Objective
We examined whether established metabolic risk genetic variants in the population confer a risk for increased waist circumference in patients with schizophrenia spectrum disorders and also an association with schizophrenia spectrum disorders irrespective of waist circumference.
Patients and methods
We analyzed the association in (i) a case–case model in which patients with schizophrenia spectrum disorder with increased waist circumference (≥80 cm for women and ≥94 cm for men) (n=534) were compared with patients with normal waist circumference (<80 cm for women; <94 cm for men) (n=124), and in (ii) a case–control model in which schizophrenia spectrum disorder patients with increased waist circumference or irrespective of waist circumference were compared with population-derived controls (n=494) adjusted for age, sex, fasting glucose, smoking, and family history of diabetes.
Results
Genetic variants in five genes (MIA3, MRAS, P2RX7, CAMKK2, and SMAD3) were associated with increased waist circumference in patients with schizophrenia spectrum disorder (P<0.046). Genetic variants in three other genes (PPARD, MNTR1B, and NOTCH2) were associated with increased waist circumference in patients when compared with control individuals (P<0.037). Genetic variants in the PPARD, MNTR1B, NOTCH2, and HNF1B were nominally associated with schizophrenia spectrum disorder irrespective of waist circumference (P<0.027). No differences in waist circumference between specific psychosis diagnoses were detected.
Conclusion
Increased waist circumference in patients with schizophrenia spectrum disorder may be explained, in part, by increased metabolic risk gene burden, and it indicates a shared genetic susceptibility to metabolic disorder and psychosis per se. Along these lines, common metabolic risk genetic variants confer a risk for increased waist circumference in patients with schizophrenia spectrum disorders.
Keywords: association study, case–case, case–control, diabetes mellitus type 2, metabolic risk genes, psychotic disorder
Introduction
An increased risk of metabolic disturbances in patients with severe mental illness, including obesity and diabetes mellitus type 2, is well documented in a large number of studies. A doubled rate of mortality from cardiovascular disease has been repeatedly shown (Osby et al., 2000; Hennekens et al., 2005; Gothefors et al., 2010). The increased mortality from cardiovascular disease is of great clinical importance because it is the main cause of death leading to reduced life expectancy in severe mental illness (Laursen et al., 2013; Nordentoft et al., 2013). Thus, further knowledge about the mechanisms of cardiovascular disease in severe mental illness is strongly warranted.
Antipsychotic medication, especially atypical antipsychotics, is well known to cause weight gain, but other factors also contribute. Studies of drug-naive patients and drug-free patients indicate increased levels of visceral fat deposition (Thakore et al., 2002), supporting the view that psychotic disorder per se is linked to metabolic disturbances (Osby et al., 2013). In the population, there is a substantial genetic vulnerability for increased body weight and increased waist circumference. Increased waist circumference is the established measure of central obesity, that is, excess adipose tissue (Pouliot et al., 1994; Janssen et al., 2004), and high values predispose for metabolic disorders irrespective of weight (Despres and Lemieux, 2006; Romero-Corral et al., 2010; Phillips, 2013). Thus, analysis of the genetic contribution to metabolic disturbances in severe mental illness patients measured by waist circumference might therefore be of substantial clinical interest.
The purpose of this study was to (i) investigate whether common metabolic genetic variants confer a risk for increased waist circumference in patients with schizophrenia spectrum disorders (SSD), and to (ii) investigate the genetic variants linked to SSD, irrespective of waist circumference.
Patients and methods
Ethical approval
Ethical approval was obtained from the Stockholm Regional Ethics Committee separately for patients and controls. All participants gave their informed consent to participate.
Patients from the Swedish study of metabolic risks in psychosis
Patients were recruited from specialized psychosis outpatient clinics, primarily in Stockholm County, Sweden, responsible for treatment of patients with long-term psychotic disorders, especially schizophrenia, between 2005 and 2009. As part of a general medical examination, all patients were asked to participate in the Swedish study of metabolic risks in psychosis (SMRP). Patients received written instructions to fast overnight before venous blood sampling. Fasting glucose, blood pressure, body weight, height, and waist circumference were measured. Patients were asked about tobacco and alcohol use, family history of diabetes, and medications and dosage. Clinical diagnoses were confirmed according to the Diagnostic and Statistical Manual of Mental disorders, Washington DC. American Psychiatric Association, 4th ed. (1994). In the present study of severe mental illness patients in clinical treatment, 658 SSD patients were included, with schizophrenia being the most common diagnosis for 356 (54%) patients, schizoaffective disorder for 68 (10%) patients, delusional disorder for 41 (6%) patients, psychosis not otherwise specified for 88 (14%) patients, bipolar disorder for 40 (6%) patients, and other psychiatric disorders for 65 (10%) patients.
