Summary
Bone healing involves complex biological pathways and interactions among various cell types and microenvironments. Among them, the monocyte–macrophage–osteoclast lineage and the mesenchymal stem cell–osteoblast lineage are critical, in addition to an initial inflammatory microenvironment. These cellular interactions induce the necessary inflammatory milieu and provide the cells for bone regeneration and immune modulation. Increasing age is accompanied with a rise in the basal state of inflammation, potentially impairing osteogenesis.
The translational potential of this article: Translational research has shown multiple interactions between inflammation, ageing, and bone regeneration. This review presents recent, relevant considerations regarding the effects of inflammation and ageing on bone healing.
Keywords: ageing, bone, inflammation, macrophages, stem cells
Introduction
The global population is ageing at a rate that the United Nations describes as “unprecedented, without parallel in human history” [1]. Indeed, by 2050, the population aged 65 years and older in the United States is expected to reach 89 million—more than double its total of 40.5 million in 2010. Along with this growing elderly population comes an inevitable proliferation of chronic ageing-related diseases, which will greatly impact health care systems for decades [2]. One such disease, osteoporosis, is responsible for over 1.5 million fractures each year, many of which necessitate total joint replacements (TJRs) as treatment. The incidence of both osteoporosis and osteoporotic fractures increases with age; the lifetime risk of a postmenopausal woman sustaining an osteoporotic fracture has been estimated as one in two. Similarly, one in every three men over the age of 75 years will suffer from osteoporosis. The total cost of short- and long-term care of these fractures is over $10 billion annually. This, combined with the ageing population, projects the economic burden of osteoporosis to reach $240 billion by 2040 [3].
Regarding the underlying mechanisms of ageing and diminishing bone mass, the previous “estrogen-centric” school of thought has been cast aside. Deficiency of sex steroids does indeed expedite the process of losing bone mass, but it has more recently been shown that bone loss commences as early as the third decade of life in both men and women. Studies suggest that age-related changes, such as oxidative stress-induced osteocyte death, are fundamental mechanisms of bone density loss and strength reduction [4]. As the body ages, the balance between removal of old bone and formation of new bone becomes reciprocally negative, leading to osteoporosis [5]. On a more microscopic level, aged mesenchymal stem cells (MSCs; precursors to the bone-forming osteoblasts) have demonstrated reduced proliferation, differentiation, and osteogenic potential [6], [7], [8].
Osteoarthritis is another ageing-related condition that will increase in prevalence with the ageing population. TJRs, such as total hip and knee replacements, are also very successful treatments for patients with end-stage joint arthritis who have failed conservative management [9]. Close to 1 million TJRs are performed annually in the United States; over 4 million are projected annually by 2030. Concurrently, total hip and knee revisions are estimated to grow by 137% and 601% in the same span, respectively [10]. With regards to complications of TJRs, advanced age is associated with both higher infection rate and more frequent implant dislocation, both of which can lead to revision surgeries [11]. The lifetime of a TJR implant is estimated at 15–25 years of use, with failure generally occurring owing to slow, progressive, subtle inflammation at the bone-implant interface. This inflammation is caused by innate immune cells such as macrophages, which are activated by implant wear debris. This slowly developing bone loss is known as “aseptic loosening” and is a common cause of TJR revision surgeries [12]. “Septic loosening” may also occur in a similarly slow manner, owing to bacterial-induced inflammation which leads to periprosthetic bone loss and implant dislodgement from the underlying bone bed [13].
Systemic inflammation has also been shown to delay fracture healing and lead to complications such as nonunions [14]. The role of inflammation in bone growth and healing should not be cast purely in a negative light, however. Though proinflammatory cytokines such as tumour necrosis factor alpha (TNF-α) and interleukin (IL)-1 are known to directly lead to joint and bone destruction in arthritis patients, a period of inflammation is unequivocally compulsory for proper fracture healing [15], [16]. Whereas the inflammation seen in severe foreign body reactions and some bone pathologies may be lengthy and unregulated, the therapeutic inflammation in fracture healing is brief and closely regulated [16].
With increased age, individuals develop a persistent, low-grade, subclinical proinflammatory status [17]. Previous studies have found elevated circulating proinflammatory marker levels with age [18]. High levels of TNF-α, inducible nitric oxide synthase, IL-1β, and interferon-γ (INF-γ) have been observed when aged macrophages were challenged with IFN-γ or lipopolysaccharide [19], [20], [21]. Likewise, Smallwood et al. [22] found that aged macrophages have increased nitric oxide production under resting conditions, and are susceptible to oxidative damage. Since inflammation is known to lead to failure of TJR implants via aseptic loosening and other causes, it is particularly worrisome that our ageing population may require many more TJR operations, while simultaneously hosting a proinflammatory state which is unfavourable for TJR implant survival. There is an obvious need for the ability to therapeutically modulate the inflammatory response postoperatively to reduce the occurrences of aseptic loosening and subsequent implant failure.
Inflammation and bone
Inflammation is generally regarded as pathologic and detrimental to wound healing. However, inflammation is a natural response to injury and stress, and, when appropriately modulated, is essential for normal bone repair and homeostasis.
