Abstract
Pregnancy-related acute kidney injury (AKI) has declined in incidence in the last three decades, although it remains an important cause of maternal and fetal morbidity and mortality. Pregnancy-related causes of AKI such as preeclampsia, acute fatty liver of pregnancy, HELLP syndrome, and the thrombotic microangiopathies (thrombotic thrombocytopenic purpura, atypical hemolytic uremic syndrome) exhibit overlapping features and often present a diagnostic conundrum. Differentiating among these conditions may be difficult or impossible based on clinical criteria only. In difficult and rare cases, a renal biopsy may need to be considered for the exact diagnosis and facilitate appropriate treatment, but the risks and benefits will need to be carefully weighed. The use of eculizumab for the treatment of atypical HUS has demonstrated efficacy in early case reports. Non-pregnancy related causes such as volume depletion and pyelonephritis require early and aggressive resuscitative and antibiotic measures. We will discuss in this review the various etiologies of AKI in pregnancy, current diagnostic approaches, and the latest treatment strategies. Given the recent trends of increasing maternal age at the time of pregnancy, as well the availability of modern reproductive methods both of which may be associated with significant co-morbidities, the issues surrounding AKI in pregnancy may become more relevant in the coming years.
Keywords: Acute kidney injury, pregnancy, preeclampsia, HELLP, thrombotic microangiopathy
Acute Kidney Injury in Pregnancy
Pregnancy-related acute kidney injury (AKI) remains an important cause of maternal and fetal morbidity and mortality. Encouraging news from the last three decades has demonstrated a dramatic decrease in its incidence in developing countries; the data from developed countries are more nuanced. For example, in India, pregnancy-associated AKI requiring dialysis has decreased from 15% from 1982–1991 to 10% from 1992–2002, with a concurrent decrease in maternal mortality from 20% to 6.4%.1 These large decreases mostly are attributable to a reduction in sepsis associated with abortion and childbirth, as well as improved management of postpartum hemorrhage and placental abruption.2 An Italian study reported that in the developed world, the incidence of AKI in pregnancy fell from 1:3000 to 1:18000 births from the years 1956–1967 to 1988–1994.3 However, a recent report from Canada revealed an increasing incidence of pregnancy-related AKI, from 1.66 per 10000 deliveries between 2003–2004, to 2.68 per 10000 deliveries between 2009–2010. The reasons for this increase may be due to higher rates of hypertensive disorders of pregnancy, however, there may be other factors. Newer standardized methods of classification of AKI using the RIFLE and AKIN criteria (see below)4 may have led to better “coding” of this condition, and hence, a seemingly higher incidence. The absolute number of cases of AKI during pregnancy is still significantly lower than in developing countries. The prevalence of AKI in pregnancy of 10% in India, for example, remains unacceptably high and leaves much room for improvement of obstetric practices.
Renal Outcomes
Renal outcomes complicated by AKI are determined by cause, demographics and availability of health care resources. A recent Canadian report showed that AKI resulting in the need for dialysis occurred in 1 per 10000 pregnant women, where 4.3% of the women died compared to 0.01% of pregnant women with no kidney injury, and 3.9% remained on dialysis four months after delivery.5 The most common reasons for AKI in this study included preeclampsia, thrombotic microangiopathy (TMA), heart failure, sepsis or postpartum hemorrhage.5 A study from India, in contrast, showed that the mortality rate for pregnant women requiring dialysis was 18.3%, of which 9% remained on dialysis six months later,6 with the most common reason for AKI being post-abortion sepsis. A Chinese study, on the other hand, determined an incidence of 0.11% of pregnancy-related AKI, with no women requiring dialysis between the years 2004–2013. Thus, outcome data are quite variable throughout the world; prevention and close monitoring remain the mainstays in the prognoses of these women.
Diagnosis
The diagnosis of AKI in pregnancy has not been standardized either in practice or research. The definitions can range from an increase in the serum creatinine to the need for dialysis. This is further confounded by the physiologic decrease in serum creatinine seen in pregnancy. The frequently cited RIFLE (Risk, Injury, Failure, Loss and End stage)7 and the AKIN (Acute Kidney Injury Network) criteria8 for the non-pregnant population have not been well validated in pregnancy. More recent obstetric studies, however, have begun to use these classifications. For example, having a high RIFLE class predicted higher mortality in obstetric patients in an intensive care unit.9 Investigators from the Mayo Clinic, using the AKIN criteria, discovered that most of the patients belonged to the AKIN stage 1 category, with only transient increases in serum creatinine.10 In addition, these women with AKI tend to have comorbid conditions such as hypertension, diabetes or chronic kidney disease, and pregnancy-related complications such as, preeclampsia/HELLP (Hemolysis, Elevated Liver function tests, Low Platelets), hemorrhage or infections. Investigators will be better able to assess whether they can be used to risk-stratify obstetric patients as more studies utilize these criteria.
