Purpose of review
Significant interest and controversy surround the use of C1q for determining risk of antibody-mediated rejection (AMR) and graft loss. Alternate models for predicting outcomes have been proposed. This review focuses on the correlation of currently utilized assays for outcome, together with the technical and theoretical limitations, to distill current thinking.
Recent findings
Results demonstrate that C1q status is significantly correlated with AMR and graft loss. There is general consensus that C1q is more clinically relevant for graft outcome than neat IgG MFI. IgG titers, subclass, and other complement assays have now been studied to determine if they are more relevant. Only IgG3 and possibly C3d fixation have shown added value to C1q for outcome correlation. Direct parallel titer comparisons of C1q and IgG are lacking and the correlation is unknown.
Summary
Overall, results confirm the correlation with C1q+ donor-specific antibody (DSA) for AMR and graft loss. The association is stronger posttransplant. C1q+ de novo antibody appears to be especially detrimental portending graft loss in about 1–2.5 years post detection. Recommendations to biopsy and treat at time of de novo C1q+ antibody detection have been suggested by several groups.
Keywords: AMR, C1q, graft loss, rejection
INTRODUCTION
The C1q assay assesses the ability of an antigen/antibody complex to bind the first component of complement potentially (but not necessarily) leading to target cell killing. It marries the sensitivity and specificity of flow or Luminex solid phase assays with the functionally relevant capacity to fix complement to define antibodies of clinical significance to human leukocyte antigen (HLA) in solid organ transplantation. Since the first reports in 2011 showing high incidence of early rejection in pediatric hearts [1], acute rejection and graft loss in pediatric and adult kidneys [2,3], and confirmed by a subsequent study of more than 1000 kidney recipients [4], most reports have shown that C1q correlates significantly with risk of antibody-mediated rejection (AMR) and/or graft loss in kidneys. C1q+ donor-specific antibody (DSA) is more clinically relevant for outcome than IgG+ DSA alone especially when de novo DSA (dnDSA) arises posttransplant. Still, use of C1q to predict graft outcome remains controversial and some attribute its correlation with clinical outcomes to other reasons, such as IgG subclass or titer. Careful attention to report details is required to determine whether the data support the conclusions as we move toward finding the best means to predict outcome.
Box 1.
no caption available
APPLICATION OF C1q
As only ∼50% of IgG+ antibodies are C1q+ [5], antibodies can be stratified based on their complement fixing ability. C1q can be used pretransplant for routine antibody screening and monitoring desensitization to strategically expand the donor pool, and posttransplant for monitoring efficacy of rejection therapies.
Technical issues
The original C1q assay [5] was far more sensitive than complement-dependent cytotoxicity (CDC) in picking up complement fixing antibody. The commercial assay is much less sensitive than the original C1q method [Fig. 1] leading to false-negative, but not false-positive, results. Schaub et al.[34] showed that the addition of antihuman globulin to the test would significantly increase its sensitivity.
FIGURE 1.
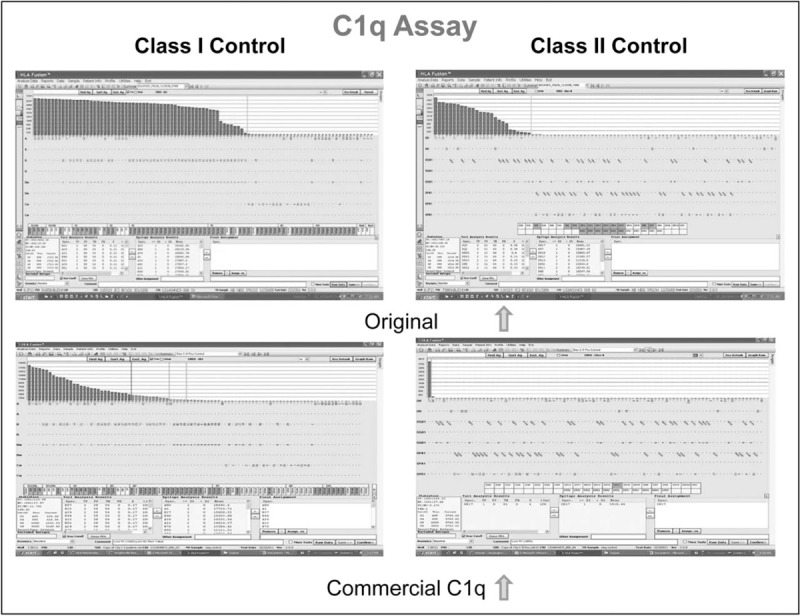
Comparison of results using commercial C1q kit vs. original method showing much weaker detection by commercial C1q of both class I (left panel) and class II (right panel) in positive control serum.
