Abstract
Diseases of glia, including both astrocytes and oligodendrocytes, are among the most prevalent and disabling, yet least appreciated, conditions in neurology. In recent years, it has become clear that besides the overtly glial disorders of oligodendrocyte loss and myelin failure, such as the leukodystrophies and inflammatory demyelinations, that a number of neurodegenerative and psychiatric disorders may also be causally linked to glial dysfunction, and derive from astrocytic as well as oligodendrocytic pathology. The relative contribution of glial dysfunction to many of these disorders may be so great as to allow their treatment by the delivery of allogeneic glial progenitor cells (GPCs), the precursors to both astroglia and myelin-producing oligodendrocytes. Given the development of new methods for producing and isolating these cells from pluripotent stem cells, both the myelin disorders and appropriate glial-based neurodegenerative conditions may now be compelling targets for cell-based therapy. As such, glial cell-based therapies may offer potential benefit to for a broader range of diseases than ever before contemplated, including disorders such as Huntington disease and the motor neuron degeneration of amyotrophic lateral sclerosis, which have traditionally been considered neuronal in nature.
Keywords: glial progenitor, oligodendrocytic progenitor, demyelinating disease, leukodystrophy, multiple sclerosis, Huntington disease, cell transplant
Oligodendrocytes are the sole source of myelin in the adult CNS, and their loss or dysfunction is at the heart of a wide variety of diseases of both children and adults. In children, the hereditary leukodystrophies accompany cerebral palsy as major sources of neurological morbidity. In adults, oligodendrocytic loss and demyelination contribute to diseases as diverse as multiple sclerosis, white matter stroke and spinal cord injury (Roy et al., 2004). In addition, demyelination is also noted in degenerative disorders as varied as normal aging, Huntington’s and Alzheimer’s diseases, while oligodendrocytic pathology has been associated with disorders as diverse as ALS (Lee et al., 2012) and schizophrenia (Tkachev et al., 2003). As a result, the myelin disorders are especially attractive targets for cell-based therapeutic strategies, as are the related disorders of astrocytes and glial progenitor cells that include dysmyelination as part of their presentation. Several recent studies have supported the readiness with which axons can remyelinate after either congenital or acquired demyelination, if provided myelinogenic cells (Duncan et al., 2009; Windrem et al., 2008). Human glial progenitor cells – also referred to as either oligodendrocyte progenitor cells or NG2 cells (Nishiyama et al., 2009) – can generate both oligodendrocytes and astrocytes, and as such are promising reagents by which to concurrently restore myelin to demyelinated regions of the diseased or injured CNS, while addressing the disorders of astrocytic function that so often attend white matter disease.
Glial progenitor cells in vivo
Glial progenitor cells (GPCs) arise from neural stem cells of the ventricular subependyma, and disperse widely throughout the central nervous system, pervading both gray and white matter (Roy et al., 1999). While the presence of GPCs in the adult human brain was inferred in several early studies that identified immature oligodendroglia in adult brain tissue (Armstrong et al., 1992; Gogate et al., 1994), mitotic bipotential GPCs were first isolated from the adult human brain using CNP2 promoter-based fluorescence-activated cell sorting (Roy et al., 1999). This study and others revealed that human glial progenitors comprise roughly 3% of all cells in the adult forebrain, and are the major mitotic neural phenotype of the adult brain (Roy et al., 1999; Scolding et al., 1998). Subsequent work demonstrated that human GPCs generate both major macroglial phenotypes, astrocytes and oligodendrocytes, and that they may do so in vivo as well, in a context-dependent fashion (Windrem et al., 2004).
As the principal source of new oligodendrocytes, GPCs are thus responsible for remyelination in the demyelinated adult CNS (Tripathi et al., 2010; Zawadzka et al., 2010). As such, human GPCs are functionally synonymous with oligodendrocyte progenitor cells (OPCs), to which they are also referred. Yet human GPCs appear to remain bipotential until their last division, and can give rise to both astrocytes and oligodendrocytes until that point. In vitro, they can manifest even broader lineage competence, as at least some fraction of adult human GPCs remain potentially neurogenic as well, in a density- and context-dependent fashion (Goldman, 2003; Nunes et al., 2003). Indeed, GPCs do not necessarily comprise a homogeneous pool; to the contrary, recent work by Castelo-Branco and colleagues has revealed substantial molecular heterogeneity within the oligodendroglial lineage in rodents (Marques et al., 2016), as has been suggested in humans as well (Leong et al., 2014). Nonetheless, since there are no compelling data arguing for oligodendrocyte-restricted progenitors in humans, for consistency’s sake in this review we will designate all of these glial progenitor phenotypes as GPCs, best identified empirically by the surface epitopes by which they have been isolated.
Identifying optimal donor cell phenotypes for treating myelin disorders
Disorders of myelin require extensive tissue repair, and in the case of the pediatric leukodystrophies, even whole neuraxis myelination. While endogenous glial progenitors can remyelinate demyelinated lesions to some degree, the mitotic exhaustion and functional depletion of endogenous glial progenitors that may occur in acquired demyelination ultimately limits the extent and utility of spontaneous remyelination (Franklin and ffrench-Constant, 2008), thus necessitating the introduction of exogenous glial progenitors as therapeutic vectors.
Yet to be safe and effective as therapeutic vectors, transplantable GPCs must be reliably deliverable in both purity and quantity (Roy et al., 2004). The surface antigen-based purification of human GPCs, based on their selective expression of gangliosides recognized by monoclonal antibody A2B5 (Nunes et al., 2003; Roy et al., 1999), first allowed the isolation of both adult and fetal GPCs, which allowed their evaluation in animal models of both adult demyelination and congenital hypomyelination (Windrem et al., 2004; Windrem et al., 2002). These studies revealed that while both fetal and adult human-derived GPCs were able to myelinate dysmyelinated brain tissue, adult GPCs did so more rapidly and efficiently, but manifested less expansion and migratory potential in vivo. In contrast, fetal GPCs emigrated more widely and engrafted more efficiently than did adult cells, and exhibited context-dependent differentiation as astrocytes or oligodendrocytes, and their tissue sources were more readily available, all of which suggested their potential therapeutic utility in a broad array of myelin disorders (Figures 2A–C). Moreover, the isolation of human tissue-derived GPCs allowed the assessment of their gene expression patterns, dominant signaling pathways and homeostatic self-renewal mechanisms (McClain et al., 2012; Sim et al., 2006; Sim et al., 2011), work that has enabled the ex vivo pre-transplant modulation of the fate of these cells, further increasing their utility as transplantable vectors.
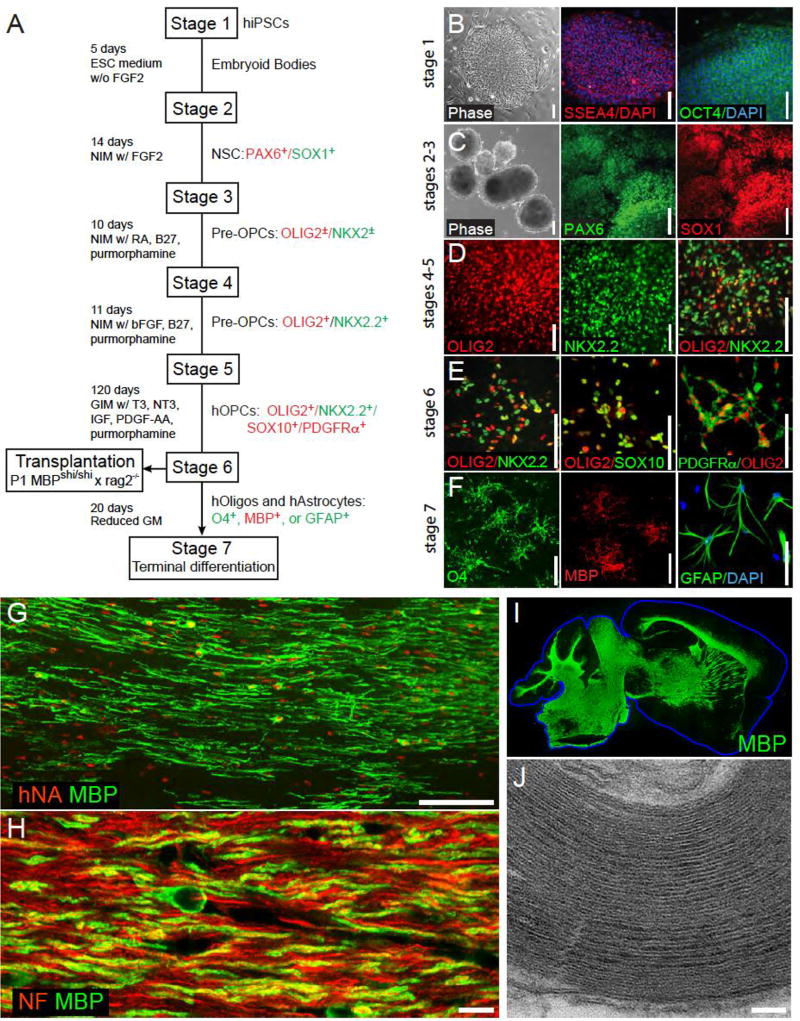
Figure 2. Stem cell-derived glial progenitor cell-mediated myelination of a dysmyelinated host.