Stockholm Diabetes Prevention Program controls
Control individuals were selected from the Stockholm Diabetes Prevention Program (SDPP) (Eriksson et al., 2008), comprising 7949 participants included from 1992 to 1998. At inclusion, only patients without known diabetes were enrolled and half of the patients had at least 1 first-degree relative with known diabetes. A follow-up was performed 9–10 years later (2002–2006) and included 5712 patients (3329 women and 2383 men) (72% of the original participants). At follow-up, 997 (17%) individuals had increased fasting glucose levels (≥5.6 mmol/l), including 289 (5%) individuals who were diagnosed with type 2 diabetes during the period between inclusion and follow-up. Data were obtained about weight, height, waist circumference, blood pressure, and fasting blood glucose both at inclusion and at follow-up.
Stockholm Diabetes Prevention Program Genetic controls
From the SDPP follow-up sample, 494 controls were selected to represent the total SDPP cohort for the genetic association study. In the control group, 404 (82%) patients had normal fasting glucose levels, 66 (13%) patients had increased fasting glucose levels, 24 (5%) patients were diagnosed with type 2 diabetes, and 185 (37%) had a family history of diabetes. Furthermore, 147 (30%) patients had normal waist circumference levels, and 347 (70%) patients had increased waist circumference (Table 1).
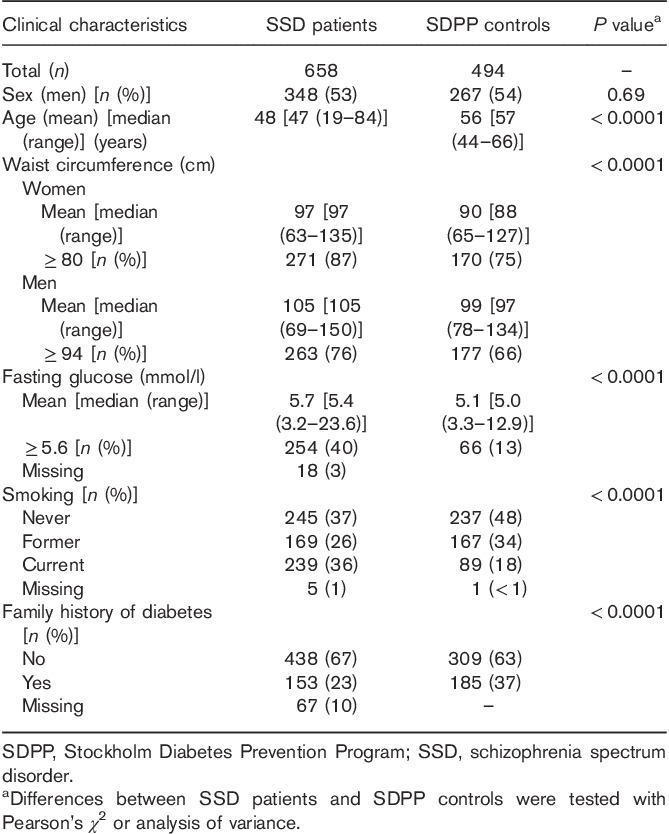
Table 1.
Clinical characteristics of the patients and controls
DNA preparation and genotyping for the patients and Stockholm Diabetes Prevention Program controls
DNA from venous blood was extracted according to standard procedures. Single-nucleotide polymorphisms (SNPs) were genotyped using an Open Array Real-Time PCR System Instrument (Applied Biosystems, Foster City, California, USA). Allelic discrimination was performed using TaqMan Genotype Software (Applied Biosystems). Genotyping success rates for the SNPs were between 85 and 98%.
Genetic variants assessed
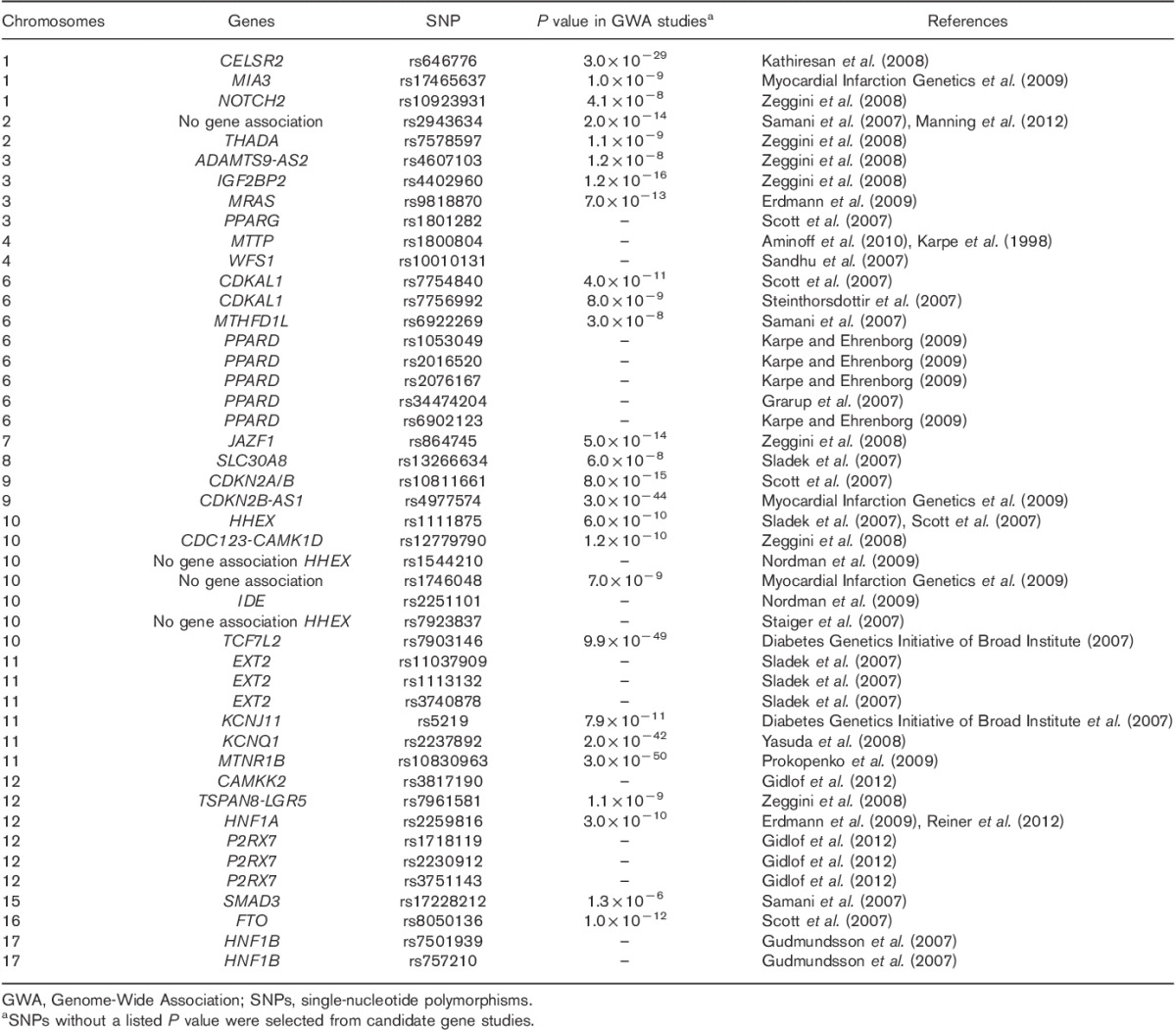
SNPs previously reported to be associated with type 2 diabetes and/or cardiovascular disease were studied. The majority of these SNPs (25 of 46) showed genome-wide significance (P<5×10−8) in Genome-Wide Association (GWA) studies (Table 2). Hardy–Weinberg equilibrium cutoff was P up to 0.05 for controls.
