Abstract
Several paradigms for rodent models of the cognitive and negative endophenotypes found in schizophrenic patients have been proposed. However, significant efforts are needed in order to study the pathophysiology of schizophrenia-related positive symptoms. Recently, it has been shown that these positive symptoms can be studied in rats by using representation-mediated learning. This learning measure the accuracy of mental representations of reality, also called ‘reality testing’. Alterations in ‘reality testing’ performance can be an indication of an impairment in perception which is a clear hallmark of positive psychotic-like states. Thus, we describe here a mouse task adapted from previous findings based on a sensory preconditioning task. With this task, associations made between different neutral stimuli (e.g., an odor and a taste) and subsequent selective devaluation of one of these stimuli have allowed us to study mental sensory representations. Thus, the interest of this task is that it can be used to model positive psychotic-like states in mice, as recently described.
Keywords: Positive symptoms, Schizophrenia, Delusions, Reality testing, Representation-mediated learning
Background
The presence of positive symptoms, such as delusions or hallucinations, is a key feature of a psychotic-like state (American Psychiatric Association, 2000 and 2013; Tandon, 2013) and represents a major challenge to rodent models (Wong and Van Tol, 2003; van den Buuse et al., 2005 ; Mouri et al., 2007 ; Jones et al., 2011 ). Indeed, these symptoms are often neglected due to the lack of suitable animal models ( Jones et al., 2011 ; Rubino and Parolaro, 2014 and 2016). Psychotogenic drug-induced hyperlocomotion in rodents has long been considered an acceptable approximation of positive symptoms of drug-induced psychotic-like states (Wong and Van Tol, 2003; van den Buuse et al., 2005 ; Mouri et al., 2007 ; Jones et al., 2011 ). However, locomotor activity cannot be used to study the mismatch between perception and reality that is the hallmark of positive psychotic-like states (Wong and Van Tol, 2003; van den Buuse et al., 2005 ). For instance, the ‘Diagnostic and Statistical Manual of Mental Disorders’ (DSM V) defines delusions as ‘erroneous beliefs that usually involve a misinterpretation of perception or experiences’ (American Psychiatric Association, 2013). To overcome this methodological limitation, recent studies have used behavioral procedures in rodents designed to measure the accuracy of mental representations of reality, also called ‘reality testing’ (McDannald and Schoenbaum, 2009; McDannald et al., 2011 ; Kim and Koh, 2016). Alterations in ‘reality testing’ performance can be an indication of an impairment in perception which, as we mentioned before, might lead to positive psychotic-like states such as delusions. Thus, we adapt these previous protocols (McDannald and Schoenbaum, 2009; McDannald et al., 2011 ; Wheeler et al., 2013 ; Kim and Koh, 2016) to design a behavioral paradigm for measuring ‘reality testing’ in mice (Busquets- Garcia et al., 2017 ). Notably, deficits in task performance induced by psychotogenic drugs (e.g., amphetamine or cannabinoids) seem to reflect the kind of perceptual alterations that are the hallmarks and early features of positive psychotic-like symptoms.
Materials and Reagents
1 ml syringe and 26 G needles (Terumo Europe, Leuven, Belgium)
Mice (C57BL6/N mice purchased from Janvier Labs, Le Genest-Saint-Isle, France)
Banana odor (isoamyl acetate) (Sigma-Aldrich, catalog number: W205508)
Almond odor (benzaldehyde) (Sigma-Aldrich, catalog number: 418099)
Maltodextrin (Sigma-Aldrich, catalog number: 419672)
Sucrose (Sigma-Aldrich, catalog number: S0389)
Lithium chloride (LiCl) (Sigma-Aldrich, catalog number: 203637)
Banana solution (0.05%) (see Recipes)
Almond solution (0.01%) (see Recipes)
Maltodextrin solution (5%) (see Recipes)
Sucrose solution (5%) (see Recipes)
Combined odor-taste solution (see Recipes)
LiCl (3 M) (see Recipes)
Note: Any psychotogenic drug (e.g., amphetamine or cannabinoids) can be used as a positive control for the test, as previously shown (Busquets-Garcia et al., 2017).