Inflammation and fracture healing
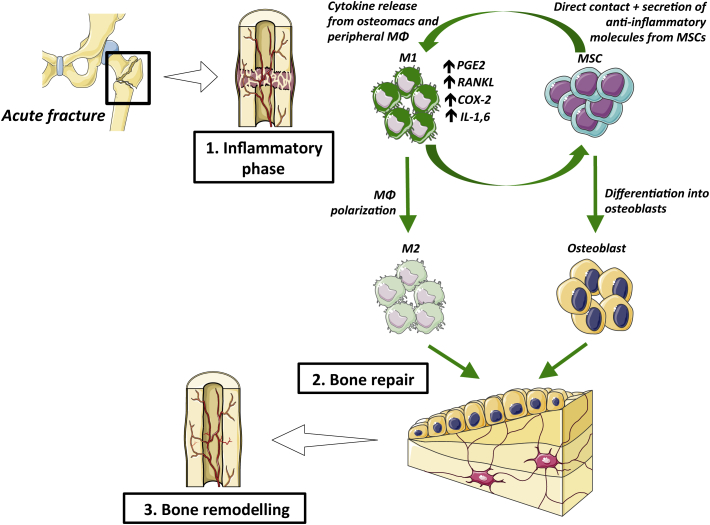
Fracture healing can be described in three characteristic phases: inflammatory, repair, and remodelling [23]. In this section, we will focus on the inflammatory phase, which lasts 3 days in mice, 4 days in rats, and up to 1 week in humans [23], [24], [25]. Acute injury results in bone, vascular, and local soft tissue damage, and resident tissue macrophages and other cells initiate an inflammatory cascade. Within the fracture gap, a fracture haematoma forms to act as a scaffold for recruited polymorphonuclear neutrophils (PMNs) to clear the area of dead cells and debris and secrete chemokines, notably CCL2 (chemokine ligand 2) and IL-6, to attract macrophages [23], [26], [27], [28], [29]. These PMNs exert their effects and die quickly, and their prolonged activation and presence is detrimental to fracture healing [28], [30].
Resident macrophages called osteomacs and recruited macrophages are pivotal for intramembranous and endochondral ossification, respectively [31], [32]. From this inflammatory milieu, macrophages are polarised to a predominantly proinflammatory M1 phenotype early, and increasingly assume the anti-inflammatory phenotype as healing progresses [33]. Macrophages can be polarised to the M1 phenotype by IFN-γ and lipopolysaccharide, and have increased expression of inducible nitric oxide synthase, CCR7 (C–C chemokine receptor type 7), and HLA-DR (Human Leukocyte Antigen – antigen D Related) [34], [35]. Macrophages can be polarized to M2 by IL-4, and M2 macrophages are characterised by increased expression of CD206 (Cluster of Differentiation 206), Ym1 (eosinophil chemotactic factor), CD163, CCL1, CCL18, FIZZ1 (Found in Inflammatory Zone protein), arginase 1, and chitotriosidase [36], [37]. During fracture healing, the M1-to-M2 transition is likely mediated by both macrophage autocrine signalling and paracrine signalling from other cells at the fracture site, including MSCs. MSCs are known to exert an anti-inflammatory effect and polarise macrophages towards the M2 phenotype for the resolution of inflammation and initiation of repair [38], [39], [40], [41]. Although the natural healing process moves towards an anti-inflammatory environment, acute inflammation is important for fracture repair, as it stimulates angiogenesis and promotes proliferation and differentiation of MSCs towards osteoblasts [31], [42], [43]. Studies have shown that M1 macrophages promote osteogenesis of MSCs and that this effect can be enhanced by precise M1-to-M2 transition either 72 h or 96 h after coculture [44], [45], [46]. Moreover, M2 macrophages survive longer than M1 macrophages, highlighting that the pro-osteogenic effects of M1 macrophages are exerted early and transiently in osteogenesis [47].
After a variable time of macrophage activity, lymphocytes migrate to the fracture site, initiate the adaptive immune response, and secrete large amounts of proinflammatory cytokines, such as IL-1, IL-6, and receptor activator of nuclear factor kappa-B ligand (RANKL) [23], [31], [42], [43]. The effects of these and other inflammatory cytokines on bone are reviewed extensively by Claes et al. [23] and Thomas and Puleo [48].