Based on more than 10 000 C1q tests, we have found that every individual has different background fluorescence in the test, some with high normalized mean fluorescence intensity (nMFI) values [Tyan, unpublished observations]. Consequently, it is not reliable to set a standard MFI cutoff for all. Prior reports have set values of 300, 500, and 1000 MFI as cutoffs with a mix of raw and nMFI values. In our experience, arranging the nMFI values from lowest to highest, finding the first increase of 300 MFI and adding 1000 to the lower MFI value at the break will yield an MFI threshold above which antibodies are clearly and reliably positive with major increases in MFI above that point. Those below the 300 MFI break are negative, and those in between are considered ‘possible.’ In contrast to published reports, C1q is also subject to prozone effects and serum treatment to eliminate the prozone can yield significantly different results.
CONTROVERSIES
There are three main controversies related to the C1q assay, namely: high IgG MFI values give the same information as C1q; IgG titer is the clinically relevant feature of the antibody and C1q results are only a function of the titer; and C1q positivity is a function of IgG subclass and the subclass can substitute for C1q.
C1q and IgG mean fluorescence intensity
Many reports have shown a correlation between C1q positivity with high IgG MFI values (threshold: 7000–10 000) and extrapolated that the C1q test is therefore not necessary. However, these same reports have shown that the relationship is not absolute because some IgG with low MFI can fix C1q whereas some with very high MFI do not, even when the serum is treated to eliminate any prozone [6–9,10▪,11▪▪]. We have observed C1q+ results with IgG MFI as low as 2000 [Tyan, unpublished observations]. Thus, it is necessary to actually do the C1q assay to be certain of the complement fixing status of any given antibody.
C1q and IgG titer
The lack of 1 : 1 correlation between IgG MFI and C1q positivity has led to the view that it is the IgG titer rather than the neat MFI that equates with C1q, especially given prozones in neat sera. Correlation between IgG and C1q improves whenever the sera are titered [6,7,12] showing titers between 1 : 16 and 1 : 32 by IgG equate to appearance of C1q reactivity; but C1q+/IgG low outliers are routinely observed [6,8]. However, in these studies, sera were only tested neat by C1q and no study to date has compared C1q to IgG at every dilution for every serum making it difficult to determine the precise correlation of C1q with titer or if it is the same for every serum/patient.
C1q and IgG subclass
IgG subclasses fix complement in the order of G3>G1>>G2>G4. Some have suggested that rather than test by C1q, determining the IgG subclass would reveal the complement fixing IgG antibodies. The rationale for doing four tests for subclass in place of one for C1q is unclear, as studies have shown that the presence of complement fixing subclasses does not correlate with C1q reactivity or graft outcome [13]. The only subclass that has been shown to have a significant impact on graft outcome is IgG3 [10▪,14▪] but C1q and IgG3 each have independent predictive value for graft outcome. Additionally, IgG subclass cannot be used to monitor patients being desensitized with intravenous immunoglobulin (IVIG) or other drugs containing human antibody (e.g, ATG) as these antibodies also contain IgG subclasses.
CLINICAL APPLICATION AND RELEVANCE OF C1q
The clinical relevance of C1q status for assessing risk for AMR and graft loss has been extensively studied in adult kidney patients both pretransplant and posttransplant with fewer studies in heart and pediatric kidney patients. Patient cohorts have been stratified according to whether they were unsensitized, sensitized or highly sensitized at transplant; developed de novo antibody; were desensitized; had early, late, or subclinical rejection; and/or experienced graft loss. Other methods have been compared with C1q to determine whether these yielded better information to help stratify risk. Serum treatment to remove prozone has varied widely or not been done.