A, This schematic outlines the multi-stage protocol by which GPCs, oligodendrocytes and astrocytes may be generated from pluripotent cells. B–F show representative images taken at serial stages of glial differentiation, with the serial expression of selected marker proteins noted at each stage. G, 3 months after neonatal transplant into hypomyelinated shiverer mice, human induced pluripotent cell (hiPSC)-derived GPCs have matured as myelinating, myelin basic protein (MBP)-expressing oligodendrocytes (MBP, green; human nuclear antigen, red). H, The hiPSC–derived oligodendrocytes ensheath mouse axons (neurofilament, red; MBP, green). I, Human iPSC-derived oligodendrocytes can myelinate the entire brain of shiverer mice, which do not otherwise express MBP (green). J, The myelin generated by hiPSC oligodendrocytes is ultrastructurally normal, exhibiting major dense lines and thick myelin sheaths. The use of such serial and distinct stages of growth factor exposure, paired with more extended periods of differentiation, have led to the production of highly enriched populations of human GPCs, that are highly efficient at myelinogenesis in vivo while manifesting no evident tumorigenesis. Scale: B–E, 100 µm; F, 25 µm; G, 100 µm; H, 10 µm; J, 100 nm. Images taken from (Wang et al., 2013); figure adapted from (Goldman and Kuypers, 2015).
Yet despite the attractiveness of fetal GPCs as therapeutic vectors, they remain finite in both initial number and expansion competence, necessitating their periodic reacquisition from new donor tissues. Fetal tissue suffers further limitations as a practicable cell source, given the unpredictability of tissue acquisition, limited gestational window of appropriate samples, and ongoing debate as to the use of fetal human-derived cells as clinical reagents. In light of these considerations, pluripotent stem cells, including both human embryonic stem cells (hESCs) and induced pluripotent cells (iPSCs), have emerged in recent years as the more feasible sources of transplantable myelinogenic GPCs (Douvaras et al., 2014; Hu et al., 2009; Wang et al., 2013) (Figure 1). Following the first report of myelination in the injured spinal cord by mouse ESCs (Brustle et al., 1999), oligodendrocytes derived from hESCs were similarly directed to generate myelin in vivo (Izrael et al., 2007; Nistor et al., 2005). Yet while ground-breaking, these studies did not isolate GPCs or oligodendrocytes prior to transplant, nor did they follow animals over the time frames required to ensure the stability of the engrafted cells, of concern since any incidentally-transplanted hESCs or undifferentiated derivatives may retain the potential for undesired expansion after implantation (Roy et al., 2006). Given this concern for tumorigenesis, stringent purification of lineage-restricted GPCs may be needed to ensure their safe use. Yet this point remains controversial; in 2009, the FDA approved a phase 1 safety trial evaluating the use of hESC-derived GPCs in spinal cord injury without such purification; although the trial was halted in 2011, its sponsor reported that its cessation was not related to safety, and described no serious adverse events attributable to the grafts (Priest et al., 2015). As a result, while the need for pre-transplant isolation of terminally-differentiated phenotypes remains unsettled, the overall safety profile of hESC-derived GPCs seems potentially acceptable.
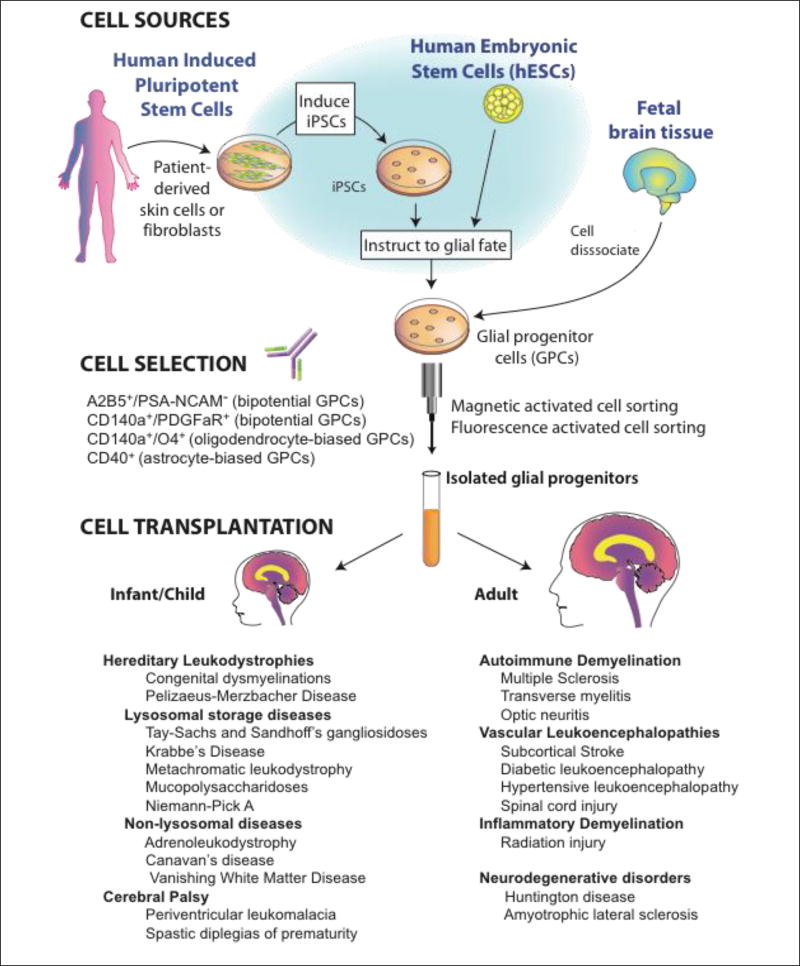
Figure 1. Glial progenitor cell sources, phenotypes and clinical targets.
Glial progenitor cells (GPCs) may be directly sorted from tissue, or produced from either human embryonic stem cells (hESCs) or induced pluripotential cells (hiPSCs), and then immunoselected based on their expression of either gangliosides recognized by monoclonal antibody (mAb) A2B5, or of CD140a/PDGFαR. The CD140a phenotype includes all potential oligodendrocytes, while the tetraspanin CD9 and sulfatide-directed mAb O4 identify progressively more oligodendrocyte-biased fractions (Douvaras et al., 2014; Sim et al., 2011). In contrast, CD44 recognizes a more astrocyte-biased fraction (Liu et al., 2004). The choice of tissue-, hESC-, or iPSC-derived GPCs depends upon whether allogeneic or autologous grafts are desired. Whereas autologous grafts of iPSC-derived GPCs might obviate the need for immunosuppression, their generation may take months, and their use in the hereditary leukodystrophies would first require correction of the underlying genetic disorder in the donor cell pool; at present, such genetic disorders of myelin would be better approached with allografted tissue- or hESC-derived GPCs. Figure adapted from (Osorio and Goldman, 2016).