Differential Diagnosis
There are numerous etiologies for AKI in pregnancy, the majority is pregnancy-related, but some are not. It is useful to categorize the types of AKI into pre-renal, renal and post-renal etiologies, as in the non-pregnant population. For pre-renal causes, it is generally a hemodynamic disturbance that starts with a reversible reduction in glomerular filtration rate, leading to ischemic acute tubular damage and resulting in irreversible cortical necrosis in the most extreme cases. These insults include, but are not limited to, massive hemorrhage (especially in the postpartum period), adrenocortical failure (in patients treated with long-term steroid therapy), amniotic fluid embolism, acute fatty liver of pregnancy (AFLP), septic abortion, chorioamnionitis, pyelonephritis or puerperal sepsis (Table 1). Acute cortical necrosis (ACN), resulting from severe hypotension, is a pathologic diagnosis based on the presence of diffuse or patchy cortical necrosis on renal biopsy, with intravascular thromboses in the interlobular and afferent arterioles, while sparing the medulla.11 Highly morbid, it usually leads to permanent kidney failure. The reasons why ACN occur in association with pregnancy are unclear. One proposed mechanism is that pregnancy is a hypercoagulable state with increased levels of coagulation factors in the face of a repressed fibrinolytic state. If an additional trigger is present, then a series of complex and self-perpetuating vasomotor and humoral phenomena follow: systems such as the coagulation, renin-angiotensin and complement pathways will be activated.12 Renal recovery is unlikely to occur if these triggers are complicated by disseminated intravascular coagulation (DIC).12 The incidence of pregnancy-associated ACN, in addition to pregnancy-related AKI, however, is decreasing. An Indian study showed a decrease in incidence from 4.7% to 0.5% between the years 1984 and 2005.13 For pregnancy related intra-renal causes, etiologies include preeclampsia, AFLP and HELLP syndrome (Table 1). Conditions that potentially are precipitated and worsened by pregnancy include pyelonephritis, sepsis, lupus nephritis, and thrombotic thrombocytopenic purpura (TTP)/ atypical hemolytic uremic syndrome (HUS). Consideration of performing a renal biopsy should be given when the laboratory evaluation is non-diagnostic in order to facilitate appropriate treatment. Though the rates of renal biopsy complications during pregnancy are similar to those in non-pregnant women,14 it is recommended to biopsy only in the first and second trimesters, and best to avoid in the third,15 as the risks of the biopsy at that time likely outweighs establishing a diagnosis so late in pregnancy. It is also only recommended if a biopsy diagnosis will alter treatment modalities in pregnancy.
Table 1.
Causes of AKI in Pregnancy
Pre-renal
|
Intra-renal
|
Post-renal
|
HELLP, hemolysis, elevated liver function tests, low platelet count;
Timing of AKI
The timing of AKI during pregnancy may serve as an important clue as to the underlying etiology (Figure 1). In developing countries, AKI that occurs in the first trimester frequently is due to septic abortions or lupus nephritis. The vast majority of AKI occurring in the second and third trimesters is due to hypertensive complications such as, preeclampsia/HELLP, TTP/HUS, abruption placentae, severe hemorrhage or DIC, or AFLP. Atypical HUS, however, generally occurs late in the third trimester or postpartum. (Table 2). Making these diagnoses may be obvious, but many exhibit overlapping features, such as preeclampsia/HELLP, lupus nephritis, TTP/HUS and AFLP, rendering making the diagnosis more difficult.
Figure 1. Main causes of pregnancy-related AKI depending on their predominant liming of occurrence during pregnancy.
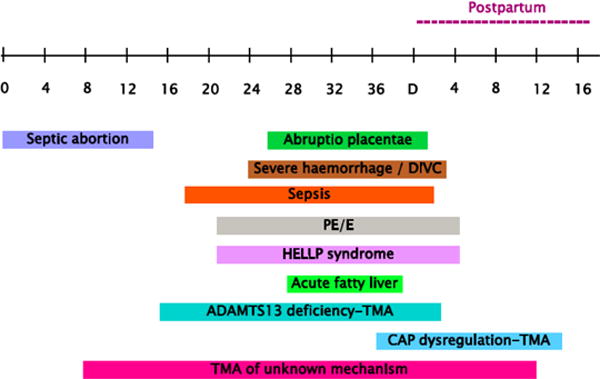
Borrowed Fakhouri F. Clin J Am Soc Nephrol. 2012 Dec; 7(12): 2100–6. doi: 10.2215/CJN.13121211. Epub 2012 Aug 9. Review
CAP, complement alternative pathway; DIVC, disseminated intravascular coagulation; PE/E, pre-eclampsia/eclampsia; TMA, thrombotic microangiopathy.
Table 2.
Signs and Symptoms of Overlapping Syndromes of Acute Kidney Injury in Pregnancy
| Preeclampsia/HELLP | TTP | aHUS | AFLP | APS | Lupus flare | |
|---|---|---|---|---|---|---|
| Timing in pregnancy | >20 weeks | Higher incidence in 2nd trimester | Higher in postpartum | Higher incidence in 3rd trimester | All gestational ages | All gestational ages |
| Blood pressure >140/90 mmHg | 3+ | 0 to 3+ | 2+ | 0 to 2+ | 0 to 3+ | 0 to 3+ |
| Neurologic involvement | 0 to 3+ | 2+ to 3+ | 0 to 1+ | 0 | 0 to 3+ | 0 to 3+ |
| Fever | 0 | 1+ to 3+ | 0 to 3+ | 0 | 2+ | 2+ |
| Schistocytes (more than 1%) | 0 to 2+ | 3+ | 2+ | 0 to 1+ | 2+ | 0 |
| Low platelet count (cells/ μL) | 0 to 3+ | 2+ to 3+ | 3+ | 1+ to 2+ | 2+ | 1+ to 2+ |
| Elevated liver enzymes | 0 to 3+ | 0 to 1+ | 0 to 1+ | 2+ to 3+ | 0 to 1+ | 0 |
| Proteinuria* | 1+ to 3+ | 1+ to 3+ | 1+ to 3+ | 1+ | 0 to 3+ | 1+ to 3+ |
| Low ADAMTS13 activity (<10%) | 0 to 1+ | 3+ | 1+ | 0 | 0 | 0 |
| Treatment | Delivery of fetus | Plasma exchange | Plasmapheresis/eculizumab | Delivery of fetus | Aspirin + anticoagulatio n | Immunos uppression |
ADAMTS13: A disintegrin and metalloprotease with a thrombospondin type 1 motif, member 13.