PRETRANSPLANT
The relevance of pretransplant C1q+ DSA is variable. Arreola-Guerra, et al.[15▪▪] reported that a negative AHG-CDC crossmatch (XM) with IgG+ DSA, C1q+ DSA predicts a positive flow XM (FXM) with an odds ratio (OR) = 27. FXM positivity is highest when the IgG MFI is less than 5000. C1q+ DSA was the most specific (95.8%) and IgG MFI = 2300 plus C1q+ DSA together the most sensitive (92%). They also found [16▪▪] a disparity in C1q positivity among loci (A = low, DQ = high; OR = 9.82 for having one of each), reporting that 48.7% of graft detrimental DQ antibodies are C1q+. They suggest a model where the locus-specific profile of DSA(s) might predict C1q positivity and susceptibility or protection for AMR/graft loss. Irure et al.[11▪▪] reported 18 kidney patients (more than 98% cPRA) tested neat and 1 : 160 for HLA class I (without serum treatment), 4 of 5 of whom were transplanted (CDC−, FXM+, C1q+, IgG+ neat and diluted) and developed AMR. Correlation between C1q and dilution was poor (r = 0.58) due to the presence of high titer IgG+/C1q− and low titer IgG−/C1q+ results when all class I beads were analyzed. The single patient without AMR was C1q and dilution negative pretransplant and posttransplant, leading the authors to recommend testing sera pretransplant by C1q that have low titer IgG to assess risk. In 60 kidney recipients with preformed DSA and high AMR frequency (30%), Malheiro et al.[17▪] found C1q and IgG MFI were both correlated with AMR. C1q was better than IgG DSA strength of at least 15 000 MFI (OR: 16.3 vs. 6.4, respectively) for predicting AMR. Multivariable logistic regression showed C1q+ DSA was a risk factor for AMR (OR = 16.80, P = 0.001) but high MFI DSAs were not. Six-year graft survival was also significantly lower in high MFI C1q+ DSA in comparison with C1q−, IgG high or low MFI DSA (38, 83 and 80%, respectively; P = 0.001). There is only one prospective study comparing C1q pretransplant and posttransplant. Viglietti et al.[14▪] found 31.8% patients had preformed C1q+ DSA at t0 with 5-year graft survival of 45.8% in the C1q+ group compared with 92.1% in the C1q− group using EDTA-treated serum. IgG3 DSA status at t0 was independently associated with graft survival and C1q+ IgG3 provided the best reclassification of risk for graft loss. On a practical note, Juhl et al.[18▪▪], found only 2.2% positive prospective CDC XM in 1432 performed. Of those, 73.1% were C1q+, leading them to suggest that by using C1q, CDC XM could be safely eliminated and replaced by VXM resulting in less shipment of donor material, shorter CIT, less DGF, and fewer false-positive XMs while not disqualifying highly sensitized patients. A single study of pretransplant allosensitization and outcomes in sensitized and unsensitized heart recipients [19] found no association of C1q with AMR, but the authors noted that a prospective negative CDC XM was required that likely eliminated any preformed C1q+ DSA patients.
POSTTRANSPLANT
Most recent C1q studies have focused on DSA posttransplant, both persistent and de novo. Fichtner et al.[20▪▪] and Susal et al.[21▪▪] studied 62 unsensitized pediatric kidney recipients with clinically indicated biopsies and found 42% were DSA+ and 15% also C1q+, independent of IgG MFI. Four-year graft survival for the DSA+ C1q+ group was 11% compared with 88% (DSA−; P < 0.001) and 82% (DSA+ C1q−; P = 0.001), with a HR = 6.4 for the DSA+ C1q+ group for chronic active AMR. None who lost their grafts were C1q− and 43% were C1q+, though not necessarily C1q+ DSA, possibly due to adsorption of C1q+ DSA onto the graft. They suggest that C1q+ DSA at the time of an indication biopsy identifies a subgroup of pediatric patients with a markedly increased risk for subsequent graft loss. Correlating C1q status with histology, Bamoulid et al.[22▪▪] found C1q+ dnDSA independently associated with AMR and graft loss in univariate and multivariate analyses. IgG MFI of dnDSA failed to show any statistical association with any parameter tested. C1q+ DSA correlated with interstitial edema but not cg scores. C1q+ dnDSA and C1q+ MFI at time of first detection of DSA (2.7–3.8 years) had a significantly higher risk of AMR within the next year and in multivariate analysis an overall risk of AMR (HR = 2.27) prompting them to suggest obtaining a biopsy at time of dnDSA detection. Wiebe et al.[23] showed C1q+ dnDSA had a specificity of 0.82 and a positive predictive value (PPV) of 0.83 for AMR and a significant correlation with graft loss (C1q+ 71%, C1q− 28%, P < 0.01) that became significant 3 years post dnDSA detection. C1q positivity correlated with tubulitis (P = 0.02) and C4d+ staining (P = 0.03). They found no correlation with sera titered between 1 : 16 and 1 : 1024 and tested by IgG even when using IgG MFI > 0 to assign a positive IgG. Despite these data, C1q results were discounted and outcomes attributed to noncompliance and clinical phenotypes that cannot be easily or reliably measured prospectively, do not translate into meaningful prognostic clinical tests and, therefore, are not useful for monitoring efficacy of intervention.