Human ESC-based therapy suffers also from the possibility of allograft rejection, and hence the need for immunosuppression in graft recipients. Enthusiasm has thus developed for the use of autologous grafts of myelinogenic GPCs derived from human induced pluripotent cells (hiPSCs), potentially – though not assuredly (Zhao et al., 2011) - obviating the need for immune suppression. These cells are generated by the reprogramming of somatic cells to a pluripotent ground state, by the forced expression of a set of transcription factors that instruct stem cell phenotype (Belmonte et al., 2009). iPSCs were first generated from mouse (Takahashi and Yamanaka, 2006) and human (Yu et al., 2007) fibroblasts, and have since been differentiated into a variety of phenotypes, including neurons (Wernig et al., 2008), astrocytes (Hu et al., 2009), and oligodendrocytes (Czepiel et al., 2011). Methods established for generating GPCs from hESCs have proven effective with human iPSCs as well, and yield GPCs that are highly myelinogenic in vivo (Wang et al., 2013). This capability allows us to reasonably anticipate the use of iPSC-derived oligodendrocytes for autologous treatment, especially for non-genetic vascular, traumatic and inflammatory demyelinations.
Importantly though, iPSC-derived GPCs share many of the risks of those derived from hESCs, including aberrant differentiation and tumorigenesis (Mattis and Svendsen, 2011). In addition, iPSCs retain epigenetic marks of the cells from which they derive (Stadtfeld et al., 2010), so that their cell type of origin may influence their differentiation competence (Polo et al., 2010). Indeed, iPSCs may differ from one another in their lineage competence even when sourced from the same individual and tissue, making their standardization difficult. Of note, recent studies have also reported the direct induction of glial progenitors and oligodendrocytes from fibroblasts as well (Najm et al., 2013; Yang et al., 2013). By thereby avoiding the need for pluripotent intermediates, such direct induction of GPCs may accelerate the production of transplantable cells, while mitigating their risk of tumorigenesis. While the lack of expandability of the largely post-mitotic oligodendrocytes generated through this approach may still limit its practical utility, future advances may permit the direct induction of mitotic GPCs, which might yet prove a clinically feasible source of autologous myelinogenic cells (Goldman, 2013).
Pediatric myelin disorders as targets of progenitor cell-based therapy
Tens of thousands of children in the U.S. suffer from diseases of myelin loss. These include the metabolic demyelinations such as adrenoleukodystrophy; the lysosomal storage disorders, such as metachromatic leukodystrophy, the neuronal ceroid lipofuscinoses, gangliosidoses, and Niemann-Pick and Krabbe’s diseases; the hypomyelinating diseases, such as Pelizaeus-Merzbacher Disease; the myelinoclastic disorders, including vanishing white matter disease, Alexander’s Disease and Canavan’s Disease (Powers, 2004); and most commonly of all, periventricular leukomalacia and cerebral palsy (Silbereis et al., 2010). Their mechanistic heterogeneity notwithstanding, all of these conditions include the prominent loss of oligodendrocytes and myelin, highlighting their attractiveness as potential targets for cell replacement (see Figure 1, table).
Metabolic and storage disorders of myelin
In some hereditary disorders of the white matter, such as Canavan’s disease and many of the lysosomal storage diseases, oligodendrocytes are principal targets of mis-accumulated toxic substrates and their abnormal metabolic products, with demyelination an early and inauspicious hallmark of disease. In others such as Krabbe’s Disease, oligodendrocytes are essentially bystanders, killed by toxic metabolites generated by cells deficient in one or more critical enzymes (Powers, 2004). In yet others, such as Alexander’s disease and vanishing white matter disease, myelin loss may be caused by astroglial pathology (Bugiani et al., 2011; Dietrich et al., 2005). Given their heterogeneous etiologies, a common treatment platform for these disorders has proven elusive. Yet since GPC engraftment is both widespread and associated with astrocytic as well as oligodendrocytic production, GPCs would seem an especially promising vehicle for dispersing astrocytes and oligodendrocytes throughout otherwise diseased and/or enzyme-deficient brain parenchyma. The lysosomal storage disorders present especially attractive targets in this regard, since wild-type lysosomal enzymes may be released by donor cells, and taken up by deficient host cells through the mannose-6-phosphate receptor pathway (Urayama et al., 2004), by which lysosomal enzymes released from wild-type donor cells may be transported to enzyme-deficient neighbors, permitting local correction of disease-specific metabolic disturbances. By this means, a relatively small number of donor glia may provide sufficient enzymatic activity to correct the underlying enzymatic deficit and storage disorder of a much larger number of host cells (Jeyakumar et al., 2005).
The cell-based rescues of enzymatically deficient host cells were first described using wild-type neural stem cells (NSCs) transplanted into a mouse model of mucopolysaccharidosis type VII, in which neonatally-implanted cells restored beta-glucuronidase enzymatic function in the recipient forebrain (Buchet et al., 2002; Meng et al., 2003; Snyder et al., 1995). Human NSCs proved similarly effective in achieving enzyme replacement in the β-hexosaminidase-deficient Sandhoff mouse, with corresponding functional benefits (Lee et al., 2007). In the same vein, NSCs engineered to over-express sphingomyelinase, engrafted into sphingomyelinase-deficient Niemann-Pick type A mice, yielded substantial reductions in mis-accumulated sphingomyelin (Shihabuddin et al., 2004). Similarly, when NSCs were engrafted into a mouse model of neuronal ceroid lipofuscinosis (NCL), the cells dispersed broadly and ameliorated the lipofuscin misaccumulation of these animals (Tamaki et al., 2009). On that basis, a clinical trial to assess the use of human NSC allografts in treating infantile and late infantile NCL was undertaken (Selden et al., 2013)., This phase 1 safety trial did not address therapeutic endpoints, but its initiation speaks to the efforts that may be anticipated in developing neural stem and glial progenitor cells as vehicles for intracerebral enzyme replacement in the metabolic leukodystrophies.
The intracerebral delivery of GPCs would thus seem an especially promising approach for treating those enzyme-deficiencies associated with early demyelination, which may require both enzyme replacement and structural remyelination. Metachromatic leukodystrophy (MLD), for example, is characterized by deficient expression of arylsulfatase A, which results in sulfatide misaccumulation and oligodendrocyte loss. Experimental models of MLD have responded well to GPC grafts, with broad dispersal and integration as well as enzymatic rescue and sulfatide clearance (Givogri et al., 2006). Krabbe’s disease, characterized by galactocerebrosidase deficiency and early demyelination, may prove similarly amenable to GPC-based enzymatic repletion and myelin restoration (Kondo and Duncan, 2016). When children with presymptomatic or early stage Krabbe’s disease were transplanted with umbilical cord stem cells, they manifested slower disease (Escolar et al., 2005). Yet the intracerebral infiltration of umbilical cord stromal derivatives is modest, suggesting that treatment of these children with GPCs, which are able to achieve parenchymal dispersal and infiltration as well as structural remyelination, might comprise an especially promising treatment strategy.