HELLP: hemolysis, elevated liver enzymes, low platelets
TTP: thrombotic thrombocytopenic purpura
AFLP: acute fatty liver of pregnancy
APS: anti-phospholipid syndrome
Proteinuria: defined as either >1+ on urinalysis or urine protein of more than 300 mg/24 hours or urine protein/creatinine ratio of > 0.3 g/g
Ratings: 0, unlikely or not present; 1+, mild or low likelihood; 2+, moderate or moderate likelihood; 3+, severe or high likelihood
Atypical Hemolytic-Uremic Syndrome in Pregnant Patients in the Age of Eculizumab
Ninety percent of HUS cases are associated with diarrhea, typically due to Shiga-like toxin producing Escherichia coli.16 The remaining 10% are classified as atypical HUS and are caused by the activation of the alternative complement pathway (ACP) that can be either familial (due to mutations in genes that code for proteins in the ACP pathway) or more commonly be sporadic (∼80% of cases). While pregnancy is one of several known triggers of abnormal complement activation leading to sporadic atypical HUS, a recent study suggested that up to 86% of these patients have an underlying mutation(s) in ACP genes; complement regulatory protein mutations in the genes encoding factor H, factor I, C3, membrane cofactor protein, or a combination of them have been described in pregnancy.17 As compared to the general population, patients with genetic complement abnormalities have worse pregnancy outcomes, i.e. higher incidence of fetal loss and preeclampsia. Overall though, women who develop pregnancy associated aHUS fared poorly with 76% of them developing ESRD by their last follow-up.
The interaction between complement activation and the clinical features of preeclampsia has been documented in pregnant mouse models of intrauterine growth restriction.18 Human studies that followed confirmed these associations by reporting complement abnormalities in 40% pregnancies complicated by the HELLP syndrome.19 Furthermore, mutations in genes that encode for complement regulatory proteins have been shown to increase the risk for preeclampsia in pregnant women with systemic lupus and those with antiphospholipid antibodies.20 As to the treatment of atypical HUS in pregnancy, the mainstay of therapy, similar to non-pregnant patients, is plasmapheresis and treatment with eculizumab, a humanized monoclonal anti-C5 antibody that selectively targets and inhibits the terminal portion of the complement cascade. Eculizumab seems to be relatively safe during pregnancy based on data indicating that it does not impair complement function in newborns when used in pregnant patients with paroxysmal nocturnal hemoglobinuria, a complement-induced hemolytic anemia.21 While its use for atypical HUS in pregnancy has been supported by isolated case reports,22,23 large-scale studies are needed to address its safety and efficacy in preventing the progression to ESRD after pregnancy in women with atypical HUS. Finally, as to the duration of treatment, the authors of this paper suggest that therapy can be discontinued in patients who have no identifiable genetic abnormalities, have had a favorable response to therapy, and for whom pregnancy was the only known triggering event.
Taken together, studies to date indicate complex interactions among complement dysregulation, pregnant state and pregnancy complications, including preeclampsia and HELLP syndrome. An intriguing question remains as to whether some forms of HELLP syndrome may be, in fact, cases of thrombotic microangiopathy due to complement dysregulation that can be treated with therapies other than induction of delivery. A case report of a woman with HELLP syndrome at 26 weeks of gestation who was treated with eculizumab reported clinical improvement and prolongation of the pregnancy for 17 days.24 Future studies should address the heterogeneity of HELLP syndrome with respect to ACP dysregulation. The medication (eculizumab) cost may be justifiable for a subset of patients with mutations in the ACP genes and recurrent pregnancy losses due to early, severe HELLP syndrome.