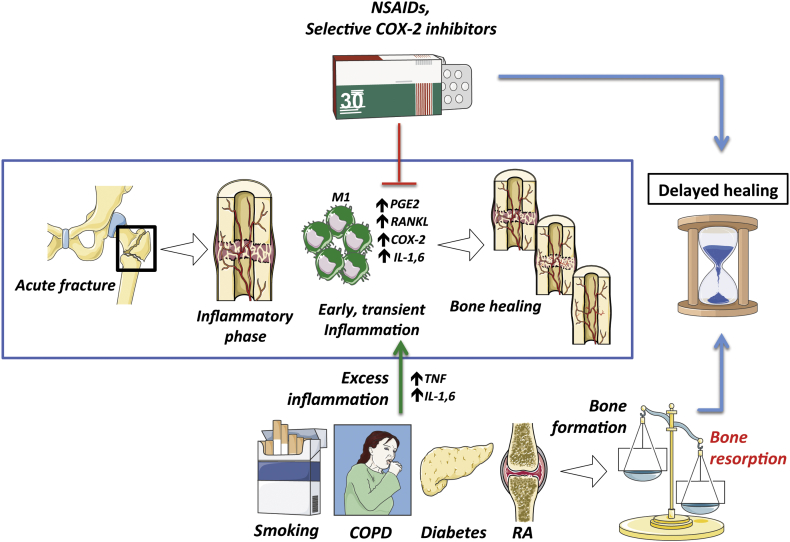
Inhibition of this inflammatory phase disrupts bone formation and increases the risk of complications (e.g., nonunion). For example, cyclooxygenase-2 (COX-2) is highly expressed after a fracture, and one of its metabolites, prostaglandin E2, is known to play a role in the inflammatory phase of healing and in promoting MSC and periosteal progenitor differentiation into osteoblasts [49], [50], [51], [52]. Studies have shown that inhibition of COX-2 and prostaglandin E2 by nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors in vitro and in the clinical setting is detrimental to bone healing [53], [54], [55]. In a retrospective review of human femoral fractures, Giannoudis et al. [56] found that patients who had nonunions of fractures were more likely to have been treated with NSAIDs. However, in the craniofacial setting, NSAID use is widely prescribed and has been shown to promote the success of endosseous implants [57]. These differences highlight the context-specific effects of COX-2 and inflammation.
In addition to these molecular factors, biomechanical conditions at the fracture site modulate the early inflammatory environment. Moderate interfragmentary movement enhances endochondral ossification, whereas fractures with flexible fixation have prolonged inflammation with an abundance of cytotoxic T cells, other leukocytes, and M1 macrophages [58], [59], [60]. Similarly, rigid fixation has been shown to increase macrophage recruitment and reduce macrophage residency at the fracture site, leading to improved bone formation [60].
This early inflammatory phase stimulates angiogenesis and promotes proliferation and differentiation of MSCs towards osteoblasts [31], [42], [43]. Studies have shown that M1 macrophages in particular promote osteogenesis of MSCs and that this effect can be enhanced by precise and timely M1-to-M2 transition [44], [45], [46]. These findings highlight that an early and transient period of inflammation is required for bone repair. Figure 1 shows an overview of the bone fracture healing process.
Figure 1.
The three phases of bone healing. COX-2 = cyclooxygenase-2; IL1,6 = interleukin 1 & 6; M1 = M1 macrophages; M2 = M2 macrophages; MΦ = macrophage; MSCs = mesenchymal stem cells; PGE2 = prostaglandin E2; RANKL = receptor activator of nuclear factor kappa-B ligand.
Excess inflammation
Although early and transient inflammation is essential for proper bone healing, chronic systemic inflammation is detrimental to bone remodelling and fracture healing. A variety of chronic inflammatory states such as rheumatoid arthritis (RA), chronic obstructive pulmonary disease, diabetes mellitus, systemic lupus erythematous, and even periprosthetic wear particles from TJRs are characterised by bone loss and secondary osteoporosis [23], [61]. With an excess of proinflammatory cytokines such as IL-1, IL-6, and TNF, the delicate balance between bone resorption and formation is disturbed and shifted towards osteoclastogenesis [23]. Figure 2 shows several factors that impair bone healing.
Figure 2.
Factors impairing bone healing. COPD = chronic obstructive pulmonary disease; COX-2 = cyclooxygenase-2; IL 1,6 = interleukin 1 & 6; M1 = M1 macrophage; NSAIDs = nonsteroidal antiinflammatory drugs; PGE2 = prostaglandin E2; RA = rheumatoid arthritis; RANKL = receptor activator of nuclear factor kappa-B ligand; TNF = tumour necrosis factor.
In inflammatory environments, immune cells from both the innate and adaptive immune responses are activated. For example, in RA and periodontal disease, dendritic cells can form aggregates with T cells in inflammatory foci that alter RANK–RANKL signalling towards bone resorption [62]. These same dendritic cells can further exacerbate bone resorption by transdifferentiating into osteoclasts [63]. Similarly, T cells are found in greater proportion in fracture calluses isolated from fractures with delayed healing [23], [60]. Conversely, Recombination activating gene 1-knockout (Rag1) mice that lack B and T cells have better fracture healing than immune-competent mice [64]. Together, these findings suggest that activation of the adaptive immune system is detrimental to fracture healing.
Disease-specific changes can also alter bone homeostasis. In RA, osteoarthritis, and diabetes mellitus, surrounding adipose tissue can dynamically contribute to inflammation and lead to dysfunctional bone homeostasis [65]. It has been shown that leptin, adiponectin, and resistin mediate inflammation in arthritis and contribute to inflammation and degeneration of the joints [65]. Similarly, the formation of advanced glycation end-products in diabetes mellitus as a result of nonenzymatic glycosylation has been shown to upregulate secretion of inflammatory cytokines, increase osteoclast-mediated resorption, and inhibit osteoblast activity [48], [66], [67], [68].
