Abstract
Diffusion-weighted MRI (DWI) holds promise to address some of the shortcomings of routine clinical breast MRI and to expand the capabilities of imaging in breast cancer management. DWI reflects tissue microstructure, and provides unique information to aid in characterization of breast lesions. Potential benefits under investigation include improving diagnostic accuracy and guiding treatment decisions. As a result, DWI is increasingly being incorporated into breast MRI protocols and multicenter trials are underway to validate single institution findings and to establish clinical guidelines. Advancements in DWI acquisition and modeling approaches are helping to improve image quality and extract additional biologic information from breast DWI scans, which may extend diagnostic and prognostic value.
Keywords: diffusion weighted imaging (DWI), apparent diffusion coefficient (ADC), breast cancer, breast lesions, diagnosis, treatment, imaging biomarkers
Introduction
Breast MRI is widely regarded as the most sensitive breast screening technique and is increasingly recommended for high-risk population screening, preoperative staging and therapy monitoring [1]. Conventional breast MRI examinations incorporate multiple pulse sequences, typically including a T2-weighted bright fluid sequence and a dynamic contrast enhanced MRI (DCE-MRI) sequence consisting of multiple T1-weighted sequences before and after contrast injection, as recommended by the American College of Radiology (ACR) [2]. This approach provides high sensitivity but moderate specificity and positive predictive value (PPV) for characterization of breast lesions when used in conjunction with the ACR MRI Breast Imaging-Reporting and Data System (BI-RADS) lexicon to assess lesion morphology (such as shape, margin and distribution) and initial and delayed contrast enhancement patterns [3].
Emerging evidence suggests that diffusion-weighted MRI (DWI) can address some of the limitations of conventional breast MRI by providing complementary information for lesion assessment [4]. Whereas DCE-MRI demonstrates tissue vascularity and provides high sensitivity for cancer detection, DWI reflects the mobility of water molecules diffusing in tissues, revealing tissue organization at the microscopic level. Numerous DWI studies have shown malignant breast lesions exhibit altered diffusion characteristics compared to normal fibroglandular tissue [5, 6]. Moreover, DWI is a short scan available on most commercial MR scanners that does not require any exogenous contrast and can be easily added to clinical breast MRI protocols. The supplementary information about the microscopic cellular environment and relative ease in implementation have made DWI an imaging tool of interest for improving breast cancer diagnosis and characterization.
Breast DWI Basics
DWI is performed using motion-sensitizing gradients applied during MR image acquisition to probe local diffusion characteristics. The resulting diffusion-weighted MRI signal is reduced in intensity proportional to the water mobility, and is commonly described by the monoexponential equation:
| [1] |
where SD is the signal intensity with diffusion weighting, S0 is the signal intensity without diffusion weighting, b is the diffusion sensitization factor (or ‘b value’) determined by the strength and timing of the applied diffusion gradients (s/mm2), and ADC is the apparent diffusion coefficient, which describes the rate of diffusion and is defined as the average area occupied by a water molecule per unit time (mm2/s) [7]. Whereas pure water exhibits random isotropic molecular diffusion, the motion of water molecules in vivo is restricted by cell membranes and other hindrances within intracellular and extracellular compartments. As a result, DWI is sensitive to microstructural tissue properties including cell density, cellular organization, and cell membrane integrity. From diffusion weighted images acquired with two or more different b-values, ADC can be calculated for each voxel in the image and presented as a parametric map. Due to increased cellularity and more restricted diffusion, breast malignancies commonly exhibit hyperintensity on DWI and lower ADC relative to normal breast parenchyma, Figure 1. While breast lesions tend to be most visible on the diffusion-weighted (SD) images (Fig 1c), such images have confounding T2-shine through effects and therefore most clinical applications rely on quantitative ADC measures for lesion characterization.
Figure 1.
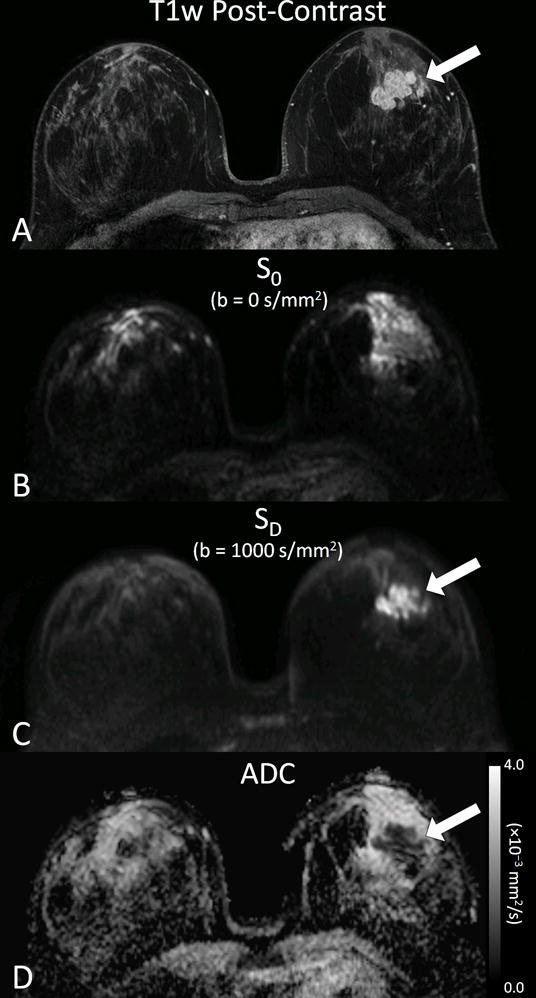
Example breast DWI images obtained in a 37 year old woman with invasive breast cancer. Shown are corresponding images from (A) post-contrast T1-weighted image, (B) DWI S0 with b = 0 s/mm2 (primarily T2-weighted), (C) DWI SD with b = 1000 s/mm2, (D) apparent diffusion coefficient (ADC) map. Invasive lobular carcinoma (arrow) exhibits reduced diffusivity on DW imaging, appearing hyperintense on the SD image and hypointense on the ADC map.
Clinical Applications
Improving Accuracy for Cancer Diagnosis
The most widely explored clinical application of DWI for breast imaging is as a supplemental diagnostic tool to DCE-MRI in differentiating between malignant versus benign findings. Because of DCE-MRI’s well-established high sensitivity (90%) yet relatively modest specificity (72%) [8], there is great interest in the addition of DWI to potentially improve PPV and reduce unnecessary biopsies. Many prior studies have shown breast malignancies have significantly lower ADC values than benign lesions, as illustrated in Figure 2. Two meta-analyses evaluating DWI performance for diagnosing breast lesions, one containing 13 studies between 2002 – 2009 [9] and another containing 14 studies between 2008 – 2014 [10], reported similar pooled sensitivities/specificities of 84%/79% and 86%/76%, respectively. More importantly, combined DCE-MRI and DWI produced superior diagnostic accuracy than either DCE-MRI or DWI alone, with resulting sensitivity/specificity of 92%/86% and area under the summary receiver operating characteristics (ROC) curve of 0.94 [10].
