed]
- Nelsen LM, Kimel M, Murray LT, et al. Qualitative evaluation of the St George’s respiratory questionnaire in patients with severe asthma. Respir Med. 2017;126:32–38. doi: 10.1016/j.rmed.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Nguyen MM, Carlini AS, Chien MP, et al. Enzyme-responsive nanoparticles for targeted accumulation and prolonged retention in heart tissue after myocardial infarction. Adv Mater. 2015;27(37):5547–5552. doi: 10.1002/adma.201502003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R, Sharma A, Zahoor A, Sharma S, Khuller GK, Prasad B. Poly (DL-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J Antimicrob Chemother. 2003;52(6):981–986. doi: 10.1093/jac/dkg477. [DOI] [PubMed] [Google Scholar]
- Paranjpe M, Müller-Goymann CC. Nanoparticle-mediated pulmonary drug delivery: a review. Int J Mol Sci. 2014;15(4):5852–5873. doi: 10.3390/ijms15045852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116(3):477–486. doi: 10.1016/j.jaci.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Patil-Gadhe, Kyadarkunte, Patole, Pokharkar et al (2014) Montelukast-loaded nanostructured lipid carriers: part II pulmonary drug delivery and in vitro-in vivo aerosol performance. Eur J Pharm Biopharm 88(1):169–77 [DOI] [PubMed]
- Patil-Gadhe, Pokharkar (2014) Montelukast-loaded nanostructured lipid carriers: part I oral bioavailability improvement. Eur J Pharm Biopharm 88(1):160–8 [DOI] [PubMed]
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139–1151. doi: 10.1016/j.rmed.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Raissy HH, Kelly HW, Harkins M, Szefler SJ. Inhaled corticosteroids in lung diseases. Am J Respir Crit Care Med. 2013;187(8):798–803. doi: 10.1164/rccm.201210-1853PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangger C, Helbok A, Sosabowski J, et al. Tumor targeting and imaging with dual-peptide conjugated multifunctional liposomal nanoparticles. Int J Nanomedicine. 2013;8:4659–4671. doi: 10.2147/IJN.S51927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratjen F, Brockhaus F, Angyalosi G. Aminoglycoside therapy against Pseudomonas aeruginosa in cystic fibrosis: a review. J Cyst Fibros. 2009;8(6):361–369. doi: 10.1016/j.jcf.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Roulet A, Armand L, Dagouassat M, et al. Intratracheally administered titanium dioxide or carbon black nanoparticles do not aggravate elastase-induced pulmonary emphysema in rats. BMC Pulm Med. 2012;12:38. doi: 10.1186/1471-2466-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph C, Lausier J, Naundorf S, Müller RH, Rosenecker J. In vivo gene delivery to the lung using polyethylenimine and fractured polyamidoamine dendrimers. J Gene Med. 2000;2(4):269–278. doi: 10.1002/1521-2254(200007/08)2:4<269::AID-JGM112>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Sadeghi L, Yousefi Babadi V, Espanani HR. Toxic effects of the Fe2O3 nanoparticles on the liver and lung tissue. Bratisl Lek Listy. 2015;116(6):373–378. doi: 10.4149/bll_2015_071. [DOI] [PubMed] [Google Scholar]
- Salem AK. A promising CpG adjuvant-loaded nanoparticle-based vaccine for treatment of dust mite allergies. Immunotherapy. 2014;6(11):1161–1163. doi: 10.2217/imt.14.97. [DOI] [PubMed] [Google Scholar]
- Salmaso S, Caliceti P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. J Drug Deliv. 2013;2013:374252. doi: 10.1155/2013/374252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders N, Rudolph C, Braeckmans K, De Smedt SC, Demeester J. Extracellular barriers in respiratory gene therapy. Adv Drug Deliv Rev. 2009;61(2):115–127. doi: 10.1016/j.addr.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CS, Xu Q, Boylan NJ, et al. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci Adv. 2017;3(4):e1601556. doi: 10.1126/sciadv.1601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Somavarapu S, Colombani A, Govind N, Taylor KM. Nebulised siRNA encapsulated crosslinked chitosan nanoparticles for pulmonary delivery. Int J Pharm. 2013;455(1–2):241–247. doi: 10.1016/j.ijpharm.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Hirahara K, Nakayama T. Maintenance of pathogenic Th2 cells in allergic disorders. Allergol Int. 2017;66(3):369–376. doi: 10.1016/j.alit.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Silva LH, da Silva JR, Ferreira GA, et al. Labeling mesenchymal cells with DMSA-coated gold and iron oxide nanoparticles: assessment of biocompatibility and potential applications. J Nanobiotechnol. 2016;14(1):59. doi: 10.1186/s12951-016-0213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocke NA, Arnold SM, Hilt JZ. Responsive hydrogel nanoparticles for pulmonary delivery. J Drug Deliv Sci Technol. 2015;29:143–151. doi: 10.1016/j.jddst.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk JS, Lai SK, Wang YY, et al. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials. 2009;30(13):2591–2597. doi: 10.1016/j.biomaterials.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MJ, Werfel TA, Crews BC, et al. Fluorocoxib A loaded nanoparticles enable targeted visualization of cyclooxygenase-2 in inflammation and cancer. Biomaterials. 2016;92:71–80. doi: 10.1016/j.biomaterials.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijt SH, Bein T, Meiners S. Medical nanoparticles for next generation drug delivery to the lungs. Eur Respir J. 2014;44(3):765–774. doi: 10.1183/09031936.00212813. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- Vij N. Nano-based theranostics for chronic obstructive lung diseases: challenges and therapeutic potential. Expert Opin Drug Deliv. 2011;8(9):1105–1109. doi: 10.1517/17425247.2011.597381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker M. Nanocarriers for intravenous injection—the long hard road to the market. Int J Pharm. 2013;457(1):50–62. doi: 10.1016/j.ijpharm.2013.08.079. [DOI] [PubMed] [Google Scholar]
- Wahajuddin. Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–3471. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ben-Jebria A, Edwards DA. Inhalation of estradiol for sustained systemic delivery. J Aerosol Med. 1999;12(1):27–36. doi: 10.1089/jam.1999.12.27. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen Y, Chen B, et al. Pharmacokinetic parameters and tissue distribution of magnetic Fe(3)O(4) nanoparticles in mice. Int J Nanomedicine. 2010;5:861–866. doi: 10.2147/IJN.S13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhu R, Xie Q, et al. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomedicine. 2012;7:3667–3677. doi: 10.2147/IJN.S30428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Huang J, Li X, Sun S, Chen X. Iron oxide nanoparticle platform for biomedical applications. Curr Med Chem. 2009;16(10):1278–1294. doi: 10.2174/092986709787846604. [DOI] [PubMed] [Google Scholar]