Lefaucheur et al.[10▪] reported that C1q+ dnDSA in the first posttransplant year had a risk of 4.8 for graft loss and adverse pathology. Neither strong IgG MFI nor IgG subclass was predictive of C1q positivity, rather the best correlation was with multiple IgG subclasses combined. Cicciarelli et al.[13] studied C1q and IgG subclasses in C4d+ DSA+ kidney patients biopsied for cause more than 7 years posttransplant. Although there was a trend toward graft loss and possibly severity of rejection in C1q+ DSA patients, neither C1q nor subclass significantly correlated with AMR. Additionally, complement fixing subclasses that should have caused C1q to be positive did not and vice versa. Though C1q correlated best with AMR, they attributed results instead to C3d, which was not tested. C1q+ DSA correlated with IgG MFI > 12 000 and C4d+ leading them to conclude that C1q is simply a function of IgG MFI and subclass.
Yamamoto et al.[24▪▪] reported that 40% of patients with dnDSA and a late biopsy have subclinical rejection. C1q+ dnDSA was an independent predictor of subclinical rejection. In contrast, Kauke et al.[25▪] did not find an association between de novo C1q+ or C1q− DSA in 110 kidney recipients with acute rejection but found significantly diminished renal function in patients with C1q+ DSA. Five-year allograft survival, when stratified according to dnDSA vs. non-DSA (nDSA) and C1q-binding status, significantly decreased from C1q− nDSA (93.5%; P = 0.4860) to C1q+ nDSA (80.9%; P = 0.0251) to C1q− DSA (76.9%; P = 0.0012) to C1q+ DSA (59.7%, P < 0.0001) as compared with controls (90.7%), with C1q+ DSA having the highest risk for graft loss. They suggested that C1q+ de novo antibody, whether DSA or not, indicated a higher risk for graft failure. Eskandary et al.[26] studied complement fixing DSA in 86 DSA+ kidney patients with stable graft function; 51% had AMR on protocol biopsy. They reported significantly higher C1q MFI (but not C3d or C4d MFI) in AMR+ vs. AMR− patients (P = 0.03). Among AMR− patients with focal C4d lesions C1q MFI levels were higher than in patients without lesions. Comoli et al.[27] reported dnDSA at median 3.6-year posttransplant in unsensitized pediatric kidney recipients (25 C1q+, 9 C3d+). They concluded that C3d correlated better with graft loss than C1q; however, they noted that the statistical power was too low for multivariate analysis. They found no AMR-free graft survival if IgG+/C1q+/C3d+ DSA was present. As graft loss occurred about 2 years post dnDSA detection, they recommended treating when dnDSA was first detected since monitoring would be expensive. In a similar cohort stratified by early (less than 1 year) and late (more than 1 year) dnDSA detection, Cioni et al.[28] reported 47% AMR+ and 20% subsequent graft loss in the early group; 80% of which were C1q+.
Interestingly, decreased graft survival was observed when C1q+ (but not IgG) antibody to denatured class I or class I+II antigen was present. This was associated with AMR and mixed rejection rather than CMR [29].
Few recent studies exist in heart transplants. Frank et al.[30▪] reported that 82% of EMB specimens taken at the time of graft dysfunction were C1q+ of which 56% were C4d+. Only one C4d+/C1q− EMB was seen. No difference in graft failure was observed related to C1q status, perhaps due to selection bias as noted by the authors.