Equipment
50-ml drinking tubes (Conical Centrifuge Tube, Thermo Fisher Scientific, Rochester, USA)
Standard balance
Standard individual plexiglas cage for each mouse
Metal tube (TD-100, 2.5” straight ball point tube, stainless steel, Ancare, New York, USA)
Rubber plug with 25 mm diameter hole (Thermo Fisher Scientific, Rochester, USA)
Note: It is important that the bottles be assembled before starting the experiment (Figure 1).
Figure 1. Drinking bottles used in the protocol.
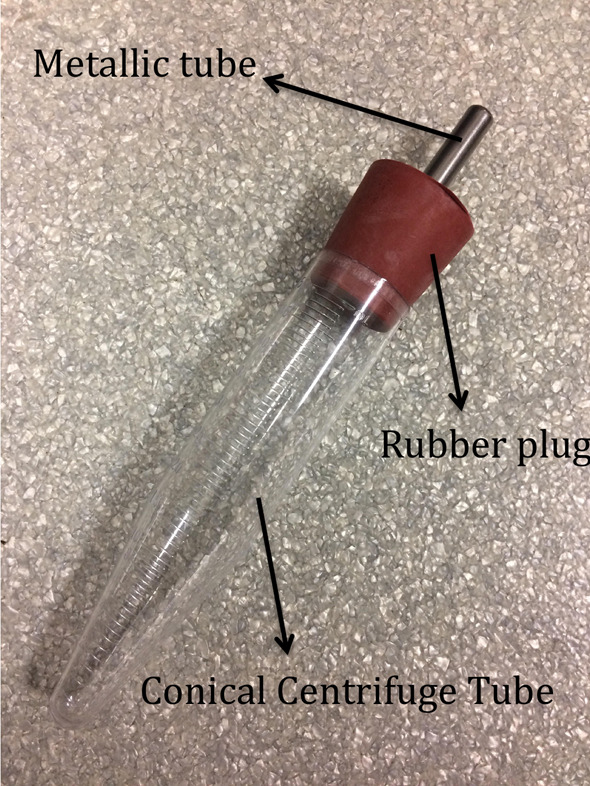
The bottles are carefully put together, as shown in the image. Note that the metal tube must pass through the rubber plug and all possible leakage must be avoided.
Procedure
Place mice in individual cages in a room with controlled temperature (21 ± 1 °C) and humidity (55 ± 10%).
Mice are water deprived 24 h before the first day of the protocol. This water deprivation lasts for the full duration of the protocol and mice weight is also controlled (maintained at around 85-90% of original).
-
All animals receive 1-h access to water (using the drinking bottles, Figure 1). The bottle is weighed before and after this period in order to precisely quantify how much the animals drink in each session. This procedure is repeated during three consecutive days as a habituation period.
Notes:
If a mouse does not drink more than 1 ml in one hour, it is given access to the bottle for one extra hour. This is particularly important the first day when animals are not yet habituated.
The position of the bottle is changed every day (right or left, see Figure 2). The height and angle of the bottles is maintained throughout the protocol.
-
Over the following days, conduct the odor-taste pairing phase (preconditioning, Figure 3) in which animals are exposed to 6 odor-taste pairings. Each pairing consists of two days: the first day, animals are given 1-h access to a flavored water-based solution containing a novel taste (either 5% sucrose or 5% maltodextrin; Taste 1, T1) and a novel odorant (0.05 % Banana or 0.01 % Almond; Odor 1, O1) in order to pair T1 with O1. On day two, animals are given access to a new taste and odor, neither of which has been presented the previous day, i.e., Taste 2 (T2) and Odor 2 (O2).
Notes:
Any new mouse strain or genetic mouse model must first be tested using 3 odor-taste pairings (instead of 6) in order to establish whether the mouse line in question is apt to present mediated aversion (Busquets-Garcia et al., 2017). In our recent work, control mice (e.g., C57BL6/N) present clear mediated aversion after 3 pairings. However, when the number of training sessions during preconditioning is increased (6 or 9 pairings), animals no longer present mediated aversion. For this reason, we used the 6 pairing protocol to study the acute effects of psychotogenic drugs (Busquets-Garcia et al., 2017). However, when using a new line (e.g., CB1 knockout mice), it must first be established that the animals are able to show mediated aversion with limited training before they are tested in long training protocols. It is likely that certain mouse models will present impaired development of mediated aversion. Indeed, odor-mediated taste learning has long been used as a learning test wherein different genotypes or mouse models present characteristic performances in mediated aversion.