Table 2.
Single-nucleotide polymorphisms studied
Study design and statistical analyses
Central obesity was measured by increased waist circumference. The cutoff level for increased waist circumference (≥80 cm women and ≥94 cm men) was defined according to criteria from the International Diabetes Federation (http://www.idf.org/webdata/docs/Metabolic_syndrome definition.pdf).
Before performing genetic analysis, differences in waist circumference between psychosis diagnoses were analyzed, separately for men and women. First, differences in waist circumference between psychosis diagnoses, for each sex, were tested for significance using analysis of variance in IBM SPSS Statistics 23 (IBM Corporation, Armonk, New York, USA).
Second, patients were analyzed for allelic association to the listed SNPs according to three models: model 1, a case–case model in which SSD patients with increased waist circumference (≥80 cm for women, ≥94 for men) were compared with SSD patients with normal waist circumference (<80 cm women, <94 cm men); model 2, a case–control model in which SSD patients with increased waist circumference were compared with SDPP controls; and model 3, another case–control model in which all SSD patients were compared with SDPP controls. Logistic regression was used in models 1 and 2, adjusted for the continuous variables age and fasting glucose, and the categorical variables family history of diabetes, sex, and smoking. The logistic regression in model 3 was adjusted for the same factors and also the categorical variable waist circumference. For multiple testing correction, false discovery rate Benjamini and Hochberg was used.
Third, to test the effect of clozapine treatment on nominal allelic associations with increased waist circumference, analyses with models 1 and 2 were performed where SSD cases were restricted to patients on clozapine (n=62).
The allelic association analyses were performed using PLINK (Center for Human Genetic Research, Massachusetts General Hospital, Boston, Massachusetts, USA; http://pngu.mgh.harvard.edu/purcell/plink/) (Purcell et al., 2007). The level of nominal significance was set to 5% (two tailed).