Preeclampsia/HELLP
AKI as a complication of preeclampsia affects only about 1% of cases.25 However, when it is associated with HELLP, AKI is much more common, occurring in 7–15% of cases.26,27 While the diagnosis of preeclampsia is commonly based on new onset hypertension and proteinuria after 20 weeks gestation, other conditions such as AFLP, TTP, aHUS, and lupus nephritis, may also exhibit these findings. The clinical clues to help make an accurate diagnosis are listed in Table 2. Furthermore, angiogenic factors such as soluble fms-like tyrosine kinase-1 (sFlt-1), placental growth factor (PlGF) and soluble endoglin (sEng) levels may prove to be helpful for the diagnosis of preeclampsia; they have been reported to have utility in discriminating between preeclampsia and chronic hypertension,28 chronic kidney disease,29 lupus nephritis30 and end-stage renal disease (ESRD) patients on dialysis.31,32
Acute Fatty Liver of Pregnancy
Acute fatty liver of pregnancy (AFLP) is a rare entity which occurs in ∼1 in 10,000 deliveries.33 Its pathogenesis is attributed to a fetal deficiency of long-chain 3-hydroxyl coenzyme A dehydrogenase (LCHAD), which leads to excess fetal free fatty acids that cross the placenta and are hepatotoxic to the mother.34 Women usually present in the third trimester with fatigue, vomiting, headache, hypoglycemia and lactic acidosis. Laboratory abnormalities include hepatic derangements such as increases in the transaminases, alkaline phosphatase and bilirubin, as well as hematologic abnormalities such as, leukocytosis, thrombocytopenia and DIC. It may also present with AKI and proteinuria,35 which may be confused with preeclampsia/HELLP.36 Distinguishing clinical findings of AFLP include hypoglycemia and abdominal ascites. Up to 50% of women with AFLP also may have concomitant preeclampsia, which adds further to the difficulty in making the correct diagnosis.37 Fortunately, the treatments are usually the same for both entities. Histologically, microvesicular steatosis and cytoplasmic ballooning are found on liver biopsy.38 Lipid accumulation has also been reported in tubular epithelial cells of the kidney.39 A liver biopsy is usually not required to make a diagnosis, and may be dangerous, especially with concomitant coagulopathy, although it may be helpful in the early phase of illness if the diagnosis is uncertain. Expedient delivery, along with supportive care and intensive monitoring, are the mainstays of treatment. There are case series that suggest that plasmapheresis40,41 and liver transplantation42 may be performed in severe cases, however, most cases resolve spontaneously after delivery. Recurrence in a subsequent pregnancy is unusual, but possible with or without the presence of the LCHAD gene mutation.43,44
Pyelonephritis
Pyelonephritis, although neither specific nor more frequent45 in pregnancy, may be much more severe. The high incidence of asymptomatic bacteriuria during pregnancy may occur due to anatomic and physiologic changes of the urinary tract. When bacteriuria becomes symptomatic, it is likely to progress to cystitis, pyelonephritis or even sepsis with severe maternal complications. Untreated bacteriuria has also been shown to lead to low birth weight and preterm delivery, while its eradication has been shown to improve outcomes.46 Treatment of asymptomatic bacteriuria has demonstrated reduction in the incidence of pyelonephritis, as well a reduction in the risk of low birth weight babies and preterm births.47 Thus, routine screening for bacteriuria at the first prenatal visit is recommended by the American College of Obstetrics and Gynecology.48 The inciting organisms are mostly gram negative in origin (Escherichia coli 70%, Klebsiella and Enterobacter species 3%, Proteus species 2%), as well as gram positive organisms (group B Streptococcus) at 10%.49 Antibiotic therapy should be tailored to the culture and sensitivities. Preferred oral antibiotics for uncomplicated urinary tract infections or cystitis are nitrofurantoin and beta-lactams (Table 3). Treatment of pyelonephritis, however, requires parenteral antibiotics (Table 3) and hospitalization due to the danger of progression to overt septicemia. Approximately 1 in 5 women with pyelonephritis will develop some criteria of the sepsis syndrome.49–53 The incidence of AKI, fortunately, has decreased significantly with early, aggressive resuscitation from 20% (50) to 2%49. When treating sepsis with aggressive hydration, there is a risk for the development of acute respiratory distress syndrome or frank pulmonary edema caused by endotoxin-mediated endothelial injury, which alters alveolar capillary membrane permeability. Supportive care with intubation and mechanical ventilation along with judicious use of furosemide may be required. The choice of antibiotics is ultimately guided by the local microbiology and susceptibility data. Classes of drugs to be avoided are the fluoroquinolones and aminoglycosides. Concerns over the safety of fluoroquinolones originated from animal reports of arthropathy,54 though the reports in humans have not substantiated this finding.55 However, given the emergence of antibiotic resistant pathogens, they are not routinely recommended as first line. Aminoglycosides carry the risk of nephrotoxicity, so they are generally not first line agents unless it is the only class of medication that the organism is susceptible to. A negative urine culture 1–2 weeks after the completion of antibiotics constitutes a “test for cure.”
Table 3.
Preferred antibiotics for treatment of asymptomatic bacteriuria, cystitis and pyelonephritis in pregnancy66
| Drug (Oral) | Dosage | Comments | FDA Category |
|---|---|---|---|
| Nitrofurantoin | 100 mg every 12 hours | Give 5–7 days Common prophylactic agent; avoid in G6PD deficiency and third trimester for risk of hemolytic anemia | B |
| Amoxicillin | 500 mg every 8 hours or 875 mg every 12 hours | Give 3–7 days May have limited utility against gram negative organisms | B |
| Cephalexin | 500 mg every 6 hours | Give 3–7 days Commonly used | B |
| Cefpodoxime | 100 mg every 12 hours | Give 3–7 days | B |
| Drug (Intravenous) | Dosage | Comments | FDA Category |
|---|---|---|---|
| Ceftriaxone | 1 gm every 24 hours | Give 7–14 days Commonly used | B |
| Cefepime | 1 gm every 12 hours | Give 7–14 days Covers Pseudomonas | B |
| Aztreonam | 1 gm every 8 hours | Give 7–14 days In setting of beta lactam allergy | B |
| Piperacillin-tazobactam | 3.375 gm every 6 hours | Give 7–14 days For severe infection | B |
| Meropenem | 1 gm every 8 hours | Give 7–14 days Limited data; for severe infection | B |
G6PD deficiency, glucose-6-phosphate dehydrogenase e deficiency; United States Federal Drug Administration Pregnancy Categories: Category A, adequate and well-controlled studies have failed to demonstrate a risk to the fetus in the first trimester of pregnancy (and there is no evidence of risk in later trimesters);Category B, animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women; Category C, animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks; Category D, there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks; Category X, studies in animals or humans have demonstrated fetal abnormalities and/or there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience, and the risks involved in use of the drug in pregnant women clearly outweigh potential benefits.