Disorders of myelin formation and maintenance
The experimental assessment of GPCs as vectors for remyelination has proceeded most aggressively in animal models of congenital hypomyelination. In an early study of cell-based myelin repair, mouse neural stem cells were transplanted into newborn shiverer mice, a hypomyelinated mutant deficient in myelin basic protein, and yielded context-dependent myelination (Yandava et al., 1999). Even earlier studies had shown that tissue grafts of fetal white matter could elaborate oligodendrocytes and myelinate the adult shiverer spinal cord, though on a more geographically limited basis (Gout et al., 1988; Gumpel et al., 1989). On the basis of these foundational studies, we transplanted immunosorted human GPCs into neonatal shiverers, so as to assess the relative myelinogenic potential of purified populations of human glial progenitors (Windrem et al., 2004). When delivered as highly-enriched isolates, fetal human GPCs spread widely throughout the brain (Figures 2A–B, 2D), developing as astrocytes and oligodendrocytes in a context-dependent fashion. The donor-derived oligodendrocytes generated ultrastructurally-mature myelin that effectively ensheathed host shiverer axons and formed nodes of Ranvier, which allowed the restoration of normal transcallosal conduction velocities in the transplanted mice (Windrem et al., 2004). By using a 5-site injection protocol to achieve broader dispersal of GPCs, we next established cell engraftment throughout the entire neuraxis, with myelination of the spinal cord and roots as well as the entire brain, brainstem, cerebellum and cranial nerve roots (Windrem et al., 2008). This was associated with substantially prolonged survivals in transplanted mice, with phenotypic recovery and frank rescue of a large minority (Figure 2C). These data strongly suggested the feasibility of neonatal GPC implantation in treating childhood disorders of myelin formation and maintenance. Later studies refined the criteria for selecting myelinogenic progenitors, by identifying the PDGFα receptor epitope CD140a as recognizing the entire population of oligodendrocyte-competent progenitors (Sim et al., 2011). CD140a–sorted GPCs proved superior to those selected on the basis of A2B5 in both their efficiency and extent of myelination, and were highly migratory; they have thus supplanted A2B5-defined cells as a preferred cellular vector for therapeutic remyelination (Figures 2D–G).
The dilemma of disease-specific dosing
The intracerebral delivery of GPCs may thus prove a viable approach to the treatment of a wide variety of enzymatic and storage diseases of myelin, as well as for the disorders of myelin misproduction or failure. In practice though, individual treatment regimens will need to be tailored to specific disease phenotypes and stages. Little data are available as to the numbers or proportion of wild-type cells required to achieve correction of enzymatic activity and substrate clearance in any storage disorder, and these values may need to be empirically derived for every disease target. Similarly, the extent of myelination required for effective treatment remains wholly speculative, as is the extent and duration of immunosuppression required for allograft acceptance; these parameters too may vary with disease phenotype. These caveats notwithstanding, neural stem cell implantation has already been assessed as a means of myelin replacement in Pelizaeus-Merzbacher disease, in a phase 1 study that reported safety albeit unclear efficacy with the dose and delivery method chosen (Gupta et al., 2012); one may anticipate that future efforts will proceed to assess the efficacy of GPC grafts in this and related disorders. As these efforts proceed, with more dedicated myelinogenic donor cell phenotypes, more refined delivery methods and increasingly informed dose estimations, one may similarly expect that both the relative efficacy and limitations of GPC delivery in alleviating the childhood myelin disorders will be definitively established.
Adult disease targets of glial progenitor cell-based treatment
In adults, oligodendrocytic loss contributes to diseases as diverse as hypertensive and diabetic white matter loss, traumatic spinal cord and brain injury, and multiple sclerosis (MS) and its variants. In addition, oligodendrocytic loss is prominent in the degenerative dementia associated with age-related white matter loss. All of these are potential targets of glial progenitor cell replacement therapy, though the adult disease environment may limit this approach in ways not encountered in pediatric disease targets. For instance, the chronically ischemic brain tissue of diabetics with small vessel disease may require aggressive treatment of the underlying vascular insufficiency before any cell replacement strategy may be considered. Similarly, the inflammatory disease environments of multiple sclerosis as well as many of the leukodystrophies present their own challenges, which need to be overcome before cell-based remyelination can succeed (Franklin and ffrench-Constant, 2008; Ip et al., 2006). Nonetheless, current disease-modifying strategies for treating both vascular and autoimmune diseases have advanced to the point where transplant-based remyelination of adult targets may now be feasible.
Progenitor cell therapy for multiple sclerosis
Interest in cell-based remyelination has focused on MS, a debilitating disease characterized by both inflammatory myelinolysis and degenerative axonal loss. The attraction of MS as a therapeutic target derives from its high incidence and prevalence, with more than 300,000 cases in the US alone. MS has been a difficult target for cell therapy, given its relapsing course and the limitations of introducing new cells into an inflammatory environment. Nonetheless, contemporary immune modulating treatments have substantially diminished disease recurrence, making cell replacement a tenable repair strategy. Natalizumab (anti-α4 integrin), alemtuzumab (anti-CD52), rituximab (anti-CD20), fingolomod (a sphingosine-1-phosphate receptor modulator), dimethylfumarate, and terflunamide (a pyrimidine synthesis inhibitor), have all been associated with significant reductions in relapse rate (Weinstock-Guttman and Ramanathan, 2012), reflecting diminished episodes of radiographically and clinically significant central inflammation.
These advances in the immunomodulatory control of MS suggest that attention may now shift from disease attenuation to the repair of demyelinated lesions, and to the prevention of the later progressive neurodegenerative phase that occurs in multiple sclerosis, designated secondary progressive MS. The latter condition, characterized by sustained axonal loss and an attendant, inexorable loss of function long after acute inflammatory events have attenuated, may reflect a loss of axonal support by local oligodendroglia (Lee et al., 2012; Saab et al., 2016). As such, progressive MS might comprise an especially attractive target for glial replacement, since oligodendrocyte engagement with otherwise denuded axons might serve to preserve neuronal viability even in the absence of remyelination. The intracerebral delivery of GPCs into demyelinated brain may thus offer tangible benefits by oligodendroglial replacement and axonal engagement per se, as well as by myelin repair.
Progenitor cell therapy for adult structural demyelinations
Besides the autoimmune demyelinations, the adult CNS is subject to demyelination from a broad variety of traumatic, vascular and metabolic insults. Traumatic brain injury, white matter stroke, age-related white matter disease and the leukomalacias associated with hypertension and diabetes are all potential targets of glial replacement for the purpose of white matter repair, and yet each of these potential disease targets needs to be separately modeled, given the vastly different disease environments and hence host constraints of each. Yet to the extent that the pathology of these disorders is limited to oligodendrocytes and astrocytes, with preserved vascular function and neuronal architecture, they too may be amenable to glial progenitor cell-based remyelination, as might be iatrogenic causes of demyelination such as radiation therapy (Fox et al., 2014; Piao et al., 2015).
To be sure, the complexity of the disease environment may make adult targets less approachable than their pediatric counterparts, especially so in aged subjects (Franklin and ffrench-Constant, 2008; Ruckh et al., 2012). Moreover, previously stably myelinated but now-demyelinated axons may have very different thresholds and permissiveness towards remyelination than their never-before myelinated, hypomyelinated or briefly myelinated pediatric counterparts. Indeed, recent studies have emphasized that improved understanding of the molecular environment of demyelinating foci, and the transcriptional response of oligodendrocyte progenitor cells to that environment, may offer significant insight into improving the efficiency and timing of glial progenitor cell transplantation (Moyon et al., 2015).
Such studies of the disease-specific tissue environment in demyelinating disorders are especially important given the relatively smaller data base regarding glial progenitor grafts into the adult CNS; whereas the dispersal and ultimate myelination of glial progenitors introduced into neonatal and young animals is now well-established, and forms the basis of the use of this strategy in the childhood myelin disorders as noted, the experimental basis for remyelination of adult-demyelinated brain is less robust. To be sure, when human GPCs were transplanted directly into lysolecithin-demyelinated lesions in the adult rat brain, the cells matured as oligodendrocytes and myelinated residual host axons, if with lower efficiency than in congenitally hypomyelinated brain (Windrem et al., 2002), and more recent studies have supported the broad migration of human GPCs introduced into the normal adult rat brain (Osorio and Goldman, 2016). Similarly, in a novel model of axon-sparing demyelination in adult cats, remyelination occurred efficiently from endogenous progenitors (Duncan et al., 2009). More recently, remyelination of demyelinated adult spinal cord lesions by human iPSC-derived GPCs has been noted by van Evercoorn and colleagues (Mozafari et al., 2015), work that establishes the permissiveness of adult-demyelinated axons to remyelination by pluripotent cell-derived GPCs. Together, these and other studies argue that remyelination of adult-demyelinated axons is clearly feasible. Nonetheless, it is equally clear that any cell-based strategies for treating adult demyelination will require not only disease modification, but rigorous stratification to define those patients with a tissue environment permissive for donor cell integration, and sufficient axonal preservation to benefit from this approach.