The position of the bottles is changed for each pairing (right or left, see Figure 2).
The preference for specific odors or tastes has been demonstrated recently (Busquets-Garcia et al., 2017).
The odor-taste solutions are prepared anew every 2-3 days and all bottles are thoroughly cleaned at the end of the preconditioning phase to avoid any residual taste persisting into the following session.
-
During the following 6 days, animals undergo the devaluation phase (Figure 3) whereby O1 is devaluated to become the conditioned stimulus (CS+). On days 1, 3 and 5 of this phase, animals receive 1-h access to O2 followed by an intraperitoneal (i.p.) injection of saline (CS-), whereas on days 2, 4 and 6, they receive 1-h access to O1 immediately followed by an i.p. injection of the emetic agent LiCl (0.3 M, 1% b.w. i.e., 10 ml/kg) (CS+) in order to induce gastric malaise.
Notes:
To assess taste-mediated odor aversion, the devaluation phase pairing is performed using the tastes. T2 is associated with a saline injection (CS-) whereas T1 is associated with a LiCl injection (CS+).
The position of the bottles is changed for each pairing (right or left, see Figure 2). The height and angle of the bottles is maintained throughout the protocol.
The LiCl injection is performed just after removal of the bottles. A few minutes after injection, animals are observed to experience dizziness and greatly reduced locomotion.
Odor or taste solutions are prepared anew every 2-3 days and all bottles are thoroughly cleaned at the end of this phase.
-
Animals are given one day for recovery in which they receive water for 1 h.
Note: The position of the water bottle is opposite that of the last pairing.
-
On the next day, mediated aversion is assessed using a 1-h two-choice test where two different bottles are presented at the same time (Figure 4). Mediated aversion is always evaluated on the first day of tests with a choice between the mCS+ and the mCS-. The placement of each bottle is counterbalanced to avoid any position bias in task performance. From our experience, control animals with intermediate training (3 pairings) during preconditioning develop mediated aversion. Notably, if the training sessions are increased (6 or 9 pairings), the animals do not present mediated aversion (see Busquets- Garcia et al., 2017 for further information).
Notes:
To assess taste-mediated odor aversion, this two-choice test is carried out using the two different odor bottles.
At the beginning of this test session, a ‘forced sampling’ procedure is used to ensure that the mice sample both fluids. Both bottles are presented: when the mouse has licked one of the bottles, this bottle is removed until the mouse licks the second bottle. When this has happened, the second bottle is also removed. Finally, both bottles are then presented simultaneously. The order of presentation of the bottles during forced sampling is counterbalanced to avoid any order bias.
On the second day, direct aversion is evaluated using a two-choice test between the CS+ and CS- with a procedure identical to that described for the mediated aversion test.
Figure 2. Position of the bottles.

The position of the bottles is changed during the protocol (see Procedure) in order to habituate the mice to drink from both positions. However, bottles must be maintained at the same height and angle throughout the protocol. Note that food is distributed in both sides of the metal grid.
Figure 3. Simple representation of the behavioural paradigm.
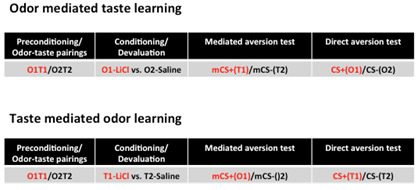
Schema of the protocol used to assess odor-mediated taste aversion (top) and taste-mediated odor aversion (bottom). For a more detailed schema refer to Busquets- Garcia et al., 2017 . Before preconditioning, all animals receive 1-h access to water during three consecutive days as water habituation. Next, conduct the odor-taste pairings phase, which consists of 6 odor-taste pairings (Busquets- Garcia et al., 2017 ). Each pairing lasts two days: the first day, animals receive 1-h access to a flavored solution containing a novel taste (T1) and a novel odorant (O1) in order to pair T1 with O1. On day two, animals receive whichever taste and odor have not been presented the previous day, i.e., T2 and O2. On the following days, animals enter the conditioning (or devaluation) phase where either O1 (top) or T1 (bottom) is devaluated. On days 1, 3 and 5 of this phase, animals receive 1-h access to O2 (top) or T2 (bottom) immediately followed by an intraperitoneal (i.p.) injection of saline, whereas on days 2, 4 and 6, they receive 1-h access to O1 (top) or T1 (bottom) immediately followed by an i.p. injection of the visceral malaise-inducing drug lithium chloride (LiCl, 0.3 M, 1% b.w.). After this conditioning, animals are given one day for recovery in which they receive water during 1 h. On the next 2 days, mediated and direct aversions are assessed using a 1-h two-choice test (top and bottom). Mediated aversion is evaluated in Test 1 by means of a choice between the mediated CS+ (mCS+: consisting of the taste [top] or odor [bottom] stimulus which has been previously associated with the LiCl-paired CS odor or taste, respectively) and the mediated CS- (mCS-: consisting of the taste [top] or odor [bottom] stimulus which has been previously associated with the saline-paired CS odor or taste, respectively). If, on the same day, researchers are interested to know the effect of a given drug on the mediated aversion test (Busquets- Garcia et al., 2017 ), acute treatments can be performed just before the test. Then, on the second day, direct aversion is evaluated by means of a choice between the conditioned LiCl-paired stimulus (CS+) and the conditioned saline-paired stimulus (CS-).