Women with frequent infections, defined as three or more symptomatic episodes over a 12-month period, may require suppressive therapy. Nitrofurantoin and trimethoprim have been shown to decrease the recurrence rate by 95% or more.56 However, there are theoretical risks of hemolytic anemia with nitrofurantoin in the fetus or newborn, especially in those with glucose-6-phosphate dehydrogenase (G6PD) deficiency,57 as well as concerns of neurotube and cardiovascular defects with trimethoprim in the first trimester; sulfonamides also has the risk of increasing unbound bilirubin due to competitive binding causing fetal jaundice.58 Hence, we recommend to avoid the use of nitrofurantoin in patients with G6PD deficiency and in the third trimester, and we suggest to avoid the use of trimethoprim/sulfamethaxozole both in the first and third trimesters, keeping in mind that practice patterns may vary throughout different institutions. Post-coital prophylaxis with a single oral dose of either cephalexin (250 mg) or nitrofurantoin (50 mg) has also significantly reduced the incidence of cystitis.59
Post-renal AKI
Post renal causes of AKI are uncommon in pregnancy. Ureteral and bladder outlet obstruction should always be considered as with the non-pregnant population. However, iatrogenic injuries to the bladder and ureters are extremely rare and are usually a result of emergent C- sections. The incidences of iatrogenic injuries range from 0.0016% to 0.94% in different parts of the world.60–62 Women who are at highest risk are those with ectopic kidneys or duplication of ureters. Another rare cause of obstructive uropathy is uterine compression of the ureters, typically at the uretero-pelvic junction. A review of 18 cases of obstructive uropathy from uterine compression showed that it has a high fetal mortality of 33%.63 Risk factors include first and twin pregnancies, solitary kidneys, polyhydramnios and nephrolithiasis; all cases were diagnosed between 20–39 weeks of gestation. Treatment depends on the underlying etiology and gestational age. Patients who were near term in this review underwent spontaneous or induced delivery.63 Patients who were too early for delivery had either an amniotomy (for polyhydramnios), placement of ureteral stents or nephrostomies (for nephrolithiasis) or hemodialysis.63 For obstructive nephrolithiasis, treatment with ureteroscopy has been shown to be successful, although its safety needs to be further validated.64
Management of AKI
General measures to treat pregnancy-related AKI include identification of the underlying source of injury, volume resuscitation, prevention of further injury, timely initiation of renal replacement therapy (RRT) and prompt delivery of fetus, if necessary. Volume repletion is crucial in pre-renal states although the rate of volume replacement needs to be carefully monitored, as women with either endotoxin-mediated injury or preeclampsia can easily develop pulmonary edema. Complications of AKI can be treated as in non-pregnant patients, i.e. hyperkalemia in most circumstances can be treated with cation exchange resins, metabolic acidosis with alkali therapy, volume overload with loop diuretics and anemia with blood transfusion. If these measures prove unsuccessful or if the renal injury progresses, initiation of renal replacement therapy will be necessary. Specific measures to treat AKI depend on the underlying etiology of the injury. Steroid and immunosuppressive therapy may be warranted for biopsy-proven cases of glomerulonephritis. For the diagnoses of severe preeclampsia, HELLP syndrome and AFLP, prompt delivery of the fetus is recommended. Treatment of the TMAs, including TTP, and atypical HUS, requires plasmapheresis and administration of eculizumab (for atypical HUS). Administration of glucocorticoids is indicated should delivery of the fetus be required prior to 34 weeks of gestation in order to reduce the risk of neonatal respiratory distress syndrome.65
Conclusion
Although the overall incidence of AKI in pregnancy in most of the world is declining, the absolute numbers of deaths from AKI remain unacceptably high. Diagnosis of pregnancy-related AKI is not always straight-forward, and can be quite challenging in those with overlapping features such as, preeclampsia/HELLP, AFLP, HUS/TTP, atypical HUS and lupus nephritis. Clinical judgment and experience become paramount in making an accurate diagnosis. Measuring angiogenic factors may prove to be helpful in making the diagnosis of preeclampsia. Sophisticated genetic or serologic testing may have the potential to help identify patients with AFLP and atypical HUS, but they are presently not available for everyday clinical use. Excluding the use of eculizumab for atypical HUS and plasma exchange for TTP, treatment of AKI in pregnancy is generally supportive, often coupled with expedient delivery. Research should focus on disease-specific diagnostic markers, with awareness that prompt availability of results is necessary to affect management decisions and impact outcomes. These issues are especially relevant if the incidence of AKI is increasing in the developed world, with the rise possibly related to the trend toward delaying child birth, which may be accompanied by increases in maternal co-morbidities. Furthermore, the increasing use of reproductive technologies that frequently result in multiple gestations may also increase the risk of AKI. Hence, the need for prompt and accurate diagnosis followed by appropriate treatment for pregnancy-related AKI continues to grow with the changes in the epidemiology of women of reproductive age.