Figure 2.
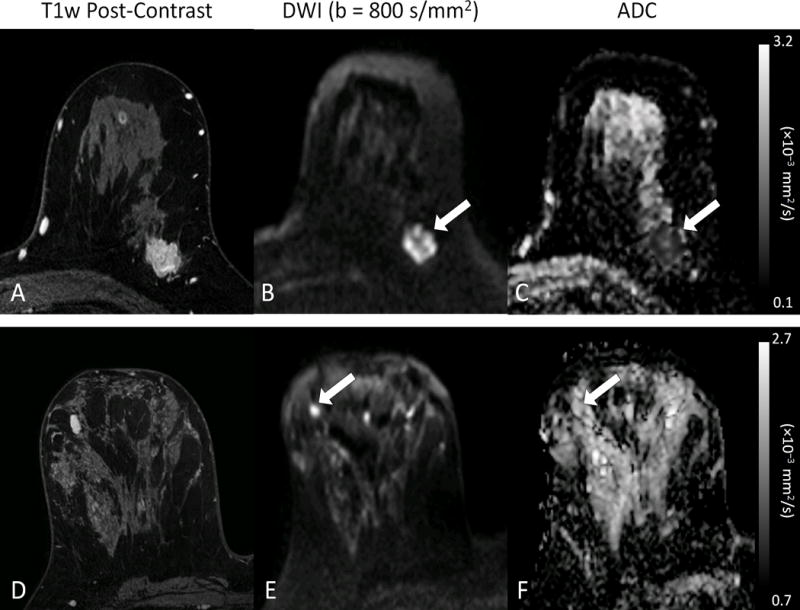
Examples of benign and malignant breast lesions. (top row) 24 mm invasive ductal carcinoma in a 52 year old woman. (bottom row) 12 mm benign fibroadenoma in a 46 year old woman. Both lesions exhibit enhancement on post-contrast T1-weighted images (A, D) and hyperintensity on diffusion-weighted images (arrows, B, E), but demonstrate marked differences on the ADC maps (C,F), where the malignant lesion (C, arrow) exhibits substantially lower ADC (ADC = 0.70 × 10−3 mm2/s) than the fibroadenoma (F, arrow, ADC = 1.81 × 10−3mm2/s).
Despite compelling evidence from multiple single center studies, DWI has not yet reached widespread utility in routine clinical settings. A major limitation to clinical implementation is the heterogeneity in approach amongst the many published studies preventing definition of generalizable diagnostic criteria. Due to dependence of lesion ADC measures on the applied b value, with ADC values generally decreasing with increasing b [11], varying selection of b values for image acquisition has resulted in a wide range of reported ‘optimal’ ADC diagnostic thresholds identified across prior studies (0.90 – 1.76 × 10−3 mm2/s [9, 10]). Study cohorts in the literature also differ with respect to lesion sizes [9], lesion morphologies (mass versus non-mass enhancement) and cancer prevalence [10]. Therefore, results from multicenter studies incorporating standardized approaches across a wide range of imaging platforms and clinical settings (e.g., American College of Radiology [ACRIN] 6702 [12]) will be fundamental in establishing guidelines for broad clinical implementation of DWI.
Selecting Appropriate Treatments
There is a spectrum of pathologies in the breast that warrant surgical management, ranging from high risk lesions to in-situ and invasive disease, one or all of which may be present in a single lesion. Ideally, a core biopsy would always reflect the most aggressive disease to stratify patients to appropriate treatment. However, in reality, this is not always true; many lesions upgrade upon surgical excision and consequently require a change in direction of management. Clinical tools that can predict final pathological results before surgery would allow for more accurate treatment planning. Literature has suggested that DWI may be one of these tools. Since DWI is a characterization of cellular density, it is reasonable to hypothesize that ADC measures would correlate to tumor aggressiveness, mirroring the pathologic spectrum. Indeed, multiple studies have supported this expected trend by demonstrating mean ADC of invasive disease to be lower than that of in-situ disease [13, 14], which is in turn lower than that of benign breast tissue [15]. ADC of high risk lesions, such as atypical ductal hyperplasia (ADH) and lobular neoplasia (lobular carcinoma in situ and atypical lobular hyperplasia) was also found to be lower compared to that of other benign lesions (mean, 1.46 ± 0.39 × 10−3 mm2/s vs 1.83 ± 0.43 × 10−3 mm2/s, respectively) [16].
DWI may provide new prognostic biomarkers for even finer stratification of cancer aggressiveness. Within invasive disease, a number of studies have shown ADC values to negatively correlate with tumor grade [17–21]. A similar correlation was seen with tumor proliferation, using Ki-67 index as a marker [13, 21–26]. The ability to detect more aggressive tumors may aid in consideration of neoadjuvant treatment prior to surgery. Similarly, within in-situ disease, DWI characteristics were able to stratify low versus high grade DCIS [27, 28]. As the scientific community continues to debate the necessity of surgically treating all DCIS, this ability to classify DCIS aggressiveness in vivo may be useful in developing new risk-based management strategies.
Given this overarching trend of decreasing ADC with increasing cancer severity, it is possible that DWI may also be helpful in predicting pathologic upgrade. Lower ADC was shown be to be associated with pathological upgrade of lesions diagnosed on core needle biopsy, both for DCIS lesions upgrading to invasive disease [29, 30] and high risk lesions upgrading to malignancy [31], Figure 3. Such findings implicate that DWI may aid in MRI-guided biopsy to preferentially target the most aggressive portion of a lesion and thereby decrease sampling error. Additionally, DWI after biopsy may help to determine appropriate management and ultimately decrease the rate of unnecessary surgical interventions.
Figure 3.