Ageing itself has also been considered a chronic inflammatory state. The phenomenon of “inflamm-ageing” has been described, as many cells, including macrophages, show increased responsiveness and hypersensitivity to inflammatory signals [69]. Beyond intrinsic cellular changes, this preactivated inflammatory resting state may contribute to impaired fracture healing with age. This topic is reviewed thoroughly by Gibon et al. [69].
In addition to chronic inflammatory diseases, wear particles from prosthetic devices (e.g., total knee and hip replacements) can cause local chronic inflammation that results in osteolysis. Macrophages, especially proinflammatory M1 macrophages, play a key role in mediating periprosthetic inflammation, cellular recruitment, and bone resorption [70], [71]. Studies have shown that immunomodulation of these macrophages with IL-4 (which promotes antiinflammatory M2 polarisation) may enhance osteogenesis by reducing the detrimental effects of prolonged inflammation mediated by M1 macrophages [45], [71].
Unlike chronic inflammatory states, acute systemic inflammation (e.g., polytrauma, sepsis) is characterised by specific activation of PMNs and macrophages [72]. As noted previously, PMNs are thought to negatively impact bone formation, while neutropenia in animals has been shown to improve fracture repair [30]. In polytrauma, a complex inflammatory cascade is initiated and is characterised by overactivation of PMNs, rapid release of proinflammatory cytokines, and complement activation [30], [72]. Because of this severe systemic inflammatory environment, fracture repair is significantly impaired in polytrauma patients.
Modulation of inflammation
Given this information, precise temporal control of inflammation is essential for normal skeletal health and repair. In addition to traditional anti-inflammatory therapies (e.g., NSAIDs, corticosteroids) that are detrimental to bone, other methods of immunomodulation show promise for the resolution of inflammation to promote osteogenesis and reestablishment of the osteoclast–osteoblast balance. Several groups have shown that MSCs have anti-inflammatory properties, and MSCs have already been used in the clinic for cardiovascular regenerative applications [38], [73], [74]. MSCs promote M2 polarisation of macrophages and show promise in mediating inflammation following injury [38], [39], [41]. Similarly, we have shown that inducing M1-to-M2 polarisation in macrophage-MC3T3 and macrophage-MSC cocultures after 72 h or 96 h can promote enhanced osteogenesis [45]. This precise timing and dynamic regulation of “good inflammation” may further be modulated by lipoxins, resolvins, and protectins, which act as inflammation “stop signals” [75]. These agents have been used to treat infection and peritonitis caused by Toxoplasma gondii and Angiostrongylus costaricensis in vitro and as protection for periodontitis [76], [77], [78], [79].
Bone and ageing
Ageing affects the bone at the macroscopic and microscopic levels. This section will focus on the effect of ageing on bone cells with an emphasis on bone marrow MSCs.
Ageing and fracture healing
Fracture in the ageing skeleton is a concerning issue, both clinically and for the health care system in general. The effects of ageing on bone healing is multifactorial [80]. Bone healing is a complex process which requires local signalling molecules, systemic signalling molecules, octeoprogenitor cells, efficient genetic machinery, and a viable blood microcirculation. However, in a study using aged rats, Prisby et al. [81] have shown that ageing reduces blood flow. The authors showed that aged rats had reduced femoral blood flow by 45% in the diaphyseal marrow. The reduction in endothelium-dependent vasodilation was mediated through impairment of the nitric oxide synthase pathway, a prominent vasodilator. In a small animal fracture model (mice), Lopas et al. [82] quantified gene expression and multiple other parameters of the integrity of the bone callus. Interestingly, Oserix and Sox9 gene expression were much more upregulated in young mice at 10 days and 20 days postfracture. However, geriatric mice produced a less robust callus, with decreased bone volume, density, and content, and a delay in peak bone formation. Similarly, Wang et al. [83] investigated age-related genes that affected bone healing using a fracture model in aged rats. Their investigation revealed four potential age-related genes: secreted phosphoprotein 1, integrin-binding sialoprotein, tenscin trophinin, and collagen type IIIα3, as well as the extracellular matrix receptor interaction pathway as a potential age-related process during bone repair. On the cellular level, Matsumoto et al. [84] showed a higher number of alkaline phosphatase positive osteoblasts in a broken rib of young mice than in older mice. Moreover, the number of tartrate-resistant acid phosphatase positive osteoclasts was significantly higher in aged mice. More importantly, they also showed a significant decrease in sonic hedgehog protein secretion in older mice. These different studies underline the complex biological processes taking place in ageing bone.