Remyelination of spinal lesions
Besides the myelinated tracts of the brain, the ascending sensory and descending motor tracts of the spinal cord are frequent victims of demyelination, whether from multiple sclerosis, neuromyelitis optica, or segmental injuries. In efforts to remyelinate the contused rat spinal cord, implanted GPCs have been found to disperse and generate both astrocytes and myelinogenic oligodendrocytes (Han et al., 2004). Similarly, hESC-derived oligodendrocytes can remyelinate demyelinated cord lesions (Nistor et al., 2005), with functional benefit in experimental models (Sharp et al., 2010). On the basis of those observations, a first-in-man safety trial was performed using hESC-derived GPCs transplanted into patients with high-grade thoracic cord lesions (Priest et al., 2015). No serious adverse events or donor-derived tumorigenesis was noted in this trial, which was thus reassuring as to the potential of hESC GPC-based neurological therapeutics. That said, the therapeutic potential of a solely remyelinative strategy in patients with such high-grade spinal lesions is unclear, given the concurrent loss of segmental neurons and long tract axons in such lesions, and the compromised vascular and inflammatory environment of the injured spinal cord. Nonetheless, such GPC grafts may hold great promise in carefully-selected patients with isolated segmental demyelination. As such, the previously noted recent demonstration of focal remyelination of the injured spinal cord by iPSC-derived GPCs (Mozafari et al., 2015), not only provides generic support for the use of these cells as therapeutic vectors, but also offers insight into the parameters that define the migration and myelination competence of human GPCs in the specific environment of the spinal cord, and which may thereby be modulated to ensure graft success.
Human glial chimeric mice reveal human-selective aspects of both glial function and dysfunction
When hypomyelinated mutant mice are engrafted neonatally with human GPCs, the donor cells mature as both myelinating oligodendrocytes and fibrous astrocytes, ultimately yielding mice with a substantially humanized white matter (Windrem et al., 2008). Large numbers of human donor cells also remain as progenitors, which over time predominate, displacing and ultimately replacing the endogenous mouse glial progenitor pool. This competitive advantage of human over murine glial progenitors is evident in wild-type as well as in hypomyelinated mice, such that in the setting of normal glial turnover the human GPCs also give rise to gray matter astrocytes, eventually resulting in substantial astrocytic as well as oligodendrocytic humanization of the recipient rodent brains (Figure 2F).
The result of these events is that the xenografted mouse brains can become substantially humanized in their glial constituents (Windrem et al., 2008; Windrem et al., 2014). These human glial chimeras lend themselves to the investigation of questions never before approachable, for lack of an appropriate in vivo model of human glial function. In particular, these mice permit assessment of the species-specific contributions of human glia to neural network function. Astrocytes clearly play a central role in synaptic efficiency and plasticity in mammals (Kang et al., 1998). Hominid evolution in particular has been attended by increasing astrocytic complexity, which may have contributed greatly to the evolution of higher cognitive functions in primates. Human astrocytes are larger and far more fibrous than those of infraprimate mammals, and include vastly more encompassed synapses within their individual geographic domains (Oberheim et al., 2006; Oberheim et al., 2009). Accordingly, when neonatal mice were engrafted with astrocyte-biased human GPCs, resulting in the colonization of the host brains with human glia, the resultant glial chimeras exhibited substantially enhanced activity-dependent plasticity and learning (Han et al., 2013). This finding strongly suggested that human astrocytes might play a much larger role in neural processing than do those of rodents. Yet by doing so, this observation also highlighted the potential for glial pathology to wreck especial havoc within human neural circuits, with attendant implications for the human neurodegenerative disorders. As such, this study suggested that human-specific astrocytic specializations might contribute not only to human cognitive evolution, but also to the appearance of human-selective neurodegenerative and psychiatric disorders.
Glial transplant-mediated amelioration of neurodegenerative disorders
Among these human-selective disorders that might reflect glial human evolution are a number of prototypic neurodegenerative and neuropsychiatric disorders. Glial pathology has already been noted to contribute to a broad set of neurodegenerative and neuropsychiatric diseases traditionally considered disorders of solely neuronal dysfunction (Di Giorgio et al., 2008; Verkhratsky and Parpura, 2015). Amyotrophic lateral sclerosis in particular was first identified as having a substantial glial component to its etiology (Di Giorgio et al., 2008; Di Giorgio et al., 2007; Meyer et al., 2014; Yamanaka et al., 2008), with a failure in astrocytic glutamate transport and disrupted potassium homeostasis, as well as defective oligodendroglial metabolite transfer (Lee et al., 2012), each potentially contributing to the loss of motor neurons and other large pyramidal neurons in this disorder. Indeed, while a number of genetic bases for ALS have been identified, glial dysfunction may be a shared trait that confers selective neuronal vulnerability across the spectrum of ALS-associated motor neuronopathies. For instance, TDP43 proteinopathy-associated ALS has been shown to exhibit primary astrocytic pathology with secondary motor neuron dysfunction (Serio et al., 2013), highlighting the non-cell autonomous nature of neuronal loss in even this quintessentially neuronal degeneration.
Huntington’s Disease is another such nominally neurodegenerative disorder, to which glial pathology also appears to make a significant causal contribution. HD is an autosomal dominant disorder characterized by abnormally long CAG repeat expansions in the first exon of the Huntingtin gene. The encoded polyglutamine expansions of mutant huntingtin protein disrupt its normal functions and protein-protein interactions, ultimately yielding widespread neuropathology, most rapidly evident in the neostriatum (Waldvogel et al., 2015). Yet despite the pronounced loss of striatal medium spiny neurons in HD, and evidence of glial dysfunction (Shin et al., 2005; Tong et al., 2014), few studies have investigated the specific contribution of glial pathology to the Huntington disease phenotype. This lack of understanding of the role of glia in HD – reflecting our broader ignorance of the role of glial dysfunction in the neurodegenerative disorders (Verkhratsky et al., 2014) - has highlighted the lack of in vivo models that permit the separate interrogation of glial and neuronal functions in HD, particularly so in humans.
To address this fundamental gap in our knowledge, we used human glial chimeric mice to assess the role of human striatal glia in the pathogenesis of HD, by comparing the behavior and MSN physiology of mice xenografted at birth with mutant HD-expressing human hGPCs to their normal HTT hGPC-engrafted controls (Benraiss et al., 2016). In this study, the motor behavior of immunodeficient mice neonatally xenografted with hGPCs produced from mutant HD human embryonic stem cells (48 CAG) was compared to that of controls engrafted with hGPCs derived from a sibling line of unaffected hESCs (18 CAG). The HD GPC-engrafted mice manifested impaired motor learning relative to control hGPC-engrafted mice, and exhibited increased neuronal input resistance and excitability, relative to those of mice engrafted with normal HTT (23 CAG)-transduced striatal glia (Figures 3A–C).