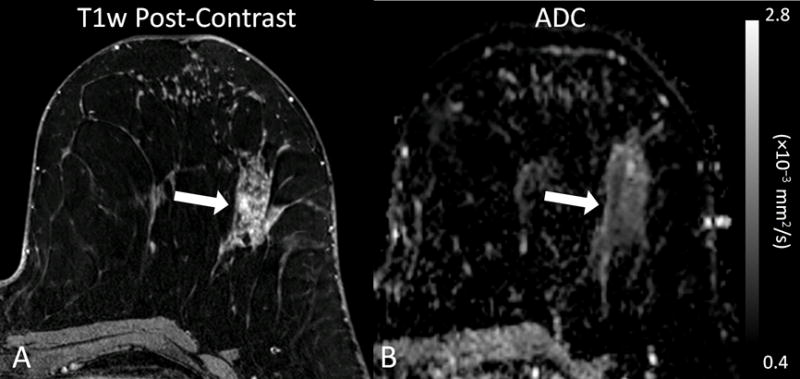
Example of a high-risk lesion (lobular carcinoma in situ) detected in a 54 year-old woman that upgraded to malignancy (ductal carcinoma in situ) on surgical excision. (A) Post-contrast T1-weighted image demonstrates a 67 mm segmental non-mass enhancement (arrow) exhibiting persistent and plateau enhancement that was assessed as BI-RADS category 4. (B) On DWI, the lesion exhibits restricted diffusion with a corresponding dark area on the apparent diffusion coefficient (ADC) map (arrow), with low ADC value measuring 1.13 × 10−3 mm2/s. (Adapted from Cheeney S, Rahbar H, Dontchos BN, et al. Apparent diffusion coefficient values may help predict which MRI-detected high-risk breast lesions will upgrade at surgical excision. J Magn Reson Imaging 2017 Feb 9; with permission.)
An additional area of active investigation is DWI’s role in identifying tumors that express specific markers, which can be targeted for personalized therapy. Because DWI reflects the tumor microenvironment, ADC measurements may vary across biologic subtypes characterized by expression of estrogen receptor (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER-2). Multiple studies report ADC values to be significantly lower in ER-positive versus ER-negative breast tumors [32–36]. A similar trend of lower ADC was observed for PR-positive versus PR-negative tumors [34–38]. Conversely, a number of studies have found HER-2-postive tumors to exhibit higher ADC than HER-2-negative tumors [24, 32, 39–41]. Of note, the observation of lower ADC values (presumably reflecting higher cell density) in ER/PR-positive tumors is counterintuitive since ER/PR expression is typically seen in slower growing, lower grade cancers, and is therefore an area warranting further investigation.
Despite the aforementioned promising results, ADC associations have been variable, even across the studies cited above, with regard to specific pathologic features. This inconsistency is a major limitation of the application of DWI as a biomarker in clinical practice. Thus, more comprehensive investigation is needed before ADC can be considered a reliable prognostic marker in breast cancer.
Evaluating Efficacy of Neoadjuvant Therapy
Another emerging role for DWI is in assessing individual response to breast cancer treatment. Cytotoxic effects of chemotherapy including cell lysis, apoptosis, and necrosis cause alterations in cell membrane integrity and reduced tumor cellularity, resulting in a less restrictive environment for diffusing water molecules. Thus, increasing tumor ADC values may reflect cell death and favorable response to treatment. Such cytotoxic changes precede changes in tumor size or perfusion, suggesting DWI has potential to provide early indication of treatment efficacy. In support of this theory, a growing number of clinical studies have demonstrated that treatment-induced changes in breast tumor ADC values can differentiate responders and non-responders early in the course of treatment, after only the first cycle of chemotherapy [42–44], Figure 4.
Figure 4.
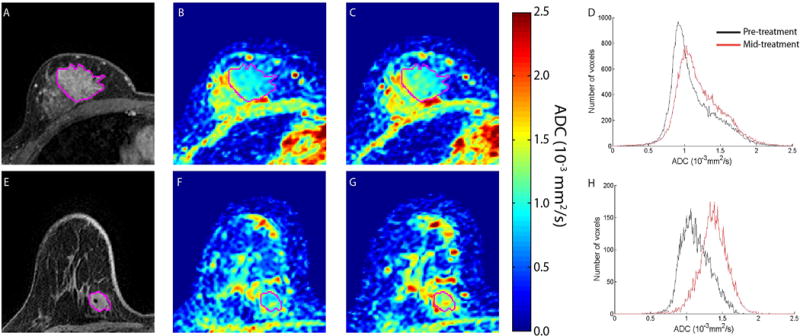
Therapeutic-induced changes in breast tumor ADC values. MRI images are depicted for non-responding (top row) and responding (bottom row) patients treated for breast cancer. (A) and (E) T1-weighted gadolinium enhanced, (B) and (F): pre-treatment ADC maps, (C) and (G): ADC maps at 8–11 days after treatment initiation, D) and (H): Histograms of ADC values in the tumor pre-treatment and post-treatment initiation. Tumor is delineated from surrounding healthy tissue in the individual images by the purple line. (Reprinted from Galban CJ, Ma B, Malyarenko D, et al. Multi-site clinical evaluation of DW-MRI as a treatment response metric for breast cancer patients undergoing neoadjuvant chemotherapy. PLoS One 2015;10(3):e0122151; with permission.)
In addition to detecting treatment response, DWI may also play a role in pre- and post-treatment management decisions. First, pre-treatment ADC measures may improve prognostic ability to identify which patients will best respond to neoadjuvant treatments. In women with triple-negative disease (ER, PR, and HER2-negative) undergoing neoadjuvant chemotherapy, several large studies have observed low pre-treatment tumor ADC values to be associated with pathological complete response [36, 45, 46]. Second, post-treatment DWI may provide a means to accurately assess extent of residual disease prior to surgery. A recent meta-analysis evaluating the utility of DWI for detecting pathologic complete response (pCR) after neoadjuvant chemotherapy reported a pooled sensitivity and specificity of 0.93 and 0.85, respectively, across eight studies with 539 total patients [47]. The performance of DWI for detecting pCR was not statistically different than for DCE-MRI, although their results suggested sensitivity to be higher by DWI and specificity to be higher by DCE-MRI and that a combination of both imaging techniques may enable more precise assessment of pathologic response to chemotherapy.
Similar to investigations of other clinical applications, wide variability exists in the current literature as to the utility of DWI to monitor breast cancer therapy, in part due to differences in study design (e.g., DWI acquisition and analysis approaches, timing of imaging examinations during treatment, and patient characteristics), and further investigation is needed to validate ADC as a predictive biomarker of therapeutic efficacy. Results of larger multisite clinical trials incorporating standardized approaches (e.g., ACRIN 6698 [48], currently underway) will provide valuable insights to the performance of DWI for assessing breast cancer response to treatment.
Considerations for Clinical Implementation
While a growing number of imaging centers are incorporating DWI into the clinical breast MR examination, there are multiple technical factors related to image acquisition and analysis that should be considered for successful implementation. Image quality can vary widely, and breast DWI acquisition protocols must be optimized to reduce artifacts and achieve adequate signal-to-noise ratio (SNR). Conventional single shot echo planar imaging (EPI)-based DWI sequences are prone to detrimental image distortions and ghosting artifacts, which are particularly problematic for breast imaging due to off-isocenter imaging, air-tissue interfaces, and high fat content in the breast. Good quality shimming and suppression of lipid signal are essential to minimize susceptibility and chemical shift artifacts for breast DWI, and use of multichannel RF coil design, parallel imaging, and higher order shimming techniques help to achieve these goals. Strategies to improve SNR include scanning at higher field strength, increasing the number of signal averages, shortening echo time (TE), increasing voxel size, and appropriate choice of b value [4].