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prakash J, Kumar H, Sinha DK, et al. Acute renal failure in pregnancy in a developing country: twenty years of experience. Ren Fail. 2006;28(4):309–313. doi: 10.1080/08860220600583658. [DOI] [PubMed] [Google Scholar]
- 2.Prakash J. The kidney in pregnancy: A journey of three decades. Indian journal of nephrology. 2012 May;22(3):159–167. doi: 10.4103/0971-4065.98750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stratta P, Besso L, Canavese C, et al. Is pregnancy-related acute renal failure a disappearing clinical entity? Ren Fail. 1996 Jul;18(4):575–584. doi: 10.3109/08860229609047680. [DOI] [PubMed] [Google Scholar]
- 4.Mehrabadi A, Liu S, Bartholomew S, et al. Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: population based retrospective cohort study. Bmj. 2014;349:g4731. doi: 10.1136/bmj.g4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hildebrand AM, Liu K, Shariff SZ, et al. Characteristics and Outcomes of AKI Treated with Dialysis during Pregnancy and the Postpartum Period. Journal of the American Society of Nephrology : JASN. 2015 May 14; doi: 10.1681/ASN.2014100954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishna A, Singh R, Prasad N, et al. Maternal, fetal and renal outcomes of pregnancy-associated acute kidney injury requiring dialysis. Indian journal of nephrology. 2015 Mar-Apr;25(2):77–81. doi: 10.4103/0971-4065.136890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative w Acute renal failure — definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care. 2004 Aug;8(4):R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamal EM, Behery MM, Sayed GA, Abdulatif HK. RIFLE classification and mortality in obstetric patients admitted to the intensive care unit with acute kidney injury: a 3-year prospective study. Reprod Sci. 2014 Oct;21(10):1281–1287. doi: 10.1177/1933719114525277. [DOI] [PubMed] [Google Scholar]
- 10.Gurrieri C, Garovic VD, Gullo A, et al. Kidney injury during pregnancy: associated comorbid conditions and outcomes. Archives of gynecology and obstetrics. 2012 Sep;286(3):567–573. doi: 10.1007/s00404-012-2323-5. [DOI] [PubMed] [Google Scholar]
- 11.Chugh KS, Jha V, Sakhuja V, Joshi K. Acute renal cortical necrosis—a study of 113 patients. Ren Fail. 1994;16(1):37–47. doi: 10.3109/08860229409044846. [DOI] [PubMed] [Google Scholar]
- 12.Matlin RA, Gary NE. Acute cortical necrosis. Case report and review of the literature. The American journal of medicine. 1974 Jan;56(1):110–118. doi: 10.1016/0002-9343(74)90756-6. [DOI] [PubMed] [Google Scholar]
- 13.Prakash J, Vohra R, Wani IA, et al. Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: a single-centre experience of 22 years from Eastern India. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association — European Renal Association. 2007 Apr;22(4):1213–1217. doi: 10.1093/ndt/gfl761. [DOI] [PubMed] [Google Scholar]
- 14.Day C, Hewins P, Hildebrand S, et al. The role of renal biopsy in women with kidney disease identified in pregnancy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association — European Renal Association. 2008 Jan;23(1):201–206. doi: 10.1093/ndt/gfm572. [DOI] [PubMed] [Google Scholar]
- 15.Lindheimer MD, Davison JM. Renal biopsy during pregnancy: ‘to b … or not to b …?’. British journal of obstetrics and gynaecology. 1987 Oct;94(10):932–934. doi: 10.1111/j.1471-0528.1987.tb02265.x. [DOI] [PubMed] [Google Scholar]
- 16.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. The New England journal of medicine. 2009 Oct 22;361(17):1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 17.Fakhouri F, Roumenina L, Provot F, et al. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. Journal of the American Society of Nephrology : JASN. 2010 May;21(5):859–867. doi: 10.1681/ASN.2009070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. The Journal of experimental medicine. 2006 Sep 4;203(9):2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fakhouri F, Jablonski M, Lepercq J, et al. Factor H, membrane cofactor protein, and factor I mutations in patients with hemolysis, elevated liver enzymes, and low platelet count syndrome. Blood. 2008 Dec 1;112(12):4542–4545. doi: 10.1182/blood-2008-03-144691. [DOI] [PubMed] [Google Scholar]