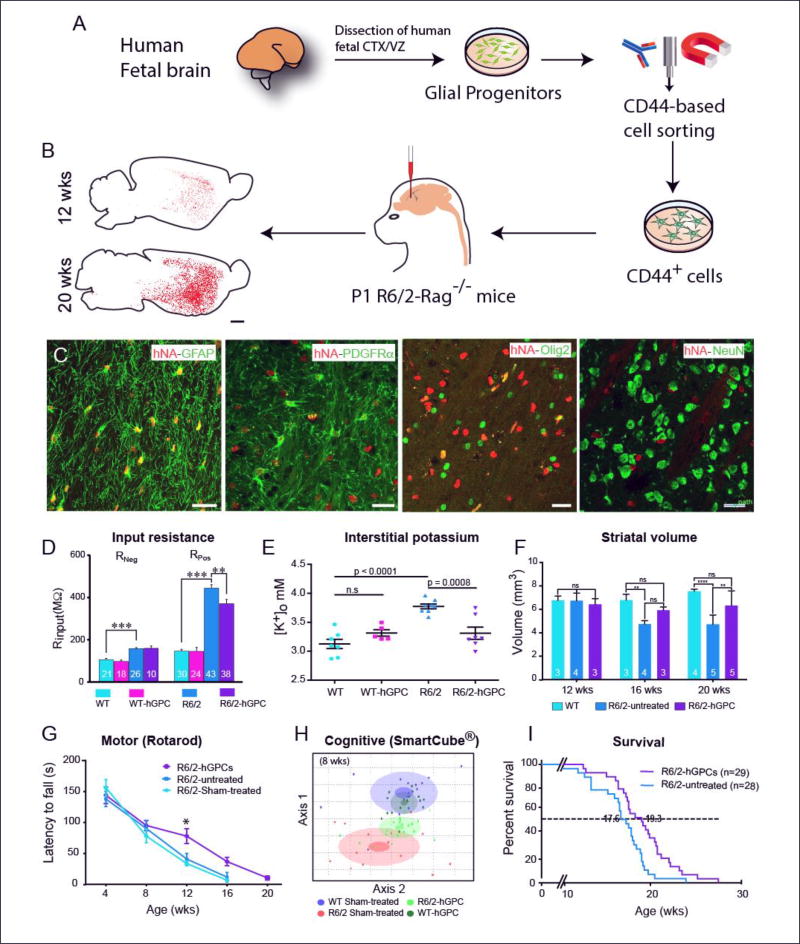
Figure 3. Glial progenitor grafts slow disease progression, stabilize neuronal excitability and extend survival in mouse models of Huntington disease.
A, Schematic outlines the preparation and delivery of normal CD44-defined astroglial progenitor cells into the striata of neonatal HTT mutant R6/2 (120Q) × rag1−/− mice. B, CD44-sorted hGPCs colonized the R6/2 × rag1−/− striatum Striatal engraftment of the R6/2 mice by CD44-sorted hGPCs was robust and dense. Donor-derived cells increased as a function of time, such that transplanted hGPCs expanded to largely replace host glia in the striata and ventral forebrains of recipient R6/2 mice by 20 weeks. Scale: 1 mm. C, By 20 weeks after neonatal graft, the donor hGPCs (human nuclear antigen, red) integrated as astrocytes (GFAP, green) or persisted as GPCs (PDGFαR and olig2, green), but did not give rise to neurons; no overlap was seen of hN and neuronal NeuN. Scale: 25 µm.
D. Chimerization with normal glia partially normalized MSN physiological function. Whole-cell I-clamp recordings from rag1−/− wild-type, CD44 hGPC-engrafted rag1−/− wild-types, R6/2 × rag1−/− mice, and CD44-engrafted rag1−/− mice revealed that the input resistance Rinput, was significantly higher in R6/2 × rag1−/− striatal neurons than in wild-type × rag1−/− controls, but was partially restored to normal in R6/2 mice chimerized with normal CD44-sorted hGPCs. E. Normal glial engraftment reduces interstitial K+ levels in the R6/2 striatum Potassium electrodes were used to measure the interstitial levels of striatal K+ in both wild-type mice and R6/2 littermates at 16 weeks of age (± 4 days), with and without neonatal intrastriatal transplants of CD44-sorted hGPCs. Untreated R6/2 mice manifested significantly higher levels of interstitial K, which were restored to normal in R6/2 mice neonatally engrafted with hGPCs. In contrast, hGPC engraftment did not influence the interstitial K+ levels of wild-type mice. F. Striatal involution of R6/2 mice was slowed by normal glial engraftment The neostriata of R6/2 HD mice typically shrink in volume with age. Human GPC engraftment attenuated this process, in that R6/2 mice manifested larger striatal volumes than unengrafted R6/2 mice by 16 weeks of age, with preservation of R6/2 striatal volumes at levels no different than wild-type controls. E–F, Means ± SEM; ** and ***, p< 0.01, and 0.0001, 1-way ANOVA.
G. Chimerization with normal glia slows motor loss and extends survival of R6/2 mice Linear regression revealed that the rate of rotarod-assessed motor deterioration of R6/2 mice was significantly slower in mice engrafted with human GPCs than in untreated mice (p<0.001 by ANOVA). H. Treatment with hGPCs slowed cognitive decline in R6/2 mice SmartCube testing (Psychogenics), a multimodal assessment of cognitive function (Benraiss et al., 2016), revealed a significant difference between sham-treated wild-type (WT) and R6/2 mice by 8 weeks of age, with significant functional preservation in the R6/2 mice when treated neonatally with normal hGPCs. In H, each dot represents a mouse. The center, small and large ellipses represent the mean, standard error and standard deviation of the composite features for each group. See (Benraiss et al., 2016) for detail. I. R6/2 mice whose striata were engrafted with human GPCs survived significantly longer than unengrafted mice (p<0.01, Mantel-Cox Log-rank test).
Since striatal chimerization with HD-derived glia proved sufficient to recapitulate aspects of disease phenotype in normal mice, one might anticipate neonatal chimerization of HD mice with normal hESC-derived glia might delay or rescue aspects of disease phenotype. Using R6/2 transgenic HD mice as hosts (Mangiarini et al., 1996), Benraiss and colleagues found that the substantial replacement of diseased striatal glia with wild-type human glia indeed resulted in a slowing of disease progression and corresponding increment in survival in transplanted R6/2 mice (Figures 3D–I) (Benraiss et al., 2016). Of note, the glial donor cells were isolated on the basis of CD44, the hyaluronan receptor, so as to capture a more astrocyte-biased donor cell population. This neonatal engraftment by CD44-defined glia yielded a transplant-associated fall in neuronal input resistance, and a corresponding drop in interstitial K+ in the R6/2 striatum, with an attendant rescue of the otherwise hyperexcitable phenotype of R6/2 striatal neurons (Figures 3D–E) (Benraiss et al., 2016). Together, these studies indicated a critical role for glial pathology in the progression of HD. As such, they suggest the potential for glial cell replacement as a therapeutic strategy in Huntington’s disease, and more broadly, to other neurodegenerative diseases in which glial pathology might be causally contributory.
Human glial involvement in - and potential rescue of - the neuropsychiatric disorders
The therapeutic benefit in animal models of glial replacement in HD raises the possibility that glial cell replacement may prove of therapeutic value not only for diseases of oligodendrocytes and myelin, but for primarily astrocytic disorders as well. HD is not alone in manifesting with relative hypomyelination and concurrent defects in cognition typically ascribed as cortical in nature. A broad variety of neurodegenerative and neuropsychiatric disorders also share concurrent deficits in central white matter structure and cortical function that might suggest glial pathology. Indeed, astroglial pathology in particular might be especially germane to those neural diseases that are fundamentally unique to humans, such as schizophrenia (Tkachev et al., 2003), whose phylogenetic appearance may parallel that of human astrocytic evolution. A number of authors have pointed to the causal contribution of both astroglial and oligodendroglial pathology, and white matter abnormalities more broadly, in schizophrenia (Davis et al., 2003; Haroutunian et al., 2014; Hof et al., 2002; Nave and Ehrenreich, 2014; Takahashi et al., 2011; Verkhratsky and Parpura, 2015; Voineskos et al., 2013; Wang et al., 2015). The previously noted observation that astroglial replacement can attenuate the behavioral phenotype of Huntington disease transgenic mice suggests the potential for such glial replacement in cognitive disorders with substantial glial pathology, especially so in those disorders such as schizophrenia that may suffer both astrocytic and oligodendrocytic dysfunction. Whether healthy donor glial progenitors can replace diseased host glia in adolescents and adults with such conditions remains unclear, and will likely need to be investigated on a disease-by-disease basis. Moreover, should this strategy be clinically feasible, the ethical issues involved in potentially ameliorating a behavioral condition, and hence potentially influencing an adult‘s personality, by means of an intracerebral graft of allogeneic stem cell-derived glia – to wit, those of another person- may prove significant (Hermeren, 2015). Nonetheless, the very possibility of changing cognition and ameliorating both neurodevelopmental and psychiatric pathology by means of glial transplantation offers both breath-taking new opportunities for cell-based therapeutics of the CNS.