The choice of b values directly affects SNR and quantitative ADC analysis. For standard spin-echo prepared DWI, a b value of approximately 1.1/ADC may provide ideal SNR [49]. For breast cancers, with typical ADC values of 0.9 to 1.5 × 10−3 mm2/s, this corresponds to an optimal diffusion weighting in the range of b = 700 to 1200 s/mm2, and these are common choices in the literature. As described above, the applied b value also directly influences lesion ADC measures, with ADC generally decreasing with increasing b [11]. However, provided ADC thresholds are adjusted according to the specific b value used, a combined analysis of 26 breast DWI studies found no significant influence of b-value on performance for differentiating benign and malignant lesions [50]. Additionally, DWI acquisitions using multiple b values (three or more) provide a more accurate sampling of signal decay and enable advanced modeling approaches. However, this extends scan time without clear diagnostic benefit and for general purposes of quantifying breast lesion ADC, the minimum requirement of two b values (e.g. b = 0, 800 s/mm2) is commonly used and appears to be sufficient.
Studies in the literature have varied in performing DWI before or after DCE-MRI during the breast MRI examination. Overall, the influence of contrast agent administration on breast lesion ADC measures was determined to be negligible [50, 51], particularly when DWI is acquired several minutes post-contrast [51], and either approach is acceptable.
Data analysis approaches including post-processing, ADC calculation, and region-of-interest (ROI) methods can also directly impact ADC measures. Image registration can be employed prior to ADC calculation to correct for patient motion as well as susceptibility and eddy-current-related EPI distortions. Adequate noise thresholding should be used when calculating ADC maps to reduce spurious values from suppressed adipose tissue signal. Lesion ADC measurements differ significantly depending on the ROI selection approach, which can range from sampling a small subregion to whole tumor 3D ROI methods, Figure 5. Differences in ADC are most dramatic in large, heterogeneous, and non mass lesions [37, 52, 53]. Some studies have reported that a smaller ROI placed over the most hypointense ADC area may provide better discriminating performance by reflecting the worst pathology within a heterogeneous lesion [37, 53]. On the other hand, whole tumor measurement may allow better reproducibility [52]. Semi-automated ROI selection techniques further show promise to improve efficiency, accuracy, and reproducibility of breast lesion ADC measures [54].
Figure 5.
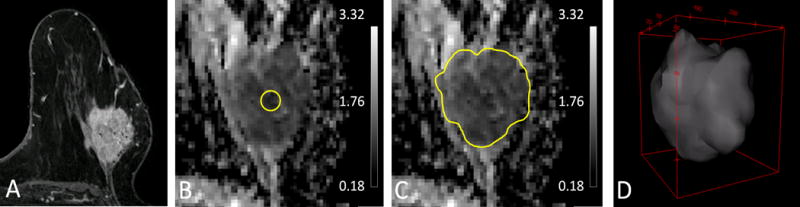
Illustration of varying tumor ADC measurement approaches. Shown are (A) Reference T1-weighted post contrast slice, (B) small minimum ADC subregion ROI, mean ADC = 0.65 × 10−3 mm2/s, (C) single slice 2D whole tumor ROI, mean ADC = 0.88 × 10−3 mm2/s, (D) 3D whole tumor ROI, mean ADC = 1.01 × 10−3 mm2/s. As shown, choice of ROI technique can substantially affect lesion ADC measures.
Advanced Techniques
Novel Acquisition Approaches
A variety of exciting advancements in DWI acquisition strategies are under development to further improve the image quality of breast DWI [55]. Aiming to increase spatial resolution and reduce distortions, these new techniques have potential to greatly improve the ability to resolve spatial heterogeneity and directly correlate lesion DCE-MRI and DWI features. Advanced EPI-based techniques such as multi-shot EPI and readout-segmented EPI employ alternative readout trajectories to shorten echo time and thereby reduce susceptibility artifacts. Reduced field-of-view (rFOV) approaches allow imaging of a target subregion, which reduces the required matrix size and both enables higher spatial resolution as well as reduces susceptibility-based distortions, Figure 6. Preliminary studies implementing these advanced EPI techniques demonstrate improved ability to assess breast lesion morphology and ADC on DWI [56–58]. It is important to note that along with the potential improvements in image quality, each of these advanced techniques incorporate some tradeoffs such as longer scan times and increased sensitivity to patient motion versus conventional single shot EPI.
Figure 6.
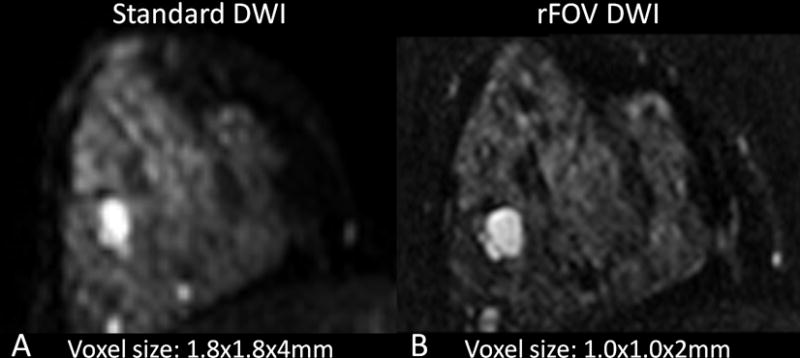
Reduced field-of-view (rFOV) breast DWI technique. Shown are corresponding images (b=0 s/mm2) from (A) standard single shot diffusion-weighted EPI and (B) rFOV diffusion-weighted EPI acquisitions. Imaging resolution (voxel size) for each DWI approach are shown. Compared to standard DWI, rFOV DWI achieves higher spatial resolution and reduces distortions, potentially improving ability to assess lesion morphology as well as diffusivity.
Alternative non-EPI sequences also offer some additional advantages for reducing susceptibility effects and artifacts of breast DWI. Fast spin echo (FSE) based sequences avoid T2*-related signal losses and susceptibility based geometric distortions by using refocused spin echoes rather than gradient echoes, and are under investigation for improving spatial accuracy for breast imaging [59, 60]. However, FSE techniques also come with drawbacks of increased T2-weighted signal loss and blurring and additional RF heating, and require further technical optimizations. A number of other emerging non-EPI approaches also show promise for overcoming current image quality challenges of breast DWI [61, 62], but are in earlier stages of development.