Results
Genetic findings
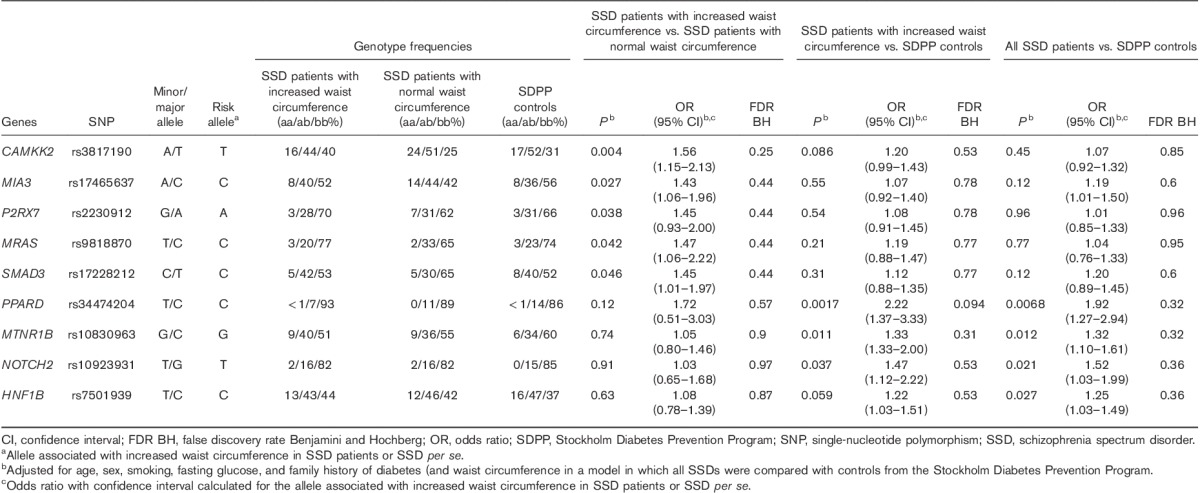
No differences in waist circumference were detected (P>0.05) between psychosis diagnoses. All SNPs except rs864745, rs12779790, rs2251101, and rs2016520 (excluded from analysis) were in Hardy–Weinberg equilibrium (P>0.05). In the case–case design, increased waist circumference was associated with SNPs located within MIA3, MRAS, P2RX7, CAMKK2, and SMAD3, and in the case–control design with SNPs in PPARD, MTNR1B, NOTCH2, and HNF1B (Table 3).
Table 3.
Allelic association between metabolic risk variants and increased waist circumference in schizophrenia spectrum disorder, and schizophrenia spectrum disorder per se
Case–case design
In the case–case analysis, the major allele T of rs3817190 in CAMKK2 [odds ratio (OR): 1.56, P=0.0040), the major allele C of rs17465637 (OR: 1.43, P=0.027) in MIA3, the major allele A of rs2230912 (OR: 1.45, P=0.038) in P2RX7, and the major allele C of rs9818870 (OR: 1.47, P=0.042) in MRAS were nominally associated with increased waist circumference among SSD patients compared with the minor allele. The minor allele C of rs17228212 in SMAD3 (OR: 1.45, P=0.046) was associated with increased waist circumference compared with the major allele.
Case–control design
In the case–control analysis, the major allele C of rs34474204 (OR: 2.22, P=0.0017) in PPARD, the minor allele G of rs10830963 (OR: 1.33, P=0.011) in MNTR1B, and the minor allele T of rs10923931 (OR: 1.47, P=0.037) in NOTCH2 were nominally associated with increased waist circumference among the SSD patients compared with the other allele (Table 2).
Genetic variants associated with schizophrenia spectrum disorder per se (irrespective of waist circumference)
In the allelic analysis of all SSD patients (n=658) compared with controls, the major allele C of rs34474204 (OR: 1.92, P=0.0068) in PPARD, the minor allele G of rs10830963 (OR: 1.32, P=0.012) in MTNR1B, the minor allele T of rs10923931 (OR: 1.52, P=0.021) in NOTCH2, and the major allele C of rs7501939 (OR: 1.25, P=0.027) in HNF1B were associated with SSD patients irrespective of waist circumference. The nine SNPs, with a signal in this study, together as predictors accounted for 1% (adjusted R2: 0.001) of the variance in psychosis per se.
The effect of clozapine treatment on allelic associations with increased waist circumference
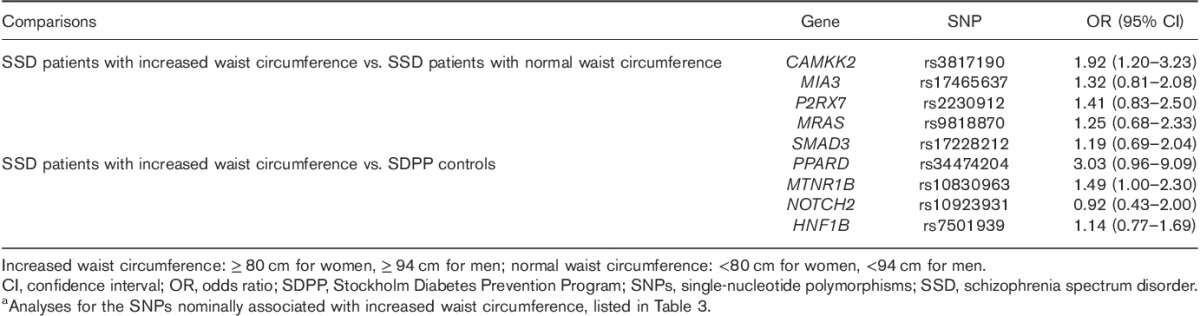
To test the effect of clozapine treatment on nominal allelic associations with increased waist circumference, the ORs were calculated including only psychosis patients on clozapine treatment. The point estimates of ORs were well within the 95% confidence intervals (CIs)of ORs based on patients irrespectively of pharmacotherapy, except for NOTCH2 (Table 4).