Figure 4. Two-choice test.

During the two-choice test two bottles (i.e., tastes or odors) are made available for duration of one hour. In order to make the animal aware of the choice, perform a ’forced sampling’ procedure following these steps: (i) both bottles are presented at the same time, (ii) when the animal drink from one bottle this bottle is removed, (iii) time is allowed for the mouse to drink from the remaining bottle, (iv) the second bottle is removed and, finally, (v) both bottles are re-introduced simultaneously, as seen in this image, in order for the two-choice test to be performed.
Data analysis
Aversion was revealed by lower consumption of mCS+ than mCS- (mediated aversion) or of CS+ than CS- (direct aversion) (Soria- Gomez et al., 2015 ; Busquets- Garcia et al., 2017 ). Data were presented as absolute liquid intake and an aversion index was calculated using the following formula: (CS- - CS+)/(CS+ + CS-) for direct aversion or (mCS- - mCS+)/(mCS+ + mCS-) for mediated aversion, respectively.
Notes
All animals were housed in individual cages for at least 5 days before the experiment.
All sessions of the experimental protocol were performed in the morning (from 9 AM to 1 PM).
In order to avoid any olfactory contamination between bottles, 3 different bottles were used: some bottles were exclusively used for banana odor or the mixture of banana odor with a taste, either sucrose or maltodextrin (banana bottles), others only for almond odors or the mixture of almond odor with a taste (almond bottles) and the remaining bottles were used for water or taste alone (water/taste bottles). Bedding was changed at the start of the protocol and after the preconditioning phase, some hours after the last session.
The task was tested in several mice lines. We found similar results between the C57/BL6N mice and the wild-type animals produced in our institute.
Recipes
-
Banana solution (0.05%)
To each 100 ml of water, we added 50 μl of isoamyl acetate. In order to prepare this solution, we put the necessary quantity of water in a container, to which we added the previously calculated quantity of odor. The solution was then mixed until the odor dissolved
-
Almond solution (0.01%)
To each 100 ml of water, we added 10 μl of benzaldehyde. In order to prepare this solution, we put the necessary quantity of water in a container, to which we added the previously calculated quantity of odor. The solution was then mixed until the odor dissolved
-
Maltodextrin solution (5%)
To each 100 ml of water, we added 5 g of maltodextrin. In order to prepare this solution, we put the necessary quantity of water in a container, to which we added the previously calculated quantity of taste. The solution was then mixed until the powder dissolved
-
Sucrose solution (5%)
To each 100 ml of water, we added 5 g of sucrose. In order to prepare this solution, we put the necessary quantity of water in a container, to which we added the previously calculated quantity of taste. The solution was then mixed until the powder dissolved
-
Combined odor-taste solution
Following the same quantities as above, we weighed out the required quantity of sucrose or maltodextrin, to which we added the necessary quantity of water and, finally, odor. The solution was then thoroughly mixed until full dissolution and transparency had been achieved
-
LiCl (3 M)
We weighed out 0.627 g of the LiCl powder and added it to 50 ml of physiological saline. The solution was then mixed thoroughly until the powder dissolved
Acknowledgments
We thank Delphine Gonzales, Nathalie Aubailly, and all the personnel of the Animal Facility of the NeuroCentre Magendie for mouse care. We would also like to thank all those who have performed experimental work using this task: Yarmo Mackenbach, Bastien Redon, Carolina Muguruza, Geoffrey Terral, Camile Pernegre, Christina Ioannidou and Marjorie Varilh. We also thank Christopher Stevens for the critical reading of the manuscript. This protocol was adapted according to McDannald MA et al. (2011). This work was supported by INSERM (to G.M.), INRA (G.F.), EU-FP7 (PAINCAGE, HEALTH-603191 to G.M. and FP7-PEOPLE-2013-IEF-623638 to A.B.-G.), European Research Council (Endofood, ERC-2010-StG-260515; CannaPreg, ERC-2014-PoC-640923, to G.M.), Fondation pour la Recherche Medicale (DRM20101220445 and DPP20151033974, to G.M.), Human Frontiers Science Program (to G.M.), Region Aquitaine (to G.M.), French State/Agence Nationale de la Recherche (BRAIN ANR-10-LABX-0043 to G.M., ANR-10-IDEX-03- 02 to A.B.-G, NeuroNutriSens ANR-13-BSV4-0006-02 to G.M. and ORUPS ANR-16-CE37-0010 to G.M. and G.F.), Fyssen Foundation (to E.S.-G.) and CONACyT (to E.S.-G.).