Advanced DWI Modeling
While the simple single compartment monoexponential decay model (given in Eq. 1) to estimate ADC is by far the most common quantitative approach utilized in clinical DWI applications, more sophisticated modeling techniques are under investigation to extract additional biological information from breast DWI scans [55]. One such approach is intravoxel incoherent motion (IVIM) modeling, which fits the DWI signal to a multicompartment biexponential decay model to separate contributions from the microvasculature and surrounding parenchyma [63]. IVIM allows measurement of tissue parameters that include the perfusion volume fraction (fp), tissue diffusivity (Dt) in the parenchyma, and pseudodiffusivity (Dp or D*) in the microvasculature. Applied to breast cancer, Dt is considered a marker of tumor cellularity, fp of blood volume, and Dp of blood velocity and vessel architecture, Figure 7. Preliminary IVIM studies have demonstrated increased fp levels in invasive breast cancers [64–66], which may reflect angiogenesis associated with more aggressive disease and may thus provide additional diagnostic and prognostic value.
Figure 7.
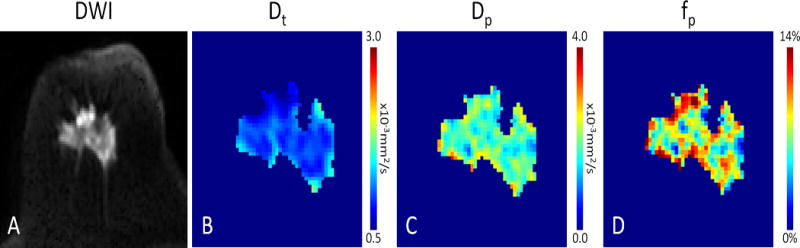
DWI with intravoxel incoherent motion (IVIM) biexponential modeling of an invasive ductal carcinoma in a 62 year old woman. Distinct physiological information is obtained by separating the IVIM components. Shown are the (A) DWI (b=800 × 10−3mm2/s) image and maps of the (B) tissue diffusivity (Dt), (C) microvasculature pseudodiffusivity (Dp), and (D) perfusion fraction (fp), which illustrate reduced diffusion (high cell density) and heterogeneous levels of perfusion within the malignancy.
Other alternative modeling approaches such as diffusion kurtosis and stretched exponential diffusion modeling characterize deviation from unrestricted single compartment (Gaussian) diffusion behavior. Non-Gaussian diffusivity is most evident in vivo at very high b-values (b >1000 s/mm2) and in theory reflects the ‘complexity’ of the tissue microenvironment creating physical barriers to diffusion (cell membranes, organelles, stromal desmoplasia, etc.). Recent studies have shown breast malignancies to exhibit more non-Gaussian diffusivity, with higher diffusional kurtosis, than benign breast lesions [67–70], which may also help to further improve lesion characterization.
Diffusion tensor imaging (DTI) is another advanced technique, more commonly used for neuroimaging, which is also being investigated for application in breast imaging. DTI characterizes both the rate (diffusivity) and directionality (anisotropy) of water diffusion and it is hypothesized it may provide further insights on glandular organization (ducts, lobules) and microarchitecture. DTI anisotropy measures have been shown to be generally lower in breast lesions versus normal parenchyma [71–73], Figure 8, thought to reflect the loss of glandular organization in the lesions, and some studies have also reported lower anisotropy values in benign versus malignant lesions [72–75].
Figure 8.
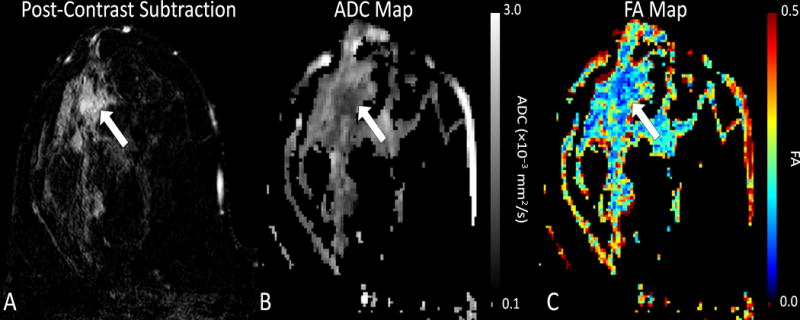
Example diffusion tensor imaging (DTI) in a 34 year old woman with invasive ductal carcinoma. Shown are (A) Post-contrast T1-weighted subtraction image, (B) ADC map, and (C) fractional anisotropy (FA) map. The enhancing tumor (arrow) exhibits restricted diffusion with reduced ADC (ADC = 0.94 × 10−3 mm2/s) and anisotropy (FA = 0.12) versus nearby normal parenchyma, suggesting increased cell density and loss of structured organization.
It is important to note that these advanced modeling approaches come with a cost of increased acquisition time (for sampling of a wider range of b values and/or diffusion directions) and post-processing requirements over standard DWI. It remains to be determined whether they can provide diagnostic advantages that would justify their clinical implementation, and so for now these techniques are primarily restricted to research applications.
Conclusion
There is growing recognition of the potential value of DWI for improving the diagnosis and management of breast cancer, and this supplemental MRI technique is increasingly being incorporated into breast MRI protocols [4]. DWI holds particular appeal due to its short acquisition time, its wide availability on most commercial MR scanners, and the lack of exogenous contrast agent needed. However, there are obstacles to routine clinical application of breast DWI related to technical challenges and the lack of standardization of imaging approaches across institutions. Novel acquisition techniques are under development to overcome commonly encountered image quality issues, and advanced modeling approaches hold potential to further expand the capabilities of DWI as an imaging biomarker. Results of prospective multicenter trials (several of which are currently underway through ACRIN [12, 48]) will help to validate promising single-institution findings and establish generalizable guidelines, which are needed to facilitate widespread implementation of DWI for breast imaging.
Acknowledgments
We would like to thank Averi Kitsch, B.S. for assistance with compiling figures and literature searches for this article.