Acknowledgments
Work discussed in the Goldman lab was supported by NIMH, NINDS, and grants from CHDI Foundation, Adelson Medical Research Foundation, Mathers Charitable Foundation, Novo Nordisk Foundation, Lundbeck Foundation, National Multiple Sclerosis Society, and the New York State Stem Cell Research Program (NYSTEM).
References
- Armstrong RC, Dorn HH, Kufta CV, Friedman E, Dubois-Dalcq ME. Pre-oligodendrocytes from adult human CNS. J Neurosci. 1992;12:1538–1547. doi: 10.1523/JNEUROSCI.12-04-01538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte JC, Ellis J, Hochedlinger K, Yamanaka S. Induced pluripotent stem cells and reprogramming: seeing the science through the hype. Nat Rev Genet. 2009;10:878–883. doi: 10.1038/nrg2700. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Wang S, Herrlinger S, Li X, Chandler-Militello D, Mauceri J, Burm H, Toner M, Osipovitch M, Xu Q, et al. Human glia can both induce and rescue aspects of phenotype in Huntington Disease. Nature communications. 2016;7 doi: 10.1038/ncomms11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science (New York, NY) 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Buchet D, Serguera C, Zennou V, Charneau P, Mallet J. Long-term expression of beta-glucuronidase by genetically modified human neural progenitor cells grafted into the mouse central nervous system. Molecular and cellular neurosciences. 2002;19:389–401. doi: 10.1006/mcne.2001.1086. [DOI] [PubMed] [Google Scholar]
- Bugiani M, Boor I, van Kollenburg B, Postma N, Polder E, van Berkel C, van Kesteren RE, Windrem MS, Hol EM, Scheper GC, et al. Defective glial maturation in vanishing white matter disease. J Neuropathol Exp Neurol. 2011;70:69–82. doi: 10.1097/NEN.0b013e318203ae74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czepiel M, Balasubramaniyan V, Schaafsma W, Stancic M, Mikkers H, Huisman C, Boddeke E, Copray S. Differentiation of induced pluripotent stem cells into functional oligodendrocytes. Glia. 2011;59:882–892. doi: 10.1002/glia.21159. [DOI] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Archives of general psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell stem cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Lacagnina M, Gass D, Richfield E, Mayer-Proschel M, Noble M, Torres C, Proschel C. EIF2B5 mutations compromise GFAP+ astrocyte generation in vanishing white matter leukodystrophy. Nat Med. 2005;11:277–283. doi: 10.1038/nm1195. [DOI] [PubMed] [Google Scholar]
- Douvaras P, Wang J, Zimmer M, Hanchuk S, O'Bara MA, Sadiq S, Sim FJ, Goldman J, Fossati V. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem cell reports. 2014;3:250–259. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan ID, Brower A, Kondo Y, Curlee JF, Jr, Schultz RD. Extensive remyelination of the CNS leads to functional recovery. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6832–6836. doi: 10.1073/pnas.0812500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolar M, Poe M, Provenzale J, Richards K, Allison J, Wood S, Wenger D, Kurtzberg J. Transplantation of umbilical cord blood in babies with infantile Krabbe's disease. New Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Daley GQ, Goldman SA, Huard J, Kamp TJ, Trucco M. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science (New York, NY) 2014;345:1247391. doi: 10.1126/science.1247391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJM, ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nature reviews Neuroscience. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Givogri M, Galbiati F, Fasano S, Amadio S, Perani L, Superchi D, Morana P, Del Carro U, Marchesini S, Brambilla R, et al. Oligodendroglial progenitor cell therapy limits central neurological deficits in mice with metachromatic leukodystrophy. Journal of Neuroscience. 2006;26:3109. doi: 10.1523/JNEUROSCI.4366-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogate N, Verma L, Zhou JM, Milward E, Rusten R, O'Connor M, Kufta C, Kim J, Hudson L, Dubois-Dalcq M. Plasticity in the adult human oligodendrocyte lineage. J Neurosci. 1994;14:4571–4587. doi: 10.1523/JNEUROSCI.14-08-04571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. Glia as neural progenitor cells. Trends in Neurosci. 2003;26:590–596. doi: 10.1016/j.tins.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Goldman SA. White matter from fibroblasts. Nature biotechnology. 2013;31:412–413. doi: 10.1038/nbt.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Kuypers NJ. How to make an oligodendrocyte. Development. 2015;142:3983–3995. doi: 10.1242/dev.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout O, Gansmuller A, Baumann N, Gumpel M. Remyelination by transplanted oligodendrocytes of a demyelinated lesion in the spinal cord of the adult shiverer mouse. Neuroscience letters. 1988;87:195–199. doi: 10.1016/0304-3940(88)90169-3. [DOI] [PubMed] [Google Scholar]
- Gumpel M, Gout O, Lubetzki C, Gansmuller A, Baumann N. Myelination and remyelination in the central nervous system by transplanted oligodendrocytes using the shiverer model. Discussion on the remyelinating cell population in adult mammals. Developmental neuroscience. 1989;11:132–139. doi: 10.1159/000111894. [DOI] [PubMed] [Google Scholar]
- Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T, et al. Neural stem cell engraftment and myelination in the human brain. Science translational medicine. 2012;4:155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SW, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, Bartzokis G. Myelination, oligodendrocytes, and serious mental illness. Glia. 2014;62:1856–1877. doi: 10.1002/glia.22716. [DOI] [PubMed] [Google Scholar]
- Hermeren G. Ethical considerations in chimera research. Development. 2015;142:3–5. doi: 10.1242/dev.119024. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res. 2002;27:1193–1200. doi: 10.1023/a:1020981510759. [DOI] [PubMed] [Google Scholar]
- Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nature protocols. 2009;4:1614–1622. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip CW, Kroner A, Bendszus M, Leder C, Kobsar I, Fischer S, Wiendl H, Nave KA, Martini R. Immune cells contribute to myelin degeneration and axonopathic changes in mice overexpressing proteolipid protein in oligodendrocytes. J Neurosci. 2006;26:8206–8216. doi: 10.1523/JNEUROSCI.1921-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, Itskovitz-Eldor J, Chebath J, Revel M. Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neuroscience. 2007;34:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Jeyakumar M, Dwek R, Butters T, Platt F. Storage solutions: Treating lysosomal disorders of the brain. Nature Reviews Neuroscience. 2005;6:1–12. doi: 10.1038/nrn1725. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nature neuroscience. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Duncan ID. Myelin repair by transplantation of myelin-forming cells in globoid cell leukodystrophy. Journal of neuroscience research. 2016;94:1195–1202. doi: 10.1002/jnr.23909. [DOI] [PubMed] [Google Scholar]
- Lee J-P, Jeyakumar M, Gonzalez R, Takahashi H, Lee P, park K-I, Butters T, Dwek R, Schwartz P, Tong G, et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nature medicine. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SY, Rao VT, Bin JM, Gris P, Sangaralingam M, Kennedy TE, Antel JP. Heterogeneity of oligodendrocyte progenitor cells in adult human brain. Annals of clinical and translational neurology. 2014;1:272–283. doi: 10.1002/acn3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Han SW, Wu Y, Fischer I, Rao MS. CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol. 2004;276:31–46. doi: 10.1016/j.ydbio.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies S, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcao A, Xiao L, Li H, Haring M, Hochgerner H, Romanov RA, et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science (New York, NY) 2016;352:1326–1329. doi: 10.1126/science.aaf6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis VB, Svendsen CN. Induced pluripotent stem cells: a new revolution for clinical neurology? Lancet Neurol. 2011;10:383–394. doi: 10.1016/S1474-4422(11)70022-9. [DOI] [PubMed] [Google Scholar]
- McClain CR, Sim FJ, Goldman SA. Pleiotrophin suppression of receptor protein tyrosine phosphatase-beta/zeta maintains the self-renewal competence of fetal human oligodendrocyte progenitor cells. J Neurosci. 2012;32:15066–15075. doi: 10.1523/JNEUROSCI.1320-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XL, Shen JS, Ohashi T, Maeda H, Kim SU, Eto Y. Brain transplantation of genetically engineered human neural stem cells globally corrects brain lesions in the mucopolysaccharidosis type VII mouse. Journal of neuroscience research. 2003;74:266–277. doi: 10.1002/jnr.10764. [DOI] [PubMed] [Google Scholar]