Ageing and periosteal cells
The role of the periosteum has been well recognized as a critical factor for bone healing [85], [86], [87]. The periosteum is a reservoir of MSCs known as periosteum-derived progenitor cells (PDPCs). They can be found in the inner cambium layer of the periosteum [88]. Their potential for bone healing is well established and studies have shown their key role in endogenous bone repair and remodelling [89], [90], [91]. Ferretti et al. [92] compared PDPCs from eight human donors aged 16–28 years and 63–92 years; aged PDPCs exhibited significant changes. Two critical cell cycle proteins (Ki67 and p53 (Tumor protein p53)) had significantly lower expression in aged PDPCs using immunohistochemistry, indicating senescence. Nitric oxide production was found to be higher in aged PDPCs as a result of elevated oxidative damage to these cells. Using Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR), the authors investigated noteworthy genes involved in osteogenesis. A significant increase in IL-6 messenger RNA was detected in samples from a 92-year-old, as well as a higher RANKL/Osteoprotegerin (OPG) ratio. Taken together, these data indicate ageing-related changes in PDPCs, with a trend towards bone resorption. In a mouse fracture model, Yukata et al. [93] assessed bone healing and the effect of recombinant human parathyroid hormone 1–34 (PTH1-34) on bone healing in young and aged mice. A transverse tibial osteotomy was performed and fixed with an intramedullary needle. Mice were then allocated into two groups receiving either hPTH1-34 or a saline solution control. Their results showed greater width of the newly formed periosteal regenerative tissue in young mice, and the effect of the PTH1-34 was higher in the young mice, both the control and PTH1-34 treated groups, compared with the old mice. Furthermore, expression of the the cell cycle regulator cyclin D1 was significantly lower in aged mice, as were the osteoblast genes Runx2, Osterix, and Osteocalcin. Al-Qtaitat et al. [94] investigated age-related changes within the periosteum microstructure. They assessed numerous periosteum characteristics in young and aged porcine mandibles. In aged animals, the periosteum was thinner, had fewer collagen type III fibres, and was more prone to calcify and harden, which can jeopardise its functional properties. Overall, the effect of ageing on periosteal MSCs tends to mitigate their bone healing function, but more studies are needed to further understand details of the various implicated mechanisms.
Conclusion
Taken together, it is clear that precise temporal, spatial, and contextual regulation of inflammation is key to normal bone homeostasis and repair. As such, a more granular understanding of the complex and often contradictory role of inflammation in skeletal health is needed to develop effective therapies for a variety of disease states. Modulation of macrophage polarisation and MSC–macrophage crosstalk through local and targeted interventions towards a microenvironment that favours bone healing represents a potential strategy to optimise bone healing in the elderly.
Conflicts of interest/Funding
The authors have no conflicts of interest relevant to this article.
References
- 1.United Nations DESA . 2001. Population division. World population aging 1950–2050. Available at: http://www.un.org/esa/population/publications/worldageing19502050/. [Accessed 17 February 2017] [Google Scholar]
- 2.Dall T.M., Gallo P.D., Chakrabarti R., West T., Semilla A.P., Storm M.V. An aging population and growing disease burden will require alarge and specialized health care workforce by 2025. Health Aff. 2013;32:2013–2020. doi: 10.1377/hlthaff.2013.0714. [DOI] [PubMed] [Google Scholar]
- 3.Dobbs M.B., Buckwalter J., Saltzman C. Osteoporosis: the increasing role of the orthopaedist. Iowa Orthop J. 1999;19:43–52. [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida M., Han L., Martin-Millan M., Plotkin L.I., Stewart S.A., Roberson P.K. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manolagas S.C., Parfitt A.M. What old means to bone. Trends Endocrinol Metab. 2010;21:369–374. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethe S., Scutt A., Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Beane O.S., Fonseca V.C., Cooper L.L., Koren G., Darling E.M. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PloS One. 2014;9 doi: 10.1371/journal.pone.0115963. e115963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asumda F.Z., Chase P.B. Age-related changes in rat bone-marrow mesenchymal stem cell plasticity. BMC Cell Biol. 2011;12:44. doi: 10.1186/1471-2121-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jämsen E., Kouri V.P., Ainola M., Goodman S.B., Nordström D.C., Eklund K.K. Correlations between macrophage polarizing cytokines, inflammatory mediators, osteoclast activity, and toll-like receptors in tissues around aseptically loosened hip implants. J Biomed Mater Res A. 2017;105:454–463. doi: 10.1002/jbm.a.35913. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Jt Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 11.Morrey B. Difficult complications after hip joint replacement: dislocation. Clin Orthop Relat Res. 1997;344:172–187. [PubMed] [Google Scholar]