Table 4.
Odds ratios for risk alleles in relation to disorder status in schizophrenia spectrum disorder patients on clozapine treatmenta (n=62)
Discussion
Main findings
The findings of this study were that increased waist circumference in patients with SSD was associated with common metabolic genetic variants in MIA3, MRAS, P2RX7, CAMKK2, and SMAD3 when compared with SSD patients with normal waist circumference, and with genetic variants in the PPARD, MNTR1B, and NOTCH2 in comparison with control individuals. The genetic variants in PPARD, MNTR1B, NOTCH2, and HNF1B were nominally associated with SSD per se.
Strengths and limitations
In the present study, we applied both a case–case and a case–control design. A case–case model takes advantage of narrow diagnostic subgroups considered to be more biologically homogeneous, and therefore may imply clinical heterogeneity differences between disease groups (Niculescu and Le-Niculescu, 2010).
At the decade around sampling, there were no solid internal genetic limits in the Swedish population, particularly the southern/middle parts of Sweden (the recruitment areas) were more genetically homogeneous (Lappalainen et al., 2009; Humphreys et al., 2011). The patient sample was recruited from specialized psychosis outpatient clinics. Participation rate was estimated from one clinic: 119 of the 155 (77%) patients participated. There was no difference in BMI between those who participated and those who declined. As waist circumference did not differ between different psychosis diagnoses, SSD patients were analyzed as one group; thus, findings from the present study may be generally applicable to increased waist circumference in SSD patients.
For the genetic association study, SDPP controls were selected in the interest of representing the whole SDPP sample at follow-up. However, at inclusion, only individuals without known diabetes were enrolled, and there was a high frequency of family history of diabetes among them.
Family history of diabetes, age, fasting glucose, and smoking may influence the risk for diabetes mellitus type 2, and we were able to adjust for these differences. As only patients, and no controls, had antipsychotic medication, this was not possible to control for. Instead, we tested the differential effect of clozapine, the antipsychotic drug associated with the greatest weight gain, previously evaluated in our patient sample (Boden et al., 2013). The low statistical power indicates a possible risk that we were not able to detect true genetic associations between the groups that may exist. No associations survived correction for multiple testing using false discovery rate assessment of the entire data set. However, given that each SNP was individually selected on the basis of previously published functionality and association with metabolic disorder, this correction might be regarded as overstringent.
Findings from other studies
Waist circumference is a convenient measure of excess adipose tissue and central obesity (Pouliot et al., 1994; Janssen et al., 2004). Excessive amounts of body adipose tissue predispose to metabolic disorders, irrespective of weight (Despres and Lemieux, 2006; Romero-Corral et al., 2010; Phillips, 2013). Persons with a family history of diabetes generally have an increased storage of fat and risk for obesity, as well as a decreased beta cell function, compared with persons without family history of diabetes (Hilding et al., 2006; Isomaa et al., 2010), which is in agreement with a genetic component in metabolic disorders. Similar metabolic disturbances have been observed in psychosis patients, although antipsychotics have been associated with weight gain. An increased prevalence of diabetes mellitus type 2 has been reported in first-episode drug-naive psychosis patients (Ryan et al., 2003), and unaffected first-degree relatives of people with schizophrenia (Fernandez-Egea et al., 2008). Psychotic disorder per se increases the risk for elevated waist circumference and fasting glucose (Osby et al., 2013).