DESENSITIZATION
Different strategies for desensitization exist. As some therapeutic drugs raise the background in the tests making lowering of antibody difficult to measure (e.g. monitoring high dose IVIG desensitization by an IgG assay), monitoring methods need to be carefully selected.
Ramon et al.[31▪] studied 30 kidney patients with biopsy-proven AMR and graft dysfunction. They showed that achieving C1q− DSA was significantly better than measuring 50% or more reduction in IgG MFI for predicting graft survival at 2 and 3 years post treatment (3 years, P = 0.02) and had better specificity (0.8 vs. 0.55) and PPV (0.83 vs. 0.44) than measuring IgG MFI reduction. Class II antibodies are especially refractory to IgG MFI decreases of at least 50%. They noted that when the treatment cannot lower the DSA below the complement fixing threshold, the mechanism of injury cannot be controlled. Tambur et al.[32] studied MFI values of sera tested neat by IgG and C1q compared with serial dilutions by IgG in a mixed population of 40 kidney recipients desensitized by plasmapheresis and low-dose IVIG either pretransplant with relatively low levels of DSA or posttransplant with AMR. Assessing all SAB+ antibodies and not DSA specifically, they concluded that titers were more informative than neat MFI values by IgG or C1q for tracking efficacy of the treatment. Unfortunately, sera were not all pretreated to remove prozones nor assessed at each dilution for C1q status to confirm whether there is a 1 : 1 correlation. No outcome data were reported. Schaefer et al.[33▪] studied 80 presensitized kidney recipients (≥85%) who had been desensitized by plasmapheresis and anti-CD20 therapy. Posttransplant, patients with C1q+ DSA had significantly higher rates of AMR and graft loss due to AMR when compared with those with C1q− or no DSA (86 vs. 33 vs. 0%, AMR) and (86 vs. 0 vs. 0%, graft loss), respectively. The positive predictive value for graft loss was 86%.
CONCLUSION
Knowing when to transplant, with which donor, when and how long to desensitize, and when to augment or discontinue augmented immunosuppression are goals shared by all. The majority of recent reports demonstrate that C1q is better correlated with AMR and graft loss than neat IgG MFI. Direct 1 : 1 titration comparisons of IgG and C1q are missing and no studies of outcomes based on titer stratification have been done. Such studies would be informative and worthwhile. Without such data, current literature suggests that C1q is more reliable for making choices. C1q is a discrete variable yielding a yes/no answer for complement fixation, whereas titer is not. The value of knowing the dilution at which the serum can no longer fix complement would be advantageous and perhaps allow transplant or discontinuation of desensitization at titers that could vary among individuals, especially in the posttransplant setting where the DSA is specifically known. Finally, C1q was never intended to be a standalone method for assessing clinical risk of rejection and/or graft failure. Combined data from C1q and IgG assays (e.g. MFI/subclass/titer) and/or other complement fixing assays (e.g. C3d) provide the most informative picture of individualized risk for the patient both before and after transplant.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
The author receives royalties for C1q licensing to One Lambda/Thermo-Fisher, Inc. and has also received travel reimbursement from them.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Chin C, Chen G, Sequeria F, et al. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant 2011; 30:158–163. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland SM, Chen G, Sequeira FA, et al. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant 2012; 16:12–17. [DOI] [PubMed] [Google Scholar]
- 3.Yabu JM, Higgins JP, Chen G, et al. C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation 2011; 91:342–347. [DOI] [PubMed] [Google Scholar]
- 4.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. New Engl J Med 2013; 369:1215–1226. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Hum Immunol 2011; 72:849–858. [DOI] [PubMed] [Google Scholar]
- 6.Zeevi A, Lunz J, Feingold B, et al. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transpl 2013; 32:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor CJ, Kosmoliaptsis V, Martin J, et al. Technical limitations of the C1q single-antigen bead assay to detect complement binding HLA-specific antibodies. Transplantation 2017; 101:1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claisse G, Absi L, Cognasse F, et al. Relationship between mean fluorescence intensity and C1q/C3d-fixing capacities of anti-HLA antibodies. Hum Immunol 2017; 78:336–341. [DOI] [PubMed] [Google Scholar]
- 9.Reinsmoen NL, Patel J, Mirocha J, et al. Optimizing transplantation of sensitized heart candidates using 4 antibody detection assays to prioritize the assignment of unacceptable antigens. J Heart Lung Transpl 2016; 35:165–172. [DOI] [PubMed] [Google Scholar]
- 10▪.Lefaucheur C, Viglietti D, Mangiola M, et al. From humoral theory to performant risk stratification in kidney transplantation. J Immunol Res 2017; 2017:8.Article 5201098. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report finds that C1q+ de novo DSA at less than 1-year post transplant is associated with a high risk of graft loss and adverse pathology.