Funding Support:
The authors acknowledge support from the following grants:
National Institutes of Health (NIH): R01CA151326
References
- 1.DeMartini W, Lehman C, Partridge S. Breast MRI for cancer detection and characterization: a review of evidence-based clinical applications. Acad Radiol. 2008;15(4):408–416. doi: 10.1016/j.acra.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 2.American College of Radiology. Breast Magnetic Resonance Imaging (MRI) Accreditation Program Requirements. doi: 10.1016/j.mri.2020.06.017. http://www.acraccreditation.org/~/media/ACRAccreditation/Documents/Breast-MRI/Requirements.pdf. [DOI] [PMC free article] [PubMed]
- 3.Morris EACC, Lee CH, et al. ACR Breast Imaging Reporting and Data System. 5th. Reston, VA: American College of Radiology; 2013. ACR BI-RADS Magnetic Resonance Imaging. [Google Scholar]
- 4.Partridge SC, McDonald ES. Diffusion weighted magnetic resonance imaging of the breast: protocol optimization, interpretation, and clinical applications. Magn Reson Imaging Clin N Am. 2013;21(3):601–624. doi: 10.1016/j.mric.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Y, Cai YQ, Cai ZL, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16(2):172–178. doi: 10.1002/jmri.10140. [DOI] [PubMed] [Google Scholar]
- 6.Sinha S, Lucas-Quesada FA, Sinha U, DeBruhl N, Bassett LW. In vivo diffusion-weighted MRI of the breast: potential for lesion characterization. J Magn Reson Imaging. 2002;15(6):693–704. doi: 10.1002/jmri.10116. [DOI] [PubMed] [Google Scholar]
- 7.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 8.Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246(1):116–124. doi: 10.1148/radiol.2461061298. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Li WL, Zhang YL, Wu Q, Guo YM, Bai ZL. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010;10:693. doi: 10.1186/1471-2407-10-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Tang M, Min Z, Lu J, Lei X, Zhang X. Accuracy of combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging for breast cancer detection: a meta-analysis. Acta Radiol. 2016;57(6):651–660. doi: 10.1177/0284185115597265. [DOI] [PubMed] [Google Scholar]
- 11.Bogner W, Gruber S, Pinker K, et al. Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis? Radiology. 2009;253(2):341–351. doi: 10.1148/radiol.2532081718. [DOI] [PubMed] [Google Scholar]
- 12.American College of Radiology Imaging Network (ACRIN) 6702: A Multi-Center Study Evaluating the Utility of Diffusion Weighted Imaging for Detection and Diagnosis of Breast Cancer. https://www.acrin.org/TabID/879/Default.aspx.
- 13.Choi SY, Chang YW, Park HJ, Kim HJ, Hong SS, Seo DY. Correlation of the apparent diffusion coefficiency values on diffusion-weighted imaging with prognostic factors for breast cancer. Br J Radiol. 2012;85(1016):e474–479. doi: 10.1259/bjr/79381464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bickel H, Pinker-Domenig K, Bogner W, et al. Quantitative apparent diffusion coefficient as a noninvasive imaging biomarker for the differentiation of invasive breast cancer and ductal carcinoma in situ. Investigative radiology. 2015;50(2):95–100. doi: 10.1097/RLI.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 15.Rahbar H, Partridge SC, Eby PR, et al. Characterization of ductal carcinoma in situ on diffusion weighted breast MRI. Eur Radiol. 2011;21(9):2011–2019. doi: 10.1007/s00330-011-2140-4. [DOI] [PubMed] [Google Scholar]
- 16.Parsian S, Rahbar H, Allison KH, et al. Nonmalignant breast lesions: ADCs of benign and high-risk subtypes assessed as false-positive at dynamic enhanced MR imaging. Radiology. 2012;265(3):696–706. doi: 10.1148/radiol.12112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cipolla V, Santucci D, Guerrieri D, Drudi FM, Meggiorini ML, de Felice C. Correlation between 3T apparent diffusion coefficient values and grading of invasive breast carcinoma. Eur J Radiol. 2014;83(12):2144–2150. doi: 10.1016/j.ejrad.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Costantini M, Belli P, Rinaldi P, et al. Diffusion-weighted imaging in breast cancer: relationship between apparent diffusion coefficient and tumour aggressiveness. Clin Radiol. 2010;65(12):1005–1012. doi: 10.1016/j.crad.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed. 2010;23(6):619–623. doi: 10.1002/nbm.1503. [DOI] [PubMed] [Google Scholar]
- 20.Nakajo M, Kajiya Y, Kaneko T, et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. Eur J Nucl Med Mol Imaging. 2010;37(11):2011–2020. doi: 10.1007/s00259-010-1529-7. [DOI] [PubMed] [Google Scholar]
- 21.Shin JK, Kim JY. Dynamic contrast-enhanced and diffusion-weighted MRI of estrogen receptor-positive invasive breast cancers: Associations between quantitative MR parameters and Ki-67 proliferation status. J Magn Reson Imaging. 2017;45(1):94–102. doi: 10.1002/jmri.25348. [DOI] [PubMed] [Google Scholar]
- 22.Molinari C, Clauser P, Girometti R, et al. MR mammography using diffusion-weighted imaging in evaluating breast cancer: a correlation with proliferation index. Radiol Med. 2015;120(10):911–918. doi: 10.1007/s11547-015-0527-z. [DOI] [PubMed] [Google Scholar]
- 23.Park EK, Cho KR, Seo BK, Woo OH, Cho SB, Bae JW. Additional Value of Diffusion-Weighted Imaging to Evaluate Prognostic Factors of Breast Cancer: Correlation with the Apparent Diffusion Coefficient. Iran J Radiol. 2016;13(1):e33133. doi: 10.5812/iranjradiol.33133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim EJ, Kim SH, Park GE, et al. Histogram analysis of apparent diffusion coefficient at 3.0t: Correlation with prognostic factors and subtypes of invasive ductal carcinoma. J Magn Reson Imaging. 2015;42(6):1666–1678. doi: 10.1002/jmri.24934. [DOI] [PubMed] [Google Scholar]
- 25.Kitajima K, Yamano T, Fukushima K, et al. Correlation of the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with pathologic prognostic factors in breast carcinoma. Eur J Radiol. 2016;85(5):943–949. doi: 10.1016/j.ejrad.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Mori N, Ota H, Mugikura S, et al. Luminal-type breast cancer: correlation of apparent diffusion coefficients with the Ki-67 labeling index. Radiology. 2015;274(1):66–73. doi: 10.1148/radiol.14140283. [DOI] [PubMed] [Google Scholar]