In this report, metabolic gene variants known to increase the risk for metabolic disorders in the population also seem to confer metabolic risk in SSD patients. The genetic variants rs17465637 in MIA3, rs9818870 in MRAS, and rs17228212 in SMAD3 have been associated with coronary artery disease in GWA studies (P<5×10−8), although explaining only a small proportion of the coronary artery disease risk (Samani et al., 2007; Erdmann et al., 2009). However, for rs9818870 in MRAS we identified the other allele as a risk allele among SSD patients, in contrast to previous findings. The MRAS gene encodes a protein that functions as a signal transducer in, for example, cell growth and differentiation. SMAD3 and MIA3 are important for vascular stability (Itoh et al., 2012) and angiogenesis (Bosserhoff and Buettner, 2002), fundamental processes for plaque development and atherosclerosis, although the mechanisms are not fully known. SMAD3 mediates transcription activity downstream of TGFB and MIA3 encodes a translation factor. The P2RX7 gene encodes an ATP-binding receptor calcium channel protein that mediates apoptosis, and its activation may also lead to changes in gene expression. CAMKK2 encodes a protein kinase that responds to increased intracellular calcium, and one of its many functions is to regulate the production of the appetite-stimulating hormone neuropeptide Y. P2RX7 and CAMKK2 have been associated with a decreased risk of cardiovascular events (Gidlof et al., 2012). In addition to metabolic disturbances, P2RX7 has also been implicated in psychiatric disorders (Backlund et al., 2012). The reported risk alleles of genetic variants rs10830963 in MTNR1B and rs10923931 in NOTCH2 were associated with diabetes mellitus type 2-related traits in GWA studies (P<5×10−8) (Zeggini et al., 2008; Prokopenko et al., 2009). We here suggest that the same risk alleles are associated with diabetes mellitus type 2-related traits among SSD patients. MNTR1B encodes a G-protein-coupled membrane protein whose variants are well known to affect fasting glucose levels. β-Cells from diabetic patients, similar to nondiabetic individuals, carrying the risk allele G of the rs10830963 have increased MTNR1B receptor expression, supported by altered insulin release in the presence of melatonin (Lyssenko et al., 2009). Recently, we reported an association for the rs10830963 allele G (OR: 1.51, 95% CI: 1.16–1.89; P=0.0039) and rs10923931 allele T (OR: 1.84, 95% CI: 1.13–2.46; P=0.011) to increased fasting glucose levels in SSD patients (Hukic et al., 2015). In addition to metabolic traits, Notch signaling has been shown to be important for neurogenesis in adult brain (Ables et al., 2011), and has been implicated in subphenotypes of psychiatric disorders (Prox et al., 2013; Monsalve et al., 2014). The NOTCH2 gene encodes a transmembrane protein that regulates interactions between physically adjacent cells. Common genetic variants in PPARD in relation to metabolic traits have generated conflicting results (Grarup et al., 2007). However, PPARD has been associated with glucose metabolism and function (Kramer et al., 2005; Karpe and Ehrenborg, 2009). The PPARD gene encodes a nuclear hormone receptor that may function as an integrator of transcription repression and nuclear receptor signaling. Changes in expression of the PPARD gene have been reported in newly diagnosed diabetics (Stoynev et al., 2014). The HNF1B gene encodes transcription factor 2, a liver-specific factor of the homeobox-containing basic helix-turn-helix family that may activate or inhibit transcription of target genes. Although it has primarily been associated with prostate cancer, mutations in this gene have been identified as the cause of maturity-onset of diabetes type 5. Here we report an association with schizophrenia per se.
The effect of clozapine treatment on the allelic associations with increased waist circumference
Treatment with clozapine is known to be associated with prominent weight gain. To explore the effect of clozapine on our nominal allelic associations reported here, analyses restricted to patients on clozapine were performed. These analyses resulted in pointwise ORs within the 95% CIs for all markers and model designs, except for NOTCH2, suggesting that the effect sizes of clozapine on observed genetic associations with increased waist circumference were limited, although NOTCH2 pointed the contrary, suggesting the other allele as a risk allele for SSD patients on clozapine treatment. Analysis of larger samples, as well as in drug-naive samples, is warranted. The severity of weight gain during treatment is also correlated with the initial weight; however, this is a cross-sectional patient group in which weight gain associated with specific antipsychotic drugs was not possible to measure.
The findings of the present study of associations between genes conferring increased metabolic risk and increased waist circumference in patients with SSD indicate that increased waist circumference in those patients may be explained, in part, by an increased genetic vulnerability for metabolic risk genes, and indicates a shared genetic susceptibility to metabolic disorder and psychosis per se. All findings indicating factors that increase the metabolic risk, for example, increasing waist circumference, in SSD patients might be of clinical interest, as increased morbidity and mortality from cardiovascular disorders is the main cause of the reduced longevity of patients with psychosis.
Our results might propose shared genetic susceptibility to metabolic disorder and psychosis per se, but replication using a polygenic risk scoring approach in GWAS data, by using SNPs linked to metabolic disturbances on a psychosis versus healthy controls data set, is required for verification.
Acknowledgements
The authors thank all the patients who participated in this study. The authors also thank research assistant Carina Schmidt for skillful assistance in managing data and biological samples.
The Swedish Study of Metabolic Risks in Psychosis (SMRP) was supported by ALF Grants 20060100 (U.Ö.), 20080022 (UÖ), 20100035 (U.Ö.), 20120262 (U.Ö.), 20150283 (U.Ö.), and 20110560 (C.L.) from Stockholm County Council and Karolinska Institutet (http://www.forskningsstod.sll.se/Ansokan/start.asp); grants from Söderström-Königska Hospital 2005 (U.Ö.), 2006 (U.Ö.), 2008 (U.Ö.) (http://www.sls.se/Forskning--utbildning/Forskningsdelegationen--Prioriteringskommitten-/Stiftelsen-Soderstrom-Konigska-sjukhemmet/); The Regional Drug and Therapeutic Committee in Stockholm (Läksak) 2004 (U.Ö.) (http://www.janusinfo.se/Global/In_English/towards%20a%20wiser%20use%20of%20drugs.pdf); the Swedish Research Council (grant 2010-3631) (C.L.) (http://vr.se).