- 11▪▪.Irure J, Asensio E, Rodrigo E, et al. Improvement in the definition of anti-HLA antibody profile in highly sensitized patients. PLoS One 2017; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article clearly demonstrates the lack of correlation between IgG and C1q even when serum is diluted.
- 12.Tambur AR, Herrera ND, Haarberg KM, et al. Assessing antibody strength: comparison of MFI, C1q, and titer information. Am J Transplant 2015; 15:2421–2430. [DOI] [PubMed] [Google Scholar]
- 13.Cicciarelli JC, Lemp NA, Chang Y, et al. Renal transplant patients biopsied for cause and tested for C4d, DSA, and IgG subclasses and C1q: which humoral markers improve diagnosis and outcomes? J Immunol Res 2017; 2017:1652931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Viglietti D, Loupy A, Vernerey D, et al. Value of donor-specific anti-HLA antibody monitoring and characterization for risk stratification of kidney allograft loss. J Am Soc Nephrol 2017; 28:702–715. [DOI] [PMC free article] [PubMed] [Google Scholar]; This prospective study shows that C1q and IgG3 independently reclassify risk (higher or lower) for graft failure at 5 years posttransplant.
- 15▪▪.Arreola-Guerra JM, Castelan N, de Santiago A, et al. C1Q assay results in complement-dependent cytotoxicity crossmatch negative renal transplant candidates with donor-specific antibodies: high specificity but low sensitivity when predicting flow crossmatch. J Transplant 2016; 2016: Article ID 2106028. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows a correlation of complement fixing C1q with positive flow crossmatch despite negative CDC-AHG results and independent of IgG MFI.
- 16▪▪.Arreola-Guerra JM, Morales-Buenrostro LE, Granados J, et al. Anti-HLA-DQ antibodies are highly and independently related to the C1q-binding capacity of HLA antibodies. Transpl Immunol 2017; 41:10–16. [DOI] [PubMed] [Google Scholar]; This study describes differential locus-specific C1q binding and suggests that certain DSA combinations may result in protection or susceptibility to AMR and graft loss depending on their loci.
- 17▪.Malheiro J, Tafulo S, Dias L, et al. Determining donor-specific antibody C1q-binding ability improves the prediction of antibody-mediated rejection in human leucocyte antigen-incompatible kidney transplantation. Transpl Int 2017; 30:347–359. [DOI] [PubMed] [Google Scholar]; This report shows that in patients with preformed antibodies, graft survival at 6 years is significantly decreased in those with C1q+ DSA.
- 18▪▪.Juhl D, Marget M, Hallensleben M, et al. Assignment of C1q-binding HLA antibodies as unacceptable HLA antigens avoids positive CDC− crossmatches prior to transplantation of deceased donor organs. Transpl Immunol 2017; 41:17–21. [DOI] [PubMed] [Google Scholar]; This study demonstrates that C1q can substitute for CDC crossmatching resulting in shorter cold ischemia time and reduced delayed graft function.
- 19.Svobodova E, Gazdic T, Kubanek M, et al. Novel insights into pretransplant allosensitization in heart transplant recipients in the contemporary era of immunosuppression and rejection surveillance. Transplant Int 2016; 29:63–72. [DOI] [PubMed] [Google Scholar]
- 20▪▪.Fichtner A, Süsal C, Höcker B, et al. Association of C1q-fixing DSA with late graft failure in pediatric renal transplant recipients. Pediatr Nephrol 2016; 31:1157–1166. [DOI] [PubMed] [Google Scholar]; C1q is reported to be the second highest predictor of graft failure 48 months post indication biopsy in unsensitized patients.
- 21▪▪.Susal C, Fichtner A, Tonshoff B, et al. Clinical relevance of HLA antibodies in kidney transplantation: recent data from the heidelberg transplant center and the collaborative transplant study. J Immunol Res 2017; 2017:5619402. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows severely compromised graft survival in C1q+/IgG+ but not C1q−/IgG+ patients.