- 27.Iima M, Le Bihan D, Okumura R, et al. Apparent diffusion coefficient as an MR imaging biomarker of low-risk ductal carcinoma in situ: a pilot study. Radiology. 2011;260(2):364–372. doi: 10.1148/radiol.11101892. [DOI] [PubMed] [Google Scholar]
- 28.Rahbar H, Partridge SC, Demartini WB, et al. In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology. 2012;263(2):374–382. doi: 10.1148/radiol.12111368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussein H, Chung C, Moshonov H, Miller N, Kulkarni SR, Scaranelo AM. Evaluation of Apparent Diffusion Coefficient to Predict Grade, Microinvasion, and Invasion in Ductal Carcinoma In Situ of the Breast. Acad Radiol. 2015;22(12):1483–1488. doi: 10.1016/j.acra.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Mori N, Ota H, Mugikura S, et al. Detection of invasive components in cases of breast ductal carcinoma in situ on biopsy by using apparent diffusion coefficient MR parameters. Eur Radiol. 2013;23(10):2705–2712. doi: 10.1007/s00330-013-2902-2. [DOI] [PubMed] [Google Scholar]
- 31.Cheeney S, Rahbar H, Dontchos BN, Javid SH, Rendi MH, Partridge SC. Apparent diffusion coefficient values may help predict which MRI-detected high-risk breast lesions will upgrade at surgical excision. J Magn Reson Imaging. 2017 doi: 10.1002/jmri.25656. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baba S, Isoda T, Maruoka Y, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med. 2014;55(5):736–742. doi: 10.2967/jnumed.113.129395. [DOI] [PubMed] [Google Scholar]
- 33.Cho GY, Moy L, Kim SG, et al. Evaluation of breast cancer using intravoxel incoherent motion (IVIM) histogram analysis: comparison with malignant status, histological subtype, and molecular prognostic factors. Eur Radiol. 2016;26(8):2547–2558. doi: 10.1007/s00330-015-4087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamitani T, Matsuo Y, Yabuuchi H, et al. Correlations between apparent diffusion coefficient values and prognostic factors of breast cancer. Magn Reson Med Sci. 2013;12(3):193–199. doi: 10.2463/mrms.2012-0095. [DOI] [PubMed] [Google Scholar]
- 35.Meng L, Ma P. Apparent diffusion coefficient value measurements with diffusion magnetic resonance imaging correlated with the expression levels of estrogen and progesterone receptor in breast cancer: A meta-analysis. J Cancer Res Ther. 2016;12(1):36–42. doi: 10.4103/0973-1482.150418. [DOI] [PubMed] [Google Scholar]
- 36.Richard R, Thomassin I, Chapellier M, et al. Diffusion-weighted MRI in pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol. 2013;23(9):2420–2431. doi: 10.1007/s00330-013-2850-x. [DOI] [PubMed] [Google Scholar]
- 37.Arponen O, Sudah M, Masarwah A, et al. Diffusion-Weighted Imaging in 3.0 Tesla Breast MRI: Diagnostic Performance and Tumor Characterization Using Small Subregions vs. Whole Tumor Regions of Interest. PLoS One. 2015;10(10):e0138702. doi: 10.1371/journal.pone.0138702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim Y, Ko K, Kim D, et al. Intravoxel incoherent motion diffusion-weighted MR imaging of breast cancer: association with histopathological features and subtypes. Br J Radiol. 2016;89(1063):20160140. doi: 10.1259/bjr.20160140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi BB, Kim SH, Kang BJ, et al. Diffusion-weighted imaging and FDG PET/CT: predicting the prognoses with apparent diffusion coefficient values and maximum standardized uptake values in patients with invasive ductal carcinoma. World J Surg Oncol. 2012;10:126. doi: 10.1186/1477-7819-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeh SK, Kim SH, Kim HS, et al. Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging. 2011;33(1):102–109. doi: 10.1002/jmri.22400. [DOI] [PubMed] [Google Scholar]
- 41.Park SH, Choi HY, Hahn SY. Correlations between apparent diffusion coefficient values of invasive ductal carcinoma and pathologic factors on diffusion-weighted MRI at 3.0 Tesla. J Magn Reson Imaging. 2015;41(1):175–182. doi: 10.1002/jmri.24519. [DOI] [PubMed] [Google Scholar]
- 42.Galban CJ, Ma B, Malyarenko D, et al. Multi-site clinical evaluation of DW-MRI as a treatment response metric for breast cancer patients undergoing neoadjuvant chemotherapy. PLoS One. 2015;10(3):e0122151. doi: 10.1371/journal.pone.0122151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li XR, Cheng LQ, Liu M, et al. DW-MRI ADC values can predict treatment response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. Med Oncol. 2012;29(2):425–431. doi: 10.1007/s12032-011-9842-y. [DOI] [PubMed] [Google Scholar]
- 44.Sharma U, Danishad KK, Seenu V, Jagannathan NR. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009;22(1):104–113. doi: 10.1002/nbm.1245. [DOI] [PubMed] [Google Scholar]
- 45.Liu S, Ren R, Chen Z, et al. Diffusion-weighted imaging in assessing pathological response of tumor in breast cancer subtype to neoadjuvant chemotherapy. J Magn Reson Imaging. 2015;42(3):779–787. doi: 10.1002/jmri.24843. [DOI] [PubMed] [Google Scholar]
- 46.Bufi E, Belli P, Costantini M, et al. Role of the Apparent Diffusion Coefficient in the Prediction of Response to Neoadjuvant Chemotherapy in Patients With Locally Advanced Breast Cancer. Clin Breast Cancer. 2015;15(5):370–380. doi: 10.1016/j.clbc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Gu Y-L, Pan S-M, Ren J, Yang Z-X, Jiang G-Q. The role of magnetic resonance imaging in detection of pathological complete remission in breast cancer patients treated with neoadjuvant chemotherapy: a meta-analysis. Clinical Breast Cancer. 2017 doi: 10.1016/j.clbc.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 48.American College of Radiology Imaging Network (ACRIN) 6698: Diffusion Weighted MR Imaging Biomarkers for Assessment of Breast Cancer Response to Neoadjuvant Treatment: A sub-study of the I-SPY 2 TRIAL (Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging And moLecular Analysis) https://www.acrin.org/TabID/825/Default.aspx.