The Stockholm Diabetes Prevention Program (SDPP) was supported by grants from the Swedish Research Council, Dnr 521-2010-3516 (C.-G.Ö.) (http://www.vr.se); The Swedish Council for Working Life and Social Research, Dnr 2008-0375 (C.-G.Ö.) (http://www.forma.se, previously http://www.fas.se); The Swedish Diabetes Association, Dnr DIA2009-061 (C.-G.Ö.) (http://www.diabetes.se); and Novo Nordisk Scandinavia (C.-G.Ö.), unrestricted grants to support SDPP (http://www.novonordisk.se).
Dr. Urban Ösby has research collaboration with Lundbeck A/S, and Dr. Eric Olsson was supported by a grant from PRIMA Child and Adult Psychiatry Inc.
Conflicts of interest
There are no conflicts of interest.
References
- Ables JL, Breunig JJ, Eisch AJ, Rakic P. (2011). Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci 12:269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff A, Ledmyr H, Thulin P, Lundell K, Nunez L, Strandhagen E, et al. (2010). Allele-specific regulation of MTTP expression influences the risk of ischemic heart disease. J Lipid Res 51:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund L, Lavebratt C, Frisen L, Nikamo P, Hukic Sudic D, Traskman-Bendz L, et al. (2012). P2RX7: expression responds to sleep deprivation and associates with rapid cycling in bipolar disorder type 1. PLoS One 7:e43057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden R, Edman G, Reutfors J, Ostenson CG, Osby U. (2013). A comparison of cardiovascular risk factors for ten antipsychotic drugs in clinical practice. Neuropsychiatr Dis Treat 9:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosserhoff AK, Buettner R. (2002). Expression, function and clinical relevance of MIA (melanoma inhibitory activity). Histol Histopathol 17:289–300. [DOI] [PubMed] [Google Scholar]
- Despres JP, Lemieux I. (2006). Abdominal obesity and metabolic syndrome. Nature 444:881–887. [DOI] [PubMed] [Google Scholar]
- Diabetes Genetics Initiative of Broad Institute of, H, Novartis Institutes of Biomedical R Mit LU, Saxena R, Voight BF, Lyssenko V, Burtt NP, De Bakker PI, et al. (2007). Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336. [DOI] [PubMed] [Google Scholar]
- Erdmann J, Grosshennig A, Braund PS, Konig IR, Hengstenberg C, Hall AS, et al. (2009). New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat Genet 41:280–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson AK, Ekbom A, Granath F, Hilding A, Efendic S, Ostenson CG. (2008). Psychological distress and risk of pre-diabetes and Type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabet Med 25:834–842. [DOI] [PubMed] [Google Scholar]
- Fernandez-Egea E, Miller B, Bernardo M, Donner T, Kirkpatrick B. (2008). Parental history of type 2 diabetes in patients with nonaffective psychosis. Schizophr Res 98:302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidlof O, Smith JG, Melander O, Lovkvist H, Hedblad B, Engstrom G, et al. (2012). A common missense variant in the ATP receptor P2X7 is associated with reduced risk of cardiovascular events. PLoS One 7:e37491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothefors D, Adolfsson R, Attvall S, Erlinge D, Jarbin H, Lindstrom K, et al. (2010). Swedish clinical guidelines – prevention and management of metabolic risk in patients with severe psychiatric disorders. Nord J Psychiatry 64:294–302. [DOI] [PubMed] [Google Scholar]
- Grarup N, Albrechtsen A, Ek J, Borch-Johnsen K, Jorgensen T, Schmitz O, et al. (2007). Variation in the peroxisome proliferator-activated receptor delta gene in relation to common metabolic traits in 7495 middle-aged white people. Diabetologia 50:1201–1208. [DOI] [PubMed] [Google Scholar]
- Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, et al. (2007). Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet 39:977–983. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. (2005). Schizophrenia and increased risks of cardiovascular disease. Am Heart J 150:1115–1121. [DOI] [PubMed] [Google Scholar]
- Hilding A, Eriksson AK, Agardh EE, Grill V, Ahlbom A, Efendic S, Ostenson CG. (2006). The impact of family history of diabetes and lifestyle factors on abnormal glucose regulation in middle-aged Swedish men and women. Diabetologia 49:2589–2598. [DOI] [PubMed] [Google Scholar]
- Hukic DS, Olsson E, Hilding A, Ostenson CG, Gu HF, Ehrenborg E, et al. (2015). Genes associated with increased fasting glucose in patients with schizophrenia spectrum disorders. Diabetes and Metabolism 6:3. [Google Scholar]
- Humphreys K, Grankvist A, Leu M, Hall P, Liu J, Ripatti S, et al. (2011). The genetic structure of the Swedish population. PLoS One 6:e22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomaa B, Forsen B, Lahti K, Holmstrom N, Waden J, Matintupa O, et al. (2010). A family history of diabetes is associated with reduced physical fitness in the Prevalence, Prediction and Prevention of Diabetes (PPP)-Botnia study. Diabetologia 53:1709–1713. [DOI] [PubMed] [Google Scholar]
- Itoh F, Itoh S, Adachi T, Ichikawa K, Matsumura Y, Takagi T, et al. (2012). Smad2/Smad3 in endothelium is indispensable for vascular stability via S1PR1 and N-cadherin expressions. Blood 119:5320–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Ross R. (2004). Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 79:379–384. [DOI] [PubMed] [Google Scholar]