- 20.Salmon JE, Heuser C, Triebwasser M, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS medicine. 2011 Mar;8(3):e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallstensen RF, Bergseth G, Foss S, et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology. 2015 Apr;220(4):452–459. doi: 10.1016/j.imbio.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Ardissino G, Wally Ossola M, Baffero GM, Rigotti A, Cugno M. Eculizumab for atypical hemolytic uremic syndrome in pregnancy. Obstetrics and gynecology. 2013 Aug;122(2 Pt 2):487–489. doi: 10.1097/AOG.0b013e31828e2612. [DOI] [PubMed] [Google Scholar]
- 23.Canigral C, Moscardo F, Castro C, et al. Eculizumab for the treatment of pregnancy-related atypical hemolytic uremic syndrome. Annals of hematology. 2014 Aug;93(8):1421–1422. doi: 10.1007/s00277-013-1970-3. [DOI] [PubMed] [Google Scholar]
- 24.Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta. 2013 Feb;34(2):201–203. doi: 10.1016/j.placenta.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstetrics and gynecology. 2009 Jun;113(6):1299–1306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 26.Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome) American journal of obstetrics and gynecology. 1993 Oct;169(4):1000–1006. doi: 10.1016/0002-9378(93)90043-i. [DOI] [PubMed] [Google Scholar]
- 27.Gul A, Aslan H, Cebeci A, Polat I, Ulusoy S, Ceylan Y. Maternal and fetal outcomes in HELLP syndrome complicated with acute renal failure. Ren Fail. 2004 Sep;26(5):557–562. doi: 10.1081/jdi-200031750. [DOI] [PubMed] [Google Scholar]
- 28.Perni U, Sison C, Sharma V, et al. Angiogenic factors in superimposed preeclampsia: a longitudinal study of women with chronic hypertension during pregnancy. Hypertension. 2012 Mar;59(3):740–746. doi: 10.1161/HYPERTENSIONAHA.111.181735. [DOI] [PubMed] [Google Scholar]
- 29.Rolfo A, Attini R, Nuzzo AM, et al. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney international. 2013 Jan;83(1):177–181. doi: 10.1038/ki.2012.348. [DOI] [PubMed] [Google Scholar]
- 30.Leanos-Miranda A, Campos-Galicia I, Berumen-Lechuga MG, et al. Circulating Angiogenic Factors and the Risk of Preeclampsia in Systemic Lupus Erythematosus Pregnancies. The Journal of rheumatology. 2015 Jul;42(7):1141–1149. doi: 10.3899/jrheum.141571. [DOI] [PubMed] [Google Scholar]
- 31.Shan HY, Rana S, Epstein FH, Stillman IE, Karumanchi SA, Williams ME. Use of circulating antiangiogenic factors to differentiate other hypertensive disorders from preeclampsia in a pregnant woman on dialysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008 Jun;51(6):1029–1032. doi: 10.1053/j.ajkd.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Cornelis T, Spaanderman M, Beerenhout C, et al. Antiangiogenic factors and maternal hemodynamics during intensive hemodialysis in pregnancy. Hemodialysis international. International Symposium on Home Hemodialysis. 2013 Oct;17(4):639–643. doi: 10.1111/hdi.12042. [DOI] [PubMed] [Google Scholar]
- 33.Reyes H, Sandoval L, Wainstein A, et al. Acute fatty liver of pregnancy: a clinical study of 12 episodes in 11 patients. Gut. 1994 Jan;35(1):101–106. doi: 10.1136/gut.35.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibdah JA, Bennett MJ, Rinaldo P, et al. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. The New England journal of medicine. 1999 Jun 3;340(22):1723–1731. doi: 10.1056/NEJM199906033402204. [DOI] [PubMed] [Google Scholar]
- 35.Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World journal of gastroenterology : WJG. 2006 Dec 14;12(46):7397–7404. doi: 10.3748/wjg.v12.i46.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vigil-De Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2001 Jun;73(3):215–220. doi: 10.1016/s0020-7292(01)00364-2. [DOI] [PubMed] [Google Scholar]
- 37.Riely CA, Latham PS, Romero R, Duffy TP. Acute fatty liver of pregnancy. A reassessment based on observations in nine patients. Annals of internal medicine. 1987 May;106(5):703–706. doi: 10.7326/0003-4819-106-5-703. [DOI] [PubMed] [Google Scholar]
- 38.Burt AD, Mutton A, Day CP. Diagnosis and interpretation of steatosis and steatohepatitis. Seminars in diagnostic pathology. 1998 Nov;15(4):246–258. [PubMed] [Google Scholar]
- 39.Slater DN, Hague WM. Renal morphological changes in idiopathic acute fatty liver of pregnancy. Histopathology. 1984 Jul;8(4):567–581. doi: 10.1111/j.1365-2559.1984.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 40.Seyyed Majidi MR, Vafaeimanesh J. Plasmapheresis in acute Fatty liver of pregnancy: an effective treatment. Case reports in obstetrics and gynecology. 2013;2013:615975. doi: 10.1155/2013/615975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin JN, Jr, Briery CM, Rose CH, Owens MT, Bofill JA, Files JC. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. Journal of clinical apheresis. 2008;23(4):138–143. doi: 10.1002/jca.20168. [DOI] [PubMed] [Google Scholar]
- 42.Amon E, Allen SR, Petrie RH, Belew JE. Acute fatty liver of pregnancy associated with preeclampsia: management of hepatic failure with postpartum liver transplantation. American journal of perinatology. 1991 Jul;8(4):278–279. doi: 10.1055/s-2007-999396. [DOI] [PubMed] [Google Scholar]