References
- 1.American Psychiatric Association(2000). DSM, 4th edition. Washington, DC: APS. [Google Scholar]
- 2.American Psychiatric Association(2013). DSM, 5th edition. Washington, DC: APS. [Google Scholar]
- 3.Busquets-Garcia A., Soria-Gomez E., Redon B., Mackenbach Y., Vallee M., Chaouloff F., Varilh M., Ferreira G., Piazza P. V. and Marsicano G.(2017). Pregnenolone blocks cannabinoid-induced acute psychotic-like states in mice. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones C. A., Watson D. J. and Fone K. C.(2011). Animal models of schizophrenia. Br J Pharmacol 164(4): 1162-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H. J. and Koh H. Y.(2016). Impaired reality testing in mice lacking phospholipase Cβ1: observed by persistent representation-mediated taste aversion. PLoS One 11(1): e0146376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDannald M. A., Whitt J. P., Calhoon G. G., Piantadosi P. T., Karlsson R. M., O'Donnell P. and Schoenbaum G.(2011). Impaired reality testing in an animal model of schizophrenia. Biol Psychiatry 70(12): 1122-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDannald M. and Schoenbaum G.(2009). Toward a model of impaired reality testing in rats. Schizophr Bull 35(4): 664-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouri A., Noda Y., Enomoto T. and Nabeshima T.(2007). Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem Int 51(2-4): 173-184. [DOI] [PubMed] [Google Scholar]
- 9.Rubino T. and Parolaro D.(2014). Cannabis abuse in adolescence and the risk of psychosis: a brief review of the preclinical evidence. Prog Neuropsychopharmacol Biol Psychiatry 52: 41-44. [DOI] [PubMed] [Google Scholar]
- 10.Rubino T. and Parolaro D.(2016). The impact of exposure to cannabinoids in adolescence: insights from animal models. Biol Psychiatry 79(7): 578-585. [DOI] [PubMed] [Google Scholar]
- 11.Soria-Gomez E., Busquets-Garcia A., Hu F., Mehidi A., Cannich A., Roux L., Louit I., Alonso L., Wiesner T., Georges F., Verrier D., Vincent P., Ferreira G., Luo M. and Marsicano G.(2015). Habenular CB1 receptors control the expression of aversive memories . Neuron 88(2): 306-313. [DOI] [PubMed] [Google Scholar]
- 12.Tandon R.(2013). Definition of psychotic disorders in the DSM-5 too radical, too conservative, or just right! Schizophr Res 150(1): 1-2. [DOI] [PubMed] [Google Scholar]
- 13.van den Buuse M., Garner B., Gogos A. and Kusljic S.(2005). Importance of animal models in schizophrenia research. Aust N Z J Psychiatry 39(7): 550-557. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler D. S., Chang S. E. and Holland P. C.(2013). Odor-mediated taste learning requires dorsal hippocampus, but not basolateral amygdala activity. Neurobiol Learn Mem 101: 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong A. H. and Van Tol H. H.(2003). Schizophrenia: from phenomenology to neurobiology. Neurosci Biobehav Rev 27(3): 269-306. [DOI] [PubMed] [Google Scholar]