- Karpe F, Ehrenborg EE. (2009). PPARdelta in humans: genetic and pharmacological evidence for a significant metabolic function. Curr Opin Lipidol 20:333–336. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, et al. (2008). Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet 40:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DK, Al-Khalili L, Perrini S, Skogsberg J, Wretenberg P, Kannisto K, et al. (2005). Direct activation of glucose transport in primary human myotubes after activation of peroxisome proliferator-activated receptor delta. Diabetes 54:1157–1163. [DOI] [PubMed] [Google Scholar]
- Lappalainen T, Hannelius U, Salmela E, Von Dobeln U, Lindgren CM, Huoponen K, et al. (2009). Population structure in contemporary Sweden – a Y-chromosomal and mitochondrial DNA analysis. Ann Hum Genet 73:61–73. [DOI] [PubMed] [Google Scholar]
- Laursen TM, Wahlbeck K, Hallgren J, Westman J, Osby U, Alinaghizadeh H, et al. (2013). Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One 8:e67133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, et al. (2009). Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 41:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, et al. (2012). A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 44:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsalve EM, Garcia-Gutierrez MS, Navarrete F, Giner S, Laborda J, Manzanares J. (2014). Abnormal expression pattern of Notch receptors, ligands, and downstream effectors in the dorsolateral prefrontal cortex and amygdala of suicidal victims. Mol Neurobiol 49:957–965. [DOI] [PubMed] [Google Scholar]
- Myocardial Infarction Genetics, C, Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, et al. (2009). Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet 41:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu AB, Le-Niculescu H. (2010). The P-value illusion: how to improve (psychiatric) genetic studies. Am J Med Genet B Neuropsychiatr Genet 153B:847–849. [DOI] [PubMed] [Google Scholar]
- Nordman S, Ostenson CG, Efendic S, Gu HF. (2009). Loci of TCF7L2, HHEX and IDE on chromosome 10q and the susceptibility of their genetic polymorphisms to type 2 diabetes. Exp Clin Endocrinol Diabetes 117:186–190. [DOI] [PubMed] [Google Scholar]
- Nordentoft M, Wahlbeck K, Hallgren J, Westman J, Osby U, Alinaghizadeh H, et al. (2013). Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS One 8:e55176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osby U, Correia N, Brandt L, Ekbom A, Sparen P. (2000). Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res 45:21–28. [DOI] [PubMed] [Google Scholar]
- Osby U, Olsson E, Edman G, Hilding A, Eriksson SV, Ostenson CG. (2013). Psychotic disorder is an independent risk factor for increased fasting glucose and waist circumference. Nord J Psychiatry 68:251–258. [DOI] [PubMed] [Google Scholar]
- Phillips CM. (2013). Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord 14:219–227. [DOI] [PubMed] [Google Scholar]
- Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. (1994). Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol 73:460–468. [DOI] [PubMed] [Google Scholar]
- Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, et al. (2009). Variants in MTNR1B influence fasting glucose levels. Nat Genet 41:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prox J, Bernreuther C, Altmeppen H, Grendel J, Glatzel M, D’Hooge R, et al. (2013). Postnatal disruption of the disintegrin/metalloproteinase ADAM10 in brain causes epileptic seizures, learning deficits, altered spine morphology, and defective synaptic functions. J Neurosci 33:12915–12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. (2010). Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 31:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Collins P, Thakore JH. (2003). Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry 160:284–289. [DOI] [PubMed] [Google Scholar]
- Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, et al. (2007). Genomewide association analysis of coronary artery disease. N Engl J Med 357:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu MS, Weedon MN, Fawcett KA, Wasson J, Debenham SL, Daly A, et al. (2007). Common variants in WFS1 confer risk of type 2 diabetes. Nat Genet 39:951–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, et al. (2007). A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316:1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, et al. (2007). A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885. [DOI] [PubMed] [Google Scholar]
- Staiger H, Machicao F, Stefan N, Tschritter O, Thamer C, Kantartzis K, et al. (2007). Polymorphisms within novel risk loci for type 2 diabetes determine beta-cell function. PLoS One 2:e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, et al. (2007). A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 39:770–775. [DOI] [PubMed] [Google Scholar]
- Stoynev N, Dimova I, Rukova B, Hadjidekova S, Nikolova D, Toncheva D, Tankova T. (2014). Gene expression in peripheral blood of patients with hypertension and patients with type 2 diabetes. J Cardiovasc Med (Hagerstown) 15:702–709. [DOI] [PubMed] [Google Scholar]
- Thakore JH, Mann JN, Vlahos I, Martin A, Reznek R. (2002). Increased visceral fat distribution in drug-naive and drug-free patients with schizophrenia. Int J Obes Relat Metab Disord 26:137–141. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, et al. (2008). Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet 40:1092–1097. [DOI] [PubMed] [Google Scholar]
- Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, et al. (2008). Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]