- 12.Landgraeber S., Jäger M., Jacobs J.J., Hallab N.J. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediat Inflamm. 2014;2014:185150. doi: 10.1155/2014/185150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pajarinen J., Jamsen E., Konttinen Y.T., Goodman S.B. Innate immune reactions in septic and aseptic osteolysis around hip implants. J Long Term Eff Med Implants. 2014;24:283–296. doi: 10.1615/jlongtermeffmedimplants.2014010564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claes L., Recknagel S., Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 15.Marsell R., Einhorn T.A. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mountziaris P.M., Mikos A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14:179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung H., Lee E., Choi Y., Kim J., Kim D., Zou Y. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 2011;90:830–840. doi: 10.1177/0022034510387794. [DOI] [PubMed] [Google Scholar]
- 18.Stranks A.J., Hansen A.L., Panse I., Mortensen M., Ferguson D.J.P., Puleston D.J. Autophagy controls acquisition of aging features in macrophages. J Innate Immun. 2015;7:375–391. doi: 10.1159/000370112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett J.P., Costello D.A., O'Sullivan J., Cowley T.R., Lynch M.A. Bone marrow-derived macrophages from aged rats are more responsive to inflammatory stimuli. J Neuroinflammation. 2015;12:67. doi: 10.1186/s12974-015-0287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Wehling-Henricks M., Samengo G., Tidball J.G. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell. 2015;14:678–688. doi: 10.1111/acel.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cecilio C.A., Costa E.H., Simioni P.U., Gabriel D.L., Tamashiro W.M. Aging alters the production of iNOS, arginase and cytokines in murine macrophages. Braz J Med Biol Res. 2011;44:671–681. doi: 10.1590/s0100-879x2011007500067. [DOI] [PubMed] [Google Scholar]
- 22.Smallwood H.S., Lopez-Ferrer D., Squier T.C. Aging enhances the production of reactive oxygen species and bactericidal activity in peritoneal macrophages by upregulating classical activation pathways. Biochemistry. 2011;50:9911–9922. doi: 10.1021/bi2011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claes L., Recknagel S., Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 24.Cho T.-J., Gerstenfeld L.C., Einhorn T.A. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Min Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 25.Rüedi T.P., Murphy W.M. Thieme; Stuttgart; New York; Davos Platz: 2007. AO principles of fracture management. [Google Scholar]
- 26.Office of the Surgeon General . Office of the Surgeon General; Rockville, MD: 2004. The burden of bone disease. Bone health and osteoporosis: a report of the surgery general. [PubMed] [Google Scholar]
- 27.Chung R., Cool J.C., Scherer M.A., Foster B.K., Xian C.J. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J Leukoc Biol. 2006;80:1272–1280. doi: 10.1189/jlb.0606365. [DOI] [PubMed] [Google Scholar]
- 28.Bastian O., Pillay J., Alblas J., Leenen L., Koenderman L., Blokhuis T. Systemic inflammation and fracture healing. J Leukoc Biol. 2011;89:669–673. doi: 10.1189/jlb.0810446. [DOI] [PubMed] [Google Scholar]
- 29.Andrew J.G., Andrew S.M., Freemont A.J., Marsh D.R. Inflammatory cells in normal human fracture healing. Acta Orthop Scand. 1994;65:462–466. doi: 10.3109/17453679408995493. [DOI] [PubMed] [Google Scholar]
- 30.Grøgaard B., Gerdin B., Reikerås O. The polymorphonuclear leukocyte: has it a role in fracture healing? Arch Orthop Trauma Surg. 1990;109:268–271. doi: 10.1007/BF00419942. [DOI] [PubMed] [Google Scholar]
- 31.Xing Z., Lu C., Hu D., Yu Y.Y., Wang X., Colnot C. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3:451–458. doi: 10.1242/dmm.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander K.A., Chang M.K., Maylin E.R., Kohler T., Müller R., Wu A.C. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Min Res. 2011;26:1517–1532. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 33.Schlundt C., El Khassawna T., Serra A., Dienelt A., Wendler S., Schell H. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone. 2015 Oct 31 doi: 10.1016/j.bone.2015.10.019. pii: S8756-3282(15)00392-0. [DOI] [PubMed] [Google Scholar]
- 34.Guihard P., Boutet M.-A., Brounais-Le Royer B., Gamblin A.-L., Amiaud J., Renaud A. Oncostatin m, an inflammatory cytokine produced by macrophages, supports intramembranous bone healing in a mouse model of tibia injury. Am J Pathol. 2015;185:765–775. doi: 10.1016/j.ajpath.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Martinez F.O., Sica A., Mantovani A., Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 36.Maresz K., Ponomarev E.D., Barteneva N., Tan Y., Mann M.K., Dittel B.N. IL-13 induces the expression of the alternative activation marker Ym1 in a subset of testicular macrophages. J Reprod Immunol. 2008;78:140–148. doi: 10.1016/j.jri.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair M.G., Du Y., Perrigoue J.G., Zaph C., Taylor J.J., Goldschmidt M. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 39.François M., Romieu-Mourez R., Li M., Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 40.Maggini J., Mirkin G., Bognanni I., Holmberg J., Piazzón I.M., Nepomnaschy I. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PloS One. 2010;5 doi: 10.1371/journal.pone.0009252. e9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Q.-Z., Su W.-R., Shi S.-H., Wilder-Smith P., Xiang A.P., Wong A. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerstenfeld L.C., Cullinane D.M., Barnes G.L., Graves D.T., Einhorn T.A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 43.Kon T., Cho T.J., Aizawa T., Yamazaki M., Nooh N., Graves D. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Min Res. 2001;16:1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 44.Guihard P., Danger Y., Brounais B., David E., Brion R., Delecrin J. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30:762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- 45.Loi F., Córdova L.A., Zhang R., Pajarinen J., Lin T.-H., Goodman S.B. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther. 2016;7:15. doi: 10.1186/s13287-016-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicolaidou V., Wong M.M., Redpath A.N., Ersek A., Baban D.F., Williams L.M. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PloS One. 2012;7 doi: 10.1371/journal.pone.0039871. e39871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang S.C.-C., Everts B., Ivanova Y., O'Sullivan D., Nascimento M., Smith A.M. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas M.V., Puleo D.A. Infection, inflammation, and bone regeneration: a paradoxical relationship. J Dent Res. 2011;90:1052–1061. doi: 10.1177/0022034510393967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naik A.A., Xie C., Zuscik M.J., Kingsley P., Schwarz E.M., Awad H. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Min Res. 2009;24:251–264. doi: 10.1359/jbmr.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon D.S., Yoo J.H., Kim Y.H., Paik S., Han C.D., Lee J.W. The effects of COX-2 inhibitor during osteogenic differentiation of bone marrow-derived human mesenchymal stem cells. Stem Cells Dev. 2010;19:1523–1533. doi: 10.1089/scd.2009.0393. [DOI] [PubMed] [Google Scholar]
- 51.Simon A.M., Manigrasso M.B., O'Connor J.P. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Min Res. 2002;17:963–976. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 52.Huang C., Xue M., Chen H., Jiao J., Herschman H.R., O'Keefe R.J. The spatiotemporal role of COX-2 in osteogenic and chondrogenic differentiation of periosteum-derived mesenchymal progenitors in fracture repair. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100079. e100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerstenfeld L.C., Thiede M., Seibert K., Mielke C., Phippard D., Svagr B. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–675. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 54.Pountos I., Georgouli T., Calori G.M., Giannoudis P.V. Do nonsteroidal anti-inflammatory drugs affect bone healing? A critical analysis. Sci World J. 2012;2012:606404. doi: 10.1100/2012/606404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu L.Y., Loi F., Nathan K., Lin T.-H., Pajarinen J., Gibon E. Pro-inflammatory M1 macrophages promote osteogenesis by mesenchymal stem cells via the COX-2-Prostaglandin E2 Pathway. J Orthop Res. 2017 doi: 10.1002/jor.23553. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giannoudis P.V., MacDonald D.A., Matthews S.J., Smith R.M., Furlong A.J., De Boer P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Jt Surg Br. 2000;82:655–658. doi: 10.1302/0301-620x.82b5.9899. [DOI] [PubMed] [Google Scholar]
- 57.Alissa R., Sakka S., Oliver R., Horner K., Esposito M., Worthington H.V. Influence of ibuprofen on bone healing around dental implants: a randomised double-blind placebo-controlled clinical study. Eur J Oral Implantol. 2009;2:185–199. [PubMed] [Google Scholar]
- 58.Claes L. Biomechanical principles and mechanobiologic aspects of flexible and locked plating. J Orthop Trauma. 2011;25(Suppl. 1):S4–S7. doi: 10.1097/BOT.0b013e318207093e. [DOI] [PubMed] [Google Scholar]
- 59.Claes L.E., Heigele C.A. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech. 1999;32:255–266. doi: 10.1016/s0021-9290(98)00153-5. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt-Bleek K., Schell H., Schulz N., Hoff P., Perka C., Buttgereit F. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res. 2012;347:567–573. doi: 10.1007/s00441-011-1205-7. [DOI] [PubMed] [Google Scholar]
- 61.Hardy R., Cooper M.S. Bone loss in inflammatory disorders. J Endocrinol. 2009;201:309–320. doi: 10.1677/JOE-08-0568. [DOI] [PubMed] [Google Scholar]
- 62.Alnaeeli M., Park J., Mahamed D., Penninger J.M., Teng Y.-T.A. Dendritic cells at the osteo-immune interface: implications for inflammation-induced bone loss. J Bone Min Res. 2007;22:775–780. doi: 10.1359/jbmr.070314. [DOI] [PubMed] [Google Scholar]
- 63.Rivollier A., Mazzorana M., Tebib J., Piperno M., Aitsiselmi T., Rabourdin-Combe C. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood. 2004;104:4029–4037. doi: 10.1182/blood-2004-01-0041. [DOI] [PubMed] [Google Scholar]
- 64.Toben D., Schroeder I., El Khassawna T., Mehta M., Hoffmann J.-E., Frisch J.-T. Fracture healing is accelerated in the absence of the adaptive immune system. J Bone Min Res. 2011;26:113–124. doi: 10.1002/jbmr.185. [DOI] [PubMed] [Google Scholar]
- 65.Toussirot E., Streit G., Wendling D. The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr Med Chem. 2007;14:1095–1100. doi: 10.2174/092986707780362826. [DOI] [PubMed] [Google Scholar]
- 66.Miyata T., Notoya K., Yoshida K., Horie K., Maeda K., Kurokawa K. Advanced glycation end products enhance osteoclast-induced bone resorption in cultured mouse unfractionated bone cells and in rats implanted subcutaneously with devitalized bone particles. J Am Soc Nephrol. 1997;8:260–270. doi: 10.1681/ASN.V82260. [DOI] [PubMed] [Google Scholar]