- 22▪▪.Bamoulid J, Roodenburg A, Staeck O, et al. Clinical outcome of patients with de novo C1q-binding donor-specific HLA antibodies after renal transplantation. Transplantation 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; This study shows de novo C1q+ DSA is independently associated with AMR and subclinical rejection in univariate and multivariate analyses.
- 23.Wiebe C, Gareau AJ, Pochinco D, et al. Evaluation of C1q status and titer of de novo donor-specific antibodies as predictors of allograft survival. Am J Transplant 2017; 17:703–711. [DOI] [PubMed] [Google Scholar]
- 24▪▪.Yamamoto T, Watarai Y, Takeda A, et al. De novo anti-HLA DSA characteristics and subclinical antibody-mediated kidney allograft injury. Transplantation 2016; 100:2194–2202. [DOI] [PubMed] [Google Scholar]; In late biopsies of stable patients, this study found C1q was an independent predictor of subclinical rejection.
- 25▪.Kauke T, Oberhauser C, Lin V, et al. De novo donor-specific anti-HLA antibodies after kidney transplantation are associated with impaired graft outcome independently of their C1q-binding ability. Transplant Int 2017; 30:360–370. [DOI] [PubMed] [Google Scholar]; This study shows that both C1q+ non-DSA and C1q+ DSA present a significant risk for graft loss.
- 26.Eskandary F, Bond G, Kozakowski N, et al. Diagnostic contribution of donor-specific antibody characteristics to uncover late silent antibody-mediated rejection – results of a cross-sectional screening study. Transplantation 2017; 101:631–641. [DOI] [PubMed] [Google Scholar]
- 27.Comoli P, Cioni M, Tagliamacco A, et al. Acquisition of C3d-binding activity by de novo donor-specific HLA antibodies correlates with graft loss in nonsensitized pediatric kidney recipients. Am J Transplant 2016; 16:2106–2116. [DOI] [PubMed] [Google Scholar]
- 28.Cioni M, Nocera A, Innocente A, et al. De novo donor-specific HLA antibodies developing early or late after transplant are associated with the same risk of graft damage and loss in nonsensitized kidney recipients. J Immunol Res 2017; 2017:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai JC, Terasaki PI, Zhu D, et al. Complement-fixing antibodies against denatured HLA and MICA antigens are associated with antibody mediated rejection. Exp Mol Pathol 2016; 100:45–50. [DOI] [PubMed] [Google Scholar]
- 30▪.Frank R, Lal P, Kearns J, et al. Correlation of circulating complement-fixing donor-specific antibodies identified by the C1q assay and presence of C4d in endomyocardial biopsy specimens. Am J Clin Pathol 2016; 145:62–68. [DOI] [PubMed] [Google Scholar]; This study found that 82% of endomyocarial biopsies at time of graft dysfunction had C1q+ DSA.
- 31▪.Ramon DS, Huang YH, Zhao LL, et al. Use of complement binding assays to assess the efficacy of antibody mediated rejection therapy and prediction of graft survival in kidney transplantation. Hum Immunol 2017; 78:57–63. [DOI] [PubMed] [Google Scholar]; This study reported that achieving C1q negativity vs. ∼50% decrease in IgG MFI after desensitization resulted in better graft survival.
- 32.Tambur AR, Glotz D, Herrera ND, et al. Can solid phase assays be better utilized to measure efficacy of antibody removal therapies? Hum Immunol 2016; 77:624–630. [DOI] [PubMed] [Google Scholar]
- 33▪.Schaefer SM, Susal C, Opelz G, et al. Pretransplant soluble CD30 in combination with total DSA but not pretransplant C1q-DSA predicts antibody-mediated graft loss in presensitized high-risk kidney transplant recipients. Hla 2016; 87:89–99. [DOI] [PubMed] [Google Scholar]; This study found that presence of C1q+ DSA post transplant associated with significantly higher rates of AMR and graft loss.
- 34.Schaub S, Honger G, Koller MT, et al. Determinants of C1q binding in the single antigen bead assay. Transplantation 2014; 98:387–393. [DOI] [PubMed] [Google Scholar]