- 49.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42(3):515–525. [PubMed] [Google Scholar]
- 50.Dorrius MD, Dijkstra H, Oudkerk M, Sijens PE. Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: a systematic review and meta-analysis. Eur Radiol. 2014;24(11):2835–2847. doi: 10.1007/s00330-014-3338-z. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen VT, Rahbar H, Olson ML, Liu CL, Lehman CD, Partridge SC. Diffusion-weighted imaging: Effects of intravascular contrast agents on apparent diffusion coefficient measures of breast malignancies at 3 Tesla. J Magn Reson Imaging. 2015;42(3):788–800. doi: 10.1002/jmri.24844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahlawat S, Khandheria P, Del Grande F, et al. Interobserver variability of selective region-of-interest measurement protocols for quantitative diffusion weighted imaging in soft tissue masses: Comparison with whole tumor volume measurements. J Magn Reson Imaging. 2016;43(2):446–454. doi: 10.1002/jmri.24994. [DOI] [PubMed] [Google Scholar]
- 53.Bickel H, Pinker K, Polanec S, et al. Diffusion-weighted imaging of breast lesions: Region-of-interest placement and different ADC parameters influence apparent diffusion coefficient values. Eur Radiol. 2016 doi: 10.1007/s00330-016-4564-3. [DOI] [PubMed] [Google Scholar]
- 54.Rahbar H, Kurland BF, Olson ML, et al. Diffusion-Weighted Breast Magnetic Resonance Imaging: A Semiautomated Voxel Selection Technique Improves Interreader Reproducibility of Apparent Diffusion Coefficient Measurements. J Comput Assist Tomogr. 2016;40(3):428–435. doi: 10.1097/RCT.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Partridge SC, Nissan N, Rahbar H, Kitsch AE, Sigmund EE. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J Magn Reson Imaging. 2017;45(2):337–355. doi: 10.1002/jmri.25479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bogner W, Pinker-Domenig K, Bickel H, et al. Readout-segmented echo-planar imaging improves the diagnostic performance of diffusion-weighted MR breast examinations at 3.0 T. Radiology. 2012;263(1):64–76. doi: 10.1148/radiol.12111494. [DOI] [PubMed] [Google Scholar]
- 57.Singer L, Wilmes LJ, Saritas EU, et al. High-resolution diffusion-weighted magnetic resonance imaging in patients with locally advanced breast cancer. Acad Radiol. 2012;19(5):526–534. doi: 10.1016/j.acra.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wisner DJ, Rogers N, Deshpande VS, et al. High-resolution diffusion-weighted imaging for the separation of benign from malignant BI-RADS 4/5 lesions found on breast MRI at 3T. J Magn Reson Imaging. 2014;40(3):674–681. doi: 10.1002/jmri.24416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baltzer PA, Renz DM, Herrmann KH, et al. Diffusion-weighted imaging (DWI) in MR mammography (MRM): clinical comparison of echo planar imaging (EPI) and half-Fourier single-shot turbo spin echo (HASTE) diffusion techniques. Eur Radiol. 2009;19(7):1612–1620. doi: 10.1007/s00330-009-1326-5. [DOI] [PubMed] [Google Scholar]
- 60.Kinoshita T, Yashiro N, Ihara N, Funatu H, Fukuma E, Narita M. Diffusion-weighted half-fourier single-shot turbo spin echo imaging in breast tumors: Differentiation of invasive ductal carcinoma from fibroadenoma. Journal of Computer Assisted Tomography. 2002;26(6):1042–1046. doi: 10.1097/00004728-200211000-00033. [DOI] [PubMed] [Google Scholar]
- 61.Granlund KL, Staroswiecki E, Alley MT, Daniel BL, Hargreaves BA. High-resolution, three-dimensional diffusion-weighted breast imaging using DESS. Magn Reson Imaging. 2014;32(4):330–341. doi: 10.1016/j.mri.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solomon E, Nissan N, Furman-Haran E, et al. Overcoming limitations in diffusion-weighted MRI of breast by spatio-temporal encoding. Magn Reson Med. 2015;73(6):2163–2173. doi: 10.1002/mrm.25344. [DOI] [PubMed] [Google Scholar]
- 63.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Lavaljeantet M. Separation of Diffusion and Perfusion in Intravoxel Incoherent Motion Mr Imaging. Radiology. 1988;168(2):497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 64.Bokacheva L, Kaplan JB, Giri DD, et al. Intravoxel incoherent motion diffusion-weighted MRI at 3.0 T differentiates malignant breast lesions from benign lesions and breast parenchyma. J Magn Reson Imaging. 2014;40(4):813–823. doi: 10.1002/jmri.24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iima M, Yano K, Kataoka M, et al. Quantitative Non-Gaussian Diffusion and Intravoxel Incoherent Motion Magnetic Resonance Imaging: Differentiation of Malignant and Benign Breast Lesions. Invest Radiol. 2014 doi: 10.1097/RLI.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 66.Liu C, Liang C, Liu Z, Zhang S, Huang B. Intravoxel incoherent motion (IVIM) in evaluation of breast lesions: comparison with conventional DWI. Eur J Radiol. 2013;82(12):e782–789. doi: 10.1016/j.ejrad.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Sun K, Chen X, Chai W, et al. Breast Cancer: Diffusion Kurtosis MR Imaging-Diagnostic Accuracy and Correlation with Clinical-Pathologic Factors. Radiology. 2015;277(1):46–55. doi: 10.1148/radiol.15141625. [DOI] [PubMed] [Google Scholar]
- 68.Wu D, Li G, Zhang J, Chang S, Hu J, Dai Y. Characterization of breast tumors using diffusion kurtosis imaging (DKI) PLoS One. 2014;9(11):e113240. doi: 10.1371/journal.pone.0113240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nogueira L, Brandao S, Matos E, et al. Application of the diffusion kurtosis model for the study of breast lesions. Eur Radiol. 2014;24(6):1197–1203. doi: 10.1007/s00330-014-3146-5. [DOI] [PubMed] [Google Scholar]
- 70.Iima M, Yano K, Kataoka M, et al. Quantitative Non-Gaussian Diffusion and Intravoxel Incoherent Motion Magnetic Resonance Imaging: Differentiation of Malignant and Benign Breast Lesions. Investigative radiology. 2015;50(4):205–211. doi: 10.1097/RLI.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 71.Partridge SC, Ziadloo A, Murthy R, et al. Diffusion tensor MRI: preliminary anisotropy measures and mapping of breast tumors. J Magn Reson Imaging. 2010;31(2):339–347. doi: 10.1002/jmri.22045. [DOI] [PubMed] [Google Scholar]
- 72.Baltzer PA, Schafer A, Dietzel M, et al. Diffusion tensor magnetic resonance imaging of the breast: a pilot study. Eur Radiol. 2011;21(1):1–10. doi: 10.1007/s00330-010-1901-9. [DOI] [PubMed] [Google Scholar]
- 73.Tsougos I, Svolos P, Kousi E, et al. The contribution of diffusion tensor imaging and magnetic resonance spectroscopy for the differentiation of breast lesions at 3T. Acta Radiol. 2014;55(1):14–23. doi: 10.1177/0284185113492152. [DOI] [PubMed] [Google Scholar]
- 74.Jiang R, Ma Z, Dong H, Sun S, Zeng X, Li X. Diffusion tensor imaging of breast lesions: evaluation of apparent diffusion coefficient and fractional anisotropy and tissue cellularity. Br J Radiol. 2016;89(1064):20160076. doi: 10.1259/bjr.20160076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teruel JR, Cho GY, Moccaldi Rt M, et al. Stimulated echo diffusion tensor imaging (STEAM-DTI) with varying diffusion times as a probe of breast tissue. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25376. [DOI] [PubMed] [Google Scholar]