- 67.Katayama Y., Akatsu T., Yamamoto M., Kugai N., Nagata N. Role of nonenzymatic glycosylation of type I collagen in diabetic osteopenia. J Bone Min Res. 1996;11:931–937. doi: 10.1002/jbmr.5650110709. [DOI] [PubMed] [Google Scholar]
- 68.Takagi M., Kasayama S., Yamamoto T., Motomura T., Hashimoto K., Yamamoto H. Advanced glycation endproducts stimulate interleukin-6 production by human bone-derived cells. J Bone Min Res. 1997;12:439–446. doi: 10.1359/jbmr.1997.12.3.439. [DOI] [PubMed] [Google Scholar]
- 69.Gibon E., Lu L., Goodman S.B. Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther. 2016;7:44. doi: 10.1186/s13287-016-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ingham E., Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 71.Rao A.J., Gibon E., Ma T., Yao Z., Smith R.L., Goodman S.B. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012;8:2815–2823. doi: 10.1016/j.actbio.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Göktürk E., Turgut A., Bayçu C., Günal I., Seber S., Gülbas Z. Oxygen-free radicals impair fracture healing in rats. Acta Orthop Scand. 1995;66:473–475. doi: 10.3109/17453679508995590. [DOI] [PubMed] [Google Scholar]
- 73.English K., Barry F.P., Mahon B.P. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115:50–58. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Choi H., Lee R.H., Bazhanov N., Oh J.Y., Prockop D.J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Serhan C.N. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 76.Bandeira-Melo C., Serra M.F., Diaz B.L., Cordeiro R.S., Silva P.M., Lenzi H.L. Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: relationship with concurrent eosinophilia. J Immunol. 2000;164:1029–1036. doi: 10.4049/jimmunol.164.2.1029. [DOI] [PubMed] [Google Scholar]
- 77.Aliberti J., Hieny S., Reis e Sousa C., Serhan C.N., Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol. 2002;3:76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- 78.Aliberti J., Serhan C., Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med. 2002;196:1253–1262. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spite M., Norling L.V., Summers L., Yang R., Cooper D., Petasis N.A. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gruber R., Koch H., Doll B.A., Tegtmeier F., Einhorn T.A., Hollinger J.O. Fracture healing in the elderly patient. Exp Gerontol. 2006;41:1080–1093. doi: 10.1016/j.exger.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 81.Prisby R.D., Ramsey M.W., Behnke B.J., Dominguez J.M., Donato A.J., Allen M.R. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Min Res. 2007;22:1280–1288. doi: 10.1359/jbmr.070415. [DOI] [PubMed] [Google Scholar]
- 82.Lopas L.A., Belkin N.S., Mutyaba P.L., Gray C.F., Hankenson K.D., Ahn J. Fractures in geriatric mice show decreased callus expansion and bone volume. Clin Orthop Relat Res. 2014;472:3523–3532. doi: 10.1007/s11999-014-3829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang W., Shen H., Xie J., Zhou Q., Chen Y., Lu H. Bioinformatics analysis of time-series genes profiling to explore key genes affected by age in fracture healing. Mol Biol Rep. 2014;41:3881–3889. doi: 10.1007/s11033-014-3255-x. [DOI] [PubMed] [Google Scholar]
- 84.Matsumoto K., Shimo T., Kurio N., Okui T., Obata K., Masui M. Expression and role of sonic hedgehog in the process of fracture healing with aging. In Vivo. 2016;30:99–105. [PubMed] [Google Scholar]
- 85.Phillips A.M. Overview of the fracture healing cascade. Injury. 2005;36(Suppl. 3):S5–S7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 86.Panetta N.J., Gupta D.M., Longaker M.T. Bone regeneration and repair. Curr Stem Cell Res Ther. 2010;5:122–128. doi: 10.2174/157488810791268618. [DOI] [PubMed] [Google Scholar]
- 87.Marsell R., Einhorn T.A. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang H., Knothe Tate M.L. Concise review: the periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl Med. 2012;1:480–491. doi: 10.5966/sctm.2011-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ringe J., Leinhase I., Stich S., Loch A., Neumann K., Haisch A. Human mastoid periosteum-derived stem cells: promising candidates for skeletal tissue engineering. J Tissue Eng Regen Med. 2008;2:136–146. doi: 10.1002/term.75. [DOI] [PubMed] [Google Scholar]
- 90.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Min Res. 2009;24:274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang X., Xie C., Lin A.S., Ito H., Awad H., Lieberman J.R. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Min Res. 2005;20:2124–2137. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferretti C., Lucarini G., Andreoni C., Salvolini E., Bianchi N., Vozzi G. Human periosteal derived stem cell potential: the Impact of age. Stem Cell Rev. 2015;11:487–500. doi: 10.1007/s12015-014-9559-3. [DOI] [PubMed] [Google Scholar]
- 93.Yukata K., Xie C., Li T.-F., Takahata M., Hoak D., Kondabolu S. Aging periosteal progenitor cells have reduced regenerative responsiveness to bone injury and to the anabolic actions of PTH 1-34 treatment. Bone. 2014;62:79–89. doi: 10.1016/j.bone.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al-Qtaitat A., Shore R.C., Aaron J.E. Structural changes in the ageing periosteum using collagen III immuno-staining and chromium labelling as indicators. J Musculoskelet Neuronal Interact. 2010;10:112–123. [PubMed] [Google Scholar]