- Meyer K, Ferraiuolo L, Miranda CJ, Likhite S, McElroy S, Renusch S, Ditsworth D, Lagier-Tourenne C, Smith RA, Ravits J, et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proceedings of the National Academy of Sciences. 2014;111:829–832. doi: 10.1073/pnas.1314085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, et al. A TALE nuclease architecture for efficient genome editing. Nature biotechnology. 2011;29:143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- Moyon S, Dubessy AL, Aigrot MS, Trotter M, Huang JK, Dauphinot L, Potier MC, Kerninon C, Melik Parsadaniantz S, Franklin RJ, et al. Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J Neurosci. 2015;35:4–20. doi: 10.1523/JNEUROSCI.0849-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozafari S, Laterza C, Roussel D, Bachelin C, Marteyn A, Deboux C, Martino G, Baron-Van Evercooren A. Skin-derived neural precursors competitively generate functional myelin in adult demyelinated mice. The Journal of clinical investigation. 2015;125:3642–3656. doi: 10.1172/JCI80437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm FJ, Lager AM, Zaremba A, Wyatt K, Caprariello AV, Factor DC, Karl RT, Maeda T, Miller RH, Tesar PJ. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nature biotechnology. 2013;31:426–433. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, Ehrenreich H. Myelination and oligodendrocyte functions in psychiatric diseases. JAMA psychiatry. 2014;71:582–584. doi: 10.1001/jamapsychiatry.2014.189. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nature Reviews Neuroscience. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Nistor G, Totoiu M, Haque NS, Carpenter M, Keirstead H. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature medicine. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Oberheim N, Wang X, Goldman S, Nedergaard SA. Astrocytic complexity distinguishes the human brain. Trends in Neurosciences. 2006;29:1–10. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio MJ, Goldman SA. Glial progenitor cell-based treatment of the childhood leukodystrophies. Experimental neurology. 2016;283:476–488. doi: 10.1016/j.expneurol.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D, et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell. 2015;16:198–210. doi: 10.1016/j.stem.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J. The Leukodystrophies: Overview and Classification. In: Lazzarini RA, editor. Myelin Biology and Disorders. San Diego: Elsevier Academic Press; 2004. pp. 663–690. [Google Scholar]
- Priest CA, Manley NC, Denham J, Wirth ED, 3rd, Lebkowski JS. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regenerative medicine. 2015;10:939–958. doi: 10.2217/rme.15.57. [DOI] [PubMed] [Google Scholar]
- Roy N, Windrem M, Goldman SA. Progenitor cells of the adult white matter. In: Lazzarini R, editor. Myelin Biology and Disorders. Amsterdam: Elsevier; 2004. pp. 259–287. [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nature medicine. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RA, Gravel M, Braun PE, Goldman SA. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell stem cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Mobius W, Goetze B, Jahn HM, Huang W, et al. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron. 2016;91:119–132. doi: 10.1016/j.neuron.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolding N, Franklin R, Stevens S, Heldin CH, Compston A, Newcombe J. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain : a journal of neurology. 1998;121:2221–2228. doi: 10.1093/brain/121.12.2221. [DOI] [PubMed] [Google Scholar]
- Selden NR, Al-Uzri A, Huhn SL, Koch TK, Sikora DM, Nguyen-Driver MD, Guillaume DJ, Koh JL, Gultekin SH, Anderson JC, et al. Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. Journal of neurosurgery Pediatrics. 2013;11:643–652. doi: 10.3171/2013.3.PEDS12397. [DOI] [PubMed] [Google Scholar]
- Serio A, Bilican B, Barmada SJ, Ando DM, Zhao C, Siller R, Burr K, Haghi G, Story D, Nishimura AL, et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4697–4702. doi: 10.1073/pnas.1300398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihabuddin LS, Numan S, Huff MR, Dodge JC, Clarke J, Macauley SL, Yang W, Taksir TV, Parsons G, Passini MA, et al. Intracerebral transplantation of adult mouse neural progenitor cells into the Niemann-Pick-A mouse leads to a marked decrease in lysosomal storage pathology. J Neurosci. 2004;24:10642–10651. doi: 10.1523/JNEUROSCI.3584-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol. 2005;171:1001–1012. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis JC, Huang EJ, Back SA, Rowitch DH. Towards improved animal models of neonatal white matter injury associated with cerebral palsy. Disease models & mechanisms. 2010;3:678–688. doi: 10.1242/dmm.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim F, Lang J, Waldau B, Roy N, Schwartz T, Chandross K, Natesan S, Merrill J, Goldman SA. Complementary patterns of gene expression by adult human oligodendrocyte progenitor cells and their white matter environment. Ann Neurology. 2006;59:763–779. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- Sim FJ, McClain CR, Schanz SJ, Protack TL, Windrem MS, Goldman SA. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nature biotechnology. 2011;29:934–941. doi: 10.1038/nbt.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Progress in neurobiology. 2011;93:13–24. doi: 10.1016/j.pneurobio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki SJ, Jacobs Y, Dohse M, Capela A, Cooper JD, Reitsma M, He D, Tushinski R, Belichenko PV, Salehi A, et al. Neuroprotection of host cells by human central nervous system stem cells in a mouse model of infantile neuronal ceroid lipofuscinosis. Cell Stem Cell. 2009;5:310–319. doi: 10.1016/j.stem.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA, Mody I, Olsen ML, Sofroniew MV, et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat Neurosci. 2014;17:694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci. 2010;30:16383–16390. doi: 10.1523/JNEUROSCI.3411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama A, Grubb JH, Sly WS, Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V. Astrogliopathology in neurological, neurodevelopmental and psychiatric disorders. Neurobiology of disease. 2015 doi: 10.1016/j.nbd.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M. Glia in the pathogenesis of neurodegenerative diseases. Biochem Soc Trans. 2014;42:1291–1301. doi: 10.1042/BST20140107. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Felsky D, Kovacevic N, Tiwari AK, Zai C, Chakravarty MM, Lobaugh NJ, Shenton ME, Rajji TK, Miranda D, et al. Oligodendrocyte genes, white matter tract integrity, and cognition in schizophrenia. Cereb Cortex. 2013;23:2044–2057. doi: 10.1093/cercor/bhs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel JP, Faull RL. The Neuropathology of Huntington's Disease. Curr Top Behav Neurosci. 2015;22:33–80. doi: 10.1007/7854_2014_354. [DOI] [PubMed] [Google Scholar]
- Wang C, Aleksic B, Ozaki N. Glia-related genes and their contribution to schizophrenia. Psychiatry and clinical neurosciences. 2015 doi: 10.1111/pcn.12290. [DOI] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock-Guttman B, Ramanathan M. Multiple sclerosis in 2011: Advances in therapy, imaging and risk factors in MS. Nature reviews Neurology. 2012;8:66–68. doi: 10.1038/nrneurol.2011.213. [DOI] [PubMed] [Google Scholar]
- Wernig M, Zhao J-P, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinsons disease. Proc Natl Acad Sci. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, Roy NS, Goldman SA. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nature medicine. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Roy NS, Wang J, Nunes M, Benraiss A, Goodman R, McKhann GM, Goldman SA. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69:966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Morrow C, Munir J, Chandler-Militello D, Wang S, Goldman SA. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J Neurosci. 2014;34:16153–16161. doi: 10.1523/JNEUROSCI.1510-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nature neuroscience. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandava BD, Billinghurst LL, Snyder EY. "Global" cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Zuchero JB, Ahlenius H, Marro S, Ng YH, Vierbuchen T, Hawkins JS, Geissler R, Barres BA, Wernig M. Generation of oligodendroglial cells by direct lineage conversion. Nature biotechnology. 2013;31:434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]