- 43.Treem WR, Shoup ME, Hale DE, et al. Acute fatty liver of pregnancy, hemolysis, elevated liver enzymes, and low platelets syndrome, and long chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. The American journal of gastroenterology. 1996 Nov;91(11):2293–2300. [PubMed] [Google Scholar]
- 44.Bacq Y, Assor P, Gendrot C, Perrotin F, Scotto B, Andres C. [Recurrent acute fatty liver of pregnancy] Gastroenterologie clinique et biologique. 2007 Dec;31(12):1135–1138. doi: 10.1016/s0399-8320(07)78351-3. [DOI] [PubMed] [Google Scholar]
- 45.Hooton TM, Scholes D, Stapleton AE, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. The New England journal of medicine. 2000 Oct 5;343(14):992–997. doi: 10.1056/NEJM200010053431402. [DOI] [PubMed] [Google Scholar]
- 46.Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstetrics and gynecology. 1989 Apr;73(4):576–582. [PubMed] [Google Scholar]
- 47.Smaill FM, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. The Cochrane database of systematic reviews. 2015;(8):CD000490. doi: 10.1002/14651858.CD000490.pub3. [DOI] [PubMed] [Google Scholar]
- 48.ACOG educational bulletin. Antimicrobial therapy for obstetric patients. Number 245, March 1998 (replaces no. 117, June 1988). American College of Obstetricians and Gynecologists. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 1998 Jun;61(3):299–308. [PubMed] [Google Scholar]
- 49.Hill JB, Sheffield JS, McIntire DD, Wendel GD., Jr Acute pyelonephritis in pregnancy. Obstetrics and gynecology. 2005 Jan;105(1):18–23. doi: 10.1097/01.AOG.0000149154.96285.a0. [DOI] [PubMed] [Google Scholar]
- 50.Cunningham FG, Leveno KJ, Bloom SI, Hauth JC, Gilstrap LC, Wenstrom KD. Renal and urinary tract disorders. 22. McGraw-Hill; 2005. [Google Scholar]
- 51.Gilstrap LC, 3rd, Cunningham FG, Whalley PJ. Acute pyelonephritis in pregnancy: an anterospective study. Obstetrics and gynecology. 1981 Apr;57(4):409–413. [PubMed] [Google Scholar]
- 52.Cunningham FG, Lucas MJ, Hankins GD. Pulmonary injury complicating antepartum pyelonephritis. American journal of obstetrics and gynecology. 1987 Apr;156(4):797–807. doi: 10.1016/0002-9378(87)90335-8. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham FG, Morris GB, Mickal A. Acute pyelonephritis of pregnancy: A clinical review. Obstetrics and gynecology. 1973 Jul;42(1):112–117. [PubMed] [Google Scholar]
- 54.Ingham BB,DW, Dale EA, McFadzean JA. Arthropathy induced by antibacterial fused N-alkyl-4-pyridone-3-carboxylic acids. Toxicology Letters. 1977;1(1):21–26. [Google Scholar]
- 55.Loebstein R, Addis A, Ho E, et al. Pregnancy outcome following gestational exposure to fluoroquinolones: a multicenter prospective controlled study. Antimicrobial agents and chemotherapy. 1998 Jun;42(6):1336–1339. doi: 10.1128/aac.42.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fihn SD. Clinical practice. Acute uncomplicated urinary tract infection in women. The New England journal of medicine. 2003 Jul 17;349(3):259–266. doi: 10.1056/NEJMcp030027. [DOI] [PubMed] [Google Scholar]
- 57.Gait JE. Hemolytic reactions to nitrofurantoin in patients with glucose-6-phosphate dehydrogenase deficiency: theory and practice. DICP : the annals of pharmacotherapy. 1990 Dec;24(12):1210–1213. doi: 10.1177/106002809002401213. [DOI] [PubMed] [Google Scholar]
- 58.C R Sulfamethoxazole/trimethoprim. Ottawa: ON2007. [Google Scholar]
- 59.Pfau A, Sacks TG. Effective prophylaxis for recurrent urinary tract infections during pregnancy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1992 Apr;14(4):810–814. doi: 10.1093/clinids/14.4.810. [DOI] [PubMed] [Google Scholar]
- 60.Kaskarelis D, Sakkas J, Aravantinos D, Michalas S, Zolotas J. Urinary tract injuries in gynecological and obstetrical procedures. International surgery. 1975 Jan;60(1):40–43. [PubMed] [Google Scholar]
- 61.Rajasekar D, Hall M. Urinary tract injuries during obstetric intervention. British journal of obstetrics and gynaecology. 1997 Jun;104(6):731–734. doi: 10.1111/j.1471-0528.1997.tb11986.x. [DOI] [PubMed] [Google Scholar]
- 62.Eisenkop SM, Richman R, Platt LD, Paul RH. Urinary tract injury during cesarean section. Obstetrics and gynecology. 1982 Nov;60(5):591–596. [PubMed] [Google Scholar]
- 63.Jena M, Mitch WE. Rapidly reversible acute renal failure from ureteral obstruction in pregnancy. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1996 Sep;28(3):457–460. doi: 10.1016/s0272-6386(96)90507-7. [DOI] [PubMed] [Google Scholar]
- 64.Johnson EB, Krambeck AE, White WM, et al. Obstetric complications of ureteroscopy during pregnancy. The Journal of urology. 2012 Jul;188(1):151–154. doi: 10.1016/j.juro.2012.02.2566. [DOI] [PubMed] [Google Scholar]
- 65.ACOG Committee Opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstetrics and gynecology. 2011 Feb;117(2 Pt 1):422–424. doi: 10.1097/AOG.0b013e31820eee00. [DOI] [PubMed] [Google Scholar]
- 66.Glaser AP, Schaeffer AJ. Urinary Tract Infection and Bacteriuria in Pregnancy. The Urologic clinics of North America. 2015 Nov;42(4):547–560. doi: 10.1016/j.ucl.2015.05.004. [DOI] [PubMed] [Google Scholar]


